Abstract
Rationale
Chronic nicotine infusion via transdermal patches has been widely shown to assist with smoking cessation. In particular, transdermal nicotine treatment prior to quitting smoking helps reduce ad libitum smoking and aids cessation (Rose et al. 2009). However, despite this success, the majority of smokers who use transdermal nicotine fail to permanently quit smoking. Additional treatments are needed. Tobacco addiction does not just depend on nicotinic receptor systems; a variety of neural systems are involved, including dopamine, norepinepherine, serotonin, histamine.
Objectives
Given the involvement of a variety of neural systems in the circuits of addiction, combination therapy may offer improved efficacy for successful smoking cessation beyond single treatments alone. We have found that pyrilamine, an H1 histamine antagonist, significantly decreases nicotine self-administration in rats.
Methods
The current study was conducted to confirm the effect of chronic nicotine infusion on ongoing nicotine self-administration and resumed access after enforced abstinence and to determine the interaction of chronic nicotine with an H1 antagonist treatment.
Results
Chronic nicotine infusion via osmotic minipump (2.5 and 5 mg/kg/day for 28 days) significantly reduced nicotine self-administration in a dose-dependent manner. Chronic nicotine infusion also reduced resumption nicotine self-administration after enforced abstinence. Chronic pyrilamine infusion (25 mg/kg/day for 14 days) also significantly reduced nicotine self-administration.
Conclusion
The combination of chronic nicotine and pyrilamine reduced nicotine self-administration to a greater extent than treatment with either drug alone.
Keywords: Nicotine, Pyrilamine, Histamine, H1, Self-administration
Introduction
Tobacco addiction is notoriously tenacious with people addicted to tobacco having a very low success rate of permanently quitting. A few medications such as nicotine replacement (transdermal patch, gum or other routes), varenicline, and bupropion have been shown to improve the success rate among smokers who want to quit, but success is still quite low (Cahill et al. 2011; Jorenby et al. 2006; Rovina et al. 2009; Stead et al. 2012). The neural systems underlying addiction are complex and include a variety of transmitter systems (for review, see Koob and Volkow 2010). Pharmacological treatment for tobacco addiction should reflect this reality. New treatment strategies for tobacco cessation are expanding beyond the sole focus on nicotinic cholinergic systems with a variety of treatments affecting dopaminergic, noradrenergic, serotonergic, glutamatergic, GABAergic and histaminergic systems as well as others (Levin et al. 2011a; Xi et al. 2009).
Given the variety of neural systems involved in tobacco addiction and the variety of promising treatments, it may be the case that combined treatments could have greater efficacy than any one treatment alone. Precedents for combination therapy include the superiority of combination NRT (e.g., patch plus lozenge), NRT plus bupropion and NRT plus varenicline (Croghan et al. 2007; Ebbert et al. 2014; Koegelenberg et al. 2014a; Piper et al. 2009) relative to monotherapy. In the current study, it is hypothesized that nicotine replacement therapy combined with chronic antihistamine treatment might improve reduction in nicotine self-administration. The recent innovative strategy of combining pharmacological treatments targeting different neurotransmitter systems has garnered increased attention at the clinical level for smoking cessation programs. It is thought that combining treatments known to reduce smoking rates on their own would produce additive effects on the behavior. Indeed, recent evidence has validated this approach in both humans and in animal models. Human studies have demonstrated that combination therapy with smoking cessation aids currently on the market result in improved outcomes for abstinence rates when compared to monotherapy (Ebbert et al. 2009; Koegelenberg et al. 2014b; Rose and Behm 2013; 2014). Our studies have validated this treatment strategy in the rat model with two FDA-approved drugs for smoking cessation. We have recently shown that combination treatment with varenicline and bupropion results in a greater reduction in nicotine self-administration in rats compared to vehicle treatment than treatment with either drug alone (Hall et al. 2015). Given these previous findings, it is clear that combination therapy targeting different neurotransmitter systems relevant to tobacco addiction remains a promising avenue for development.
