Abstract
Binge eating behavior involves rapid consumption of highly palatable foods leading to increased weight gain. Feeding in binge disorders resembles other compulsive behaviors, many of which are responsive to N-acetyl cysteine (NAC), which is a cysteine prodrug often used to promote non-vesicular glutamate release by a cystine-glutamate antiporter. To examine the potential for NAC to alter a form of compulsive eating, we examined the impact of NAC on binge eating in a rodent model. Specifically, we monitored consumption of standard chow and a high-fat, high carbohydrate western diet (WD) in a rodent limited-access binge paradigm. Prior to each session, rats received either a systemic or intraventricular injection of NAC. Both systemic and central administration of NAC resulted in significant reductions of binge eating the WD without decreasing standard chow consumption. The reduction in WD was not attributable to general malaise since NAC did not produce condition taste aversion. These results are consistent with the clinical evidence of NAC to reduce or reverse compulsive behaviors such as drug addiction, skin picking, and hair pulling.
INTRODUCTION
Binge eating is one of the most prevalent eating disorders in the United States and contributes to the obesity epidemic that is currently endangering the health of approximately 30% of all Americans.1, 2 Feeding in binge disorders is characterized by rapid consumption of large amounts of highly palatable food that occur in very short periods of time.3 These maladaptive alterations in feeding behavior culminate in a destructive state that often parallels other compulsive disorders, such as drug addiction.4, 5 In many instances, binge eating is reported to occur in the absent of hunger, suggesting that this compulsive feeding state could arise from excessive hedonic drive rather than unmet homeostatic needs.6, 7
Many compulsive disorders involve abnormal glutamatergic signaling.8 Likely because of this, the cysteine prodrug NAC has been shown to reduce many forms of compulsive behaviors ranging from addiction to trichotillomania.9, 10 The mechanism of action of NAC typically involves increasing the activity of the cystine-glutamate antiporter, system xc-,11 which has been shown to be a key component of glutamate homeostasis by maintaining extrasynaptic glutamate tone via non-vesicular release and ,in turn, affecting receptor function (ie synaptic signaling) in regions implicated in hedonic motivated eating.12, 13 System xc- has been shown to be an effective target to inhibit cocaine-induced behaviors and reinstatement in pre-clinical studies.14 Because of the compulsive aspect of binge eating, an overeating behavior analogous to drug abuse, we examine the potential for systemic or intraventricular administration of NAC to alter binge eating. In the current study, we utilized a frequently employed pre-clinical model of binge eating in which rodents are permitted daily limited-access to a highly palatable food as an adjunct to their ad lib standard chow and recapitulates several characteristics of human binge eating disorder.15
METHODS
Animals
Male Sprague-Dawley rats (225-250g; 53-58 days old; Harlan; Indianapolis, IN) were housed individually under a 12 hour light/dark cycle (2PM: Lights OFF; 2AM: Lights ON) with ad lib access to a Harlan standard diet (8604 formula) for the duration of all experiments. Intake was monitored daily via a BioDAQ Food Intake Monitor (Research Diets, New Brunswick, NJ) or by pre-weighing food bins prior to and after experimental sessions. Body weights were also recorded daily. All procedures using animals were approved by the Marquette University Institutional Animal Care and Use Committee.
Cannulation Surgery & Microinjections
Animals were anesthetized with a ketamine/xylazine/acepromazine (77:1.5:1.5 mg/ml/kg; i.p.) cocktail and placed in a stereotaxic apparatus. A 26 gauge unilateral guide cannula (Plastics One; Roanoke, VA) was placed into a lateral ventricle and secured to the skull via an acrylic resin. The stereotaxic coordinates were anterior/posterior, −0.8 mm from bregma; medial/lateral, ± 1.5 mm from midline; dorsal/ventral, −3.0 mm from the surface of the skull, whereas the injector extended 0.5mm further beyond the tip of the guide cannula. Stereotaxic coordinates were based on The Rat Brain in Stereotaxic Coordinates, 6th Edition.16
Limited access binge-eating model
Animals with ad lib access to standard chow (Harlan 8604 formula; SC; 32% of total kcal protein, 54% of total kcal carbohydrate, 14% of total kcal fat; 3.0 kcal/g) were additionally allowed daily limited access (30 min/day) to a highly palatable western diet (WD; Research Diets; New Brunswick, NJ; 17% of total kcal protein, 43% of total kcal carbohydrate, 41% of total kcal fat; 4.7 kcal/g) shortly after lights out (45 minutes). Chronic systemic (i.p.; 90mg/kg) and intermittent central (ICV; 10ug/5ul) NAC was administered 45-60 minutes prior to gaining limited access to WD based on previous work examining the pre-clinical therapeutic potential of systemic and centrally administered NAC in attenuating cocaine relapse behavior in rodents.17
Chronic systemic N-acetylcysteine
Animals (n=12-17/treatment) received chronic intraperitoneal (ip) injections of either vehicle (saline) or N-acetylcysteine (90 mg/kg) 45-60 minutes prior to gaining limited access (30 min/day) to the pre-weighed bin of WD for 14 days.
