Abstract
In spite of significant advances in anti-retroviral (ARV) therapy, the elimination of human immunodeficiency virus (HIV) reservoirs from the periphery and the CNS remains a formidable task. The incapability of ARV to go across the blood-brain-barrier (BBB) after systemic administration makes the brain one of the dominant HIV reservoirs. Thus, screening, monitoring, and elimination of HIV reservoirs from the brain remain a clinically daunting and key task. The practice and investigation of nanomedicine possesses potentials for therapeutics against neuroAIDS. This review highlights the advancements in nanoscience and nanotechnology to design and develop specific sized therapeutic cargo for efficient navigation across BBB so as to recognize and eradicate HIV brain reservoirs. Different navigation and drug release strategies, their biocompatibility and efficacy with related challenges and future prospects are also discussed. This review would be an excellent platform to understand nano-enable multidisciplinary research to formulate efficient nanomedicine for the management of neuroAIDS.
Keywords: Anti-retroviral (ARV) therapy, Nanotechnology, HIV-1, Blood-Brain-Barrier (BBB), CNS drug delivery, neuroAIDS, Magnetic Nanoparticle, HIV-Monitoring
Graphical Abstract
Introduction
1. Brain pathogenesis of HIV and neuroAIDS
Human immunodeficiency virus (HIV) neuroinvasion associated neurologic condition i.e. neuroAIDS prevails among acquired immune deficiency syndrome (AIDS) patients. The HIV presence in the brain jeopardizes the health and function of nerve cells resulting from inflammation mediated damage of the brain region and spinal cord involved in learning and information processing[1]. Nearly ~ 50% HIV patients demonstrate neuropathological signs or symptoms such as loss of sensation, cognitive impairment, seizures, behavioral changes [2], etc., and nearly 80% autopsies shows a range of neuropathology in AIDS patients [3]. AIDS associated neurologic condition is caused by HIV infection to the brain cells, by opportunistic infections via bacteria, fungi or other viruses, toxic effects of antiretroviral drugs or by HIV associated oncogenesis. Major neurological complications associated with AIDS are: HIV-associated dementia [4, 5] (HAD), central nervous system (CNS) lymphomas, chronic meningitis, peripheral neuropathies, neurosyphilis, vacuolar myelopathy, progressive multifocal leukoencephalopathy etc. [6]. Some neurological disorders of unknown origin has also been reported during HIV infection [7, 8]. Nonetheless, the onset of neuroAIDS condition in HIV infected patients remains debatable among scientific group due to multifaceted symptoms and pathologies and lack of specific diagnosis tools or protocols. Many studies suggest that neuroAIDS may develop as soon as HIV infects the brain. Contrary to initial belief, it has been proven that HIV infects the brain during early phase when HIV concentration is as high as the late infection stage. As such, HIV particles, its DNA and proteins can be detected in early during the infection. The HIV entry to the brain is mediated via mononuclear phagocytes (‘Trojan horse’ mechanism) i.e. monocytes and blood-borne macrophages in response to specific cytokines/chemokines (e.g. monocyte chemotactic protein-1)[6, 9]. Initial HIV infection in brain triggers production of factors that alter the integrity of the blood-brain-barrier (BBB) (e.g. matrix metalloproteinase) which induces movement of infected/non-infected leukocytes across BBB from peripheral circulation [1, 10]. This intensifies the HIV infection in various brain cells. While HIV infection of astrocytes and microglia has been established, the direct or indirect invasion mechanism executed by HIV in nerve cells remains debated among scientific group. It is believed that HIV in subpopulations of infected brain cells acquires latency and, in turn, escapes the deleterious effect of antiretroviral therapy (ART) and immune response. Latency can persist for years where no or little virus is produced due to low transcription of host-integrated HIV genome. An appropriate endogenous or exogenous stimulus can reactivate the latent cell causing production of fresh infectious virions [11]. Thus, latent cells are the primary cause of HIV persistence and are reservoirs of rebound viremia.
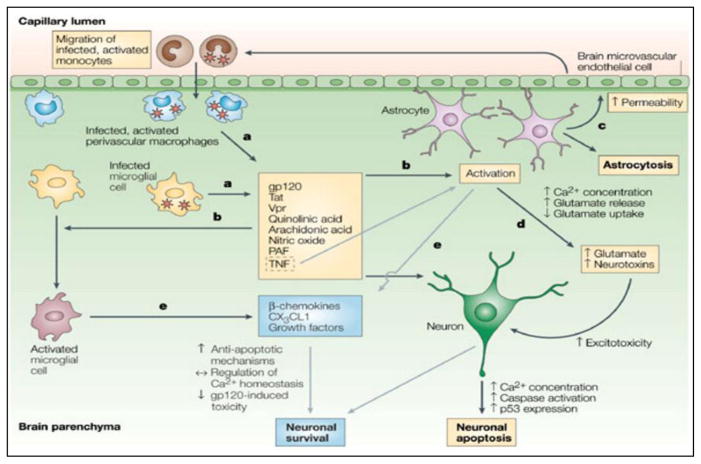
The HIV virion and its neurotoxic proteins induce significant pathology across various brain cells (Figure 1) [11]. HIV-infected monocytes differentiation into macrophages activates astrocytes and resting microglia which elicits severe neuroinflammation and releases neuron-damaging products such as Tumor necrosis factor (TNF-α), Interleukin (IL-1β), reactive oxygen species (ROS), nitric oxide (NO), quinolinic acid, etc. The envelope protein gp-120/gp-41 activates neuronal chemokine receptors (CXCR4 or CCR5) and subsequent elevation of intracellular Ca2+ leads to apoptosis. In astrocytes, gp120 downregulates glutamate uptake to cause excitotoxicity and nitric oxide synthase production leading to cell death. In macrophages and microglia, gp120 elevates the level of proinflammatory factors such as IL-1β, TNF-α, β-chemokines, arachidonic acid, etc. The gp120 also inhibits proliferation and migration of neural progenitor cells (NPCs) and affect the component of BBB by inducing apoptosis in brain microvascular endothelial cells (BMVECs). The p-53 pathway mediates the activation of apoptosis by gp120/gp41 occurring in all brain cells i.e., neurons, astrocytes, and macrophages/microglia. Similar to gp120, HIV-Tat protein induces multiple adverse effects on neurons: (i) induce N-methyl-D-aspartate (NMDA) receptors, (ii) decrease dopamine (iii) inhibits tyrosine hydroxylase, and (iv) activate nitrous oxide (NO) and Calcium (Ca)-release. All of these lead to neuronal death by apoptosis or other cytotoxicity means. HIV Tat mediated upregulation of MCP-1 and reductions of glutamate uptake are seen in astrocytes; in macrophages and microglia, the levels of proinflammatory factors such as TNF-α and IP-10 are elevated. Tat exposure also induces apoptosis in Brain microvascular endothelial cells (BMVECs) and inhibits neural progenitor cells (NPCs) neurogenesis. The HIV Vpr (Viral Protein-R) and Nef proteins induce apoptosis in neurons, astrocytes, and BMVECs. Furthermore, the Vpr modulates ion channels and H2O2 upregulation in neurons. Vpr exposure to NPCs causes mitochondrial dysfunction and impaired neuron maturation. The Nef in neurons modulates [K+] channels and induces proinflammatory factors such as TNF-α, Macrophage inflammatory protein-1-alpha (MIP-1), IL-6, and elevates superoxide release in macrophages and microglia. In astrocytes, Nef upregulate complement factor C3, IP-10, monocyte chemotactic protein-1 (MCP-1), and Matrix metallopeptidase-9 (MMP-9) activity. Thus, HIV induced injury of brain cells is mediated via various cell-specific mechanisms [12]. These pathological mechanisms aggravate the neuroAIDS condition. Nonetheless, synaptodendritic injury by HIV is believed to be a primary cause of HIV-associated neurocognitive disorders (HAND) symptoms in HIV patients[13]. Recently we reported that HIV infection to the brain causes significant damage to synaptic plasticity. Atluri et al showed remarkable loss of spines, dendrite diameter, decreased spine density, and dendrite and spine area in HIV-1 clade B and clade C infected neuroblastoma cells [14, 15]. The inter-clade variation in density and morphology of spine and dendrite was seen where HIV clade C was found less injurious than Clade B. This inter-clade variation can be attributed to difference in potency of HIV neurotoxic peptides between both clades. Such as, Thangavel et al found that HIV Tat of clade B and C exert non-similar effect on morphology and spine density, with clade C Tat being less potent [16]. The change in synaptic plasticity was substantiated by the HIV clade and protein specific differential expression of Immediate-early genes (IEGs), long-term potentiation (LTP) genes, and long-term depression (LTD) genes. These are three major groups of genes that play a central role in synaptic plasticity regulations. Moreover, cell specific variations in the expression of synaptic plasticity genes are seen during HIV infection. This may reflect infectivity and latency intensity where one cell type establishes persistence HIV infection while the other is active. The changes in these genes adversely affect synaptic connections. In fact, subjects with HIV associated neurological disorders exhibit decreased synaptic and dendritic density with atrophy of grey and white matter [17]. As such, ARV and anti-latency agents will not be sufficient to improve the neuroAIDS condition; neuro-protection or neuron-resuscitating therapeutics would be required.
Figure 1. Mechanisms of neurodegeneration and neuroprotection in AIDS [6].
a) Infected perivascular macrophages and microglia are responsible for producing HIV but might also release viral proteins that can be deleterious to the central nervous system. The HIV-envelope protein gp120 (glycoprotein 120), Tat (transcriptional transactivator) and Vpr (viral protein R) have all been shown to be toxic in vitro to neurons and/or astrocytes, although their relevance in vivo is unknown. Infected and activated cells also produce other factors — such as cytokines (including tumour-necrosis factor, TNF), quinolinic and arachidonic acid, platelet-activating factor (PAF) and nitric oxide-which are known to have neurotoxic effects; b)Importantly, they promote the further activation (and to some extent, proliferation) of macrophages and/or microglia, as well as the proliferation and activation of astrocytes; c) Activated astrocytes modify the permeability of the blood–brain barrier and promote the migration of more monocytes into the brain; d) In addition, through increases in release of intracellular Ca2+ and glutamate and through decreases in glutamate uptake, the brain concentration of glutamate and other neurotoxins increases and results in excitotoxic death of neurons; e) Activation of macrophages and/or microglia, and TNF-mediated activation of astrocytes, also results in the release of beta-chemokines, CX3C-chemokine ligand 1 (CX3CL1) and growth factors, all of which are known to regulate Ca2+ homeostasis in neurons, to promote anti-apoptotic signaling pathways and to decrease gp120-mediated and excitotoxic neuronal cell death, thereby promoting neuronal survival. Grey arrows indicate neuroprotective pathways. (Reproduce with permission from authors- González-Scarano and Martín-García - Nature Reviews Immunology, 2005).
2. State of the art for Current HIV/AIDS treatment
The present state-of-the-art treatment for HIV/AIDS is highly active antiretroviral therapy (HAART), which consist of combination or single formulation of different classes of ARV drugs. In spite of the significant success using the current HAART treatment, numerous challenges are still to be resolved. One of the main drawback in the successful eradication in HIV treatment is poor patient compliance with drug therapy [18]. Due to lifelong continuous medication, patients did not sticks to the treatment schedule and leads to ineffective drug concentration which promotes the ricochet of viral reproduction [19–21]. Despite the best efforts on the part of the healthcare professionals, non-adherence of patients with HAART remains very high. Clinical trial data on HIV drugs shows that missed doses of ARV drugs were occur due to forgetfulness and being away from home (43%), sleeping (36%), busyness to take the dose (22%), feeling sick (11%), and depression (9%) [22, 23]. Also, neurocognitive diseases in PLWH/A (people living with HIV/AIDS) can be greatly aggravated by concurrent intake of drugs abuse e.g. cocaine, methamphetamine etc., which decreases likelihood of adherence to HAART regimens [24]. No single intervention approach will likely improve the adherence; efforts to increase patient adherence will likely rest on set of key factors. Among all strategies, decreasing the frequency of medication administration may have the greatest potential to improve compliance and achieve better treatment outcomes [25, 26].
Moreover, the other issues with HAART are toxic side effects and virological resistance with high economic burden [27]. The key mechanism of ARV drug resistance is due to the great genetic diversity of HIV-1 and their constant mutation during infection. Resistant issue can be solved by developing personalized therapy, wherein the resistance testing can be performed to decide the right kind of therapy with respect each individual patients [28]. Once the body is infected with HIV, due to weak immune system, chances of other diseases progression increases e.g. heart disease, diabetes, neurodegeneration, and immunological disorders [19]. Main reason for above mentioned diseases could be due to the HIV infection in combination with other co-infection (e.g. hepatitis C) and cytotoxicity due to HAART [27]. The main perpetrator for the HIV cure is the presence of resilient viral reservoirs known as “latent reservoirs”. [19, 20]. Mainly these latent cells resides in explicit anatomical location (e.g. lymphoid tissue, testes, liver, kidney, gut and CNS) [29–32]. Along with the presence of latent reservoir in presence of HAART, additional cryptic viral sources also exist along with small amount of active virus replication that follows even during the therapy [33–35]. Therefore, there is a great need to discover new approaches/methods/drugs for developing non-toxic, low-dosage treatment that provide more sustained dosing coverage and efficient eradication of the virus from the reservoirs, circumventing the need for life long treatments [21, 27]. Current HAART regimen is sufficient for complete eradication of the virus from the body, therefore, understanding the HIV persistence mechanism and development of novel purging strategies of viral reservoirs should be the key areas of research center for complete HIV eradication.
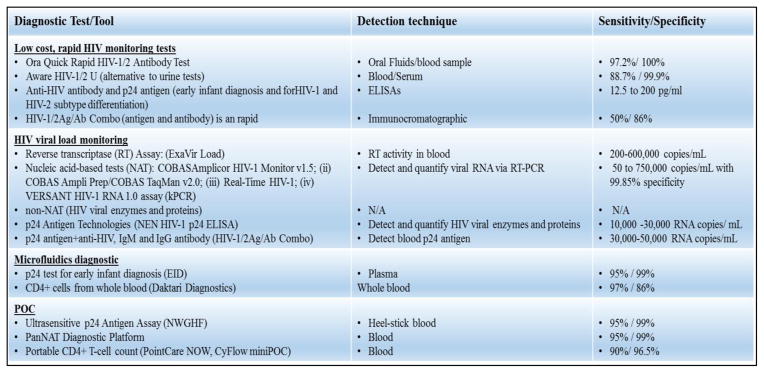
2.1 Constraint for CNS delivery
The available recommended drugs or therapeutics can be delivered to the body in general via several routes of administrations. (E.g. oral, intravenous, dermal, subcutaneous, inhalational or intraperitoneal). Drugs absorption process starts immediately once it interact with receptor or biological components and subsequently reaches to the target site in the body. By the time drug is administer and reached to the target site, it may or may not be actively modified or metabolized and due to this it may or may not be recognized by receptors to show its effects at target organs/cells [36]. The unique properties of each therapeutic agent may have desirable or undesirable properties at each of these steps and may affect the pharmacological activity. Throughout this expedition, (i.e. from the time of administration to the site of action/delivery), ARV drugs or nanoformulation (NF) may come across various biological “barriers” as shown in Fig. 2.
