SUMMARY
Ethanolamine glycerophospholipids are ubiquitous cell membrane components. Trypanosomatid parasites of the genus Leishmania synthesize the majority of their ethanolamine glycerophospholipids as 1-O-alk-1′-enyl-2-acyl-sn-glycero-3-phosphoethanolamine or plasmenylethanolamine (PME) through the Kennedy pathway. PME is a subtype of ether phospholipids also known as ethanolamine plasmalogen whose functions are not well characterized. In this study, we investigated the role of PME synthesis in Leishmania major through the characterization of an ethanolamine phosphotransferase (EPT) mutant. EPT-null parasites are largely devoid of PME and fully viable in regular medium but fail to proliferate in the absence of fetal bovine serum. They exhibit significant abnormalities in the synthesis and localization of GPI-anchored surface molecules. EPT-null mutants also show attenuated virulence in BALB/c mice. Furthermore, in addition to PME synthesis, ethanolamine also contributes to the production of phosphatidylcholine, the most abundant class of lipids in Leishmania. Together, these findings suggest that ethanolamine production is likely required for Leishmania promastigotes to generate bulk phospholipids, to handle stress, and to control the expression of membrane bound virulence factors.
Keywords: Leishmania, phospholipid, virulence, choline, starvation
Graphical abstract
Leishmania parasites synthesize a high level of plasmenylethanolamine (PME), a special class of glycerophospholipids. Effects of PME synthesis on Leishmania survival and virulence are investigated in this study.
INTRODUCTION
Leishmania parasites are trypanosomatid protozoans responsible for a spectrum of serious diseases known as leishmaniasis (WHO, 2015). During their life cycle, these pathogens alternate between motile, slender-shaped promastigotes in sandflies and non-motile, oval-shaped amastigotes in mammalian phagocytes (Podinovskaia & Descoteaux, 2015). Current drugs are inadequate and no safe vaccine is available. To develop new treatments, it is important to understand how Leishmania synthesize vital cellular components to sustain growth.
Leishmania parasites possess a unique combination of lipids including sphingolipids, glycerophospholipids, and ergostane-based sterols (Wassef et al., 1985, Zhang & Beverley, 2010). Previous studies indicate that a primary role of sphingoid base metabolism in Leishmania is to generate ethanolamine-phosphate, an important metabolite for promastigote growth and differentiation (Zhang et al., 2007). In most eukaryotes, ethanolamine or ethanolamine-phosphate is used to synthesize ethanolamine glycerophospholipids (EGP), mainly in the form of phosphatidylethanolamine (PE) via the Kennedy pathway: ethanolamine ⇒ ethanolamine-phosphate ⇒ CDP-ethanolamine ⇒ PE (Fig. S1A) (Gibellini & Smith, 2010, Kennedy, 1956). A parallel branch of the pathway incorporates choline into phosphatidylcholine (PC): choline ⇒ choline-phosphate ⇒ CDP-choline ⇒ PC (Fig. S1A).
In Leishmania major and Trypanosoma brucei (another trypanosomatid parasite), 80–90% of EGP belongs to plasmenylethanolamine (PME) while the rest consists of PE and lyso-PE (Richmond et al., 2010, Zufferey et al., 2003). The structural difference between PME and PE is indicated in Fig. S1B. The Kennedy pathway is responsible for nearly all EGP synthesis in L. major and T. brucei whereas contributions from other enzymes, such as phosphatidylserine synthase 2 and phosphatidylserine decarboxylase, are negligible (Gibellini et al., 2009, Signorell et al., 2008b) (Zhang et al., 2007). Two enzymes catalyze the final step of the Kennedy pathway: an ethanolamine phosphotransferase (EPT) which combines CDP-ethanolamine with 1-alkyl-2-acyl-glycerol to form 1-alkyl-2-acyl-phosphoethanolamine, the precursor for PME (Fig. S1A); and a choline/ethanolamine phosphotransferase (C/EPT) that condenses CDP-ethanolamine and 1,2-diacylglycerol into PE (Ford, 2003, Signorell et al., 2008b). C/EPT also catalyzes the production of PC (Signorell et al., 2008b, Henneberry & McMaster, 1999) (Fig. S1A).
PME (1-O-1′-alkenyl-2-acyl-sn-glycero-3-phosphoethanolamine) belongs to a family of ether phospholipids also known as ethanolamine plasmalogen (Fig. S1B) (Nagan & Zoeller, 2001). In mammals, PME is highly abundant in brain and heart tissues. The vinyl ether bond at the sn-1 position may confer protection against oxidants and hydrolysis (Lessig & Fuchs, 2009). In addition, the slightly lower melting temperature of PME (comparing to PE) and its tendency to form nonbilayer lipid structures such as reverse hexagon suggest it can facilitate membrane fusion and vesicle trafficking (Lohner, 1996, Nagan & Zoeller, 2001). Furthermore, PME is implicated in a number of disease states in humans including certain peroxisomal disorders, aging, Alzheimer’s disease, and heart disease (Nagan & Zoeller, 2001, Braverman & Moser, 2012). Functions of PME or other plasmalogen lipids in protozoans are poorly understood.
It is worth mentioning that EGP (PME and/or PE) can serve as intermediates to produce other molecules. For example, PE may be converted into PC through three consecutive N-methylation reactions, a process found in mammalian hepatocytes (Walkey et al., 1998, Vance et al., 2007) and recently in Leishmania (Bibis et al., 2014). Additionally, PE is involved in synthesis of GPI-anchored proteins in trypanosomatids by providing the ethanolamine-phosphate bridge that links proteins to glycan anchors (Menon et al., 1993, Signorell et al., 2008a). Furthermore, protein modification by EGP is observed in the formation of autophagosome during macroautophagy (Ichimura et al., 2000, Besteiro et al., 2006, Williams et al., 2012) and the posttranslational modification of eukaryotic elongation factor 1A in T. brucei (Signorell et al., 2008a).
In Leishmania, PME abundance increases 2–4-fold when the replicative procyclics differentiate into infective metacyclics (Zhang et al., 2007), yet its precise roles remain to be determined. It is also not known whether ethanolamine serves any other functions beyond EGP synthesis. To address these questions, we generated an EPT-null mutant in L. major, which is largely devoid of PME. Results showed that PME production is crucial for the stress response and virulence of Leishmania promastigotes.
RESULTS
Targeted deletion and cellular localization of EPT in L. major
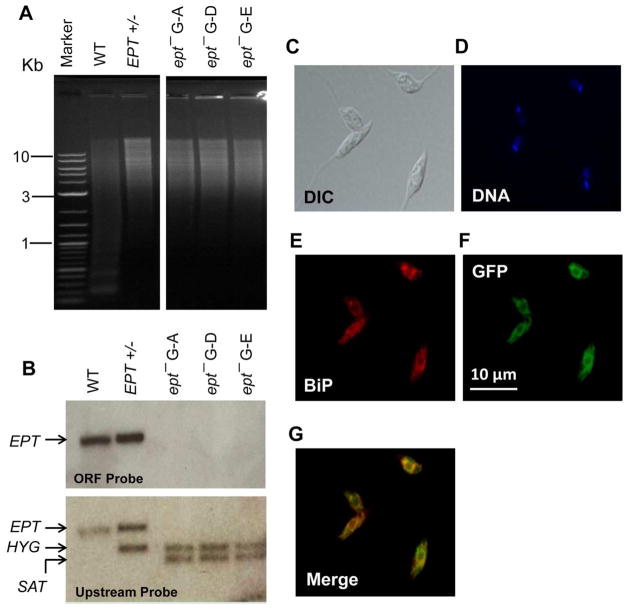
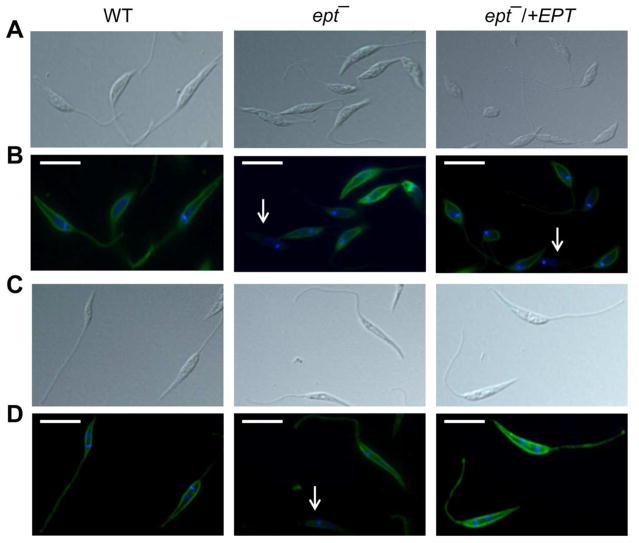
In Leishmania, the majority of EGP belongs to PME, a specific class of ether phospholipid with a vinyl ether linkage at the sn-1 position and an ester linkage at the sn-2 position (Fig. S1B). To explore the function of PME in L. major, we focused on a putative EPT protein (LmjF.18.0810, 415 aa) which was predicted to catalyze the formation of 1-alkyl-2-acyl PE, the second to last step of PME synthesis (Fig. S1A). This protein contains a CDP-alcohol phosphatidyltransferase domain and nine transmembrane regions. No obvious signal peptide was detected but a potential ER membrane retention signal “RKGA” (predicted by PSORT) was present at the C-terminus. To generate the EPT-null mutant, endogenous EPT alleles were deleted from L. major LV39 parasites and the resulting ept− parasite was confirmed by Southern blot (Fig. 1A–B). The mutant was then complemented with a high-copy number episome (ept−/+EPT) to restore EPT expression (Fig. S2). To examine the cellular localization of EPT, an EPT-GFP chimeric protein was introduced into the ept− mutant (Fig. S3). In fluorescence microscopy, EPT-GFP exhibited a diffused, membranous pattern extending from the perinuclear region (Fig. 1C–G). It showed partial overlap with BiP, which resides in the lumen of peripheral ER (Fig. 1E–G) (Bangs et al., 1993). Since EPT has multiple transmembrane helices and a putative ER-membrane retention signal, we speculate that this enzyme is in a subdomain of ER mainly at the perinuclear region. A similar localization was recently reported for the EPT orthologue in T. brucei (Farine et al., 2015).
