Abstract
Objective
To evaluate the cardiovascular (CV) prognostic value of adipokines in a large prospective cohort of patients participating in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial.
Patients and Methods
The effects of the adipokine levels at baseline and change from baseline on the composite outcome (CV death, myocardial infarction and stroke) were analyzed using unadjusted and fully adjusted Cox models in 2330 patients with type 2 diabetes (DM) and coronary artery disease (CAD) who had participated in BARI2D trial (January 2001, through November 2008).
Results
In a fully adjusted model, baseline leptin and change from baseline leptin were protective for CV events, while baseline adiponectin, baseline tumor necrosis factor-alpha (TNF- α), change from baseline TNF-α, baseline C-reactive protein (CRP), and change from baseline CRP were harmful. The effect of baseline leptin on CV events depended on the body mass index (BMI), such that the hazard ratios (HR) varied between 0.6 and 1.4 across the BMI quintiles (interaction P=.03). The same was true for baseline adiponectin (HR varied between 0.7 and 1.7, interaction P=.01), change from baseline monocyte chemoattractant protein-1 (MCP-1) (HR varied between 0.8 and 1.8, interaction P=.03), change from baseline TNF-α (HR varied between 0.9 to 1.4, interaction P=.02), and change from baseline CRP (HR varied between 0.7 to 1.2, interaction P=.02).
Conclusions
Adipokines are independent predictors of CV events in patients with DM and CAD. The association between the specific adipokines and CV outcome varies depending on BMI. This reflects the complex pathophysiology of CV disease in obesity and may help explain the “obesity paradox”.
Clinical Trial Registration
Keywords: cardiovascular, prognosis, obesity, adipokines, coronary disease, type 2 diabetes
INTRODUCTION
Among many comorbid conditions of obesity, cardiovascular (CV) disease and risk factors for CV disease play a prominent role.1,2 Of note, the relationship between body mass and CV disease appears to be continuous and there is evidence of increased CV risk even at mildly elevated body mass index (BMI) levels.3,4 The correlation between BMI and CV risk is independent of other traditional metabolic and CV risk factors,3,4 suggesting that BMI may predispose to CV disease due to other unique adipose tissue-related variables.
Obesity has also been linked to increased mortality.5,6 However, the association between body mass and mortality appears to be complex. The term “obesity paradox” has been used to describe a U- or J-shaped relationship between the BMI and clinical outcome in people with CV diseases, which reflects the phenomenon where overweight individuals appear to have better survival compared to the leaner ones.2,5,7
Adipose tissue releases bioactive hormones, known as adipokines.8 Adipokines may mediate the association between obesity and CV disease, 9,10 but clinical reports on the relationship between adipokines and CV outcome have been inconsistent. For example, higher leptin levels have been associated with unfavorable 11–14 or favorable 15,16 CV effects. Similarly divergent results have been reported for adiponectin.17–29 Some studies did not observe any significant or independent associations.30,31 To better understand the differences between various clinical reports, we evaluated independent prognostic value of baseline levels and longitudinal changes of several adipokines in a large prospective cohort of patients participating in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) outcome trial.32
RESEARCH DESIGN AND METHODS
The design and primary outcome results from the BARI 2D trial have been previously published.32 The protocol was approved by the institutional ethics committee of all participating sites and all subjects provided informed consent. BARI 2D enrolled 2368 participants with type 2 diabetes (DM) and coronary artery disease (CAD) between January 2001 and March 2005. Participants were randomized using a 2x2 factorial design to simultaneously place them into a CV randomized group and a DM randomized group. The CV randomization was to either revascularization and aggressive medical therapy, or aggressive medical therapy alone with deferred revascularization as needed. The DM randomization was to either a primarily insulin sensitizing (IS) or primarily insulin providing (IP) glycemic control strategies. No differences were found in baseline characteristics across groups.32 All participants had concomitant risk factor control for hypertension, dyslipidemia and obesity, and a goal HbA1c of < 7.0% regardless of randomization assignment. Follow-up ended in November 2008.
This analysis is based on an ancillary study to the BARI 2D trial. The study evaluated blood levels of selected adipokines and cytokines: leptin, adiponectin, monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-alpha (TNF-α) and interleukin 6 (IL-6). CRP was also assessed. All samples were analyzed in the Laboratory of Clinical Biochemistry Research at the University of Vermont. IL-6, leptin, TNF-α and MCP-1 were analyzed using a bead-based multiplex assay system (Millipore Adipokine Panel B). Total adiponectin was measured by a validated enzyme-linked immunoassay (R&D Systems) and high sensitivity CRP by Nephelometry (Siemens). The interassay coefficients of variation were between 5.0% and 8.5%.
Up to three stored fasting blood samples were obtained from each participant: a baseline, a year 1, and a unique last measurement. For some of the participants, a unique last measurement was not possible and the baseline or year 1 measurement was the final measurement available. Participants needed to have a baseline stored blood sample available for analysis (N=2330 of the 2368 participants). Annual adipokine values between year 1 and the last measurement (or baseline and the last measurement if there was no first year measurement) were estimated with linear interpolation.
The data handling and the first year change in adipokine values based on the IS or IP randomization were previously published.33 Briefly, log transformations were used to obtain approximately normal distributions of the skewed adipokine measures. The adipokine variables were standardized based on the sex-specific log baseline mean and standard deviation (SD). There were 26 values that were more than 5 SDs away from the mean and they were removed from the analysis.
The primary outcome was the composite of CV death, non-procedural myocardial infarction (MI) and stroke. During the follow-up period, there were 146 CV deaths, 206 nonprocedural MIs, 66 strokes and 364 composite events (as some patients had multiple events and 54 events occurred after an initial composite event).
