Abstract
Organophosphate (OP) compounds which include nerve agents and pesticides are considered chemical threat agents. Currently approved antidotes are crucial in limiting OP mediated acute mortality. However, survivors of lethal OP exposure exhibit delayed neuronal injury and chronic behavioral morbidities. In this study, we investigated neuroprotective capabilities of dantrolene and carisbamate in a rat survival model of paraoxon (POX) induced status epilepticus (SE). Significant elevations in hippocampal calcium levels were observed 48-h post POX SE survival, and treatment with dantrolene (10 mg/kg, i.m.) and carisbamate (90 mg/kg, i.m.) lowered these protracted calcium elevations. POX SE induced delayed neuronal injury as characterized by Fluoro Jade C labeling was observed in critical brain areas including the dentate gyrus, parietal cortex, amygdala, and thalamus. Dantrolene and carisbamate treatment provided significant neuroprotection against delayed neuronal damage in these brain regions when administered one-hour after POX-SE. These results indicate that dantrolene or carisbamate could be effective adjuvant therapies to the existing countermeasures to reduce neuronal injury and behavioral morbidities post OP SE survival.
Keywords: Paraoxon, Status Epilepticus, Cell death, Calcium, Dantrolene, Carisbamate
Introduction
Organophosphate (OP) compounds are classified as lethal chemicals that include nerve gas and pesticides. Both the civilian and military population has been exposed to nerve agents under acts of war and terrorism [1–3]. In addition, civilians are also exposed to OP compounds occupationally, intentionally (suicide) or due to accidents [4–6]. Paraoxon (POX) is an active metabolite of parathion and is used in laboratory research to reliably model OP pesticide toxicity [7]. POX and other OP chemicals are potent inhibitors of the enzyme acetylcholine esterase (AChE) [8]. Inhibition of AChE prevents breakdown of acetylcholine (ACh) and rapidly builds up its level at the synapses. Overt stimulation of ACh receptors leads to the classical “cholinergic crisis” followed by respiratory depression, bradycardia and status epilepticus (SE). This prolonged seizure activity represents a clinical emergency and if left untreated results in the death [9]. The current FDA approved OP treatment protocol involves administration of an anticholinergic drug atropine to manage hypercholinergic symptoms, an oxime pralidoxime to reactivate AChE, and a benzodiazepine midazolam to stop SE [10]. Despite the effectiveness of the standard three-drug regimen in limiting immediate mortality following OP exposure, OP/ SE survivors are vulnerable to the development of chronic neurological morbidities [11–17].
Our laboratory has developed SE survival models of OP toxicity using POX [7,12] and DFP [18]. The mortality, behavioral manifestations and EEG profile for these OP SE models mimicked the signs and symptoms of human OP intoxication. Significant neuronal damage was observed throughout the limbic system in the brain of OP SE rats [7,18]. Subsequently, symptoms of chronic depression and memory impairments were also observed in these lethal OP exposed rats [7,12]. These models provide a reproducible method to mimic the human survival of OP toxicity and are useful to screen novel medical countermeasures and also identify molecular mechanisms underlying the mortality and morbidity following OP intoxication.
One of the long standing interest of our laboratory has been studying role of Ca2+ homeostatic mechanisms following brain injuries [19]. Ca2+ ions are major second messenger molecules and they participate in a variety of signaling cascades critical for learning, memory, neuronal injury and other vital cellular functions. We have demonstrated significant elevations in neuronal calcium levels ([Ca2+]i) that lasted for weeks following the termination of brain injuries, such as SE, stroke or TBI. These protracted elevations in [Ca2+]i known as the Ca2+ plateau [7,18,20–22] could underlie the neuronal injury and the chronic neurological morbidities following survival from OP SE. Interestingly, Ca2+ influx from NMDA receptors was required for induction of this Ca2+ plateau [18], but the protracted Ca2+ elevations were mediated by intracellular Ca2+ induced Ca2+ release (CICR) from endoplasmic reticulum. Indeed, we have recently shown that treatments with the CICR antagonist dantrolene, and carisbamate, but not the NMDA antagonist MK-801 lowered OP-SE mediated Ca2+ elevations [7,21]. In this study, we investigated whether inhibition of this Ca2+ plateau with dantrolene and carisbamate could provide neuroprotection when administered after the termination of OP SE. Given the role of Ca2+ signaling in modulating behavior and cell death mechanisms [23,24], preventing the development of Ca2+ plateau could be a critical target to improve outcome following lethal OP exposure.
Materials and methods
Animals
All animal use procedures were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by VCU’s Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing ~250–280 g and 10-weeks age were housed two per cage at 20–22°C with a 12 Light: 12 Dark hour cycle and free access to food and water.