Our research has previously shown that systemic administration of pyrilamine, an histamine H1 receptor antagonist, reduces nicotine self-administration in rats (Levin et al. 2011c); (Cousins et al. 2014). The rationale for targeting H1 receptors arose from the finding that the antipsychotic drug clozapine reduces smoking in schizophrenic patients (McEvoy et al. 1995). Clozapine is a multifaceted drug with substantial H1 action (Schotte et al. 1993). There is also recent evidence demonstrating that pyrilamine blocks nicotine effects promoting catecholamine release (Kim et al. 2014). H1 receptors appear to play a role in cholinergic activity with regard to spatial cognition (Chen et al. 2001). Nicotine itself has even been shown to act as a weak competitive antagonist at H1 receptors (Ercan and Turker 1985). Combining nicotinic with pyrilamine may offer an alternative strategy for smoking cessation treatment.
The current study was conducted to determine how nicotine replacement therapy (NRT) combined with chronic antihistamine treatment would affect nicotine self-administration in rats. We hypothesized that combining these treatments would result in a more efficacious reduction in nicotine self-administration than with either treatment alone. Combinations of effective treatments might provide mutually augmenting effects in aiding smoking cessation.
Methods
Subjects
Young adult female Sprague-Dawley rats (Charles River Laboratories, Raleigh, NC, USA) were use for the current studies. The rats started training at eight weeks of age and finished testing at 14 weeks of age. The rats were singly housed and kept on a reverse 12:12 hr day/night cycle (lights off from 7:00 a.m. till 7:00 p.m.),. Rats were fed daily after behavioral testing to maintain a lean health weight adjusted for growth, and were given ad lib access to water. The rats were housed and cared for in conditions in accordance with university, state, and federal regulations.
Behavioral Training
Prior to jugular catheterization surgery and nicotine (Sigma-Aldrich, Inc., St. Louis MO, USA) self-administration sessions, rats were trained to lever-press for self-administration of food reinforcement with approximately twelve hours of food restriction. The operant conditioning chambers had one active and one inactive lever. The active lever was vertically paired with a cue light, which illuminated when a food pellet was available. Correct lever pressing caused a food pellet to be delivered, a 0.5s feedback tone was also sounded, and the light went out until another food pellet was available by lever press. The inactive lever did not have an illuminated cue light, and had no effect when pressed. Counts of both lever presses were scored. Prior to food self-administration sessions, rats were placed in chambers for one overnight session, during which the rats were periodically delivered food pellets paired with illumination or darkening of the cue light until the rat learned to associate the illuminated lever with food pellet delivery. A rat passed an overnight session when it successfully pressed the active lever 100 times. Following overnight sessions, the rat must self-administer at least 50 food pellets during three 30-min pellet sessions to proceed to the nicotine self-administration phase of training.
During self-administration sessions, nicotine was administered to rats via catheter tubing implanted into the jugular vein. Jugular vein catheterization surgery was performed in a sterile, aseptic environment. For surgery, a general anesthesia mix of dexmedetomidine (0.15 mg/kg, i.p.) and ketamine (60 mg/kg i.p.) was delivered. A catheter was placed in the jugular vein. The catheter was flushed with heparin in sterile saline and the antibiotic gentamicin to prevent coagulation and infection following surgery. Solutions of 0.03-mg/kg nicotine ditartrate (expressed as nicotine base) were was dissolved in sterile saline and adjusted to a standard pH between 7.0 and 7.2. and passed through a 0.22 μ filter to ensure sterilization. The rats transitioned from self-administration of food pellets to nicotine by a similar mechanism. The same delivery chamber was used, and the lever that previously administered a food pellet when pressed now delivered a 0.03-mg/kg/infusion dose of nicotine solution. As before, the opposing lever had no effect. Following each lever press and nicotine delivery, the cue light turned off for one minute, the house light illuminated, and the lever was inactivated until the cue light illuminated again. Prior to the start of the drug treatment studies, the rats were given five baseline training nicotine self-administration sessions. Before sessions, catheters were flushed with 0.3-ml of a 100 units/ml heparinized saline solution. Sessions lasted for 45-min, and responses were measured using MED-PC software (Med Associated, Georgia, VT, USA). Following sessions, nicotine was drawn out of the delivery port and replaced with 0.3-ml of a saline solution containing 8-mg/ml of the antibiotic gentamicin and 500-units/ml of heparin.