Figure 2.
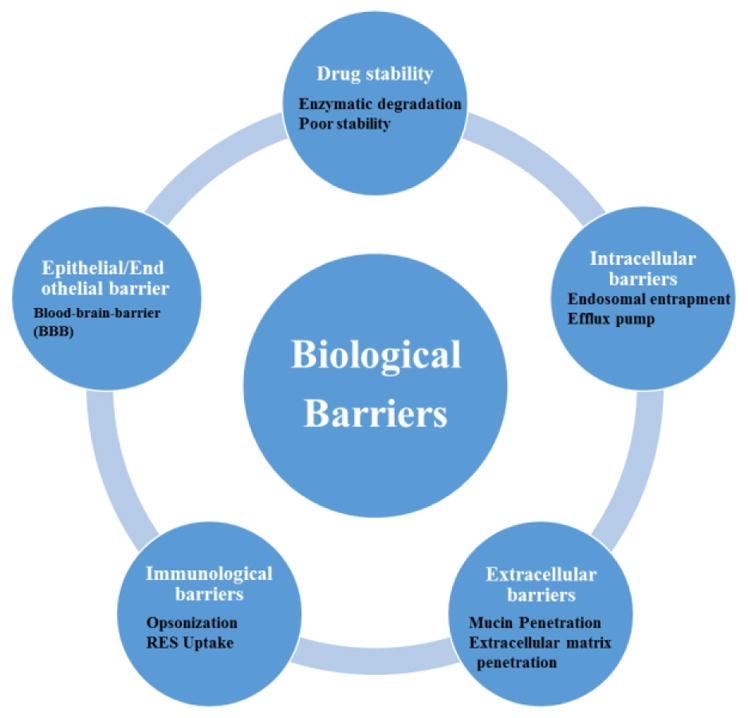
Different types of biological barriers for ARV drugs for CNS drug delivery
For the CNS drugs delivery, the key hindrance is the presence of the BBB tight junction [11, 31, 32, 37–43]. The BBB is an active interface between the blood and the CNS, which rigorously controls the molecular and cellular traffic between the blood and the brain, thus maintains the proper homeostasis of neuronal function [44–47]. Scientists have explored many novel strategies to improve the permeability of ARVs across BBB and to study these parameters of drug transport various types of in-vitro BBB model have been developed [11, 36, 48, 49]. To mimic the real in-vivo BBB scenario, various in-vitro models have been developed using various type of cell line of different origins and culture environments. To achieve more realistic situation, constantly new improvements have been done in the well characterized and established protocols by the scientists. For detailed information on BBB model developments, please read review by Wilhelm and Krizbai [50]. Based on their observation, we have summarized (Table-1) the list of most commonly used in-vitro BBB model to study the drug transport or nanoformulations (NF) for CNS delivery.
Table 1.
ypes of in-vitro BBB models for drugs/nanoformulation transport. [50]
| Model Type | Advantages | Disadvantage |
|---|---|---|
| Epithelial cells overexpressing Transporters models |
|
|
Transwell monoculture models
|
|
|
Co-cultures models
|
|
|
| Dynamic in-vitro (DIV) models |
|
|
| Microfluidic models |
|
|
2.2 Therapeutics approaches for HIV treatment
To achieve the effective treatment for an HIV-infected patient, all sources of virus replication must be abolished. To achieve this objective, we may need the amalgamation of diverse methods, to counter attack the replication of virus from active infection along with the supply from viral reservoirs [21, 51, 52]. To overcome the limitations of the CNS delivery, a number of different strategies have been explored to increase the penetrability of ARVs through the BBB. Each of these strategies presents strengths and limitations for the CNS delivery [53]. At preclinical levels, ARVs delivery to the brain using nanotechnology has shown promising results so far [27, 31, 32, 36, 38, 39, 49, 53–59]. Based on the delivery mechanism, the chemical modifications of ARVs may or may not be needed for effective delivery. The following sections discuss the strategies for enhanced brain delivery using different approaches.
2.3 Approaches for ARV penetration across the BBB
Over the years, conventional and non-conventional approaches have been developed to overcome the BBB constraints to allow drug administration into the brain. The well-known techniques or approaches that have been explored for BBB delivery of drugs or other therapeutic agent are described below.
2.3.1. ATP-binding cassette (ABC) transporters blocking approach
Most of ARVs are large, high molecular weight lipophilic compounds. They seem probable candidates to be an ABC transporter substrate. Over the years, numerous new chemical entities (NCEs) capable of blocking specific ABC-transporters have been developed [60] and their efficacies have been tested at in-vivo level using different animal studies [61]. E.g., treatment with P-gp inhibitor LY-335979 in mice have resulted in higher concentration for many Protease Inhibitors (PIS) (i.e., saquinavir, indinavir, amprenavir, and nelfinavir) in CNS [62]. Similar in-vivo results were also observed for Saquinavir using other class of agents i.e. GF120918 (P-gp/ABCG2 blocker)and MK571 (MRP inhibitor) [63]. Another study conducted by Megard et al also proved that P-gp is the key player and plays an vital part in regulating the penetrability of PIs across the BBB [64]. Since, the abundant presence of ABC transporters, these blockers are not very CNS-specific and due to increase ARV delivery across BBB it may results in higher chances of drug toxicity/drug-drug interactions.
2.3.2. The BBB opening approach for navigation of drug to the brain
The BBB functions to maintain a delicate homeostatic environment by regulating ion and neurotransmitter concentrations, while simultaneously preventing the access of toxins, immune cells and pathogens from the peripheral circulatory system [65]. The BBB regulates the transport of nutrients into the brain and assists with removing waste products. While these functions are necessary for maintaining the health of the brain, the BBB prevents the access of therapeutic agents when brain diseases develop, thus makes brain diseases treatment difficult. It has been estimated that the BBB prevents the access of over 98% of potential therapeutics from passing into the brain [66]. To overcome these issues, an alternate strategy known as hyper-osmotic opening of the BBB has been explored for transient opening. Mannitol and urea hypertonic solution have been extensively used for opening of BBB tight junction transitorily due to shrinkage of capillary endothelial cells which induces water efflux and successively paracellular transport of the drugs or nanoformulation materials across the BBB [67, 68]. This approach has been explored in-vivo with some success due to the risky nature of this procedure, it is not well accepted in general. Most common side effects includes seizures and impulsive long-term neurological complications, thus restricts it application in day to day procedure. Another alternate strategy used for the transient BBB opening is the usage of cytotoxic agents, e.g. alkylating agents (etoposide and cisplatin), they main works by disrupting the BBB tight junctions and generate small opening with in the endothelial cells [69]. Likewise, intra-arterial delivery of vasoactive agents (e.g. bradykinin, peptidase inhibitors and angiotensin-II) may also transitorily upsurges the BBB permeability [70–72]. The enhanced brain concentration of testing agents, therapeutic and drug nanoformulations has been detected after systemic delivery. But due to toxic nature of these compounds, this strategy is not recommended for the enhanced delivery of ARVs at the CNS. Another minimal invasive approach of focused ultrasound and microbubble (fine gas bubbles with diameter of > 50 μm) are also being explored for the BBB opening for therapeutic delivery [73–75]. Microbubbles on exposure to focused ultrasound, help the cavitation nuclei to focus and transduce the acoustic energy into mechanical power and this force at the BBB interface induces the transitory opening of endothelial tight junctions [76–78]. Chen et al have used focused ultrasound transiently to allow the permeability of the BBB to increase as to allow more drug diffusion. They have used non-invasive external magnetic field for the improved localization of therapeutic agent immobilized on the magnetic nanoparticle. Results showed that this combinative approach significantly improved the in-vivo delivery of anti-cancer drug [BCNU: 1,3-bis(2-chloroethyl)-1-nitrosourea] for the treatment of brain tumor [79]. Due to safety concerns, this strategy is also not suggested for long term application and to make this strategy works more standardization is required. In addition, the biggest challenge with the above technique is that the BBB opening is uncontrolled and may allow unwanted or other neuro damaging entities entry into the brain [75, 80, 81]. Especially in case of HIV infected patient, the passage of virus from periphery to CNS is unwanted.
2.3.3. Prodrug based approaches
Presently, only two traditional prodrugs have been developed in HIV therapy, fosamprenavir and tenofovir disoproxil fumarate [82]. Recently, Tenofovir alafenamide fumarate ( TAF, before known as GS-7340), a new pro-drug of the widely used tenofovir (Nucleoside reverse transcriptase inhibitor-NRTI), have similar antiviral efficacy reaches and better penetration power in HIV harboring cells than the parent tenofovir disoproxil fumarate (TDF) molecule [83]. The prodrugs with the good lipophilic profile, once reaches to the endothelial cells can be hydrolyzed and releases the parent ARVs molecule and crosses the cell membrane of the endothelial cells by passive diffusion [84]. Although the BBB penetration is restricted, TDF dose is capable of passing the blood-CSF barrier of the choroid plexus gaining access to perivascular and meningeal macrophages [85]. Generally a prodrug is recognized as a separate chemical entity than the parent molecule, considerably more drug purification steps are needed along with the screening tests and clinical studies to prove the efficacy of the drugs are foreseen, which is generally not lucrative or possible to derive all pharmaceutical agents to prodrug states. Hence this technique may not be as effective as parent ARV molecule delivery approach.
2.3.4. Nanomaterials based approach
There are numerous valid reasons for which nano based drug delivery systems (NDDS) are attractive option for the development of formulation towards the treatment of neuroAIDS. The most important reason is the increased availability of charged surface molecules on carriers with respect to the total number of drug molecules to design efficient nanoformulation systems. Due to the higher surface area of nanoparticles, it results in higher drug binding/adsorption and can also be used to carry for diverse kinds of compounds such as biological molecules (siRNA, gene or plasmids) and even large proteins. The nanomaterials/nanoformulations used for the CNS drug delivery should have following salient features
Soluble, safe or non-toxic, and biocompatible
Have good bioavailability and long circulation life
Size of nanoparticle should not block blood vessel
Protects or avoid the drug from enzymatic and hydrolytic degradation
Can be used to deliver short half-life drugs in sustained or controlled manner
Increase or should not delay the dissolution rate of drug and onset of therapeutic action
Helps in avoiding loss of drug through rapid RES clearance and its metabolism
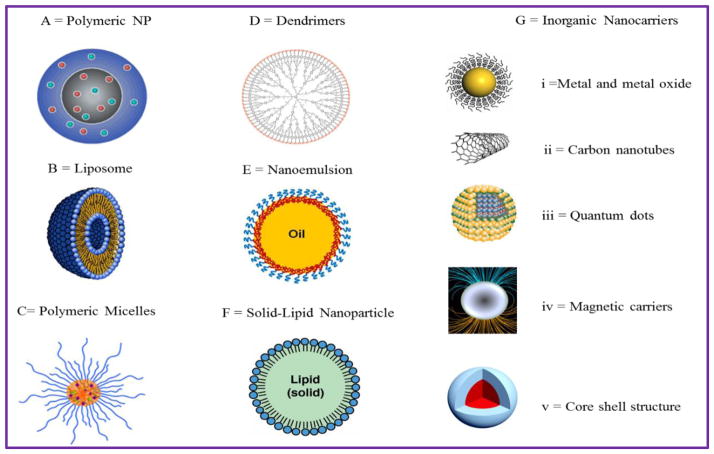
Nanoscale drug delivery systems are available in variety of forms e.g. nanoparticles, liposomes, dendrimers, fullerene, nanotubes, quantum dots, nanocapsule, nanosphere, nanocrystals etc. are believed to have promises to transform drug delivery systems [Fig. 3]. Additionally nanomaterials also used for device or instruments development in variety of industries e.g. lab-on-chips, nano-robotics etc. Nanoparticle can be made using organic or inorganic materials have certain advantage and disadvantages, but can be used smartly to achieve desired therapeutic action. Thus, nanomaterials can be explored for the development of novel drug delivery systems and redeveloping the current drugs delivery techniques to enhancement the efficacy, patient-compliance, better safety of drugs and economic burden of health care system [86]. The next section discusses each type of nanomaterial explored for the ARV drug delivery to CNS and its state of the art with respect to neuroAIDS treatment.
Figure 3. Illustration of various nanocarriers (NCs) exploited for CNS drug delivery.
A) Polymeric nanoparticle (PLGA, PLA PBCA); B) Liposomes; C) Polymeric micelle; D) Dendrimers; E) Lipid micro & nanoemulsion; F) Solid lipid nanoparticle and G) Inorganic NCs including: (i) Metal & metal oxide nanoparticles; (ii) Carbon nanotubes; (iii) Quantum dots; (iv) Magnetic nanoparticles (MNP) and (v) Core-shell nanoparticles (MENP)
2.4 NanoART for neuroAIDS treatment
The ARVs can be efficiently delivered to the brain using drug loaded on nanocarriers (NCs) [11, 38, 53, 58]. Depending on the chemical/physical nature of NCs, chemical modifications of ARVs may or may not be essential for effective loading and its targeted delivery. There is an extensive variety of NCs are available (as shown in Fig. 3) that are in practice and being tested for the brain drug delivery applications to cure CNS diseases. Their versatility of tuning properties allows them to deliver ARVs across the BBB [49, 55, 58, 87, 88]. The following sections will discuss the application NCs for ARVs delivery to the brain in details.
2.4.1 Magnetic nanoformulations for neuroAIDS treatment
The MNPs certainly possess advantage over other NCs because of its inherent super-paramagnetic property which allows the control over its magnetization i.e. movement/speed and, in turn, attached drug can be delivered to specific body locations by applying non-invasive magnetic force from exterior. Simultaneously, neuroimaging techniques such as magnetic resonance imaging (MRI) can be applied and based on quantification analysis of MNPs-associated drugs can lead to determine site-specific optimal or suboptimal dosing [38, 89–93]. Nonetheless, applications of the MNPs for the brain drug delivery has been explored a little. The release of loaded drugs from MNPs, in entirety, depends on cell or tissue based physiological phenomenon such as change in temperature, pH, intracellular Ca2+ level, etc., which cannot be manually controlled. Biomolecules such as proteins, enzymes, drugs, etc. can be tagged on MNPs and navigated magnetically to targeted sites including brain pathologies [94]. Recent improvement in synthesis techniques allow achieving super paramagnetic MNPs size of as small as 10 nm which can cross the BBB without affecting its integrity [95]. Moreover, MNPs encapsulations into liposomes i.e. “magnetoliposomes” can be excellent module to protect loaded drug from peripheral enzymatic decomposition and can reduce the entrapment by reticuloendothelial systems as well [38, 96]. Our group (Saiyed et al) already shown that magnetoliposomes used packed in monocytes/macrophage and used for the drugs delivery across BBB [58]. The movement/speed of these magnetized carriers can be operated in the same way as for naked MNPs. In recent years, our laboratory has investigated on the transendothelial delivery of MNPs bound anti-retroviral drugs. Nanoformulation of 3′-azido-3′-deoxythymidine-5′-triphosphate (AZTTP) showed a comparable efficiency to the free drug in suppressing HIV replication. An AZTTP-magnetoliposomes were developed which showed sustained AZTTP release for 14 days with intact anti-HIV potency [58]. Both, AZTTP-magnetic and AZTTP-magnetoliposomes resulted in nearly 3 fold increased in vitro BBB transmigration when compared to free AZTTP and did not affect the BBB integrity. Wen et al also have explored the magneto-liposomal formulation [Magnetic-PLGA/lipid nanoparticles (MPLs)] for trans-activating transcriptor (Tat peptide) delivery across BBB. Therapeutic efficiency of the drug-loaded Tat-MPLs versus drug-loaded MPLs was compared in bEnd.3 cells (endothelial cell line). Results showed dose and time dependent accumulation of higher concentration of Tat-MPLs than MPLs in bEnd.3 cells and proves that Tat-conjugated MPLs may serve as an effective drug delivery approach that crosses the BBB [97].