Chemicals
All the chemicals were obtained from Sigma Aldrich Company (St. Louis, MO). POX was prepared by dissolving in ice-cold phosphate buffered saline, while atropine sulfate and pralidoxime chloride (2-PAM) were dissolved in saline (0.9% NaCl). Midazolam was obtained from VCU Health System Pharmacy. Carisbamate (RWJ 333369) was a gift from Johnson & Johnson PRD (Titusville, NJ, USA), and was suspended in 40% polyethylene glycol and 30% ethanol solution. Dantrolene solution (10 mg/mL) was prepared in saline and the solution was subjected to 10-min of sonication just before the injection.
Acute Isolation of Hippocampal CA1 Neurons and Loading with Fura-2
Acute isolation of CA1 hippocampal neurons was performed by established procedures [7,18,20]. Briefly, 48-h following POX SE, rats were anesthetized with isoflurane and decapitated. Brains were rapidly dissected and placed in 4°C oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid (aCSF) consisting of (in mM): 201.5 sucrose, 10 glucose, 1.25 NaH2PO4, 26 NaHCO3, 3 KCl, 7 MgCl2, and 0.2 CaCl2). MK-801 (1 µM) was added to all solutions to increase cell viability and was removed 15 min prior to imaging. Hippocampal slices (450 µm) were cut on a vibrating microtome (Leica Microsystems, Wetzlar, Germany) and then equilibrated for 10 min at 34°C in a piperazine-N,N'-bis[2-ethanesulfonic acid] (PIPES)-aCSF solution containing (in mM): 120 NaCl, 25 glucose, 20 PIPES, 5 KCl, 7 MgCl2, and 0.1 CaCl2. Slices were then treated with mg/ml protease in PIPES-aCSF for 6 min at 34°C and rinsed. Enzyme treated slices were visualized on a dissecting microscope to excise the CA1 hippocampal layer which was then triturated with a series of Pasteur pipettes of decreasing diameter in cold (4°C) PIPES-aCSF solution containing 1 µM Fura-2 AM (Invitrogen, Carlsbad, CA). The cell suspension was placed in the middle of 2 well glass-bottomed chambers (Nunc, Thermo Scientific). These glass chambers were previously treated overnight with 0.05 mg/ml poly-L-lysine followed by multiple rinses with distilled water and then further treated with Cell-Tak™ (BD-Biosciences, San Jose, CA) biocompatible cellular adhesive (3.5 µg/cm2) for 30-min, rinsed and air-dried. Neuronal suspension placed in the center of adhesive coated dishes when settled firmly adhered to the bottom. This technique simplified further manipulations on the dissociated neurons. Plates were then incubated at 37°C in a 5% CO2/95% air atmosphere for 45 min. Fura-2 was washed off with PIPES-aCSF and plates were incubated an additional 15 min to allow for complete cleavage of the AM moiety from Fura-2. Plates were then incubated at 37°C in a 5% CO2/95% air atmosphere for 45 min. Fura-2 was washed off with PIPES-aCSF and plates were incubated an additional 15 min to allow for complete cleavage of the AM moiety from Fura-2.
Measurement of [Ca2+]i
Following the incubation period, Fura-2 loaded cells were transferred to a 37°C heated stage (Harvard Apparatus, Hollington, MA) on an Olympus IX-70 inverted microscope coupled to a fluorescence imaging system for [Ca2+]i measurements [18,20]. All experiments were performed using a 20×, 0.7 N.A. water immersion objective and images were recorded by an ORCA-ER high-speed digital CCD camera (Hammamatsu Photonics K.K., Japan). Fura-2 was excited with a 75 W xenon arc lamp (Olympus America, Center Valley, PA). Ratio images were acquired by alternating excitation wavelengths (340/380 nm) by using a Lambda 10-2 filter wheel (Sutter Instruments Co., Novato, CA) and a Fura filter cube at 510/540 emission with a dichroic at 400 nm. All image acquisition and processing was controlled by a computer connected to the camera and filter wheel using Metafluor Software ver 7.6 (MDS Analytical Technologies, Downington, PA). Image pairs were captured every 5 s and the images at each wavelength were averaged over 10 frames. Background fluorescence was obtained by imaging a field lacking Fura-2. Hippocampal CA1 neurons were identified based on their distinct morphology. These neurons displayed pyramidal shaped cell body, long axon and dendrites. The process of enzymatic treatment and mechanical trituration can add minimal stress during acute dissociation of neurons. However, neurons isolated using these procedures exhibit electrophysiological properties identical neurons in slices or in cultures are viable, and not apoptotic or necrotic.
Calcium calibration
We performed Ca2+ calibration determinations as described previously [18,20] to provide estimates of [Ca2+]i concentrations from the 340/380 ratio values. A Ca2+ calibration curve was constructed using solutions of calibrated Ca2+ buffers ranging from 0 Ca2+ (Ca2+ free) to 39 µM Ca2+ (Invitrogen, Carlsbad, CA). Values from the calibration curve were used to convert fluorescent ratios to [Ca2+]i. Final [Ca2+]i were calculated from the background corrected 340/380 ratios using the equation [25]:
where R is the 340/380 ratio at any time; Rmax is the maximum measured ratio in saturating Ca2+ solution (39 µM free Ca2+); Rmin is the minimal measured ratio Ca2+ free solution; Sf2 is the absolute value of the corrected 380-nm signal at Rmin; Sb2 is the absolute value of the corrected 380-nm signal at Rmax; the Kd value for Fura 2 is 224 nM.
