Abstract
Impaired cardiorespiratory fitness is associated with inferior survival in patients preparing to undergo hematopoietic cell transplantation (HCT). Exercise training based on short, higher-intensity intervals has the potential to efficiently improve cardiorespiratory fitness. We studied home-based interval exercise training (IET) in 40 patients prior to autologous (N=20) or allogeneic (N=20) HCT. Each session consisted of 5, three-minute intervals of walking, jogging, or cycling at 65-95% maximal heart rate (MHR) with 3 minutes of low intensity exercise (<65% MHR) between intervals. Participants were asked to perform sessions at least 3 times weekly. The duration of the intervention was at least 6 weeks, depending on each patient’s scheduled transplantation date. Cardiorespiratory fitness was assessed from a peak oxygen consumption test (VO2peak) and a 6 minute walk (6MWD) before and after the intervention period. For the autologous HCT cohort, improvements in VO2peak (p=0.12) and 6MWD (p=0.19) were not statistically significant. For the allogeneic cohort, the median VO2peak improvement was 3.7ml/kg*min (p=0.005) and the median 6MWD improvement was 34 meters (p=0.006). Home-based, interval exercise training can be performed prior to HCT and has the potential to improve cardiorespiratory fitness.
Keywords: hematopoietic stem cell transplantation, outcome assessment, physical fitness, quality of life, exercise
Introduction
There is mounting evidence that cardiorespiratory fitness is strongly associated with survival in cancer and non-cancer populations 1-4. Impaired cardiorespiratory fitness may also be associated with the development of malignancy 5-7. Within the field of exercise physiology, cardiopulmonary exercise testing performed using indirect calorimetry is considered the gold standard for the assessment of maximal oxygen uptake, and may predict who is more likely to survive hematopoietic cell transplantation (HCT) 8-10. Due to the association of cardiorespiratory fitness with mortality, several investigators have developed exercise programs for cancer patients, with results suggesting a net positive impact of exercise upon health-related quality of life, treatment toxicity, relapse incidence, and overall mortality in several cancer settings 11-15.
Currently, most exercise recommendations for cancer patients are directed toward survivors, and statements about intensity are general in nature 16. Many of the exercise studies in cancer have been performed in patients with breast cancer, and transplant exercise studies have usually been performed in the post-transplant setting 17. However, peri-transplant exercise interventions have potential to mitigate post-transplant toxicity. Wiskemann et al. 18 demonstrated significant improvements in post-transplant fatigue and physical fitness/functioning as a result of moderate peri-transplant exercise, and also showed that exercise after allogeneic transplant may improve mortality.19 Though a large, recently conducted study of peri-transplant exercise training did not demonstrate an improvement in post-transplant health-related quality of life, this study had a very different design than the above studies and was an intervention of significantly lower intensity. 20
Recent evidence supports the use of higher intensity exercise as an approach for inducing rapid improvements in cardiorespiratory fitness 21, 22. As a result of the intense treatment utilized in HCT, and the accompanying effects of HCT treatment upon cardiorespiratory fitness, pulmonary function, and skeletal muscle capacity, exercise intensity may be an important factor in this population to mitigate transplant toxicity 23, 24. Higher intensity exercise approaches have begun to be explored in cancer patients 25-27. Home-based training utilizing higher-intensity intervals of exercise may offer several advantages, including modifiability to fit the needs of patients, adaptability to the home setting, and requirement of less overall time than traditional approaches to improve fitness 28-30.
The purpose of this study was to evaluate the feasibility and physiological effects of a home-based interval exercise training (IET) intervention for cancer patients in the pre-hematopoietic stem cell transplant period. We sought to address a key limitation in the field of exercise oncology research, namely that home-based exercise prescriptions are insufficiently personalized and/or lacking the appropriate adaptability and intensity necessary to achieve improvements in fitness and long-term cancer outcomes. We also wished to evaluate the physiological effects from a tailored program that patients could realistically complete in the pre-transplant period. We drew upon data from supervised high-intensity interval training in other populations suggesting that interval-based exercise efficiently improves cardiorespiratory fitness within a period of weeks 30, 31. We hypothesized that a home-based, interval training intervention, personalized by targeting participant heart rate percentages derived from individual maximal heart rates during exercise testing, could be feasibly performed by cancer patients in the weeks leading up to HCT. This hypothesis was tested in separate cohorts of autologous and allogeneic pre-transplant patients.