Figure 1. Targeted deletion and cellular localization of EPT in L. major.
(A) Genomic DNA samples (5 μg each) were digested with NcoI and XbaI, separated on a 0.8% agarose gel, and stained with ethidium bromide. (B) Southern blot was then performed using probes recognizing either the ORF (top) or an upstream region (bottom) of EPT. Replacement of EPT by HYG and SAT resistance genes was indicated in B. In A–B, WT: L. major LV39 wild type, EPT +/−: EPT heterologous mutant, ept− G-A, G-D, and G-E: three independent clones of EPT homozygous mutants. (C–G) Log phase promastigotes of ept−/+EPT-GFP were labeled with an ER marker (rabbit anti-T. brucei BiP antiserum followed by goat anti-rabbit IgG-Texas Red) and subjected to immunofluorescence microscopy. (C) Differential interference contrast (DIC) images; (D) Hoechst staining of DNA; (E) Anti-BiP staining; (F) GFP fluorescence; (G) Merge of E and F.
The EPT-null mutants (ept−) are viable in culture but deficient in PME synthesis
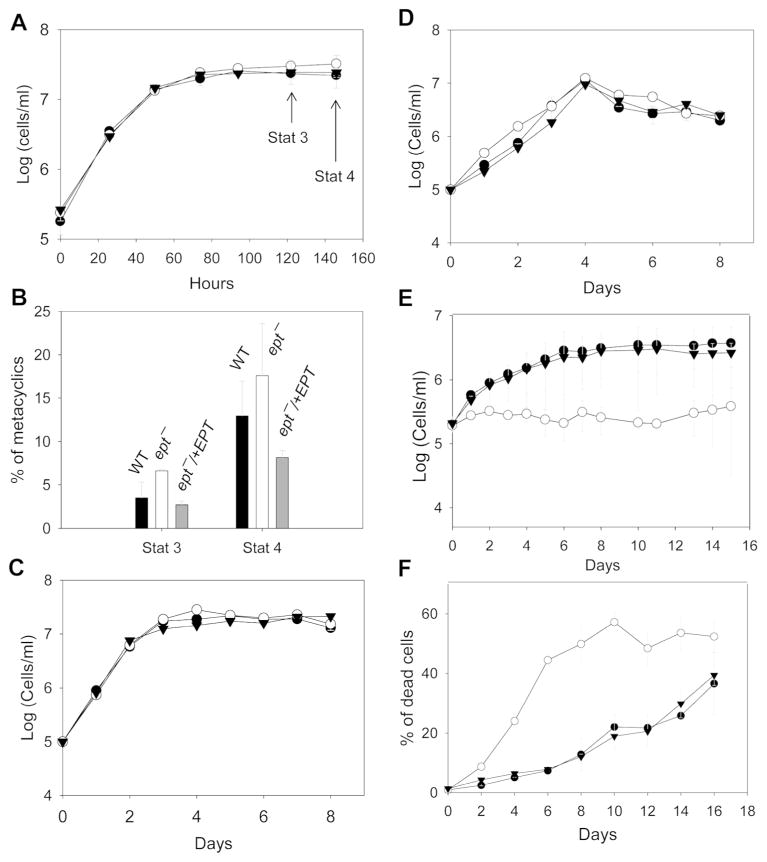
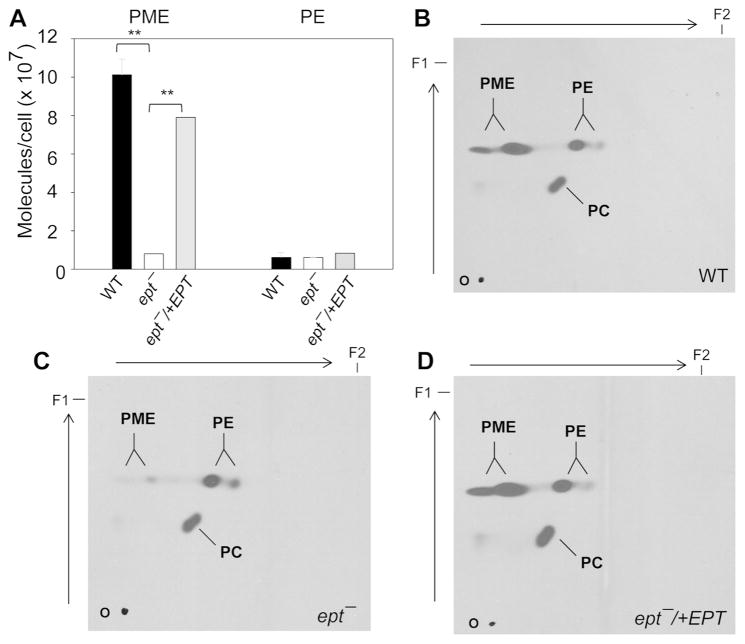
When inoculated in complete M199 medium (containing 10% fetal bovine serum or FBS), ept− mutants were fully viable and grew at a similar rate as WT and ept−/+EPT promastigotes (Fig. 2A). These parasites showed normal morphology and were able to differentiate into infective metacyclics in stationary phase (Sacks & Perkins, 1984) (Fig. 2B and data not shown). Mass spectrometry analysis revealed that WT and ept−/+EPT parasites contained 8–11 × 107 molecules/cell of PME (mainly as p18:0/18:1-PE and p18:0/18:2-PE) (Fig. S4 and Fig. 3A). In ept− parasites, the abundance of PME was reduced to less than 4 × 106 molecules/cell (Fig. S4 and Fig. 3A), and the decrease in PME coincided with an increase in alkyl-acyl-phosphatidylinositol, as the ratio of a18:0/18:1-PI (the dominant alkyl-acyl-phosphatidylinositol) to 18:0/18:1-PI (the dominant diacyl-phosphatidylinositol) is higher than WT or ept−/+EPT (Fig. S4). In addition, ept− mutants contained normal levels of PE and sterols but more PC than WT and ept−/+EPT parasites (summarized in Fig. S5 and Table S1; the difference between WT and ept− is statistically significant in stationary phase). These changes may arise from the redirection of EPT substrates, CDP-ethanolamine and 1-alkyl-2-acyl-glycerol, towards PC and alkylacyl-PI synthesis in ept− (Fig. S1A). Together, these analyses allude to a significant change in the membrane lipid composition of ept− mutants.
Figure 2. EPT-null mutants grow normally in complete M199 medium but struggle to survive in the absence of FBS.
Promastigotes (●: WT, ○: ept−, ▼: ept−/+EPT) were cultured in M199 medium with 10% FBS (A–B), M199 with 10% delipidated FBS (C), M199 with 0.4% BSA (D), or M199 without FBS or BSA (E–F). Culture densities were determined daily (A, C, D, and E) using a hemacytometer. In B, metacyclics were isolated and quantified from day 3 and day 4 stationary phase parasites. In F, percentages of dead cells were examined every two days by flow cytometry. Error bars represent standard deviations from three independent experiments.
Figure 3. Ept− mutants are defective in PME synthesis.
(A) Total lipids from day 2 stationary phase promastigotes were analyzed by ESI/MS in the negative ion mode. Portions of [M-H]− spectra are shown in Fig. S4. The abundance of PME and PE was summarized and error bars represent standard deviations from three independent experiments. **: p<0.01. (B–D) Log phase promastigotes were labeled with 3H-ethanolamine for 48 hours in complete M199 medium and total lipids (from 107 cells each) were separated by 2D-TLC. First dimension (from bottom to top) was run in chloroform:methanol:water (65:25:4, v/v/v). Second dimension (from left to right) was run in chloroform:methanol:29% ammonium hydroxide (65:25:4, v/v/v). Autoradiography was used to visualize the incorporation of 3H-ethanolamine into PME, PE, and PC. O: origin. F1/F2: solvent front for dimension 1/2.