Central N-acetylcysteine
After a one week period of recovery from guide cannula surgery, animals (n=6-8/dose) were placed on the limited access binge paradigm for 7 days during which NAC was injected into the cerebral ventricles on alternating days. On days 1, 3, 5, and 7 animals received central infusions of NAC (10μg/5μl) while control animals received an equal volume of vehicle (saline); microinjections were preformed 1 hr prior to WD access. Animals were given daily access (30min/day) to WD to avoid associating bin access with any protocol associated with microinjections.
Conditioned Taste Aversion
A separate group of animals were habituated to restricted water access (1HR/day) and systemic injections (i.p.) of saline 45-60 minutes prior to water access for 1 week. Subsequently, animals were separated into three treatment groups (n=5/group): saline, NAC (90 mg/kg), or LiCl (2%; IP) and offered two bottles each containing a 0.15% saccharin solution for 24 hours then returned to their previous restricted water conditions. Two days later, animals were provided a two-bottle choice test with one bottle containing 0.15% saccharin solution and the other containing water for 1 hr.
Statistics
Data are presented as means ± standard errors of the mean, and were analyzed statistically (Sigma Plot 11; SystatSoftware Inc.; San Jose, CA) by analysis of variance (with repeated measures when appropriate) or student’s t-test. Fischer LSD analysis was used for all post-hoc group comparisons. P values less than 0.05 were considered statistically significant.
RESULTS
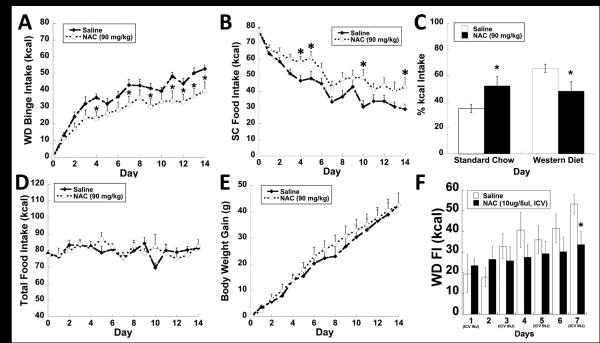
Daily systemic injections of N-acetyl cysteine (NAC; 90 mg/kg; ip) or vehicle (saline) injections were administered 45 minutes prior to limited access (30 min/day) to a highly palatable western diet (WD) as an adjunct to ad lib standard chow (SC) for 14 days. Animals treated with NAC ate significantly less WD (Fig. 1A; treatment F(1,405)= 5.478; p<0.03), and significantly more SC (Fig 1B; treatment F(1,405)= 4.893; p<0.04) over the 2 week study. As expected, NAC treated animals displayed a significantly larger percentage of daily kilocalorie ingested from SC and a concomitant reduction in WD intake than vehicle treated rats by the 14th day of experimentation (Fig. 1C; Left; p<0.05). The compensatory increase in SC consumption paired with the attenuation in WD consumption resulted in no significant change in total calories consumed or body weight gain (Fig. 1D-E). In a separate study, NAC administered centrally every 48 hours (prior to WD access) at the onset of dark via intraventricular (ICV) injections displayed a similar suppression in the escalation of WD consumption over a 7 day period (Fig. 1F; treatment X time F(6, 97)= 2.281; p<0.05).