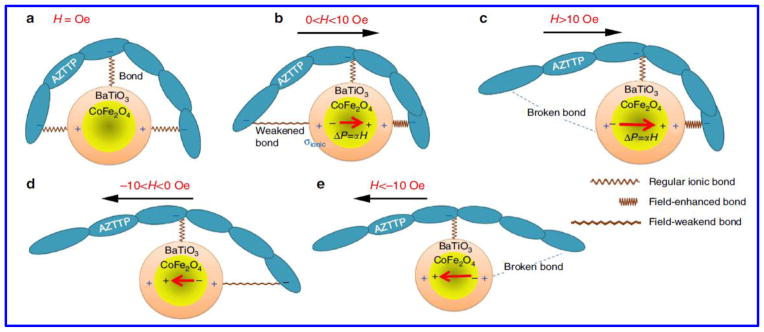
Furthermore, to address the issue of non-adherence in HIV infected subjects, it is necessary to develop long-term release of drug from formulation to achieve better patient compliance and therapy adherence. Our group (Jayant et al) have recently explored this aspect and investigated a novel ARV loaded magnetic nanoformulation for sustained release by assembling layer-by-layer (LbL) of polymer molecules (dextran sulphate) and ARV drug (Tenofovir) on MNPs [55, 98]. The sustained release of tenofovir showed 2.8 times higher drug loading with 30-fold increase in the drug release period. Also, nanoformulation showed a higher BBB transmigration ability (37.95%±1.5%) and improved in vitro antiviral efficacy (~33% reduction of p24 level) over a period of 5 days after HIV infection in primary human astrocytes compared to free tenofovir. Also our group (Raymond et al) have explored MNP based delivery of Nef peptides containing the Nef myrisolation site across an in vitro BBB, which results in reduced nef-transfected microglia release of Nef exosomes and prevented the loss of BBB integrity and permeability. This study shows the preliminary evidence of the role of exNef in HIV neuroimmune pathogenesis and the possibility of a nanomedicine-based therapeutics targeting exNef to treat HIV-associated neuropathogenesis [99]. Recently, Fiandra et al investigated amphiphilic polymer (MYTS) to improve the permeation of a high-weighted ARV drug [Enfuvirtide-Enf)] across the BBB both in in-vitro and in-vivo models. Results showed good efficacy and trans-BBB permeation of nanoformulation across BBB in mice model [100]. The advantage of MYTS coating on nanoparticles is that it help in loading of multiple drugs of different classes. Notably, cell viability of all aforementioned magnetic nanoformulations has been > 95%, making them very promising therapeutic agents. To overcome this constraint of uncontrolled release of drug from the MNPs, our group have discovered a novel magneto–electric nanoparticle (MENPs). MENP exhibits dual effect (magnetic and electronic) and therefore possesses both ferroelectric and ferromagnetic parameters in a single Nanocarrier. The biggest advantage of MENPs is that there is no heat dissipation on application alternate current (a.c.) field and thus leads to unprecedented high release efficacy of the drug without any side effects or toxic effect to target or neighboring cells. Our group have explored this novel dual property (Magnetic and electrical property) nanomaterial for the delivery of ARV drug i.e., AZTTP across in-vitro BBB model for the potential application of neuroAIDS treatment [49]. MENPs under the influence of external ac trigger allows on-demand drug release via disturbing the original symmetry of ionic bonding (charge distribution) between drugs molecules and MENPs as shown in Fig. 4. The salient features of MENPs to prove it as a potential CNS drug delivery agent were explained by Kaushik et al. [39]. MENP-AZTTP nanoformulation showed 100% drug release at low alternating current (44 Oe at 100 HZ) without losing the drug integrity due to ME (magneto-electro) effect and showed excellent HIV-p24 inhibition at in vitro level with very good transendothelial BBB transmigration efficiency [49].
Figure 4.
A simplified (one directional) illustration of the concept of on-demand drug release by MENPs stimulated by a uniform a.c. magnetic field in X direction. (a) At zero field, only the ionic charge is present in the MEN shell. (b) An additional dipole moment (proportional to the magnetic field) breaks the original symmetry of the charge distribution in the shell. (c) As the field is increased above the threshold value (σionic~σME), the bond on one side is broken. (d,e) The field is reversed to break the bond on the opposite side of the nanoparticle. The red arrows show the electric dipole due to the ME effect. In practice, owing to the random configurations of nanoformulations with respect to the field, the effect is present along every central bond orientation. (Reproduce with author permission, Nair et al, Nature Communication, [49])
The results are encouraging but at the moment this novel technique needs more validation at in-vivo levels to prove its future clinical application. Currently we are testing these MENP nanoformulation at in vivo levels (both at rodent and non-primate levels) to prove it clinical efficacy for the treatment of neuroAIDS. While these anti-retroviral magnetic nanoformulations certainly show tremendous promise in reducing or eliminating HIV load from the brain, still lot of process optimization and technique validation at in-vivo level is required before it can be adapted in clinical settings.
2.4.2 Polymeric NCs for ARV delivery
Polymers are the important and well-explored NCs utilized for drug delivery system. Most of (>90%) controlled and sustained drug delivery systems basically contain polymers as important formulation constituent. Due to versatile tunable features such as safety profiles, biodegradability, high encapsulation/entrapment efficiency, anticipated physical properties e.g. controlled rate of disassociation, permeability, degradation rate along with targeting capabilities and easy modulation of release kinetics polymeric NCs (synthetic or natural) have been explored for ARV drug delivery across the BBB [11, 21, 53, 101, 102]. Polymers like Poly (butyl cyanoacryalate) (PBCA) and methylmethacrylate-sulfopropylmethacrylate (MMA-SPM) have been explored for Zidovudine, Lamivudine and stavudine delivery at in-vitro and in-vivo levels. Kau et al reported delivery of stavudine, zidovudine and lamivudine using MMA-SPM polymer nanoformulation leads to increase in ARV permeability (8–20 folds) across in-vitro BBB model [103]. Same group also studied SLNs (tripalmitin and cocoa butter mixture) as carriers for stavudine, delavirdine and saquinavir delivery across the in-vitro BBB model and showed enhanced permeability of the ARV compared to free forms [104]. Poly- D-L-lactide-co-glycolide (PLGA) and polylactide (PLA) nanoparticle formulations also have been explored for various ARVs (e.g. zidovudine (AZT), lamivudine) delivery across mice brain [105]. Mainardes et al showed higher bioavailability (2.7 times) AZT in PLA-PEG nanoparticle compared to AZT-loaded PLA nanoparticle and 1.3 times higher relative to free drug solution, thereby showing PLA-PEG blend NPs as potential carrier of drug to delivery drugs across BBB and also suggested intranasal route as a better route of delivery as a potential therapeutic approach for HIV-1 infection treatment [106]. Table. 2 summarized all type of polymeric nanotherapeutics that have been explored for the CNS delivery of ARV drug for the treatment of HIV-1.
Table 2.
Summary of preclinical NCs/Nanomaterials based CNS delivery of ARV drugs or other therapeutic compounds for prevention/treatment of HIV-1
| Type of Therapy | Therapeutic agents | Nanomaterials/Nanocarriers | References |
|---|---|---|---|
| Antiretroviral therapy (ART) | ddCTP, Zidovudine, Stavudine, Didanosine, Zalcitabine, Foscarnet & Indinavir | Liposome | [104, 121, 122, 124, 132, 133] |
| Stavudine | Liposome-laden macrophages | [124, 125] | |
| Zidovudine | Mannose- and galactose-targeted liposome Mannose-targeted liposome |
[27, 134] | |
| Lamivudine | Mannose-targeted dendrimer | [111, 135] | |
| Efavirenz | Tuftsin dendrimers | [111, 136] | |
| Ritonavir, lopinavir, zidovudine & Stavudine | PLGA nanoparticles | [105, 106, 137] | |
| Nevirapine Ritonavir & Efavirenz | Tat-conjugated nanoparticle | [53, 97, 138, 139] | |
| Ritonavir, Darunavir & Atazanavir | Poly(ε-caprolactone) nanoparticles | [140, 141] | |
| Dapivirine & Saquinavir | RMP-7/MMA-SPM nanoparticles | [132] | |
| Saquinavir, Stavudine, delavirdine & Atazanavir | Solid lipid nanoparticles (SLN) | [104, 131] | |
| Tenofovir, Enfuvirtide & AZTTP | Magnetic nanoparticle | [55, 58, 100] | |
| AZTTP | Magneto-electric nanoparticle | [49] | |
| Efavirenz, Lamivudine | Cyclodextrins | [113, 142] | |
| Rilpivirine | Poloxamer 338/TPGS 1000 | 116 | |
| Zidovudine, Lamivudine, Efavirenz, Nelfinavir & Ritonavir | Micelles P85 | [112–114] | |
| Zidovudine, Lamivudine, Efavirenz, Indinavir, Ritonavir & Atazanavir | Monocyte-derived macrophages-nanoparticle | [111, 139, 143–145] | |
| Stavudine, Zidovudine, lamivudine & Delavirdine | PBCA, MMA-SPM | [103, 104, 132, 146] | |
| Ampenavir & Saquinavir | Transferrin (Tf)-conjugated QD | [138, 147] | |
| Lamivudine | CNT | 122 | |
| HIV gene therapy (RNA- and DNA-based therapies) | Antisense RNAs, aptamers, siRNA therapeutics to HIV infected cells | PLGA nanoparticles CNT; Dendrimers |
[27, 138, 148–153] |
| Protein or peptide vaccine (Immunotherapy) | Gp-41, 120, 160, p24 protein, Env, Gag, Tat | PLGA nanoparticles | [153–155] |
| DNA Vaccine | Env, rev, gag, tat, ODN | Liposomes, nanoemulsion, PLA nanoparticles | [154–158] |
PBCA-Poly(butylcyanoacryalate); MMA_SPM-Methylmethacrylatesulfopropylmethacrylate; PLA-Polylactide; PLGA-Poly (D,L-lactide-co-glycolide); Tf-Transferrin; ddCTP- 2′,3′-Dideoxycytidine-5′-Triphosphate; AZTTP -3′-Azido-2′,3′-dideoxy thymidine-5′-Triphosphate; RMP-7: Bradykinin agonist; Tat- Transactivator of transcription; TPGS- D-α-Tocopherol polyethylene glycol succinate; P85- Pluronics; CNT- Carbon Nanotubes; QD- Quantum dots; RNA- Ribonucleic acid; DNA- Deoxyribonucleic acid; siRNA- Small interfering RNA; gp-HIV envelope proteins e.g. gp 41, 120, 160; p24- HIV-1 viral protein; ODN- CpG oligodeoxynucleotides; rev- transactivating protein HIV-1; Env- HIV-1 envelope; gag- Protein of HIV-1
2.4.3 Dendrimer based BBB ARV delivery
Dendrimers have been used as carriers of antiretroviral peptides and genes for HIV inhibition and more astonishingly, many recent studies indicated that they themselves can be used as antiretroviral agents [107–109]. Mannose-capped [poly (propyleneimine)-PPI] and polyamidoamine [PAMAM] dendrimers loaded with lamivudine were evaluated in-vitro for antiviral activity in HIV-1 infected MT-2 cells. Lamivudine loaded dendrimer NCs showed 21-fold increase in cellular uptake and 2.6-fold reduction in the viral p24 levels when compared to the group treated with free drug solution [110], again proving the importance of nanoformulation for BBB delivery. Dutta et al also have prepared efavirenz loaded tufstin conjugated 5th generation poly (propyleneimine) dendrimers (TuPPI). Tuftsin [natural macrophage activator tetrapeptide (Thr-Lys-Pro-Arg)] bind specifically to mononuclear phagocytic cells and enhance their phagocytic activity. They reported that the dendrimer were able to extend the in vitro drug release up to 144 hr in comparison to 24h of the PPI polymer. Furthermore, a 34.5 times higher cellular uptake and reduced viral load by 99% at a concentration of 0.625 ng/ml was also reported; this activity was more significant in HIV infected macrophages than uninfected cells. In a similar study the same group prepared t-Boc-glycine conjugated PPI dendrimer (TPPI) and mannose conjugated dendrimer [111]. The mannose conjugated dendrimer exhibited a higher (12 times) cellular uptake of efavirenz by monocyte/macrophage cells compared to free drug solution. Regardless of these promising results, unpredictable drug release kinetics and long-term safety issues hampers their wide use in BBB delivery like other polymers (e.g. PLGA). More modification in the parent molecule are needed to further authenticate the use of dendrimeric nano-systems for ARVs delivery to the CNS.
2.4.4 Micelle-based NCs and ARV delivery
Due to unique properties of smaller size and higher drug solubilization make micelles a promising candidate for drug and protein delivery across BBB. Amongst all the dendrimers, Pluronic micelles have shown very good promise in the BBB drug transport across in-vitro and in-vivo model [11, 60, 112]. The influence of Pluronic P85 on the permeability of a broad range of structurally unrelated ARV compounds was examined by Kabanov’s group in vitro using bovine brain microvascular endothelial cell (BMVECs) model [113]. Results showed 19-fold increases in the drug permeability in in-vitro BBB model compared to free drug solution. Most remarkable permeability results were obtained for PI class of drugs e.g. ritonavir due to strong bond formation with P85. Batrakova and co-workers investigated the co-administration of ARV drugs such as zidovudine, nelfinavir, and lamivudine with P85 and reported an improvement of the drugs permeability in-vitro in BMVECs and macrophages [113]. At in vivo levels, Pluronics have shown increased ARV delivery to the brain of wild-type mice, but results were opposite in mdr1a/b knockout mice, showing that the drug permeability effect by Pluronics is facilitated in part by P-gp inhibition at the BBB [112]. The enhancement of the drug’s efficacy upon co-administration with P85 was also confirmed in-vivo in SCID mouse model of HIV-1 encephalitis [114]. Along with so many promising advantaged, micelles biological stability and their slower rate of drug dissociation, long drug retention time limits their clinical use for CNS drug delivery.