Metabolic labeling of WT promastigotes with [3H]-ethanolamine led to robust incorporation into both EGP and PC, whereas [14C]-choline was only incorporated into PC (Fig. S6). In agreement with mass spectrometry results, incorporation of [3H]-ethanolamine into EGP was reduced in ept− mutants by ~80% in comparison to WT parasites (Fig. S6A and C). In a similar labeling experiment, PC synthesis from choline was largely unaltered in ept− (Fig. S6B and D). Complementation of ept− with EPT or EPT-GFP restored EGP synthesis from ethanolamine (Fig. S6A and C). To distinguish PME from PE, [3H]-ethanolamine-labeled lipids were analyzed by two-dimensional TLC and results further confirmed the role of EPT in PME synthesis (Fig. 3B–D). Together, these data indicate that EPT is responsible for producing the bulk of PME. Residual amount of PME in ept− may be produced by salvage or the overlapping activity of C/EPT, which is predicted to catalyze the synthesis of PE and PC (Fig. S1A) (Signorell et al., 2008b).
Ept− mutants exhibit defects in the expression of GPI-anchored virulence factors
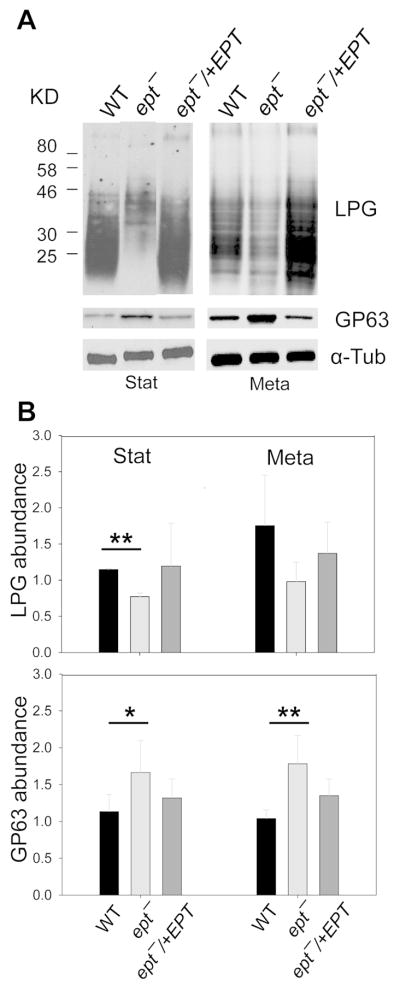
Membrane-bound glycoconjugates such as lipophosphoglycan (LPG) and GP63 are important virulence factors for Leishmania promastigotes. Since EGP is required for the synthesis of GPI-anchored proteins in T. brucei (Menon et al., 1993), we examined whether EPT is involved in the production of LPG or GP63. Western-blot (using a LPG-specific monoclonal antibody WIC79.3) revealed low levels of cell-bound LPG in ept− during log, stationary, and metacyclic stages (Fig. 4A–B and Fig. S7A). The release of LPG was also compromised in ept− mutants (Fig. S7B). Similar finding was observed by flow cytometry using the same antibody (Fig. S7C). In contrast, the cellular level of GP63 in ept− was ~two-fold higher than those in WT and ept−/+EPT parasites (Fig. 4A and B). We also examined the localization of LPG and GP63 in ept− by immunofluorescence microscopy. While WT and ept−/+EPT parasites showed uniform, plasma membrane staining for LPG, ept− mutants exhibited a more heterogeneous distribution as 24–30% of cells were undetectable for LPG (Fig. 5 and Table S2). Localization of GP63 was not altered in ept− mutants (Fig. S8). We also investigated the association of GP63 with detergent resistant membrane (DRM) fractions, which reflects the integrity of ordered membrane microdomains or lipid rafts (Brown & Rose, 1992). As previously described for WT parasites (Denny et al., 2001), GP63 showed a strong association with DRM fractions (40–50%) in ept− at 4 °C but not at 37 °C (Fig. S9). Thus, the propensity of ept− to form DRM/rafts appeared to be unaltered. Collectively, our findings reveal a profound change in the expression of GPI-anchored virulence factors in PME-deficient Leishmania parasites.
Figure 4. Abnormal expression of LPG and GP63 in ept− promastigotes.
(A) Whole cell lysates from stationary phase (left) and metacyclic parasites (right) were probed with antibodies against LPG, GP63 or α-tubulin. In B, the relative abundance of LPG (top) and GP63 (bottom) was determined by phosphoimaging using α-tubulin as a loading control. Error bars represent standard deviations from three experiments. *: p<0.05; **: p<0.01.
Figure 5. Uneven distribution of LPG in ept− mutants.
Log phase (A, B) or stationary phase (C, D) promastigotes of WT, ept−, and ept−/+EPT were labeled with the anti-LPG monoclonal antibody WIC79.3 and subjected to immunofluorescence microscopy. A and C: DIC images; B and D: overlay of LPG labeling (green) and DNA staining (blue). White arrows indicate cells with low or undetectable LPG. Scale bars: 10 μm.
Role of PME synthesis in Leishmania stress response
In the regular medium (M199 with 10% FBS), ept− promastigotes grew normally without any obvious defects (Fig. 2A–B). To determine whether PME synthesis is required for Leishmania growth under lipid-limiting conditions, parasites were inoculated in M199 with 10% delipidated FBS or M199 with 0.4% bovine serum albumin (BSA). As shown in Fig. 2C–D, ept− mutants proliferated as well as WT parasites in these media, suggesting that lipid salvage is not essential for ept−. In contrast, M199 without either FBS or BSA failed to support the replication of ept− and these mutants died more rapidly than WT and ept−/+EPT parasites (Fig. 2E–F). Without FBS or BSA, the osmotic pressure of culture medium is significantly changed. We postulate that the altered membrane composition in ept− makes them more susceptible to such stress.
In addition, we examined whether PME synthesis played roles in adaptation to conditions found in the mammalian host such as elevated temperature (33–37 °C), acidic pH, and increased oxidative/nitrosative stress. Overall, the lack of PME does not significantly affect parasite’s ability to tolerate heat, H2O2, or SNAP (a slow releaser of nitric oxide) (Fig. S10–S11). However, when stationary phase promastigotes were incubated in a pH 5.0 medium, ept− mutants survived much better (8–30% dead) than WT and ept−/+EPT parasites (65–92% dead) after 48 hours (Fig. S11D).
Autophagosome formation is normal in ept−
Autophagy is a cellular remodeling process that involves the sequestration and degradation of existing cellular components for energy and nutrients. In Leishmania, autophagy-like process is initiated during stationary phase when promastigotes undergo metacyclogenesis (Besteiro et al., 2006, Williams et al., 2013). Covalent conjugation of PE to autophagy protein 8 (ATG8) on autophagic membrane is critical for autophagosome formation, maturation, and its eventual fusion with the lysosome (Ichimura et al., 2000, Hanada et al., 2007). Since the predominant form of EGP in Leishmania is PME, we examined whether autophagy was affected in PME-deficient parasites by monitoring the distribution of a GFP-ATG8 fusion protein. As previously shown, GFP-ATG8 was mainly cytosolic during the replicative log phase and early stationary phase, but was then reorganized into intense, punctuated spots indicative of autophagosome-like structures in mid-late stationary phase (Fig. S12A–D) (Williams et al., 2012). This change of localization coincided with the detection of lipidated GFP-ATG8 by western-blot (the lower band in Fig. S12E). No significant difference was observed between WT and ept− in these analyses (Fig. S12). Similar results on autophagosome formation and GFP-ATG8 modification were obtained when log phase promastigotes were incubated in phosphate buffered saline to induce starvation for 0–24 hours (Fig. S13). Overall, these findings indicate that ept− mutants are fully capable of initiating autophagy response, despite the lack of PME.
Ept− promastigotes show attenuated virulence in mice
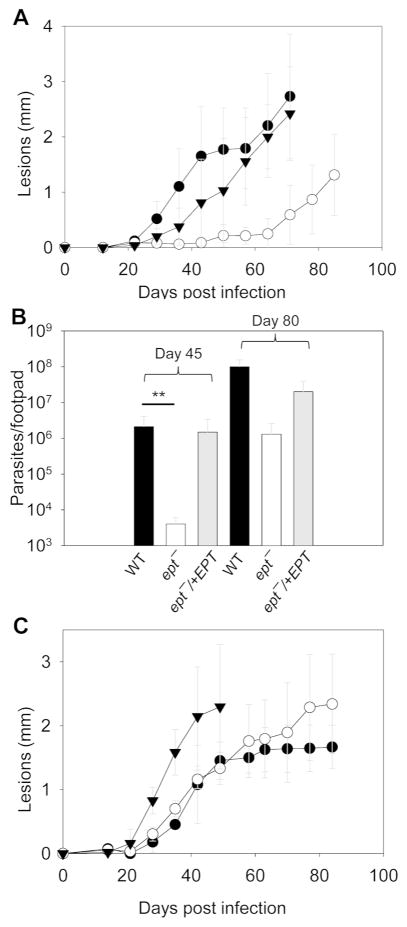
To study the role of PME synthesis in Leishmania virulence, BALB/c mice were infected subcutaneously in the footpad with late stationary phase WT, ept−, and ept−/+EPT promastigotes. With WT and ept−/+EPT parasites, mice developed lesions ~2 mm in size about eight weeks post infection (Fig. 6A). In comparison, ept− mutants required almost twice as long to produce lesions of similar sizes and to reach similar levels of parasite burden (Fig. 6A–B). We then isolated amastigotes from footpad lesions and injected them directly into another group of BALB/c mice. In this case, ept− amastigotes acted similarly as WT amastigotes and did not exhibit any defects in pathogenesis or proliferation (Fig. 6C and data not shown). Thus, PME synthesis is important for the virulence of L. major promastigotes but not amastigotes.