Drug Treatments
Nicotine ditartrate (Sigma-Aldrich, Inc., St. Louis MO, USA) was chronically infused for four weeks via osmotic minipump implanted subcutaneously. The four-week nicotine infusion was accomplished with the Alzet 2ML4 pump and the two-week pyrilamine infusion was accomplished by the Alzet 2ML2 pump (Durect, Inc, Cupertino, CA, USA). In the first study, nicotine was infused at doses of 0, 2.5 and 5 mg/kg/day with group sizes of N=10, 11, and 11 rats respectively. The 2.5 and 5 mg/kg/day doses of nicotine were selected because this dose range in the rat provides pharmacokinetically and dynamically equivalent doses as moderate smoking in humans (for review see (Matta et al. 2007)). In the second study with separate rats, two minipumps were implanted. As shown in the timeline below, pyrilamine was infused for two weeks with a 2ML2 pump at a dose of 25 mg/kg/day and nicotine was infused for four weeks with a 2ML4 pump at a dose of 2.5 mg/kg/day. There were four treatment groups: placebo control (N=8), pyrilamine only (N=10), nicotine only (N=11) and the combination of nicotine and pyrilamine (N=10). The drug doses were measured as the base weight.
| Nicotine-Pynlamine Study Sequence | |||||
| Pellet Training | Nicotine SA Training | Nicotine SA | Enforced Abstinence | Resumed SA Access | |
|
| |||||
| Week 1 | Week 2 | ||||
| Nicotine-------------------------------------------------------- | |||||
| Pyrilamine------------------ | |||||
|
| |||||
Data Analysis
The effects of chronic nicotine and pyrilamine infusions on the dependent measure of nicotine infusions taken per session were assessed by analysis of variance for between subjects factors, which were nicotine and pyrilamine dose and repeated measures of daily test sessions or average weekly response. An alpha level of p<0.05 was the threshold for significance. Post hos Dunnett's tests (2-tailed) were used to compare nicotine and pyrylamine doses to control and control alpha level for multiple comprisons.
Results
Experiment 1: Chronic Nicotine Dose-Effect
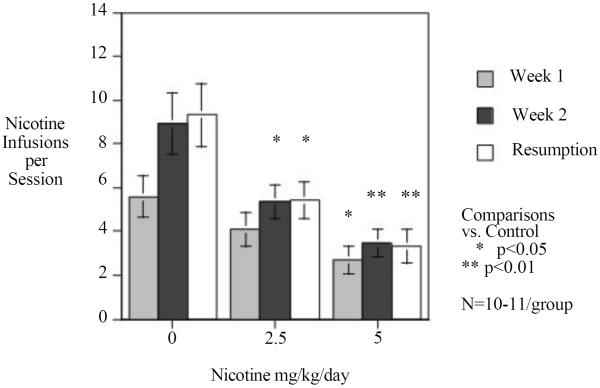
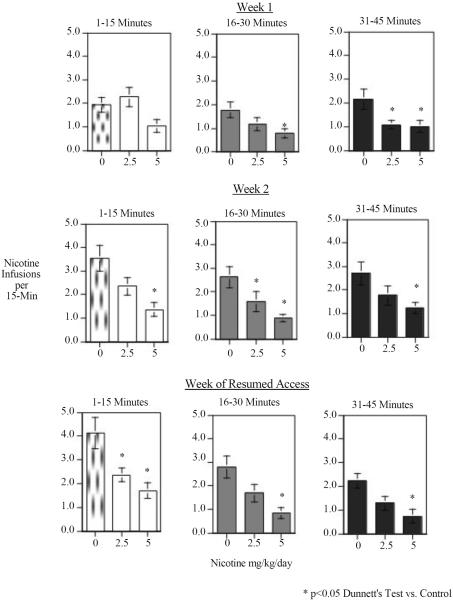