The impact of baseline adipokine levels and their change from baseline on the composite outcome were analyzed using unadjusted and fully adjusted Cox models. The adjustment variables included: age, sex, BMI, systolic blood pressure, left ventricular ejection fraction < 50%, number of coronary vessels with ≥50% stenosis, prior revascularization, history of MI, history of stroke, HbA1c, low density lipoprotein, high density lipoprotein and triglyceride levels, micro- and macro- albuminuria, glomerular filtration rate, current smoking status and randomization groups. All adjustment variables were measured at baseline, with the exception of BMI, which was updated each time when adipokine measures were available. All models included the baseline adipokine value as well as the change from baseline as a time-varying covariate. If an event occurred prior to the first follow-up adipokine measure at year 1, then it was assumed that there was no change in the adipokine levels between baseline and the event. A fully adjusted model containing all the adipokines (but not the ratios involving adipokines, as they would induce collinearity with the other variables in the model) was estimated. Interactions for the baseline and the change from baseline are reported separately. All reported p-values are nominal. The analysis was performed using SAS/STAT software, Version 9.3 of the SAS System for Windows.
RESULTS
The baseline demographic and clinical characteristics of the study population and the baseline adipokine/cytokine values are summarized in Table 1. Trial participants were mostly White middle-aged males with DM. All had angiographically proven CAD; a third had experienced a prior MI and most received antihypertensive and statin therapy.
Table 1.
Baseline characteristics of the study population (N=2330*).
| Age (years), mean (SD) | 62 (9) | |
| Male, n (%) | 1637 (70) | |
| White, n (%) | 1535 (66) | |
| BMI (kg/m2), mean (SD) * | 32 (6) | |
| Waist circumference (cm), mean (SD) | 108 (14) | |
| Systolic blood pressure (mmHg), mean (SD) * | 132 (20) | |
| Diastolic blood pressure (mmHg), mean (SD) * | 75 (11) | |
| Left ventricular ejection fraction <50%, n (%) * | 391 (17) | |
| Number of coronary vessels with ≥50% stenosis, n (%) | ||
| 0 | 159 (7) | |
| 1 | 724 (31) | |
| 2 | 827 (36) | |
| 3 | 617 (27) | |
| Leptin (ng/ml), median (Q1, Q3) | 17.8 (8.7, 34.3) | |
| Adiponectin (µg/ml), median (Q1, Q3) * | 4.8 (3.0, 7.9) | |
| MCP-1 (pg/ml), median (Q1, Q3) | 196.1 (150.5, 245.7) | |
| TNF-α (pg/ml), median (Q1, Q3) | 4.9 (3.6, 6.6) | |
| CRP (µg/ml), median (Q1, Q3) * | 2.2 (0.9, 5.6) | |
| IL-6 (pg/ml), median (Q1, Q3) | 2.3 (1.3, 4.0) | |
| HbA1c (%), mean (SD) | 7.7 (1.6) | |
| Total cholesterol (mg/dl), mean (SD) * | 169 (41) | |
| LDL (mg/dl), mean (SD) | 89 (41) | |
| HDL (mg/dl), mean (SD) * | 38 (10) | |
| Triglycerides (mg/dl), mean (SD) * | 181 (136) | |
| GFR (MDRD Algorithm), mean (SD) * | 79 (29) | |
| Macro-albuminuria (albumin/creatinine ratio >300 mg/g), n (%) * | 210 (10) | |
| Micro-albuminuria, n (%) * | 495 (23) | |
| Prior Revascularization, n (%) | 546 (23) | |
| History of MI, n (%) * | 734 (32) | |
| History of stroke, n (%) * | 227 (10) | |
| History of CHF requiring treatment, n (%) * | 151 (7) | |
| Hypertension requiring treatment, n (%) * | 1901 (83) | |
| Aspirin, n (%) * | 2045 (88) | |
| Beta blocker, n (%) * | 1695 (73) | |
| ACEi or ARB, n (%) * | 1796 (77) | |
| Statin, n (%) * | 1740 (75) | |
| Biguanide, n (%) * | 1259 (54) | |
| Thiazolidinedione, n (%) * | 442 (19) | |
| Sulfonylurea, n (%) * | 1248 (54) | |
| Insulin, n (%) * | 646 (28) | |
The following measures had the associated number of missing values: 20 for BMI, 22 for systolic blood pressure, 23 for diastolic blood pressure, 3 for left ventricular ejection fraction, 13 for adiponectin, 5 for CRP, 40 for total cholesterol and triglycerides, 57 for HDL, 11 for GFR, 168 for the albuminuria category, 37 for history of an MI, 10 for history of a stroke, 15 for CHF requiring treatment, 27 for hypertension requiring treatment, 9 for aspirin usage, 5 for beta blocker and statin usage, 4 for ACEi or ARB usage, 2 for biguanide, thiazolidinedione, sulfonylurea and insulin usage.