Figure 6. Ept− promastigotes show attenuated virulence in mice.
BALB/c mice were infected with day 3stationary phase promastigotes (A and B; 1 × 106 per mouse) or lesion-derived amastigotes (C; 1 × 104 per mouse). Footpads lesions were measured weekly in A and C (●: WT, ○: ept−, ▼: ept−/+EPT). Limiting dilution assay was performed at 45 and 80 days post infection to determine parasites burden in B. Error bars represent standard deviations. **: p < 0.01.
DISCUSSION
Leishmania parasites synthesize a high level of PME especially in the stationary phase (Zhang et al., 2007). An ER-localized EPT is responsible for the bulk of PME production. While the ept− mutants are viable and replicative in culture, their membrane composition is significantly altered, as evidenced by the increased abundance of PC and alkyl-acyl-PI. Despite the lack of PME, ept− mutants contain more GP63 than WT parasites (Fig. 4B), suggesting that PE alone is sufficient to provide the ethanolamine-phosphate linkage in GPI-anchored glycoproteins (Menon et al., 1993, Rifkin & Fairlamb, 1985). Interestingly, ept− mutants have less LPG (which does not have the ethanolamine-phosphate moiety) (Turco & Descoteaux, 1992, McConville et al., 1990) than WT parasites. We suspect that the accumulation of alkyl-acyl-PI in ept− may lead to more anchors for GP63 (anchored through alkyl-acyl-PI) and less for LPG (anchored through alkyl-lyso-PI) (Ralton & McConville, 1998). Ept− mutants also show an uneven distribution of LPG (Fig. 5 and Table S2), indicating greater cell-to-cell variation in the sorting of GPI-anchored molecules. This is unlikely due to pleiotropic defects in vesicular trafficking since changes in lipid composition do not affect the localization of GP63 or its association with DRM in ept−.
Our growth experiments with various media suggest that exogenous protein (BSA) but not lipid is essential for ept− mutants (Fig. 2). BSA is known to confer osmotic pressure and facilitate nutrient uptake in tissue culture cells (Brunner et al., 2010). It is possible that the altered membrane lipid composition in ept− mutants makes them vulnerable under protein-poor conditions. The lack of PME has little effect on parasite’s ability to form autophagosomes or lipidated ATG8 (Fig. S11–12) (Besteiro et al., 2006, Williams et al., 2013). It is not known if the ATG8-conjugation machinery (ATG12-ATG5) can discriminate between PME and PE (Ichimura et al., 2004, Williams et al., 2012). The combination of PE and a low level of PME may be sufficient for the modification of ATG8 and maturation of autophagosomes (Fig. S11–12).
Although PME has been implicated in membrane stability and defense against oxidants (Lessig & Fuchs, 2009), our ept− mutants show normal sensitivity to heat, SNAP, and H2O2 when grown in regular medium (Fig. S10–S11). A somewhat curious observation is that these PME-deficient promastigotes are highly resistant to acidic pH (Fig. S11D). One possibility is that mutant cells accumulate ethanolamine-derived metabolites which may serve as weak bases to buffer acidic stress (Fig. S1A). Or, changes in membrane lipid composition may lead to reduced permeability to protons.
The reduced level of LPG likely contributes to the attenuated virulence exhibited by ept− promastigotes (Fig. 6A–B). Alternatively, sensitivity to protein deprivation may play a role as well. Infectivity of ept− amastigotes, however, is at least as potent as that of WT amastigotes (Fig. 6C). This is consistent with the previous report that LPG is not required for the survival and proliferation of L. major amastigotes in mice (Spath et al., 2000). In addition, ept− mutants possess more GP63 which affects various signaling pathways and nuclear physiology in the host cell (Olivier et al., 2012, Isnard et al., 2015) and their increased tolerance to acidic pH may improve their fitness as amastigotes. Furthermore, Leishmania amastigotes are known to salvage sphingolipids, phospholipids, glycolipids and sterols from the host (Zhang et al., 2005, Zhang et al., 2009, Xu et al., 2014), making them less dependent on the de novo synthesis during the mammalian stage.
Metabolic labeling of Leishmania promastigotes with [3H]-ethanolamine led to robust incorporation of [3H] into both EGP and PC, indicating a link between the ethanolamine branch and choline branch of the Kennedy pathway, probably through the activity of PE N-methyltransferase (Bibis et al., 2014). This conversion is common in mammalian hepatocytes (Vance et al., 2007) but absent from T. brucei where ethanolamine is only used to synthesize PE and PE derivatives (Smith & Butikofer, 2010, Gibellini et al., 2009), suggesting a lack of cross-talk between the ethanolamine and choline branches in that organism. PC is the most abundant lipid accounting for ~30% of total lipids in Leishmania (Zhang & Beverley, 2010). Thus, being able to generate PC from two branches of the Kennedy pathway (Fig. S1A) may allow Leishmania to sustain growth under various nutrient-restricting conditions.
In summary, PME synthesis in L. major promastigotes is important for the proper production of GPI-anchored molecules, survival under nutrient limiting conditions, and the establishment of infection in mammals. Future work will elucidate the impact of PME on the functions of plasma membrane and intracellular organelles in Leishmania. In addition, the significance of PC synthesis from the choline branch of Kennedy pathway and the potential of targeting phospholipid metabolism to control leishmaniasis will be explored.
EXPERIMENTAL PROCEDURES
Materials
Ethanolamine [1-3H] hydrochloride (40 Ci/mmol) was purchased from the American Radiolabeled Chemicals, Inc. Choline [methyl-14C] chloride (52 mCi/mmol) and [α-32P]-dCTP (3000 Ci/mmol) were purchased from Perkin Elmer. Phospholipid standards for mass spectrometry studies including 1-stearoyl-2-arachidonoyl-sn-glycero-3-phosphoethanolamine (18:0-20:4-PE), 1-(1Z-octadecenyl)-2-arachidonoyl-sn-glycero-3-phosphoethanolamine (p18:0-20:4-PE), and 1,2-dimyristyl-sn-glycero-3-phosphocholine (14:0-14:0-PC) were purchased from Avanti Polar Lipids. Cholesta-3,5-diene was purchased from Sigma Aldrich as the standard for sterol analysis. The anti-LPG monoclonal antibody was acquired from the culture supernatant of mouse hybridoma WIC79.3 cells (de Ibarra et al., 1982). The mouse anti-Leishmania GP63 monoclonal antibody (for immunofluorescence microscopy) was purchased from the Cedarlane Laboratories. The mouse-anti-α-tubulin, rabbit-anti-GFP, goat anti-rabbit IgG-HRP, goat anti-mouse IgG-HRP, and goat anti-mouse IgG-FITC antibodies were purchased from Life Technologies. All other reagents were purchased from VWR International or Fisher Scientifics unless specified otherwise.
Molecular cloning
The 1248 bp open reading frame (ORF) of L. major EPT (trytripdb ID: LmjF18.0810) was amplified by PCR and cloned into the pXG1 vector, generating pXG1-EPT. To study localization, a modified version of EPT ORF without the stop codon was amplified and cloned into pXG1-‘GFP to generate pXG1-EPT-GFP to drive the expression of EPT-GFP fusion protein. For knockout constructs, the upstream and downstream flanking regions (~1 Kb each) of EPT ORF were amplified and cloned into the pUC18 vector. Subsequently, genes conferring resistance to nourseothricin (SAT) and hygromycin (HYG) were inserted between the upstream and downstream flanking regions to generate pUC18-KO-EPT:SAT and pUC18-KO-EPT:HYG.
For autophagy studies, the L. major ATG8 ORF (LmjF.19.1630) was PCR amplified from L. major genomic DNA and cloned into pXG-GFP2+’ to generate pXG-GFP-ATG8 (with a G418 resistance marker). The GFP-ATG8 fusion was also subcloned into pXGBSD to produce pXGBSD-GFP-ATG8 (with a blasticidin resistance marker). All constructs were confirmed by restriction digestion and/or DNA sequencing.
Leishmania promastigote culture and transfection
Unless otherwise specified, L. major LV39 clone 5 (Rho/Su/59/P) promastigotes were cultured at 27 °C in M199 medium with 10% heat-inactivated fetal bovine serum (FBS) and other supplements as previously described (Kapler et al., 1990). Culture density was determined daily using a hemacytometer. Metacyclics were isolated and enumerated from day 3 or day 4 stationary phase promastigotes as previously described (Spath & Beverley, 2001). The endogenous EPT alleles were sequentially deleted from wild type (WT) L. major by two rounds of targeted replacement as described (Zhang, 2004) using linearized knockout constructs from pUC18-KO-EPT:SAT and pUC18-KO-EPT:HYG. The resulting ept− mutant (ΔEPT::SAT/ΔEPT::HYG) was confirmed by Southern blot using [α-32P]-labeled DNA probes corresponding to the ORF or an upstream region of EPT. To restore EPT expression, pXG1-EPT was transfected into ept− and referred to as ept−/+EPT (ΔEPT::SAT/ΔEPT::HYG/+pXG1-EPT). No detectable phenotypic differences were observed among ept− clones, so ept− clone G-A and its add-back parasites were used in the following experiments. For localization studies, pXG1-EPT-GFP was introduced into ept− to generate ept−/+EPT-GFP. For autophagy studies, WT and ept− were transfected with pXG-GFP-ATG8 and ept−/+EPT parasites were transfected with pXGBSD-GFP-ATG8.