As shown in figure 1 chronic nicotine infusion significantly reduced nicotine self-administration relative to the control group that was implanted with minipumps delivering the saline vehicle. There was a significant min effect of nicotine (F(2,29)=8.68, p<0.005). Dunnett's tests showed that the higher 5 mg/kg/day nicotine treatment significantly decreased rates of IV nicotine self-administration (p<0.01) and the lower 2.5 mg/kg/day dose significantly (p<0.05) reduced it as well. There was a significant three-way interaction of nicotine treatment × week of testing × 15-min block within each session (F(8,116)=7.60, p<0.025). Tests of the simple main effects showed that there were significant effects of nicotine at all of the 15-minute time blocks during each of the weeks of testing (F(2,29) Week 1 Block 1=3.82, p<0.05, Week 1 Block 2=3.36, p<0.05, Week 1 Block 3=4.61, p<0.025, Week 2, Block 1=6.97, p<0.01, Week 2, Block 2=5.57, p<0.01, Week 2, Block 3=3.77, p<0.05, resumption, Block 1=7.99, p<0.005, Resumption, Block 2=7.21, p<0.005, Resumption, Block 3= 6.16, p<0.01) The effect of chronic nicotine during the first week of administration was seen most prominently during the later parts of the test session (Fig. 2). During week 2 and during the resumption period, chronic nicotine self-administration via minipump reduced nicotine self-administration during the early part of the test session (Fig. 2).
Figure 1.
Chronic continuous nicotine infusion (4 weeks at 0, 2.5 or 5 mg/kg/day, sc. N=10, 11 and 11 respectively) effects on IV nicotine self-administration, weekly mean response (mean±sem).
Figure 2.
Chronic continuous nicotine infusion (4 weeks at 2.5 or 5 mg/kg/day, sc) effects on IV nicotine self-administration, 15-min blocks within each session weekly mean response (mean±sem).
Experiment 2: Chronic Nicotine-Pyrilamine Interactions
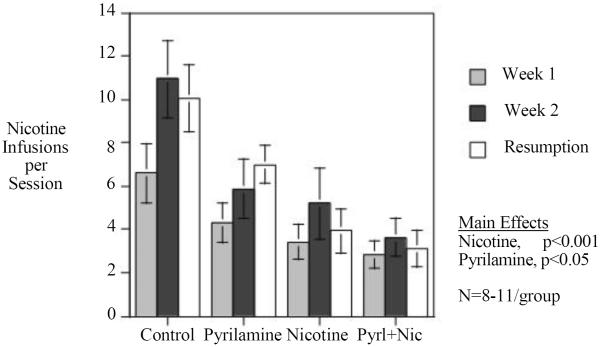
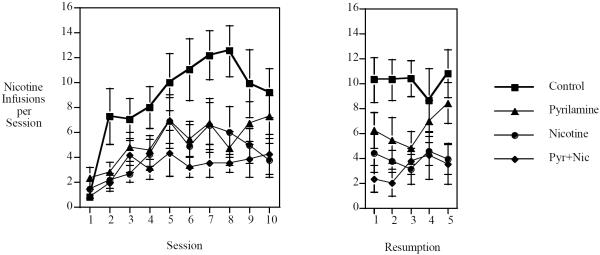
This study replicated the finding that chronic SC nicotine infusion significantly reduced IV nicotine self-administration (Fig. 3). In addition, our previous finding that the H1 antagonist pyrilamine reduced nicotine self-administration (Levin et al. 2011d) was replicated by the finding of a significant reduction during the two weeks of chronic pyrilamine infusion (25 mg/kg/day for 14 days). The main effects of Nicotine (F(1,34)=14.69, p<0.001) and Pyrilamine (F(1,34)=5.12, p<0.05) were both significant. The combined treatment with nicotine and pyrilamine resulted in the lowest level of nicotine self-administration as shown in the figures of weekly and daily nicotine self-administration (Figs. 3 and 4).
Figure 3.