Abbreviations: SD (standard deviation), HbA1c (glycated hemoglobin), MCP-1 (monocyte chemoattractant protein-1), TNF-α (tumor necrosis factor-alpha), CRP (C-reactive protein), IL-6 (interleukin 6), LDL (low density lipoprotein), HDL (high density lipoprotein), GFR (glomerular filtration rate), MI (myocardial infarction), CHF (chronic heart failure), ACEi (angiotensin-converting-enzyme inhibitor), ARB (angiotensin II receptor blocker)
Table 2 contains the results for CV outcome. In unadjusted models with only one adipokine (including both baseline and time-varying change) per model, all adipokines had significant associations with outcome, with the exception of MCP-1 and leptin/BMI ratio. Change in leptin and baseline adiponectin had significant associations, as did both baseline and change values for leptin/adiponectin ratio, TNF-α, CRP, and IL-6. When adding adjustment variables, the significance and direction of the associations remained unchanged, except for the addition of significance for baseline leptin, baseline leptin/BMI and change in leptin/BMI, and the loss of significance for change in IL-6. When combining multiple adipokines into one fully adjusted model, baseline leptin (hazard ratio [HR]=0.9; 95% confidence intervals [CIs]: 0.8, 1.0; P=.02) and change from baseline leptin (HR=0.8; 95% CIs: 0.7, 0.9; P < .001) was protective, while baseline adiponectin (HR=1.2; 95% CIs: 1.0, 1.4; P=.01), baseline TNF-α (baseline HR=1.1; 95% CIs: 1.0, 1.3; P=.01), change from baseline TNF-α (HR=1.2; 95% CIs: 1.0, 1.3; P=.02), baseline CRP (HR=1.4; 95% CIs: 1.2, 1.6; P < .001) and change from baseline CRP (HR=1.2; 95% CIs: 1.1, 1.4; P=.004) were harmful.
Table 2.
Effect of adipokines on the composite endpoint of CV death, non-procedural MI and stroke.
| Outcome: CV Death/Non-Procedural MI/Stroke |
Unadjusted Model | Adjusted Model* | Final Fully Adjusted Model* |
|||
|---|---|---|---|---|---|---|
| Covariate | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Baseline Log leptin | 0.9 (0.8, 1.0) | .22 | 0.8 (0.7, 1.0) | .02 | 0.9 (0.8, 1.0) | .02 |
| Change from Baseline Log leptin | 0.8 (0.8, 1.0) | .01 | 0.8 (0.7, 0.9) | <.001 | 0.8 (0.7, 0.9) | <.001 |
| Baseline Log adiponectin | 1.2 (1.1, 1.3) | .001 | 1.2 (1.0, 1.3) | .01 | 1.2 (1.0, 1.4) | .01 |
| Change from Baseline Log adiponectin | 1.1 (1.0, 1.2) | .20 | 1.0 (0.9, 1.2) | .69 | 1.0 (0.9, 1.2) | .53 |
| Baseline Log MCP-1 | 1.1 (0.9, 1.2) | .35 | 0.9 (0.8, 1.1) | .34 | 0.9 (0.8, 1) | .14 |
| Change from Baseline Log MCP-1 | 1.1 (1, 1.3) | .19 | 1.0 (0.9, 1.2) | .60 | 1.0 (0.9, 1.2) | .84 |
| Baseline Log leptin/adiponectin | 0.9 (0.8, 1.0) | .01 | 0.8 (0.7, 0.9) | .001 | ||
| Change from Baseline Log leptin/adiponectin | 0.8 (0.8, 1.0) | .008 | 0.8 (0.7, 0.9) | .003 | ||
| Baseline Log leptin/BMI | 0.9 (0.8, 1.0) | .21 | 0.9 (0.8, 1.0) | .02 | ||
| Change from Baseline Log leptin/BMI | 0.9 (0.8, 1.0) | .09 | 0.8 (0.7, 0.9) | .003 | ||
| Baseline Log TNF-α | 1.4 (1.2, 1.5) | <.001 | 1.2 (1.1, 1.4) | <.001 | 1.1 (1.0, 1.3) | .01 |
| Change from Baseline Log TNF-α | 1.2 (1.1, 1.3) | <.001 | 1.2 (1.1, 1.3) | .002 | 1.2 (1.0, 1.3) | .02 |
| Baseline Log CRP | 1.4 (1.3, 1.6) | <.001 | 1.5 (1.3, 1.7) | <.001 | 1.4 (1.2, 1.6) | <.001 |
| Change from Baseline Log CRP | 1.2 (1.1, 1.4) | .003 | 1.3 (1.1, 1.5) | <.001 | 1.2 (1.1, 1.4) | .004 |
| Baseline Log IL-6 | 1.4 (1.3, 1.6) | <.001 | 1.3 (1.1, 1.5) | <.001 | 1.1 (0.9, 1.3) | .28 |
| Change from Baseline Log IL-6 | 1.1 (1.0, 1.3) | .04 | 1.1 (1.0, 1.3) | .10 | 1.0 (0.9, 1.2) | .82 |
Unadjusted and adjusted models contain only the baseline and change of one adipokine measure at a time, thus each major row is a different model. The adjusted models are adjusted for : age, sex, BMI, systolic blood pressure, left ventricular ejection fraction <50%, number of coronary vessels with ≥50% stenosis, prior revascularization, history of MI, history of stroke, HbA1c, low density lipoprotein, high density lipoprotein and triglyceride levels, micro- and macro- albuminuria, glomerular filtration rate, current smoking status and randomization groups. All adjustment variables were measured at baseline, with the exception of BMI, which was updated each time when adipokine measures were available. The final fully adjusted model is a joint model, with all adipokines in the final model column in one model. HR reflects a one SD change in the sex standardized log value of the adipokine measure on the composite endpoint.
20 participants were excluded due to missing baseline BMI values
Effects of the adipokines on outcome were also evaluated using three different subgroups: IS vs. IP randomization groups, above or below the median BMI, and females vs. males (Supplemental Tables 1S, 2S). No interactions of the IS/IP randomization groups were detected, and the only interactions of sex were with baseline IL-6 and with baseline and change in BMI. However, several interactions were detected between BMI and the adipokines, specifically for baseline leptin, change in adiponectin, baseline leptin/BMI ratio, change in TNF- α, and baseline IL-6. An interaction was also seen between BMI and baseline CRP. Of note, in the subgroup of patients with BMI values above the median, higher baseline BMI (HR=0.4; 95% CIs: 0.2, 0.8; P=.01) and increase in BMI (HR=0.4; 95% CIs: 0.3, 0.6; P < .001) were protective (Supplementary Table 1S), which was not seen in those with BMI values below the median. Furthermore, in the subgroup of patients with BMI values above the median, higher baseline waist circumference (HR=0.7; 95% CIs: 0.5, 0.9; P=.008) and increase in waist circumference (HR=0.6; 95% CIs: 0.4, 0.9; P=.02) were protective, whereas in those with BMI values below the median, higher baseline circumference was harmful (HR=1.5; 95% CIs: 1.1, 1.9; P=.01). To further evaluate these interactions, additional analyses were performed by quintiles of BMI (Supplemental Table 3S) and quintiles of leptin/adiponectin ratio (Supplemental Table 4S).