Materials and Methods
Participants
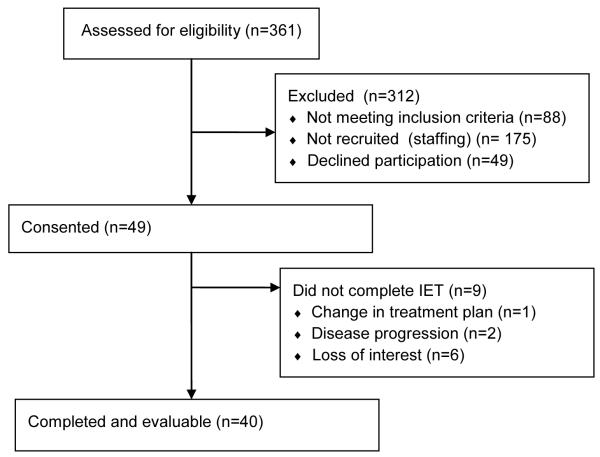
Participants between the ages of 18-75 years were recruited from among patients preparing to undergo autologous or allogeneic HCT at the University of North Carolina from 07/2013 through 10/2014 (Table 1). In order to participate, patients had an upcoming planned autologous or allogeneic transplant with enough time to accommodate a six week exercise intervention. Participants could not have received erythropoiesis-stimulating agents (ESAs) within four weeks prior to enrollment, nor could they have a comorbid illness that would preclude participation in maximal effort exercise testing or regular exercise, as determined by the treating physicians. Of the 98 patients approached for enrollment and meeting eligibility criteria, 49 agreed to participate, signing an approved consent form (Figure 1). Of the 49 that consented, 9 withdrew from the study as a result of a change in treatment plan, disease progression, or lack of interest. The final cohort analyzed was a total of 40 patients (20 autologous and 20 allogeneic transplant recipients). This protocol was approved by the Biomedical Institutional Review Board at the University of North Carolina and all procedures are in accordance with the Helsinki Declaration of 1975. All participants provided written informed consent prior to participation in any study-related activities
Table 1.
Patient characteristics at baseline testing. Values are presented as median [IQR].
| Gender | Age (yrs) | Height (cm) | Weight (kg) | Body Mass Index (kg/m2) |
|
|---|---|---|---|---|---|
| Autologous (n=20) | 14 Male 6 Female |
60.5 [54.5 - 68.0] |
172.8 [165.5 - 177.25] |
85.0 [73.4 - 97.8] |
28.6 [26.5 - 31.0] |
| Allogeneic (n=20) | 12 Male 8 Female |
52.5 [45.0 - 63.0] |
171.6 [162.6 - 180.0] |
75.4 [68.7 - 97.9] |
27.0 [23.6 - 31.2] |
Figure 1.
Flow diagram.
Physiological Testing
Physiological testing occurred before and after the 6-week home-based IET program. All participants underwent baseline maximal cardiopulmonary exercise testing (CPET) with cycle ergometry (VO2peak) as previously described 9. Briefly, this graded exercise test was completed on an electronically braked cycle ergometer (Corival 400, Lode, Gronigen, The Netherlands). Participants were fitted with a facemask (NRB1, Hans Rudolph Inc., Kansas City, MO) in order to ensure a secure seal around the nose and mouth. Participants completed a 2 minute warm-up with no resistance, maintaining pedal cadence between 50-70 rpm. The initial stage was set to 25 watts (W) for all participants. Subsequent stages were increased based on the subject’s fitness level and leg strength (10-25W), as determined by an exercise physiologist. Workloads were kept consistent for pre- and post-testing. Respiratory gases were monitored continuously and analyzed with open-circuit spirometry using a calibrated metabolic cart (True One 2400, Parvomedics, Provo, UT). Data was averaged over 15-seconds, with the three highest 15-second oxygen consumption values from the final minute identified as VO2peak. Heart rate was monitored continuously throughout the duration of the protocol using a polar heart rate strap (Model FT1, Polar, Inc., Lake Success, NY). The VO2peak test was conducted to determine cardiorespiratory fitness and to establish participant-specific maximal heart rates (MHR). A 6-minute walk distance (6MWD) was also obtained at baseline using a standardized protocol. Patients walked a 15 meter flat corridor unaccompanied, turning 180° every 15 m in the allotted 6 minute time frame. Patients were allowed to rest if needed, and time remaining was called every 2 minutes. After the 6 minutes, total distance travelled was calculated, and was reported as the 6MWD.