Interaction of chronic continuous nicotine infusion (4 weeks at 2.5 mg/kg/day, sc) and chronic continuous pyrilamine infusion (2 weeks at 25 mg/kg/day, sc) weekly mean response (mean±sem). Control N=8, Nicotine only N=11, Pyrilamine only N=10, Nicotine+Pyrilamine N=10
Figure 4.
Interaction of chronic continuous nicotine infusion (4 weeks at 2.5 mg/kg/day, sc) and chronic continuous pyrilamine infusion (2 weeks at 25 mg/kg/day, sc) daily response (mean±sem).
Pyrilamine was shown to have a preferential effects on active lever responding, causing a significant decrease (p<0.05) in correct side lever press (no pyrilamine = 14.2±3.0 and pyrilamine = 7.8±1.2) whereas only a trend toward an effect was seen (p=0.29) with incorrect side lever press (no pyrilamine=8.0±2.1 and pyrilamine= 6.1±1.4).
Discussion
The results of the current study demonstrate that combining chronic nicotine and pyrilamine treatment reduces nicotine self-administration in rats. The combination of nicotine and pyrilamine also resulted in a reduced resumption of self-administration activity after a week of enforced abstinence from nicotine. This study served as a replication of previous studies that have shown that chronic nicotine infusion (LeSage et al. 2002) and chronic pyrilamine infusion (Levin et al. 2011c) in rats seperately cause significant reductions in nicotine self-administration. The current study shows that these two treatments have mutually augmenting effects. Chronic nicotine infusion via sc implanted osmotic minipumps is functionally similar to the zero order kinetic of steady nicotine infusion achieved by nicotine skin patches. As with nicotine skin patches in smokers, chronic nicotine sc infusions with osmotic minipumps significantly reduced nicotine self-administration. The higher nicotine infusion dose (5 mg/kg/day) significantly reduced nicotine self-administration from the first week of treatment and continued for the second week with an even stronger effect, preventing the rise in self-administration seen in controls during the second week of the treatment phase of the study. Importantly, the efficacy of 5 mg/kg/day of nicotine infusion in significantly suppressing nicotine self-administration continued during the resumed access period after a week of enforced abstinence, which modeled efficacy against relapse. The lower nicotine infusion dose of 2.5 mg/kg/day had a more modest effect, but did provide protection against the rise in nicotine self-administration seen in controls during the second week of treatment, and like the higher nicotine dose, continued to cause a significant reduction in nicotine self-administration during the resumed access period after a week-long enforced abstinence period. During the first week of treatment the most reliable effects of chronic nicotine infusion was during the final third of the test session. During the second week and the resumed access period, the chronic nicotine showed expanded effectiveness to include all parts of the test session.
With the use of female rats the question of the potential role of estrus phase on nicotine self-administration arises. This factor is likely not a factor in the interpretation of the current study. First of all the study took place over several weeks during which all the rats in all the groups went through all the phases of the four-day rat estrus cycle several times. The weekly averages included data from all phases of the cycle. Analysis on this time scale showed the significant drug treatment results as reported. Finally, several studies have directly examined the potential relationship between rat estrus cycle and nicotine self-administration and have not detected a relationship (Donny et al. 2000; Levin et al. 2011b; Rezvani et al. 2008).
In the study of the combination of chronic nicotine and chronic pyrilamine, both treatments individually caused significant reductions in nicotine self-administration, and together had additive effects. The main effects of both treatments showed significant reductions in nicotine self-administration with no interaction indicating simple additivity rather than sub-or supra-additive effects. This replicated the finding of the first study in this series as well as our previous finding that chronic pyrilamine infusion significantly reduces nicotine self-administration Combined treatment with both drugs also prevented significant elevations in nicotine self-administration during the second week of testing sessions as well as resumption after a week of enforced abstinence; self-administration rates for these phases of the study remained similar to those during the first week of testing. While there appeared to be greater increases in nicotine self-administration during the second week of testing for rats treated with nicotine or pyrilamine alone than with combined treatment, the results for combined treatment were not found to rise.