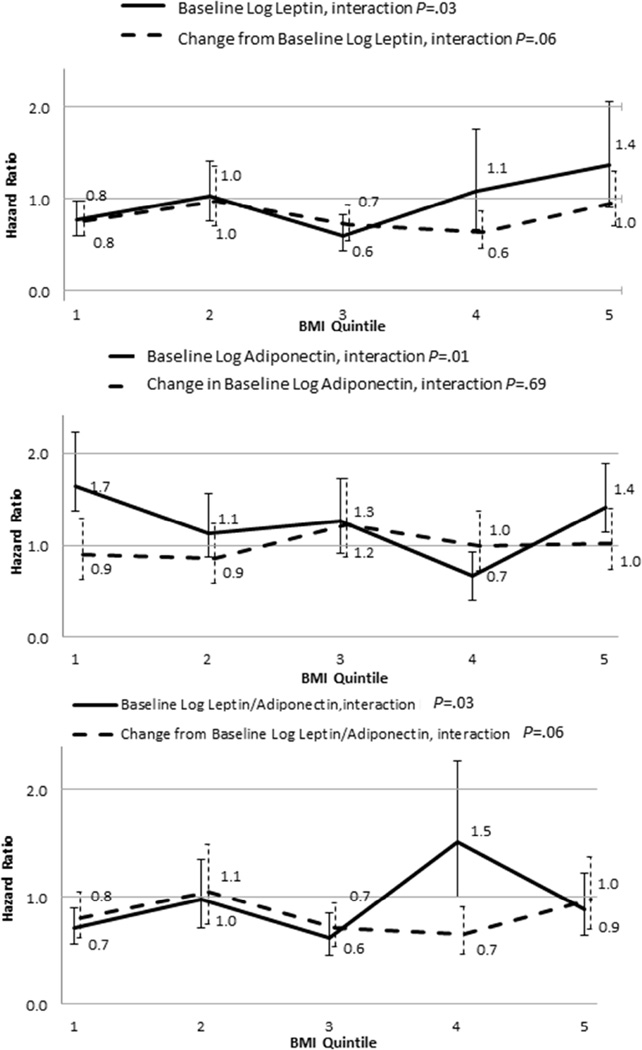
Figure 1 and Table 3S summarize the HRs (and corresponding 95% CIs) for the associations of leptin and adiponectin with outcome in the BMI quintiles. The effect of baseline leptin on CV events depended on the BMI quintile, such that the hazard ratio (HR) varied between 0.6 and 1.4 across the BMI quintiles (interaction P=.03). The same was true for baseline adiponectin (HR varied between 0.7 and 1.7, interaction P=.01), and baseline leptin/adiponectin ratio (HR varied between 0.6 and 1.5, interaction P=.03). At lower BMIs leptin was protective, while at higher BMIs it tended to be harmful. Adiponectin was harmful in the lower and higher BMI quintiles compared to the mid-range of BMIs. At lower BMIs higher leptin/adiponectin ratio was protective, but in the 32–36 BMI range it appeared harmful.
Figure 1.
Hazard Ratios (HRs) for baseline (solid line) or change from baseline (dotted line) in leptin (a), adiponectin (b) and leptin/adiponectin ratio (c) by BMI quintiles. Error bars indicate 95% confidence intervals (CIs). When the CIs do not include 1 for a given HR estimate, the effect is statistically significant.
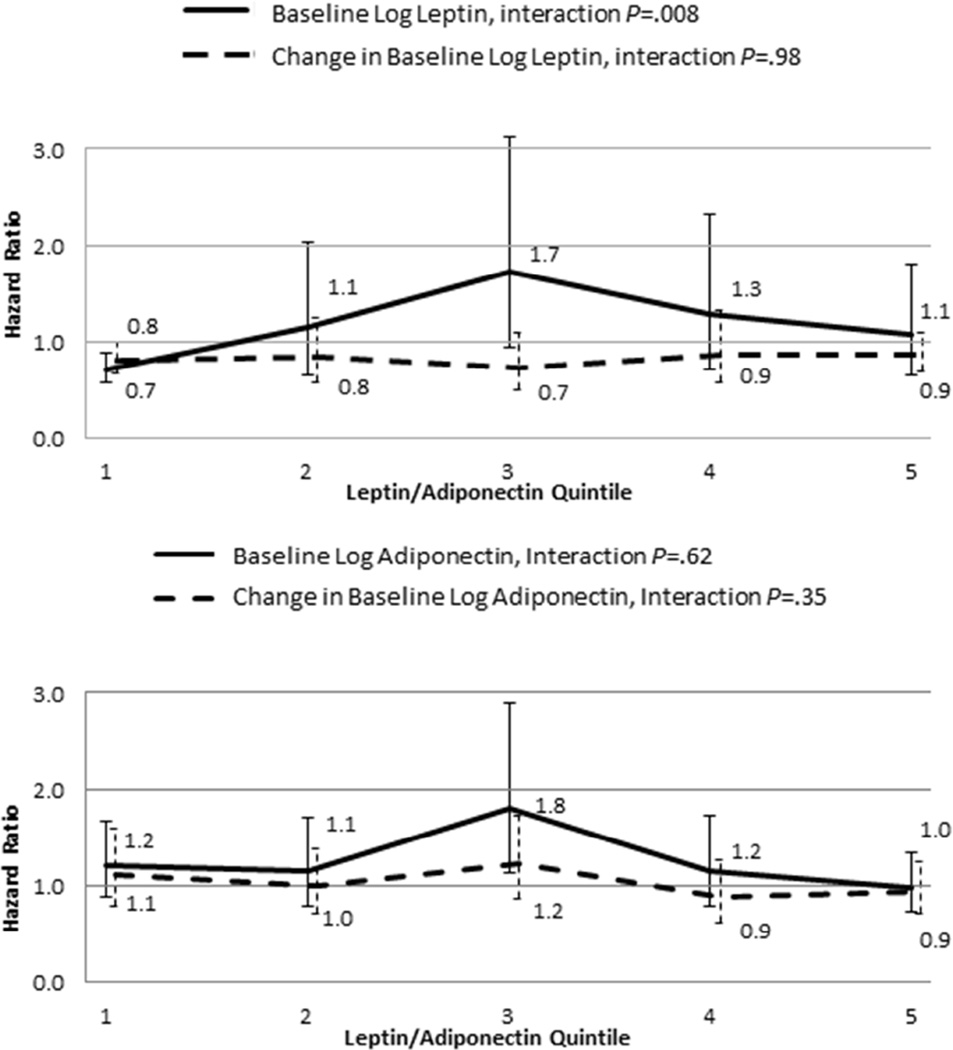
Additional insights in the interrelationship between leptin and adiponectin were obtained by evaluating the HRs for the association of leptin and adiponectin with outcome in the leptin/adiponectin ratio quintiles (Figure 2 and Table 4S). The interaction with the leptin/adiponectin quintiles was significant for baseline leptin (P=.008). Leptin was protective in the lowest leptin/adiponectin ratio quintile, but not in the higher leptin/adiponectin ratio range.
Figure 2.
Hazard Ratios (HRs) for baseline (solid line) or change from baseline (dotted line) in leptin (a) and adiponectin (b) by leptin/adiponectin ratio quintiles. Error bars indicate 95% confidence intervals (CIs). When the CIs do not include 1 for a given HR estimate, the effect is statistically significant.
With regard to other adipokines/cytokines (see Supplemental Tables 3S and 4S for HRs and 95% CIs), the effect of baseline MCP-1 on CV events varied by BMI quintile (HR range from 0.8 to 1.8, interaction P=.03). Similar interactions were observed for change from baseline TNF-α (HR varied from 0.9 to 1.4, interaction P=.02) and change from baseline CRP (HR varied from 0.7 to 1.2, interaction P=.02). Change from baseline MCP-1 was harmful in the lowest BMI quintile, while change in TNF-α was harmful except for the mid-range of BMIs (Table 3S). There were significant interactions indicating that the association between MCP-1 change from baseline varied by leptin/adiponectin quintiles (HR range from 0.8 to 1.6, interaction P=.02), which was also true for baseline CRP (HR varied from 1.1 to 2.1, interaction P=.007) and CRP change from baseline (HR varied from 1.1 to 1.6, interaction P=.03). Change in MCP-1 was harmful in the lowest leptin/adiponectin ratio quintile, while CRP and change in CRP were harmful in the lowest quintile as well as in the mid-range of the leptin/adiponectin ratios (Table 4S).
An analysis was also performed in clinically defined BMI strata (Table 3). Of note, specific adipokines/cytokines were identified whose levels were associated with outcome in normal weight or overweight patients, but not obese or very obese patients.
Table 3.
Effect of adipokines on the composite endpoint of CV death, non-procedural MI and stroke by Baseline BMI Categories.
| Baseline BMI Quintiles BMI Range Events/N in subgroup at baseline* |
Q1 | Q2 | Q3 | Q4 | |
|---|---|---|---|---|---|
| 18<=BMI<25 | 25<=BMI<30 | 30<=BMI<35 | 35<=BMI<66 | Interaction | |
| 33/219 | 124/797 | 108/735 | 97/559 | ||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | P | |
| Baseline Log leptin | 0.6 (0.4, 0.8) |
0.9 (0.7, 1.1) |
0.9 (0.7, 1.3) |
1.2 (0.9, 1.8) |
.06 |