Interval Exercise Training
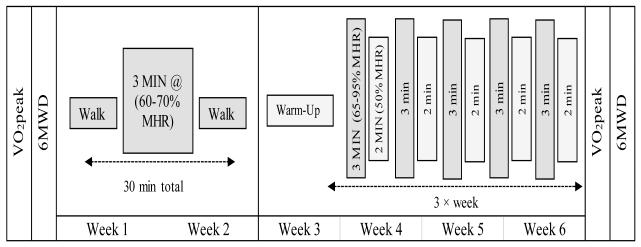
Following baseline exercise testing, participants were educated about how to participate in IET at home using target heart rates established from the VO2peak test. An exercise physiologist discussed which mode might be most advantageous for that patient to achieve target heart rates. Modes performed by the participants included variations in walking, running, and/or cycling; participants were encouraged to maintain the same mode throughout the duration of the study. All participants were provided with heart rate monitors (FTI, Polar Inc.) to facilitate training. Monitors were set according to calculated training zones, providing a beep when a patient achieved the HR zone. For weeks one and two of the 6-week home-based intervention, participants were asked to perform three, 30 minute walking sessions with one, 3-minute higher intensity interval (60-70% MHR) per session. For the subsequent 4 weeks, participants were asked to perform a greater volume and intensity of IET training consisting of five, 3-minute higher intensity intervals (65-95% MHR) during each 30 minute session, 3 times per week. Participants were instructed to reduce intensity or rest between intervals (Figure 2). Because participants were allowed to train at an MHR as low as 65%, this was not strictly a high intensity training program by American College of Sports Medicine (ACSM) standards,32 though most participants did achieve a high percentage of MHR (see results). All training sessions were recorded on a paper log, including maximum heart rate achieved per session and date of completion. Additionally, all participants were provided with accelerometers (FitBit Flex, Fitbit, Inc., San Francisco, CA) and were instructed to wear these throughout the duration of the home-based exercise intervention, through hospitalization for transplant, and for four weeks post discharge. Participants were also instructed to record completion of each session and maximum heart rate achieved per session on an interval training log. Weekly calls were provided by the study team in order to provide motivation, answer questions, and to address potential issues such as a declining health status, equipment dysfunction, or intervention related adverse events. Participants were also encouraged to contact the study team at any time to discuss any issues encountered during the IET program. After the completion of the IET program and prior to transplantation, participants underwent the same fitness assessments performed at baseline which included a maximal cardiopulmonary exercise test (VO2peak) and 6MWD.
Figure 2.
Overview of Exercise Intervention
Statistical Analysis
Descriptive statistics (including medians, interquartile ranges (IQR), and percentages) were calculated for both cohorts separately. Feasibility of the program was evaluated by adherence data, including number of weeks completed and number of IET sessions initiated and completed, respectively. Physiological effects (e.g. VO2peak, 6MWD) were evaluated on the change scores from pre to post-training using Wilcoxon Signed Rank tests, with a p-value of less 0.05 considered significant. Sample size for this study was based on previous data from the authors 30, anticipating a 2 ml/kg*min change in VO2peak with a standard deviation of 2.5 ml/kg*min. A total of 20 patients, in this single arm, non-randomized intervention, were needed to achieve 92% power. Due to the novelty of the intervention and the ability to evaluate autologous and allogeneic groups separately, 20 patients per group were recruited. Analyses were conducted using SAS Statistical Software (version 9.3, Cary, NC).
Results
Of the 40 total patients enrolled, disease classification consisted of acute myeloid leukemia (n=6 allo); acute lymphoblastic leukemia (n=2 allo); multiple myeloma (n=13 auto; n=1 allo), chronic myeloid leukemia (n=1 auto; n=2 allo); myelodysplastic syndrome (n=4 allo); non hodgkin lymphoma (n=3 auto; n=1 allo); myelofibrosis (n=1 allo); aplastic anemia (n=1 allo) and other (n=3 auto; n=1 allo).
