Abstract
Ever since proangiogenic growth factors have been used as a vascular medicine to treat tissue ischemia, efforts have been increasingly made to develop a method to enhance efficacy of growth factors in recreating microvascular networks, especially at low dose. To this end, we hypothesized that polysaccharides substituted with sulfate groups would amplify growth factor receptor activation and stimulate phenotypic activities of endothelial cells involved in neovascularization. We examined this hypothesis by modifying alginate with a controlled number of sulfates and using it to derive a complex with vascular endothelial growth factor (VEGF), as confirmed with fluorescence resonance energy transfer (FRET) assay. Compared with the bare VEGF and with a mixture of VEGF and unmodified alginates, the VEGF complexed with alginate sulfates significantly reduced the dissociation rate with the VEGFR-2, elevated VEGFR-2 phosphorylation level, and increased the number of endothelial sprouts in vitro. Furthermore, the VEGF-alginate sulfate complex improved recovery of perfusion in an ischemic hindlimb of a mouse due to the increase of the capillary density. Overall, this study not only demonstrates an important cofactor of VEGF but also uncovers an underlying mechanism by which the cofactor mitigates the VEGF-induced signaling involved in the binding kinetics and activation of VEGFR. We therefore believe that the results of this study will be highly useful in improving the therapeutic efficacy of various growth factors and expediting their uses in clinical treatments of wounds and tissue defects.
Keywords: vascular endothelial growth factor, fluorescence resonance energy transfer, binding kinetics, angiogenesis, alginate
Graphical Abstract
INTRODUCTION
The circulatory system consists of a network of arteries, veins, and capillaries tasked with moving blood throughout a body. Blood supplies cells with essential nutrients including oxygen, amino acids, and electrolytes. The loss or lack of blood supply to the cells within a tissue or organ is defined as ischemia and can cause cell death and dysfunction, and ultimately tissue/organ damage.1 The resulting ischemia often causes a number of vascular diseases, including ischemic heart disease and peripheral artery disease.2–4 Mild ischemic diseases are treated by noninvasive therapies such as diet, exercise, or prescription of certain cholesterol-lowering or anticoagulant drugs. More severe cases of ischemic disease, however, require invasive, surgical therapies such as endarterectomy or grafting/bypasses.5,6 These invasive therapies possess significant side effects and costs. Over the past 30 years, less-invasive treatments have been explored to restore or improve blood flow to ischemic tissues by stimulating angiogenesis and recreating new capillary or midsized blood vessels.7
Angiogenesis involves multiple steps controlled by multiple growth factors known to stimulate vascular sprouting from preexisting vessels.8,9 Specifically, vascular endothelial growth factor (VEGF) binds to one of the VEGF receptors (VEGFR) on the surface of endothelial cells.10 The binding of VEGF to VEGFR-2 activates internal cell signaling, which controls proliferation and migration of endothelial cells, and ultimately the formation of new blood vessels.11 Additionally, platelet-derived growth factor and angiopoietin attract smooth muscle cells and pericytes necessary for the new blood vessels to become mature and stable.12,13
Initially, angiogenesis-based revascularization therapies utilized VEGF delivered systemically through the bloodstream or locally to the target tissue.14 However, the quick clearance from the body and short half-life of VEGF led to substandard outcomes.15 To resolve these challenges, VEGF was frequently administered at high dosages, which caused severe side effects and resulted in dysfunctional, immature vasculature.16 Therefore, extensive efforts have been made to incorporate VEGF into a variety of materials that can release VEGF in a sustained manner and subsequently improve the efficacy of VEGF. These materials are fabricated in a form of biodegradable micro- and nanoparticles, microporous scaffolds, and hydrogels.17–19 Whereas these approaches reported some degrees of success, there are still grand needs to improve the ability of VEGF to activate blood vessel-forming cells with a reduced dosage.20 Our method, coupled with previously developed VEGF carriers, will substantially improve VEGF-based revascularization therapies.
