Abstract
Background
In previous studies female monkeys self-administered more oral phencyclidine (PCP) than males, and PCP intake differed by phase of menstrual cycle.
Objectives
The purpose of this study was to examine sex and hormonal influences on oral cocaine self-administration in male and female rhesus monkeys in the follicular vs. luteal phases of the menstrual cycle, with concurrent access to an alternative nondrug reward, saccharin (SACC) vs. water.
Materials and methods
Concurrent access to cocaine (0.2, 0.4 and 0.8 mg/ml) and SACC or water was available from two drinking spouts under concurrent fixed-ratio (FR) 2, 4, and 8 schedules during daily 3-h sessions.
Results
Cocaine deliveries were similar in males and females in the females’ luteal phase, but cocaine deliveries were higher in females during the follicular phase than the luteal phase and compared to males. When SACC was available cocaine deliveries were reduced in females in the follicular phase of the cycle, and cocaine intake (mg/kg) was reduced in males and in females’ follicular and luteal phases.
Conclusions
Access to concurrent SACC (vs. water) reduced cocaine intake (mg/kg) in males and in females during both menstrual phases, and the magnitude of the reduction in cocaine intake was greatest during the females’ follicular phase. Thus, a nondrug alternative reward, SACC, is a viable alternative treatment for reducing cocaine’s rewarding effects on male and female monkeys, and reductions in cocaine-seeking were optimal in the females’ luteal phase.
Keywords: cocaine, environmental enrichment, follicular phase, luteal phase, menstrual cycle, oral drug intake, rhesus monkeys, saccharin (SACC), self-administration, sex
Introduction
Addiction to cocaine and other illegal drugs has an estimated cost to society of over 180 billion dollars per year (Surgeon General’s Report, 2004). Cocaine use is more prevalent in men than women (SAMHSA 2013), but women progress more rapidly than men to cocaine- and other substance-abuse disorders (Cotto et al. 2010). Women are less successful in treatment than men, due to relapse and lower retention rates (e.g., Hyman et al. 2008). The purpose of the present study was to examine the effect of sex and female hormonal cycles on oral cocaine self-administration in rhesus monkeys and the effect of treatment with concurrent access to a nondrug alternative reward, saccharin (SACC). Methods developed by Meisch (Meisch et al. 1990; Meisch and Stewart 1995) were used to train oral cocaine self-administration in rhesus monkeys. While this is not the route of administration used in humans, it involved similar features as smoked or insufflated cocaine, such as absorption in oral and nasal mucosa. As the oral method offers an opportunity for chronic drug self-administration, it was used in the present study to examine menstrual cycle effects while observing other behavioral economic factors such as availability of a nondrug alternative reward as well as drug concentration and fixed-ratio (FR) variations to determine the effects of response cost.
In human laboratory studies sex and hormonal differences were found in the subjective effects of cocaine, especially in those who smoke cocaine (Evans and Foltin 2006; Evans et al. 2002; Sofuoglu et al. 1999) vs. those who use intranasal cocaine (Collins et al. 2007; Lukas et al. 1996). Subjective effects of smoked cocaine were reported to be similar in men and women in the follicular phase of the menstrual cycle when estrogen is peaking (Evans and Foltin 2010), compared to the luteal phase when progesterone (PRO) rises and falls. In other studies, exogenous PRO administration attenuated the effects of smoked cocaine in women but not in men (Evans and Foltin 2006) and decreased the ratings of ‘high’ for iv cocaine in men and women in the follicular but not the luteal phase (Sofuoglu et al. 2004). Sofuoglu et al. (2002) found that PRO given early in the follicular phase in women attenuated some of the subjective effects of cocaine. Thus, using nonhuman primates to study menstrual cycle effects and long-term treatments is a productive approach because hormonal cycles are similar in monkeys and humans (28 days).
Animal models provide valuable information on vulnerability to drug abuse with respect to hormonal status, during the application of pharmacological and behavioral treatments (e.g., Mello et al. 2007, 2011). Rodent studies confirm a role for ovarian hormones in these sex differences, with estrogen increasing and PRO decreasing drug-seeking and -taking behavior (Anker and Carroll 2011; Becker and Hu 2008; Becker et al. 2012, Jackson et al. 2006, Kippen et al. 2005, Roberts et al. 1989). However, fewer studies on sex differences have been conducted with nonhuman primates (vs. rats). Initial results in female cynomologous monkeys indicated higher rates of cocaine self-administration under a progressive-ratio (PR) schedule than males, but there was no effect of menstrual cycle phase (Mello et al. 2007). A subsequent study in rhesus monkeys Mello et al. (2008) revealed that exogenously- administered estradiol failed to alter cocaine self-administration in female gonadally-intact or ovariectomized rhesus monkeys, and a later study indicated that PRO reduced iv self-administration of a low dose of cocaine in intact or ovariectomized rhesus monkeys (Mello et al. 2011). In a recent study of iv cocaine self-administration comparing endogenous hormone levels in rhesus monkeys using a progressive ratio (PR) schedule Cooper et al. (2013) reported that cocaine self-administration at doses ranging from 0.0125 to 0.05 mg/kg did not vary across 5 stages of the menstrual cycle. While there is not a clear pattern in the influence of hormonal factors in cocaine’s effects in nonhuman primates, initial results suggest a role for PRO in reducing stimulant abuse and the potential of PRO as a medication for treatment of cocaine addiction in humans. These initial studies highlight the importance of examining potential treatment effects in both sexes and during females’ hormonal cycle phases. Initial results with cocaine self-administration studies with monkeys suggest that sex and hormonal differences in cocaine’s effects are behaviorally-based, as pharmacokinetic differences have not been reported (Evans and Foltin 2010; Lucas et al. 1996; Mendelson et al. 1999).
In the present study a nondrug alternative reward, saccharin (SACC) that reduces drug intake in male and female rhesus monkeys (Carroll et al. 2009), was used to reduce drug intake in male and female monkeys. Intake of cocaine (and SACC or water) was compared during phases of the menstrual cycle; mid-follicular (when estrogen peaks) and late luteal (when PRO has peaked and started to decline), as changes in phencyclidine (PCP) self-administration in rhesus monkeys has been reported in these phases (Carroll et al. 2013b). It has been well established that SACC and other nondrug rewards reduce drug-taking in rats (see reviews by Bardo et al. 2001; Carroll et al. 2001), rhesus monkeys (Cosgrove and Carroll 2003) and humans (Carroll et al. 2001; Solinas et al. 2010), but sex differences and hormonal influences on the use of nondrug rewards to reduce drug self-administration have not been examined. Initial data suggest that a sweet substance, SACC, is more protective against cocaine self-administration in female than in male rats (Cason & Grigson 2013), and SACC previously reduced oral PCP self-administration more in female than male rhesus monkeys in the acquisition (Carroll et al. 2005) and maintenance (Cosgrove and Carroll 2003) phases of self-administration. Thus, SACC was used in the present study as a prototype nondrug alternative reward for reducing stimulant drug intake with the goal of applying this treatment to behavioral interventions in treatment of human stimulant abusers (e.g., Higgins et al. 2003, 2008).