2.4.5 Lipid-based NCs for the brain Delivery
Lipid-based NCs have shown strong potential for delivery of ARVs drugs to the CNS or other target specific delivery. There are a wide range of biological lipids and phospholipids accessible for lipid NCs development {please refer these reviews for more details [115–118]}. Liposomes are vesicles made of phospholipids bilayers, biocompatible in nature and have very good biodegradable profile. Due to unique lipophilic nature they possess the natural affinity to target the BBB and thus presents the usefulness for CNS delivery of ARVs [118]. Lipid based NCs exists in many forms including liposomes, microemulsion, nanoemulsion and solid lipid nanoparticles (SLN). The unique and advantageous property of liposomes lies in their recognition as a foreign body by the cells of the mononuclear phagocytic system (MPS) e.g. monocytes/macrophages [119, 120]. Since HIV largely exist in the macrophages and MPS, the liposome based nanoformulations of ARV drugs helps in decreasing cytotoxicity associated with free drug solution both at in-vitro and in-vivo levels. Largely, there are four types of the liposomes (cationic, anionic, sterically stabilized and immune-liposomes) largely explored for the anti-HIV/AIDS drug delivery so far [101]. Liposomes have been mostly used for the delivery of hydrophobic anti-HIV drugs like ddCTP, zidovudine, didanosine and zalcitabine, which is not possible with magnetic or other polymeric nanoparticle. In one of the study, liposomal foscarnet (antiviral used as a rescue therapy for late-stage HIV patients with multidrug resistance) nanoformulation helps in increasing the drug level in rat brains by 13-fold when compared to the free foscarnet solution [121]. Study on encapsulated 2′,3′-dideoxycytidine-5′-triphosphate (ddCTP) in liposomes and compared its antiviral effect with the dideoxycytidine and dideoxycytidine-triphosphate in cultured human monocyte-macrophages (M/M) infected with HIV-1 by Szebeni et al [122] showed better drug stability and cell uptake compared to free drug, thus suggest the ability of liposomal formulation for targeting drugs to macrophages in-vivo and can be a better choice to increase the therapeutic index of ARV drugs. AZT-loaded liposomes was also studied by Phillips et al [123] for the antiviral effect and bone marrow toxicity of in C57BL/6 mice, results showed improved antiviral activity for liposomal nanoformulation when compared with free drug solution in the HIV infected mice. Also, Mannosylated anggalactosylated liposomes were also investigated for the delivery of stavudine in order to improve the stability of liposomes, which is an intrinsic limitation for these carriers [124, 125]. Makabi-Panzu et al. have investigated the cellular accumulation, tissue distribution, and antiviral efficacy of liposomal and free zalcitabine in RAW264.7 and U937 cell lines and reported a considerably higher drug uptake of the liposomal formulation compared to free drug in both the cell lines [126, 127]. Ramana et al developed have nepiravine (NVP) loaded liposomal formulation and studied its different parameters with respect drug loading, release kinetic and BBB transmigration [128]. The in-vitro results showed an efficient targeted delivery of the NVP to the selected compartments with reduced systemic toxic effects. All the studies support the encapsulating ARV drugs in liposomes is more effective approach for delivering drugs across BBB for the treatment of HIV-associated CNS complications. Vyas et al evaluated oral formulation of flaxseed oil-based nanoemulsion of saquinavir for CNS delivery. Results showed 3 folds increase in the drug concentration in the systemic circulation and 5-fold increase in the area-under-the curve (AUC) values and maximum drug concentration in the brain for saquinavir nanoemulsion when compared with free drug solution in male balb/c mice, respectively. Results indicates due to small size of the nanoemulsion formulation, it has better BBB permeability and may also help in bypassing gastrointestinal tract barriers when administered orally [129]. The SLN are another relatively new class of lipid-based NCs. As per one of the study, SLN showed better profiles drug release profile and showed less non-specific cell toxicity when compared to PLGA nanoparticles formulation [130], which is FDA approved and more standard biomaterial. Chattopadhyay et al have examined role of SLN for atazanavir delivery using a human brain microvessel endothelial cell line in-vitro BBB model. Results showed significant improvement in the accumulation of SLN-[3H]-atazanavir when compared with free drug [131]. Thus suggest that SLN clearly have strong potential as nanocarrier for brain delivery of ARVs, especially for highly lipid soluble compound e.g. PIs. In table 2, we have complied the most recent strategies for the HIV-1 nanotherapeutics for BBB delivery and treatment of neuroAIDS. Other than the HIV-1 nanotherapeutics discussed above, different types of therapeutics (genes, proteins, peptide, DNA vaccine etc.,) and modifications of carriers [cyclodextrins (CDs), carbon nanotubes (CNT) etc.,] are being investigated for the treatment of HIV-1 in CNS. However, we are not discussing them here as they falls outside the scope of present review manuscript, but the related references and the strategies for the CNS delivery have been cited in Table 2 for the benefit of readers. For more details about the advantage, disadvantages and BBB transmigration potentials and suggested improvement all type of NCs systems, their physico-chemical properties, cytotoxicity and immunologic responses to other neighboring cell/organs have been described in details in our previously published review by Sagar et al [11].
3. Monitoring and management of NeuroAIDS
Effective prevention of HIV/AIDS needs timely diagnosis, introduction of therapy, and routine plasma viral load monitoring of the infected patient; viral rebound rate assessment by precise and sensitive assays is desirable to increase HIV prevention [159]. Hence, more reasonable and user-friendly technologies capable of providing continuous monitoring and early diagnosis is very much needed. In the past few years, we have seen massive scientific and technical developments to achieve simple, cost-effective, and rapid diagnostic tests for HIV detection. HIV/AIDS has quickly risen and thus there is a high demand globally to find improved and simplified diagnostic tools while maintaining good patient care. In this regards, the monitoring of HIV-1 at CNS is crucial to manage early diagnosis and treatment strategy for neuroAIDS treatment [39, 160, 161]. Qualitative and quantitative strategies have been used for the diagnostics of HIV-1, staging of HIV-1, progression and selection of ARV therapy [162, 163]. Point-of-care (POC) technologies and other novel detection assays are increasing access to HIV monitoring services in developing countries. These technologies are endurable, portable, easy to use, and provide adequate accuracy in ARV therapy. This section highlights the recent advancements and potentials to combat HIV using POC, image guided HIV therapy, smart optical and electrical assay based on nanotechnology.
3.1 Biomarkers for HIV-1 in CNS for disease detection and its progression
Currently biomarkers of CNS diseases are being used to study the progression of HIV as the brain and spinal cord cannot be assessed due to its inaccessibility. Unfortunately, biomarkers with high accuracy and quantifiable assessments have not been found. Due to these limitations, a combination of different markers or multi-marker assay or technique is needed to predict the HIV-1 disease progression [164, 165]. CD4+ cell count (CD4) and RNA viral loads (RNA) are the two most frequently used as analytical markers in the clinical assessment of HIV infection progression [166, 167].
However, these markers have variable predictive values that depend on which stage the disease is in and cannot explain all discrepancies of disease progression. As a result, further studies investigating markers of immune activation has occurred. Additionally, other types of HIV progression markers exist e.g. p24 antigen, CD8+ cell count, platelet concentration, anti-HIV antibodies, erythrocyte sedimentation rate (ESR) and (Hb) hemoglobin concentration that can be used to along with main markers to predict the diagnosis. In addition to all these markers, the serum concentrations of beta-2-microglobulin [168], IgA, interleukin-2 receptors and p24 antigen (as shown in Fig. 5) have also been utilized. Cellular activation and inflammation, in conjunction with the discovery of the above markers, have been used to assess HIV infection progression; consequently, focus has been turned to these markers and their function in the CNS. In order to monitor progress, which is thought to serve as the substrate for neuropathology in HAND, soluble CSF markers of macrophage activation (neopterin), chemokines stimulators of macrophages and lymphocytes across the BBB (CCL2/MCP1 and CXCL10/IP10), and molecules involved at various phases in the pathways for cell turnover and activation within the CNS compartment are used. Brew et al observed that progression to HAD can be predicted with the monitoring of CSF neopterin, CD4 cell count and cerebrospinal fluid concentrations of β-2-microglobulin [169]. However, immune activation for HIV infection lacks specificity and so although moderately high levels of the previous markers have been correlated to disease activity, they have not been clinically used for diagnosis or monitoring of NeuroAIDS. Next section deals with techniques and assays that have been used for detection of HIV in laboratory setting for disease detection and its progression.
Fig. 5.
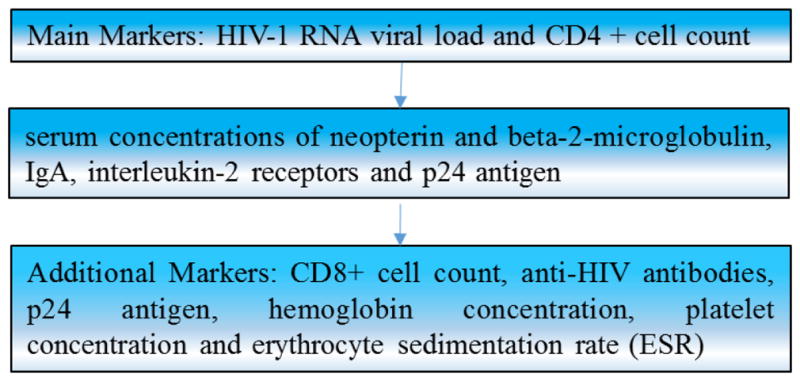
Types for biomarkers used to detect and monitor HIV-1 progression
3.2 Techniques and technology used HIV-1 diagnosis and monitoring
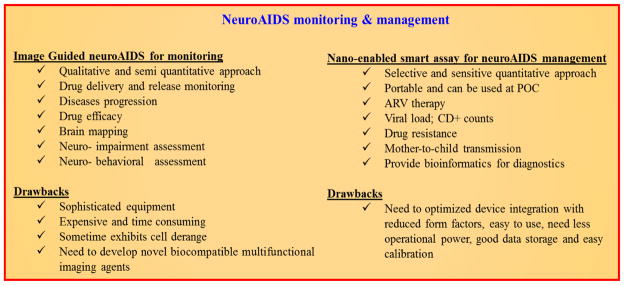
HIV/AIDS diagnosis can be categorized into three different stages/test: (i) tests to enable preliminary diagnosis, (ii) tests to stage the patient, and (iii) tests to monitor the patient (before and after commencement of ART) [159]. Currently, delivering therapeutics across the BBB for treatment of CNS disorders signifies the most challenging and emergent field in neuro-pharmaceuticals. The image-guided therapy in combination with nanotechnology is emerging as a novel tool for HIV-1 detection, monitoring and treatment (theranostics) for CNS diseases, but this type of therapy has not been implemented with the management of neuroAIDS. We believe that the combination of personalized nanomedicine with non-invasive imaging techniques (anatomical imaging modalities) e.g. magnetic resonance imaging (MRI), positron emission tomography (PET), ultrasound (US), single-photon emission computed tomography (SPECT), fluorescence molecular tomography (FMT-based on fluorescence), optical and thermal imaging, X-ray computed tomography (CT), and NIR (Near infrared) [89, 160, 170]. Comprehension and treatment of CNS diseases has been further advanced due to drug and diagnostic molecules that can be delivered to the brain across the BBB as personalized nanomedicine with the help of highly specific and multifunctional NPs using drug delivery images. Recently, to cure neuroAIDS, drug delivery across the BBB is being developed with NFs consisting of MNP, imaging agents and optimized ratio of therapeutic agent [56]. This efficient therapeutic approach can be coupled with a suitable imaging technique to monitor HIV progression, drug efficacy and neurobehavioral changes. For example, magnetic NCs and anti-HIV drug based NFs have demonstrated HIV eradication across BBB [55, 171]. Such investigated NFs can be navigated cross to BBB in mice by applying external magnetic field and ultrasound. An MRI technique can be coupled with such systems to monitor stimuli responsive targeted drug release and its efficacy [56, 172]. This brain imaging research technology has potential for real time tracking of therapeutic cargo and therapeutic mechanism. However, wide application is sometimes limited because these methods possess neuronal alteration (CT) and low penetration (NIR based imaging); furthermore, they require high expertise, and a laboratory set-up of sophisticated equipment such as cryogenic, cyclotron, sensitive probes. Fig. 6 explains the advantages and disadvantages of image guided and nano-enabling POC devices for neuroAIDS monitoring and management.
Figure 6.
Comparative analysis of images guided and nano-enabled techniques for neuroAIDS monitoring and management
Diagnostics assays such as flow cytometry, western blot (WB), polymerase chain reaction (PCR), and enzyme-linked immunosorbent assay (ELISA) are being used to detect and monitor HIV progression in spite of high costs. Shafiee et al have summarized potential emergent technologies for Point-of-care (POC) management of HIV infection and proposed standards of ASSURED (affordable, sensitive, specific, user-friendly, rapid, robust, equipment-free and deliverable) to patients and clinics [162]. This ASSURES criteria should be a minimum landmark to technologist for developing advanced analytical devices for HIV management. Exploring POC detection system for HIV monitoring do not need expensive laboratory set-up, multi-step detection protocol, complicated operational parameters, and high expertise [162, 163]. Thus, developing such devices for POC will facilitate the gathering of on-site bioinformatics to decide timely treatment. The POC detection of HIV is performed via estimation of CD+ T lymphocytes count, viral load detection, and drug resistance measurements. Various tools have been utilized for detection of physiological range and to generate enough informatics to manage and cure HIV [162]. Details of state-of-the-art of the technologies involved in HIV screening and its detection is summarized in Table-3.
In consideration with the aims of this review, we are summarizing the performance of recent advancements in selected technologies that are capable of detecting HIV at an early stage. To begin with, flow cytometry, a conventional reliable technique to detect and quantify CD+ T lymphocytes has been well explored [173]. The captured CD+ T lymphocytes from whole blood (3μL) [174] is detected using specific antibody that binds with cell surface markers and is quantified by using a bright field or fluorescent image processing method integrated with a microfluidic system [175–177]. A sensitive (97%) and selective (80%) ELISA integrated with an automated reader was also developed for CD4+ T lymphocytes count up to 350 cell/μL using whole blood as real sample within 40 min [178]. ELISA was used to estimate viral load < 200 RNA copies/mL [179]. This ELISA system needs a high volume of real sample i.e., 1 mL of plasma, and a long assay time of 48 hours to perform a test [180]. These issues limit this tool to be used at POC application. The nucleic acid based PCR approach extracts HIV-RNA from blood and amplifies RNA detection at 100–200 copies/mL in the case of HIV-1 and HIV-2 category [181]. This PCR system coupled with microfluidic system for precise and accurate RNA extraction from real sample (100μL) can detect DNA at 5,000 copies/mL, which seems suitable for diagnosing HIV at a mature stage [182]. Thus investigating novel tools for rapid selective screening and monitoring of HIV at CNS would be of use for timely diagnostics to optimize cure. According to ASSURED landmark, nano-enabled biosensors emerged as a potential analytical device to detect HIV-viral load. The nano-plasmonic resonance based sensing method was able to quantify (~100 copies/mL-HIV-1 subtype D) with a smaller volume (100 μL) of whole blood and plasma [183]. Meanwhile, Nanostructure photonic crystals based biosensors have been found suitable for label free optical detection (104 to 106 copies/mL) and capturing of HIV-1 in blood plasma [184]. Both bio-sensing methods do not need pre-processing of sample and can be integrated in a POC system. Miniaturized nano-enable electro-chemical biosensor surfaced recently as a potential approach to detect CD+ T lymphocytes and viral load in real samples [185]. Lab-on-chip (LOC) based on highly sensitive electrochemical biosensor on MEMS integration are capable of capturing cells to quantify and detect CD+ lymphocytes [186]. These microchips exhibited a detection limit of 9 cells/μL and a detection range from 100 to 700 cells/μL [187]. LOC functionalized with specific antibodies demonstrate viral lysate detection in an automated manner [188].