| Change from Baseline Log leptin | 0.7 (0.5, 1.0) |
0.8 (0.6, 1.0) |
0.8 (0.6, 1.0) |
0.9 (0.7, 1.1) |
.67 |
| Baseline Log adiponectin | 1.5 (1.0, 2.4) |
1.2 (1.0, 1.6) |
0.9 (0.7, 1.2) |
1.3 (1, 1.7) |
.40 |
| Change from Baseline Log adiponectin | 1.2 (0.7, 2.2) |
0.9 (0.7, 1.1) |
1.2 (0.9, 1.5) |
1.0 (0.8, 1.3) |
.43 |
| Baseline Log MCP-1 | 1.4 (0.9, 2.2) |
1.0 (0.8, 1.2) |
0.9 (0.7, 1.1) |
0.9 (0.7, 1.1) |
.66 |
| Change from Baseline Log MCP-1 | 3.0 (1.5, 6) |
1.2 (0.9, 1.5) |
0.8 (0.6, 1.0) |
1.0 (0.8, 1.4) |
.007 |
| Baseline Log leptin/adiponectin | 0.6 (0.4, 0.9) |
0.8 (0.6, 1.0) |
1.0 (0.8, 1.4) |
0.9 (0.7, 1.2) |
.22 |
| Change from Baseline Log leptin/adiponectin | 0.7 (0.5, 1.1) |
0.9 (0.7, 1.1) |
0.7 (0.6, 0.9) |
0.9 (0.7, 1.2) |
.42 |
| Baseline Log leptin/BMI | 0.7 (0.5, 0.9) |
0.9 (0.7, 1.1) |
1.0 (0.8, 1.3) |
1.2 (0.9, 1.6) |
.14 |
| Change from Baseline Log leptin/BMI | 0.8 (0.6, 1.2) |
0.8 (0.6, 1.0) |
0.8 (0.6, 1.1) |
0.8 (0.6, 1) |
.98 |
| Baseline Log TNF-α | 1.4 (0.8, 2.6) |
1.3 (1.0, 1.6) |
1.1 (0.9, 1.4) |
1.2 (0.9, 1.5) |
.35 |
| Change from Baseline Log TNF-α | 1.3 (0.9, 1.8) |
1.2 (1, 1.5) |
1.0 (0.8, 1.2) |
1.3 (1.1, 1.5) |
.12 |
| Baseline Log CRP | 1.5 (0.9, 2.4) |
1.8 (1.4, 2.2) |
1.6 (1.2, 2) |
1.0 (0.7, 1.3) |
.03 |
| Change from Baseline Log CRP | 0.9 (0.5, 1.5) |
1.5 (1.2, 1.8) |
1.4 (1.1, 1.8) |
1.5 (1.1, 2.0) |
.47 |
| Baseline Log IL-6 | 2.1 (1.3, 3.5) |
1.4 (1.1, 1.8) |
1.1 (0.9, 1.4) |
1.2 (0.9, 1.6) |
.04 |
| Change from Baseline Log IL-6 | 1.1 (0.6, 2) |
1.4 (1.1, 1.7) |
0.9 (0.7, 1.1) |
1.2 (0.9, 1.6) |
.05 |
All models fully adjusted.
Eighteen (18) participants without events were missing BMI at baseline, and 2 participants with events were missing BMI at baseline. Thus this table represents 2310 participants and 362 events.
Discussion
The results of this study illustrate the complexity of the pathophysiology of adipokines as related to CV disease and outcome. First, we confirmed in a very large CV outcome trial that plasma levels of several adipokines are related to CV outcome, independent of a very broad range of demographic, clinical, angiographic, echocardiographic and biochemical CV risk factors previously identified to be related to CV prognosis. This further establishes the role of adipokines as novel CV risk factors. Second, the relationship of adipokines to CV outcome is non-linear and differs in various BMI strata.
The novel finding of the non-linear association between adipokine levels and CV outcome can be explained when one considers complex interactions between adipose tissue and adipokines based on multiple feedback loops. For example, leptin is secreted by adipose tissue and its levels increase in obesity. It then acts as a negative feedback mediator to suppress appetite and affect energy homeostasis in such a way as to prevent further fat gain. The finding that in obese individuals excessive adiposity coexists with high leptin levels has been interpreted as evidence for leptin resistance in obesity.34 In addition, studies in animal models suggest interactions between leptin and adiponectin.35 A recent study has shown that leptin increases adiponectin expression and therefore impaired leptin signaling may contribute to paradoxically low adiponectin levels in human obesity.36 Consequently, the leptin/adiponectin ratio may serve as an index of leptin resistance in that reduced leptin sensitivity in obesity would lead to a decrease in leptin-induced adiponectin expression and an increase in the leptin/adiponectin ratio.