Another goal of the present study was to compare the combined effect of SACC, and hormonal conditions when PRO (luteal phase) is elevated, to reduce cocaine intake compared to SACC or the luteal phase alone. Progesterone and testosterone have reduced cocaine self-administration in female rhesus monkeys, but the effects in males are unknown (Mello et al. 2011). Previous research shows that the effectiveness of SACC or other nondrug rewards (e.g., exercise) in reducing cocaine and other drug self-administration has been enhanced by adding hormonal or medication treatments such as PRO (Carroll and Zlebnik 2014) or atomoxetine (Zlebnik et al. 2014) to produce additive reductions in drug seeking. Examining combined treatments with animal models has had important implications for humans, and parallels in the clinical literature provide consistent results (Stoops and Rush, 2014). Thus, in the present study we compared cocaine intake during the luteal phase when serum PRO peaks in rhesus monkeys to the follicular phase when estrogen peaks (Cooper et al. 2013), and we compared cocaine intake during the luteal and follicular phases. Recently it was shown that physical exercise reduced methamphetamine use in humans undergoing a residential treatment program (Rawson et al. 2015). Others have shown that nondrug alternative rewards reduce drug-seeking in animals (Foltin et al. 2015) and humans (Foltin et al. 2015; Higgins et al. 2008). Instead of SACC, a more healthy alternative, such as physical exercise, would be a desirable intervention to use with monkeys, but it is difficult to implement with the limitations of existing primate laboratory conditions. Thus, SACC was used in the present study as a prototype alternative nondrug reward that could be extended for treatment in humans with more healthy alternative rewards (e.g., Higgins et al. 2003, 2008).
Finally, the present study was designed to examine the effect of response cost (responses/mg of drug self-administered) of cocaine and the nondrug alternative, SACC (vs. water), by independently varying the FR from 2 to 4 to 8 responses per delivery and the drug concentration from 0.2 to .4 to .8 mg/ml. Recent research with rhesus monkeys showed a greater effect of a nondrug alternative reward with higher response cost for the drug with monkeys (Foltin et al. 2015) and humans (Vosberg et al. 2010). A behavioral economic analysis of demand for drug was compared across 5 unit prices (responses/mg) for males vs. females in their follicular vs. luteal phase of the menstrual cycle, previous work revealed that medication and behavioral-based treatments for cigarette smoking varied depending on whether women initiated quitting in the follicular (Franklin et al. 2008) vs. luteal (Allen et al. 2008; Carpenter et al. 2008; Mazure et al. 2011) phase. The present study was designed to last several months – years to examine chronic access to cocaine, a self-sustaining nondrug alternative reward (SACC), and the effect of repeated menstrual cycles with high (luteal) vs. low (follicular) PRO. It was hypothesized that the effects of SACC would add to those of the luteal phase to produce a maximum reduction in cocaine intake in females. This approach would have relevance for long-term treatment of human drug abuse and demonstrate that nondrug alternative reinforcement is self-sustaining over the long term. The oral cocaine self-administration methodology used (see Meisch et al. 1990) has yielded long-term, stable self-administration that allows chronic drug access (7 days/week over months) to study factors that modify that behavior. As a means of further emulating human drug-taking culture, monkeys were housed in long-standing social groups with minimal procedures involving human laboratory or veterinary staff.
Material and methods
Animals
Eleven adult male and 9 adult female rhesus monkeys were used in this study. Initially, 2 other females were started on the study, but they were discontinued because one developed endometriosis and was treated with Depo-provera, and the other was amenorrheic. All of the monkeys in this study had a history of phencyclidine (PCP) self-administration (Carroll et al., 2009b; 2013b). The monkeys were maintained at 85–90% of their free-feeding body weight, and their reduced weights ranged from 9.1 to 12.7 kg (mean=11.2) in males and 6.5 to 8.6 kg (mean=7.5) in females. Prior to introduction of the oral cocaine solution the monkeys had exposure to PCP (0.25 mg/ml) and ethanol (8% wt/vol) in previous studies (Carroll et al. 2009b, 2013). Water was always available during the daily 3-h drug access sessions, and intersession periods. Male and female monkeys were used in all conditions, and females’ menstrual cycles were of similar length and were often synchronized. Each monkey was housed individually in visual, auditory, and social contact with monkeys in neighboring cages. There were 10 monkeys in each of 2 rooms that contained males and females in each room containing well-established groups of males and females. Temperature (23°C) and humidity were controlled, and the lights were on from 0600 to 1800 h. Enrichment objects such as foraging devices, primate toys, movies, fruit, and vegetables were available during the intersession period. The experiment was conducted in accordance with the Principles of Laboratory Animal Care (National Research Council 2011) and approved by the University of Minnesota Institutional Care and Use Committee. Laboratory facilities were accredited by the American Association for the Assessment and Accreditation of Laboratory Animal Care.
Apparatus
Monkeys were housed individually in 4-plex quad-units with 2 upper and 2 lower attached cages (Lab Products, Maywood, NJ, USA). Each animal’s cage had an operant conditioning response panel mounted to the inside of one side wall of the cage that had holes allowing a removable panel equipped with drinking spouts and stimulus lights affixed to the panel through punched holes in the side walls. This allowed the monkeys to access the response apparatus, a view of the stimulus conditions, and easy removal of the panel for cage washing. Access to drug and water was computer-controlled 7 days per week. Two brass drinking spouts were mounted approximately 45 cm apart on a clear Plexiglas plate with 2 small white lights mounted behind them to indicate water or SACC availability, and 2 small green lights behind the plate signaled cocaine access. Above each spout a single larger green light that was steadily illuminated when water or SACC was available, and this light flashed when cocaine was available at the spout and was solid-on to indicate water. The operant response panels were controlled, and data were recorded by Med-PC software (Med-PC for Windows), and interfaces (Med Associates, St. Albans, VT, USA) and PC-compatible computers located in an adjacent room controlled the experiment.
Procedure
The experimental design consisted of 3 phases; 1) Training cocaine self-administration, 2) Cocaine vs water self-administration, and 3) Cocaine vs SACC self-administration
Training cocaine self-administration
Each day from 8–10 am there was a time-out period when no food or liquids were available, and data from the previous intersession period (1 pm – 8 am) were collected. From 10 am – 12 pm the monkeys had concurrent access to cocaine and water (or SACC) and FRs for liquid deliveries were varied according to the experimental procedure. Another time out period was scheduled between 12 pm and 1 pm when no food or liquids were available. Finally, from 2 pm to 8 am the next day there was an intersession period when water was available from both spouts under an FR 1 schedule. The fading procedure used to obtain cocaine self-administration is described in Table 1. Initially, water and PCP (0.25 mg/ml) were concurrently available, (Carroll et al. 2009b, 2013), and monkeys were then stabilized at a PCP concentration of 0.25 mg/ml with water available from a second electronic spout under concurrent fixed-ratio (FR) 16 schedules (see Carroll et al. 2009b, 2013). Next, the PCP concentration was reduced, while increasing concentrations of ethanol (ETOH) were added to the PCP (Table 1). Subsequently a procedure modified from Meisch et al. (1990) was used whereby ethanol was faded out by decreasing concentrations, and cocaine was faded in with increasing concentrations in the ethanol-cocaine mixture (see Table 1). When stable behavior was obtained, the cocaine concentration was changed to 0.4 mg/ml and the FR 4 to begin the study. This FR and cocaine concentration yielded cocaine intakes (mg/kg) similar to those self-administered by humans (Hatsukami et al. 1994) and monkeys smoking cocaine base (Carroll et al. 1990). The stability criterion, 5 days of no increasing or decreasing trend, was based on previous studies of smoked cocaine (Carroll et al. 1990) and oral PCP self-administration in monkeys (Carroll et al. 2009b, 2013).
Table 1.