To examine cell growth under nutrient-limiting conditions, we prepared M199 medium without FBS but containing other supplements as described (Kapler et al., 1990). Leishmania promastigotes were inoculated at 1–2 × 105 cells ml−1 in M199 with10% delipidated FBS, M199 with 0.4% fatty acid free BSA or M199 only. Delipidation of FBS was performed as previously described by Cham and Knowles (Cham & Knowles, 1976). To measure the percentage of dead cells, parasites were labeled with 5 μg/ml of propidium iodide and analyzed by flow cytometry. Response to high temperature (37 °C), acidic pH (pH 5.0), hydrogen peroxide, and S-nitroso-N-acetylpenicillamine (SNAP) were determined as previously described (Xu et al., 2011).
Mass spectrometry analysis of lipids
Promastigote lipids (from 1 × 108 cells per sample) were extracted as described (Xu et al., 2014). Half of the samples contained Leishmania lipids only (for comparison) while the other half had lipid standards added to cell lysate prior to lipid extraction (1 × 107 molecules cell−1 for 18:0-20:4-PE, 1 × 108 molecules cell−1 for p18:0-20:4-PE, and 5 × 107 molecules cell−1 for 14:0/14:0-PC). For sterol analysis, cholesta-3,5-diene was added at 2 × 107 molecules cell−1. These lipid standards are not found in normal Leishmania samples. Extracted lipids were dissolved in chloroform:methanol (1:2, v/v) and subjected to electrospray ionization mass spectrometry (ESI/MS) for phospholipid analysis (Hsu et al., 2014). Quantitation of EGP (PME and PE species) and PC was performed using precursor ion scan of m/z 196 (negative ion mode) and m/z 184 (positive ion mode), respectively. Sterol analysis was performed using electron ionization gas chromatography mass spectrometry (EI GC-MS) as previously described (Xu et al., 2014).
Metabolic labeling and TLC
To examine the de novo synthesis of phospholipids, promastigotes were inoculated at 1 × 105 cells ml−1 in complete M199 medium in the presence of 1 μCi ml−1 of [1-3H]-ethanolamine hydrochloride or 1 μCi ml−1 of [methyl-14C] choline chloride. The medium contains 23.8 μM (25 mg L−1) of serine which can be converted into ethanolamine-phosphate (Zhang et al., 2007) and 3.6 μM (0.5 mg L−1) of choline. After incubation at 27°C for 48 hours, total lipids were extracted from 1–1.5 × 108 cells and dissolved in chloroform:methanol (1:2, v/v) at the equivalence of 2.0×109 cells/ml. The incorporation of [1-3H]-ethanolamine or [methyl-14C] choline into Leishmania lipids was determined by scintillation counting. One dimensional thin layer chromatography (TLC) was performed in a solvent of chloroform:methanol:water (65:25:4, v/v/v). For two dimensional TLC, lipids were first separated as described above, dried, and then plates were rotated 90 ° before running in chloroform:methanol:29% ammonium hydroxide (65:25:4, v/v/v). The TLC plates were sprayed with EN3HANCE (Perkin Elmer) and visualized by autoradiography.
Western-blot
To examine the level of lipophosphoglycan (LPG) and GP63, whole cell lysates and culture supernatants of Leishmania promastigotes were collected and resolved by SDS-PAGE, followed by immunoblotting with mAb WIC79.3 (1:1000), rabbit anti-GP63 (1:1000), or mAb anti-tubulin (1:5000) as previously described (Zhang, 2004). To analyze detergent resistant membrane (DRM) fractions, 1 × 108 cells were lysed in 1 ml of 0.1% TritonX-100 prepared in PBS; samples were incubated at either 4 °C or 37 °C and vortexed every 2 minutes for a total of 10 minutes; DRM fractions were isolated by centrifugation at 14,000 × g for 2 minutes; both the soluble and insoluble fractions were boiled in SDS sample buffer and processed for western-blot. Signals from western-blot were quantified using a FluorChem E system (Protein Simple).
To detect GFP-ATG8 (unconjugated and lipidated) and EPT-GFP, parasite lysates were boiled in SDS sample buffer and preceded for western-blot using a rabbit anti-GFP serum (Life Technologies, 1:1000) as previously described (Besteiro et al., 2006).
Fluorescence microscopy
An Olympus BX50 Upright Fluorescence Microscope equipped with a digital camera was used to visualize the expression and localization of EPT-GFP, GFP-ATG8, LPG, and GP63. For immunofluorescence microscopy, Leishmania promastigotes were attached to poly-L-lysine coated coverslips, fixed with 3.7% formaldehyde, and then permeabilized on ice with 100% ethanol. Incubation with primary antibodies against LPG (WIC79.3, 1:1000), GP63 (mAb anti-GP63 #235, 1:1000), or BiP (rabbit anti-T. brucei BiP, 1:1000) were carried out at ambient temperature for 30 minutes. After washing, coverslips were incubated with goat-anti-mouse-FITC (1:3000) or goat anti-rabbit-Texas Red (1:2000) antiserum for 30 minutes, followed by washing and staining with Hoechst 33242 (2.5 μg ml−1).
To measure the percentage of cells with autophagosomes, promastigotes expressing GFP-ATG8 were monitored daily by microscopy. For each time point, ~ 200 cells were counted per group and percentages of cytosolic and autophagosomal GFP-ATG8 localization were determined.
Mouse infection
The use of mice in this study was approved by the Animal Care and Use Committee at Texas Tech University (US PHS Approved Animal Welfare Assurance NO. A3629-01). BALB/c mice (female, 8 weeks old) were purchased from Charles River Laboratories International. Mice were housed and cared for in the facility operated by the Animal Care and Resources Center at Texas Tech University adhering to the Guide for the Care and Use of Laboratory Animals (the 8th Edition, NRC 2011) for animal husbandry.
Promastigotes were injected into the footpads of BALB/c mice and recovered after 3–4 weeks to start low passage in vitro cultures. To assess virulence, day 3 stationary phase promastigotes (cultured for less than 5 passages after recovery from mice) or lesion-derived amastigotes were resuspended in DMEM and injected into the left hind footpads of BALB/c mice (1.0 × 106 cells per mouse for promastigotes, 1.0 × 104 cells per mouse for amastigotes, 5–6 mice per group). Lesion sizes were measured weekly using a Vernier caliper and parasite loads were determined by limiting dilution assay (Titus et al., 1985).
Statistical analysis
Difference between two groups was determined by the unpaired Student’s t test using Sigmaplot 11.0 (Systat Software Inc, San Jose, CA). P values indicating statistical significance were grouped into values of <0.05 and <0.01.
Supplementary Material
Figure S1. EGP and PC synthesis in Leishmania. (A) De novo synthesis of EGP (PME + PE) and PC. Dashed arrows represent potential links between the ethanolamine branch and choline branch. SPT: serine palmitoyltransferase; SK: sphingosine kinase; SPL: sphingosine-1-phosphate lyase; EK: ethanolamine kinase; EPCT: ethanolaminephosphate cytidylyltransferase; EPT: ethanolamine phosphotransferase; CK: choline kinase; CPCT: cholinephosphate cytidylyltransferase; C/EPT: choline/ethanolamine phosphotransferase. EtN: ethanolamine; EtN-P: ethanolamine phosphate; Cho: choline; Cho-P: choline phosphate; DAG: diacylglycerol; CMP: cytidine-monophosphate; CDP: cytidine-diphosphate; PME: plasmenylethanolamine; PE: (1,2-diacyl-)phosphatidylethanolamine; PC: (1,2-diacyl-) phosphatidylcholine. (B) Structures and full chemical names of a PME (top) and a PE (bottom).
Figure S2. Southern blot to confirm EPT deletion mutants and episomal add-backs. (A) Genomic DNA samples were digested with restriction enzymes (NcoI and XbaI), separated on a 0.8% agarose gel, and stained with ethidium bromide. (B) Southern blot was performed using a probe recognizing the open reading frame (ORF) of EPT. Expected sizes for pXG1-EPT (~8 Kb) and EPT (~ 4 Kb) were marked by arrows. M: 1 Kb DNA ladder, 1: LV39WT (5 μg), 2: ept− G-A (5 μg), 3: ept−/+EPT (0.5 μg), 4: ept−/+EPT (0.1 μg), 5: ept−/+EPT (0.05 μg).
Figure S3. Detection of EPT-GFP by western-blot. Cell lysates from log phase promastigotes were probed with either anti-GFP antibody (A) or anti-α-tubulin antibody (B). Lane 1: ept−/+EPT-GFP; lane 2: WT/+GFP. Predicted sizes for EPT-GFP and GFP are 72 KD and 27 KD, respectively.