In our study, at lower BMI levels (i.e., in the absence of obesity and, presumably, the absence of leptin resistance) higher leptin levels were associated with a better outcome (it is also notable that in patients with BMI values below the median, higher baseline waist circumference [a measure of central adiposity] was also protective). This may be due to several potentially beneficial effects of leptin, including coronary artery vasodilation, activation of endothelial nitric oxide production, activation of endothelial progenitor cells, decreased lipid accumulation, to mention only a few.37,38 In the same lower BMI range, higher adiponectin was associated with worse outcome. Even though several in vitro and animal studies have suggested atheroprotective effects of adiponectin, clinical studies have been inconsistent and it has been proposed that in patients with established vascular disease (such as our study population) there may be a compensatory increase in adiponectin levels, in which case elevated adiponectin may be just a disease marker.23 Furthermore, adiponectin has also been postulated to inhibit leptin signaling,39 potentially suggesting that high adiponectin levels could abrogate the beneficial effects of leptin. This explanation is consistent with the finding in our study that, at lower BMI levels, the higher leptin/adiponectin ratio is associated with better CV outcome (Figure 1).
By contrast, at higher BMI levels, the pathophysiological milieu is significantly different. Higher BMI (or obesity) is associated with leptin resistance. As discussed earlier, in the presence of obesity, the leptin/adiponectin ratio may serve as an index of leptin resistance, with the higher leptin/adiponectin ratio indicative of decreased sensitivity to leptin. In this setting, both higher leptin and higher leptin/adiponectin ratio are associated with worse CV outcome (Figure 1), as they reflect greater resistance to the beneficial effects of leptin. Indeed, as illustrated in Figure 2, leptin is protective when the leptin/adiponectin ratio is low, but not when the leptin/adiponectin ratio is high. Finally, part of the explanation could be the dose dependence of the favorable and unfavorable effects of leptin as well as the phenomenon of selective leptin resistance, whereby in the obese state some unfavorable effects of leptin (such as sympathetic excitation, platelet activation, etc.) may be selectively preserved despite resistance to some other effects.37
Our findings have several important implications. First, they provide a strong rationale for considering specific adipokine levels in optimizing CV risk stratification, especially in normal weight or overweight (but not yet obese) individuals. Second, our results begin to unravel the “obesity paradox”, namely that overweight individuals with CV disease have better outcomes than the leaner ones, before outcomes worsen again with more severe obesity. The following explanation can be offered based on our findings. Important effects of body fat on CV outcome are mediated through adipokines, such as leptin and others. A moderate increase in body fat (with the BMI in the “overweight” range) will lead to an increase in leptin levels. The latter will be associated with improved CV outcome in the absence of leptin resistance. As leptin resistance develops with progressing obesity, the beneficial effects of leptin will be eliminated and the negative CV effects of obesity will prevail. The fact that the previous clinical studies evaluating effects of adipokines on CV outcome did not take into account the BMI and leptin resistance strata may explain their inconsistent and contradictory results. It should be noted, however, that our results were obtained from a specific population of patients with DM and angiographically confirmed CAD; it remains to be evaluated whether they also apply to other populations. Additionally, physical fitness has been suggested to alter the relationship between adiposity and prognosis, in that the “obesity paradox” may be apparent mostly in patients with low fitness;7 in the BARI 2D trial, usable physical activity information was not collected.
