Abstract
A combination of advances spanning from isolation to delivery of potent HIV-specific antibodies have begun to revolutionize understandings of antibody-mediated antiviral activity. As a result, the set of broadly neutralizing and highly protective antibodies have grown in number, diversity, potency, and breadth of viral recognition and neutralization. These antibodies are now being further enhanced by rational engineering of their anti-HIV activities and coupled to cutting edge gene delivery and strategies to optimize their pharmacokinetics and biodistribution. As a result, the prospects for clinical use of HIV-specific antibodies to treat, clear, and prevent HIV infection are gaining momentum. Here we discuss the diverse methods whereby antibodies are being optimized for neutralization potency and breadth, biodistribution, pharmacokinetics, and effector function with the aim of revolutionizing HIV treatment and prevention options.
Graphical abstract
1. Introduction
Antibodies have demonstrated therapeutic utility since at least the 1890s in the form of “serum therapy” used to combat infectious diseases such as diphtheria, tetanus, influenza, measles, and polio. Although the first clinically approved monoclonal antibody (mAb), palivizumab [1, 2], similarly found utility in an infectious disease setting (RSV), the majority (28 out of 30) of current therapeutic mAb therapies have been developed for non-infectious applications in allergy, autoimmune diseases, and cancer. The demonstration of both clinical safety and efficacy of this treatment modality time and again across diverse disease settings has led to rapid growth in therapeutic mAb development in recent years. This renewed interest in antibody therapies has led to rapid technological advances in the isolation and characterization of mAbs, ushering in an era in which mAb engineering strategies are routinely used to translate basic understandings of antibody feature contributions into more efficacious and creative treatment and prevention strategies.
Against HIV, anti-viral antibodies have demonstrated the capacity to reduce viremia and protect from infection in several animal models [3–13] and in humans [14–16]. Humoral responses to HIV may be classified as non-neutralizing, neutralizing, or broadly neutralizing based on their ability to inhibit viral attachment and cell entry via binding to the HIV envelope glycoprotein (Env). The extensive pathogenic diversity and rapid evolution of HIV poses a considerable challenge to the natural development of neutralizing antibodies (NAbs) before establishment of a permanent viral reservoir.
Although the two HIV types (HIV-1 and HIV-2) share ~30–60% genetic similarity, they differ greatly in their epidemiology: HIV-1 dominates public attention as the most widespread and increasingly prevalent global health issue, whereas HIV-2 predominates in West Africa and is currently declining in prevalence [17, 18]. Furthermore, HIV-2 infection progresses to AIDS much more slowly than HIV-1 [19, 20] and is significantly less transmissible [21]. In addition to stronger virus-specific T-cell mediated immune responses [22–24], cellular resistance to infection [25], lower viral replication kinetics [26, 27], and an immune protective function of part of the HIV-2 envelope [28, 29], increased and broader NAb responses are implicated in the significant viral control and slower disease progression found in the majority of HIV-2 infected individuals (recently reviewed in [30]). From HIV-1 infection, broadly neutralizing abs (bNAbs) may appear after several years of persistent antigen exposure in a subset of patients with high viral load and progressive disease [31], at which point they are too late to offer any clinical benefit. In contrast, HIV-2 infection frequently results in the development of bNAb responses [32–36], and many infected individuals maintain low viral loads and normal CD4 T cell counts without antiviral therapy [19, 37–39]. In this review, we focus on engineering bNAbs against HIV-1 infection but much can be learned from natural HIV-2 humoral immunity. At the level of the infected individual, HIV-2 Env evolves at equivalent/faster rates than HIV-1 [40, 41], likely due to selective pressure from NAb responses [42]. Antigenic differences allowing for the greater generation of NAb responses in HIV-2 as compared to HIV-1 include greater stability of the Env trimer [43], less glycan shielding and a more ‘open’ conformation allowing for greater accessibility of NAb epitopes [36, 44], and greater structural and functional constraints to diversity in some NAb epitopes as compared to HIV-1 [45]. Differences in humoral responses to HIV-2 infection as compared to HIV-1 infection include 1) an increased frequency of both IgG and IgA responses [32, 46–48], 2) a predominance of IgG1 & IgG3 subclasses [48] (whereas the more inert IgG2 & IgG4 subclasses are additionally found in viremic HIV-1 infected individuals [49]), and 3) a greater contribution of antibody-dependent complement-dependent cytotoxicity to antiviral activity [50].
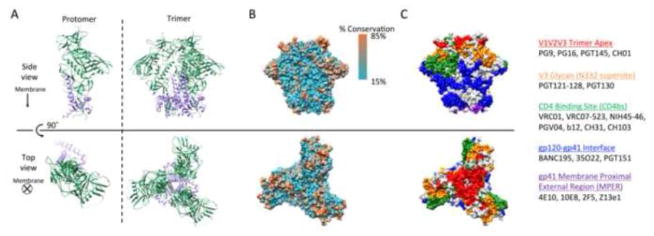
Despite the apparently greater challenges to mounting a natural antibody response to HIV-1 with high neutralization and effector capability, engineered bNAbs may similarly offer significant therapeutic potential to HIV-1 infected individuals. Rapid progress in screening HIV-1-infected donors for serum neutralizing activity [51–53] and in efficient antigen-specific B-cell sorting [54, 55] has led to the discovery of new, highly potent bNAbs [54, 56–65], as well as the structural definition of major sites vulnerable to neutralization on HIV-1 Env [60, 61, 66–81]. HIV-1 Env assembly entails heterodimerization of gp120 and gp41 subunits to form gp140 protomers, and trimerization of gp140 protomers (Figure 1A). Trimeric Env is inherently unstable, heavily glycosylated, expressed at low levels on the viral surface, and provides limited access to functionally critical epitopes [82, 83], features that contribute to viral fitness, immune escape, and the propensity to generate non-neutralizing antibody responses. Despite extensive viral diversity in Env sequences, bNAbs target conserved epitopes (Figure 1B), often involving both amino acid residues and/or glycosylation motifs, shared across several viral strains. Recent advances in next generation sequencing and antibody isolation have allowed researchers to better understand and identify both bNAb structural features contributing to potency and more broadly conserved Env epitopes targeted by bNAbs. These findings are catalogued on two publicly available databases: CATNAP (LANL) [84] and bNAber [85]. bNAb target epitopes comprise a broad continuum along the Env glycoprotein [86] but are commonly divided into 5 domains including 1) the trimer apex and variable loops V1, V2, and V3, 2) the V3 glycan supersite at Asn332, 3) the CD4 binding site (CD4bs), 4) the gp120-gp41 interface, and 5) the gp41 membrane-proximal external region (MPER) (Figure 1C).
Figure 1.
Structure of the HIV Envelope glycoprotein based on the crystal structure of BG505 SOSIP.664 (PDB ID: 4TVP). A] Ribbon representation of HIV gp140 protomer and trimers composed of gp120 (green) and gp41 (purple) subunits. B] Surface structure of HIV Env trimer colored by degree of conservation in the 2014 HIV Sequence Compendium from the LANL database [331]. C] Surface epitope domains colored by contact residues and residues ≤ 4Å from contact residues: V1V2V3 loop/trimer apex (red); V3 glycan-N332 supersite (orange); CD4 binding site (green); gp120-gp41 interface (blue); gp41 membrane-proximal external region (purple). Common bNAbs against each epitope domain are listed. Glycans are not shown, but importantly mask a significant portion of the viral Env surface. Images were generated using the UCSF Chimera package.[332]
Shared features of bNAbs isolated from HIV-1-infected individuals include high levels of somatic hypermutation [87–89], with 40–100 nucleotide mutations present in the heavy chain variable region (VH) alone [57, 58, 61, 72, 89–92], high levels of insertions/deletions [93], and long CDR-H3 regions which facilitate penetration of the Env glycan shield and access to functionally conserved regions [67, 71]. Whether these and other unusual features are generally required for broad neutralization or simply arise from extended periods of antibody maturation and selection is currently unknown, but has inspired rational protein engineering efforts based on sequence information from germline ancestors and clonal families of bNAbs. Generally, bNAbs exhibit characteristics reflective of natural trade-offs between potency, breadth, and specificity.
2. HIV-specific mAb therapeutic applications
HIV-specific mAbs have potential clinical roles in prevention, therapy, and even functional cure of HIV infection. Each of these applications may require antibodies optimized for different properties based on the different mechanisms of action that might be most effective in each setting.
2.1 Prevention of Infection
Naturally-raised neutralizing antibodies have been implicated in preventing infection in HIV-exposed but uninfected individuals [94–97] and in reducing the risk of mother-to-child transmission (MTCT) [98–102]. Beyond neutralization capacities, effector functions of antibodies have correlated with protection or reduced viral loads in vaccine trials in macaques [103–107] and reduced risk of infection in human VAX004 and RV144 trials [108–110]. Direct evidence for bNAb protection from acquisition of infection has been limited to pre-clinical models, which demonstrate that passive administration of bNAbs provides sterilizing immunity against simian HIV (SHIV) infection in macaques [5–7, 10, 111]. However, ex vivo studies of the neutralization sensitivity of human infant transmitted/founder strains to bNAbs have demonstrated encouraging results for MTCT prophylaxis [112–114], including the finding that the combination of PG9 and NIH45-46(G54W) neutralized all tested viruses from almost 90% of mother-infant pairs [114], and a Phase I clinical trial to evaluate the use of VRC01 in HIV-1 exposed infants is currently recruiting (clinicaltrials.gov identifier: NCT02256631).
2.2 Therapeutic Suppression of Viremia
Particular features of naturally-raised, anti-HIV antibodies are associated with spontaneous control of viremia without antiretroviral therapy (ART) in a subset of infected individuals known as elite controllers [115–117]. Passively administered bNAbs have resulted in suppresion of viremia after established infection in macaques [106, 118], humanized mice [119–123], and humans [14–16]. While clinical trials of first generation bNAbs, F105, 2F5, 2G12, 4E10, KD-247, and p2G12, have been completed in humans with largely underwhelming results of transient decreases in viral load [14, 15], the recent surge in identification/isolation of more potent bNAbs, combined with impressive demonstrations of protective effects in preclinical animal models, has inspired the return of anti-HIV mAbs to clinical trials with several currently recruiting to evaluate candidates 3BNC117, 10-1074, PG9, and VRC01 (clinicaltrials.gov).
Most recently, the results from the first Phase I dose-escalation studies of CD4bs-specific bNAbs VRC01 [124] and 3BNC117 [16] in humans have demonstrated clinical safety and, in the case of 3BNC117, the capacity to transiently reduce viral load for as long as therapeutic concentrations were maintained in serum in a dose-dependent manner. High doses resulted in up to 2.5log10 reductions in viral load. Additionally, the single individual who did not respond was infected with a virus resistant to 3BNC117. The single IV dose of 3BNC117 exerted selective pressure on patient viral populations and resulted in the development of highly resistant strains in some, but not all individuals, as demonstrated by 3NBC117 sensitivity testing of patient viral isolates and sequence analysis of viral Env. This landmark study demonstrates the significant therapeutic potential of bNAbs for treatment of HIV-infected individuals, although combinations of mAbs to avoid resistance or adjunctive therapy with ARV [106, 118, 119, 125] are expected to be required.
Additionally, passively administered anti-HIV antibodies can demonstrate indirect ‘vaccine-like effects’ whereby stimulation of the endogenous host immune response contributes to antiviral activity lasting well beyond the treatment period itself (reviewed [126]). In macaque studies of SIV and SHIV infection, SIV-/SHIV-IG treatment not only delayed disease onset and increased survival rates, but also accelerated de novo production of autologous anti-SIV antibodies [127, 128] and elevated virus-specific CD4+ and CD8+ T cell responses during both acute and chronic infection phases [129]. Similarly, administration of monoclonal anti-HIV bNAbs after SHIV infection to macaques also correlated with a slight increase in neutralizing antibody titers and significantly improved functionality of Gag-specific CD4+ and CD8+ T cell responses, with a subset of animals maintaining long-term virologic control in the absence of further mAb infusions [106]. While viral escape from single bNAb treatment does occasionally occur in macaques, as demonstrated by studies administering 3BNC117 and 10-1074 [118], single mAb treatment in humanized mice invariably leads to rapid viral escape from mAb neutralization [119, 125]. A recent critical study elucidated these results by demonstrating that autologous host humoral responses present in macaques (but impaired in humanized mice) contribute to the control of viremia by synergizing with passively administered bNAbs to prevent the emergence of viral escape variants [130]. Thus, therapeutic administration of bNAbs after established HIV infection may suppress viremia through both direct antibody-mediated functions and indirect stimulation of endogenous antiviral immune responses.
2.3 Functional Cure: Persistent surveillance and eradication of latent reservoirs
As opposed to sterilizing protection to prevent establishment of viral reservoirs at the time of initial viral exposure, a cure for chronic HIV infection requires a durable therapeutic to continuously surveil and destroy newly activated reservoir cells and/or clear or compromise nascent virions [131]. The only example of complete viral eradication thus far has been the Berlin patient, a formerly HIV-infected individual with acute myelogenous leukemia who underwent bone marrow/stem cell transplantation from a donor lacking expression of viral coreceptor CCR5 [132]. Less drastic approaches that preserve autologous immunity may instead implement mAbs to target viral reservoirs: 1) “kick and kill” strategies employ a combination of viral inducers to activate viral reservoirs and bNAbs to eliminate newly activated cells [131, 133], and 2) gene delivery strategies induce long-lasting expression and production of mAbs to continuously protect against newly activated reservoir cells and virion release [134]. For these strategies, understanding the dynamics of viral Env epitope expression on the surface of latently infected cells will be critical: current studies suggest that early in infection prior to virion release, cells will more likely express trimeric Env proteins [135] whereas later in the virus infection cycle, cells will more likely express monomeric gp140 and gp41 stumps as the dissociated remnants of viral Env [133]. Thus, neutralizing Abs may be best suited for acutely infected and recently reactivated cells and non-neutralizing Abs recognizing gp41 epitopes for latent, chronically infected cells. Furthermore, viral reservoirs may exist in multiple tissues [136], and concerns regarding compartmental differences in innate immune cell populations and access to immunologically privileged sites [133] may require the addition of nonnative functions to bNAbs to fully eradicate reservoirs.
Engineering strategies to enhance the clinical utility of bNAbs in each of these indications will be discussed according to approaches aimed at the different structural aspects of antibody activity: 1) Fv engineering to improve neutralization potency and breadth and to decrease polyreactivity and viral escape (Table 1), 2) Fc engineering to improve half-life, biodistribution, and recruitment of innate immunity (Table 2), and 3) combination with additional functional agents (Table 3), such as effector recruitment signals (T-cell engaging scFvs), and even cellular therapies (Chimeric Antigen Receptors). Finally, delivery strategies that can be applied to any engineered bNAb to further improve bioavailability and durability of response will be discussed.
Table 1.
Summary of HIV bNAb Fv engineering strategies.
| ↑ Potency and/or breadth of neutralization | Directed evolution | b12 | ↑ Affinity ~400x & ↑ breadth | 139,140 |
| m9 | ↑ Breadth through sequential antigen panning | 141 | ||
| Rational mutations | PG9_N100(F)Y | Stabilize CDR-H3 in active conformation | 142 | |
| NIH45-46_G54W, VRC07-523 | Improve Hydrophobic interactions | 65, 143 | ||
| 45–46m2 | Leverage glycan contacts | 144 | ||
| 45–46m2, 45–46m7, 45–46m25, and 45–46m28 | Avoid steric clashes between Ab & Ag escape variants | 144 | ||
| 10–1074&PGT121, PG9-PG16-RSH, 4E10&10E8 | Combine CDRs of bNAbs targeting similar epitopes | 60, 69, 145 | ||
| CD4-Ig | Replace Fv with extracellular domain of CD4 | 147–150, 153, 154 | ||
| Restrict viral escape | Rational mutations | 45–46m7, 45–46m25, and 45–46m28 | Bias antigen escape towards detrimental mutations | 144 |
| Combining mAbs | Combine mAbs with complementary resistance patterns | 156–158 | ||
| Target host cellular receptors | Ibalizumab (iMAb) | Target CD4 receptor | 159–162 | |
| PRO140 | Target CCR5 coreceptor | 159, 163–166 | ||
| bNAb + iMab / PRO140 | Bispecifics combining bNAbs with iMab or PRO140 | 167, 168 | ||
| ↓Polyreacvity/aggreggation propensity | Rational mutations | NIH45-46_G54W, 10E8 | Determine acceptable mutations based on clonal relatives | 65, 143 |
Table 2.