Comparisons of the effects of all four drug treatment groups would suggest that chronic nicotine treatment is the primary driver of the reduction in nicotine self-administration, at least at the doses used; pyrilamine augmented this effect in what would appear to be an additive fashion. This outcome was predicted because the animals were already receiving nicotine before they self-administered additional nicotine, which would be expected to attenuate the reinforcing effects of the additional nicotine dose. Interactions of pyrilamine with the system may continue to suppress the effect of self-administered nicotine. From a human treatment standpoint, these results demonstrate that supplementing nicotine therapy with additional treatment options should result in better outcomes. Indeed, this has been shown in previous studies augmenting nicotine therapy with bupropion (Jorenby et al. 1999; Rose and Behm 2013) and varenicline (Koegelenberg et al. 2014b). However, given the high degree of variability regarding the efficacy for bupropion and varenicline in the human population, a broader range of options should be available to individuals who may not respond to these treatments.
The mechanism by which pyrilamine lowers nicotine self-administration may involve pharmacokinetic or pharmacodynamic actions. Pyrilamine has been shown to slow nicotine transport across the blood brain barrier (Tega et al. 2013). Pharmacodynamic effects include non-specific sedative effects which would lower all motor activity. Higher dose pyrilamine infusion (50 mg/kg/day) was shown in an earlier study to cause significant reduction in food self-administration (Levin et al. 2011c). Because of this, a lower dose of 25 mg/kg/day was chosen for this study. The potential impact of non-specific sedative effects of pyrilamine on operant responding was assessed by analysis of correct side and incorrect side lever responding. The pyrilamine caused a significant decrease in correct side responding with no significant effect on incorrect side responding. The H1 histamine antagonist pyrilamine used in this study has been found to inhibit dopamine release when infused into the nucleus accumbens (Galosi et al. 2001), an effect that may be key in reducing nicotine self-administration.
In conclusion, the results of this study confirm that chronic, combined treatment with nicotine and pyrilamine significantly reduces nicotine self-administration in the rat model. Our results also demonstrate proof of concept that combination treatment, which augments nicotine therapy may provide a more efficacious avenue for smoking cessation programs. Further research should be directed towards targeting the histaminergic system, which has long been known to have a modulatory role in reward processes in the brain (Cohn et al. 1973; Frisch et al. 1998; Zimmermann et al. 1999). Indeed, many previous studies have shown a role for this system in the reinforcing properties of other drugs of abuse including cocaine, amphetamine, and alcohol (for review, see Brabant et al. 2010). The histaminergic system offers a possible alternative pathway for the development of new smoking cessation aids for individuals for whom varenicline and bupropion are ineffective. The relative safety of selective histaminergic agents should render them appropriate candidates for combination treatment with which to augment nicotine therapy.
Acknowledgements
This research was supported by P50 grant DA027840 from NIDA.
References
- Brabant C, Alleva L, Quertemont E, Tirelli E. Involvement of the brain histaminergic system in addiction and addiction-related behaviors: a comprehensive review with emphasis on the potential therapeutic use of histaminergic compounds in drug dependence. Prog Neurobiol. 2010;92:421–41. doi: 10.1016/j.pneurobio.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2011:CD006103. doi: 10.1002/14651858.CD006103.pub2. [DOI] [PubMed] [Google Scholar]
- Chen Z, Chen JQ, Kamei C. Effect of H1-antagonists on spatial memory deficit evaluated by 8-arm radial maze in rats. Acta Pharmacol Sin. 2001;22:609–13. [PubMed] [Google Scholar]