Adherence and Efficacy
Autologous Transplant Cohort
Among pre- autologous transplant recipients, most patients (n=16) had at least 6 weeks before their transplant, with the remaining having 3-5 weeks available prior to transplant. For the 20 patients in this group, 6 (30%) participated in more than 75% of the training sessions. The entire group completed a median of 39% of the IET sessions. Among all autologous recipients, initiation of a median of 7 total IET sessions (IQR 3-15) were recorded during the intervention period. The median duration of each IET session was 30 minutes. The highest recorded maximal heart rate percentage (MHR, from the Karvonen Prediction Equation) achieved at any time during IET sessions was a median of 94.6% MHR. Recorded daily activity of participants using accelerometry was a median of 5546 steps per week (IQR 3929-7469) throughout the intervention period.
Evaluation of the physiological change from pre- to post-intervention for VO2peak demonstrated a median increase of 1.1 ml/kg*min (p=0.121), and a median increase of 30.1m for the 6MWD (p=0.19) (Table 2 and Figure 3).
Table 2.
Adherence and efficacy data for autologous and allogeneic patients. Values are reported as medians.
| Autologous Cohort | Allogeneic Cohort | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Pre | Change | p-value | Pre | Change | p-value | |
|
|
|
|||||
| VO2peak (ml/kg*min) | 16.1 | +1.1 | 0.12 | 18.1 | +3.7 | 0.005 |
|
| ||||||
| VO2peak (L/min) | 1.23 | +0.15 | 0.09 | 1.33 | +0.31 | 0.004 |
|
| ||||||
| 6MWD (m) | 525 | +30 | 0.19 | 495 | +34 | 0.006 |
|
| ||||||
| RER (pre-post) | 1.15-1.19 | 1.23-1.27 | ||||
|
| ||||||
| Number of weeks participated |
4.0 | 5.0 | ||||
|
| ||||||
| Number of IET sessions participated |
7.0 | 11.0 | ||||
|
| ||||||
| Median duration of IET sessions (minutes) |
30 | 30 | ||||
|
| ||||||
| Maximum HR achieved (bpm, % MHR) |
159, 91% | 161, 89% | ||||
|
| ||||||
| Daily Steps | 5547 | 5178 | ||||
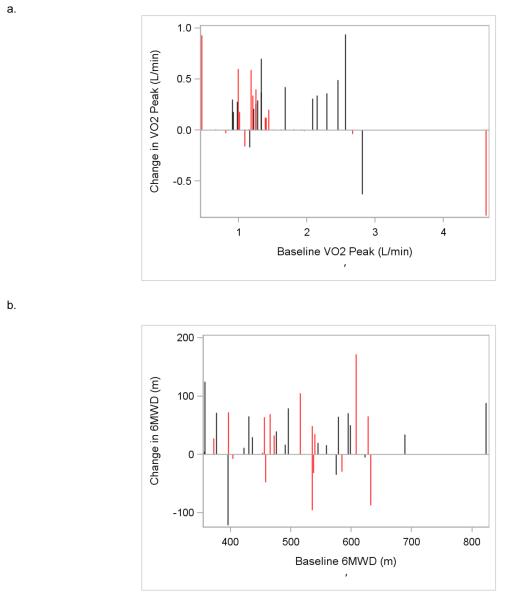
Figure 3.
VO2peak (a) and 6MWD (b) changes on an individual participant basis. Red vertical lines represent participants from the autologous transplant cohort, and black vertical lines represent participants from the allogeneic transplant cohort.
Allogeneic Transplant Cohort
Among pre-allogeneic transplant recipients, all patients had 6 weeks available prior to transplant. Of the 18 total sessions available, 7 (35%) completed more than 75% of the IET sessions; the entire group completed a median of 47% of the IET sessions. Allogeneic recipients recorded initiation of a median of 11 total IET sessions (IQR 5-16). The median duration of each IET session was 30 minutes. The highest recorded maximal heart rate (MHR) percentage achieved at any time during IET sessions was a median of 91.3% MHR for the allogeneic cohort. Allogeneic recipients walked a median of 5178 steps per week (IQR 4186-6554) throughout the intervention period.
Analysis of VO2peak from pre- to post-intervention demonstrated a significant median improvement of 3.7 ml/kg*min (p=0.005) and a significant median improvement in 6MWD of 34m (p=0.006) in this cohort.
Safety and Adverse Events
There were no study-related adverse events identified in either the autologous or allogeneic cohort that were directly related to the exercise testing or exercise intervention. Adverse events could be reported by the study team, nursing staff, treating clinicians, or the patients themselves.