Summary of HIV bNAb Fc engineering strategies. Note the listed references for modifications represent a subset of applicable Fc engineering strategies.
| ↑ Half-life | ↑FcRn binding | CH2-CH3 domain | longer serum half-life, increased localization to mucosal epithelial surfaces | 184–186, 190 | 181 |
| ↑ Mucosal immunity | Reformatting as IgA | Isotype switching | enhanced mucosal localization & effector function | 192–193 | 192, 193 |
| ↑binding to FcαR | “Cross-isotype” IgG-IgA: IgGA | ↑macrophage-mediated ADCP & Neutrophil-mediated ADCC | 242 | ||
| ↑ Effector function | IgG Subclass Switching | IgG Fc switching | skewing towards particular effector functions | 215–216 | 214 |
| ↑ Binding to activating FcRs by protein engineering | ↑ FcγRIIIa binding | ↑ ADCC | 222, 231–234 | 214, 229–230 | |
| ↑FcγRIIa:FcγRIIb | ↑ ADCP & ADCC | 235, 237 | |||
| ↑ C1q binding | ↑ CDC | 239, 240 | |||
| Mixed IgG1-IgG3 Fc | ↑ CDC & ADCC | 241 | |||
| “Cross-isotype” IgG-IgA: IgGA | ↑ ADCC, ADCP, ADCDC | 242 | |||
| Self-assembling hexameric IgG | ↑ CDC | 243 | |||
| ↑ Binding to activating FcγRs by glycoengineering N297 | ↓Fucosylation | ↑ FcγRIIIa binding --> ↑ADCC | 245, 252 | 258 | |
| ↓Sialylation | ↓lectin receptor-mediated anti-inflammatory cascades --> ↑ ADCC | 253 | |||
| Mixed protein & glycoengineering | Mixed IgG1-IgG3 Fc + afucosylated | ↑ CDC & ADCC | 241 | ||
| Aglycosylated (bacterial expressed) + ↑FcγRI binding, no FcγRIIa/IIb binding | ↑ ADCC | 260 | |||
| Engineering the protein-carbohydrate interface | ↓ ADCC | 263 |
Table 3.
Summary of HIV bNAb engineering strategies to introduce non-native functions.
| Restrict viral escape | Bispecific molecules | 3BNC60, b12, 10-1074, PG16 | (Fab)2 molecules: both homo- and hetero-epitopic, permit intra-spike crosslinking | 273 |
| VRC07 & 10E8; PGT121 & PG9-PG16-RSH; bNAb & iMab/PRO140 | CrossMab heterodimerization bispecifics, potential synergism | 275, 276 | ||
| HY + 7B2 | Tetravalent dual variable domain Ig molecules (DVD-Igs), potential synergism | 277 | ||
| two epitopes on CCR5; PG9/PG16-iMAb | Tetravalent Morrison-type bispecifics, potential synergism | 167, 168 | ||
| Engage T-cell responses: targeting viral reservoirs | “Kick and kill” bispecifics | A32 (CD4i) / 7B2 (gp41) & anti-CD3 | Dual-Affinity Re-Targeting Proteins (DARTs) | 287 |
| VRC07 & anti-CD3 | Bispecific immunomodulatory protein | 288 | ||
| Chimeric antigen receptors (CAR) | CD4EC Domain; 98.6; F105; CD4-17b bispecific | CAR T-cells | 296–298, 196, 299, 300 | |
| CD4EC Domain | CAR embryonic stem cells | 305 |
3. Fv engineering
A recent study detailing the incomplete neutralization profiles of several bNAbs [137] reveals shortcomings in Fv regions of naturally elicited bNAbs that may be improved through engineering strategies (summarized in Table 1). For prevention of infection, broad and potent neutralization are particularly crucial to prevent establishment of a viral reservoir. After infection, additional measures to avoid viral escape are necessary to suppress viremia therapeutically. Towards functional cure, identification of the most prevalent epitopes on latent reservoir cells is most critical, but additional engineering to enhance potency may be necessary to target the Env expressed on reactivated, latently infected cells. In all of these cases, engineering strategies to reduce the polyreactivity common to many bNAbs will improve therapeutic efficacy and reduce the risk of adverse reactions.
3.1 To improve Potency & Breadth
Protein engineering strategies to improve the breadth and potency of bNAbs (reviewed in [138]) include both combinatorial directed evolution techniques and rational computational strategies. Early directed evolution of bNAb b12 through phage display enhanced affinity nearly 400-fold [139] and demonstrated that improved affinity could lead to increased breadth of neutralization [140]. This study demonstrated that affinity can in some cases be used as a surrogate marker for both potency and breadth in evolutionary strategies, suggesting the value of affinity maturation as a generalizable strategy for enhancing bNAb activity. In another approach, the neutralization breadth of the HIV-1 m9 scFv was evolved from a previously isolated X5 bNAb from phage display libraries by selecting for binders to sequentially changing antigens [141].
Computational strategies instead use information provided by both clonally related bNAbs and more extensive antibody databases and molecular modeling to inform mutational choices. The variable fragment (Fv) of antibodies consists of complementarity-determining residues (CDRs) involved in antigen-binding specificity and framework residues (FWRs) contributing to antibody structure. Rational protein engineering approaches to enhance potency and breadth of Env binding commonly manipulate CDR residues to enhance stability, conformation, orientation, energetics, or kinetics of Ab:antigen interactions. For example, stabilizing the CDR3 of the heavy chain (CDR-H3) of bNAb PG9 in its active conformation led to the increased potency and breadth of engineered variant PG9_N100(F)Y, which neutralizes diverse PG9-resistant HIV strains, some of which lack the Env N160 glycan critical for PG9 binding [142]. Rational mutations to improve hydrophobic interactions [65, 143], leverage glycan contacts [144], and to avoid steric clashes [144] between antibody and antigen have similarly generated VRC01-class Ab variants with increased potency and breadth and have resulted in neutralization of strains resistant to the original antibody. Combining CDR features of bNAbs targeting similar epitopes has conferred increased breadth of neutralization and accommodation of glycan heterogeneity and/or proximal membrane lipids to antibody hybrids of 10-1074 & PGT121 [60], PG9 & PG16 [69], and 4E10 & 10E8 [145]. Although often overlooked in antibody-antigen interactions, FWRs may present another strategy through which to enhance antibody Fv’s, as some FWR mutations directly contribute to breadth and neutralization [89, 146].
An alternative strategy replaces the Fv region of an antibody entirely with the extracellular domain of CD4, the primary receptor of HIV-1 Env. These CD4-Ig immunoadhesin molecules achieve broad neutralization, irreversible inactivation of Env, and selection for less-fit escape variants with impaired receptor binding [147–150] but suffer from a lower affinity for Env compared to bNAbs [151] and a simultaneous capacity to promote infection at subneutralizing concentrations (undetermined mechanism) [152]. Nevertheless, CD4-Ig molecule PRO542 has demonstrated safety and efficacy (80% response rate, ~0.5log10 reduction in viral load 4–6wks post-treatment) as salvage therapy in advanced HIV-1 disease [153] and a recently improved version fusing CD4-Ig with a CCR5 mimetic peptide at the C-terminus of the Fc, named eCD4-Ig, has demonstrated unmatched neutralization breadth (including strains resistant to CD4bs bNAbs NIH45-46, VRC01, 3BNC117) and potency thought to be due in part to higher avidity from the CCR5 mimetic, a decreased ability to promote infection, and enhanced antibody-dependent cellular cytotoxicity (ADCC) [154]. Significantly, AAV delivery of eCD4-Ig to rhesus macaques protected all inoculated macaques from multiple infectious doses, lasting for at least 34 weeks after inoculation. Thus, eCD4-Ig represents one of the broadest “bNAbs” tested to date and may prove beneficial in combination therapy with other bNAbs or as sole AAV therapy if anti-eCD4-Ig responses, or other aspects of dosing subjects with recombinant CD4 receptor, do not preclude its use.
3.2 Restricting viral escape
Even with the engineered improvements in potency and breadth of VRC01-class engineered antibody NIH45-46(G54W), some HIV-1 clones are naturally resistant [143] and escape mutants can emerge during exposure [125]. Rational engineering of NIH45-46(G54W) based on the structure of the NIH45-46—gp120 complex and Env sequences of NIH45-46-resistant viral strains resulted in variants 45–46m2 and 45–46m7 [144]. Whereas viral escape mutants in mice treated with NIH45-46(G54W) exhibited mutations anywhere along the highly conserved N/DNGG (aa279-282) consensus sequence identified as involved in resistance to this Ab [155], escape mutants from mice treated with the enhanced variants 45–46m2 and 45–46m7 were limited to substitutions to introduce a potential N-linked glycosylation site (PNGS) at N279 that introduces a fitness cost that may be sufficiently severe to account for its rare or nonexistent presence in natural HIV-1 strains catalogued in the Los Alamos database [144]. By restricting the pathways for HIV-1 escape with the new 45–46m2/m7 variants and imposing a fitness cost for escape mutations, the authors demonstrated control of viremia in humanized mice using only three antibodies—45–46m2, 45–46m7, and 10-1074—targeting only two epitopes. Thus, protein engineering strategies to restrict viral escape can allow for better viral control with fewer mAbs.
A second strategy to combat resistance involves the identification and combination of mAbs with complementary resistance patterns, where mutations leading to viral escape from one mAb render it more susceptible to the complementary mAb. Studies investigating the in vitro neutralizing activities of combinations of 2–4 mAbs targeting four distinct epitopes (CD4bs, V1V2-glycan, V3-glycan, gp41MPER) across a panel of 125 Env-pseudotyped viruses found that combinations of bNAbs with complementary neutralization profiles recognizing distinct epitopes resulted in improved neutralization breadth closely predicted by an additive effect model [156]. At 50% inhibitory concentration (IC50) cutoffs of 1 μg/mL per antibody, combinations of two mAbs neutralized 89–98% of viruses and combinations of three neutralized 98–100% of viruses. Statistically significant, albeit weak, synergy was further observed in 15 out of 22 mAb combinations, consistent with earlier reports combining earlier generation bNAbs [157, 158]. By targeting multiple epitope specificities, combinations of mAbs increase the breadth of viruses neutralized and may thereby prevent resistance by outpacing viral evolution and escape.
In addition to targeting various Env epitopes, therapeutic anti-HIV mAbs may target host cellular receptors necessary for viral entry (recently reviewed in [159]). Ibalizumab (iMab), a humanized IgG4 against the CD4 receptor, has demonstrated broad inhibition against a panel of >100 HIV-1 Env pseudoviruses in vitro, inhibiting 92% of viruses, but has substantially lower potency than bNAbs PG9 or VRC01. Although the mechanism of action remains uncertain, recent single-molecule force spectroscopic analysis of the CD4-gp120 interaction suggests that gp120 binding to CD4 induces a mechanical extension of CD4 domains 1 and 2 that is inhibited by iMab [160]. iMab is currently in phase 3 clinical trials for HIV-1 infection and has received orphan drug designation from the US FDA Office of Orphan Products Development. The predominant resistance mechanism against iMAb entails the loss of V5 PNGS which enhance sensitivity to neutralization by VRC01 [161], and other resistant variants have demonstrated enhanced sensitivity to soluble CD4 (sCD4) [162]. Thus, combining iMAb with CD4bs-targeting bNAbs or sCD4 may also prevent viral escape.
Similarly, PRO140, a humanized IgG4 against the CCR5 coreceptor, has demonstrated broad anti-HIV-1 activity in PBMC and macrophage neutralization assays against R5- and dual-tropic viruses [163]. PRO140 acts noncompetitively to allosterically inhibit viral attachment [164] and is currently in phase 2b studies. Viral resistance to therapy develops less rapidly for PRO140 compared to traditional ARV: R5 viral susceptibility to PRO140 remained intact following 3 weeks of subcutaneous monotherapy [165] and two weeks after single IV dose of PRO140 [166]. Furthermore, bispecific IgG combining the specificities of bNAbs with iMab or PRO140 exhibited highly synergistic effects [167]. One tetravalent, bispecific CCR5 antibody blocking two alternative docking sites of R5-tropic HIV strains on the CCR5 coreceptor has demonstrated 18–57 fold improved potency with one combination demonstrating antiviral activity against virus strains resistant to each parental Ab alone [168].
3.3 To Decrease Polyreactivity and Improve Pharmacokinetics
Polyreactive/autoreactive antibodies have been associated with HIV-1 infection for more than 25 years [169–171], and higher frequencies of polyreactivity are found among bNAbs compared to non-neutralizing Abs [172]. Importantly, multiple studies have reported that increased polyreactivity and/or reduced solubility and increased aggregation propensity can accompany increasing breadth/potency [65, 173, 174]. Polyreactivity is correlated with increased rates of clearance in vivo [175]. Efforts to combat polyreactivity and aggregation propensities that could lead to polyreactivity include isotype switching, inserting N-linked glycosylation sites, and reducing the number of surface hydrophobic residues [138]. In engineered variants of NIH45-46 and VRC07-523 [65, 143], a G54W mutation enhanced potency but also increased polyreactivity. Substitution to histidine, which was observed in clonal relatives and thereby likely acceptable to Ab function, decreased polyreactivity and improved pharmacokinetics while maintaining the improved potency of G54W [65]. Sequence and structural analysis of clonal relatives of 10E8 similarly led to new variants with equivalent potency and nearly 10-fold increased solubility [176].
4. Fc Engineering
Antibody Fc’s engage a wide range of soluble and cellular receptors (recently reviewed in [177]) with varying preferences dependent upon antibody isotype, subclass, and glycosylation status. By binding to transport receptors, the antibody Fc directs the trafficking of antibodies and immune complexes and determines both the serum half-life and biodistribution of therapeutic mAbs. By binding to Fc Receptors (FcRs) on innate immune cells or complement receptors (such as C1q), the Fc serves as a mediator between host innate and adaptive immune responses and stimulates effector cells to contribute not only to direct antibody-mediated responses such as Ab-dependent cellular cytotoxicity (ADCC), Ab-dependent cellular phagocytosis (ADCP), and antibody-dependent complement-dependent cytotoxicity (ADCDC), but also to the generation of durable endogenous adaptive immunity [126]. Thus, engineering the Fc domains of HIV-specific antibodies (reviewed in [178] and [179]) to enhance half-life, biodistribution, and effector functions can significantly improve bNAbs’ clinical efficacy (summarized in Table 2).
4.1 To increase half-life
Most clinical mAbs are of the IgG isotype (Figure 2), which can interact with the neonatal Fc Receptor (FcRn) in a pH-dependent manner to allow for transport of maternal antibodies to fetuses in utero and recycling of antibodies after internalization in numerous cell and tissue types. FcRn binds IgG tightly in acidic endosomal vesicles, but weakly at the neutral cell surface where it is released [180]. FcRn binding can additionally direct antibody transport across epithelial surfaces [181, 182] or immune complex targeting to lysosomes for degradation [183]. A recent key study demonstrated that anti-HIV bNAb variants engineered for enhanced FcRn binding exhibited longer serum half-life, enhanced localization to and persistence at mucosal epithelial surfaces, and ultimately superior protection from mucosal intrarectal SHIV challenge in macaques [181]. In addition to protecting from mucosal transmission, FcRn-binding enhanced variants may prove especially useful in MTCT for in utero prophylaxis to deliver bNAbs to the fetus.
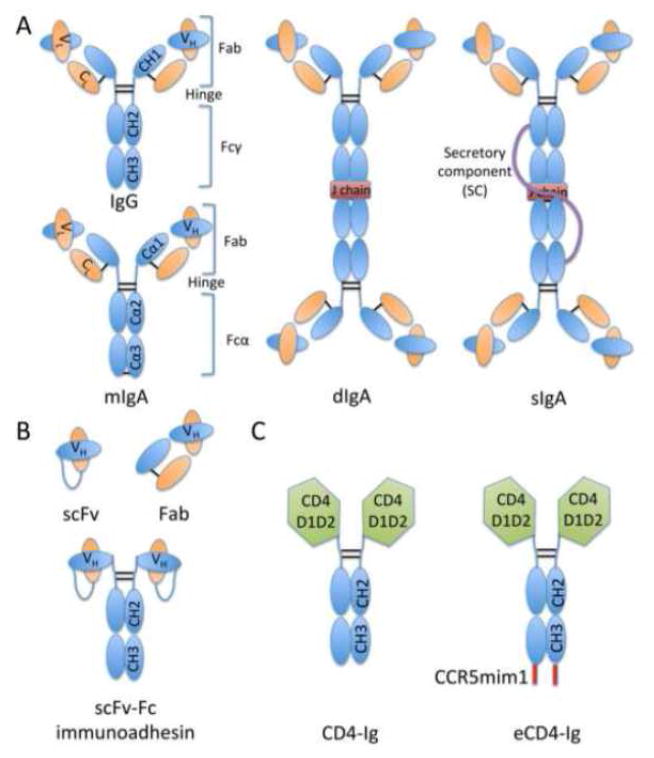
Figure 2.