Moreover, paper-based sensors based on microfluidic principle or Microelectromechanical systems (MEMS) are made of a disposable sensing chip that has been integrated to detect early stage HIV-1 infection at POC application. Regrettably, accuracy and sensitivity limit paper-based sensors; nevertheless, future advances in the improvement of analytical techniques can develop more state-of-the-art technology based on these sensors. Figure 7 summarizes the commercially available diagnostics kit/tools that are used commonly for the detection and monitoring of HIV-1 in rural and urban clinical settings. However, there is always a considerable scope to explore engineered novel sensing strategies and the integration of miniaturized portable sensing devices of reduced form factor to detect all possible biomarkers related with HIV progression before and after therapeutics. The outcomes of such devices that satisfy ASSURED criteria would be valuable bioinformatics, which will be of use for complete HIV management.
Fig. 7.
Summary of marketed available diagnostic test or tools for HIV-1 detection &monitoring
4. Challenges and Future prospects
As a proof-of-concept, varieties of nanoformulations consist of therapeutics agents and nanocarriers (NCs) have been navigated across BBB for eradication of HIV. Unfortunately, mostly such investigated nanoformulations (NFs) and approached are limited to only in-vitro model. The potential of NCs for developing personalized nanomedicine to achieve targeted or on-demand release of therapeutics without depending on the physiological conditions has been demonstrated. The natural integrity of BBB found as a major obstacle to achieve the CNS delivery of nanomedicine due to BBB permeability size constraints, technically NFs of size > 150 nm has less chances to cross BBB. Thus scientists are exploring surface bioengineering and formulation strategies to develop nanomedicine with size limitation (< 120–150 nm) for higher efficacy. To achieve this, efforts are being made to fine tune the particles size, surface engineering, toxicity profiles, and BBB transmigration ability of NCs for effective brain delivery without loss of drug payload. These NCs can be promoted to develop non-invasive deep brain stimulation for the treatment of neurological disorders such as Parkinson’s, Alzheimer’s, dementia, etc. Beside this, few transient BBB opening approaches as discussed above have been demonstrated for the CNS delivery of therapeutics (ARVs) cargo. To overcome related issues with transient BBB opening, efforts are being made to explore novel CNS navigation methods which can delivery NFs to the brain non-invasively and without generating side effect. For, example, our group extensively working on magnetically guided CNS delivery of magnetic NFs can be used as a potential alternate delivery strategy to achieve combat against HIV infection at CNS. Recent investigation of on-demand drug release of therapeutic agent in the CNS has also shown significance step towards the eradication and cure of neuroAIDS treatment. MENPs based NFs for magnetically responsive, MNP-LBL based NFs approach to sustained release, and optical responsive based NFs have demonstrated site-specific on-demand drug release for the eradication of HIV. However, execution of these approaches using animal model is not yet well established. Another challenge in the CNS nanotechnology is the optimization of toxicity associated with the NCs and or its NFs for in-vivo application. A low dose of NCs such as metal, metal oxides, polymers, and composites are generally non-toxic but continuous delivery such agent may lead to long term accumulation and its related toxic effects. Thus, more comprehensive studies are required to assess the short and long term toxicity using in-vivo models (small animals to non-human primates). Advantage of such type of studies will be that they will help in understanding the organ specific toxicity and also alteration in neuro-behavioral to access changes in motor coordination and associated factors. The outcomes of these finding will helps in confirming the clinical application the developed NF and safety for human subjects. Due to the limitation of ARV in penetrating into the latent reservoirs, an alternate strategy would be to deliver latency reactivating agents (LRAs) along with ARV therapy. This dual agent delivery will be helpful in complete eradiation of HIV-1 from the CNS reservoir as shown by us previously [55]. Also prolong delivery of multiple ARV agents to the brain may cause neurotoxicity and may lead to neuroinflammation. To avoid these challenge with ARV therapy, recently efforts are being made to investigate a single therapeutic agent which is capable of recognition and eradication of HIV-1 from the CNS reservoirs. One of such recent discovery was the advancement of clustered regulatory interspaced short palindromic repeat [CRISPR], a powerful type of genome editing technology appears promising. A Cas9/gRNA system has been designed to target highly specific sequences within the HIV-1 LTR U3 region that were proficiently modified by Cas9/gRNA [189–191]. However, delivery of this powerful Cas9/gRNA complex across the BBB is limited and an effective delivery and release will be high significance in eliminate the HIV-1 from the latent and active reservoir in the brain with any neuroinflammatory side effects. With the help of nanotechnology, the delivery of CAS9/gRNA to the brain could be possible and may be a novel future research direction. We believe that targeting latent virus and permanent elimination of integrated DNA proviral copies of HIV-1 in brain is possible using our newly invented [MENP+Cas9/gRNA] on-demand release technology. Additionally, considerable efforts are being to develop novel sensing technologies, novel signaling transduction, and imaging pathways to monitor HIV infection progression and mechanism of HIV eradication. Nano-enable sensing immunoassays and biosensor have been used for screening and detection HIV markers at pM (picomolar) level. At clinical level, such investigated nano-enabling sensing systems technologies on integrated with drug delivery systems can be promoted for the management of neuroAIDS. We proposed that a multidisciplinary nano-engineered approaches must be investigated to develop site-specific targeted delivery of NFs to brain to combat against HIV. Thus, highly specific on-demand drug release and HIV progression monitoring devices would be a 3-D diagnostics approach towards developing a personalized nanomedicine approach for the treatment and management of neuroAIDS.
5. Conclusions
This review has summarized some of the significant progresses and prospects of nanomedicine to investigate an effective therapeutics to screen, recognize and eradicate HIV-1 infection from the CNS. State-of-the-art of HIV eradication using nanotechnology suggested that developing a nanomedicine for site-specific controlled targeted drug delivery to CNS and smart assaying to assess or monitor HIV progression is in high demand for neuroAIDS management. Thus, significant efforts should be made to accelerate fundamental and applied nanoscience research to investigate smart biocompatible NCs, site-specific navigation approaches, stimuli responsive release of desired drug without side-effects to combat against neuroAIDS. On the other hand, high-quality diagnostics are also crucial to fight against HIV and to diminish its transmission and its management. Currently available diagnostic needs more advancement in without increasing the cost of treatment and its availability at underdeveloped countries. Hence, timely advancement of proposed delivery systems/testing strategies will be important to move into a new era of HIV free world. In summary, getting medicine in to the brain seems possible on exploring and optimizing nano-enable compartmentalization based personalized nanomedicine along with suitable monitoring technologies for the management of neuroAIDS. Once well characterized and validated, an optimized NFs strategy will be developed, it may be explored to cure CNS diseases/neurological disorders other than neuroAIDS e.g. Huntington’s disease, Amyotrophic lateral sclerosis, Parkinson’s disease and Alzheimer’s disease, wherein site-specific drug delivery is the key to manage diagnosis and treatment.
Table 3.
Summary of various HIV detection techniques useful for HIV management
| Techniques | CD+ T Lymphocytes Counting | Viral Load Estimation | Remarks |
|---|---|---|---|
| Flow Cytometry | Conventional well-established for both |
|
|
| Image Processing | Bright field or fluorescent image of CD+ T lymphocytes |
|
|
| ELISA | Centrifuged based approach | Nano-enabled ELISA |
|
| RT-PCR | DNA based Virus capture based |
|
|
| Electrical Sensing | Best suitable for HIV detection and monitoring |
|
|
ELISA-Enzyme-linked immunosorbent assay; RT-PCR- Reverse transcription polymerase chain reaction; CD- Cluster of differentiation; POC- Point-of-care; AC-Alternate current; min-minutes; μL-microliters; mL- milliliters.
Acknowledgments
Authors acknowledge NIH grants namely R01-DA037838, RO1-DA 034547, R21-MH 101025, RO1-DA027049, and 1R01-DA-040537.
Footnotes
Chemical compounds studied in this article
Ferrous Oxide or Iron (II) oxide (PubChem CID: 14945); Pluronic-P85 (PubChem CID: 24751); Mannitol (PubChem CID: 6251); Bradykinin (PubChem CID: 439201); Saquinavir Mesylate (PubChem CID: 60934); Tenofovir disoproxil fumarate (PubChem CID: 6398764); Foscarnet Sodium (PubChem CID: 44561); Enfuvirtide (PubChem CID: 16130199), Lamivudine & Zidovudine (PubChem CID: 160352); Neopterin (PubChem CID: 4455)
Conflict of interest: Authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaul M, Zheng J, Okamoto S, Gendelman H, Lipton S. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death & Differentiation. 2005;12:878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- 2.McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. The Lancet Neurology. 2005;4:543–555. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- 3.Almeida SMd, Letendre S, Ellis R. Human immunodeficiency virus and the central nervous system. Brazilian Journal of Infectious Diseases. 2006;10:41–50. doi: 10.1590/s1413-86702006000100009. [DOI] [PubMed] [Google Scholar]
- 4.McArthur JC. HIV dementia: an evolving disease. Journal of neuroimmunology. 2004;157:3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 5.McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: an evolving disease. Journal of neurovirology. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- 6.González-Scarano F, Martín-García J. The neuropathogenesis of AIDS. Nature Reviews Immunology. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 7.Letendre SL, Ellis RJ, Everall I, Ances B, Bharti A, McCutchan JA. Neurologic complications of HIV disease and their treatment. Topics in HIV medicine: a publication of the International AIDS Society USA. 2009;17:46. [PMC free article] [PubMed] [Google Scholar]
- 8.Singer EJ, Valdes-Sueiras M, Commins D, Levine A. Neurologic presentations of AIDS. Neurologic clinics. 2010;28:253–275. doi: 10.1016/j.ncl.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams KC, Hickey WF. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annual review of neuroscience. 2002;25:537–562. doi: 10.1146/annurev.neuro.25.112701.142822. [DOI] [PubMed] [Google Scholar]
- 10.Atluri V, Hidalgo M, Samikkannu T, Kurapati KRV, Jayant RD, Sagar V, Nair M. Effect of HIV infection and its proteins on Blood-Brain Barrier integrity and function: An update. Name: Frontiers in Cellular Neuroscience. 2015;9:212. doi: 10.3389/fncel.2015.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sagar V, Pilakka-Kanthikeel S, Pottathil R, Saxena SK, Nair M. Towards nanomedicines for neuroAIDS. Reviews in medical virology. 2014;24:103–124. doi: 10.1002/rmv.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace DR. HIV neurotoxicity: potential therapeutic interventions. BioMed Research International. 2006;2006 doi: 10.1155/JBB/2006/65741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindl KA, Marks DR, Kolson DL, Jordan-Sciutto KL. HIV-associated neurocognitive disorder: pathogenesis and therapeutic opportunities. Journal of neuroimmune pharmacology. 2010;5:294–309. doi: 10.1007/s11481-010-9205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atluri V, Kanthikeel SP, Reddy P, Yndart A, Nair M. Human synaptic plasticity gene expression profile and dendritic spine density changes in HIV-infected human CNS cells: role in HIV-associated neurocognitive disorders (HAND) PLoS One. 2013;8:e61399. doi: 10.1371/journal.pone.0061399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atluri V, Pilakka-Kanthikeel S, Samikkannu T, Sagar V, Kurapati K, Saxena SK, Yndart A, Raymond A, Ding H, Hernandez O. Vorinostat positively regulates synaptic plasticity genes expression and spine density in HIV infected neurons: role of nicotine in progression of HIV-associated neurocognitive disorder. Mol Brain. 2014;7:37. doi: 10.1186/1756-6606-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samikkannu T, Atluri VSR, Arias AY, Rao KVK, Mulet CT, Jayant RD, Nair MPN. HIV-1 Subtypes B and C Tat Differentially Impact Synaptic Plasticity Expression and Implicates HIV-Associated Neurocognitive Disorders §. Current HIV research. 2014;12:397–405. doi: 10.2174/1570162x13666150121104720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avdoshina V, Bachis A, Mocchetti I. Synaptic dysfunction in human immunodeficiency virus type-1-positive subjects: inflammation or impaired neuronal plasticity? Journal of internal medicine. 2013;273:454–465. doi: 10.1111/joim.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrigan PR, Hogg RS, Dong WW, Yip B, Wynhoven B, Woodward J, Brumme CJ, Brumme ZL, Mo T, Alexander CS. Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. Journal of Infectious Diseases. 2005;191:339–347. doi: 10.1086/427192. [DOI] [PubMed] [Google Scholar]
- 19.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 20.Chun TW, Davey RT, Engel D, Lane HC, Fauci AS. AIDS: re-emergence of HIV after stopping therapy. Nature. 1999;401:874–875. doi: 10.1038/44755. [DOI] [PubMed] [Google Scholar]
- 21.Marsden MD, Zack JA. Eradication of HIV: current challenges and new directions. Journal of antimicrobial chemotherapy. 2009;63:7–10. doi: 10.1093/jac/dkn455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin LR, Williams SL, Haskard KB, DiMatteo MR. The challenge of patient adherence. Therapeutics and clinical risk management. 2005;1:189. [PMC free article] [PubMed] [Google Scholar]
- 23.Machtinger EL, Bangsberg DR. HIV InSite Knowledge Base Chapter. USA: 2005. Adherence to HIV antiretroviral therapy. [Google Scholar]