Discussion
This is one of the first reports of a home-based, personalized, interval exercise training program in cancer patients preparing to undergo hematopoietic cell transplantation. Our data show that the personalized IET program, based on maximal heart rate, could be performed at home and appeared to be safe. The IET intervention also resulted in improvements in cardiorespiratory fitness in patients, most notably in the pre-allogeneic transplant group. These findings are of clinical relevance, as pre-transplant cardiorespiratory fitness has been demonstrated to predict post-transplant survival 9.
Interestingly, the present results demonstrate that VO2peak values significantly improved after the exercise intervention in the pre-allogeneic transplant population, but cardiorespiratory fitness changes were not significant in the pre-autologous transplant population. Rates of adherence, and achievement of target heart rates, did not appear to differ significantly between these two cohorts, and despite the non-significance, the pre-autologous group did demonstrate a small median improvement in VO2peak. However, the differences between groups may be partially explained by a number of the autologous transplant patients undergoing stem cell mobilization and collection during or overlapping with the IET program. This observation is important for a few reasons. First, our data show that IET appears to be feasible and safe around the time of mobilization and collection. Second, it is possible that stem cell mobilization has a detrimental impact upon cardiorespiratory fitness as measured by CPET. On the other hand, the autologous transplant cohort appeared to experience rapid collection and robust stem cell yields. In this cohort, 100% underwent successful stem cell mobilization on the first attempt, using either etoposide chemo-mobilization or G-CSF mobilization per institutional protocol. 74% of patients collected adequate cells within 1 day of collection, with all other patients, except for 1 collecting within 2 days (the exception was a patient with a higher collection target to support 3 auto transplants for germ cell tumor, who required a third day). The median total stem cell dose collected for the autologous cohort was 6.2 × 106/kg CD34+ cells, with 5 patients collecting more than 10 × 106/kg and 1 patient collecting 32.4 × 10 6/kg CD34+ cells. Pre-clinical and clinical data suggests that exercise may enhance progenitor cell mobilization, lending some biological plausibility to these findings and suggesting further study in this area 33-35.
In contrast to the autologous cohort, the pre-allogeneic transplant cohort experienced a significant improvement in VO2peak measurements. The median improvement of 3.7 ml/kg*min represents a significant benefit in cardiorespiratory fitness for a short term intervention. It is possible that the interval-based nature of the intervention might help to explain this observation, as supervised interval training programs have been associated with efficient improvements in cardiorespiratory fitness in short periods of time in other, non-cancer populations 30, 36, 37. A randomized study would be required to demonstrate that the IET protocol was ultimately responsible for the fitness improvements that were seen in our pre-allogeneic transplant population, and to determine whether cardiorespiratory fitness improvements translate into improved post-transplant outcomes. Of note, it was apparent that responses and non-responses in the allogeneic transplant cohort could not be attributed solely to the degree of participation in the interval exercise training sessions. It is possible that factors related to underlying individual physiology or disease- or treatment-related effects may have contributed to differential responses among participants with similar levels of participation (or non-participation) in the intervention. Larger studies will be needed to adequately account for other potential predictors of response to this exercise intervention.
We acknowledge several limitations to this pilot study, and opportunities for improvement in follow-up work that will test the hypothesis that pre-transplant interval exercise training improves pre-transplant fitness and potentially post-transplant outcomes and survival. First, this was a non-randomized study with a relatively small sample, with findings that need validation in larger, randomized cohorts. Second, though we allowed a relatively wide range of targeted % MHR for the interval training, supported by the observation that exercise intensity ranges may be defined differently in cancer patients undergoing transplant than in healthy individuals 38. Third, the recruitment rate of eligible patients was not as high as observed in previous pre-transplant exercise studies. It is possible that the intensity of our intervention may have dissuaded potential participants, though other reversible workflow limitations in our clinical environment may have also influenced recruitment, which we plan to address in future studies. For example, improving the logistics of testing sessions around convenient times for patients, many of whom were traveling significant distances to our cancer center, would have facilitated better recruitment and will be addressed in subsequent work. Fourth, while feasible and safe, participation in the weekly interval sessions was lower than desired. However, participants reported maximal heart rates during their interval training that that were within the prescribed intensity range for the intervention. Additionally, because of the frequency and complexity of the home-based intervention, adherence rates may have been underestimated, as it was challenging for participants to remember to reliably record each completed session using paper diaries. Regardless, the observed improvements in cardiorespiratory fitness in the allogeneic cohort suggest that a low “dose” of interval training may be sufficient to achieve improvement in VO2peak. Further improvements to the study procedures to increase and reliably ascertain adherence to the home-based intervention may provide more confidence around the dose effect estimates of the intervention, and may potentially lead to even greater improvements in cardiorespiratory fitness.