- Cohn CK, Ball GG, Hirsch J. Histamine: effect on self-stimulation. Science. 1973;180:757–8. doi: 10.1126/science.180.4087.757. [DOI] [PubMed] [Google Scholar]
- Cousins V, Rose JE, Levin ED. IV nicotine self-administration in rats using a consummatory operant licking response: sensitivity to serotonergic, glutaminergic and histaminergic drugs. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:200–5. doi: 10.1016/j.pnpbp.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croghan IT, Hurt RD, Dakhil SR, Croghan GA, Sloan JA, Novotny PJ, Rowland KM, Bernath A, Loots ML, Le-Lindqwister NA, Tschetter LK, Garneau SC, Flynn KA, Ebbert LP, Wender DB, Loprinzi CL. Randomized comparison of a nicotine inhaler and bupropion for smoking cessation and relapse prevention. Mayo Clinic Proceedings. 2007;82:186–195. doi: 10.4065/82.2.186. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology. 2000;151:392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Burke MV, Hays JT, Hurt RD. Combination treatment with varenicline and nicotine replacement therapy. Nicotine Tob Res. 2009;11:572–6. doi: 10.1093/ntr/ntp042. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Hatsukami DK, Croghan IT, Schroeder DR, Allen SS, Hays JT, Hurt RD. Combination Varenicline and Bupropion SR for Tobacco-Dependence Treatment in Cigarette Smokers: A Randomized Trial. Journal of the American Medical Association. 2014;311:155–163. doi: 10.1001/jama.2013.283185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan ZS, Turker RK. Nicotine is a competitive antagonist for H1-receptors in smooth muscles. Arch Int Pharmacodyn Ther. 1985;274:24–30. [PubMed] [Google Scholar]
- Frisch C, Hasenohrl RU, Haas HL, Weiler HT, Steinbusch HW, Huston JP. Facilitation of learning after lesions of the tuberomammillary nucleus region in adult and aged rats. Exp Brain Res. 1998;118:447–56. doi: 10.1007/s002210050301. [DOI] [PubMed] [Google Scholar]
- Galosi R, Lenard L, Knoche A, Haas H, Huston JP, Schwarting RK. Dopaminergic effects of histamine administration in the nucleus accumbens and the impact of H1-receptor blockade. Neuropharmacology. 2001;40:624–633. doi: 10.1016/s0028-3908(00)00181-7. [DOI] [PubMed] [Google Scholar]
- Hall BJ, Slade S, Wells C, Rose JE, Levin ED. Bupropion-varenicline interactions and nicotine self-administration behavior in rats. Pharmacol Biochem Behav. 2015;130:84–9. doi: 10.1016/j.pbb.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR, Varenicline Phase 3 Study G Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, Fiore MC, Baker TB. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med. 1999;340:685–91. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Kim DC, Yun SJ, Park YS, Jun DJ, Kim D, Jiten Singh N, Kim S, Kim KT. Pyrilamine inhibits nicotine-induced catecholamine secretion. Neurochem Int. 2014;74:42–5. doi: 10.1016/j.neuint.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Koegelenberg CF, Noor F, Bateman ED, van Zyl-Smit RN, Bruning A, O'Brien JA, Smith C, Abdool-Gaffar MS, Emanuel S, Esterhuizen TM, Irusen EM. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: A randomized clinical trial. Journal of the American Medical Association. 2014a;312:155–161. doi: 10.1001/jama.2014.7195. [DOI] [PubMed] [Google Scholar]
- Koegelenberg CF, Noor F, Bateman ED, van Zyl-Smit RN, Bruning A, O'Brien JA, Smith C, Abdool-Gaffar MS, Emanuel S, Esterhuizen TM, Irusen EM. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: a randomized clinical trial. JAMA. 2014b;312:155–61. doi: 10.1001/jama.2014.7195. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Shoeman D, Raphael D, Collins G, Pentel PR. Continuous nicotine infusion reduces nicotine self-administration in rats with 23-h/day access to nicotine. Pharmacol Biochem Behav. 2002;72:279–89. doi: 10.1016/s0091-3057(01)00775-4. [DOI] [PubMed] [Google Scholar]