POX induced SE
One minute following POX injection (2 mg/kg, s.c.) animals received human-dose equivalents of 2-PAM (25 mg/kg, i.m.) and atropine (0.5 mg/kg, i.m.). Within 5–7 minutes following POX administration, rats displayed overt cholinergic symptoms and rapidly underwent SE-like activity. One hour following onset of POX SE, animals were injected with midazolam (2 mg/kg, i.m.) to terminate seizures. For neuroprotection studies, dantrolene (10 mg/kg, i.m.) or carisbamate (90 mg/kg, i.m.) or appropriate vehicle was injected at 1-h and 6-h following onset of SE and then at 8:00 AM/ 6:00 PM the next day.
Fluoro-Jade staining
Animals were sacrificed 48-h following POX exposure. Briefly, deep anesthesia was induced in rats with ketamine/xylazine (75mg/kg/7.5mg/kg i.p.) mixture. Anesthetized animals were flushed transcardially with saline and perfused with 4% paraformaldehyde in a 100 mM sodium phosphate buffer (pH 7.4). Fixed brains were removed and post-fixed in 4% paraformaldehyde/phosphate buffer overnight, cryoprotected in 30% sucrose/phosphate buffer (pH 7.4) (48 h), flash frozen in isopentane and stored at −80°C until used for sectioning. Coronal sections (40 µm) were cut on a cryostat (Leica Microsystems, Wetzlar, Germany) and mounted onto microscope slides (Trubond 380; Tru Scientific LLC, Bellingham, WA). Slides were dried in a desiccant chamber at 55°C for 30 min prior to staining. Slides were first incubated in a solution of 1% NaOH in 80% ethanol for 5 minutes followed by hydration in a 70% ethanol and then ddH2O for 2 minutes each. Slides were then incubated in a 0.06% KMnO4 solution for 10 min followed by washing in ddH2O for 2 min. Slides were then stained in a 0.0004% Fluoro-Jade C (FJC) solution in 0.1% acetic acid for 20 min [7,26]. Stained slides underwent 3× washes in ddH2O for 2 min each and then dried in a desiccant chamber at 55°C for 30 min. Stained slides were then cleared with xylene for 5 min and cover slipped with DPX mounting agent. Stained sections were evaluated with a fluorescent inverted microscope with a 20× (UApo 340, 0.7 n.a., water) objective and excitation/emission filters for visualization of FITC. Greyscale digital images (1324×1024, 16-bit, 1X1 binning) of FJC staining for select brain regions were acquired.