Figure S4. Ept− mutants possess diminished amount of PME. Total lipids from day 2 stationary phase promastigotes (A: WT, B: ept−, C: ept−/+EPT) were analyzed by ESI/MS in the negative ion mode. A PE standard (std) at m/z 750.5 was added to each sample prior to lipid extraction. Portions of [M-H]− spectra are shown with PME (p18:0/18:2-PE and p18:0/18:1-PE at m/z 726.5 and 728.5, respectively) and PE (16:0/20:4-PE, 18:1/18:2-PE, [18:0/18:2 + 18:1/18:1]-PE, and 18:0/18:1-PE at m/z 738.4, 740.5, 742.5 and 744.5, respectively) marked by arrows. Peaks representing IPC (d16:1/18:0-IPC at m/z 778.5), alkyl-acyl-PI (a18:0/18:1-PI at m/z 849.6), and diacyl-PI (18:0/18:1-PI at m/z 863.6) are also indicated.
Figure S5. Ept− mutants contain elevated level of PC. Total lipids from WT, ept−, and ept−/+EPT promastigotes (log or stationary phase) were analyzed by ESI/MS in the positive ion mode. The abundance of PC was determined based on comparison to an internal standard (14:0/14:0-PC, provided at 5 × 107 molecules cell−1). Values from three independent experiments are averaged (error bars represent standard deviations). *: p < 0.05.
Figure S6. Ept− mutants show reduced EGP synthesis. Log phase promastigotes were labeled with [3H]-ethanolamine (A, C) or [14C]-choline (B, D) for 48 hours in complete M199 medium. Total lipids were extracted and dissolved in chloroform:methanol (1:2 v/v) at 2.0 × 106 cells/μl. Incorporation of [3H]-ethanolamine or [14C]-choline into Leishmania lipids was determined by scintillation counting and summarized in A and B. Error bars represent standard deviations from three experiments. *: p < 0.05. In C and D, lipids were separated by TLC (chloroform:methanol:water 65:25:4 v/v/v) and visualized by autoradiography. Each lane contained lipids from 1.0 × 107 cells. O: origin. F: solvent front.
Figure S7. Ept− mutants produce less LPG than WT parasites. Whole cell lysates (A) or culture supernatants (B) from log phase promastigotes were probed with anti-LPG or anti-α-tubulin antibodies in western-blots. (C) Flow cytometry analysis of promastigotes labeled with the WIC79.3 monoclonal antibody followed by a FITC-conjugated anti-mouse IgG secondary antibody. Black: WT labeled with secondary antibody only, red: WT, blue: ept−, yellow: ept−/+EPT.
Figure S8. GP63 localization is not affected in ept− mutants. WT (A), ept− (B), and ept−/+EPT (C) promastigotes (day 3 stationary phase) were labeled with an anti-GP63 monoclonal antibody and subjected to immunofluorescence microscopy. Left column: anti-GP63; Middle column: DIC; Right column: DNA staining. Scale bars: 10 μm.
Figure S9. GP63 association with DRM is not affected in ept− mutants. (A) Western-blot of GP63 in detergent insoluble (I) and detergent soluble (S) membrane fractions. Samples were prepared at either 4 °C or 37 °C. (B) Percentages of GP63 in detergent insoluble (black bars) and detergent soluble (gray bars) fractions were quantified. Error bars represent standard deviations from 3 experiments.
Figure S10. Sensitivity of ept− mutants to oxidative and nitrosative stress. Log phase promastigotes (●: WT, ○: ept−, ▼: ept−/+EPT) were inoculated in various concentrations of hydrogen peroxide (A) or SNAP (B). Culture densities were determined after 48 hours. Experiments were repeated three times and error bars represent standard deviations.
Figure S11. Sensitivity of ept− mutants to heat and acidic pH. (A–B) Stationary phase promastigotes were cultured at 27 °C or 37 °C/5% CO2. (C–D) Stationary phase promastigotes were incubated under either neutral (pH 7.4) or acidic (pH 5.0) conditions. Percentages of dead cells were determined every 12 or 24 hours. Experiments were repeated three times and error bars represent standard deviations. **: p < 0.01.
Figure S11. Lack of PME has little effect on autophagy during promastigote differentiation. (A–C) Promastigotes expressing GFP-ATG8 were cultured from log phase to stationary phase (day 1–day 4) in complete M199 medium. Localization of GFP-ATG8 was monitored by fluorescence microscopy in A and B (scale bars: 10 μm). Percentages of cells with punctuated autophagosomes were summarized in C (black bars: WT + GFP-ATG8; gray bars: ept− +GFP-ATG8; error bars represent standard deviations from three experiments). (D) Western-blot using an anti-GFP antibody to examine the presence of free GFP-ATG8 (cytosolic) and lipid-conjugated GFP-ATG8 (autophagosomal).
Figure S12. PME depletion does not affect autophagy during promastigote differentiation. (A–C) Promastigotes expressing GFP-ATG8 were cultured from log phase to stationary phase (day 1–day 4) in complete M199 medium. Localization of GFP-ATG8 was monitored by fluorescence microscopy in A–C (scale bars: 10 μm). Percentages of cells with punctuated autophagosomes were quantified in D (black bars: WT + GFP-ATG8; white bars: ept− + GFP-ATG8; gray bars: ept−/+EPT +GFP-ATG8; error bars represent standard deviations from three experiments). (E) Western blot using an anti-GFP antibody to examine the presence of free GFP-ATG8 (cytosolic) and lipid-conjugated GFP-ATG8 (*: autophagosomal).
Figure S13. Starvation-induced autophagy is normal in ept− mutants. Log phase promastigotes were transferred to phosphate buffered saline (PBS) and incubated for 4, 8, or 24 hours; percentages of cells with autophagosomes were determined by fluorescence microscopy (A). Percentages dead cells were determined by flow cytometry (B). Error bars represent standard deviations from two experiments. (C) Western blot was performed to detect the formation of lipid-conjugated GFP-ATG8 (*) during starvation.
Table S1. Sterol abundance in ept− mutants
Table S2. Uneven distribution of LPG in ept− mutants
Acknowledgments
We thank Dr. Stephen Beverley (Washington University School of Medicine) for providing the pXG-GFP-ATG8 plasmid, Dr. Robert McMaster (University of British Colombia) for providing the rabbit anti-Leishmania GP63 #235 antibody for Western blot, and Dr. Jay Bangs (University at Buffalo, SUNY) for providing the rabbit anti-T. brucei BiP antiserum.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- Bangs JD, Uyetake L, Brickman MJ, Balber AE, Boothroyd JC. Molecular cloning and cellular localization of a BiP homologue in Trypanosoma brucei. Divergent ER retention signals in a lower eukaryote. Journal of cell science. 1993;105(Pt 4):1101–1113. doi: 10.1242/jcs.105.4.1101. [DOI] [PubMed] [Google Scholar]
- Besteiro S, Williams RA, Morrison LS, Coombs GH, Mottram JC. Endosome sorting and autophagy are essential for differentiation and virulence of Leishmania major. The Journal of biological chemistry. 2006;281:11384–11396. doi: 10.1074/jbc.M512307200. [DOI] [PubMed] [Google Scholar]
- Bibis SS, Dahlstrom K, Zhu T, Zufferey R. Characterization of Leishmania major phosphatidylethanolamine methyltransferases LmjPEM1 and LmjPEM2 and their inhibition by choline analogs. Molecular and biochemical parasitology. 2014;196:90–99. doi: 10.1016/j.molbiopara.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman NE, Moser AB. Functions of plasmalogen lipids in health and disease. Biochimica et biophysica acta. 2012;1822:1442–1452. doi: 10.1016/j.bbadis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Brunner D, Frank J, Appl H, Schoffl H, Pfaller W, Gstraunthaler G. Serum-free cell culture: the serum-free media interactive online database. Altex. 2010;27:53–62. doi: 10.14573/altex.2010.1.53. [DOI] [PubMed] [Google Scholar]
- Cham BE, Knowles BR. A solvent system for delipidation of plasma or serum without protein precipitation. Journal of lipid research. 1976;17:176–181. [PubMed] [Google Scholar]
- de Ibarra AA, Howard JG, Snary D. Monoclonal antibodies to Leishmania tropica major: specificities and antigen location. Parasitology. 1982;85(Pt 3):523–531. doi: 10.1017/s0031182000056304. [DOI] [PubMed] [Google Scholar]
- Denny PW, Field MC, Smith DF. GPI-anchored proteins and glycoconjugates segregate into lipid rafts in Kinetoplastida. FEBS Lett. 2001;491:148–153. doi: 10.1016/s0014-5793(01)02172-x. [DOI] [PubMed] [Google Scholar]
- Farine L, Niemann M, Schneider A, Butikofer P. Phosphatidylethanolamine and phosphatidylcholine biosynthesis by the Kennedy pathway occurs at different sites in Trypanosoma brucei. Scientific reports. 2015;5:16787. doi: 10.1038/srep16787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford DA. Separate myocardial ethanolamine phosphotransferase activities responsible for plasmenylethanolamine and phosphatidylethanolamine synthesis. Journal of lipid research. 2003;44:554–559. doi: 10.1194/jlr.M200426-JLR200. [DOI] [PubMed] [Google Scholar]
- Gibellini F, Hunter WN, Smith TK. The ethanolamine branch of the Kennedy pathway is essential in the bloodstream form of Trypanosoma brucei. Molecular microbiology. 2009;73:826–843. doi: 10.1111/j.1365-2958.2009.06764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibellini F, Smith TK. The Kennedy pathway--De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB life. 2010;62:414–428. doi: 10.1002/iub.337. [DOI] [PubMed] [Google Scholar]
- Hanada T, Noda NN, Satomi Y, Ichimura Y, Fujioka Y, Takao T, Inagaki F, Ohsumi Y. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. The Journal of biological chemistry. 2007;282:37298–37302. doi: 10.1074/jbc.C700195200. [DOI] [PubMed] [Google Scholar]
- Henneberry AL, McMaster CR. Cloning and expression of a human choline/ethanolaminephosphotransferase: synthesis of phosphatidylcholine and phosphatidylethanolamine. The Biochemical journal. 1999;339(Pt 2):291–298. [PMC free article] [PubMed] [Google Scholar]