Figure 1.
System (90mg/kg; IP) and central (10μg/5μl) NAC alters binge behavior produced by daily limited access to a palatable food. (A) NAC treated animals ate significantly less WD (30 min/day) compared to saline injected control animals. (B) Subsequently, NAC treated animals ate significantly more SC over the duration of the study. (C) Percent kilocalorie intake of SC on the 14th day is significantly higher for the NAC treated group, while WD consumption was significantly decreased compared to the control group. (D,E) There is no significant difference in cumulative food intake or body weight gained between the two experimental groups. (F) In a separate study, animals received infusions (NAC or saline; ICV) 30 minutes prior to accessing WD, central administration of NAC significantly suppressed WD consumption over the 7 day study. Data expressed as mean ± SEM. * = P < .05 compared to control group.
The hypophagic effects of NAC appear to be specific to palatable WD consumption as it does not significantly alter the feeding behavior of animals maintained on ad lib SC only (Fig. 2A). Moreover, there were no effects on standard chow consumption over the first 6 hours or 24 hours after administration of NAC (data not shown). Additionally, a conditioned taste aversion revealed that suppression of WD by NAC was not due to malaise (Fig. 2B).
Figure 2.
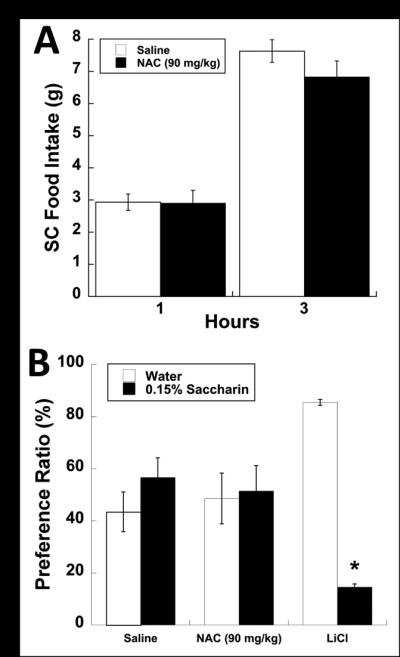
Systemic administration of NAC (90mg/kg; IP) does not alter ad libitum SC consumption or produce any conditioned taste aversion. (A) Animals maintained on ad libitum SC and not subjected to a limited-access binge paradigm show no changes in feeding behavior measured 1 and 3 hours post i.p. injection. (B) Saccharin preference ratio (%) of control (saline), NAC and LiCl (2% bw) treated animals produced only a significant suppression of saccharin consumption in the LiCl treated group. Data expressed as mean ± SEM. * = P < 0.05 compared to controls.
DISCUSSION
Chronic systemic N-acetylcysteine significantly attenuates binge eating of a WD over a 14 day study (Fig 1). Although chronic NAC administration did not alter total caloric intake or weight gain (Fig. 1D;1E), systemic or central administration was sufficient to reduce intake of highly caloric and palatable food without inducing malaise (Fig 2B; 2C). This selectivity of NAC to regulate only WD consumption is quite notable as it suggests that system xc- activation does not impact overall caloric homeostasis but may lessen the hedonic drive for food. Future studies extending chronic NAC administration beyond two weeks may be necessary to demonstrate effects on long-term calorie consumption and body weight.
NAC, a cysteine prodrug, has been demonstrated to drive the activity of the cystine-glutamate antiporter, system xc-, which promotes non-vesicular glutamate release and consequently impact synaptic glutamate signaling, which is critical to the development and maintenance of a wide variety of compulsive behaviors.18, 19 In the case of long-term use of drugs of abuse there is a reduction in system xc- activity in the nucleus accumbens which is thought to contribute to the pathological glutamate signaling contributing to addiction.20 Subsequently, both preclinical and clinical studies of NAC treatment have shown inhibition of cocaine-induced behaviors in animals and reduction in craving and or use of cocaine and tobacco in humans.9, 20, 21 The effectiveness of NAC to reduce binge eating of a highly caloric palatable food is consistent with other studies demonstrating the attenuation of compulsive behaviors such as drug taking, hair pulling and skin picking, and suggests that system xc- is a potential therapeutic target for the treatment of compulsive feeding disorders.9, 10, 22 Pursuing effective treatments for compulsive eating disorders will require further understanding of the neural mechanisms that underlie hedonic drive.7
AKNOWLEDGMENTS
This work was supported by the US National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; DK074734) and the US National Institute on Drug Abuse (NIDA: DA035088).