Conclusion
Specific adipokines are independent predictors of CV outcome in patients with DM and CAD. The associations between adipokines and CV outcome vary depending on BMI. This reflects the complex pathophysiology of CV disease in obesity and may help explain the “obesity paradox”.
Supplementary Material
Acknowledgments
None
FUNDING: This work was supported by National Heart, Lung and Blood Institute and National Institute of Diabetes and Digestive and Kidney Disease [grant numbers R01HL087214, U01HL061744, U01HL061746, U01HL061748, U01HL063804, R21HL121495]; American Heart Association [11SDG7260046 to PS]; Glaxo Smith Kline; Lantheus Medical Imaging, Inc. (formerly Bristol-Myers Squibb Medical Imaging, Inc.); Astellas Pharma US, Merck & Co.; Abbott Laboratories, Pfizer; MediSense Productions, Bayer Diagnostics; Becton Dickinson and Company; JR Carlson Laboratories, Centocor, Eli Lilly and Company; LipoScience, Merck; Sante, Novartis Pharmaceuticals Corporation; NovoNordisk. The sponsors had no role in the conduct and/or preparation, collection, analysis and interpretation of the data, in writing of the report, and in the decision to submit this manuscript.
Abbreviations
- BARI 2D
Bypass Angioplasty Revascularization Investigation 2 Diabetes
- BMI
body mass index
- CAD
coronary artery disease
- CRP
C-reactive protein
- CV
cardiovascular
- DM
type 2 diabetes
- HR
hazard ratio
- IL-6
interleukin 6
- IP
insulin providing
- IS
insulin sensitizing
- MCP-1
monocyte chemoattractant protein-1
- MI
myocardial infarction
- SD
standard deviation
- TNF-α
tumor necrosis factor-alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST:
Authors report no conflict of interest in connection with this manuscript.
References
- 1.Patterson RE, Frank LL, Kristal AR, White E. A comprehensive examination of health conditions associated with obesity in older adults. Am J Prev Med. 2004;27(5):385–390. doi: 10.1016/j.amepre.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Bastien M, Poirier P, Lemieux I, Despres JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis. 2014;56(4):369–381. doi: 10.1016/j.pcad.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Cho E, Manson JE, Stampfer MJ, et al. A prospective study of obesity and risk of coronary heart disease among diabetic women. Diabetes Care. 2002;25(7):1142–1148. doi: 10.2337/diacare.25.7.1142. [DOI] [PubMed] [Google Scholar]
- 4.Wolk R, Berger P, Lennon RJ, Brilakis ES, Somers VK. Body mass index: a risk factor for unstable angina and myocardial infarction in patients with angiographically confirmed coronary artery disease. Circulation. 2003;108(18):2206–2211. doi: 10.1161/01.CIR.0000095270.85646.E8. [DOI] [PubMed] [Google Scholar]
- 5.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masters RK, Reither EN, Powers DA, Yang YC, Burger AE, Link BG. The impact of obesity on US mortality levels: the importance of age and cohort factors in population estimates. Am J Public Health. 2013;103(10):1895–1901. doi: 10.2105/AJPH.2013.301379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavie CJ, McAuley PA, Church TS, Milani RV, Blair SN. Obesity and cardiovascular diseases: implications regarding fitness, fatness, and severity in the obesity paradox. JACC. 2014;63(14):1345–1354. doi: 10.1016/j.jacc.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316(2):129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Van de Voorde J, Pauwels B, Boydens C, Decaluwe K. Adipocytokines in relation to cardiovascular disease. Metabolism. 2013;62(11):1513–1521. doi: 10.1016/j.metabol.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. J Cardiol. 2014;63(4):250–259. doi: 10.1016/j.jjcc.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace AM, McMahon AD, Packard CJ, et al. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS) Circulation. 2001;104(25):3052–3056. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- 12.Wolk R, Berger P, Lennon RJ, Brilakis ES, Johnson BD, Somers VK. Plasma leptin and prognosis in patients with established coronary atherosclerosis. JACC. 2004;44(9):1819–1824. doi: 10.1016/j.jacc.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 13.Romero-Corral A, Sierra-Johnson J, Lopez-Jimenez F, et al. Relationships between leptin and C-reactive protein with cardiovascular disease in the adult general population. Nature clinical practice Cardiovascular medicine. 2008;5(7):418–425. doi: 10.1038/ncpcardio1218. [DOI] [PubMed] [Google Scholar]
- 14.Soderberg S, Colquhoun D, Keech A, et al. Leptin, but not adiponectin, is a predictor of recurrent cardiovascular events in men: results from the LIPID study. Int J Obes. 2009;33(1):123–130. doi: 10.1038/ijo.2008.224. [DOI] [PubMed] [Google Scholar]
- 15.Piemonti L, Calori G, Mercalli A, et al. Fasting plasma leptin, tumor necrosis factor-alpha receptor 2, and monocyte chemoattracting protein 1 concentration in a population of glucose-tolerant and glucose-intolerant women: impact on cardiovascular mortality. Diabetes Care. 2003;26(10):2883–2889. doi: 10.2337/diacare.26.10.2883. [DOI] [PubMed] [Google Scholar]