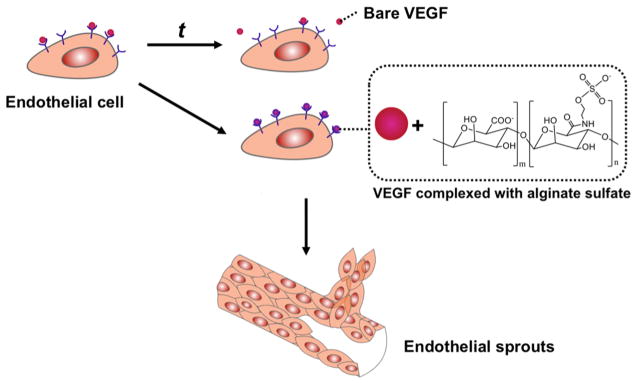
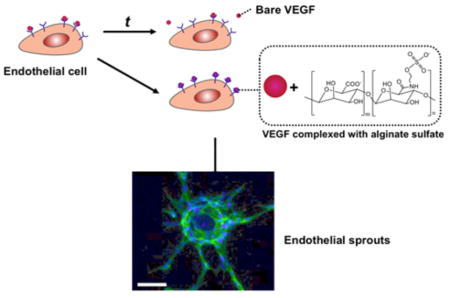
We hypothesize that the coadministration of VEGF and chemically modified alginate would significantly improve the binding of VEGF with VEGFR, activation of VEGFR phosphorylation and, ultimately, the efficacy of VEGF in treating ischemic tissue (Figure 1). To examine this hypothesis, alginate was modified with a controlled number of sulfate groups using carbodiimide chemistry.21,22 Association of the resulting alginate sulfates with VEGF was examined with fluorescence resonance energy transfer (FRET) between FRET donor and acceptors, each of which were conjugated to the alginate sulfates and VEGF, respectively. We then examined the extent by which the alginate sulfates enhanced VEGF-induced capillary sprouting by utilizing an in vitro angiogenesis assay. In parallel, we examined the effect of sulfated alginate on the binding kinetics of VEGF with cellular receptors using surface plasmon resonance (SPR) and the phosphorylation activation of VEGFR-2 of endothelial cells. Finally, the mixture of alginate sulfates and VEGF were injected in the ischemic hindlimb of a mouse to evaluate the role of alginate sulfates to improving recovery of perfusion.
Figure 1.
Schematic describing a strategy to enhance the binding of VEGF with VEGFR-2 using alginate sulfates and ultimately improve efficacy of VEGF to stimulating angiogenesis.
MATERIALS AND METHODS
Synthesis of Alginate Sulfates
Alginate was modified with sulfate groups using carbodiimide chemistry, as previously described.22 In brief, alginate with a large fraction of guluronic acid residues (LF20/40, FMC Technologies) was dissolved at a concentration of 1% in 0.1 M (2-(N-morpholino) ethanesulfonic acid) (MES) buffer (Sigma-Aldrich). Then, 1-hydroxybenzotriazole (HOBt, Fluka), 1-ethyl-3-(3-(dimethylamino)-propyl) carbodiimide (EDC, Thermo Scientific), and 2-aminoethyl hydrogen sulfate (Sigma-Aldrich) were dissolved in the alginate solution at a molar ratio of 2:2:1, respectively, and stirred overnight. The solution was dialyzed, sterilized via filtration, and lyophilized. The degree of sulfate incorporation was determined with inductively coupled plasma (ICP-MS; PerkinElmer–SciEx ELAN DRC). The resulting alginate sulfates were reconstituted in deionized water at a concentration of 4% (w/w).
FRET Assay To Evaluate Alginate Sulfate-VEGF Complex Formation
The alginate sulfates and VEGF were fluorescently labeled by attaching fluorescein (Invitrogen) as previously described.22 In brief, 5-(aminomethyl)fluorescein hydrochloride was attached to alginate sulfate by aqueous carbodiimide chemistry. Separately, Alexa Fluor 555 fluorophores containing succinimidyl ester groups were coupled to VEGF using a protein labeling kit (Invitrogen) according to the manufacturer’s protocol. Then, alginate sulfate-FITC and VEGF-Alexa Fluor 555 samples were mixed together and excited at 488 nm to evaluate the fluorescence emission intensity between 500 to 650 nm using a FluoroMax-4 spectrofluorometer (HORIBA JOBI-YVON). Pure alginate sulfate-FITC and VEGF-Alexa Fluor 555 solutions were tested as controls.