Antibodies and antibody-based molecules. A] Cartoon representations of antibody isotypes IgG, monomeric IgA (mIgA), dimeric IgA (dIgA), and secretory IgA (sIgA) are depicted and color coded as follows: heavy chain (blue); light chain (orange); disulfide bonds (black lines); J chain (red); secretory component (purple line). Constant heavy domains 1–3 (CH1-CH3), constant light domain (CL), variable heavy (VH), variable light (VL), antigen-binding fragment (Fab), and crystallizable fragment (Fc) domains are also annotated. B] Antibody-based molecules include the single-chain variable fragment (scFv), antigen-binding fragment (Fab), and scFv-Fc immunoadhesin molecules. Linkers are represented by blue lines. C] CD4-Ig molecules replace scFv/Fab regions with CD4 extracellular domains 1 & 2 (CD4 D1D2 in green). eCD4-Ig additionally includes a C-terminal CCR5 mimetic peptide (CCR5mim1).
Several human IgG Fc variants have been developed to enhance FcRn binding at acidic pH and maintain no or moderate binding at neutral pH [184–186] by targeting Fc residues in the CH2 and CH3 domains responsible for FcRn binding [187, 188]. While these domains are physically distinct from the CH1 and hinge regions bound by FcγRs for effector function, a recent study examining four human IgG1 variants engineered for enhanced FcRn binding found that in most cases, Fc engineered mutants bound human C1q and FcγRs less strongly than the wild type IgG1 and demonstrated varying abilities to induce ADCP and ADCC in vitro [189]. The authors noted that differences in glycosylation were found between WT and mutant variants, even when mutations were introduced distally from the N297 glycosylation site. Thus, mutations at the CH2-CH3 interface may impact the glycosylation profile and/or flexibility of the domains and thereby directly affect interaction with C1q and FcγRs to explain the unpredictable changes in effector functions. This study suggests that Fc engineering for enhanced binding to FcRn may indirectly affect FcγR binding and effector functions, so mAb engineering to optimize mutations to balance increased half-life and clinical efficacy, as has been done for ADCC [190], may be critical.
4.2 To improve mucosal immunity
In addition to IgG, IgA is found at mucosal surfaces through binding of its Fc to polymeric IgA Receptors (pIgR). The role of IgA in HIV infection remains controversial [191], with findings of HIV-specific mucosal IgA in exposed but uninfected subjects [94–97] supporting a potentially protective role of mucosal IgA in HIV infection while associations of HIV-specific plasma IgA with increased risk of infection in a vaccine trial [110] suggest a negative role in protection. Passive transfer studies in macaques allowing for more systematic and controlled evaluation of antibody Fc/dose/localization and route of viral challenge clarify the protective potential of mucosal IgA: intrarectal administration of dimeric IgA1 (dIgA1) afforded greater protection from intrarectal viral challenge than dimeric IgA2 or IgG1 bearing the same neutralizing Fv [192], and the combination of intravenous IgG1 with mucosal administration of dIgA1 demonstrated superior protection to either antibody alone [193]. Thus, in addition to the development of bNAbs as FcRn-binding enhanced IgG1 variants, formulation as IgA (Figure 2) may prove beneficial to protect against mucosal infection.
For mucosal immunity, dimeric IgA (dIgA) binds to the polymeric Ig receptor (pIgR) for transport to mucosal surfaces where it is released as secretory IgA (sIgA) [177]. The localization and polymeric tendencies of IgA enable it to aggregate and trap virions at mucosal sites [194, 195], with enhanced inhibitory effects observed from bNAbs expressed as dIgA compared to monomeric IgA (mIgA) on mucosal transmission of HIV in humanized mice [196]. Successful recombinant protein engineering of mIgA [197], dIgA [198], sIgA [199], and a stabilized IgA2m(2) allotype [200] has been described and avails the opportunity to better understand the protective mechanisms of IgA variants when delivered intravenously vs. mucosally. Further engineering efforts to enhance binding to activating FcαRs to increase ADCP or to polymeric Ig receptor (pIgR) to facilitate excretion of HIV from mucosal lamina propria through transcytosis [201] could additionally enhance protection of bNAbs re-engineered as IgA.
4.3 To enhance effector functions
Antibodies can engage a wide range of innate immune cells to exert antiviral activity through ADCC, ADCP, and ADCDC. Elevated levels of ADCC-inducing Abs correlate positively with spontaneous control of HIV without ARV in elite controllers [117] and reduced mortality in infant cases of MTCT when passively acquired through breast milk [202] and inversely with rate of progression to AIDS [203–206] and infection risk in the VAX004 vaccine trial [109] and among individuals with lower IgA responses in the RV144 trial [110]. Similarly, evidence of a role for ADCP in controlling HIV disease progression has been documented [207–209] and higher antibody phagocytic capacities have been found in HIV elite controllers compared to chronic progressors [115]. The role of antibody-dependent CDC is less well defined since initiation of the complement cascade may occur in the absence of antibody, making it difficult to separate ADCDC from CDC present in HIV-infection [210]. However, ADCDC has been associated with decreased viral load [211] and neutralization-independent protection from heterologous virus challenge in primary infection [212].
Through antibody effector functions, non-neutralizing antibodies may also derive therapeutic value and have been demonstrated to reduce viral load upon topical vaginal application in macaques challenged with Simian/human immunodeficiency virus (SHIV), although no prevention of SHIV acquisition was seen in these animals [213]. Furthermore, a landmark study found that the in vivo protective activity of anti-HIV bNAbs more precisely correlates with their ability to engage activating Fc-gamma receptors (FcγRs) than their in vitro neutralization activity [214]. Thus, as bNAbs exert their in vivo efficacy through Fc-mediated effector functions, an opportunity arises to engineer existing bNAb Fc regions to enhance binding to activating Fc receptors or to skew towards particular Fc-mediated responses.
4.3.1 IgG Subclass
IgG antibodies dominate the anti-HIV response and are responsible for the effector functions ADCC, ADCP, and ADCDC. The relative prevalence of IgG subclasses IgG1-IgG4 in plasma correspond to their numbering, but each subclass’ distinct profile for FcR binding determines its relative contribution to effector functions [215, 216], with IgG1 and IgG3 preferentially engaging activating FcRs for ADCC, IgG2 for ADCP, and IgG4 better engaging inhibitory receptors. By harnessing these natural variations in effector function preferences, IgG subclass switching has been demonstrated to alter the effector functions and in vivo efficacy of anti-HIV mAbs in mouse models [214], as well as in numerous other settings.
Particular subclass profiles have been associated with viremic control in natural infection: elite controllers tend to exhibit higher IgG1 titers to p24 and gp120 and higher IgG3 titers to Env gp120 compared to chronic progressors [116], corresponding to increased engagement of activating FcRs for ADCC. Similarly, vaccine trials have also provided associations of particular antibody subclass profiles with reduced risk of infection. RV144 and VAX003 are two different vaccine trials that included administration of the bivalent recombinant gp120 AIDSVAX B/E protein. Both trials elicited gp120-specific antibodies, but poor antibody neutralization activity [217] and negligible cytotoxic T cell responses [218]. However, the RV144 vaccine regimen successfully, albeit moderately, reduced the risk of infection among vaccinees [219]. A recent study demonstrated that antibodies induced by the protective RV144 trial were capable of eliciting multiple effector functions and included induction of IgG3 Abs, whereas VAX003-induced antibodies were more ‘monofunctional’ and skewed towards the more inert IgG2 and IgG4 subclasses [220]. Even though both trials elicited humoral responses largely composed of IgG1, depletion of IgG3 from RV144 samples significantly reduced ADCC and ADCP activity, and depletion of IgG4 from VAX003 samples significantly increased ADCP activity and trended toward increased ADCC activity. Thus, even low levels of particular antibody subclasses significantly altered the effector profiles of humoral responses in these two vaccine trials, emphasizing the importance of the functional quality of antibodies. These findings encourage anti-HIV mAb engineering efforts to generate therapeutic IgG3s in addition to the typical IgG1 molecules that have dominated clinical mAb development thus far.
4.3.2 IgG Fc Engineering
Combinations of finer amino acid mutations and glycoengineering strategies to enhance Fc participation in ADCC (reviewed in [179, 221]), CDC [221], and ADCP [222] have generated an arsenal of Fc variants to incorporate into clinical mAbs. Studies have demonstrated that the in vivo efficacy of antibody therapeutics is determined by selective interactions of the Fc domain with activating and inhibitory Fc Receptors (FcRs) expressed on the surface of effector cells [216, 223]. Previous studies have implicated FcγRs in the protection afforded by HIV-specific antibodies [224–228] and demonstrated that Fc effector activity is necessary for the protective activity of anti-HIV mAbs in macaques [229] and humanized mice [230]. A recent study employing Fc domain engineering to selectively enhance or diminish engagement of activating FcγRs has demonstrated that the capacity to engage activating FcγRs predicts the in vivo efficacy of anti-HIV mAbs more accurately than in vitro neutralization activity: poorly neutralizing anti-HIV mAbs gained potent in vivo activity when expressed as IgG subclass variants capable of engaging activating FcγRs, and bNAbs exhibited reduced in vivo potency when their ability to engage activating FcγRs was compromised [214].
Protein engineering efforts to improve ADCC have roots in improving cancer mAb therapies and utilize a combination of rational engineering based on Fc/FcR co-crystal structures and directed evolution by yeast and bacterial display to generate Fc variants with enhanced FcγRIIIa binding [222, 231–233]. The most potent engineered variants (S239D-I332E) exhibit stronger binding to FcγRIIa and FcγRIIb in addition to FcγRIIIa, increase NK cell-mediated ADCC and macrophage-mediated ADCP [222], and potently improved the in vivo efficacy of tumor immunotherapy anti-CD19 antibodies in monkeys [234]. A newly described approach to enhance ADCC combines amino acid mutations asymmetrically to generate Fc heterodimers with similar or superior potency in ADCC, increased stability in the CH2 domain compared to conventional Fc variants, and selectively improved binding to activating FcγRIIa over inhibitory FcγRIIb [235].
Efforts to improve ADCP are motivated by studies demonstrating that the affinity ratio of immune complex binding to activating FcγRIIa vs. inhibitory FcγRIIb determines whether inflammatory or attenuated immune responses are set into motion [236]. Within the realm of HIV, antibodies from elite controllers and untreated progressors exhibit greater phagocytic activity, altered Fc domain glycosylation, and higher ratios of FcγRIIa: FcγRIIb binding compared to untreated progressors [115]. Identification of an Fc variant, G236A, with 15-fold enhanced FcγRIIa:FcγRIIb binding ratios, mediated enhanced ADCP by macrophages, and combinations with other mutations resulted in Fc variants with both high NK-cell-mediated ADCC and macrophage-mediated ADCP in the setting of cancer [237]. Such variants may be especially relevant for mAb targeting of viral tissue reservoirs where macrophages reside [133], in addition to viral suppression by phagocytosis of opsonized viral particles.
Lastly, strategies to improve CDC activity have similarly relied upon structural identification of C1q binding to the CH2 domain of IgG1 [238]. Whereas many engineered, CDC-enhanced variants exhibited decreases in ADCC activity [239, 240], the most potent variant to date (S276E-H268F-S234T + G236A-I332E) demonstrates 23-fold enhanced CDC activity and maintains ADCC activity similar to wild-type [240]. Alternative approaches to enhance CDC include 1) mixed IgG1-IgG3 Fc variants: one variant (1133) consisting of the CH1, hinge, and a portion of the COOH-terminal CH3 domain from IgG1 and the Fc from IgG3 enhanced both CDC and ADCC activity of antitumor antibodies against CD52 in a cynomolgus monkey model [241], 2) mixed IgG-IgA “cross-isotype” variants which additionally binds to FcαRI comparably to IgA and mediates greater CDC than IgG1 or IgA Abs [242], and 3) the creation of self-assembling hexameric IgG [243].
4.3.3 IgG Fc Glycoengineering
Within an IgG subclass, further variation in immune activation results from >30 different glycoforms possibly present on the Fc domain at Asn297 of the heavy chain [244] which can affect the conformation of the Fc and its ability to interact with FcgRs. Three sugar residues in particular dramatically alter antibody Fc binding to FcRs: 1) fucosylation of the mannose core decreases binding affinity to FcgRIIIA [245], 2) sialylation of terminal galactose groups allows for lectin receptor binding to initiate anti-inflammatory cascades [246], and 3) outer arm galactosylation enhances binding to C1q and FcγRI [247–249], decreases binding to FcγRIIIA [228], and can implement anti-inflammatory responses through immune complex-mediated associations of FcγRIIB with dectin-1 [250]. Antibody glycosylation profiles of HIV infected individuals exhibiting spontaneous control and improved antiviral activity have demonstrated a global shift toward agalactosylated glycoforms and an even more dramatic shift of HIV-specific antibodies toward agalactosylated, afucosylated, and asialylated glycans [228]. Glycoengineering efforts to improve therapeutic antibodies are still relatively new but supported by studies demonstrating that preparations of antibodies from different expression cell lines bear distinct glycosylation profiles and different capacities to trigger effector functions (reviewed in [251]). Glycoengineering efforts to decrease fucosylation [245, 252] and/or sialylation [253] may be accomplished through antibody production in modified cell lines, including mammalian (CHO), insect, yeast, and plant cell lines [254, 255], or through the use of specific enzymes that allow rational remodeling of antibodies’ Fc bound N-glycan structures [256, 257]. A non-fucosylated variant of HIV bNAb b12 predictably increased FcγRIIIA binding and ADCC activity in vitro but unexpectedly did not enhance protection against viral challenge in vivo [258], possibly due to maximal ADCC activity already achieved by the wild type b12, or due to a greater relevance of other FcγR-mediated functions to in vivo protection. Because non-fucosylated antibodies have often led to higher efficacy in other settings, this study highlights the importance of understanding the in vivo contributions of a given bnAb and its Ab effector functions to prevention, therapy, and cure to better direct Fc engineering efforts towards clinically relevant goals.
Combined protein and glyco-engineering efforts can improve effector functions [241, 245, 259, 260] but in some cases improve binding affinity to FcγRs without eliciting stronger effector function [258, 261, 262]. These examples suggest that affinity thresholds for effector function exist that may already be met by single protein- or glyco-engineered variants, so enhanced affinity does not improve activity [221]. However, combining the two strategies can expand the breadth of mAb binding to FcγRs and C1q to increase the range of effector mechanisms through which therapeutic mAbs can recruit innate immunity [241, 260, 263], which may be particularly useful in combatting viral reservoirs in various tissue compartments, each with potentially different profiles of innate immune cell populations.
5. Adding non-native functions to bNAbs
Engineering anti-HIV mAb therapies offers the opportunity to move a step beyond naturally occurring bNAbs to add non-native functions for enhanced clinical utility in HIV prevention, therapy, and cure. Many such strategies exist and include conjugation of antiviral mAbs or mAb-based molecules to: 1) cholesterol to enhance potency through cholesterol-mediated interactions with the viral membrane [264], 2) small molecule inhibitors of viral entry to enhance neutralization potency [265], 3) CD4 domains to expose CD4-inducible epitopes on viral Env [266–268], and 4) recombinant immunotoxins to enhance cytotoxicity [269–271]. In this review, we focus on the recent development of various bispecific formats (Figure 3) to combat viral resistance and T-cell engaging molecules/cells to eradicate latent HIV reservoirs (summarized in Table 3).
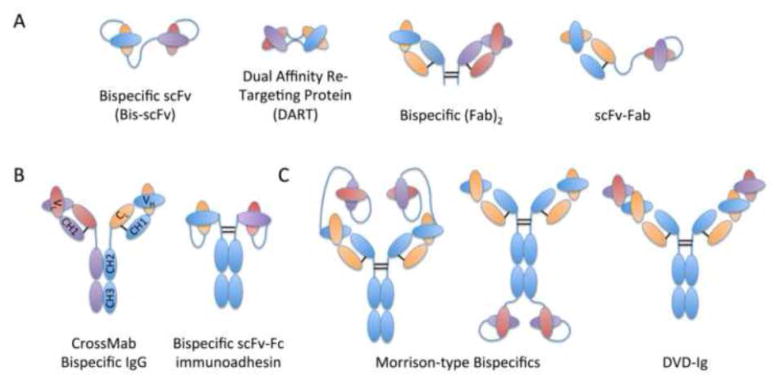
Figure 3.
Bispecific antibody formats. Cartoon representations of Fab- and scFv-based bispecifics (A), divalent Fc-containing bispecifics (B), and tetravalent bispecific antibody formats (C) are depicted and color-coded by parental mAbs: mAb 1 HC (blue) and LC (orange); mAb 2 HC (purple) and LC (red).