Data analysis
For comparison of [Ca2+]i between control, POX-SE and POX + drug treated animals, One Way Analysis of Variance (ANOVA) was applied followed by the post-hoc Tukey test. Statistical tests were run and graphs generated with SigmaPlot 12.5 (SPSS Inc, Chicago, IL). p<0.05 was considered significant. Analysis of digital images to count FJC positive cell staining was carried out with Image-J (NIH, Bethesda, MD) by thresholding for specific stain and obtaining positive cell counts using the particle analysis component (size range in pixel: 25–1000). Digital acquisition and staining analysis parameters remained constant throughout.
Results
Blockade of the calcium plateau following POX induced SE
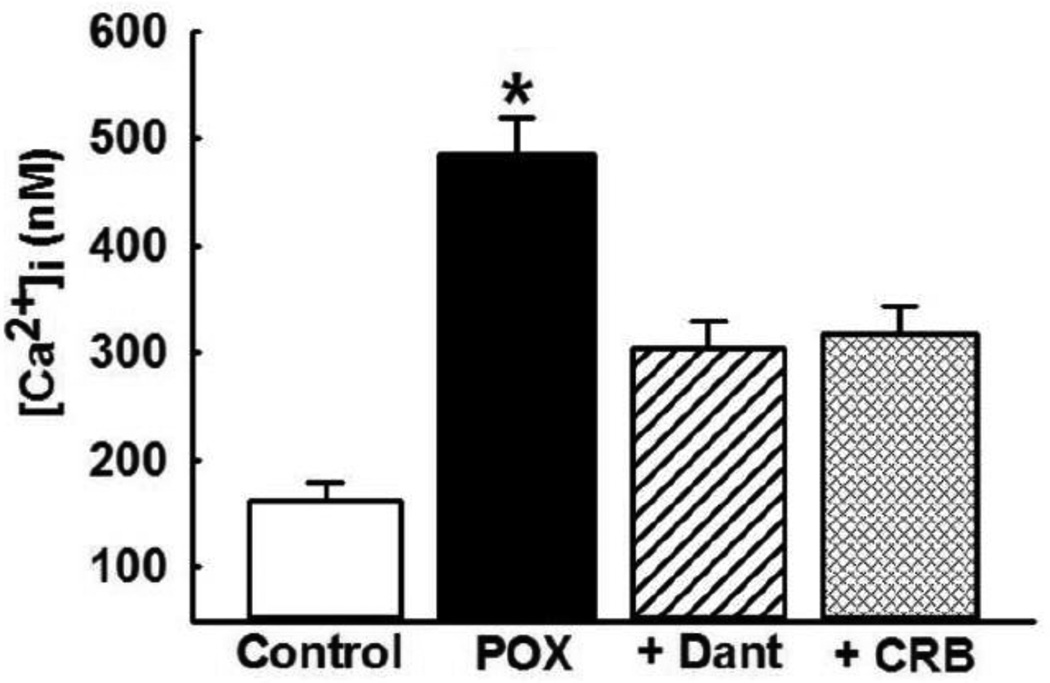
We have previously shown that hippocampal [Ca2+]i levels increased 1-h post POX SE and they remained elevated when evaluated at 1-d, 1-week, and 1-month post SE [7]. In this study, to coincide with cell-death estimation following dantrolene and carisbamate administration, we determined [Ca2+]i at 48-h post POX SE. As shown in Fig. 1, at 2-days following SE termination, [Ca2+]i was 485 ± 35 nM which was significantly higher than [Ca2+]i in neurons harvested from age-matched control rats. To investigate the effects of dantrolene and carisbamate treatment regimens (materials and methods) on [Ca2+]i, levels hippocampal neurons were isolated from rats treated with these drugs. As shown in Fig. 1, [Ca2+]i in hippocampal neurons isolated from rats treated with dantrolene (10 mg/kg, i.m) were 304 ± 24 nM, while [Ca2+]i in hippocampal neurons isolated from rats treated with carisbamate (90 mg/kg, i.m.) were 318 ± 26 nM. These values were significantly lower than [Ca2+]i in neurons isolated from untreated POX SE rats (p<0.05, one-way ANOVA, n= 6 rats per treatment).
Figure 1. Delayed [Ca2+]i elevations following POX SE.