- 24.Attonito JM, Dévieux JG, Lerner BD, Hospital MM, Rosenberg R. Exploring substance use and HIV treatment factors associated with neurocognitive impairment among people living with HIV/AIDS. Frontiers in public health. 2014;2 doi: 10.3389/fpubh.2014.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.P.o.A.G.f. Adults, Adolescents, Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2009. pp. 1–161. [Google Scholar]
- 26.Sabaté E. Adherence to long-term therapies: evidence for action. World Health Organization; 2003. [PubMed] [Google Scholar]
- 27.Mamo T, Moseman EA, Kolishetti N, Salvador-Morales C, Shi J, Kuritzkes DR, Langer R, Andrian Uv, Farokhzad OC. Emerging nanotechnology approaches for HIV/AIDS treatment and prevention. Nanomedicine. 2010;5:269–285. doi: 10.2217/nnm.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sax PE, Cohen CJ, Kuritzkes DR. HIV Essentials 2013. Jones & Bartlett Publishers; 2013. [Google Scholar]
- 29.Lamers SL, Salemi M, Galligan DC, De Oliveira T, Fogel GB, Granier SC, Zhao L, Brown JN, Morris A, Masliah E. Extensive HIV-1 intra-host recombination is common in tissues with abnormal histopathology. PLoS One. 2009;4:e5065. doi: 10.1371/journal.pone.0005065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGee B, Smith N, Aweeka F. HIV pharmacology: barriers to the eradication of HIV from the CNS. HIV clinical trials. 2006;7:142–153. doi: 10.1310/AW2H-TP5C-NP43-K6BY. [DOI] [PubMed] [Google Scholar]
- 31.Vyas TK, Shah L, Amiji MM. Nanoparticulate drug carriers for delivery of HIV/AIDS therapy to viral reservoir sites. 2006 doi: 10.1517/17425247.3.5.613. [DOI] [PubMed] [Google Scholar]
- 32.Nowacek A, Gendelman HE. NanoART, neuroAIDS and CNS drug delivery. Nanomedicine. 2009;4:557–574. doi: 10.2217/nnm.09.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharkey ME, Teo I, Greenough T, Sharova N, Luzuriaga K, Sullivan JL, Bucy RP, Kostrikis LG, Haase A, Veryard C. Persistence of episomal HIV-1 infection intermediates in patients on highly active anti-retroviral therapy. Nature medicine. 2000;6:76–81. doi: 10.1038/71569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailey JR, Sedaghat AR, Kieffer T, Brennan T, Lee PK, Wind-Rotolo M, Haggerty CM, Kamireddi AR, Liu Y, Lee J. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. Journal of virology. 2006;80:6441–6457. doi: 10.1128/JVI.00591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer S, Maldarelli F, Wiegand A, Bernstein B, Hanna GJ, Brun SC, Kempf DJ, Mellors JW, Coffin JM, King MS. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proceedings of the National Academy of Sciences. 2008;105:3879–3884. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parboosing R, Maguire GE, Govender P, Kruger HG. Nanotechnology and the treatment of HIV infection. Viruses. 2012;4:488–520. doi: 10.3390/v4040488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Upadhyay RK. Drug delivery systems, CNS protection, and the blood brain barrier. BioMed research international. 2014;2014 doi: 10.1155/2014/869269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding H, Sagar V, Agudelo M, Pilakka-Kanthikeel S, Atluri VSR, Raymond A, Samikkannu T, Nair MP. Enhanced blood–brain barrier transmigration using a novel transferrin embedded fluorescent magneto-liposome nanoformulation. Nanotechnology. 2014;25:055101. doi: 10.1088/0957-4484/25/5/055101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaushik A, Jayant RD, Sagar V, Nair M. The potential of magneto-electric nanocarriers for drug delivery. Expert opinion on drug delivery. 2014;11:1635–1646. doi: 10.1517/17425247.2014.933803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood–brain barrier: structural components and function under physiologic and pathologic conditions. Journal of Neuroimmune Pharmacology. 2006;1:223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 41.Kanmogne GD, Schall K, Leibhart J, Knipe B, Gendelman HE, Persidsky Y. HIV-1 gp120 compromises blood–brain barrier integrity and enhance monocyte migration across blood–brain barrier: implication for viral neuropathogenesis. Journal of Cerebral Blood Flow & Metabolism. 2007;27:123–134. doi: 10.1038/sj.jcbfm.9600330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ricardo-Dukelow M, Kadiu I, Rozek W, Schlautman J, Persidsky Y, Ciborowski P, Kanmogne GD, Gendelman HE. HIV-1 infected monocyte-derived macrophages affect the human brain microvascular endothelial cell proteome: new insights into blood–brain barrier dysfunction for HIV-1-associated dementia. Journal of neuroimmunology. 2007;185:37–46. doi: 10.1016/j.jneuroim.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaudhuri A, Duan F, Morsey B, Persidsky Y, Kanmogne GD. HIV-1 activates proinflammatory and interferon-inducible genes in human brain microvascular endothelial cells: putative mechanisms of blood–brain barrier dysfunction. Journal of Cerebral Blood Flow & Metabolism. 2008;28:697–711. doi: 10.1038/sj.jcbfm.9600567. [DOI] [PubMed] [Google Scholar]
- 44.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-oncology. 2005;7:452. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hill J, Rom S, Ramirez SH, Persidsky Y. Emerging roles of pericytes in the regulation of the neurovascular unit in health and disease. Journal of Neuroimmune Pharmacology. 2014;9:591–605. doi: 10.1007/s11481-014-9557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Persidsky Y, Hill J, Zhang M, Dykstra H, Winfield M, Reichenbach NL, Potula R, Mukherjee A, Ramirez SH, Rom S. Dysfunction of brain pericytes in chronic neuroinflammation. Journal of Cerebral Blood Flow & Metabolism. 2015 doi: 10.1177/0271678X15606149. 0271678X15606149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Persidsky Y, Rom S, Dykstra H, Reichenbach N, Winfield M, Ramirez S. Pericyte dysfunction in blood brain barrier impairment caused by HIV infection (278.1) The FASEB Journal. 2014;28:278.271. [Google Scholar]
- 48.Guduru R, Liang P, Runowicz C, Nair M, Atluri V, Khizroev S. Magneto-electric nanoparticles to enable field-controlled high-specificity drug delivery to eradicate ovarian cancer cells. Scientific reports. 2013;3 doi: 10.1038/srep02953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nair M, Guduru R, Liang P, Hong J, Sagar V, Khizroev S. Externally controlled on-demand release of anti-HIV drug using magneto-electric nanoparticles as carriers. Nature communications. 2013;4:1707. doi: 10.1038/ncomms2717. [DOI] [PubMed] [Google Scholar]
- 50.Wilhelm I, Krizbai InA. In vitro models of the blood–brain barrier for the study of drug delivery to the brain. Molecular pharmaceutics. 2014;11:1949–1963. doi: 10.1021/mp500046f. [DOI] [PubMed] [Google Scholar]
- 51.Eisele E, Siliciano RF. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity. 2012;37:377–388. doi: 10.1016/j.immuni.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Churchill MJ, Deeks SG, Margolis DM, Siliciano RF, Swanstrom R. HIV reservoirs: what, where and how to target them. Nature Reviews Microbiology. 2015 doi: 10.1038/nrmicro.2015.5. [DOI] [PubMed] [Google Scholar]
- 53.Gomes MJ, das Neves J, Sarmento B. Nanoparticle-based drug delivery to improve the efficacy of antiretroviral therapy in the central nervous system. International journal of nanomedicine. 2014;9:1757. doi: 10.2147/IJN.S45886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu CT, Zhao YZ, Wong HL, Cai J, Peng L, Tian XQ. Current approaches to enhance CNS delivery of drugs across the brain barriers. International journal of nanomedicine. 2014;9:2241. doi: 10.2147/IJN.S61288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jayant RD, Atluri VS, Agudelo M, Sagar V, Kaushik A, Nair M. Sustained-release nanoART formulation for the treatment of neuroAIDS. International journal of nanomedicine. 2015;10:1077. doi: 10.2147/IJN.S76517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koffie RM, Farrar CT, Saidi LJ, William CM, Hyman BT, Spires-Jones TL. Nanoparticles enhance brain delivery of blood–brain barrier-impermeable probes for in vivo optical and magnetic resonance imaging. Proceedings of the National Academy of Sciences. 2011;108:18837–18842. doi: 10.1073/pnas.1111405108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pilakka-Kanthikeel S, Atluri VSR, Sagar V, Saxena SK, Nair M. Targeted brain derived neurotropic factors (BDNF) delivery across the blood-brain barrier for neuro-protection using magnetic nano carriers: an in-vitro study. 2013 doi: 10.1371/journal.pone.0062241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saiyed ZM, Gandhi NH, Nair MP. Magnetic nanoformulation of azidothymidine 5′-triphosphate for targeted delivery across the blood–brain barrier. International journal of nanomedicine. 2010;5:157. doi: 10.2147/ijn.s8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang H. Nanoparticle-Mediated Brain-Specific Drug Delivery, Imaging, and Diagnosis. Pharmaceutical Research. 2010;27:1759–1771. doi: 10.1007/s11095-010-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong HL, Chattopadhyay N, Wu XY, Bendayan R. Nanotechnology applications for improved delivery of antiretroviral drugs to the brain. Advanced drug delivery reviews. 2010;62:503–517. doi: 10.1016/j.addr.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 61.Sun H, Dai H, Shaik N, Elmquist WF. Drug efflux transporters in the CNS. Advanced drug delivery reviews. 2003;55:83–105. doi: 10.1016/s0169-409x(02)00172-2. [DOI] [PubMed] [Google Scholar]
- 62.Choo EF, Leake B, Wandel C, Imamura H, Wood AJ, Wilkinson GR, Kim RB. Pharmacological inhibition of P-glycoprotein transport enhances the distribution of HIV-1 protease inhibitors into brain and testes. Drug Metabolism and Disposition. 2000;28:655–660. [PubMed] [Google Scholar]
- 63.Park S, Sinko PJ. P-glycoprotein and mutlidrug resistance-associated proteins limit the brain uptake of saquinavir in mice. Journal of Pharmacology and Experimental Therapeutics. 2005;312:1249–1256. doi: 10.1124/jpet.104.076216. [DOI] [PubMed] [Google Scholar]
- 64.Megard I, Garrigues A, Orlowski S, Jorajuria S, Clayette P, Ezan E, Mabondzo As. A co-culture-based model of human blood–brain barrier: application to active transport of indinavir and in vivo–in vitro correlation. Brain research. 2002;927:153–167. doi: 10.1016/s0006-8993(01)03337-6. [DOI] [PubMed] [Google Scholar]
- 65.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiology of disease. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 66.Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salahuddin T, Johansson B, Kalimo H, Olsson Y. Structural changes in the rat brain after carotid infusions of hyperosmolar solutions: a light microscopic and immunohistochemical study. Neuropathology and applied neurobiology. 1988;14:467–482. doi: 10.1111/j.1365-2990.1988.tb01338.x. [DOI] [PubMed] [Google Scholar]
- 68.Neuwelt EA, Hill SA, Frenkel EP. Osmotic blood-brain barrier modification and combination chemotherapy: concurrent tumor regression in areas of barrier opening and progression in brain regions distant to barrier opening. Neurosurgery. 1984;15:362–366. doi: 10.1227/00006123-198409000-00011. [DOI] [PubMed] [Google Scholar]
- 69.van der Sandt IC, Gaillard PJ, Voorwinden HH, de Boer AG, Breimer DD. P-glycoprotein inhibition leads to enhanced disruptive effects by anti-microtubule cytostatics at the in vitro blood-brain barrier. Pharmaceutical research. 2001;18:587–592. doi: 10.1023/a:1011016923346. [DOI] [PubMed] [Google Scholar]
- 70.Qin LJ, Gu YT, Zhang H, Xue YX. Bradykinin-induced blood–tumor barrier opening is mediated by tumor necrosis factor-α. Neuroscience letters. 2009;450:172–175. doi: 10.1016/j.neulet.2008.10.080. [DOI] [PubMed] [Google Scholar]
- 71.Sood RR, Taheri S, Candelario-Jalil E, Estrada EY, Rosenberg GA. Early beneficial effect of matrix metalloproteinase inhibition on blood–brain barrier permeability as measured by magnetic resonance imaging countered by impaired long-term recovery after stroke in rat brain. Journal of Cerebral Blood Flow & Metabolism. 2008;28:431–438. doi: 10.1038/sj.jcbfm.9600534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fleegal-DeMotta MA, Doghu S, Banks WA. Angiotensin II modulates BBB permeability via activation of the AT1 receptor in brain endothelial cells. Journal of Cerebral Blood Flow & Metabolism. 2009;29:640–647. doi: 10.1038/jcbfm.2008.158. [DOI] [PubMed] [Google Scholar]
- 73.McDannold N, Vykhodtseva N, Raymond S, Jolesz FA, Hynynen K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: histological findings in rabbits. Ultrasound in medicine & biology. 2005;31:1527–1537. doi: 10.1016/j.ultrasmedbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 74.Burgess A, Hynynen K. Drug delivery across the blood-brain barrier using focused ultrasound. Expert opinion on drug delivery. 2014;11:711–721. doi: 10.1517/17425247.2014.897693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burgess A, Shah K, Hough O, Hynynen K. Focused ultrasound-mediated drug delivery through the blood-brain barrier. Expert review of neurotherapeutics. 2015;15:477–491. doi: 10.1586/14737175.2015.1028369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vykhodtseva N, McDannold N, Hynynen K. Progress and problems in the application of focused ultrasound for blood–brain barrier disruption. Ultrasonics. 2008;48:279–296. doi: 10.1016/j.ultras.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burgess A, Hynynen K. Noninvasive and targeted drug delivery to the brain using focused ultrasound. ACS chemical neuroscience. 2013;4:519–526. doi: 10.1021/cn300191b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meairs S, Alonso A. Ultrasound, microbubbles and the blood–brain barrier. Progress in biophysics and molecular biology. 2007;93:354–362. doi: 10.1016/j.pbiomolbio.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 79.Chen P-Y, Liu H-L, Hua M-Y, Yang H-W, Huang C-Y, Chu P-C, Lyu L-A, Tseng I-C, Feng L-Y, Tsai H-C. Novel magnetic/ultrasound focusing system enhances nanoparticle drug delivery for glioma treatment. Neuro-oncology. 2010 doi: 10.1093/neuonc/noq054. noq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nyborg WL. Biological effects of ultrasound: development of safety guidelines. Part II: general review. Ultrasound in medicine & biology. 2001;27:301–333. doi: 10.1016/s0301-5629(00)00333-1. [DOI] [PubMed] [Google Scholar]
- 81.Aryal M, Vykhodtseva N, Zhang YZ, McDannold N. Multiple sessions of liposomal doxorubicin delivery via focused ultrasound mediated blood–brain barrier disruption: A safety study. Journal of Controlled Release. 2015;204:60–69. doi: 10.1016/j.jconrel.2015.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Palombo M, Singh Y, Sinko P. Prodrug and conjugate drug delivery strategies for improving HIV/AIDS therapy. Journal of drug delivery science and technology. 2009;19:3–14. doi: 10.1016/s1773-2247(09)50001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zolopa A, Ortiz R, Sax P, Brar I, Elion R, Wang H, Callebaut C, Ramanathan S, Fordyce M, McCallister S. Comparative study of tenofovir alafenamide vs tenofovir disoproxil fumarate, each with elvitegravir, cobicistat, and emtricitabine, for HIV treatment. 20th Conference on Retroviruses and Opportunistic Infections; 2013. pp. 3–6. [Google Scholar]