5.1 Bispecifics
Encouraging results from studies combining mAbs to optimize neutralization potency and breadth and to limit viral resistance have inspired the development of bispecifics which allow targeting of two epitopes with a single therapeutic agent (reviewed in [138]). Furthermore, low surface densities of Env and the trimeric Env structure represent viral evasion strategies to prevent bivalent inter- or intra-spike binding of natural IgGs [272] which can be overcome by divalent (Fab)2 molecules with linker lengths permitting bivalent intra-spike binding [273]: mono- and bi-specific (Fab)2 molecules demonstrated linker length-dependent enhancement of neutralization potencies and, in some cases, neutralization of viral strains resistant to conventional IgGs [273]. Thus, bispecifics may benefit from avidity effects to enhance potency and prevent viral escape beyond that observed from combinations of mAbs.
A recent study applied the CrossMab heavy chain heterodimerization strategy [274] to generate four bispecific combinations of HIV bNAbs exhibiting high breadth (VRC07 & 10E8) and high potency (PGT121 & PG9-PG16-RSH) [275]. All four bispecifics neutralized 94–97% of viruses in a panel of 206 HIV-1 strains and generally demonstrated potencies intermediate to that of the two parental mAbs, with the exception of VRC07xPG9-16-RSH which was 6.9 and 2.2-fold more potent than VRC07 and PG9-16-RSH respectively against dual-sensitive viruses. The physical combinations of the two parental mAbs were more potent than equivalent concentrations of the bispecific IgGs except for the VRC07xPG9-16-RSH combination, which demonstrates that some bispecifics may demonstrate enhanced clinical efficacy compared to combination mAb therapy. Similarly, CrossMab generated anti-HIV bispecifics targeting HIV-1 Env and cellular receptors, such as PRO140-10E8, have demonstrated exceptional breadth and potency of neutralization [276].
Alternative bispecific formats include two variable domains from each of two mAbs to form tetravalent molecules (Figure 3): 1) tetravalent dual variable domain Ig molecules (DVD-Igs), in which a second specificity VH&VL domain is linked directly to a full-length IgG, as was done to combine HY (an affinity matured b12) and 7B2 (anti-gp41) [277], and 2) Morrison-type bispecifics in which scFvs are linked via (G4S)n linkers to either the C or N terminus of the LC or HC of an IgG, as was done to generate tetravalent anti-CCR5 bispecifics [168] and of PG9-/PG16-iMAb [167]. PG9-iMab and PG16-iMab DVD-Igs exhibited picomolar potency and were able to neutralize viruses resistant to both parental mAbs—the authors hypothesized that iMab bound to CD4 and thereby concentrated PG9/16 at sites of viral entry to increase the potency of PG9/16 domains. Similarly, tetravalent anti-CCR5 bispecifics exhibited 18–57 fold potency compared to parental Abs, with one bispecific demonstrating activity against strains resistant to each parental Ab. Both of these examples demonstrate the synergistic effects resulting from tetravalent bispecific combinations but again were only observed for certain combinations of mAb specificities. On the other hand, HY-7B2 DVD-Igs simultaneously targeting gp120 CD4bs and gp41 epitopes generally performed only equally to the more effective parental Ab HY in neutralizing virus, although they did expectedly improve binding avidity and could more effectively deliver cytotoxic immunoconjugates than either parental Ab alone. Other bispecifics simultaneously targeting gp120 and gp41 have demonstrated enhanced neutralization activity compared to the parental Abs alone and are thought to hinder the function of Env by cross-linking gp120 and gp41 [278]. Although these two studies utilized different parental mAbs, they suggest that the format of DVD-Igs and bispecifics can be optimized to bind both epitopes on either the same or adjacent viral Env for enhanced neutralization activity.
5.2 Engaging T-cell responses
Two antibody-based strategies, bispecific T-cell engaging molecules and chimeric antigen receptor T cells, harness the power of cytotoxic T cells to eradicate latent HIV-infected cells. Combining humoral and cytotoxic responses in this way may be especially relevant for HIV given associations of high circulating levels of HIV-1-specific cytotoxic T-lymphocytes (CTLs) in long-term non-progressors [279, 280] and HIV-exposed seronegative subjects [281].
5.2.1 Bispecific T-cell engagers
Most recently, bispecifics with one specificity for T-cell engagement have shown significant promise in combatting viral reservoirs in “kick & kill” strategies. Bispecific T-cell engaging molecules have largely been designed as immunotherapy in the treatment of various cancers, and the first US FDA approved bispecific T-cell engaging antibody, blinatumomab, was recently approved in 2014 for use in refractory acute lymphoblastic leukemia, further demonstrating the clinical safety and efficacy of these engineered molecules [282]. In HIV-targeted “kick and kill” strategies, avid binding effects from both HIV Env- and T Cell CD3-specific arms of the bispecific to infected cells may allow for cross-linking of CD3 to trigger latent HIV-infected CD3+ T cells to reactivate and produce viral particles and concurrently display more viral Env on their surface. Alternatively, the addition of HIV latency reversing agents may provide or boost the “kick” to induce viral production. After induction of increased Env expression on the surface of latently infected cells, the anti-CD3 moiety of the bispecific may then cross-link CD3 on cytotoxic CD8+ T cells to induce cytolysis.
Historically, bispecific molecules to target HIV latent reservoir cells paired soluble versions of the CD4 receptor to anti-CD3 T cell engaging antibody fragments in a variety of formats [283–285]. Previous attempts to employ anti-HIV mAb specificities in bispecific formats with anti-CD3 demonstrated limited efficacy, likely due to limited breadth [284–286]. More recently, two groups have incorporated anti-HIV mAbs with broader recognition of diverse viral strains into bispecific T-cell engaging molecules: one group employed non-neutralizing mAbs recognizing CD4i (A32) and gp41 (7B2) epitopes [287], while another utilized bNAb VRC07 recognizing the CD4bs epitope [288]. As mentioned previously, non-neutralizing antibodies recognizing epitopes not normally present on viral surfaces may be helpful against latently infected cells, which often display monomeric gp140 or gp41 stumps. Both of these bispecific T-cell engaging molecules directed autologous T-cell-mediated cytolysis of primary HIV latently infected cells in vitro using patient PBMCs. Furthermore, one study demonstrated in vivo safety in ART-treated macaques and an absence of increased viral replication despite activation of latent cells in the presence of ART [288]. Additionally, a combination of T-cell engaging bispecifics targeting different viral Env epitopes demonstrated increased potency in vitro [287], and supports the incorporation of additional anti-HIV mAb specificities for potential use in combination therapy. These early promising results support future in vivo testing to determine the extent of bispecific permeation of tissue compartments where viral reservoir cells are likely to be found, as well as whether physiologic effector:target cell ratios in reservoir compartments of HIV-infected individuals influences the effectiveness of bispecific T-cell engaging molecules.
5.2.2 Chimeric Antigen Receptors (CARs)
Clinically, CAR T cells are increasingly demonstrating success for the treatment of cancers [289–292] and have been proposed for use in combatting HIV viral reservoirs [293, 294]. Furthermore, the first clinical use of “off-the-shelf” CAR T-cells in an infant who was administered the therapy on a compassionate basis in the UK was recently reported to demonstrate safety and efficacy [295], and makes the universal applicability and potential of HIV-specific CAR T cell therapy more viable.
HIV Env-targeting extracellular domains are fused to T cell CD3ζ cytoplasmic domains, which may contain additional costimulatory signal intracellular domains to activate T-cell cytolysis of Env-expressing cells. Env-targeting CAR T-cells thus far have utilized components from the CD4 receptor [296–298], gp41-specific mAb 98.6 [296], CD4bs mAb F105 [299], and, most recently, a CD4-17b bispecific (17b targets a CD4-inducible epitope) [300]. The CD4-based CAR, composed of the CD4 extracellular domain linked to the CD3ζ signaling chain (termed CD4ζ), is the most well-studied and has undergone clinical trials demonstrating durability of CAR expression and CAR T-cell proliferation in patients with predicted t1/2>16 years [301]. Encouragingly, CAR T-cell trafficking to rectal tissue and decreased viral load within rectal tissues was observed in patients with acute viremia [302], but, ultimately, no significant antiviral effects were found on plasma viral levels in these individuals, nor in the more long-lived viral reservoirs of patients with chronic infection [303]. However, these studies used first-generation CARs containing only the CD3ζ intracellular domain. In contrast, the recently described CD4-17b bispecific CAR represents a second generation CAR with the addition of costimulatory signaling domains from CD28 [300], and third-generation CARs with further incorporation of survival signals from 4-1BB (CD137) or OX40 (CD134) to enhance clinical efficacy, as demonstrated in various cancer settings [304], could similarly enhance HIV-specific CAR candidates. Furthermore, candidates from recently isolated, more potent bNAbs could be incorporated into CARs, either alone or in bispecific formats to combat resistance similar to mAb therapy. Interestingly, the authors of the CD4-17b bispecific CAR observed linker length-dependent effects on potency of viral suppression [300], which could further be tuned in bispecific CARs employing bNAbs targeting different epitopes. Similar to bispecific T-cell engagers, identification of Env epitopes that are most widely expressed and accessible on reservoir cells will be critical to HIV CAR development, which may give new purpose to non-neutralizing antibodies that recognize monomeric gp140 or gp41 stumps.
Introducing CARs to human embryonic stem cells and induced pluripotent stem cells has also recently been described and demonstrated to produce NK cells with the ability to inhibit HIV replication in CD4+ T cells in vitro, although in vivo HIV suppression was not significantly different for engineered cells compared to unmodified cells [305]. However, similar to CD4-CAR T cell studies, redesign as 2nd or 3rd generation CARs containing additional costimulatory domains or incorporation of bNAb extracellular domains could be beneficial. Interestingly, hematopoietic stem progenitor cells have previously been engineered to express bNAbs prior to directed differentiation into Ab-secreting plasmablasts with demonstrated protection in vitro and in vivo [306, 307]. Thus, combining both CAR-engineered NK-/T-cells and bNAb-secreting B cells/plasmablasts from stem progenitor cells offers a chance to simultaneously direct cytotoxic, humoral, and innate immune responses against HIV.
6. Engineering Delivery Strategies
Targeted and durable administration may further improve the clinical efficacy of bNAbs for protection, therapy, and cure of HIV infection. Delivery of bNAbs to mucosal surfaces may better prevent infection by sexual transmission, whereas systemic circulation may better ensure broad surveillance and targeting of infected cells and latent reservoirs. In all applications, longer-lived responses decrease the frequency of administrations required and decrease the burden placed on patients. In this review, we discuss strategies to deliver recombinant bNAb proteins and bNAb-encoding genes for endogenous production.
6.1 Protein Delivery
Preventing sexual transmission of HIV in high-risk populations such as that found in sub-saharan Africa has been difficult for a variety of social, economic, and cultural factors. Women are particularly vulnerable and often unable to negotiate safer sex with their partners [308]. Thus, female-initiated pre-exposure prophylaxis methods are in great need, and thus far development efforts have largely focused on topical microbicides and oral ART. Both of these methods require daily or coitus-dependent application and have suffered from patient non-adherence in multiple clinical trials [309]. bNAb immunotherapies offer a longer-acting (months), coitus-independent alternative to current strategies. Intravenous or intramuscular injections of bNAbs could deliver months of protection at a time but would require clinic visits and may be less available to wider populations as a result. A recent NHP study administering simianized bNAb VRC01 demonstrated durable protective antibody concentrations lasting for 108 days and protecting from viral challenge 52 days after the last dose, serving as a model for clinical dosing schedules of passively-delivered bNAbs: six antibody infusions (loading phase every 2 weeks followed by maintenance phase every 2 months) could provide complete protection for a year [310].
While topical formulations have demonstrated efficacy in humanized mice with direct application to the exposure site [123], microbicide trials suggest that the reliance on adherence may undercut the effectiveness of this delivery strategy. As an alternative, intravaginal rings (IVR) have previously been developed for sustained delivery of small molecule drugs to combat non-adherence [311]. A recent study has developed an IVR for controlled release of IgG antibodies and demonstrated sustained release for 14 days in vitro [312], although translation to the in vivo mucosal environment remains to be seen. As the realm of recombinant protein therapeutics expands, additional delivery strategies for controlled release of such “biopharmaceuticals” (reviewed in [313]), such as injectable polymer depot strategies, could additionally be applied to HIV bNAb pre-exposure prophylaxis.
6.2 Gene delivery (Plasmid, lentiviral, AAV)
An alternative delivery strategy to achieve durable plasma circulation is to induce long-term expression of monoclonal bNAbs via plasmid, lentiviral, or adeno-associated virus vector based gene delivery strategies (reviewed in [134]). In vivo electroporation of Fab-encoding plasmids has demonstrated rapid generation of functional VRC01 Fab molecules in mouse sera lasting for at least 7 days after injection [314]. While this system suffers from relatively short-lived expression, it may significantly streamline the developmental process required to deliver bNAbs compared to traditional recombinant production/purification schemes.
Gene delivery using viral vectors enables more durable expression. Lentiviral delivery of genes encoding HIV-specific antibodies to human hematopoietic stem/progenitor cells (HSPCs), with their capacities for unlimited regeneration and multilineage differentiation, has demonstrated successful neutralization activity in vitro [307] and in vivo protection from HIV challenge in humanized mouse models [196, 306]. However, ex vivo transduction of human HSPCs faces challenges in isolating HSPCs in sufficient numbers, matching donors to patients, and may be difficult to scale up for production as a broadly applicable therapeutic.
Recent gene delivery approaches have largely focused on recombinant AAV (rAAV) vector gene delivery to skeletal muscle where the rAAV delivered genome can form stable non-integrating circular episomes that persist in non-dividing muscle cells [315–317]. Over 100 clinical trials demonstrate safety of transduction [318] and the first gene therapy product clinically approved for use in humans uses rAAV vectors to treat lipoprotein lipase deficiency [319].
AAV delivery of HIV-/SIV-specific neutralizing mAbs (reviewed in [320]) as full length-antibodies [321, 322] and immunoadhesin molecules (scFv+(G4S)3+Fc) [320, 323] have demonstrated high expression levels with serum concentrations of delivered transgene proteins of up to 1000 μg/mL [322, 324] and significant durability of expression—one study found sustained serum concentrations of 20 μg/mL immunoadhesins for the past four years in macaques [320]. Significantly, 6 out of 9 macaques receiving an AAV-delivered SIV-specific immunoadhesin were completely protected from IV challenge with 40 macaque infectious doses of SIVmac316 [320, 323], with the lack of protection in the remaining three thought to result from significant immune responses to the immunoadhesin observed before viral challenge [323]. Similar protection from viral challenge has been observed from AAV delivered human bNAb transgenes in a dose-dependent manner in humanized mice [120, 310, 325].
AAV-delivered bNabs also protect from mucosal HIV transmission: AAV vector delivery of VRC01 family bNAbs protected the majority of humanized mice from 15 consecutive weekly vaginal challenges with the JR-CSF molecular clone of HIV and from 21 weekly vaginal exposures to a transmitted founder strain of HIV (REJO.c) [325]. Another study delivering a simian version of CD4bs bNAb VRC07 demonstrated protection against SHIV infection in monkeys 5.5 weeks after treatment and circulating antibody levels up to 66 μg/mL with the addition of immune suppression drugs (cyclosporine) [326]. AAV-delivery of an immunoadhesin scFv-Fc based on HIV bNAb PG9 has recently entered phase I clinical trials (www.clinicaltrials.gov NCT01937455).
Furthermore, AAV-delivery of a non-neutralizing anti-SIV mAb, 5L7, in full IgG1 format to rhesus monkeys resulted in endogenously produced anti-SIV mAb concentrations ranging from 1–270 μg/mL, with lower concentrations reflecting host antibody responses to the delivered mAb observed in 9 out of 12 cases, as well as lower peak and setpoint viral load and delay in time to peak viral load from time of exposure [327]. One monkey with the highest serum concentrations of 5L7 completely resisted six successive SIVmac239 IV challenges and exhibited extraordinarily high serum ADCC activity on a per μg basis greater than the equivalent concentration of recombinantly produced 5L7. The authors postulated that this extraordinarily high ADCC activity likely results from differences in post-translational modifications of the endogenously produced 5L7, further supporting glycoengineering efforts to tune Fc effector function and an additional potential advantage of AAV-delivered bNAb gene therapy over passive transfer of protein.
One major potential limitation of AAV therapy arises from preexisting immunity [328, 329] since ~80% of people are seropositive for either the AAV1 or AAV2 capsid [52]. Thus, efforts to identify capsids from other species [55] or to engineer entirely new capsids [56,57] may be important for the clinical utility of AAV therapy. Potential immunogenicity and polyreactivity of delivered bNAbs may also require bNAb engineering strategies, although no anti-antibody responses have been observed in passive administration of anti-HIV mAbs in human trials thus far [14, 330].