Hippocampal intra neuronal CA1 [Ca2+]i from control (white bar) and POX rats (black bar) were isolated 48-h after SE. [Ca2+]i in neurons isolated from POX-SE rats were significantly higher than control values. CA1 neurons were isolated 30-mins post the last drug injection with either Dantrolene (Dant, 10 mg/kg, i.m.) or carisbamate (CRB, 90 mg/kg, i.m.) [Ca2+]i in neurons isolated from POX-SE rats treated with either DANT or CRB were significantly lower than comparable POX SE rats (no drugs) values at the respective time point. (*p<0.05, compared to POX, one-way ANOVA, post-hoc Tukey test, n= 6 rats/ treatment). Data represented as mean ± SEM.
Multifocal neuronal injury following POX induced SE
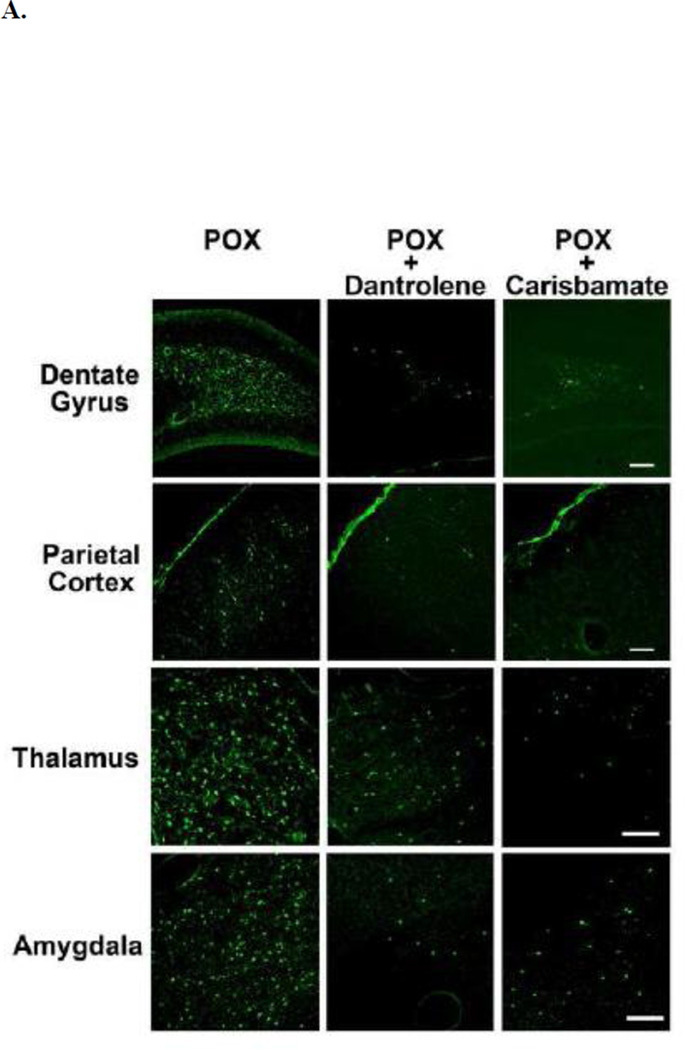
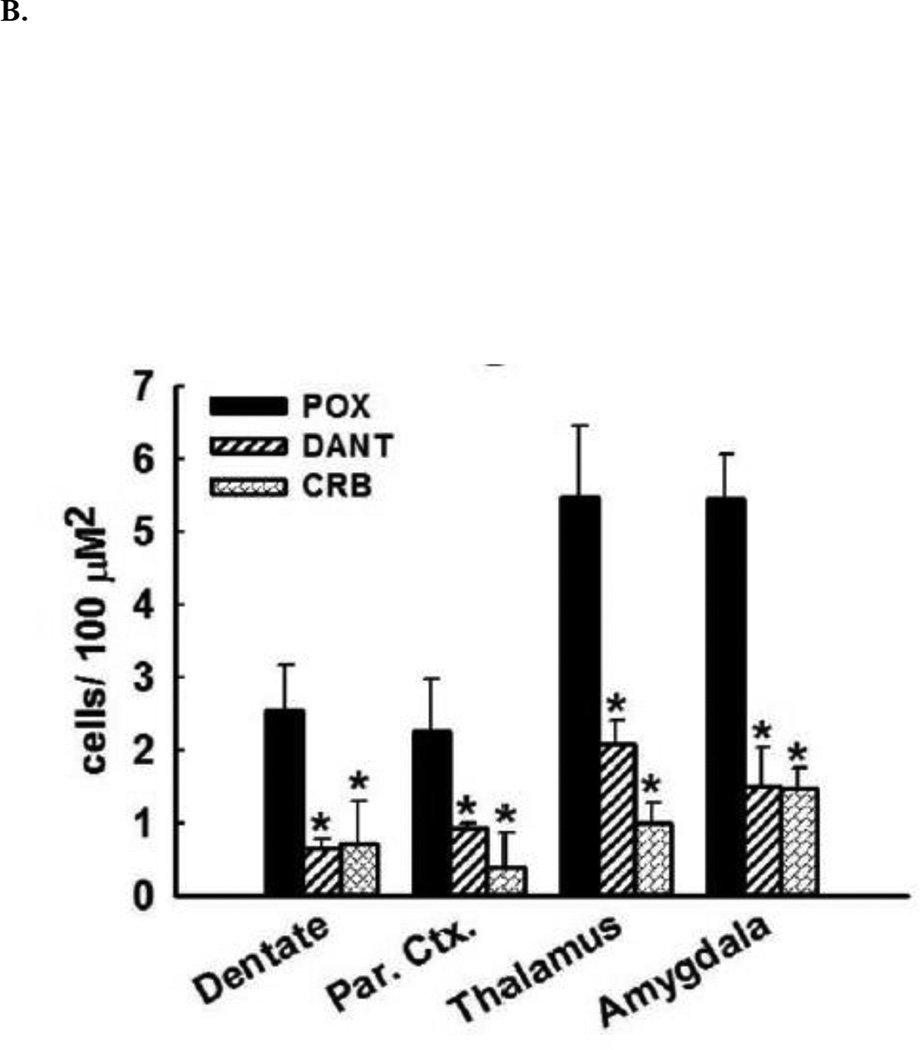
Across all brain regions examined, there was negligible FJC labeling in brain sections obtained from vehicle controls. In contrast, after POX treatment, FJC-positive cells were observed in select regions throughout the forebrain. Within the hippocampus, FJC-positive staining was observed in the polymorphic layer and along the hilus/granule cell border of the dentate gyrus. FJC-positive stained neurons were observed throughout layers II and III of the parietal cortex. Additionally, FJC-positive cells were also observed in amygdala and thalamic nuclear regions (Fig. 2A). The quantification of the neuronal injury expressed as FJC positive cells is shown in Fig. 2B. POX SE rat brains showed FJC positive cells in brain sections obtained from both sides of brain hemispheres.
Figure 2. Neuroprotective effects of dantrolene and carisbamate following POX SE.
A. Representative photomicrographs of Fluoro-Jade C (FJC) staining in the dentate gyrus-hilus region, parietal cortex, amygdala, and thalamus of a POX rat 2 days after POX SE, and POX + dantrolene, and + carisbamate treated rats. Scale bars, 200 µm.
B. Quantitative analyses of FJC labeling. Control rats did not exhibit any FJC labeling. FJC positive cells indicative of neuronal injury were observed in hilus, amygdala, thalamus and cortex of POX rats 48-h after SE termination. Rats treated with dantrolene or carisbamate showed significantly less FJC labeling in these brain regions (*p<0.05 compared to control, t-test, n= 6 rats).