Several strategies are available to improve motivation and adherence to this intervention in future work. For example, the rapid development and improvement of wearable technology offers the opportunity to further facilitate the uptake of home-based, personalized exercise prescriptions in transplant and non-transplant populations 39. Several commercially-available wearable devices now provide continuous heart rate tracking capabilities without the need for additional equipment, as was required in our protocol, and enable users to customize exercise bouts and heart rate targets. Importantly, the data tracking capabilities of these devices have significant potential to improve estimates of adherence, and potentially to eliminate the need for participants to manually log each completed IET session in a separate exercise diary. We anticipate that these capabilities may facilitate better intervention adherence in future personalized, home-based exercise prescription protocols.
Home-based, personalized intensive exercise programs have the potential to improve cardiorespiratory fitness in patients preparing to undergo hematopoietic cell transplantation. These programs may be applicable in many settings in which rapid improvement in physiological reserve is a key goal, including the pre-transplant and pre-surgical settings, but also among cancer survivors in general and potentially in the setting of certain types of chemotherapy. Just as there is a national movement towards the application of precision medicine within cancer treatment, the time is also right for the personalized application of exercise programming to improve long-term cancer outcomes.
Acknowledgements
Financial disclosure: The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR001109. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest statement: There are no conflicts of interest to report.
Authorship statement: All authors designed the study, collected and analyzed data, and wrote the manuscript. DW and AD performed the statistical analysis and edited the manuscript. All authors critically revised the manuscript for important intellectual content and approved the manuscript for publication.
References
- 1.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr., Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262(17):2395–2401. doi: 10.1001/jama.262.17.2395. e-pub ahead of print 1989/11/03. [DOI] [PubMed] [Google Scholar]
- 2.Jones LW, Watson D, Herndon JE, 2nd, Eves ND, Haithcock BE, Loewen G, et al. Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer. 2010;116(20):4825–4832. doi: 10.1002/cncr.25396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laukkanen JA, Lakka TA, Rauramaa R, Kuhanen R, Venalainen JM, Salonen R, et al. Cardiovascular fitness as a predictor of mortality in men. Arch Intern Med. 2001;161(6):825–831. doi: 10.1001/archinte.161.6.825. [DOI] [PubMed] [Google Scholar]
- 4.Slattery ML, Jacobs DR., Jr Physical fitness and cardiovascular disease mortality. The US Railroad Study. Am J Epidemiol. 1988;127(3):571–580. doi: 10.1093/oxfordjournals.aje.a114832. [DOI] [PubMed] [Google Scholar]
- 5.Lee CD, Blair SN. Cardiorespiratory fitness and smoking-related and total cancer mortality in men. Med Sci Sports Exerc. 2002;34(5):735–739. doi: 10.1097/00005768-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Sawada SS, Muto T, Tanaka H, Lee IM, Paffenbarger RS, Jr., Shindo M, et al. Cardiorespiratory fitness and cancer mortality in Japanese men: a prospective study. Med Sci Sports Exerc. 2003;35(9):1546–1550. doi: 10.1249/01.MSS.0000084525.06473.8E. [DOI] [PubMed] [Google Scholar]
- 7.Zhang P, Sui X, Hand GA, Hebert JR, Blair SN. Association of changes in fitness and body composition with cancer mortality in men. Med Sci Sports Exerc. 2014;46(7):1366–1374. doi: 10.1249/MSS.0000000000000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumann FT, Kraut L, Schule K, Bloch W, Fauser AA. A controlled randomized study examining the effects of exercise therapy on patients undergoing haematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45(2):355–362. doi: 10.1038/bmt.2009.163. [DOI] [PubMed] [Google Scholar]