- Hsu FF, Kuhlmann FM, Turk J, Beverley SM. Multiple-stage linear ion-trap with high resolution mass spectrometry towards complete structural characterization of phosphatidylethanolamines containing cyclopropane fatty acyl chain in Leishmania infantum. J Mass Spectrom. 2014;49:201–209. doi: 10.1002/jms.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura Y, Imamura Y, Emoto K, Umeda M, Noda T, Ohsumi Y. In vivo and in vitro reconstitution of Atg8 conjugation essential for autophagy. The Journal of biological chemistry. 2004;279:40584–40592. doi: 10.1074/jbc.M405860200. [DOI] [PubMed] [Google Scholar]
- Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M, Noda T, Ohsumi Y. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408:488–492. doi: 10.1038/35044114. [DOI] [PubMed] [Google Scholar]
- Isnard A, Christian JG, Kodiha M, Stochaj U, McMaster WR, Olivier M. Impact of Leishmania infection on host macrophage nuclear physiology and nucleopore complex integrity. PLoS pathogens. 2015;11:e1004776. doi: 10.1371/journal.ppat.1004776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapler GM, Coburn CM, Beverley SM. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol Cell Biol. 1990;10:1084–1094. doi: 10.1128/mcb.10.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy EP. The synthesis of cytidine diphosphate choline, cytidine diphosphate ethanolamine, and related compounds. The Journal of biological chemistry. 1956;222:185–191. [PubMed] [Google Scholar]
- Lessig J, Fuchs B. Plasmalogens in biological systems: their role in oxidative processes in biological membranes, their contribution to pathological processes and aging and plasmalogen analysis. Current medicinal chemistry. 2009;16:2021–2041. doi: 10.2174/092986709788682164. [DOI] [PubMed] [Google Scholar]
- Lohner K. Is the high propensity of ethanolamine plasmalogens to form non-lamellar lipid structures manifested in the properties of biomembranes? Chem Phys Lipids. 1996;81:167–184. doi: 10.1016/0009-3084(96)02580-7. [DOI] [PubMed] [Google Scholar]
- McConville MJ, Homans SW, Thomas-Oates JE, Dell A, Bacic A. Structures of the glycoinositolphospholipids from Leishmania major. A family of novel galactofuranose-containing glycolipids. The Journal of biological chemistry. 1990;265:7385–7394. [PubMed] [Google Scholar]
- Menon AK, Eppinger M, Mayor S, Schwarz RT. Phosphatidylethanolamine is the donor of the terminal phosphoethanolamine group in trypanosome glycosylphosphatidylinositols. EMBO J. 1993;12:1907–1914. doi: 10.1002/j.1460-2075.1993.tb05839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagan N, Zoeller RA. Plasmalogens: biosynthesis and functions. Prog Lipid Res. 2001;40:199–229. doi: 10.1016/s0163-7827(01)00003-0. [DOI] [PubMed] [Google Scholar]
- Olivier M, V, Atayde D, Isnard A, Hassani K, Shio MT. Leishmania virulence factors: focus on the metalloprotease GP63. Microbes and infection/Institut Pasteur. 2012;14:1377–1389. doi: 10.1016/j.micinf.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Podinovskaia M, Descoteaux A. Leishmania and the macrophage: a multifaceted interaction. Future microbiology. 2015;10:111–129. doi: 10.2217/fmb.14.103. [DOI] [PubMed] [Google Scholar]
- Ralton JE, McConville MJ. Delineation of three pathways of glycosylphosphatidylinositol biosynthesis in Leishmania mexicana. Precursors from different pathways are assembled on distinct pools of phosphatidylinositol and undergo fatty acid remodeling. The Journal of biological chemistry. 1998;273:4245–4257. doi: 10.1074/jbc.273.7.4245. [DOI] [PubMed] [Google Scholar]
- Richmond GS, Gibellini F, Young SA, Major L, Denton H, Lilley A, Smith TK. Lipidomic analysis of bloodstream and procyclic form Trypanosoma brucei. Parasitology. 2010;137:1357–1392. doi: 10.1017/S0031182010000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin MR, Fairlamb AH. Transport of ethanolamine and its incorporation into the variant surface glycoprotein of bloodstream forms of Trypanosoma brucei. Molecular and biochemical parasitology. 1985;15:245–256. doi: 10.1016/0166-6851(85)90088-x. [DOI] [PubMed] [Google Scholar]
- Sacks DL, Perkins PV. Identification of an infective stage of Leishmania promastigotes. Science. 1984;223:1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- Signorell A, Jelk J, Rauch M, Butikofer P. Phosphatidylethanolamine is the precursor of the ethanolamine phosphoglycerol moiety bound to eukaryotic elongation factor 1A. The Journal of biological chemistry. 2008a;283:20320–20329. doi: 10.1074/jbc.M802430200. [DOI] [PubMed] [Google Scholar]
- Signorell A, Rauch M, Jelk J, Ferguson MA, Butikofer P. Phosphatidylethanolamine in Trypanosoma brucei is organized in two separate pools and is synthesized exclusively by the Kennedy pathway. The Journal of biological chemistry. 2008b;283:23636–23644. doi: 10.1074/jbc.M803600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Butikofer P. Lipid metabolism in Trypanosoma brucei. Molecular and biochemical parasitology. 2010;172:66–79. doi: 10.1016/j.molbiopara.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spath GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp Parasitol. 2001;99:97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- Spath GF, Epstein L, Leader B, Singer SM, Avila HA, Turco SJ, Beverley SM. Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc Natl Acad Sci USA. 2000;97:9258–9263. doi: 10.1073/pnas.160257897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus RG, Marchand M, Boon T, Louis JA. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7:545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Turco SJ, Descoteaux A. The lipophosphoglycan of Leishmania parasites. Annual review of microbiology. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- Vance DE, Li Z, Jacobs RL. Hepatic phosphatidylethanolamine N-methyltransferase, unexpected roles in animal biochemistry and physiology. The Journal of biological chemistry. 2007;282:33237–33241. doi: 10.1074/jbc.R700028200. [DOI] [PubMed] [Google Scholar]
- Walkey CJ, Yu L, Agellon LB, Vance DE. Biochemical and evolutionary significance of phospholipid methylation. The Journal of biological chemistry. 1998;273:27043–27046. doi: 10.1074/jbc.273.42.27043. [DOI] [PubMed] [Google Scholar]
- Wassef MK, Fioretti TB, Dwyer DM. Lipid analyses of isolated surface membranes of Leishmania donovani promastigotes. Lipids. 1985;20:108–115. doi: 10.1007/BF02534216. [DOI] [PubMed] [Google Scholar]
- WHO, M.c. 2015 http://www.who.int/mediacentre/factsheets/fs375/en/
- Williams RA, Mottram JC, Coombs GH. Distinct roles in autophagy and importance in infectivity of the two ATG4 cysteine peptidases of Leishmania major. The Journal of biological chemistry. 2013;288:3678–3690. doi: 10.1074/jbc.M112.415372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RA, Smith TK, Cull B, Mottram JC, Coombs GH. ATG5 is essential for ATG8-dependent autophagy and mitochondrial homeostasis in Leishmania major. PLoS pathogens. 2012;8:e1002695. doi: 10.1371/journal.ppat.1002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Hsu FF, Baykal E, Huang J, Zhang K. Sterol Biosynthesis Is Required for Heat Resistance but Not Extracellular Survival in Leishmania. PLoS pathogens. 2014;10:e1004427. doi: 10.1371/journal.ppat.1004427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Xin L, Soong L, Zhang K. Sphingolipid degradation by Leishmania major is required for its resistance to acidic pH in the mammalian host. Infection and immunity. 2011;79:3377–3387. doi: 10.1128/IAI.00037-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Barron T, Turco SJ, Beverley SM. The LPG1 gene family of Leishmania major. Mol Biochem Parasitol. 2004;136:11–23. doi: 10.1016/j.molbiopara.2004.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Beverley SM. Phospholipid and sphingolipid metabolism in Leishmania. Molecular and biochemical parasitology. 2010;170:55–64. doi: 10.1016/j.molbiopara.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Hsu FF, Scott DA, Docampo R, Turk J, Beverley SM. Leishmania salvage and remodelling of host sphingolipids in amastigote survival and acidocalcisome biogenesis. Molecular microbiology. 2005;55:1566–1578. doi: 10.1111/j.1365-2958.2005.04493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Pompey JM, Hsu FF, Key P, Bandhuvula P, Saba JD, Turk J, Beverley SM. Redirection of sphingolipid metabolism toward de novo synthesis of ethanolamine in Leishmania. EMBO J. 2007;26:1094–1104. doi: 10.1038/sj.emboj.7601565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang O, Wilson MC, Xu W, Hsu FF, Turk J, Kuhlmann FM, Wang Y, Soong L, Key P, Beverley SM, Zhang K. Degradation of host sphingomyelin is essential for Leishmania virulence. PLoS pathogens. 2009;5(12):e1000692. doi: 10.1371/journal.ppat.1000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R, Allen S, Barron T, Sullivan DR, Denny PW, Almeida IC, Smith DF, Turco SJ, Ferguson MA, Beverley SM. Ether phospholipids and glycosylinositolphospholipids are not required for amastigote virulence or for inhibition of macrophage activation by Leishmania major. The Journal of biological chemistry. 2003;278:44708–44718. doi: 10.1074/jbc.M308063200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. EGP and PC synthesis in Leishmania. (A) De novo synthesis of EGP (PME + PE) and PC. Dashed arrows represent potential links between the ethanolamine branch and choline branch. SPT: serine palmitoyltransferase; SK: sphingosine kinase; SPL: sphingosine-1-phosphate lyase; EK: ethanolamine kinase; EPCT: ethanolaminephosphate cytidylyltransferase; EPT: ethanolamine phosphotransferase; CK: choline kinase; CPCT: cholinephosphate cytidylyltransferase; C/EPT: choline/ethanolamine phosphotransferase. EtN: ethanolamine; EtN-P: ethanolamine phosphate; Cho: choline; Cho-P: choline phosphate; DAG: diacylglycerol; CMP: cytidine-monophosphate; CDP: cytidine-diphosphate; PME: plasmenylethanolamine; PE: (1,2-diacyl-)phosphatidylethanolamine; PC: (1,2-diacyl-) phosphatidylcholine. (B) Structures and full chemical names of a PME (top) and a PE (bottom).