7. Conclusion
Anti-HIV antibodies offer significant promise for prophylactic protection from establishment of infection, therapeutic control of viremia after infection, and even functional cure of infection through combinations of virus neutralization, antibody-mediated effector functions, and added functionalities from conjugation to drugs, proteins, or whole cells. Recent technological advances have enabled rapid isolation of bNAbs with increased potency and breadth that have demonstrated exciting successes in several preclinical animal models and inspired the return of anti-HIV mAbs to human clinical trials. Sequence and structural information from isolated bNAbs and viral escape mutations inform Fv-engineering strategies to increase Ab potency and breadth, counter viral resistance, and decrease polyreactivity and solubility issues. Deeper understanding of extra-neutralizing roles for antibodies redefine the clinical utility of non-neutralizing antibodies and greatly inform engineering efforts to improve Fc regions of clinical anti-HIV mAbs to increase antibody half-life, improve mucosal immunity, and enhance recruitment and activation of innate immunity. Addition of small molecules, multiple antibody specificities, soluble receptors, toxins, or T-cell-engaging scFv’s/cells may prevent viral escape or even provide a functional cure through targeting of latent HIV reservoirs. Finally, various delivery strategies to localize recombinant mAbs at sites of infection or to deliver mAb-encoding genes for long-lived therapy significantly increase the clinical utility and feasibility of anti-HIV mAbs for prevention, therapy, and functional cure of HIV infection. While further studies to evaluate viral resistance, immunogenicity, and adverse reactions to several of the strategies discussed in this review remain to be conducted, the current arsenal of tools with which to enhance anti-HIV antibodies for clinical use is expansive and offers opportunities to exercise ingenuity and innovation to outpace HIV infection.
Acknowledgments
The authors are supported by NIAID NIH 1R01AI102691 and the Bill and Melinda Gates Foundation OPP1114729 and OPP1032144
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnson S, et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176(5):1215–24. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- 2.Malley R, et al. Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. J Infect Dis. 1998;178(6):1555–61. doi: 10.1086/314523. [DOI] [PubMed] [Google Scholar]
- 3.Baba TW, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6(2):200–6. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 4.Haigwood NL, et al. Passive immune globulin therapy in the SIV/macaque model: early intervention can alter disease profile. Immunol Lett. 1996;51(1–2):107–14. doi: 10.1016/0165-2478(96)02563-1. [DOI] [PubMed] [Google Scholar]
- 5.Hessell AJ, et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15(8):951–4. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hessell AJ, et al. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 2009;5(5):e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hessell AJ, et al. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J Virol. 2010;84(3):1302–13. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofmann-Lehmann R, et al. Postnatal passive immunization of neonatal macaques with a triple combination of human monoclonal antibodies against oral simian-human immunodeficiency virus challenge. J Virol. 2001;75(16):7470–80. doi: 10.1128/JVI.75.16.7470-7480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mascola JR, et al. Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73(5):4009–18. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mascola JR, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6(2):207–10. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 11.Ng CT, et al. Passive neutralizing antibody controls SHIV viremia and enhances B cell responses in infant macaques. Nat Med. 2010;16(10):1117–9. doi: 10.1038/nm.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parren PW, et al. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;75(17):8340–7. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibata R, et al. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5(2):204–10. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 14.Trkola A, et al. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med. 2005;11(6):615–22. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- 15.Mehandru S, et al. Adjunctive passive immunotherapy in human immunodeficiency virus type 1-infected individuals treated with antiviral therapy during acute and early infection. J Virol. 2007;81(20):11016–31. doi: 10.1128/JVI.01340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caskey M, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522(7557):487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamel DJ, et al. Twenty years of prospective molecular epidemiology in Senegal: changes in HIV diversity. AIDS Res Hum Retroviruses. 2007;23(10):1189–96. doi: 10.1089/aid.2007.0037. [DOI] [PubMed] [Google Scholar]
- 18.da Silva ZJ, et al. Changes in prevalence and incidence of HIV-1, HIV-2 and dual infections in urban areas of Bissau, Guinea-Bissau: is HIV-2 disappearing? AIDS. 2008;22(10):1195–202. doi: 10.1097/QAD.0b013e328300a33d. [DOI] [PubMed] [Google Scholar]
- 19.Marlink R, et al. Reduced rate of disease development after HIV-2 infection as compared to HIV-1. Science. 1994;265(5178):1587–90. doi: 10.1126/science.7915856. [DOI] [PubMed] [Google Scholar]
- 20.Whittle H, et al. HIV-2-infected patients survive longer than HIV-1-infected patients. AIDS. 1994;8(11):1617–20. doi: 10.1097/00002030-199411000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Kanki PJ, et al. Slower heterosexual spread of HIV-2 than HIV-1. Lancet. 1994;343(8903):943–6. doi: 10.1016/s0140-6736(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 22.Duvall MG, et al. Maintenance of HIV-specific CD4+ T cell help distinguishes HIV-2 from HIV-1 infection. J Immunol. 2006;176(11):6973–81. doi: 10.4049/jimmunol.176.11.6973. [DOI] [PubMed] [Google Scholar]
- 23.Leligdowicz A, et al. Direct relationship between virus load and systemic immune activation in HIV-2 infection. J Infect Dis. 2010;201(1):114–22. doi: 10.1086/648733. [DOI] [PubMed] [Google Scholar]
- 24.de Silva TI, et al. Correlates of T-cell-mediated viral control and phenotype of CD8(+) T cells in HIV-2, a naturally contained human retroviral infection. Blood. 2013;121(21):4330–9. doi: 10.1182/blood-2012-12-472787. [DOI] [PubMed] [Google Scholar]
- 25.Duvall MG, et al. Dendritic cells are less susceptible to human immunodeficiency virus type 2 (HIV-2) infection than to HIV-1 infection. J Virol. 2007;81(24):13486–98. doi: 10.1128/JVI.00976-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchant D, Neil SJ, McKnight A. Human immunodeficiency virus types 1 and 2 have different replication kinetics in human primary macrophage culture. J Gen Virol. 2006;87(Pt 2):411–8. doi: 10.1099/vir.0.81391-0. [DOI] [PubMed] [Google Scholar]
- 27.MacNeil A, et al. Direct evidence of lower viral replication rates in vivo in human immunodeficiency virus type 2 (HIV-2) infection than in HIV-1 infection. J Virol. 2007;81(10):5325–30. doi: 10.1128/JVI.02625-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavaleiro R, et al. Monocyte-mediated T cell suppression by HIV-2 envelope proteins. Eur J Immunol. 2007;37(12):3435–44. doi: 10.1002/eji.200737511. [DOI] [PubMed] [Google Scholar]
- 29.Cavaleiro R, et al. Marked immunosuppressive effects of the HIV-2 envelope protein in spite of the lower HIV-2 pathogenicity. AIDS. 2000;14(17):2679–86. doi: 10.1097/00002030-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 30.Makvandi-Nejad S, Rowland-Jones S. How does the humoral response to HIV-2 infection differ from HIV-1 and can this explain the distinct natural history of infection with these two human retroviruses? Immunol Lett. 2015;163(1):69–75. doi: 10.1016/j.imlet.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 31.Stamatatos L, et al. Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat Med. 2009;15(8):866–70. doi: 10.1038/nm.1949. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez SK, et al. Comparison of heterologous neutralizing antibody responses of human immunodeficiency virus type 1 (HIV-1)- and HIV-2-infected Senegalese patients: distinct patterns of breadth and magnitude distinguish HIV-1 and HIV-2 infections. J Virol. 2007;81(10):5331–8. doi: 10.1128/JVI.02789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozkaya Sahin G, et al. Potent intratype neutralizing activity distinguishes human immunodeficiency virus type 2 (HIV-2) from HIV-1. J Virol. 2012;86(2):961–71. doi: 10.1128/JVI.06315-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Silva TI, et al. Potent autologous and heterologous neutralizing antibody responses occur in HIV-2 infection across a broad range of infection outcomes. J Virol. 2012;86(2):930–46. doi: 10.1128/JVI.06126-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bjorling E, et al. Autologous neutralizing antibodies prevail in HIV-2 but not in HIV-1 infection. Virology. 1993;193(1):528–30. doi: 10.1006/viro.1993.1160. [DOI] [PubMed] [Google Scholar]
- 36.Kong R, et al. Broad and potent neutralizing antibody responses elicited in natural HIV-2 infection. J Virol. 2012;86(2):947–60. doi: 10.1128/JVI.06155-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Loeff MF, et al. Undetectable plasma viral load predicts normal survival in HIV-2- infected people in a West African village. Retrovirology. 2010;7:46. doi: 10.1186/1742-4690-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berry N, et al. Low peripheral blood viral HIV-2 RNA in individuals with high CD4 percentage differentiates HIV-2 from HIV-1 infection. J Hum Virol. 1998;1(7):457–68. [PubMed] [Google Scholar]
- 39.Jaffar S, et al. Rate of decline of percentage CD4+ cells is faster in HIV-1 than in HIV-2 infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16(5):327–32. doi: 10.1097/00042560-199712150-00003. [DOI] [PubMed] [Google Scholar]
- 40.Skar H, et al. HIV-2 genetic evolution in patients with advanced disease is faster than that in matched HIV-1 patients. J Virol. 2010;84(14):7412–5. doi: 10.1128/JVI.02548-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borrego P, et al. The role of the humoral immune response in the molecular evolution of the envelope C2, V3 and C3 regions in chronically HIV-2 infected patients. Retrovirology. 2008;5:78. doi: 10.1186/1742-4690-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rocha C, et al. Evolution of the human immunodeficiency virus type 2 envelope in the first years of infection is associated with the dynamics of the neutralizing antibody response. Retrovirology. 2013;10:110. doi: 10.1186/1742-4690-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bohl C, et al. A twin-cysteine motif in the V2 region of gp120 is associated with SIV envelope trimer stabilization. PLoS One. 2013;8(7):e69406. doi: 10.1371/journal.pone.0069406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi Y, et al. Evolution of human immunodeficiency virus type 2 coreceptor usage, autologous neutralization, envelope sequence and glycosylation. J Gen Virol. 2005;86(Pt 12):3385–96. doi: 10.1099/vir.0.81259-0. [DOI] [PubMed] [Google Scholar]
- 45.Barroso H, et al. Evolutionary and structural features of the C2, V3 and C3 envelope regions underlying the differences in HIV-1 and HIV-2 biology and infection. PLoS One. 2011;6(1):e14548. doi: 10.1371/journal.pone.0014548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lizeng Q, et al. Serum immunoglobulin A (IgA)-mediated immunity in human immunodeficiency virus type 2 (HIV-2) infection. Virology. 2003;308(2):225–32. doi: 10.1016/s0042-6822(02)00088-0. [DOI] [PubMed] [Google Scholar]
- 47.Ozkaya Sahin G, et al. Frequent intratype neutralization by plasma immunoglobulin a identified in HIV type 2 infection. AIDS Res Hum Retroviruses. 2013;29(3):470–8. doi: 10.1089/aid.2012.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marcelino JM, et al. Envelope-specific antibody response in HIV-2 infection: C2V3C3-specific IgG response is associated with disease progression. AIDS. 2008;22(17):2257–65. doi: 10.1097/QAD.0b013e3283155546. [DOI] [PubMed] [Google Scholar]
- 49.Ackerman ME, et al. Polyfunctional HIV-Specific Antibody Responses Are Associated with Spontaneous HIV Control. PLoS Pathog. 2016;12(1):e1005315. doi: 10.1371/journal.ppat.1005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ozkaya Sahin G, et al. Effect of complement on HIV-2 plasma antiviral activity is intratype specific and potent. J Virol. 2013;87(1):273–81. doi: 10.1128/JVI.01640-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walker LM, et al. A limited number of antibody specificities mediate broad and potent serum neutralization in selected HIV-1 infected individuals. PLoS Pathog. 2010;6(8):e1001028. doi: 10.1371/journal.ppat.1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simek MD, et al. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol. 2009;83(14):7337–48. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doria-Rose NA, et al. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J Virol. 2010;84(3):1631–6. doi: 10.1128/JVI.01482-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scheid JF, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458(7238):636–40. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 55.Sok D, et al. Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. Proc Natl Acad Sci U S A. 2014;111(49):17624–9. doi: 10.1073/pnas.1415789111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang J, et al. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491(7424):406–12. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walker LM, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477(7365):466–70. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker LM, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326(5950):285–9. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu X, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329(5993):856–61. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mouquet H, et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc Natl Acad Sci U S A. 2012;109(47):E3268–77. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scheid JF, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333(6049):1633–7. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sok D, et al. Promiscuous glycan site recognition by antibodies to the high-mannose patch of gp120 broadens neutralization of HIV. Sci Transl Med. 2014;6(236):236ra63. doi: 10.1126/scitranslmed.3008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doria-Rose NA, et al. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. 2014;509(7498):55–62. doi: 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang J, et al. Broad and potent HIV-1 neutralization by a human antibody that binds the gp41-gp120 interface. Nature. 2014;515(7525):138–42. doi: 10.1038/nature13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rudicell RS, et al. Enhanced potency of a broadly neutralizing HIV-1 antibody in vitro improves protection against lentiviral infection in vivo. J Virol. 2014;88(21):12669–82. doi: 10.1128/JVI.02213-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Julien JP, et al. Asymmetric recognition of the HIV-1 trimer by broadly neutralizing antibody PG9. Proc Natl Acad Sci U S A. 2013;110(11):4351–6. doi: 10.1073/pnas.1217537110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McLellan JS, et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480(7377):336–43. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pancera M, et al. Crystal structure of PG16 and chimeric dissection with somatically related PG9: structure-function analysis of two quaternary-specific antibodies that effectively neutralize HIV-1. J Virol. 2010;84(16):8098–110. doi: 10.1128/JVI.00966-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pancera M, et al. Structural basis for diverse N-glycan recognition by HIV-1-neutralizing V1-V2-directed antibody PG16. Nat Struct Mol Biol. 2013;20(7):804–13. doi: 10.1038/nsmb.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pejchal R, et al. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science. 2011;334(6059):1097–103. doi: 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pejchal R, et al. Structure and function of broadly reactive antibody PG16 reveal an H3 subdomain that mediates potent neutralization of HIV-1. Proc Natl Acad Sci U S A. 2010;107(25):11483–8. doi: 10.1073/pnas.1004600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou T, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010;329(5993):811–7. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou T, et al. Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature. 2007;445(7129):732–7. doi: 10.1038/nature05580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou T, et al. Multidonor analysis reveals structural elements, genetic determinants, and maturation pathway for HIV-1 neutralization by VRC01-class antibodies. Immunity. 2013;39(2):245–58. doi: 10.1016/j.immuni.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Julien JP, et al. Broadly neutralizing antibody PGT121 allosterically modulates CD4 binding via recognition of the HIV-1 gp120 V3 base and multiple surrounding glycans. PLoS Pathog. 2013;9(5):e1003342. doi: 10.1371/journal.ppat.1003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ofek G, et al. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J Virol. 2004;78(19):10724–37. doi: 10.1128/JVI.78.19.10724-10737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cardoso RM, et al. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity. 2005;22(2):163–73. doi: 10.1016/j.immuni.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 78.Julien JP, et al. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science. 2013;342(6165):1477–83. doi: 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lyumkis D, et al. Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science. 2013;342(6165):1484–90. doi: 10.1126/science.1245627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bartesaghi A, et al. Prefusion structure of trimeric HIV-1 envelope glycoprotein determined by cryo-electron microscopy. Nat Struct Mol Biol. 2013;20(12):1352–7. doi: 10.1038/nsmb.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pancera M, et al. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature. 2014;514(7523):455–61. doi: 10.1038/nature13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoxie JA. Toward an antibody-based HIV-1 vaccine. Annu Rev Med. 2010;61:135–52. doi: 10.1146/annurev.med.60.042507.164323. [DOI] [PubMed] [Google Scholar]