Neuroprotective effects of dantrolene and carisbamate following POX induced SE
Treatments with either dantrolene or carisbamate (material and methods) that blocked or reduced the Ca2+ plateau (Fig. 1) significantly inhibited POX-induced neuronal injury in the hilar region of dentate gyrus as shown in representative photomicrographs of FJC labeling (Fig. 2A). Dantrolene or carisbamate administered 60-mins post POX SE resulted in approximately 75% reduction in FJC labeling in the dentate gyrus. In the parietal cortex, dantrolene produced approximately 60% neuroprotection, while carisbamate treatment produced approximately 80% reduction in FJC labeling respectively. Dantrolene and carisbamate treatment also produced an approximately 60% and 80% neuroprotection in thalamic region respectively. Strong neuroprotective effects of these drugs as evidenced by approximately 70% reduction in FJC labeling in the amygdala when administered 1-h post POX SE (Fig. 2B). No differences in neuroprotection by dantrolene and carisbamate were observed between the ipsilateral and contralateral brain hemispheres.
Discussion
In this study, we tested the possibility whether treatment with the CICR inhibitors under conditions that blocked the OP-SE induced Ca2+ plateau could provide effective neuroprotective therapy. Indeed, both dantrolene and carisbamate blocked the protracted hippocampal Ca2+ elevations, and extended significant neuroprotection in critical brain areas following POX SE. Thus, a significantly lower numbers of FJC positive cells were observed in hippocampus, cortex, amygdala, and thalamus in the brains of POX rats treated with either dantrolene or carisbamate. This neuroprotective effect was observed when dantrolene or carisbamate were administered 60 mins post POX SE, demonstrating a significant therapeutic window after control of SE for employing these neuroprotective agents.
The POX model of OP SE mimics the acute mortality and chronic morbidity associated with OP intoxication [7,12]. At 48-h post OP SE, an optimal time-point for noting OP mediated delayed neuronal damage [26], significant elevations in hippocampal Ca2+ levels were noted. These findings are in-line with our previous data where protracted increases in hippocampal [Ca2+]i were observed that lasted for weeks (Ca2+ plateau) following the termination of OP SE with currently approved countermeasure therapies [7,18]. Interestingly, the development of Ca2+ plateau was NMDA receptor dependent during SE [18,20], but once induced it was independent of NMDA receptor activation and dependent on the CICR mechanisms [21], such that treatments with dantrolene or carisbamate significantly lowered OP-SE mediated protracted Ca2+ elevations [7].
In agreement with previous findings [7,26,27], we observed wide-spread neuronal injuries in OP SE rats rescued with FDA approved medical therapies. Neuronal damage in these critical brain areas have been implicated in the development of neurological impairments. For example, the hippocampus is the seat of learning and memory [28]. It also plays a major role in the pathophysiology of chronic depression [29]. Lesion studies have shown that damage to specific hippocampal neurons produce memory impairments and anxiety [30]. Neuronal injury in the amygdala and thalamus has also been observed in mood disorders including depression and anxiety [31]. Brain injuries such as TBI or stroke that produces damage in these same brain areas as SE [32], and which we have demonstrated to be associated with delayed Ca2+ dysregulations [33] are also associated cognitive deficits and depressive symptoms [34,35]. Thus, neuronal damage in these brain areas could underlie the chronic morbidities of depression and cognitive deficits in POX SE survivor rats [12].
In this study, pharmacological blockade of Ca2+ plateau using the CICR inhibitors dantrolene and carisbamate afforded significant neuroprotection when administered after the induction of POX-induced SE. Dantrolene is a FDA approved drug used for the treatment of malignant hyperthermia. Experimental studies have demonstrated its neuroprotective effects in both in vitro and in vivo models of SE [21,36]. Dantrolene has also been shown to be neuroprotective following experimental SE [36] and ameliorate cognitive deficits in a mouse model of Alzheimer’s disease [37]. Carisbamate is an orphan drug for the treatment of infantile spasms. We have demonstrated carisbamate to be neuroprotective and antiepileptogenic in a hippocampal neuronal culture model of acquired epilepsy [38]. Behavior modifying effects of carisbamate have also been reported in experimental epilepsy [39]. We are currently investigating whether treatments with these agents shown to be neuroprotective in this study would also prevent the development of chronic morbidities in OP SE survivors. The results in this study indicate that dantrolene and carisbamate or possibly other inhibitors/ mechanisms targeting the CICR could be effective adjuvant therapies to the existing medical countermeasures regimen to reduce neuronal injury and possibly prevent OP SE induced behavioral morbidities.
Highlights.