Figure S2. Southern blot to confirm EPT deletion mutants and episomal add-backs. (A) Genomic DNA samples were digested with restriction enzymes (NcoI and XbaI), separated on a 0.8% agarose gel, and stained with ethidium bromide. (B) Southern blot was performed using a probe recognizing the open reading frame (ORF) of EPT. Expected sizes for pXG1-EPT (~8 Kb) and EPT (~ 4 Kb) were marked by arrows. M: 1 Kb DNA ladder, 1: LV39WT (5 μg), 2: ept− G-A (5 μg), 3: ept−/+EPT (0.5 μg), 4: ept−/+EPT (0.1 μg), 5: ept−/+EPT (0.05 μg).
Figure S3. Detection of EPT-GFP by western-blot. Cell lysates from log phase promastigotes were probed with either anti-GFP antibody (A) or anti-α-tubulin antibody (B). Lane 1: ept−/+EPT-GFP; lane 2: WT/+GFP. Predicted sizes for EPT-GFP and GFP are 72 KD and 27 KD, respectively.
Figure S4. Ept− mutants possess diminished amount of PME. Total lipids from day 2 stationary phase promastigotes (A: WT, B: ept−, C: ept−/+EPT) were analyzed by ESI/MS in the negative ion mode. A PE standard (std) at m/z 750.5 was added to each sample prior to lipid extraction. Portions of [M-H]− spectra are shown with PME (p18:0/18:2-PE and p18:0/18:1-PE at m/z 726.5 and 728.5, respectively) and PE (16:0/20:4-PE, 18:1/18:2-PE, [18:0/18:2 + 18:1/18:1]-PE, and 18:0/18:1-PE at m/z 738.4, 740.5, 742.5 and 744.5, respectively) marked by arrows. Peaks representing IPC (d16:1/18:0-IPC at m/z 778.5), alkyl-acyl-PI (a18:0/18:1-PI at m/z 849.6), and diacyl-PI (18:0/18:1-PI at m/z 863.6) are also indicated.
Figure S5. Ept− mutants contain elevated level of PC. Total lipids from WT, ept−, and ept−/+EPT promastigotes (log or stationary phase) were analyzed by ESI/MS in the positive ion mode. The abundance of PC was determined based on comparison to an internal standard (14:0/14:0-PC, provided at 5 × 107 molecules cell−1). Values from three independent experiments are averaged (error bars represent standard deviations). *: p < 0.05.
Figure S6. Ept− mutants show reduced EGP synthesis. Log phase promastigotes were labeled with [3H]-ethanolamine (A, C) or [14C]-choline (B, D) for 48 hours in complete M199 medium. Total lipids were extracted and dissolved in chloroform:methanol (1:2 v/v) at 2.0 × 106 cells/μl. Incorporation of [3H]-ethanolamine or [14C]-choline into Leishmania lipids was determined by scintillation counting and summarized in A and B. Error bars represent standard deviations from three experiments. *: p < 0.05. In C and D, lipids were separated by TLC (chloroform:methanol:water 65:25:4 v/v/v) and visualized by autoradiography. Each lane contained lipids from 1.0 × 107 cells. O: origin. F: solvent front.
Figure S7. Ept− mutants produce less LPG than WT parasites. Whole cell lysates (A) or culture supernatants (B) from log phase promastigotes were probed with anti-LPG or anti-α-tubulin antibodies in western-blots. (C) Flow cytometry analysis of promastigotes labeled with the WIC79.3 monoclonal antibody followed by a FITC-conjugated anti-mouse IgG secondary antibody. Black: WT labeled with secondary antibody only, red: WT, blue: ept−, yellow: ept−/+EPT.
Figure S8. GP63 localization is not affected in ept− mutants. WT (A), ept− (B), and ept−/+EPT (C) promastigotes (day 3 stationary phase) were labeled with an anti-GP63 monoclonal antibody and subjected to immunofluorescence microscopy. Left column: anti-GP63; Middle column: DIC; Right column: DNA staining. Scale bars: 10 μm.
Figure S9. GP63 association with DRM is not affected in ept− mutants. (A) Western-blot of GP63 in detergent insoluble (I) and detergent soluble (S) membrane fractions. Samples were prepared at either 4 °C or 37 °C. (B) Percentages of GP63 in detergent insoluble (black bars) and detergent soluble (gray bars) fractions were quantified. Error bars represent standard deviations from 3 experiments.
Figure S10. Sensitivity of ept− mutants to oxidative and nitrosative stress. Log phase promastigotes (●: WT, ○: ept−, ▼: ept−/+EPT) were inoculated in various concentrations of hydrogen peroxide (A) or SNAP (B). Culture densities were determined after 48 hours. Experiments were repeated three times and error bars represent standard deviations.
Figure S11. Sensitivity of ept− mutants to heat and acidic pH. (A–B) Stationary phase promastigotes were cultured at 27 °C or 37 °C/5% CO2. (C–D) Stationary phase promastigotes were incubated under either neutral (pH 7.4) or acidic (pH 5.0) conditions. Percentages of dead cells were determined every 12 or 24 hours. Experiments were repeated three times and error bars represent standard deviations. **: p < 0.01.
Figure S11. Lack of PME has little effect on autophagy during promastigote differentiation. (A–C) Promastigotes expressing GFP-ATG8 were cultured from log phase to stationary phase (day 1–day 4) in complete M199 medium. Localization of GFP-ATG8 was monitored by fluorescence microscopy in A and B (scale bars: 10 μm). Percentages of cells with punctuated autophagosomes were summarized in C (black bars: WT + GFP-ATG8; gray bars: ept− +GFP-ATG8; error bars represent standard deviations from three experiments). (D) Western-blot using an anti-GFP antibody to examine the presence of free GFP-ATG8 (cytosolic) and lipid-conjugated GFP-ATG8 (autophagosomal).
Figure S12. PME depletion does not affect autophagy during promastigote differentiation. (A–C) Promastigotes expressing GFP-ATG8 were cultured from log phase to stationary phase (day 1–day 4) in complete M199 medium. Localization of GFP-ATG8 was monitored by fluorescence microscopy in A–C (scale bars: 10 μm). Percentages of cells with punctuated autophagosomes were quantified in D (black bars: WT + GFP-ATG8; white bars: ept− + GFP-ATG8; gray bars: ept−/+EPT +GFP-ATG8; error bars represent standard deviations from three experiments). (E) Western blot using an anti-GFP antibody to examine the presence of free GFP-ATG8 (cytosolic) and lipid-conjugated GFP-ATG8 (*: autophagosomal).
Figure S13. Starvation-induced autophagy is normal in ept− mutants. Log phase promastigotes were transferred to phosphate buffered saline (PBS) and incubated for 4, 8, or 24 hours; percentages of cells with autophagosomes were determined by fluorescence microscopy (A). Percentages dead cells were determined by flow cytometry (B). Error bars represent standard deviations from two experiments. (C) Western blot was performed to detect the formation of lipid-conjugated GFP-ATG8 (*) during starvation.
Table S1. Sterol abundance in ept− mutants
Table S2. Uneven distribution of LPG in ept− mutants