- 84.Anderson BD, Morgan ME, Singhal D. Enhanced oral bioavailability of DDI after administration of 6-Cl-ddP, an adenosine deaminase-activated prodrug, to chronically catheterized rats. Pharmaceutical research. 1995;12:1126–1133. doi: 10.1023/a:1016299507382. [DOI] [PubMed] [Google Scholar]
- 85.Anthonypillai C, Gibbs JE, Thomas SA. The distribution of the anti-HIV drug, tenofovir (PMPA), into the brain, CSF and choroid plexuses. Cerebrospinal Fluid Res. 2006;3:1. doi: 10.1186/1743-8454-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Couvreur P. Nanoparticles in drug delivery: past, present and future. Advanced drug delivery reviews. 2013;65:21–23. doi: 10.1016/j.addr.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 87.Sagar V, Pilakka-Kanthikeel S, Atluri VS, Ding H, Arias AY, Jayant RD, Kaushik A, Nair M. Therapeutical Neurotargeting via Magnetic Nanocarrier: Implications to Opiate-Induced Neuropathogenesis and NeuroAIDS. 2015 doi: 10.1166/jbn.2015.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nair M. Personalized NanoMedicine: Towards new Theranostic Approach. Journal of personalized nanomedicine. 2015;1:1. [PMC free article] [PubMed] [Google Scholar]
- 89.Ding H, Wu F. Image guided biodistribution of drugs and drug delivery. Theranostics. 2012;2:1037. doi: 10.7150/thno.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tomitaka A, Arami H, Gandhi S, Krishnan KM. Lactoferrin conjugated iron oxide nanoparticles for targeting brain glioma cells in magnetic particle imaging. Nanoscale. 2015;7:16890–16898. doi: 10.1039/c5nr02831k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tomitaka A, Ferguson RM, Khandhar AP, Kemp SJ, Ota S, Nakamura K, Takemura Y, Krishnan KM. Variation of magnetic particle imaging tracer performance with amplitude and frequency of the applied magnetic field, Magnetics. IEEE Transactions on. 2015;51:1–4. doi: 10.1109/TMAG.2014.2341570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tomitaka A, Jo J-i, Aoki I, Tabata Y. Preparation of biodegradable iron oxide nanoparticles with gelatin for magnetic resonance imaging. Inflammation and Regeneration. 2014;34:045–055. [Google Scholar]
- 93.Arami H, Khandhar AP, Tomitaka A, Yu E, Goodwill PW, Conolly SM, Krishnan KM. In vivo multimodal magnetic particle imaging (MPI) with tailored magneto/optical contrast agents. Biomaterials. 2015;52:251–261. doi: 10.1016/j.biomaterials.2015.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Estelrich J, Escribano E, Queralt J, Busquets MA. Iron Oxide Nanoparticles for Magnetically-Guided and Magnetically-Responsive Drug Delivery. International journal of molecular sciences. 2015;16:8070–8101. doi: 10.3390/ijms16048070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kong SD, Lee J, Ramachandran S, Eliceiri BP, Shubayev VI, Lal R, Jin S. Magnetic targeting of nanoparticles across the intact blood–brain barrier. Journal of Controlled Release. 2012;164:49–57. doi: 10.1016/j.jconrel.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tomitaka A, Takemura Y, Roy ZHU, Nair M. Recent Advances in Magnetoliposomes as Drug Delivery Carriers. Journal of Personalized NanoMedicine. 2015;1:51–57. [Google Scholar]
- 97.Wen X, Wang K, Zhao Z, Zhang Y, Sun T, Zhang F, Wu J, Fu Y, Du Y, Zhang L. Brain-targeted delivery of trans-activating transcriptor-conjugated magnetic PLGA/lipid nanoparticles. 2014 doi: 10.1371/journal.pone.0106652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jayant R. Layer-by-Layer (LbL) Assembly of Anti HIV Drug for Sustained Release to Brain Using Magnetic Nanoparticle. JOURNAL OF NEUROIMMUNE PHARMACOLOGY, SPRINGER 233 SPRING ST, NEW YORK, NY 10013 USA. 2014:25–25. [Google Scholar]
- 99.Raymond A, Diaz P, Chevelon S, Agudelo M, Yndart-Arias A, Ding H, Kaushik A, Jayant RD, Nikkhah-Moshaie R, Roy U. Microglia-derived HIV Nef+ exosome impairment of the blood–brain barrier is treatable by nanomedicine-based delivery of Nef peptides. Journal of neurovirology. 2015:1–11. doi: 10.1007/s13365-015-0397-0. [DOI] [PubMed] [Google Scholar]
- 100.Fiandra L, Colombo M, Mazzucchelli S, Truffi M, Santini B, Allevi R, Nebuloni M, Capetti A, Rizzardini G, Prosperi D. Nanoformulation of antiretroviral drugs enhances their penetration across the blood brain barrier in mice. Nanomedicine: Nanotechnology, Biology and Medicine. 2015 doi: 10.1016/j.nano.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 101.Iannazzo D, Pistone A, Romeo R, Giofrè S. Nanotechnology Approaches for Antiretroviral Drugs Delivery. Journal of AIDS and HIV Infections. 2015;1:1–13. [Google Scholar]
- 102.Mukherjee B, Dey NS, Maji R, Bhowmik P, Das PJ, Paul P. Current Status and Future Scope for Nanomaterials in Drug Delivery. 2014 [Google Scholar]
- 103.Kuo YC. Loading efficiency of stavudine on polybutylcyanoacrylate and methylmethacrylate-sulfopropylmethacrylate copolymer nanoparticles. International journal of pharmaceutics. 2005;290:161–172. doi: 10.1016/j.ijpharm.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 104.Kuo YC, Su FL. Transport of stavudine, delavirdine, and saquinavir across the blood–brain barrier by polybutylcyanoacrylate, methylmethacrylate-sulfopropylmethacrylate, and solid lipid nanoparticles. International journal of pharmaceutics. 2007;340:143–152. doi: 10.1016/j.ijpharm.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 105.Basu S, Mukherjee B, Chowdhury SR, Paul P, Choudhury R, Kumar A, Mondal L, Hossain CM, Maji R. Colloidal gold-loaded, biodegradable, polymer-based stavudine nanoparticle uptake by macrophages: an in vitro study. International journal of nanomedicine. 2012;7:6049. doi: 10.2147/IJN.S38013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mainardes RM, Gremião MPD, Brunetti IL, Da Fonseca LM, Khalil NM. Zidovudine-loaded PLA and PLA–PEG blend nanoparticles: Influence of polymer type on phagocytic uptake by polymorphonuclear cells. Journal of pharmaceutical sciences. 2009;98:257–267. doi: 10.1002/jps.21406. [DOI] [PubMed] [Google Scholar]
- 107.Lenjisa JL, Woldu MA, Satessa GD. New hope for eradication of HIV from the body: the role of polymeric nanomedicines in HIV/AIDS pharmacotherapy. Journal of nanobiotechnology. 2014;12:9. doi: 10.1186/1477-3155-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rao KS, Ghorpade A, Labhasetwar V. Targeting anti-HIV drugs to the CNS. 2009 doi: 10.1517/17425240903081705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Peng J, Wu Z, Qi X, Chen Y, Li X. Dendrimers as potential therapeutic tools in HIV inhibition. Molecules. 2013;18:7912–7929. doi: 10.3390/molecules18077912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huang RQ, Qu YH, Ke WL, Zhu JH, Pei YY, Jiang C. Efficient gene delivery targeted to the brain using a transferrin-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. The FASEB Journal. 2007;21:1117–1125. doi: 10.1096/fj.06-7380com. [DOI] [PubMed] [Google Scholar]
- 111.Dutta T, Agashe HB, Garg M, Balasubramanium P, Kabra M, Jain NK. Poly (propyleneimine) dendrimer based nanocontainers for targeting of efavirenz to human monocytes/macrophages in vitro: Research Paper. Journal of drug targeting. 2007;15:89–98. doi: 10.1080/10611860600965914. [DOI] [PubMed] [Google Scholar]
- 112.Kabanov AV, Alakhov VY. Pluronic® block copolymers in drug delivery: From micellar nanocontainers to biological response modifiers. Critical Reviews™ in Therapeutic Drug Carrier Systems. 2002;19 doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.10. [DOI] [PubMed] [Google Scholar]
- 113.Batrakova EV, Li S, Miller DW, Kabanov AV. Pluronic P85 increases permeability of a broad spectrum of drugs in polarized BBMEC and Caco-2 cell monolayers. Pharmaceutical research. 1999;16:1366–1372. doi: 10.1023/a:1018990706838. [DOI] [PubMed] [Google Scholar]
- 114.Spitzenberger TJ, Heilman D, Diekmann C, Batrakova EV, Kabanov AV, Gendelman HE, Elmquist WF, Persidsky Y. Novel delivery system enhances efficacy of antiretroviral therapy in animal model for HIV-1 encephalitis. Journal of Cerebral Blood Flow & Metabolism. 2007;27:1033–1042. doi: 10.1038/sj.jcbfm.9600414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gershkovich P, Wasan KM, Barta CA. A review of the application of lipid-based systems in systemic, dermal/transdermal, and ocular drug delivery. Critical Reviews™ in Therapeutic Drug Carrier Systems. 2008;25 doi: 10.1615/critrevtherdrugcarriersyst.v25.i6.20. [DOI] [PubMed] [Google Scholar]
- 116.Webb MS, Rebstein P, Lamson W, Bally MB. Liposomal drug delivery: recent patents and emerging opportunities. Recent patents on drug delivery & formulation. 2007;1:185–194. doi: 10.2174/187221107782331593. [DOI] [PubMed] [Google Scholar]
- 117.Sawant KK, Dodiya SS. Recent advances and patents on solid lipid nanoparticles. Recent patents on drug delivery & formulation. 2008;2:120–135. doi: 10.2174/187221108784534081. [DOI] [PubMed] [Google Scholar]
- 118.Micheli MR, Bova R, Magini A, Polidoro M, Emiliani C. Lipid-based nanocarriers for CNS-targeted drug delivery. Recent patents on CNS drug discovery. 2012;7:71–86. doi: 10.2174/157488912798842241. [DOI] [PubMed] [Google Scholar]
- 119.Lameijer MA, Tang J, Nahrendorf M, Beelen RH, Mulder WJ. Monocytes and macrophages as nanomedicinal targets for improved diagnosis and treatment of disease. 2013 doi: 10.1586/14737159.2013.819216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. International journal of nanomedicine. 2006;1:297. [PMC free article] [PubMed] [Google Scholar]
- 121.Dusserre N, Lessard C, Paquette N, Perron S, Poulin L, Tremblay M, Beauchamp D, Désormeaux A, Bergeron MG. Encapsulation of foscarnet in liposomes modifies drug intracellular accumulation, in vitro anti-HIV-1 activity, tissue distribution, and pharmacokinetics. AIDS. 1995;9:833–842. doi: 10.1097/00002030-199508000-00002. [DOI] [PubMed] [Google Scholar]
- 122.SZEBENI J, WAHL SM, BETAGERI GV, WAHL LM, GARTNER S, POPOVIC M, PARKER RJ, BLACK CD, WEINSTEIN JN. Inhibition of HIV-1 in Monocyte/Macrophage Cultures by 2′, 3′-Dideoxycytidine- 5′-Triphosphate, Free and in Liposomes*. AIDS research and human retroviruses. 1990;6:691–702. doi: 10.1089/aid.1990.6.691. [DOI] [PubMed] [Google Scholar]
- 123.Phillips NC, Skamene E, Tsoukas C. Liposomal encapsulation of 3′-azido-3′-deoxythymidine (AZT) results in decreased bone marrow toxicity and enhanced activity against murine AIDS-induced immunosuppression. JAIDS Journal of Acquired Immune Deficiency Syndromes. 1991;4:959–966. [PubMed] [Google Scholar]
- 124.Garg M, Asthana A, Agashe HB, Agrawal GP, Jain NK. Stavudine-loaded mannosylated liposomes: in-vitro anti-HIV-I activity, tissue distribution and pharmacokinetics. Journal of pharmacy and pharmacology. 2006;58:605–616. doi: 10.1211/jpp.58.5.0005. [DOI] [PubMed] [Google Scholar]
- 125.Garg M, Dutta T, Jain NK. Reduced hepatic toxicity, enhanced cellular uptake and altered pharmacokinetics of stavudine loaded galactosylated liposomes. European journal of pharmaceutics and biopharmaceutics. 2007;67:76–85. doi: 10.1016/j.ejpb.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 126.Makabi-Panzu B, Gourde P, Desormeaux A, Bergeron M. Intracellular and serum stability of liposomal 2′, 3′-dideoxycytidine. Effect of lipid composition. Cellular and molecular biology (Noisy-le-Grand France) 1998;44:277–284. [PubMed] [Google Scholar]
- 127.Makabi-Panzu B, Lessard C, Perron S, Désormeaux A, Tremblay M, Poulin L, Beauchamp D, Bergeron M. Comparison of cellular accumulation, tissue distribution, and anti-HIV activity of free and liposomal 2′, 3′-dideoxycytidine. AIDS research and human retroviruses. 1994;10:1463–1470. doi: 10.1089/aid.1994.10.1463. [DOI] [PubMed] [Google Scholar]
- 128.Ramana LN, Sethuraman S, Ranga U, Krishnan UM. Research Development of a liposomal nanodelivery system for nevirapine. Journal of biomedical science. 2010 doi: 10.1186/1423-0127-17-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vyas TK, Shahiwala A, Amiji MM. Improved oral bioavailability and brain transport of Saquinavir upon administration in novel nanoemulsion formulations. International journal of pharmaceutics. 2008;347:93–101. doi: 10.1016/j.ijpharm.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Müller RH, Rühl D, Runge S, Schulze-Forster K, Mehnert W. Cytotoxicity of solid lipid nanoparticles as a function of the lipid matrix and the surfactant. Pharmaceutical research. 1997;14:458–462. doi: 10.1023/a:1012043315093. [DOI] [PubMed] [Google Scholar]
- 131.Chattopadhyay N, Zastre J, Wong HL, Wu XY, Bendayan R. Solid lipid nanoparticles enhance the delivery of the HIV protease inhibitor, atazanavir, by a human brain endothelial cell line. Pharmaceutical research. 2008;25:2262–2271. doi: 10.1007/s11095-008-9615-2. [DOI] [PubMed] [Google Scholar]
- 132.Kuo YC, Lee CL. Methylmethacrylate–sulfopropylmethacrylate nanoparticles with surface RMP-7 for targeting delivery of antiretroviral drugs across the blood–brain barrier. Colloids and Surfaces B: Biointerfaces. 2012;90:75–82. doi: 10.1016/j.colsurfb.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 133.Jain S, Tiwary A, Jain N. PEGylated elastic liposomal formulation for lymphatic targeting of zidovudine. Current drug delivery. 2008;5:275–281. doi: 10.2174/156720108785915078. [DOI] [PubMed] [Google Scholar]
- 134.Kaur CD, Nahar M, Jain NK. Lymphatic targeting of zidovudine using surface-engineered liposomes. Journal of drug targeting. 2008;16:798–805. doi: 10.1080/10611860802475688. [DOI] [PubMed] [Google Scholar]
- 135.Wan L, Zhang X, Pooyan S, Palombo MS, Leibowitz MJ, Stein S, Sinko PJ. Optimizing size and copy number for PEG-fMLF (N-formyl-methionyl-leucyl-phenylalanine) nanocarrier uptake by macrophages. Bioconjugate chemistry. 2007;19:28–38. doi: 10.1021/bc070066k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dutta T, Garg M, Jain NK. Targeting of efavirenz loaded tuftsin conjugated poly (propyleneimine) dendrimers to HIV infected macrophages in vitro. European journal of pharmaceutical sciences. 2008;34:181–189. doi: 10.1016/j.ejps.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 137.Destache CJ, Belgum T, Christensen K, Shibata A, Sharma A, Dash A. Combination antiretroviral drugs in PLGA nanoparticle for HIV-1. BMC infectious diseases. 2009;9:198. doi: 10.1186/1471-2334-9-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mahajan SD, Aalinkeel R, Law WC, Reynolds JL, Nair BB, Sykes DE, Yong KT, Roy I, Prasad PN, Schwartz SA. Anti-HIV-1 nanotherapeutics: promises and challenges for the future. International journal of nanomedicine. 2012;7:5301. doi: 10.2147/IJN.S25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Borgmann K, Rao KS, Labhasetwar V, Ghorpade A. Efficacy of Tat-conjugated ritonavir-loaded nanoparticles in reducing HIV-1 replication in monocyte-derived macrophages and cytocompatibility with macrophages and human neurons. AIDS research and human retroviruses. 2011;27:853–862. doi: 10.1089/aid.2010.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jain S, Sharma JM, Jain AK, Mahajan RR. Surface-stabilized lopinavir nanoparticles enhance oral bioavailability without coadministration of ritonavir. Nanomedicine. 2013;8:1639–1655. doi: 10.2217/nnm.12.181. [DOI] [PubMed] [Google Scholar]