- 83.McElrath MJ, Haynes BF. Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity. 2010;33(4):542–54. doi: 10.1016/j.immuni.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yoon H, et al. CATNAP: a tool to compile, analyze and tally neutralizing antibody panels. Nucleic Acids Res. 2015;43(W1):W213–9. doi: 10.1093/nar/gkv404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eroshkin AM, et al. bNAber: database of broadly neutralizing HIV antibodies. Nucleic Acids Res. 2014;42(Database issue):D1133–9. doi: 10.1093/nar/gkt1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wibmer CK, Moore PL, Morris L. HIV broadly neutralizing antibody targets. Curr Opin HIV AIDS. 2015;10(3):135–43. doi: 10.1097/COH.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kwong PD, Mascola JR, Nabel GJ. Broadly neutralizing antibodies and the search for an HIV-1 vaccine: the end of the beginning. Nat Rev Immunol. 2013;13(9):693–701. doi: 10.1038/nri3516. [DOI] [PubMed] [Google Scholar]
- 88.West AP, Jr, et al. Structural insights on the role of antibodies in HIV-1 vaccine and therapy. Cell. 2014;156(4):633–48. doi: 10.1016/j.cell.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Klein F, et al. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell. 2013;153(1):126–38. doi: 10.1016/j.cell.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Corti D, et al. Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PLoS One. 2010;5(1):e8805. doi: 10.1371/journal.pone.0008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu X, et al. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science. 2011;333(6049):1593–602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiao X, et al. Maturation Pathways of Cross-Reactive HIV-1 Neutralizing Antibodies. Viruses. 2009;1(3):802–17. doi: 10.3390/v1030802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kepler TB, et al. Immunoglobulin gene insertions and deletions in the affinity maturation of HIV-1 broadly reactive neutralizing antibodies. Cell Host Microbe. 2014;16(3):304–13. doi: 10.1016/j.chom.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaul R, et al. Mucosal IgA in exposed, uninfected subjects: evidence for a role in protection against HIV infection. AIDS. 2001;15(3):431–2. doi: 10.1097/00002030-200102160-00026. [DOI] [PubMed] [Google Scholar]
- 95.Hirbod T, et al. HIV acquisition is associated with increased antimicrobial peptides and reduced HIV neutralizing IgA in the foreskin prepuce of uncircumcised men. PLoS Pathog. 2014;10(10):e1004416. doi: 10.1371/journal.ppat.1004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seaton KE, et al. HIV-1 specific IgA detected in vaginal secretions of HIV uninfected women participating in a microbicide trial in Southern Africa are primarily directed toward gp120 and gp140 specificities. PLoS One. 2014;9(7):e101863. doi: 10.1371/journal.pone.0101863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shacklett BL. Understanding the “lucky few”: the conundrum of HIV-exposed, seronegative individuals. Curr HIV/AIDS Rep. 2006;3(1):26–31. doi: 10.1007/s11904-006-0005-2. [DOI] [PubMed] [Google Scholar]
- 98.Dickover R, et al. Role of maternal autologous neutralizing antibody in selective perinatal transmission of human immunodeficiency virus type 1 escape variants. J Virol. 2006;80(13):6525–33. doi: 10.1128/JVI.02658-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Permar SR, et al. Maternal HIV-1 envelope-specific antibody responses and reduced risk of perinatal transmission. J Clin Invest. 2015;125(7):2702–6. doi: 10.1172/JCI81593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goo L, et al. Neutralizing antibody escape during HIV-1 mother-to-child transmission involves conformational masking of distal epitopes in envelope. J Virol. 2012;86(18):9566–82. doi: 10.1128/JVI.00953-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rainwater SM, et al. Cloning and characterization of functional subtype A HIV-1 envelope variants transmitted through breastfeeding. Curr HIV Res. 2007;5(2):189–97. doi: 10.2174/157016207780076986. [DOI] [PubMed] [Google Scholar]
- 102.Wu X, et al. Neutralization escape variants of human immunodeficiency virus type 1 are transmitted from mother to infant. J Virol. 2006;80(2):835–44. doi: 10.1128/JVI.80.2.835-844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hidajat R, et al. Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques. J Virol. 2009;83(2):791–801. doi: 10.1128/JVI.01672-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gomez-Roman VR, et al. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J Immunol. 2005;174(4):2185–9. doi: 10.4049/jimmunol.174.4.2185. [DOI] [PubMed] [Google Scholar]
- 105.Barouch DH, et al. HIV-1 vaccines. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science. 2015;349(6245):320–4. doi: 10.1126/science.aab3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barouch DH, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503(7475):224–8. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fouts TR, et al. Balance of cellular and humoral immunity determines the level of protection by HIV vaccines in rhesus macaque models of HIV infection. Proc Natl Acad Sci U S A. 2015;112(9):E992–9. doi: 10.1073/pnas.1423669112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bonsignori M, et al. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol. 2012;86(21):11521–32. doi: 10.1128/JVI.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Forthal DN, et al. Recombinant gp120 vaccine-induced antibodies inhibit clinical strains of HIV-1 in the presence of Fc receptor-bearing effector cells and correlate inversely with HIV infection rate. J Immunol. 2007;178(10):6596–603. doi: 10.4049/jimmunol.178.10.6596. [DOI] [PubMed] [Google Scholar]
- 110.Haynes BF, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366(14):1275–86. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shingai M, et al. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J Exp Med. 2014;211(10):2061–74. doi: 10.1084/jem.20132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fouda GG, et al. Postnatally-transmitted HIV-1 Envelope variants have similar neutralization-sensitivity and function to that of nontransmitted breast milk variants. Retrovirology. 2013;10:3. doi: 10.1186/1742-4690-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mabuka J, et al. HIV-1 maternal and infant variants show similar sensitivity to broadly neutralizing antibodies, but sensitivity varies by subtype. AIDS. 2013;27(10):1535–44. doi: 10.1097/QAD.0b013e32835faba5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nakamura KJ, et al. Coverage of primary mother-to-child HIV transmission isolates by second-generation broadly neutralizing antibodies. AIDS. 2013;27(3):337–46. doi: 10.1097/QAD.0b013e32835cadd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ackerman ME, et al. Enhanced phagocytic activity of HIV-specific antibodies correlates with natural production of immunoglobulins with skewed affinity for FcgammaR2a and FcgammaR2b. J Virol. 2013;87(10):5468–76. doi: 10.1128/JVI.03403-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Banerjee K, et al. IgG subclass profiles in infected HIV type 1 controllers and chronic progressors and in uninfected recipients of Env vaccines. AIDS Res Hum Retroviruses. 2010;26(4):445–58. doi: 10.1089/aid.2009.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lambotte O, et al. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS. 2009;23(8):897–906. doi: 10.1097/QAD.0b013e328329f97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shingai M, et al. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013;503(7475):277–80. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Horwitz JA, et al. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc Natl Acad Sci U S A. 2013;110(41):16538–43. doi: 10.1073/pnas.1315295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Balazs AB, et al. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012;481(7379):81–4. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gauduin MC, et al. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat Med. 1997;3(12):1389–93. doi: 10.1038/nm1297-1389. [DOI] [PubMed] [Google Scholar]
- 122.Parren PW, et al. Protection against HIV-1 infection in hu-PBL-SCID mice by passive immunization with a neutralizing human monoclonal antibody against the gp120 CD4-binding site. AIDS. 1995;9(6):F1–6. doi: 10.1097/00002030-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 123.Veselinovic M, et al. Topical gel formulation of broadly neutralizing anti-HIV-1 monoclonal antibody VRC01 confers protection against HIV-1 vaginal challenge in a humanized mouse model. Virology. 2012;432(2):505–10. doi: 10.1016/j.virol.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ledgerwood JE, et al. Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin Exp Immunol. 2015;182(3):289–301. doi: 10.1111/cei.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Klein F, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012;492(7427):118–22. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pelegrin M, Naranjo-Gomez M, Piechaczyk M. Antiviral Monoclonal Antibodies: Can They Be More Than Simple Neutralizing Agents? Trends Microbiol. 2015;23(10):653–65. doi: 10.1016/j.tim.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Haigwood NL, et al. Passive immunotherapy in simian immunodeficiency virus-infected macaques accelerates the development of neutralizing antibodies. J Virol. 2004;78(11):5983–95. doi: 10.1128/JVI.78.11.5983-5995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jaworski JP, et al. Neutralizing polyclonal IgG present during acute infection prevents rapid disease onset in simian-human immunodeficiency virus SHIVSF162P3-infected infant rhesus macaques. J Virol. 2013;87(19):10447–59. doi: 10.1128/JVI.00049-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yamamoto H, et al. Post-infection immunodeficiency virus control by neutralizing antibodies. PLoS One. 2007;2(6):e540. doi: 10.1371/journal.pone.0000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Klein F, et al. Enhanced HIV-1 immunotherapy by commonly arising antibodies that target virus escape variants. J Exp Med. 2014;211(12):2361–72. doi: 10.1084/jem.20141050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dey B, Berger EA. Towards an HIV cure based on targeted killing of infected cells: different approaches against acute versus chronic infection. Curr Opin HIV AIDS. 2015;10(3):207–13. doi: 10.1097/COH.0000000000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Levy JA. Not an HIV cure, but encouraging new directions. N Engl J Med. 2009;360(7):724–5. doi: 10.1056/NEJMe0810248. [DOI] [PubMed] [Google Scholar]
- 133.Euler Z, Alter G. Exploring the potential of monoclonal antibody therapeutics for HIV-1 eradication. AIDS Res Hum Retroviruses. 2015;31(1):13–24. doi: 10.1089/aid.2014.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Deal CE, Balazs AB. Vectored antibody gene delivery for the prevention or treatment of HIV infection. Curr Opin HIV AIDS. 2015;10(3):190–7. doi: 10.1097/COH.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Checkley MA, Luttge BG, Freed EO. HIV-1 envelope glycoprotein biosynthesis, trafficking, and incorporation. J Mol Biol. 2011;410(4):582–608. doi: 10.1016/j.jmb.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Svicher V, et al. Understanding HIV compartments and reservoirs. Curr HIV/AIDS Rep. 2014;11(2):186–94. doi: 10.1007/s11904-014-0207-y. [DOI] [PubMed] [Google Scholar]
- 137.McCoy LE, et al. Incomplete Neutralization and Deviation from Sigmoidal Neutralization Curves for HIV Broadly Neutralizing Monoclonal Antibodies. PLoS Pathog. 2015;11(8):e1005110. doi: 10.1371/journal.ppat.1005110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sievers SA, et al. Antibody engineering for increased potency, breadth and half-life. Curr Opin HIV AIDS. 2015;10(3):151–9. doi: 10.1097/COH.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang WP, et al. CDR walking mutagenesis for the affinity maturation of a potent human anti-HIV-1 antibody into the picomolar range. J Mol Biol. 1995;254(3):392–403. doi: 10.1006/jmbi.1995.0626. [DOI] [PubMed] [Google Scholar]
- 140.Barbas CF, 3rd, et al. In vitro evolution of a neutralizing human antibody to human immunodeficiency virus type 1 to enhance affinity and broaden strain cross-reactivity. Proc Natl Acad Sci U S A. 1994;91(9):3809–13. doi: 10.1073/pnas.91.9.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang MY, et al. Improved breadth and potency of an HIV-1-neutralizing human single-chain antibody by random mutagenesis and sequential antigen panning. J Mol Biol. 2004;335(1):209–19. doi: 10.1016/j.jmb.2003.09.055. [DOI] [PubMed] [Google Scholar]
- 142.Willis JR, et al. Redesigned HIV antibodies exhibit enhanced neutralizing potency and breadth. J Clin Invest. 2015;125(6):2523–31. doi: 10.1172/JCI80693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Diskin R, et al. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science. 2011;334(6060):1289–93. doi: 10.1126/science.1213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Diskin R, et al. Restricting HIV-1 pathways for escape using rationally designed anti-HIV-1 antibodies. J Exp Med. 2013;210(6):1235–49. doi: 10.1084/jem.20130221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen J, et al. Mechanism of HIV-1 neutralization by antibodies targeting a membrane-proximal region of gp41. J Virol. 2014;88(2):1249–58. doi: 10.1128/JVI.02664-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Georgiev IS, et al. Antibodies VRC01 and 10E8 neutralize HIV-1 with high breadth and potency even with Ig-framework regions substantially reverted to germline. J Immunol. 2014;192(3):1100–6. doi: 10.4049/jimmunol.1302515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fletcher CV, et al. Nonlinear pharmacokinetics of high-dose recombinant fusion protein CD4-IgG2 (PRO 542) observed in HIV-1-infected children. J Allergy Clin Immunol. 2007;119(3):747–50. doi: 10.1016/j.jaci.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hussey RE, et al. A soluble CD4 protein selectively inhibits HIV replication and syncytium formation. Nature. 1988;331(6151):78–81. doi: 10.1038/331078a0. [DOI] [PubMed] [Google Scholar]
- 149.Jacobson JM, et al. Single-dose safety, pharmacology, and antiviral activity of the human immunodeficiency virus (HIV) type 1 entry inhibitor PRO 542 in HIV-infected adults. J Infect Dis. 2000;182(1):326–9. doi: 10.1086/315698. [DOI] [PubMed] [Google Scholar]
- 150.Haim H, et al. Soluble CD4 and CD4-mimetic compounds inhibit HIV-1 infection by induction of a short-lived activated state. PLoS Pathog. 2009;5(4):e1000360. doi: 10.1371/journal.ppat.1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Moebius U, et al. The human immunodeficiency virus gp120 binding site on CD4: delineation by quantitative equilibrium and kinetic binding studies of mutants in conjunction with a high-resolution CD4 atomic structure. J Exp Med. 1992;176(2):507–17. doi: 10.1084/jem.176.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sullivan N, et al. Determinants of human immunodeficiency virus type 1 envelope glycoprotein activation by soluble CD4 and monoclonal antibodies. J Virol. 1998;72(8):6332–8. doi: 10.1128/jvi.72.8.6332-6338.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jacobson JM, et al. Treatment of advanced human immunodeficiency virus type 1 disease with the viral entry inhibitor PRO 542. Antimicrob Agents Chemother. 2004;48(2):423–9. doi: 10.1128/AAC.48.2.423-429.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gardner MR, et al. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature. 2015;519(7541):87–91. doi: 10.1038/nature14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.West AP, Jr, et al. Structural basis for germ-line gene usage of a potent class of antibodies targeting the CD4-binding site of HIV-1 gp120. Proc Natl Acad Sci U S A. 2012;109(30):E2083–90. doi: 10.1073/pnas.1208984109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kong R, et al. Improving neutralization potency and breadth by combining broadly reactive HIV-1 antibodies targeting major neutralization epitopes. J Virol. 2015;89(5):2659–71. doi: 10.1128/JVI.03136-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mascola JR, et al. Potent and synergistic neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12. J Virol. 1997;71(10):7198–206. doi: 10.1128/jvi.71.10.7198-7206.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zwick MB, et al. Neutralization synergy of human immunodeficiency virus type 1 primary isolates by cocktails of broadly neutralizing antibodies. J Virol. 2001;75(24):12198–208. doi: 10.1128/JVI.75.24.12198-12208.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pace C, Markowitz M. Monoclonal antibodies to host cellular receptors for the treatment and prevention of HIV-1 infection. Curr Opin HIV AIDS. 2015;10(3):144–50. doi: 10.1097/COH.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 160.Perez-Jimenez R, et al. Probing the effect of force on HIV-1 receptor CD4. ACS Nano. 2014;8(10):10313–20. doi: 10.1021/nn503557w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pace CS, et al. Anti-CD4 monoclonal antibody ibalizumab exhibits breadth and potency against HIV-1, with natural resistance mediated by the loss of a V5 glycan in envelope. J Acquir Immune Defic Syndr. 2013;62(1):1–9. doi: 10.1097/QAI.0b013e3182732746. [DOI] [PubMed] [Google Scholar]