Footnotes
Conflict of interest statement: The authors declare no conflict of interest.
REFERENCES
- 1.OECD. OECD Health Data: Non-medical determinants of health. OECD Health Statistics. 2013 [Google Scholar]
- 2.Hudson JI, Hiripi E, Pope HG, Kessler RC. The Prevalence and Correlates of Eating Disorders in the National Comorbidity Survey Replication. Biological Psychiatry. 2007;61(3):348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric A. Diagnostic and statistical manual of mental disorders : DSM-5. 2013 doi: 10.1590/s2317-17822013000200017. [DOI] [PubMed] [Google Scholar]
- 4.Gearhardt AN, White MA, Potenza MN. Binge Eating Disorder and Food Addiction. Current drug abuse reviews. 2011;4(3):201–207. doi: 10.2174/1874473711104030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13(5):635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher JO, Birch LL. Eating in the absence of hunger and overweight in girls from 5 to 7 y of age. The American journal of clinical nutrition. 2002;76(1):226–231. doi: 10.1093/ajcn/76.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcus MD, Kalarchian MA. Binge eating in children and adolescents. Int J Eat Disord. 2003;34(57):S47–57. doi: 10.1002/eat.10205. [DOI] [PubMed] [Google Scholar]
- 8.Kalivas PW, LaLumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Supplement 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amen SL, Piacentine LB, Ahmad ME, Li SJ, Mantsch JR, Risinger RC, et al. Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology. 2011;36(4):871–8. doi: 10.1038/npp.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant JE, Odlaug BL, Kim SW. N-acetylcysteine, a glutamate modulator, in the treatment of trichotillomania: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2009;66(7):756–63. doi: 10.1001/archgenpsychiatry.2009.60. [DOI] [PubMed] [Google Scholar]
- 11.McClure EA, Gipson CD, Malcolm RJ, Kalivas PW, Gray KM. Potential Role of N-Acetylcysteine in the Management of Substance Use Disorders. CNS drugs. 2014;28(2):95–106. doi: 10.1007/s40263-014-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bridges R, Lutgen V, Lobner D, Baker DA. Thinking outside the cleft to understand synaptic activity: contribution of the cystine-glutamate antiporter (System xc-) to normal and pathological glutamatergic signaling. Pharmacol Rev. 2012;64(3):780–802. doi: 10.1124/pr.110.003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin P, Pratt WE. Inactivation of the Nucleus Accumbens Core or Medial Shell Attenuates Reinstatement of Sugar-Seeking Behavior following Sugar Priming or Exposure to Food-Associated Cues. PLoS One. 2014;9(6):e99301. doi: 10.1371/journal.pone.0099301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, et al. Neuroadaptations in cystine glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6(7):743–9. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 15.Corwin RL, Wojnicki FHE, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited Access to a Dietary Fat Option Affects Ingestive Behavior But Not Body Composition in Male Rats. Physiology & behavior. 1998;65(3):545–553. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- 16.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; 2007. [DOI] [PubMed] [Google Scholar]
- 17.Kupchik YM, Moussawi K, Tang X-C, Wang X, Kalivas BC, Kolokithas R, et al. The effect of N-acetylcysteine in the nucleus accumbens on neurotransmission and relapse to cocaine. Biological Psychiatry. 2012;71(11):978–986. doi: 10.1016/j.biopsych.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22(20):9134–41. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;1:169–73. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker DA, McFarland K, Lake RW, Shen H, Tang X-C, Toda S, et al. Neuroadaptations in cystine glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6(7):743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 21.Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65(10):841–5. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odlaug BL, Grant JE. N-acetyl cysteine in the treatment of grooming disorders. J Clin Psychopharmacol. 2007;27(2):227–9. doi: 10.1097/01.jcp.0000264976.86990.00. [DOI] [PubMed] [Google Scholar]