- Levin ED, Johnson JE, Slade S, Wells C, Cauley M, Petro A, Rose JE. Lorcaserin, a 5-HT2C agonist, decreases nicotine self-administration in female rats. J Pharmacol Exp Ther. 2011a;338:890–6. doi: 10.1124/jpet.111.183525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Slade S, Wells C, Cauley M, Petro A, Vendittelli A, Johnson M, Williams P, Horton K, Rezvan AH. Threshold of adulthood for the onset of nicotine self-administration in male and female rats. Behavioural Brain Research. 2011b;225:473–481. doi: 10.1016/j.bbr.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Slade S, Wells C, Pruitt M, Cousins V, Cauley M, Petro A, Hampton D, Rose J. Histamine H(1) antagonist treatment with pyrilamine reduces nicotine self-administration in rats. Eur J Pharmacol. 2011c;650:256–60. doi: 10.1016/j.ejphar.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Levin ED, Slade S, Wells C, Pruitt M, Cousins V, Cauley M, Petro A, Hampton D, Rose JE. Histamine H1 antagonist treatment with pyrilamine reduces nicotine self-administration in rats. European Journal of Pharmacology. 2011d;650:256–260. doi: 10.1016/j.ejphar.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Corrigall WA, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines for nicotine dose selection for in vivo research. Psychopharmacology. 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- McEvoy J, Freudenreich O, McGee M, VanderZwaag C, Levin E, Rose J. Clozapine decreases smoking in patients with chronic schizophrenia. Biol Psychiatry. 1995;37:550–2. doi: 10.1016/0006-3223(94)00365-A. [DOI] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fiore M, PhD, Jorenby DE, Fraser D, Baker TB. A Randomized Placebo-Controlled Clinical Trial of 5 Smoking Cessation Pharmacotherapies. Archives of General Psychiatry. 2009;66:1253–1262. doi: 10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Eddins D, Slade S, Hampton DS, Christopher NC, Petro A, Horton K, Johnson M, Levin ED. Neonatal 6-hydroxydopmine lesions of the frontal cortex in rats: Persisting effects on locomotor activity, learning and nicotine self-administration. Neuroscience. 2008;154:885–897. doi: 10.1016/j.neuroscience.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Adapting smoking cessation treatment according to initial response to precessation nicotine patch. Am J Psychiatry. 2013;170:860–7. doi: 10.1176/appi.ajp.2013.12070919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Combination Treatment With Varenicline and Bupropion in an Adaptive Smoking Cessation Paradigm. Am J Psychiatry. 2014 doi: 10.1176/appi.ajp.2014.13050595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Herskovic JE, Behm FM, Westman EC. Precessation treatment with nicotine patch significantly increases abstinence rates relative to conventional treatment. Nicotine and Tobacco Research. 2009;11:1067–1075. doi: 10.1093/ntr/ntp103. [DOI] [PubMed] [Google Scholar]
- Rovina N, Nikoloutsou I, Katsani G, Dima E, Fransis K, Roussos C, Gratziou C. Effectiveness of pharmacotherapy and behavioral interventions for smoking cessation in actual clinical practice. Ther Adv Respir Dis. 2009;3:279–87. doi: 10.1177/1753465809350653. [DOI] [PubMed] [Google Scholar]
- Schotte A, Janssen PF, Megens AA, Leysen JE. Occupancy of central neurotransmitter receptors by risperidone, clozapine and haloperidol, measured ex vivo by quantitative autoradiography. Brain Res. 1993;631:191–202. doi: 10.1016/0006-8993(93)91535-z. [DOI] [PubMed] [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Hartmann-Boyce J, Cahill K, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;11:CD000146. doi: 10.1002/14651858.CD000146.pub4. [DOI] [PubMed] [Google Scholar]
- Tega Y, Akanuma S, Yoshiyuki K, Terasaki T, Hosoya K. Blood-to-brain influx transport of nicotine at the rat blood-brain barrier: Involvement of a pyrilamine-sensitive organic cation transport process. Neurochemistry International. 2013;62:173–181. doi: 10.1016/j.neuint.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Spiller K, Gardner EL. Mechanism-based medication development for the treatment of nicotine dependence. Acta Pharmacol Sin. 2009;30:723–39. doi: 10.1038/aps.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Privou C, Huston JP. Differential sensitivity of the caudal and rostral nucleus accumbens to the rewarding effects of a H1-histaminergic receptor blocker as measured with place-preference and self-stimulation behavior. Neuroscience. 1999;94:93–103. doi: 10.1016/s0306-4522(99)00309-7. [DOI] [PubMed] [Google Scholar]