- 162.Toma J, et al. Loss of asparagine-linked glycosylation sites in variable region 5 of human immunodeficiency virus type 1 envelope is associated with resistance to CD4 antibody ibalizumab. J Virol. 2011;85(8):3872–80. doi: 10.1128/JVI.02237-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Trkola A, et al. Potent, broad-spectrum inhibition of human immunodeficiency virus type 1 by the CCR5 monoclonal antibody PRO 140. J Virol. 2001;75(2):579–88. doi: 10.1128/JVI.75.2.579-588.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pugach P, et al. Neutralizing antibody and anti-retroviral drug sensitivities of HIV-1 isolates resistant to small molecule CCR5 inhibitors. Virology. 2008;377(2):401–7. doi: 10.1016/j.virol.2008.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jacobson JM, et al. Anti-HIV-1 activity of weekly or biweekly treatment with subcutaneous PRO 140, a CCR5 monoclonal antibody. J Infect Dis. 2010;201(10):1481–7. doi: 10.1086/652190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jacobson JM, et al. Antiviral activity of single-dose PRO 140, a CCR5 monoclonal antibody, in HIV-infected adults. J Infect Dis. 2008;198(9):1345–52. doi: 10.1086/592169. [DOI] [PubMed] [Google Scholar]
- 167.Pace CS, et al. Bispecific antibodies directed to CD4 domain 2 and HIV envelope exhibit exceptional breadth and picomolar potency against HIV-1. Proc Natl Acad Sci U S A. 2013;110(33):13540–5. doi: 10.1073/pnas.1304985110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schanzer J, et al. Development of tetravalent, bispecific CCR5 antibodies with antiviral activity against CCR5 monoclonal antibody-resistant HIV-1 strains. Antimicrob Agents Chemother. 2011;55(5):2369–78. doi: 10.1128/AAC.00215-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev. 2002;1(6):329–37. doi: 10.1016/s1568-9972(02)00086-1. [DOI] [PubMed] [Google Scholar]
- 170.Kopelman RG, Zolla-Pazner S. Association of human immunodeficiency virus infection and autoimmune phenomena. Am J Med. 1988;84(1):82–8. doi: 10.1016/0002-9343(88)90012-5. [DOI] [PubMed] [Google Scholar]
- 171.Solinger AM, et al. Acquired immune deficiency syndrome (AIDS) and autoimmunity--mutually exclusive entities? J Clin Immunol. 1988;8(1):32–42. doi: 10.1007/BF00915154. [DOI] [PubMed] [Google Scholar]
- 172.Liu M, et al. Polyreactivity and autoreactivity among HIV-1 antibodies. J Virol. 2015;89(1):784–98. doi: 10.1128/JVI.02378-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wu H, et al. Development of motavizumab, an ultra-potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract. J Mol Biol. 2007;368(3):652–65. doi: 10.1016/j.jmb.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 174.Pepinsky RB, et al. Improving the solubility of anti-LINGO-1 monoclonal antibody Li33 by isotype switching and targeted mutagenesis. Protein Sci. 2010;19(5):954–66. doi: 10.1002/pro.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hotzel I, et al. A strategy for risk mitigation of antibodies with fast clearance. MAbs. 2012;4(6):753–60. doi: 10.4161/mabs.22189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kwon YD, et al. Enhancing the solubility of HIV-1 neutralizing antibody 10E8. AIDS Res Hum Retroviruses. 2014;30(Suppl 1) [Google Scholar]
- 177.Boesch AW, Brown EP, Ackerman ME. The role of Fc receptors in HIV prevention and therapy. Immunol Rev. 2015;268(1):296–310. doi: 10.1111/imr.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Boesch AW, Alter G, Ackerman ME. Prospects for engineering HIV-specific antibodies for enhanced effector function and half-life. Curr Opin HIV AIDS. 2015;10(3):160–9. doi: 10.1097/COH.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nimmerjahn F. A constant threat for HIV: Fc-engineering to enhance broadly neutralizing antibody activity for immunotherapy of the acquired immunodeficiency syndrome. Eur J Immunol. 2015;45(8):2183–90. doi: 10.1002/eji.201445386. [DOI] [PubMed] [Google Scholar]
- 180.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715–25. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 181.Ko SY, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514(7524):642–5. doi: 10.1038/nature13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gupta S, et al. The Neonatal Fc receptor (FcRn) enhances human immunodeficiency virus type 1 (HIV-1) transcytosis across epithelial cells. PLoS Pathog. 2013;9(11):e1003776. doi: 10.1371/journal.ppat.1003776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Weflen AW, et al. Multivalent immune complexes divert FcRn to lysosomes by exclusion from recycling sorting tubules. Mol Biol Cell. 2013;24(15):2398–405. doi: 10.1091/mbc.E13-04-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hinton PR, et al. An engineered human IgG1 antibody with longer serum half-life. J Immunol. 2006;176(1):346–56. doi: 10.4049/jimmunol.176.1.346. [DOI] [PubMed] [Google Scholar]
- 185.Vaccaro C, et al. Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nat Biotechnol. 2005;23(10):1283–8. doi: 10.1038/nbt1143. [DOI] [PubMed] [Google Scholar]
- 186.Dall’Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn) J Biol Chem. 2006;281(33):23514–24. doi: 10.1074/jbc.M604292200. [DOI] [PubMed] [Google Scholar]
- 187.Kim JK, et al. Mapping the site on human IgG for binding of the MHC class I-related receptor, FcRn. Eur J Immunol. 1999;29(9):2819–25. doi: 10.1002/(SICI)1521-4141(199909)29:09<2819::AID-IMMU2819>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 188.West AP, Jr, Bjorkman PJ. Crystal structure and immunoglobulin G binding properties of the human major histocompatibility complex-related Fc receptor(,) Biochemistry. 2000;39(32):9698–708. doi: 10.1021/bi000749m. [DOI] [PubMed] [Google Scholar]
- 189.Grevys A, et al. Fc Engineering of Human IgG1 for Altered Binding to the Neonatal Fc Receptor Affects Fc Effector Functions. J Immunol. 2015;194(11):5497–508. doi: 10.4049/jimmunol.1401218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Monnet C, et al. Combined glyco- and protein-Fc engineering simultaneously enhance cytotoxicity and half-life of a therapeutic antibody. mAbs. 2014;6(2):422–436. doi: 10.4161/mabs.27854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhou M, Ruprecht RM. Are anti-HIV IgAs good guys or bad guys? Retrovirology. 2014;11:109. doi: 10.1186/s12977-014-0109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Watkins JD, et al. Anti-HIV IgA isotypes: differential virion capture and inhibition of transcytosis are linked to prevention of mucosal R5 SHIV transmission. AIDS. 2013;27(9):F13–20. doi: 10.1097/QAD.0b013e328360eac6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sholukh AM, et al. Defense-in-depth by mucosally administered anti-HIV dimeric IgA2 and systemic IgG1 mAbs: complete protection of rhesus monkeys from mucosal SHIV challenge. Vaccine. 2015;33(17):2086–95. doi: 10.1016/j.vaccine.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Stieh DJ, et al. Aggregate complexes of HIV-1 induced by multimeric antibodies. Retrovirology. 2014;11:78. doi: 10.1186/s12977-014-0078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fahrbach KM, et al. Differential binding of IgG and IgA to mucus of the female reproductive tract. PLoS One. 2013;8(10):e76176. doi: 10.1371/journal.pone.0076176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hur EM, et al. Inhibitory effect of HIV-specific neutralizing IgA on mucosal transmission of HIV in humanized mice. Blood. 2012;120(23):4571–82. doi: 10.1182/blood-2012-04-422303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Brunke C, et al. Effect of a tail piece cysteine deletion on biochemical and functional properties of an epidermal growth factor receptor-directed IgA2m(1) antibody. MAbs. 2013;5(6):936–45. doi: 10.4161/mabs.26396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lohse S, et al. Recombinant dimeric IgA antibodies against the epidermal growth factor receptor mediate effective tumor cell killing. J Immunol. 2011;186(6):3770–8. doi: 10.4049/jimmunol.1003082. [DOI] [PubMed] [Google Scholar]
- 199.Moldt B, et al. Simplifying the synthesis of SIgA: combination of dIgA and rhSC using affinity chromatography. Methods. 2014;65(1):127–32. doi: 10.1016/j.ymeth.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lohse S, et al. Characterization of a mutated IgA2 antibody of the m(1) allotype against the epidermal growth factor receptor for the recruitment of monocytes and macrophages. J Biol Chem. 2012;287(30):25139–50. doi: 10.1074/jbc.M112.353060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wright A, Lamm ME, Huang YT. Excretion of human immunodeficiency virus type 1 through polarized epithelium by immunoglobulin A. J Virol. 2008;82(23):11526–35. doi: 10.1128/JVI.01111-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Milligan C, et al. Passively acquired antibody-dependent cellular cytotoxicity (ADCC) activity in HIV-infected infants is associated with reduced mortality. Cell Host Microbe. 2015;17(4):500–6. doi: 10.1016/j.chom.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ahmad R, et al. Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. J Clin Immunol. 2001;21(3):227–33. doi: 10.1023/a:1011087132180. [DOI] [PubMed] [Google Scholar]
- 204.Baum LL, et al. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol. 1996;157(5):2168–73. [PubMed] [Google Scholar]
- 205.Forthal DN, et al. Antibody-dependent cellular cytotoxicity independently predicts survival in severely immunocompromised human immunodeficiency virus-infected patients. J Infect Dis. 1999;180(4):1338–41. doi: 10.1086/314988. [DOI] [PubMed] [Google Scholar]
- 206.Forthal DN, Landucci G, Keenan B. Relationship between antibody-dependent cellular cytotoxicity, plasma HIV type 1 RNA, and CD4+ lymphocyte count. AIDS Res Hum Retroviruses. 2001;17(6):553–61. doi: 10.1089/08892220151126661. [DOI] [PubMed] [Google Scholar]
- 207.Forthal DN, et al. FcgammaRIIa genotype predicts progression of HIV infection. J Immunol. 2007;179(11):7916–23. doi: 10.4049/jimmunol.179.11.7916. [DOI] [PubMed] [Google Scholar]
- 208.French MA, et al. Vaccine-induced IgG2 anti-HIV p24 is associated with control of HIV in patients with a ‘high-affinity’ FcgammaRIIa genotype. AIDS. 2010;24(13):1983–90. doi: 10.1097/QAD.0b013e32833c1ce0. [DOI] [PubMed] [Google Scholar]
- 209.Ngo-Giang-Huong N, et al. HIV type 1-specific IgG2 antibodies: markers of helper T cell type 1 response and prognostic marker of long-term nonprogression. AIDS Res Hum Retroviruses. 2001;17(15):1435–46. doi: 10.1089/088922201753197105. [DOI] [PubMed] [Google Scholar]
- 210.Ackerman ME, Dugast AS, Alter G. Emerging concepts on the role of innate immunity in the prevention and control of HIV infection. Annu Rev Med. 2012;63:113–30. doi: 10.1146/annurev-med-050310-085221. [DOI] [PubMed] [Google Scholar]
- 211.Huber M, et al. Complement lysis activity in autologous plasma is associated with lower viral loads during the acute phase of HIV-1 infection. PLoS Med. 2006;3(11):e441. doi: 10.1371/journal.pmed.0030441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Aasa-Chapman MM, et al. Detection of antibody-dependent complement-mediated inactivation of both autologous and heterologous virus in primary human immunodeficiency virus type 1 infection. J Virol. 2005;79(5):2823–30. doi: 10.1128/JVI.79.5.2823-2830.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Moog C, et al. Protective effect of vaginal application of neutralizing and nonneutralizing inhibitory antibodies against vaginal SHIV challenge in macaques. Mucosal Immunol. 2014;7(1):46–56. doi: 10.1038/mi.2013.23. [DOI] [PubMed] [Google Scholar]
- 214.Bournazos S, et al. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell. 2014;158(6):1243–53. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nimmerjahn F, Ravetch JV. Translating basic mechanisms of IgG effector activity into next generation cancer therapies. Cancer Immun. 2012;12:13. [PMC free article] [PubMed] [Google Scholar]
- 216.Nimmerjahn F, Ravetch JV. Divergent immunoglobuling subclass activity through selective Fc receptor binding. Science. 2005;310(5753):1510–2. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 217.Gilbert P, et al. Magnitude and breadth of a nonprotective neutralizing antibody response in an efficacy trial of a candidate HIV-1 gp120 vaccine. J Infect Dis. 2010;202(4):595–605. doi: 10.1086/654816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Jones NG, et al. AIDSVAX immunization induces HIV-specific CD8+ T-cell responses in high-risk, HIV-negative volunteers who subsequently acquire HIV infection. Vaccine. 2009;27(7):1136–40. doi: 10.1016/j.vaccine.2008.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Rerks-Ngarm S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 220.Chung AW, et al. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med. 2014;6(228):228–38. doi: 10.1126/scitranslmed.3007736. [DOI] [PubMed] [Google Scholar]
- 221.Kellner C, et al. Boosting ADCC and CDC activity by Fc engineering and evaluation of antibody effector functions. Methods. 2014;65(1):105–13. doi: 10.1016/j.ymeth.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 222.Lazar GA, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A. 2006;103(11):4005–10. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li F, Ravetch JV. Inhibitory Fcgamma receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science. 2011;333(6045):1030–4. doi: 10.1126/science.1206954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Forthal DN, et al. Fc-glycosylation influences Fcgamma receptor binding and cell-mediated anti-HIV activity of monoclonal antibody 2G12. J Immunol. 2010;185(11):6876–82. doi: 10.4049/jimmunol.1002600. [DOI] [PubMed] [Google Scholar]
- 225.Forthal DN, et al. IgG2 inhibits HIV-1 internalization by monocytes, and IgG subclass binding is affected by gp120 glycosylation. AIDS. 2011;25(17):2099–104. doi: 10.1097/QAD.0b013e32834b64bd. [DOI] [PubMed] [Google Scholar]
- 226.Lai JI, et al. Divergent antibody subclass and specificity profiles but not protective HLA-B alleles are associated with variable antibody effector function among HIV-1 controllers. J Virol. 2014;88(5):2799–809. doi: 10.1128/JVI.03130-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Perez LG, Zolla-Pazner S, Montefiori DC. Antibody-DEPENDENT, FcgammaRI-mediated neutralization of HIV-1 in TZM-bl cells occurs independently of phagocytosis. J Virol. 2013;87(9):5287–90. doi: 10.1128/JVI.00278-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ackerman ME, et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest. 2013;123(5):2183–92. doi: 10.1172/JCI65708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Halper-Stromberg A, et al. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. 2014;158(5):989–99. doi: 10.1016/j.cell.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hessell AJ, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449(7158):101–4. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 231.Shields RL, et al. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem. 2001;276(9):6591–604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 232.Bowles JA, et al. Anti-CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood. 2006;108(8):2648–54. doi: 10.1182/blood-2006-04-020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Stavenhagen JB, et al. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fcgamma receptors. Cancer Res. 2007;67(18):8882–90. doi: 10.1158/0008-5472.CAN-07-0696. [DOI] [PubMed] [Google Scholar]
- 234.Zalevsky J, et al. The impact of Fc engineering on an anti-CD19 antibody: increased Fcgamma receptor affinity enhances B-cell clearing in nonhuman primates. Blood. 2009;113(16):3735–43. doi: 10.1182/blood-2008-10-182048. [DOI] [PubMed] [Google Scholar]
- 235.Liu Z, et al. Asymmetrical Fc engineering greatly enhances antibody-dependent cellular cytotoxicity (ADCC) effector function and stability of the modified antibodies. J Biol Chem. 2014;289(6):3571–90. doi: 10.1074/jbc.M113.513366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Indik Z, et al. Human Fc gamma RII, in the absence of other Fc gamma receptors, mediates a phagocytic signal. J Clin Invest. 1991;88(5):1766–71. doi: 10.1172/JCI115496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Richards JO, et al. Optimization of antibody binding to FcgammaRIIa enhances macrophage phagocytosis of tumor cells. Mol Cancer Ther. 2008;7(8):2517–27. doi: 10.1158/1535-7163.MCT-08-0201. [DOI] [PubMed] [Google Scholar]
- 238.Painter RH. The C1q receptor site on human immunoglobulin G. Can J Biochem Cell Biol. 1984;62(6):418–25. doi: 10.1139/o84-057. [DOI] [PubMed] [Google Scholar]
- 239.Idusogie EE, et al. Engineered antibodies with increased activity to recruit complement. J Immunol. 2001;166(4):2571–5. doi: 10.4049/jimmunol.166.4.2571. [DOI] [PubMed] [Google Scholar]
- 240.Moore GL, et al. Engineered Fc variant antibodies with enhanced ability to recruit complement and mediate effector functions. MAbs. 2010;2(2):181–9. doi: 10.4161/mabs.2.2.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Natsume A, et al. Engineered antibodies of IgG1/IgG3 mixed isotype with enhanced cytotoxic activities. Cancer Res. 2008;68(10):3863–72. doi: 10.1158/0008-5472.CAN-07-6297. [DOI] [PubMed] [Google Scholar]
- 242.Kelton W, et al. IgGA: a “cross-isotype” engineered human Fc antibody domain that displays both IgG-like and IgA-like effector functions. Chem Biol. 2014;21(12):1603–9. doi: 10.1016/j.chembiol.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 243.Diebolder CA, et al. Complement is activated by IgG hexamers assembled at the cell surface. Science. 2014;343(6176):1260–3. doi: 10.1126/science.1248943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lux A, Nimmerjahn F. Impact of differential glycosylation on IgG activity. Adv Exp Med Biol. 2011;780:113–24. doi: 10.1007/978-1-4419-5632-3_10. [DOI] [PubMed] [Google Scholar]
- 245.Shields RL, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277(30):26733–40. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 246.Anthony RM, Ravetch JV. A novel role for the IgG Fc glycan: the anti-inflammatory activity of sialylated IgG Fcs. J Clin Immunol. 2010;30(Suppl 1):S9–14. doi: 10.1007/s10875-010-9405-6. [DOI] [PubMed] [Google Scholar]