- 9.Wood WA, Deal AM, Reeve BB, Abernethy AP, Basch E, Mitchell SA, et al. Cardiopulmonary fitness in patients undergoing hematopoietic SCT: a pilot study. Bone Marrow Transplant. 2013;48(10):1342–1349. doi: 10.1038/bmt.2013.58. [DOI] [PubMed] [Google Scholar]
- 10.Kelsey CR, Scott JM, Lane A, Schwitzer E, West MJ, Thomas S, et al. Cardiopulmonary exercise testing prior to myeloablative allo-SCT: a feasibility study. Bone Marrow Transplant. 2014;49(10):1330–1336. doi: 10.1038/bmt.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimeo F, Fetscher S, Lange W, Mertelsmann R, Keul J. Effects of aerobic exercise on the physical performance and incidence of treatment-related complications after high-dose chemotherapy. Blood. 1997;90(9):3390–3394. [PubMed] [Google Scholar]
- 12.Dimeo FC, Tilmann MH, Bertz H, Kanz L, Mertelsmann R, Keul J. Aerobic exercise in the rehabilitation of cancer patients after high dose chemotherapy and autologous peripheral stem cell transplantation. Cancer. 1997;79(9):1717–1722. [PubMed] [Google Scholar]
- 13.Jones LW, Eves ND, Mackey JR, Peddle CJ, Haykowsky M, Joy AA, et al. Safety and feasibility of cardiopulmonary exercise testing in patients with advanced cancer. Lung Cancer. 2007;55(2):225–232. doi: 10.1016/j.lungcan.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Jones LW, Peddle CJ, Eves ND, Haykowsky MJ, Courneya KS, Mackey JR, et al. Effects of presurgical exercise training on cardiorespiratory fitness among patients undergoing thoracic surgery for malignant lung lesions. Cancer. 2007;110(3):590–598. doi: 10.1002/cncr.22830. [DOI] [PubMed] [Google Scholar]
- 15.Speed-Andrews AE, Courneya KS. Effects of exercise on quality of life and prognosis in cancer survivors. Curr Sports Med Rep. 2009;8(4):176–181. doi: 10.1249/JSR.0b013e3181ae98f3. [DOI] [PubMed] [Google Scholar]
- 16.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 18.Wiskemann J, Dreger P, Schwerdtfeger R, Bondong A, Huber G, Kleindienst N, et al. Effects of a partly self-administered exercise program before, during, and after allogeneic stem cell transplantation. Blood. 2011;117(9):2604–2613. doi: 10.1182/blood-2010-09-306308. [DOI] [PubMed] [Google Scholar]
- 19.Wiskemann J, Kleindienst N, Kuehl R, Dreger P, Schwerdtfeger R, Bohus M. Effects of physical exercise on survival after allogeneic stem cell transplantation. Int J Cancer. 2015;137(11):2749–2756. doi: 10.1002/ijc.29633. [DOI] [PubMed] [Google Scholar]
- 20.Jacobsen PB, Le-Rademacher J, Jim H, Syrjala K, Wingard JR, Logan B, et al. Exercise and stress management training prior to hematopoietic cell transplantation: Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0902. Biol Blood Marrow Transplant. 2014;20(10):1530–1536. doi: 10.1016/j.bbmt.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gremeaux V, Drigny J, Nigam A, Juneau M, Guilbeault V, Latour E, et al. Long-term lifestyle intervention with optimized high-intensity interval training improves body composition, cardiometabolic risk, and exercise parameters in patients with abdominal obesity. Am J Phys Med Rehabil. 2012;91(11):941–950. doi: 10.1097/PHM.0b013e3182643ce0. e-pub ahead of print 2012/08/03. [DOI] [PubMed] [Google Scholar]
- 22.Kessler HS, Sisson SB, Short KR. The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Med. 2012;42(6):489–509. doi: 10.2165/11630910-000000000-00000. e-pub ahead of print 2012/05/17. [DOI] [PubMed] [Google Scholar]
- 23.Lakoski SG, Eves ND, Douglas PS, Jones LW. Exercise rehabilitation in patients with cancer. Nat Rev Clin Oncol. 2012;9(5):288–296. doi: 10.1038/nrclinonc.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elbl L, Vasova I, Tomaskova I, Jedlicka F, Kral Z, Navratil M, et al. Cardiopulmonary exercise testing in the evaluation of functional capacity after treatment of lymphomas in adults. Leuk Lymphoma. 2006;47(5):843–851. doi: 10.1080/10428190500402559. [DOI] [PubMed] [Google Scholar]
- 25.Thijs KM, de Boer AG, Vreugdenhil G, van de Wouw AJ, Houterman S, Schep G. Rehabilitation using high-intensity physical training and long-term return-to-work in cancer survivors. Journal of occupational rehabilitation. 2012;22(2):220–229. doi: 10.1007/s10926-011-9341-1. [DOI] [PubMed] [Google Scholar]