Cocaine oral self-administration training procedures Drugs (conc/dose)
| PCP (mg/ml) | .25 | .25 | .125 | .0625 | 0 | |||||||||||
| ETOH (% wt/vol) | 0 | .5 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | .5 | .25 | 0 | 0 | 0 |
| Cocaine (mg/ml) | 0 | .0125 | .025 | .05 | .1 | .2 | .2 | .2 | .2 | .2 | .4* | .8* |
0 = Water
Cocaine concentrations were presented in nonsystematic order
Cocaine vs water self-administration
After behavior stabilized with cocaine and water concurrently available from the two drinking spouts during daily 3-h sessions, with 3 FRs (2, 4 and 8) and 3 cocaine concentrations (0.2, 0.4, and 0.8 mg/ml. Each FR and cocaine concentration was held constant until 4 days of stable behavior were obtained during the mid-follicular and late-luteal phases of the menstrual cycle. The FR values and cocaine concentrations were based on previous work by Meisch and Stewart (1995). However, due to changes in feeding guidelines over the last 10 years the FRs achieved in the present study were not as high as Meisch and Stewart (1995). Males were maintained under the same experimental conditions for the same period of time as the females, and their data were collected at times of the month that corresponded with the females’ menstrual cycle days that were used (Days 7–10 and 24–27).
Cocaine vs SACC self-administration
During the third phase of the experiment, cocaine continued to be available, under the same concentration and FR parameters, but SACC (0.3 % wt/vol) replaced water during the 3-h sessions, while cocaine concentrations and FRs were again varied as when concurrent water was available. The monkeys all had previous experience with PCP and SACC in earlier studies (Carroll et al. 2009b, 2013b); however, cocaine was novel. Instead of counterbalancing the order of concurrent SACC and water, water was tested first and then SACC, as previous studies indicated that monkeys show a short-term reduction in drug self-administration after SACC access has terminated (Campbell and Carroll 2000). This phase began with 0.4 mg/ml cocaine and SACC (0.3 % wt/vol), each concurrently available under FR 4 schedules, and the FR for both was then changed in nonsystematic order to FR 2, 4 and 8 while different cocaine concentrations were tested. Changes took place after data were obtained under both the follicular and luteal phases for females and at comparable times in males. Each monkey was tested with both the concurrent water and SACC conditions, with water always available (FR 1) from both spouts during the intersession period.
Menstrual cycle phase determination
The methods used to determine cycle phase were similar to those previously reported (Newman et al. 2006; Mello et al. 2007). Monkeys were observed daily for bleeding, and vaginal swabbing was performed 1 h before the daily sessions to confirm visual observation of menses onset and stage of menstrual cycle by cytology (see Table 2). Data for the mid-follicular phase were taken on Days 7–10 post menses, and the late luteal phase on Days 24–27 post menses, based on rationale and results obtained in our previous study with oral PCP intake during menstrual cycle phases in female rhesus monkeys (Newman et al. 2006).
Table 2.
Percent of total cytology slides for which cell types matched the predicted phase of the menstrual cycle based on onset of menses (number of observations)
| Menstrual phase | Water | SACC |
|---|---|---|
| Follicular | 90.56 (58) | 87.5 (8) |
| Luteal | 94.73 (59) | 88.23 (17) |
To verify menstrual phase, cytology of the vaginal epithelium was classified by the method of Mauro et al. (1970). Menses was defined as predominance of parabasal and anucleated/cornified cell types. The follicular phase was defined by superficial, intermediate, and anucleated/cornified cell types, and the luteal phase was indicated by the presence of parabasal, superficial, and intermediate and anucleated/cornified epithelial cells. We did not take blood samples to analyze estradiol, leutinizing hormone, and PRO, as the monkeys’ behavioral testing occurred 7 days per week, and blood draws involving food and water restriction for anesthesia, and subsequent substitution of water for cocaine for 1–2 days would have caused disruptions in behavior for the next day or two. Menstrual cycles ranged from 27–29 days across animals, but cycle length was typically the same number of days within each female and for most monkeys 28 days. Monkeys’ cycles became synchronized with other females in the room. Cytology data were classified by research assistants blinded to the menstrual cycle phases and experimental conditions. While samples were obtained almost every day, slides were viewed for verification of menstrual phase only during Days 7–10 (follicular) and 24–27 (luteal) post menses. Number of samples obtained per monkey ranged from 24 to 43. Table 2 indicates that under these conditions the histological data matched the phase that was counted from onset of menses.
Drugs
Cocaine HCl was obtained from the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, NC). The cocaine solutions were prepared with tap water, stored in a locked refrigerator, and delivered to the monkeys at 0.6 ml/delivery at room temperature. Cocaine intake (mg/kg) was calculated by the amount of drug (mg) delivered per session, divided by the monkey’s most recent body weight (kg). Monkeys were weighed twice monthly.
Data Analysis
The initial design of this study was to compare the self-administration measures (liquid deliveries, or mg/kg intake) across cocaine concentration, FR size, concurrent liquid (water or SACC), and sex/hormonal conditions (male, female follicular, female luteal). However, there were no significant differences or interactions with the FR variable; thus, data were collapsed across the 3 FRs (2, 4, and 8) at each of the other conditions. Dependent measures such as cocaine, water, and SACC deliveries (Fig 1), mg/kg cocaine intake (Fig 2), and cocaine deliveries as a function of unit price (responses/mg) (Fig 3) were analyzed across the independent variables, cocaine concentration, concurrent liquid (water vs SACC), and sex/hormonal conditions in a three-factor, mixed ANOVA with sex as the between subjects’ factor and hormonal status, cocaine concentration and environmental enrichment (water vs SACC) as the within-subjects conditions. The data in Fig 3 was unit price of cocaine (responses/mg), which calculated responses emitted per session divided by mg intake (intake volume (ml) x concentration in (mg/ml) x liquid delivery amount, 0.6 ml). Post-hoc comparisons were made using Newman-Keuls, and results were considered significant if p < 0.05. Statistical analyses were based on GB Stat (Dynamic Microsystems, Inc., Silver Spring, MN, USA).
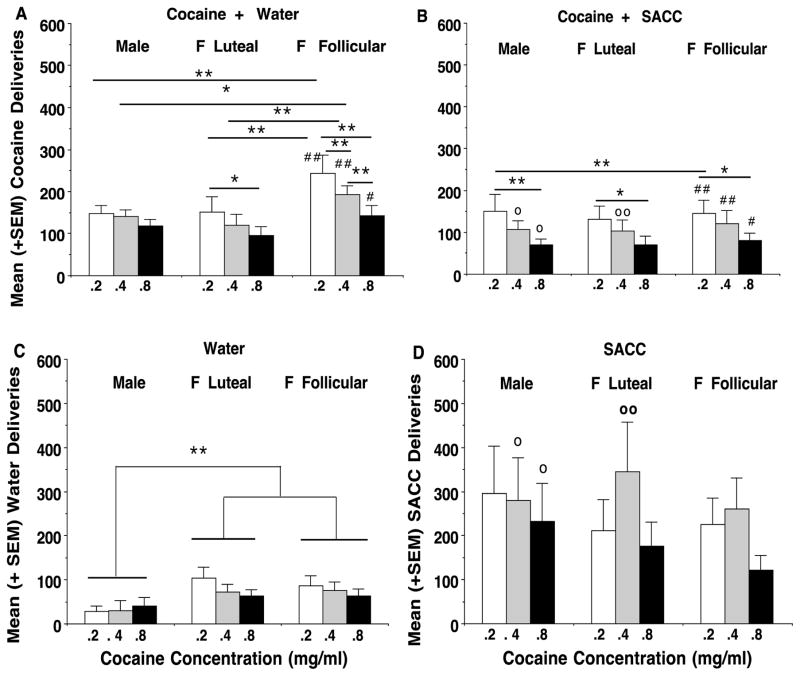
Fig 1.