- 247.Boyd PN, Lines AC, Patel AK. The effect of the removal of sialic acid, galactose and total carbohydrate on the functional activity of Campath-1H. Mol Immunol. 1995;32(17–18):1311–8. doi: 10.1016/0161-5890(95)00118-2. [DOI] [PubMed] [Google Scholar]
- 248.Lund J, et al. Oligosaccharide-protein interactions in IgG antibody molecules: structural and functional consequences. Biochem Soc Trans. 1995;23(1):102S. doi: 10.1042/bst023102s. [DOI] [PubMed] [Google Scholar]
- 249.Tsuchiya N, et al. Effects of galactose depletion from oligosaccharide chains on immunological activities of human IgG. J Rheumatol. 1989;16(3):285–90. [PubMed] [Google Scholar]
- 250.Karsten CM, et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1. Nat Med. 2012;18(9):1401–6. doi: 10.1038/nm.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov. 2009;8(3):226–234. doi: 10.1038/nrd2804. [DOI] [PubMed] [Google Scholar]
- 252.Shinkawa T, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278(5):3466–73. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 253.Naso MF, et al. Engineering host cell lines to reduce terminal sialylation of secreted antibodies. mAbs. 2010;2(5):519–527. doi: 10.4161/mabs.2.5.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Loos A, Steinkellner H. IgG-Fc glycoengineering in non-mammalian expression hosts. Arch Biochem Biophys. 2012;526(2):167–73. doi: 10.1016/j.abb.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Yin B, et al. Glycoengineering of Chinese hamster ovary cells for enhanced erythropoietin N-glycan branching and sialylation. Biotechnol Bioeng. 2015;112(11):2343–51. doi: 10.1002/bit.25650. [DOI] [PubMed] [Google Scholar]
- 256.Hodoniczky J, Zheng YZ, James DC. Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnol Prog. 2005;21(6):1644–52. doi: 10.1021/bp050228w. [DOI] [PubMed] [Google Scholar]
- 257.Huang W, et al. Chemoenzymatic glycoengineering of intact IgG antibodies for gain of functions. J Am Chem Soc. 2012;134(29):12308–18. doi: 10.1021/ja3051266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Moldt B, et al. A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcgammaRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. J Virol. 2012;86(11):6189–96. doi: 10.1128/JVI.00491-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Sazinsky SL, et al. Aglycosylated immunoglobulin G1 variants productively engage activating Fc receptors. Proc Natl Acad Sci U S A. 2008;105(51):20167–72. doi: 10.1073/pnas.0809257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Jung ST, et al. Aglycosylated IgG variants expressed in bacteria that selectively bind FcgammaRI potentiate tumor cell killing by monocyte-dendritic cells. Proc Natl Acad Sci U S A. 2010;107(2):604–9. doi: 10.1073/pnas.0908590107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Repp R, et al. Combined Fc-protein- and Fc-glyco-engineering of scFv-Fc fusion proteins synergistically enhances CD16a binding but does not further enhance NK-cell mediated ADCC. J Immunol Methods. 2011;373(1–2):67–78. doi: 10.1016/j.jim.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 262.Masuda K, et al. Enhanced binding affinity for FcgammaRIIIa of fucose-negative antibody is sufficient to induce maximal antibody-dependent cellular cytotoxicity. Mol Immunol. 2007;44(12):3122–31. doi: 10.1016/j.molimm.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 263.Yu X, et al. Engineering hydrophobic protein-carbohydrate interactions to fine-tune monoclonal antibodies. J Am Chem Soc. 2013;135(26):9723–32. doi: 10.1021/ja4014375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Lacek K, et al. Dramatic potentiation of the antiviral activity of HIV antibodies by cholesterol conjugation. J Biol Chem. 2014;289(50):35015–28. doi: 10.1074/jbc.M114.591826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Gavrilyuk J, et al. Antibody conjugation approach enhances breadth and potency of neutralization of anti-HIV-1 antibodies and CD4-IgG. J Virol. 2013;87(9):4985–93. doi: 10.1128/JVI.03146-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Dey B, Del Castillo CS, Berger EA. Neutralization of human immunodeficiency virus type 1 by sCD4–17b, a single-chain chimeric protein, based on sequential interaction of gp120 with CD4 and coreceptor. J Virol. 2003;77(5):2859–65. doi: 10.1128/JVI.77.5.2859-2865.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.West AP, Jr, et al. Evaluation of CD4-CD4i antibody architectures yields potent, broadly cross-reactive anti-human immunodeficiency virus reagents. J Virol. 2010;84(1):261–9. doi: 10.1128/JVI.01528-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Chen W, et al. Exceptionally potent and broadly cross-reactive, bispecific multivalent HIV-1 inhibitors based on single human CD4 and antibody domains. J Virol. 2014;88(2):1125–39. doi: 10.1128/JVI.02566-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Bera TK, et al. Specific killing of HIV-infected lymphocytes by a recombinant immunotoxin directed against the HIV-1 envelope glycoprotein. Mol Med. 1998;4(6):384–91. [PMC free article] [PubMed] [Google Scholar]
- 270.Kennedy PE, et al. Anti-HIV-1 immunotoxin 3B3(Fv)-PE38: enhanced potency against clinical isolates in human PBMCs and macrophages, and negligible hepatotoxicity in macaques. J Leukoc Biol. 2006;80(5):1175–82. doi: 10.1189/jlb.0306139. [DOI] [PubMed] [Google Scholar]
- 271.Denton PW, et al. Targeted cytotoxic therapy kills persisting HIV infected cells during ART. PLoS Pathog. 2014;10(1):e1003872. doi: 10.1371/journal.ppat.1003872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Klein JS, Bjorkman PJ. Few and far between: how HIV may be evading antibody avidity. PLoS Pathog. 2010;6(5):e1000908. doi: 10.1371/journal.ppat.1000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Galimidi RP, et al. Intra-spike crosslinking overcomes antibody evasion by HIV-1. Cell. 2015;160(3):433–46. doi: 10.1016/j.cell.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Schaefer W, et al. Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc Natl Acad Sci U S A. 2011;108(27):11187–92. doi: 10.1073/pnas.1019002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Asokan M, et al. Bispecific antibodies targeting different epitopes on the HIV-1 envelope exhibit broad and potent neutralization. J Virol. 2015 doi: 10.1128/JVI.02097-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ho DD, Huang Y, Yu J. Improved hiv-1-neutralizing antibody potency and breadth via cell receptor anchoring using bispecific antibodies with native architecture. 2015 Google Patents. [Google Scholar]
- 277.Craig RB, et al. Anti-HIV double variable domain immunoglobulins binding both gp41 and gp120 for targeted delivery of immunoconjugates. PLoS One. 2012;7(10):e46778. doi: 10.1371/journal.pone.0046778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Mouquet H, et al. Enhanced HIV-1 neutralization by antibody heteroligation. Proc Natl Acad Sci U S A. 2012;109(3):875–80. doi: 10.1073/pnas.1120059109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Rinaldo C, et al. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J Virol. 1995;69(9):5838–42. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Klein MR, et al. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181(4):1365–72. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Pinto LA, et al. ENV-specific cytotoxic T lymphocyte responses in HIV seronegative health care workers occupationally exposed to HIV-contaminated body fluids. J Clin Invest. 1995;96(2):867–76. doi: 10.1172/JCI118133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Stieglmaier J, Benjamin J, Nagorsen D. Utilizing the BiTE (bispecific T-cell engager) platform for immunotherapy of cancer. Expert Opin Biol Ther. 2015;15(8):1093–9. doi: 10.1517/14712598.2015.1041373. [DOI] [PubMed] [Google Scholar]
- 283.Berg J, et al. Bispecific antibodies that mediate killing of cells infected with human immunodeficiency virus of any strain. Proc Natl Acad Sci U S A. 1991;88(11):4723–7. doi: 10.1073/pnas.88.11.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Traunecker A, Lanzavecchia A, Karjalainen K. Bispecific single chain molecules (Janusins) target cytotoxic lymphocytes on HIV infected cells. EMBO J. 1991;10(12):3655–9. doi: 10.1002/j.1460-2075.1991.tb04932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Chamow SM, et al. A humanized, bispecific immunoadhesin-antibody that retargets CD3+ effectors to kill HIV-1-infected cells. J Immunol. 1994;153(9):4268–80. [PubMed] [Google Scholar]
- 286.Fernandez-Sesma A, et al. Superantigen-activated T cells redirected by a bispecific antibody inhibit vesicular stomatitis virus replication in vitro and in vivo. J Immunol. 1998;160(4):1841–9. [PubMed] [Google Scholar]
- 287.Sung JA, et al. Dual-Affinity Re-Targeting proteins direct T cell-mediated cytolysis of latently HIV-infected cells. J Clin Invest. 2015;125(11):4077–90. doi: 10.1172/JCI82314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Pegu A, et al. Activation and lysis of human CD4 cells latently infected with HIV-1. Nat Commun. 2015;6:8447. doi: 10.1038/ncomms9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Kochenderfer JN, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116(20):4099–102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Porter DL, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Frey NV, Porter DL. CAR T-cells merge into the fast lane of cancer care. Am J Hematol. 2015 doi: 10.1002/ajh.24238. [DOI] [PubMed] [Google Scholar]
- 293.Lam S, Bollard C. T-cell therapies for HIV. Immunotherapy. 2013;5(4):407–14. doi: 10.2217/imt.13.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Zhen A, Kitchen S. Stem-cell-based gene therapy for HIV infection. Viruses. 2013;6(1):1–12. doi: 10.3390/v6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Qasim W, et al. First Clinical Application of Talen Engineered Universal CAR19 T Cells in B-ALL. 57th American Society of Hematology (ASH) Annual Meeting; 2015; Orlando, FL. [Google Scholar]
- 296.Roberts MR, et al. Targeting of human immunodeficiency virus-infected cells by CD8+ T lymphocytes armed with universal T-cell receptors. Blood. 1994;84(9):2878–89. [PubMed] [Google Scholar]
- 297.Sahu GK, et al. Anti-HIV designer T cells progressively eradicate a latently infected cell line by sequentially inducing HIV reactivation then killing the newly gp120-positive cells. Virology. 2013;446(1–2):268–75. doi: 10.1016/j.virol.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Romeo C, Seed B. Cellular immunity to HIV activated by CD4 fused to T cell or Fc receptor polypeptides. Cell. 1991;64(5):1037–46. doi: 10.1016/0092-8674(91)90327-u. [DOI] [PubMed] [Google Scholar]
- 299.Masiero S, et al. T-cell engineering by a chimeric T-cell receptor with antibody-type specificity for the HIV-1 gp120. Gene Ther. 2005;12(4):299–310. doi: 10.1038/sj.gt.3302413. [DOI] [PubMed] [Google Scholar]
- 300.Liu L, et al. Novel CD4-Based Bispecific Chimeric Antigen Receptor Designed for Enhanced Anti-HIV Potency and Absence of HIV Entry Receptor Activity. J Virol. 2015;89(13):6685–94. doi: 10.1128/JVI.00474-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Scholler J, et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med. 2012;4(132):132ra53. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Mitsuyasu RT, et al. Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects. Blood. 2000;96(3):785–93. [PubMed] [Google Scholar]
- 303.Deeks SG, et al. A phase II randomized study of HIV-specific T-cell gene therapy in subjects with undetectable plasma viremia on combination antiretroviral therapy. Mol Ther. 2002;5(6):788–97. doi: 10.1006/mthe.2002.0611. [DOI] [PubMed] [Google Scholar]
- 304.Hombach AA, Holzinger A, Abken H. The weal and woe of costimulation in the adoptive therapy of cancer with chimeric antigen receptor (CAR)-redirected T cells. Curr Mol Med. 2013;13(7):1079–88. doi: 10.2174/1566524011313070003. [DOI] [PubMed] [Google Scholar]
- 305.Ni Z, et al. Expression of chimeric receptor CD4zeta by natural killer cells derived from human pluripotent stem cells improves in vitro activity but does not enhance suppression of HIV infection in vivo. Stem Cells. 2014;32(4):1021–31. doi: 10.1002/stem.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Joseph A, et al. Inhibition of in vivo HIV infection in humanized mice by gene therapy of human hematopoietic stem cells with a lentiviral vector encoding a broadly neutralizing anti-HIV antibody. J Virol. 2010;84(13):6645–53. doi: 10.1128/JVI.02339-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Luo XM, et al. Engineering human hematopoietic stem/progenitor cells to produce a broadly neutralizing anti-HIV antibody after in vitro maturation to human B lymphocytes. Blood. 2009;113(7):1422–31. doi: 10.1182/blood-2008-09-177139. [DOI] [PubMed] [Google Scholar]
- 308.Abdool KS, Abdool KQ, Baxter C. Antibodies for HIV prevention in young women. Curr Opin HIV AIDS. 2015;10(3):183–9. doi: 10.1097/COH.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Gengiah TN, et al. Adherence challenges with drugs for pre-exposure prophylaxis to prevent HIV infection. Int J Clin Pharm. 2014;36(1):70–85. doi: 10.1007/s11096-013-9861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Saunders KO, et al. Sustained Delivery of a Broadly Neutralizing Antibody in Nonhuman Primates Confers Long-Term Protection against Simian/Human Immunodeficiency Virus Infection. J Virol. 2015;89(11):5895–903. doi: 10.1128/JVI.00210-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Thurman AR, et al. Intravaginal rings as delivery systems for microbicides and multipurpose prevention technologies. Int J Womens Health. 2013;5:695–708. doi: 10.2147/IJWH.S34030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Gunawardana M, et al. An intravaginal ring for the sustained delivery of antibodies. J Pharm Sci. 2014;103(11):3611–20. doi: 10.1002/jps.24154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13(9):655–72. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Muthumani K, et al. Optimized and enhanced DNA plasmid vector based in vivo construction of a neutralizing anti-HIV-1 envelope glycoprotein Fab. Hum Vaccin Immunother. 2013;9(10):2253–62. doi: 10.4161/hv.26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Nowrouzi A, et al. Integration frequency and intermolecular recombination of rAAV vectors in non-human primate skeletal muscle and liver. Mol Ther. 2012;20(6):1177–86. doi: 10.1038/mt.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Penaud-Budloo M, et al. Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. J Virol. 2008;82(16):7875–85. doi: 10.1128/JVI.00649-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Schnepp BC, et al. Genetic fate of recombinant adeno-associated virus vector genomes in muscle. J Virol. 2003;77(6):3495–504. doi: 10.1128/JVI.77.6.3495-3504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Jiang H, et al. Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol Ther. 2006;14(3):452–5. doi: 10.1016/j.ymthe.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 319.Yla-Herttuala S. Endgame: glybera finally recommended for approval as the first gene therapy drug in the European union. Mol Ther. 2012;20(10):1831–2. doi: 10.1038/mt.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Schnepp BC, Johnson PR. Adeno-associated virus delivery of broadly neutralizing antibodies. Curr Opin HIV AIDS. 2014;9(3):250–6. doi: 10.1097/COH.0000000000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Lewis AD, et al. Generation of neutralizing activity against human immunodeficiency virus type 1 in serum by antibody gene transfer. J Virol. 2002;76(17):8769–75. doi: 10.1128/JVI.76.17.8769-8775.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Fang J, et al. Stable antibody expression at therapeutic levels using the 2A peptide. Nat Biotechnol. 2005;23(5):584–90. doi: 10.1038/nbt1087. [DOI] [PubMed] [Google Scholar]
- 323.Johnson PR, et al. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat Med. 2009;15(8):901–6. doi: 10.1038/nm.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Fang J, et al. An antibody delivery system for regulated expression of therapeutic levels of monoclonal antibodies in vivo. Mol Ther. 2007;15(6):1153–9. doi: 10.1038/sj.mt.6300142. [DOI] [PubMed] [Google Scholar]
- 325.Balazs AB, et al. Vectored immunoprophylaxis protects humanized mice from mucosal HIV transmission. Nat Med. 2014;20(3):296–300. doi: 10.1038/nm.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Saunders KO, et al. Broadly Neutralizing Human Immunodeficiency Virus Type 1 Antibody Gene Transfer Protects Nonhuman Primates from Mucosal Simian-Human Immunodeficiency Virus Infection. J Virol. 2015;89(16):8334–45. doi: 10.1128/JVI.00908-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Fuchs SP, et al. AAV-Delivered Antibody Mediates Significant Protective Effects against SIVmac239 Challenge in the Absence of Neutralizing Activity. PLoS Pathog. 2015;11(8):e1005090. doi: 10.1371/journal.ppat.1005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Wang L, et al. Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum Gene Ther. 2011;22(11):1389–401. doi: 10.1089/hum.2011.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Martinez-Navio JM, et al. Host Anti-antibody Responses Following Adeno-associated Virusmediated Delivery of Antibodies Against HIV and SIV in Rhesus Monkeys. Mol Ther. 2015 doi: 10.1038/mt.2015.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Joos B, et al. Long-term multiple-dose pharmacokinetics of human monoclonal antibodies (MAbs) against human immunodeficiency virus type 1 envelope gp120 (MAb 2G12) and gp41 (MAbs 4E10 and 2F5) Antimicrob Agents Chemother. 2006;50(5):1773–9. doi: 10.1128/AAC.50.5.1773-1779.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Foley BLT, Apetrei C, Hahn B, Mizrachi I, Mullins J, Rambaut A, Wolinksy S, Korber B. HIV Sequence Compendium. Theoretical Biology and Biophysics Group: Los Alamos National Laboratory; NM, LA-UR 13–26007: 2014. [Google Scholar]
- 332.Pettersen EF, et al. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–12. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]