- 26.Persoon S, Kersten MJ, Chinapaw MJ, Buffart LM, Burghout H, Schep G, et al. Design of the EXercise Intervention after Stem cell Transplantation (EXIST) study: a randomized controlled trial to evaluate the effectiveness and cost-effectiveness of an individualized high intensity physical exercise program on fitness and fatigue in patients with multiple myeloma or (non-) Hodgkin's lymphoma treated with high dose chemotherapy and autologous stem cell transplantation. BMC Cancer. 2010;10:671. doi: 10.1186/1471-2407-10-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Weert E, Hoekstra-Weebers JE, May AM, Korstjens I, Ros WJ, van der Schans CP. The development of an evidence-based physical self-management rehabilitation programme for cancer survivors. Patient education and counseling. 2008;71(2):169–190. doi: 10.1016/j.pec.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 28.Lunt H, Draper N, Marshall HC, Logan FJ, Hamlin MJ, Shearman JP, et al. High intensity interval training in a real world setting: a randomized controlled feasibility study in overweight inactive adults, measuring change in maximal oxygen uptake. PLoS One. 2014;9(1):e83256. doi: 10.1371/journal.pone.0083256. e-pub ahead of print 2014/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith-Ryan AE. Enjoyment of high-intensity interval training in an overweight/obese cohort: A short report. Physiological Measurement. 2015 doi: 10.1111/cpf.12262. e-pub ahead of print In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith-Ryan AE, Melvin MN, Wingfield HL. High-intensity interval training: Modulating interval duration in overweight/obese men. Phys Sportsmed. 2015;43(2):107–113. doi: 10.1080/00913847.2015.1037231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keteyian SJ, Hibner BA, Bronsteen K, Kerrigan D, Aldred HA, Reasons LM, et al. Greater improvement in cardiorespiratory fitness using higher-intensity interval training in the standard cardiac rehabilitation setting. J Cardiopulm Rehabil Prev. 2014;34(2):98–105. doi: 10.1097/HCR.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 32.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 33.De Lisio M, Baker JM, Parise G. Exercise promotes bone marrow cell survival and recipient reconstitution post-bone marrow transplantation, which is associated with increased survival. Exp Hematol. 2013;41(2):143–154. doi: 10.1016/j.exphem.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 34.De Lisio M, Parise G. Characterization of the effects of exercise training on hematopoietic stem cell quantity and function. J Appl Physiol (1985) 2012;113(10):1576–1584. doi: 10.1152/japplphysiol.00717.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keser I, Suyani E, Aki SZ, Sucak AG. The positive impact of regular exercise program on stem cell mobilization prior to autologous stem cell transplantation. Transfus Apher Sci. 2013;49(2):302–306. doi: 10.1016/j.transci.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Talanian JL, Galloway SD, Heigenhauser GJ, Bonen A, Spriet LL. Two weeks of high-intensity aerobic interval training increases the capacity for fat oxidation during exercise in women. J Appl Physiol. 2007;102(4):1439–1447. doi: 10.1152/japplphysiol.01098.2006. [DOI] [PubMed] [Google Scholar]
- 37.Whyte LJ, Gill JM, Cathcart AJ. Effect of 2 weeks of sprint interval training on health-related outcomes in sedentary overweight/obese men. Metabolism. 2010;59(10):1421–1428. doi: 10.1016/j.metabol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Kuehl R, Scharhag-Rosenberger F, Schommer K, Schmidt ME, Dreger P, Huber G, et al. Exercise intensity classification in cancer patients undergoing allogeneic HCT. Med Sci Sports Exerc. 2015;47(5):889–895. doi: 10.1249/MSS.0000000000000498. [DOI] [PubMed] [Google Scholar]
- 39.Wood WA, Bennett AV, Basch E. Emerging uses of patient generated health data in clinical research. Mol Oncol. 2015;9(5):1018–1024. doi: 10.1016/j.molonc.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]