Paraoxon induced status epilepticus produces calcium plateau in hippocampal neurons
This calcium plateau is dependent on calcium release from intracellular stores
Paraoxon induced status epilepticus produces widespread neuronal death
Blockade of intracellular calcium release is neuroprotective upon paraoxon toxicity
Acknowledgments
This work was supported by the CounterACT Program, National Institutes of Health Office of the Director, and the National Institute of Neurological Disorders and Stroke Grant No. (U01NS058213-10) to RJD. This work was also supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Gulf War Illness Research Program under Award No. (W81XWH-14-1-0478) to LSD. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the federal government or the Department of Defense.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haley RW, Tuite JJ. Epidemiologic evidence of health effects from long-distance transit of chemical weapons fallout from bombing early in the 1991 Persian Gulf War. Neuroepidemiology. 2013;40(3):178–189. doi: 10.1159/000345124. [DOI] [PubMed] [Google Scholar]
- 2.Hood E. The Tokyo attacks in retrospect: sarin leads to memory loss. Environ Health Perspect. 2001;109(11):A542. doi: 10.1289/ehp.109-a542a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sellstrom A, Cairns S, Barbeschi M. Report of the United Nations Mission to Investigate Allegations of the Use of Chemical Weapons in the Syrian Arab Republic on the alleged use of chemical weapons in the Ghouta area of Damascus on 21 August 2013. 2013 [Google Scholar]
- 4.Ajdacic-Gross V, Weiss MG, Ring M, Hepp U, Bopp M, Gutzwiller F, Rossler W. Methods of suicide: international suicide patterns derived from the WHO mortality database. Bulletin of the World Health Organization. 2008;86(9):726–732. doi: 10.2471/BLT.07.043489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konradsen F. Acute pesticide poisoning--a global public health problem. Danish medical bulletin. 2007;54(1):58–59. [PubMed] [Google Scholar]
- 6.Than K. Organophosphates: A Common But Deadly Pesticide. National Geographic. 2013 [Google Scholar]
- 7.Deshpande LS, Carter DS, Phillips KF, Blair RE, DeLorenzo RJ. Development of status epilepticus, sustained calcium elevations and neuronal injury in a rat survival model of lethal paraoxon intoxication. Neurotoxicology. 2014;44C:17–26. doi: 10.1016/j.neuro.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuovinen K. Organophosphate-induced convulsions and prevention of neuropathological damages. Toxicology. 2004;196(1–2):31–39. doi: 10.1016/j.tox.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Bajgar J. Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Adv Clin Chem. 2004;38:151–216. doi: 10.1016/s0065-2423(04)38006-6. [DOI] [PubMed] [Google Scholar]
- 10.Chemical Hazards Emergency Medical Management. Nerve Agents - Emergency Department/Hospital Management. 2013 [Google Scholar]
- 11.de Araujo Furtado M, Rossetti F, Chanda S, Yourick D. Exposure to nerve agents: from status epilepticus to neuroinflammation, brain damage, neurogenesis and epilepsy. Neurotoxicology. 2012;33(6):1476–1490. doi: 10.1016/j.neuro.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Deshpande LS, Phillips K, Huang B, DeLorenzo RJ. Chronic behavioral and cognitive deficits in a rat survival model of paraoxon toxicity. Neurotoxicology. 2014;44:352–357. doi: 10.1016/j.neuro.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helmstaedter C. Cognitive outcome of status epilepticus in adults. Epilepsia. 2007;48(Suppl 8):85–90. doi: 10.1111/j.1528-1167.2007.01360.x. [DOI] [PubMed] [Google Scholar]
- 14.Neligan A, Shorvon SD. Prognostic factors, morbidity and mortality in tonicclonic status epilepticus: a review. Epilepsy Res. 2011;93(1):1–10. doi: 10.1016/j.eplepsyres.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Rod CS. Status epilepticus in the developing brain: Long-term effects seen in humans. Epilepsia. 2009;50(s12):32–33. doi: 10.1111/j.1528-1167.2009.02374.x. [DOI] [PubMed] [Google Scholar]
- 16.Phillips KF, Deshpande LS. Repeated low-dose organophosphate DFP exposure leads to the development of depression and cognitive impairment in a rat model of Gulf War Illness. Neurotoxicology. 2016;52:127–133. doi: 10.1016/j.neuro.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Savage EP, Keefe TJ, Mounce LM, Heaton RK, Lewis JA, Burcar PJ. Chronic neurological sequelae of acute organophosphate pesticide poisoning. Arch Environ Health. 1988;43(1):38–45. doi: 10.1080/00039896.1988.9934372. [DOI] [PubMed] [Google Scholar]