- 16.Ku IA, Farzaneh-Far R, Vittinghoff E, Zhang MH, Na B, Whooley MA. Association of low leptin with cardiovascular events and mortality in patients with stable coronary artery disease: the Heart and Soul Study. Atherosclerosis. 2011;217(2):503–508. doi: 10.1016/j.atherosclerosis.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhee CM, Nguyen DV, Moradi H, et al. Association of Adiponectin With Body Composition and Mortality in Hemodialysis Patients. Am J Kidney Dis. 2015;66(2):313–321. doi: 10.1053/j.ajkd.2015.02.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumada M, Kihara S, Sumitsuji S, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23(1):85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 19.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291(14):1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 20.Wolk R, Berger P, Lennon RJ, Brilakis ES, Davison DE, Somers VK. Association between plasma adiponectin levels and unstable coronary syndromes. Eur Heart J. 2007;28(3):292–298. doi: 10.1093/eurheartj/ehl361. [DOI] [PubMed] [Google Scholar]
- 21.Ai M, Otokozawa S, Asztalos BF, et al. Adiponectin: an independent risk factor for coronary heart disease in men in the Framingham offspring Study. Atherosclerosis. 2011;217(2):543–548. doi: 10.1016/j.atherosclerosis.2011.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Mo X, Hao Y, et al. Adiponectin levels and risk of coronary heart disease: a meta-analysis of prospective studies. Am J Med Sci. 2013;345(6):455–461. doi: 10.1097/MAJ.0b013e318262dbef. [DOI] [PubMed] [Google Scholar]
- 23.Cavusoglu E, Ruwende C, Chopra V, et al. Adiponectin is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction in patients presenting with chest pain. Eur Heart J. 2006;27(19):2300–2309. doi: 10.1093/eurheartj/ehl153. [DOI] [PubMed] [Google Scholar]
- 24.Pilz S, Mangge H, Wellnitz B, et al. Adiponectin and mortality in patients undergoing coronary angiography. J Clin Endocrinol Metab. 2006;91(11):4277–4286. doi: 10.1210/jc.2006-0836. [DOI] [PubMed] [Google Scholar]
- 25.Wannamethee SG, Whincup PH, Lennon L, Sattar N. Circulating adiponectin levels and mortality in elderly men with and without cardiovascular disease and heart failure. Arch Intern Med. 2007;167(14):1510–1517. doi: 10.1001/archinte.167.14.1510. [DOI] [PubMed] [Google Scholar]
- 26.Dekker JM, Funahashi T, Nijpels G, et al. Prognostic value of adiponectin for cardiovascular disease and mortality. J Clin Endocrinol Metab. 2008;93(4):1489–1496. doi: 10.1210/jc.2007-1436. [DOI] [PubMed] [Google Scholar]
- 27.Hascoet S, Elbaz M, Bongard V, et al. Adiponectin and long-term mortality in coronary artery disease participants and controls. Arterioscler Thromb Vasc Biol. 2013;33(1):e19–e29. doi: 10.1161/ATVBAHA.112.300079. [DOI] [PubMed] [Google Scholar]
- 28.Wu ZJ, Cheng YJ, Gu WJ, Aung LH. Adiponectin is associated with increased mortality in patients with already established cardiovascular disease: a systematic review and meta-analysis. Metabolism. 2014;63(9):1157–1166. doi: 10.1016/j.metabol.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Poehls J, Wassel CL, Harris TB, et al. Association of adiponectin with mortality in older adults: the Health, Aging, and Body Composition Study. Diabetologia. 2009;52(4):591–595. doi: 10.1007/s00125-009-1261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sattar N, Wannamethee G, Sarwar N, et al. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation. 2006;114(7):623–629. doi: 10.1161/CIRCULATIONAHA.106.618918. [DOI] [PubMed] [Google Scholar]
- 31.Sattar N, Wannamethee G, Sarwar N, et al. Leptin and coronary heart disease: prospective study and systematic review. JACC. 2009;53(2):167–175. doi: 10.1016/j.jacc.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 32.Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360(24):2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolk R, Bertolet M, Brooks MM, et al. Differential effects of insulin sensitization and insulin provision treatment strategies on concentrations of circulating adipokines in patients with diabetes and coronary artery disease in the BARI 2D trial. Eur J Prev Cardiol. 2014 doi: 10.1177/2047487314544046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 35.Lindstrom P. The physiology of obese-hyperglycemic mice [ob/ob mice] TheScientificWorldJournal. 2007:7666–7685. doi: 10.1100/tsw.2007.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh P, Sharma P, Sahakyan KR, et al. Differential effects of leptin on adiponectin expression with weight gain versus obesity. Int J Obes. 2015 doi: 10.1038/ijo.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolk R, Somers VK. Leptin and vascular function: Friend or foe? Eur Heart J. 2006;27(19):2263–2265. doi: 10.1093/eurheartj/ehl246. [DOI] [PubMed] [Google Scholar]
- 38.Wolk R, Deb A, Caplice NM, Somers VK. Leptin receptor and functional effects of leptin in human endothelial progenitor cells. Atherosclerosis. 2005;183(1):131–139. doi: 10.1016/j.atherosclerosis.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Z, Wang F, Wang BJ, et al. Inhibition of leptin-induced vascular extracellular matrix remodelling by adiponectin. J Mol Endocrinol. 2014;53(2):145–154. doi: 10.1530/JME-14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.