Mean (±SEM) cocaine (A, B), water (C), and SACC (D) deliveries are presented for male monkeys and female monkeys in the follicular and luteal phases of the menstrual cycle when they had concurrent access to cocaine and water (A, C) or cocaine and SACC (B, D). Data are presented for 3 cocaine concentrations: 0.2 (white bars), 0.4 (striped bars), and 0.8 mg/ml (black bars). Each point represents a mean of 9 (female) or 11 (male) monkeys, and data for each monkey consisted of a mean of 4 consecutive daily sessions during the mid-follicular (Days 7–10) phase or the late-luteal phase (Days 24–27) of the menstrual cycle (Day 1 = onset of menses). Data for males were collected at similar times. Significant differences between cocaine deliveries with concurrent water (A) vs. concurrent SACC (B) are indicated by * = p<0.05, ** = p<0.01 across A and B, and in A vs. C. ** = significant differences (p< 0.01) in water intake across males, and females in the luteal vs. follilcular phases. Significant differences in concurrent SACC deliveries with cocaine vs. water are indicated by o = p< 0.05, and oo = p< 0.01 across B and D.
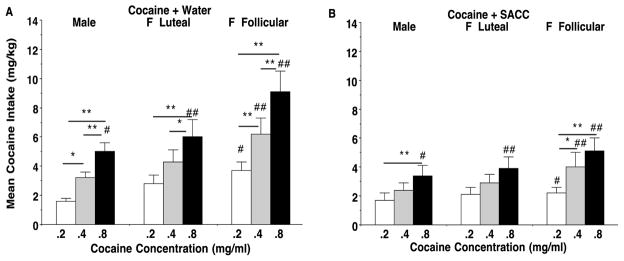
Fig 2.
Mean (±SEM) cocaine intake (mg/kg) is presented for female monkeys in the follicular and luteal phases of their menstrual cycle and male monkeys when they had concurrent access to cocaine and water (A) or cocaine and SACC (B). Data correspond to the liquid delivery data shown in Fig 1A and 1B and are presented for 3 cocaine concentrations: 0.2 mg/ml (white bars), 0.4 mg/ml (light gray bars), and 0.8 mg/ml (black bars). Each point represents a mean of 9 (female) or 11 (male) monkeys, and data for each monkey consisted of a mean of 4 consecutive sessions/days during the mid-follicular (Days 7–10) phase or the late-luteal phase (Days 24–27) of the menstrual cycle (Day 1 = onset of menses). Significant differences between cocaine intake (mg/kg) when water (A) or SACC (B) was concurrently available are indicated by ## = water >SACC (A vs. B) p<0.01; # = p<0.05. Differences in cocaine intake between concentrations (mg/ml) are indicated by * = p< 0.05, ** = p< 0.01 above the lines connecting the concentrations within A or B.
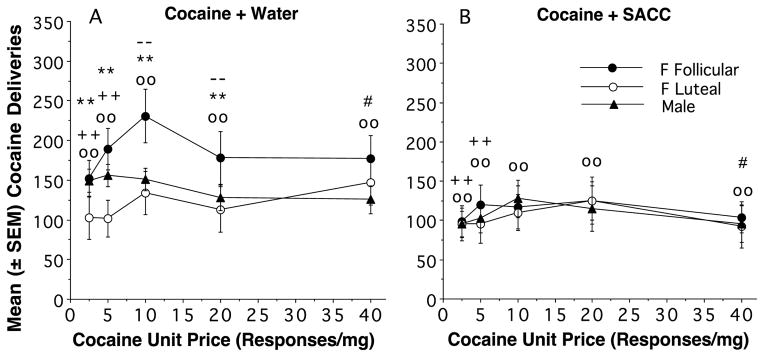
Fig 3.
Mean (±SEM) cocaine deliveries are compared when water (A) or SACC (B) was concurrently available with cocaine are presented for 5 unit prices (2.5, 5, 10, 20, and 40 responses/mg) and 3 sex/female hormonal phase conditions: males (filled triangles) and females in the follicular phase (filled circles), and females in the luteal phase (open circles). Within cocaine + water (A) and cocaine + SACC (B) conditions, ** indicates that females in the follicular phase > males p< 0.01, and - - indicates that females in the follicular phase > females in the luteal phase p<0.01. There were also significant differences across the A and B conditions when cocaine was available with concurrent water (A) or SACC (B): oo = p< .01 (Coc with Water > Coc with SACC) in females in the follicular phase, # =p < 0.05, and ++ = p < 0.01 in males.
Results
Cocaine vs water, cocaine vs SACC deliveries
Initial analyses indicated no significant effects for FR value; thus, the data for each animal were averaged over the 3 FR values (FR 2, FR 4, FR 8). Liquid deliveries were compared on sex and menstrual cycle phase (follicular vs. luteal), concurrent water vs. SACC conditions and the 3 cocaine concentrations (0.2, 0.4, and 0.8 mg/ml). Figures 1A and 1B show cocaine deliveries when water (A) or SACC (B) was concurrently available with 3 cocaine concentrations (0.2, 0.4, or 0.8 mg/ml) in males and in females during the luteal and follicular phases. There was a main effect of cocaine vs. water deliveries, and cocaine deliveries were significantly higher when water was concurrently available (Fig 1A) compared to SACC was concurrently available (Fig 1B) (F1,173 = 30.02, p<0.0001). There was also a significant main effect of sex and menstrual cycle phase during the follicular phase (F2,173 = 4.0984, p<0.05) and a significant interaction with concurrent liquid (water vs SACC) and sex/menstrual cycle phase (F2,173 = 5.8881, p<0.005), indicating that SACC reduced cocaine deliveries in the follicular phase, but not in the luteal phase in females and not in males. Post-hoc analyses revealed that cocaine deliveries were significantly higher when water (vs SACC was available during the follicular phase in females at the 0.2 (p< 0.01), 0.4 (p< 0.01), and 0.8 (p< 0.05) mg/lml cocaine concentration. A comparison of liquid deliveries at the 3 cocaine concentrations revealed a significant main effect of cocaine concentration (mg/ml) (F2,173 = 8.6954, p<0.0005), and post hoc analyses revealed that increasing the cocaine concentration as shown in Fig. 1A and 1B under all sex/menstrual phase conditions except in males in Fig. 1A.
In Fig 1C and 1D there was a significant main effect of the water (Fig 1C) vs SACC (Fig 1D) deliveries that were concurrently available with cocaine (F1,173 = 9.3117, p<0.0005). SACC deliveries (D) were significantly higher than water (C) deliveries (p<0.01). There were no interactions with sex/menstrual phase or cocaine concentration on these measures. Also, when water deliveries (Fig 1C) were compared to cocaine deliveries (Fig 1A), cocaine deliveries were significantly higher than the concurrent water deliveries (F1,173 = 30.0218, p<0.0001), indicating that cocaine was functioning as a reinforcer. When SACC deliveries (Fig 1D) were compared to concurrently available cocaine deliveries (Fig 1B), a significant main effect showed that SACC deliveries exceeded cocaine deliveries (F1,173 = 52.3529, p< 0.0001). These results suggested that SACC functioned as a competing reward for cocaine, and at these concentrations SACC deliveries exceeded cocaine deliveries. There was no significant effect of cocaine concentration on water (Fig 1C) or SACC (Fig. 1D) deliveries, nor was there a significant group effect indicating differences between males and females in either menstrual phase. However, females in both the luteal and follicular phases showed significantly higher intake of water than males (F2,173 = 9.3117, p < 0.0002) in Fig 1C.