- 18.Deshpande LS, Carter DS, Blair RE, DeLorenzo RJ. Development of a prolonged calcium plateau in hippocampal neurons in rats surviving status epilepticus induced by the organophosphate diisopropylfluorophosphate. Toxicol Sci. 2010;116(2):623–631. doi: 10.1093/toxsci/kfq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeLorenzo RJ, Sun DA, Deshpande LS. Cellular mechanisms underlying acquired epilepsy: the calcium hypothesis of the induction and maintainance of epilepsy. Pharmacol Ther. 2005;105(3):229–266. doi: 10.1016/j.pharmthera.2004.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raza M, Blair RE, Sombati S, Carter DS, Deshpande LS, DeLorenzo RJ. Evidence that injury-induced changes in hippocampal neuronal calcium dynamics during epileptogenesis cause acquired epilepsy. Proc Natl Acad Sci U S A. 2004;101(50):17522–17527. doi: 10.1073/pnas.0408155101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagarkatti N, Deshpande LS, Carter DS, DeLorenzo RJ. Dantrolene inhibits the calcium plateau and prevents the development of spontaneous recurrent epileptiform discharges following in vitro status epilepticus. Eur J Neurosci. 2010;32(1):80–88. doi: 10.1111/j.1460-9568.2010.07262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filbert M, Levine E, Ballough G. Neuroprotection for nerve agent-induced brain damage by blocking delayed calcium overload: a review. J Med CBR Def. 2005;3 [Google Scholar]
- 23.Bengtson CP, Bading H. Nuclear calcium signaling. Adv Exp Med Biol. 2012;970:377–405. doi: 10.1007/978-3-7091-0932-8_17. [DOI] [PubMed] [Google Scholar]
- 24.Baker KD, Edwards TM, Rickard NS. The role of intracellular calcium stores in synaptic plasticity and memory consolidation. Neurosci Biobehav Rev. 2013;37(7):1211–1239. doi: 10.1016/j.neubiorev.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J BiolChem. 1985;260(6):3440–3450. [PubMed] [Google Scholar]
- 26.Li Y, Lein PJ, Liu C, Bruun DA, Tewolde T, Ford G, Ford BD. Spatiotemporal pattern of neuronal injury induced by DFP in rats: a model for delayed neuronal cell death following acute OP intoxication. Toxicol Appl Pharmacol. 2011;253(3):261–269. doi: 10.1016/j.taap.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Lein PJ, Liu C, Bruun DA, Giulivi C, Ford GD, Tewolde T, Ross-Inta C, Ford BD. Neuregulin-1 is neuroprotective in a rat model of organophosphate-induced delayed neuronal injury. Toxicol Appl Pharmacol. 2012;262(2):194–204. doi: 10.1016/j.taap.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Battaglia FP, Benchenane K, Sirota A, Pennartz CM, Wiener SI. The hippocampus: hub of brain network communication for memory. Trends Cogn Sci. 2011;15(7):310–318. doi: 10.1016/j.tics.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29(6):417–426. [PMC free article] [PubMed] [Google Scholar]
- 30.Barkus C, McHugh SB, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM. Hippocampal NMDA receptors and anxiety: at the interface between cognition and emotion. Eur J Pharmacol. 2010;626(1):49–56. doi: 10.1016/j.ejphar.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Truitt WA, Johnson PL, Dietrich AD, Fitz SD, Shekhar A. Anxiety-like behavior is modulated by a discrete subpopulation of interneurons in the basolateral amygdala. Neuroscience. 2009;160(2):284–294. doi: 10.1016/j.neuroscience.2009.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain pathology. 2004;14(2):215–222. doi: 10.1111/j.1750-3639.2004.tb00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeLorenzo RJ, Sun DA, Blair RE, Sombati S. An in vitro model of stroke-induced epilepsy: elucidation of the roles of glutamate and calcium in the induction and maintenance of stroke-induced epileptogenesis. Int Rev Neurobiol. 2007;81:59–84. doi: 10.1016/S0074-7742(06)81005-6. [DOI] [PubMed] [Google Scholar]
- 34.Deshpande LS, Sun DA, Sombati S, Baranova A, Wilson MS, Attkisson EA, Hamm RJ, Delorenzo RJ. Alterations In Neuronal Calcium Levels Are Associated With Cognitive Deficits After Traumatic Brain Injury. Neurosci Lett. 2008;441(1):115–119. doi: 10.1016/j.neulet.2008.05.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loubinoux I, Kronenberg G, Endres M, Schumann-Bard P, Freret T, Filipkowski RK, Kaczmarek L, Popa-Wagner A. Post-stroke depression: mechanisms, translation and therapy. Journal of cellular and molecular medicine. 2012;16(9):1961–1969. doi: 10.1111/j.1582-4934.2012.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niebauer M, Gruenthal M. Neuroprotective effects of early vs. late administration of dantrolene in experimental status epilepticus. Neuropharmacology. 1999;38(9):1343–1348. doi: 10.1016/s0028-3908(99)00059-3. [DOI] [PubMed] [Google Scholar]
- 37.Peng J, Liang G, Inan S, Wu Z, Joseph DJ, Meng Q, Peng Y, Eckenhoff MF, Wei H. Dantrolene ameliorates cognitive decline and neuropathology in Alzheimer triple transgenic mice. Neurosci Lett. 2012;516(2):274–279. doi: 10.1016/j.neulet.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deshpande LS, Nagarkatti N, Ziobro JM, Sombati S, Delorenzo RJ. Carisbamate prevents the development and expression of spontaneous recurrent epileptiform discharges and is neuroprotective in cultured hippocampal neurons. Epilepsia. 2008;49(10):1795–1802. doi: 10.1111/j.1528-1167.2008.01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faure JB, Marques-Carneiro JE, Akimana G, Cosquer B, Ferrandon A, Herbeaux K, Koning E, Barbelivien A, Nehlig A, Cassel JC. Attention and executive functions in a rat model of chronic epilepsy. Epilepsia. 2014;55(5):644–653. doi: 10.1111/epi.12549. [DOI] [PubMed] [Google Scholar]