- 141.Meshram SM, Kumar V, Dodoala S, Sampathi S, Gera S. Biodegradable Polymeric Nanoparticles for Delivery of Combination of Antiretroviral Drugs [Google Scholar]
- 142.Sathigari S, Chadha G, Lee YP, Wright N, Parsons DL, Rangari VK, Fasina O, Babu RJ. Physicochemical characterization of efavirenz–cyclodextrin inclusion complexes. Aaps Pharmscitech. 2009;10:81–87. doi: 10.1208/s12249-008-9180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nowacek AS, Miller RL, McMillan J, Kanmogne G, Kanmogne M, Mosley RL, Ma Z, Graham S, Chaubal M, Werling J. NanoART synthesis, characterization, uptake, release and toxicology for human monocyte-macrophage drug delivery. Nanomedicine. 2009;4:903–917. doi: 10.2217/nnm.09.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Puligujja P, McMillan J, Kendrick L, Li T, Balkundi S, Smith N, Veerubhotla RS, Edagwa BJ, Kabanov AV, Bronich T. Macrophage folate receptor-targeted antiretroviral therapy facilitates drug entry, retention, antiretroviral activities and biodistribution for reduction of human immunodeficiency virus infections, Nanomedicine: Nanotechnology. Biology and Medicine. 2013;9:1263–1273. doi: 10.1016/j.nano.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rossi L, Franchetti P, Pierigé F, Cappellacci L, Serafini S, Balestra E, Perno CF, Grifantini M, Caliò R, Magnani M. Inhibition of HIV-1 replication in macrophages by a heterodinucleotide of lamivudine and tenofovir. Journal of antimicrobial chemotherapy. 2007;59:666–675. doi: 10.1093/jac/dkm011. [DOI] [PubMed] [Google Scholar]
- 146.Kuo YC, Chen HH. Effect of nanoparticulate polybutylcyanoacrylate and methylmethacrylate–sulfopropylmethacrylate on the permeability of zidovudine and lamivudine across the in vitro blood–brain barrier. International journal of pharmaceutics. 2006;327:160–169. doi: 10.1016/j.ijpharm.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 147.Mahajan SD, Roy I, Xu G, Yong KT, Ding H, Aalinkeel R, Reynolds JL, Sykes DE, Nair BB, Lin EY. Enhancing the delivery of anti retroviral drug “Saquinavir” across the blood brain barrier using nanoparticles. Current HIV research. 2010;8:396. doi: 10.2174/157016210791330356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Eguchi A, Meade BR, Chang YC, Fredrickson CT, Willert K, Puri N, Dowdy SF. Efficient siRNA delivery into primary cells by a peptide transduction domain–dsRNA binding domain fusion protein. Nature biotechnology. 2009;27:567–571. doi: 10.1038/nbt.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Song E, Zhu P, Lee SK, Chowdhury D, Kussman S, Dykxhoorn DM, Feng Y, Palliser D, Weiner DB, Shankar P. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nature biotechnology. 2005;23:709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 150.Weber N, Ortega P, Clemente MI, Shcharbin D, Bryszewska M, de la Mata FJ, Gómez R, Muñoz-Fernández MA. Characterization of carbosilane dendrimers as effective carriers of siRNA to HIV-infected lymphocytes. Journal of Controlled Release. 2008;132:55–64. doi: 10.1016/j.jconrel.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 151.Steinbach JM, Weller CE, Booth CJ, Saltzman WM. Polymer nanoparticles encapsulating siRNA for treatment of HSV-2 genital infection. Journal of Controlled Release. 2012;162:102–110. doi: 10.1016/j.jconrel.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schleifman EB, McNeer NA, Jackson A, Yamtich J, Brehm MA, Shultz LD, Greiner DL, Kumar P, Saltzman WM, Glazer PM. Site-specific genome editing in PBMCs with PLGA nanoparticle-delivered PNAs confers HIV-1 resistance in humanized mice. Molecular Therapy—Nucleic Acids. 2013;2:e135. doi: 10.1038/mtna.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nature materials. 2009;8:526–533. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Aline F, Brand D, Pierre J, Roingeard P, Séverine M, Verrier B, Dimier-Poisson I. Dendritic cells loaded with HIV-1 p24 proteins adsorbed on surfactant-free anionic PLA nanoparticles induce enhanced cellular immune responses against HIV-1 after vaccination. Vaccine. 2009;27:5284–5291. doi: 10.1016/j.vaccine.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 155.Lori F, Calarota S, Lisziewicz J. Nanochemistry-based immunotherapy for HIV-1. Current medicinal chemistry. 2007;14:1911–1919. doi: 10.2174/092986707781368513. [DOI] [PubMed] [Google Scholar]
- 156.Elamanchili P, Diwan M, Cao M, Samuel J. Characterization of poly (D, L-lactic-co-glycolic acid) based nanoparticulate system for enhanced delivery of antigens to dendritic cells. Vaccine. 2004;22:2406–2412. doi: 10.1016/j.vaccine.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 157.Fahmy TM, Demento SL, Caplan MJ, Mellman I, Saltzman WM. Design opportunities for actively targeted nanoparticle vaccines. 2008 doi: 10.2217/17435889.3.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang Y, Gao S, Ye WH, Yoon HS, Yang YY. Co-delivery of drugs and DNA from cationic core–shell nanoparticles self-assembled from a biodegradable copolymer. Nature materials. 2006;5:791–796. doi: 10.1038/nmat1737. [DOI] [PubMed] [Google Scholar]
- 159.Haleyur Giri Setty MK, Hewlett IK. Point of care technologies for HIV. AIDS research and treatment. 2014;2014 doi: 10.1155/2014/497046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ding H, Wu F, Nair MP. Image-guided drug delivery to the brain using nanotechnology. Drug discovery today. 2013;18:1074–1080. doi: 10.1016/j.drudis.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kaushik A, Yndart A, Jayant RD, Sagar V, Atluri V, Bhansali S, Nair M. Electrochemical sensing method for point-of-care cortisol detection in human immunodeficiency virus-infected patients. International journal of nanomedicine. 2015;10:677. doi: 10.2147/IJN.S75514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shafiee H, Wang S, Inci F, Toy M, Henrich TJ, Kuritzkes DR, Demirci U. Emerging technologies for point-of-care management of HIV infection. Annual review of medicine. 2015;66:387–405. doi: 10.1146/annurev-med-092112-143017. [DOI] [PubMed] [Google Scholar]
- 163.Yager P, Domingo GJ, Gerdes J. Point-of-care diagnostics for global health. Annu Rev Biomed Eng. 2008;10:107–144. doi: 10.1146/annurev.bioeng.10.061807.160524. [DOI] [PubMed] [Google Scholar]
- 164.Fahey JL, Taylor JM, Detels R, Hofmann B, Melmed R, Nishanian P, Giorgi JV. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. New England Journal of Medicine. 1990;322:166–172. doi: 10.1056/NEJM199001183220305. [DOI] [PubMed] [Google Scholar]
- 165.Saves M, Morlat P, Chene G, Peuchant E, Pellegrin I, Bonnet F, Bernard N, Lacoste D, Salamon R, Beylot J. Prognostic value of plasma markers of immune activation in patients with advanced HIV disease treated by combination antiretroviral therapy. Clinical Immunology. 2001;99:347–352. doi: 10.1006/clim.2001.5033. [DOI] [PubMed] [Google Scholar]
- 166.Koller M, Fatti G, Chi BH, Keiser O, Hoffmann CJ, Wood R, Prozesky H, Stinson K, Giddy J, Mutevedzi P. Implementation and Operational Research: Risk Charts to Guide Targeted HIV-1 Viral Load Monitoring of ART: Development and Validation in Patients From Resource-Limited Settings. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2015;70:e110–e119. doi: 10.1097/QAI.0000000000000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Graham NM. The role of immunologic and viral markers in predicting clinical outcome in HIV infection. Aids. 1996;10:S21–S25. doi: 10.1097/00002030-199612005-00004. [DOI] [PubMed] [Google Scholar]
- 168.Verhofstede C, Reniers S, Van Wanzeele F, Plum J. Evaluation of proviral copy number and plasma RNA level as early indicators of progression in HIV-1 infection: correlation with virological and immunological markers of disease. AIDS (London, England) 1994;8:1421–1427. doi: 10.1097/00002030-199410000-00008. [DOI] [PubMed] [Google Scholar]
- 169.Brew BJ, Dunbar N, Pemberton L, Kaldor J. Predictive markers of AIDS dementia complex: CD4 cell count and cerebrospinal fluid concentrations of β2-microglobulin and neopterin. Journal of Infectious Diseases. 1996;174:294–298. doi: 10.1093/infdis/174.2.294. [DOI] [PubMed] [Google Scholar]
- 170.Ding H, Wu F. Image guided biodistribution and pharmacokinetic studies of theranostics. Theranostics. 2012;2:1040. doi: 10.7150/thno.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ruiz A, Nair M, Kaushik A. Recent update in NanoCure of NeuroAIDS. Science Letters Journal. 2015;4:172. [Google Scholar]
- 172.Liu HL, Hua MY, Yang HW, Huang CY, Chu PC, Wu JS, Tseng IC, Wang JJ, Yen TC, Chen PY, Wei KC. Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proceedings of the National Academy of Sciences. 2010;107:15205–15210. doi: 10.1073/pnas.1003388107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang S, Xu F, Demirci U. Advances in developing HIV-1 viral load assays for resource-limited settings. Biotechnology advances. 2010;28:770–781. doi: 10.1016/j.biotechadv.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang Z, Chin SY, Chin CD, Sarik J, Harper M, Justman J, Sia SK. Microfluidic CD4+ T-cell counting device using chemiluminescence-based detection. Analytical chemistry. 2009;82:36–40. doi: 10.1021/ac902144w. [DOI] [PubMed] [Google Scholar]
- 175.Alyassin MA, Moon S, Keles HO, Manzur F, Lin RL, Hæggstrom E, Kuritzkes DR, Demirci U. Rapid automated cell quantification on HIV microfluidic devices. Lab on a Chip. 2009;9:3364–3369. doi: 10.1039/b911882a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gurkan UA, Moon S, Geckil H, Xu F, Wang S, Lu TJ, Demirci U. Miniaturized lensless imaging systems for cell and microorganism visualization in point-of-care testing. Biotechnology journal. 2011;6:138–149. doi: 10.1002/biot.201000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Moon S, Gurkan UA, Blander J, Fawzi WW, Aboud S, Mugusi F, Kuritzkes DR, Demirci U. Enumeration of CD4+ T-cells using a portable microchip count platform in Tanzanian HIV-infected patients. PLoS One. 2011;6:e21409. doi: 10.1371/journal.pone.0021409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang S, Tasoglu S, Chen PZ, Chen M, Akbas R, Wach S, Ozdemir CI, Gurkan UA, Giguel FF, Kuritzkes DR. Micro-a-fluidics ELISA for Rapid CD4 Cell Count at the Point-of-Care. Scientific reports. 2014;4 doi: 10.1038/srep03796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Labbett W, Garcia-Diaz A, Fox Z, Clewley GS, Fernandez T, Johnson M, Geretti AM. Comparative evaluation of the ExaVir Load version 3 reverse transcriptase assay for measurement of human immunodeficiency virus type 1 plasma load. Journal of clinical microbiology. 2009;47:3266–3270. doi: 10.1128/JCM.00715-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mine M, Bedi K, Maruta T, Madziva D, Tau M, Zana T, Gaolathe T, Moyo S, Seipone K, Ndwapi N. Quantitation of human immunodeficiency virus type 1 viral load in plasma using reverse transcriptase activity assay at a district hospital laboratory in Botswana: a decentralization pilot study. Journal of virological methods. 2009;159:93–97. doi: 10.1016/j.jviromet.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 181.Tanriverdi S, Chen L, Chen S. A rapid and automated sample-to-result HIV load test for near-patient application. Journal of Infectious Diseases. 2010;201:S52–S58. doi: 10.1086/650387. [DOI] [PubMed] [Google Scholar]
- 182.Jangam SR, Agarwal AK, Sur K, Kelso DM. A point-of-care PCR test for HIV-1 detection in resource-limitedsettings. Biosensors and Bioelectronics. 2013;42:69–75. doi: 10.1016/j.bios.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 183.Inci F, Tokel O, Wang S, Gurkan UA, Tasoglu S, Kuritzkes DR, Demirci U. Nanoplasmonic quantitative detection of intact viruses from unprocessed whole blood. ACS nano. 2013;7:4733–4745. doi: 10.1021/nn3036232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Shafiee H, Lidstone EA, Jahangir M, Inci F, Hanhauser E, Henrich TJ, Kuritzkes DR, Cunningham BT, Demirci U. Nanostructured optical photonic crystal biosensor for HIV viral load measurement. Scientific reports. 2014;4 doi: 10.1038/srep04116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cheng X, Liu Y-s, Irimia D, Demirci U, Yang L, Zamir L, Rodriguez WR, Toner M, Bashir R. Cell detection and counting through cell lysate impedance spectroscopy in microfluidic devices. Lab on a Chip. 2007;7:746–755. doi: 10.1039/b705082h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Holmes D, Morgan H. Single cell impedance cytometry for identification and counting of CD4 T-cells in human blood using impedance labels. Analytical chemistry. 2010;82:1455–1461. doi: 10.1021/ac902568p. [DOI] [PubMed] [Google Scholar]
- 187.Watkins NN, Sridhar S, Cheng X, Chen GD, Toner M, Rodriguez W, Bashir R. A microfabricated electrical differential counter for the selective enumeration of CD4+ T lymphocytes. Lab on a Chip. 2011;11:1437–1447. doi: 10.1039/c0lc00556h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shafiee H, Jahangir M, Inci F, Wang S, Willenbrecht R, Giguel FF, Tsibris A, Kuritzkes DR, Demirci U. Acute On-Chip HIV Detection Through Label-Free Electrical Sensing of Viral Nano-Lysate. Small. 2013;9:2553–2563. doi: 10.1002/smll.201202195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hu W, Kaminski R, Yang F, Zhang Y, Cosentino L, Li F, Luo B, Alvarez-Carbonell D, Garcia-Mesa Y, Karn J. RNA-directed gene editing specifically eradicates latent and prevents new HIV-1 infection. Proceedings of the National Academy of Sciences. 2014;111:11461–11466. doi: 10.1073/pnas.1405186111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Khalili K, Kaminski R, Gordon J, Cosentino L, Hu W. Genome editing strategies: potential tools for eradicating HIV-1/AIDS. Journal of neurovirology. 2015;21:310–321. doi: 10.1007/s13365-014-0308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gori JL, Hsu PD, Maeder ML, Shen S, Welstead GG, Bumcrot D. Delivery and Specificity of CRISPR/Cas9 Genome Editing Technologies for Human Gene Therapy. Human gene therapy. 2015;26:443–451. doi: 10.1089/hum.2015.074. [DOI] [PubMed] [Google Scholar]