Cocaine vs. water and cocaine vs. SACC intake (mg/kg)
Fig 2 expresses the amount of cocaine intake by males and females based on their body weight (mg/kg), since males weigh nearly 50% more than females. Cocaine intake (mg/kg) was compared across cocaine concentrations to determine the effect of alternative reinforcement with SACC (vs. water). Results of the ANOVA indicated that cocaine intake (mg/kg) was significantly higher overall with concurrent water (Fig 2A) than with concurrent SACC (Fig 2B) (F1,173 = 43.4012, p<0.0001). There was also a significant sex/menstrual phase effect (F2,173 = 13.7271, p< .0001) with significant interactions of cocaine concentration and concurrent liquid (water vs SACC) (F2,173 = 7.6754, p < 0.001), and sex and menstrual phase X concurrent liquid, (F2,173 = 6.1818, p < 0.005). In addition, there was a significant main effect of cocaine concentration in both the concurrent water (Fig 2A) vs. SACC (Fig 2B) conditions (F2,173 = 23.9828, p<0.001) with cocaine intake (mg/kg) generally increasing with cocaine concentration. Cocaine intake (mg/kg) was reduced by concurrent SACC at the high cocaine concentrations and when intake was elevated during the follicular vs. luteal phase in females or in males. Thus, the effects of SACC are rate dependent; higher cocaine intake in females during the follicular phase was more prone to reduction by concurrent SACC than lower cocaine intake in females in the luteal phase or males. Overall, these results indicate that treatment with a nondrug alternative reinforcer, SACC, was more effective in females during their follicular phase than in their luteal phase or in males.
Cocaine vs. water and cocaine vs. SACC deliveries x unit price (responses per mg)
Fig 3 describes the interaction of cocaine concentration, FR response requirement, and treatment with SACC in a data in a behavioral economic format whereby cocaine intake (deliveries earned) is plotted as a function of unit price (responses/mg cocaine intake). Unit price was derived from using 3 cocaine concentrations (0.2, 0.4, and 0.8 mg/ml) and 3 FR requirements (FR 2, 4, and 8) that yielded 5 unit prices (2.5, 5, 10, 20, and 40 responses/mg). There was a significant effect of unit price on cocaine deliveries (F4,329 = 3.7221, p< 0.05), indicating that females in the follicular phase showed elevated demand for cocaine compared with those in the luteal phase or males. Also, SACC significantly reduced the demand for cocaine (F1,329 = 8.1543, p<0.05). There was a significant interaction between the SACC vs. water condition and the sex/hormonal condition (F2,329 = 6.1731, p< 0.001) indicating a greater reduction in the demand for cocaine in the follicular females vs. the luteal females or males. A 3-way interaction (F8,329 = 2.089, p<0.05) indicated that SACC treatment was more effective at reducing demand when unit prices (responses/mg) were low. Overall, this analysis confirms previous cocaine response (Fig 1) and intake (Fig 2) data that optimal conditions for reducing the demand for cocaine is in follicular phase females (vs. luteal phase or males), at high drug concentrations.
Discussion
In the present study oral cocaine self-administration was sustained in male and female rhesus monkeys during daily 3-h sessions for 1.5 to 2 years. The monkeys had histories of chronic ethanol and PCP self-administration prior to this study. While the duration of cocaine exposure was longer in the present study than in previous iv drug studies (e.g., Cooper et al. 2013) results were similar to those obtained during shorter periods in monkeys using oral (Meisch et al. 1990) or iv (Cooper et al. 2013; Mello et al. 2007) routes. Sex differences were found in cocaine intake, and cocaine intake varied by phase of the menstrual cycle in females. Cocaine was reduced when concurrent SACC (vs. water) was available, and that varied with sex and menstrual cycle phase. Overall, the results indicated that: 1) cocaine intake (ml) was higher in females than males and higher during the follicular than the luteal phase of the menstrual cycle, 2) SACC, reduced cocaine intake (mg/kg) in males and females during both menstrual phases. 3) when FR and cocaine concentration were varied, increased response cost (responses/mg) elevated drug seeking, and this interacted with sex, hormonal status, and the effectiveness of SACC in reducing chronic drug seeking. Thus, the present study revealed several factors: that sex, hormonal cycle phase, access to nondrug alternative rewards, and response cost (FR) are all involved in drug seeking and treatment effectiveness. The FR value did not affect drug intake, as it had previously with larger FR values (Meisch et al. 1990, Meisch and Stewart 1995), possibly due to differences in daily food intake between studies, but it interacted with other factors in the present study. These factors are discussed in more detail below.
Sex and hormonal status influence cocaine self-administration
Females had more cocaine deliveries than males during the follicular phase of their menstrual cycle, when estrogen hypothetically peaks, than in the luteal phase when PRO peaks (Reddy et al. 2009; Schiller et al. 2012). Although hormone levels were not measured, cytological data (Table 2) illustrated a high concordance of the predicted phase of the menstrual cycle (follicular vs. luteal) and the cytology results. The present results agree with our previous work with PCP showing that female rhesus monkeys had higher levels of PCP intake than males (Carroll et al. 2000) and with a previous study of iv cocaine self-administration in cynomologous macaques indicating higher drug intake in females than males (Mello et al. 2008). In contrast, other hormonal studies have not shown significant differences in cocaine intake in rhesus monkeys (e.g., Cooper et al. 2013; Mello et al. 2007). The hormonal cycle effects found with cocaine in the present study agree with rat studies indicating that estrogen and PRO were related to cocaine-seeking behavior (e.g., Becker and Hu 2008; Becker et al. 2012) Anker and Carroll, 2011).
In contrast, in a previous study of oral PCP self-administration, intake was higher in the luteal than the follicular phase of the menstrual cycle (Carroll et al. 2013; Newman et al. 2006). This difference may have been due to different pharmacological actions of cocaine, a stimulant, vs. PCP, a dissociative anesthetic, that has sedative and dysphoric effects. For example, COC’s rewarding stimulant effects seen in the follicular phase could have been counteracted by PRO’s anxiolytic effects (Nyberg et al. 2006; Schneider and Popik 2007) to reduce intake during the follicular phase. In contrast, higher PCP intake in the luteal phase may have been due to alleviation of dysphoric effects of PCP by putative anxiolytic effects of PRO (Llaneza and Frye 2009; Schneider and Popik 2007) or its metabolite, allopregnanolone (ALLO) (Milivojevic et al. 2016). Elevated PRO did not decrease cocaine self-administration in previous studies (Mello et al. 2007; Cooper et al. 2013), but exogenously-administered PRO decreased cocaine self-administration in female rhesus monkeys (Mello et al. 2011). In the present study the effects of PRO on cocaine intake were supported by data from the monkey with endometriosis who received long-term PRO (Depo-Provera) to relieve her symptoms. Her cocaine intake remained reduced from a previous baseline for almost 2 years. Studies of subjective responses to cocaine in humans (Evans and Foltin 2010; Hudson and Stamp 2011) also support the hypothesis that PRO has therapeutic potential for reducing drug seeking in human and nonhuman primates.
Nondrug alternative rewards for treatment of drug abuse
In the present study concurrent access to SACC reduced COC administration on a chronic basis. This method of reducing drug abuse, providing a substitute, in behavioral economic terms, has been extensively examined (Carroll and Bickel 1998; Bickel et al. 1991, 2000; Carroll and Campbell 2000; Carroll et al. 2001). In the present study, chronic, concurrent access to SACC (vs. water) reduced cocaine deliveries (mg/kg) in the follicular phase but not in the luteal phase in females or in males. These results were consistent with SACC-induced reductions in PCP self-administration that were reported in female rhesus monkeys (Cosgrove and Carroll 2003; Carroll et al. 2009b) and reduced drug seeking in rats (Carroll et al. 2000; Cason and Grigson 2014).
The present results with monkeys concur with a general theme of sex differences with females showing more vulnerability in drug seeking than males in various phases of addiction, and females respond more favorably to treatment for drug seeking with nondrug alternative reinforcers and pharmacological treatments. The present findings show the durability of the SACC treatment as a sustainable, nondrug reward to achieve voluntary abstinence from illicit drug use on a long-term basis in humans. However, while SACC was used in our monkey laboratory to achieve reduced drug intake (see reviews by Carroll and Lynch 2016, Carroll and Smethells 2016), a healthy nondrug reward, such social/community reinforcement (Higgins et al. 2003, 2008), physical exercise (Bardo and Compton 2015; Zhou et al. 2015; Rawson et al. 2015), or an employment-based therapeutic workplace (e.g., Silverman et al. 2002) are suitable nondrug alternatives for application to humans.
Combining treatments is also of interest because in rat studies physical exercise reduced cocaine self-administration more in females than males (Cosgrove and Carroll 2004), and exercise added to medication treatments such as progesterone (Zlebnik and Carroll 2014) or atomoxetine (Zlebnik et al. 2014) to reduce drug-seeking more than either treatment alone. There is support for this combination strategy in human treatment research (Stoops and Rush, 2014).
Response cost (FR value; responses/mg) and treatment of drug abuse: interaction with sex, hormonal status and concurrent access to SACC (vs. water)
A consistent finding in this study was that responding for cocaine and cocaine intake (mg/kg) increased as FR values increased indicating that higher demand for cocaine interacted with response cost (FR 8 > FR 4 > FR 2 in mg/kg intake), sex (F > M), and phase of the menstrual cycle (follicular > luteal). For example, SACC (vs. water) was more effective at high unit prices (responses/mg) for for reducing cocaine at a low concentration and/or high response requirement – FR). When drug-seeking behavior in the present study was translated into a behavioral economic analysis of demand (response x unit price, or responses per mg) for cocaine with concurrent water vs. SACC (Fig 3A vs 3B), cocaine intake was highest for females in the follicular phase (when estrogen levels peak in humans and other primates) vs. the luteal phase or in males, and SACC reduced the demand for cocaine in females during the follicular phase and males. These demand functions were similar to those reported by Comer et al. (1994) and Rodefer et al. (1996, 1997) showing that SACC reduced the demand for smoked cocaine base when a range of FR values were used, especially when demand for drug was high. SACC also reduced motivation to seek smoked cocaine under a PR schedule (Rodefer and Carroll 1997).
Results of this study indicated that behavioral economic factors such as response cost and the availability of a nondrug alternative reinforcer, sex, and hormonal status, are all important factors modulating cocaine seeking. These findings are consistent with previous studies, mainly in rats, showing additive vulnerability factors for enhanced drug seeking as well as better treatment outcome in reward-seeking individuals (Carroll et al. 2009, 2013, 2014, Carroll and Lynch 2016, Carroll and Smethells 2016). When considering treatment outcome and sex differences, previous studies in rats and monkeys have also shown that medications (Sershen et al. 1998; Carroll et al. 2001; Campbell et al. 2002; Cosgrove and Carroll 2004) or SACC (Cosgrove et al. 2002; Cosgrove and Carroll 2003) are more effective treatment in females than males across several different self-administered drugs and routes of self-administration. The present results suggest that PRO, which peaks during the luteal phase, SACC availability (EE), and FR requirement as drug cost (FR/mg) add to the effectiveness of addiction treatment.
Overall, these recent rat data and the current monkey results indicate that a nondrug reward (e.g., SACC, exercise) combined with a pharmacological agent (e.g., progesterone, atomoxetine) has an additive effect, and optimal behavioral economic conditions for treatment are defined. However, in the present study a key novel aspect of the success of the combined treatments may have been the long duration of self-administration and SACC treatment. Access to SACC in the present study occurred over a year, while previous treatment studies with monkeys have lasted for days – weeks. A recent study conducted with rats and treatment with physical exercise indicated that extended treatment durations may be needed to prevent incubation of craving and relapse that can occur several weeks to months after termination of drug access, as drug cues are not always extinguished and animals move to different environments (Zlebnik and Carroll 2015).
Limitations of the present study that may constrict interpretation of the results were that PRO was not administered or measured, but inferred from the phase of the menstrual cycle. Thus, dose-response functions were not available. Also, drug access was not suspended in order to determine whether there was incubation of craving over time or if treatment prevented the renewal of drug seeking during exposure to contextual cues after an abstinence period. Another limitation was the narrow range of response costs that was not comparable to a wider range that might have occurred with iv self-administration (e.g., Mello et al. 2008, 2011). Finally, substitution of SACC for water during the 3-hr sessions, does not have translational significance for humans, as would other healthier nondrug rewards such as exercise or social contact that are difficult to administer to monkeys. However, exercise and other forms of alternative reinforcement have been extensively studied, and increasing evidence supports their value in the prevention and treatment of drug abuse and other pathological processes, especially those like addiction that involve stress (Solinas et al. 2010; Bardo and Compton 2015; Zhou et al. 2015).
In conclusion, single-housed male and female monkeys, maintained in groups in a stable, social environment, self-administered orally-delivered cocaine (0.2, 0.4, and 0.8 mg/ml) under FR 2, 4, and 8 schedules for 3 h daily for up to 2 years. Females showed higher cocaine intake (mg/kg) in the follicular phase compared to their luteal phase and to males. Cocaine deliveries and intake (mg/kg) increased with cocaine concentration when concurrent SACC or water was available on a second drinking spout during the 3-hr sessions under the same FR schedules. Concurrrent SACC availability reduced cocaine deliveries and intake, especially at the higher cocaine concentrations. The results indicated that in females’ cocaine was reduced both during the luteal phase (when PRO peaks) and when SACC was concurrently available, suggesting that the luteal phase of the menstrual cycle and SACC add to reduce cocaine seeking in females at the highest concentration (0.8 mg/ml).
Acknowledgments
The authors are grateful to Troy Velie and Krista Johnson for their technical assistance and to Dr. Krista Walkowiak, Dr. Nellie Hegge, and Jeff Davis for their assistance with animal care. This research was supported by the National Institute on Drug Abuse grants R01 DA002486 and K05 DA015267 (MEC), and the University Medical Foundation, University of Minnesota.
References
- Allen SS, Bade T, Center B, Finstad D, Hatsukami D. Menstrual phase effects on smoking relapse. Addiction. 2008;103:809–821. doi: 10.1111/j.1360-0443.2008.02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and role of ovarian hormones. In: Neill JC, Kulkarni J, editors. Biological Basis of Sex Differences in Psychopharmacology. Vol. 8. Springer; London, UK: 2011. pp. 73–96. Current Topics in Behavioral Neurosciences. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Compton WM. Does physical activity protect against drug abuse vulnerability? Drug Alcohol Depend. 2015;153:3–13. doi: 10.1016/j.drugalcdep.2015.05.037. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology. 2001;155:278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Becker JB, Perry AN, Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biology of Sex Differences. 2012;3:14–35. doi: 10.1186/2042-6410-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Frontiers in Neurobiology. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Hughes JR, Higgins ST. Behavioral economics of drug self-administration I. Functional eqivalence of response requirement and drug dose. Life Sci. 1991;47:1501–1510. doi: 10.1016/0024-3205(90)90178-t. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA, Carroll ME. Deconstructing relative reinforcing efficacy and situating the measures of pharmacological reinforcement with behavioral economics: A theoretical proposal. Psychopharmacology. 2000;153:44–56. doi: 10.1007/s002130000589. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Carroll ME. Reduction of drug self-administration by an alternative non-drug reinforcer in rhesus monkeys magnitude and temporal effects. Psychopharmacology. 2000;147:418–425. doi: 10.1007/s002130050011. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Morgan AD, Carroll ME. Sex differences in the effects of baclofen on the acquisition of intravenous cocaine self-administration in rats. Dr Alc Dep. 2002;66:61–69. doi: 10.1016/s0376-8716(01)00185-5. [DOI] [PubMed] [Google Scholar]
- Carpenter MJ, Saladin ME, Leinback AS, Larowe SD, Upadhyaya HP. Menstrual phase effects on smoking cessation: a pilot feasibility study. J Womens Health (Larchmt) 2008;17:293–301. doi: 10.1089/jwh.2007.0415. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ, Perry JL. Modeling risk factors for nicotine and other drug abuse in the preclinical laboratory, Drug and Alcohol Dependence, Supplemental Issue: Women and Smoking: Understanding Socioeconomic Influences. Dr Alc Dep. 2009a;93:343–348. doi: 10.1016/j.drugalcdep.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Batulis DA, Landry KL, Morgan AD. Sex differences in the escalation of oral phencyclidine (PCP) self-administration under FR and PR schedules in rhesus monkeys. Psychopharmacology. 2005;180:414–426. doi: 10.1007/s00213-005-2182-x. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Campbell UC. A behavioral economic analysis of the reinforcing effects of drugs: transition states of addiction. In: Bickel WK, Vuchinich R, editors. Reframing Health Behavior Change with Behavioral Economics. Lawrence Erlbaum Associates, Inc; New Jersey: 2000. pp. 63–87. [Google Scholar]
- Carroll ME, Holtz NA, Zlebnik NE. The relationship between feeding and drug-seeking behaviors. In: Brewerton TD, Dennis AB, editors. Eating Disorders, Addictions and Substance Use Disorders: Research, Clinical and Treatment Aspects. Springer-Verlag; New York: 2014. [Google Scholar]
- Carroll ME, Kohl EA, Johnson KM, LaNasa RM. Increased impulsive choice for saccharin during PCP withdrawal in female monkeys: influence of menstrual cycle phase. Psychopharmacology. 2013b;227:413–424. doi: 10.1007/s00213-012-2963-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Krattiger KL, Gieske D, Sadoff DA. Cocaine-base smoking in rhesus monkeys: Reinforcing and physiological effects. Psychopharmacology. 1990;102:443–450. doi: 10.1007/BF02247123. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WD. How to study sex differences using animal models. Addiction Biology. 2016 doi: 10.1111/adb.12400. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Mach JL, LaNasa RM, Newman JL. Impulsivity as a behavioral measure of withdrawal of orally delivered PCP and nondrug rewards in male and female monkeys. Psychopharmacology. 2009b;207:85–98. doi: 10.1007/s00213-009-1636-y. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Roth ME, Voeller RK, Nguyen PD. Acquisition of oral phencyclidine self-administration in rhesus monkeys: effect of sex. Psychopharmacology. 2000;149:401–408. doi: 10.1007/s002130000389. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Smethells JR. Sex differences in behavioral dyscontrol: Role in drug addiction and novel treatments. Front Psychiatry. 2016;6:175. doi: 10.3389/fpsyt.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Grigson PS. Prior access to a sweet is more protective against cocaine self-administration in female rats than in male rats. Physiol Behav. 2013;113:96–103. doi: 10.1016/j.physbeh.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SL, Evans SM, Foltin RW, Haney M. Intranasal cocaine in humans: effects of sex and menstrual cycle. Pharmacol Biochem Behav. 2007;86:117–124. doi: 10.1016/j.pbb.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Hunt VR, Carroll ME. Effects of concurrent saccharin availability and buprenorphine pretreatment on demand for smoked cocaine base in rhesus monkeys. Psychopharmacology. 1994;115:15–23. doi: 10.1007/BF02244746. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Foltin W, Evans SM. Effects of menstrual cycle phase on cocaine self-adminsitration in rhesus macaques. Hormones and Behavior. 2013;63:105–113. doi: 10.1016/j.yhbeh.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Carroll ME. Effects of a non-drug reinforcer, saccharin, on oral self-administration of in male and female rhesus monkeys. Psychopharmacology. 2003;170:9–16. doi: 10.1007/s00213-003-1487-x. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Carroll ME. Differential effects of bremazocine on oral phencyclidine (PCP) self-administration in male and female rhesus monkeys. Exp Clin Psychopharm. 2004;12:111–117. doi: 10.1037/1064-1297.12.2.111. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Hunter R, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharm Biochem Behav. 2002;73:663–671. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Cotto JH, Davis E, Dowling GJ, Elcano JC, Staton AB, Weiss SR. Gender effects on drug use, abuse, and dependence: a special analysis of results from the National Survey on Drug use and Health. Gend Med. 2010;7:402–413. doi: 10.1016/j.genm.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Does the response to cocaine differ as a function of sex or hormonal status in human and non-human primates? Horm Behav. 2010;58:13–21. doi: 10.1016/j.yhbeh.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Byrd EA, Henderson AR, See RE. Attenuation of cocaine-seeking by progesterone treatment in female rats. Psychoneuroendocrinology. 2009;34:343–352. doi: 10.1016/j.psyneuen.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Haney M, Rubin E, Reed SC, Vadhan N, Balter R, Evans SM. Development of translational preclinical models in substance abuse: Effects of cocaine administration on cocaine choice in humans and nonhuman primates. Pharmacol Biochem Behav. 2015;134:12–21. doi: 10.1016/j.pbb.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Ehrman R, Lynch KG, Harper D, Sciortino N, O’Brien CP, Childress AR. Menstrual cycle phase at quit date predicts smoking status in an NRT treatment trial: a retrospective analysis. J Women’s Health. 2008;17:287–292. doi: 10.1089/jwh.2007.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Thompson T, Pentel PR, Flygaare B, Carroll ME. Self-administration of smoked cociane. Exp Clin Psychopharmacol. 1994;2:115–125. [Google Scholar]
- Hecht GS, Spear NE, Spear LP. Changes in progressive ratio responding for intravenous cocaine throughout the reproductive process in female rats. Devel Psychobiol. 1999;35:136–145. [PubMed] [Google Scholar]
- Higgins ST, Sigmon SC, Won CJ, Heil SH, Badger GJ, Donham R, Dantona RL, Anthony S. Community reinforcement therapy for cocaine-dependent outpatients. Arch Gen Psy. 2003;60:1043–1052. doi: 10.1001/archpsyc.60.9.1043. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Silverman K, Heil SH. Contingency Management in Substance Abuse Treatment. Guilford Press; New York: 2008. p. 380. [Google Scholar]
- Hudson A, Stamp JA. Ovarian hormones and propensity to drug relapse: a review. Neurosci Biobehav Rev. 2011;35:427–436. doi: 10.1016/j.neubiorev.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Paliwel, Chaplin TM, Mazure CM, Rounsaville BJ, Sinha R. Severity of childhood trauma is predictive of cocaine relapse outcomes in women but not men. Dr Alc Dep. 2008;92:208–216. doi: 10.1016/j.drugalcdep.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of coaine self-administraiton in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Kippen TE, Kapur S, van der Kooy D. Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology (Berl) 2005;182:245–252. doi: 10.1007/s00213-005-0071-y. [DOI] [PubMed] [Google Scholar]
- Llaneza DC, Frye CA. Progestogens and estrogen influence impulsive burying and avoidant freezing behavior of naturally cycling and ovariectomized rats. Pharmacol Biochem Behav. 2009;93:337–342. doi: 10.1016/j.pbb.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas SE, Sholar M, Lundahl LH, Lamas X, Kouri E, Wines JD, Kragie L, Mendelson JH. Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology (Berl) 1996;125:346–354. doi: 10.1007/BF02246017. [DOI] [PubMed] [Google Scholar]
- Mauro J, Serrone D, Somsin P, Stein AA. Cyclic vaginal cytologic patterns in the Macaca mulatta. Acto Cytol. 1970;14:348–352. [PubMed] [Google Scholar]
- Mazure CM, Toll B, McKee SA, Wu R, O’Malley SS. Menstrual cycle phase at quit date and smoking abstinence at 6 weeks in an open label trail of bupropion. Drug Alcohol Depend. 2011;114:68–72. doi: 10.1016/j.drugalcdep.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisch RA, George FR, Lemaire GA. Orally delivered cocaine as a reinforcer for rhesus monkeys. Pharmacol Biochem Behav. 1990;35:245–249. doi: 10.1016/0091-3057(90)90233-8. [DOI] [PubMed] [Google Scholar]
- Meisch RA, Stewart RB. Relative reinforcing effects of different doses of orally delivered cocaine. Dr Alc Dep. 1995;37:141–147. doi: 10.1016/0376-8716(94)01068-v. [DOI] [PubMed] [Google Scholar]
- Mello NK, Knudson IM, Kelly M, Fivel PA, Mendelson JH. Effects of progesterone and testosterone on cocaine self-administration and cocaine discrimination by female rhesus monkeys. Neuropsychopharm. 2011;36:2187–2199. doi: 10.1038/npp.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Knudson IM, Mendelson JH. Sex and menstrual cycle effects on progressive ratio measures of cocaine self-administration in cynomolgus monkeys. Neuropsychopharm. 2007;32:1956–1966. doi: 10.1038/sj.npp.1301314. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS, Knudson IM, Kelly M, Mendelson JH. Effects of estradiol on cocaine self-administration and cocaine discrimination by female rhesus monkeys. Neuropsychopharmacology. 2008;33:783–795. doi: 10.1038/sj.npp.1301451. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Kaufman MJ, Levin JM, Renshaw PF, Cohen BM. Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle. Neuropsychopharmacology. 1999;21:94–303. doi: 10.1016/S0893-133X(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Milivojevic V, Fox HC, Sofuoglu M, Covault J, Sinja R. Effects of progesterone stimulated allopregnanolone on craving and stress response in cocaine dependent men and women. Psychoneuroendocrinology. 2016;65:44–53. doi: 10.1016/j.psyneuen.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the care and use of laboratory animals. 8. The National Academies Press; Washington, DC: 2011. [Google Scholar]
- Newman JL, Carroll ME. Reinforcing effects of smoked methamphetamine in rhesus monkeys. Psychopharmacology. 2006;188:193–200. doi: 10.1007/s00213-006-0479-z. [DOI] [PubMed] [Google Scholar]
- Newman JL, Thorne JJ, Batulis DK, Carroll ME. Effects of menstrual cycle phase on the reinforcing effects of phencyclidine (PCP) in rhesus monkeys. Pharmacol Biochem Behav. 2006;85:584–591. doi: 10.1016/j.pbb.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg S, Wahlstrom G, Backstrom T, Sundstrom Poromaa I. Altered sensitivity to alcohol in the late luteal phase among patients with premenstrual dysphoric disorder. Psychoneuroendocrinology. 2004;29:767–777. doi: 10.1016/S0306-4530(03)00121-5. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Chudzynski J, Mooney L, Gonzales R, Ang A, Dickerson D, Penate J, Salem BA, Dolezal B, Cooper CB. Impact of an exercise intervention on methamphetamine use outcomes post-residential treatment care. Dr Alc Dep. 2015;56:21–28. doi: 10.1016/j.drugalcdep.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. The role of neurosteroids in the pathophysiology andtreatment of catamenial epilepsy. Epilepsy Res. 2009;85:1–30. doi: 10.1016/j.eplepsyres.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SC, Evans SM, Bedi G, Rubin E, Foltin RW. The effects of oral micronized progesterone on smoked cocaine self-administration in women. Horm Behav. 2011;59:227–235. doi: 10.1016/j.yhbeh.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DCS, Bennett SL, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Rodefer JS, Carroll ME. A comparison of progressive ratio schedules vs behavioral economic measures: Effect of an alternative reinforcer on the reinforcing efficacy of phencyclidine. Psychopharmacology. 1997;132:95–103. doi: 10.1007/s002130050325. [DOI] [PubMed] [Google Scholar]
- Rodefer JS, Carroll ME. Progressive ratio and behavioral economic evaluation of the reinforcing efficacy of orally-delivered phencyclidine and ethanol in monkeys: Effects of feeding conditions. Psychopharmacology. 1996;128:265–274. doi: 10.1007/s002130050134. [DOI] [PubMed] [Google Scholar]
- Rodefer JS, Mattox AJ, Thompson SS, Carroll ME. Effects of buprenorphine and an alternative nondrug reinforcer, alone and in combination on smoked cocaine self-administration in monkeys. Dr Alc Dep. 1997;45:21–29. doi: 10.1016/s0376-8716(97)01341-0. [DOI] [PubMed] [Google Scholar]
- Schiller CE, Saladin ME, Gray KM, Hartwell KJ, Carpenter MJ. Association between ovarian hormones and smoking behavior in women. Exp lin Psychopharmacol. 2012;20:251–257. doi: 10.1037/a0027759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T, Popik P. Attenuation of estrous cycle-dependent marble burying in female rats by acute treatment with progesterone and antidepressants. Psychoneuroendocrinology. 2007;32:651–659. doi: 10.1016/j.psyneuen.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Sershen H, Hashim A, Lajtha A. Gender differences in kappa-opioid modulation of cocaine-induced behavior and NMDA-evoked dopamine release. Brain Res. 1998;801:67–71. doi: 10.1016/s0006-8993(98)00546-0. [DOI] [PubMed] [Google Scholar]
- Silverman K, Svikis D, Wong CJ, Hampton J, Stitzer ML, Bigelow GE. A reinforcement-based therapeutic workplace for the treatment of drug abuse: three-year abstinence outcomes. Exp Clin Psychopharmacol. 2002;10:228–240. doi: 10.1037//1064-1297.10.3.228. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav. 2002;72:431–435. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol Biochem Behav. 2004;78:699–705. doi: 10.1016/j.pbb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Solinas M, Thiriet N, Chauvet C, Jaber M. Prevention and treatment of drug addiction by environmental enrichment. Prog Neurobiol. 2010;92:572–592. doi: 10.1016/j.pneurobio.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Rush CR. Combination pharmacotherapies forstimulant use disorder: a review of clinical findings and recommendations for future research. Expert Rev Cliln Pharmacol. 2014;7:363–374. doi: 10.1586/17512433.2014.909283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. [Google Scholar]
- Surgeon General’s Report ONDCP, 2004
- Zhou Y, Zhao M, Zhou C, Li R. Sex differences in drug addition and response to exercise intervention: From human to animals studies. Frontiers in Neuroendocrinology. 2015 Jul 13; doi: 10.1016/j.yfrne.2015.07.001. S0091-3022(15)30001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Carroll ME. Effects of the combination of wheel running and atomoxetine on cue- and cocaine-primed reinstatement in rats selected for high or low impulsivity. Psychopharmacology (Berl) 2015;232:1049–59. doi: 10.1007/s00213-014-3744-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NA, Saykao AT, Carroll ME. Effects of combined exercise and progesterone treatments on cocaine seeking in male and female rats. Psychopharmacology (Berl) 2014;231:3787–98. doi: 10.1007/s00213-014-3513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]