In Vitro Endothelial Sprouting Assay
Primary human vein endothelial cells (HUVECs) (Lonza, Walkersville, MD) were cultured in endothelial growth media-2 (EGM-2) in accordance with the manufacturer’s instructions. HUVECs, passaged less than six times, were utilized in the sprouting assay, as previously described. In brief, dextran microcarriers (Sigma-Aldrich, St. Louis, MO) were swollen over 4 h and sterilized by autoclaving. Roughly 1500 microcarriers were suspended in a test tube with 1 × 107 HUVECs and intermittently agitated for 4 h to allow the HUVECs to adhere to the microcarriers. The coated microcarriers were mixed with a solution of 4 mg/mL fibrin and 4 U/mL aprotinin. In a 96-well plate, the fibrinogen and microcarrier solution was mixed with 25 units/mL of human thrombin at a ratio of 1:1. The alginate or alginate sulfate was also mixed with fibrinogen solution to load them in the fibrin gel. The concentration of the alginate sulfates in the fibrin gel was varied from 0.0001 to 1.5 μM. The solution was incubated at 37 °C for 30 min to form a hydrogel, and fresh media supplemented with 15 ng/mL of VEGF was placed on top of the hydrogels. Bright-field images were taken intermittently to monitor the sprouting process (Leica DMI 4000), and measurements were taken using image processing software (ImageJ).
Cellular Imaging
After incubation, the fibrin gels were washed three times in PBS and fixed with a formaldehyde solution (Sigma-Aldrich) overnight at 4 °C. The fixed gels were washed twice with PBS for 1 h, and the cells were permeabilized with 0.5% Triton X-100 (Sigma-Aldrich) and 5% dry milk in PBS for 2 h. The intracellular actin fibers were stained using 5 U/mL Oregon Green 514 phalloidin (Invitrogen), and the nuclei were stained with 300 nM/mL DAPI (Invitrogen) in PBS for 4 h. The cells were subsequently washed with PBS every 2 to 4 h for 12 h and observed with a Leica TCS SP2 confocal microscope.
VEGFR-2 Activation Assay
HUVECs were serum starved for 12 h prior to treatment. The cells were exposed to 20 ng/mL of VEGF for 5 min. The cells were then rinsed with PBS, and all the liquid was aspirated from the well. Then, 330 μL of lysis buffer was added to the wells, and the cells were kept ice cold and agitated for 20 min. The surface was scraped and all liquid was removed from the well. The insoluble material was removed via centrifuge at 4 °C for 10 min (Eppendorf). The activation of the cellular VEGFR-2 was quantified with a Human pVEGFR-2 ELISA kit (R&D Systems), according to the manufacturer’s instructions. In short, a sandwich-based ELISA was conducted, and the absorbance of the samples was measured at 450 nm using a plate reader (Biotek Instruments). The activation of the cellular VEGFR-2 was normalized to the total amount of VEGFR-2. The total amount of VEGFR-2 was measured with a Human VEGFR-2 ELISA kit (R&D Systems) in parallel to the pVEGFR-2 ELISA kit.
Analysis of VEGF–VEGFR2 Binding Kinetics Using Surface Plasmon Resonance
A gold sensor chip (GE Healthcare, USA) was modified to present an 11-mercaptoundecanoic acid (MUA, Aldrich) monolayer by injecting MUA solution into the flow cell in a Biacore 3000 (GE Healthcare, USA) for 30 min at 25 °C. The carboxylic groups of the MUA monolayer were then activated by flowing 0.2 M EDC and 0.05 M N-hydroxysuccinimide (NHS, Aldrich) solutions through the flow cell for 7 min. After activation, 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE, Sigma) was chemically linked to the MUA layer by flowing DPPE solution until the response unit was saturated. The remaining NHS-ester groups on the MUA surface were blocked by injecting 1.0 M ethanolamine hydrochloride into the flow cell. Then the top layer was built by injecting a solution of recombinant human VEGFR-2 for 5 min. Finally, the mixture of VEGF and alginate sulfates or unmodified alginate suspended in PBS at a concentration of 2 μg/mL were injected into the flow cell to examine the association and dissociation rates of the VEGF complex with the gold sensor chip modified with DPPC and VEGFR-2. The media flow rate was kept constant at 5.0 μL/min. The kinetic data from SPR sensorgrams were obtained with the assistance of BIA evaluation version 4.1, where a 1:1 Langmuir binding model was applied to quantify the association and dissociation rates.23
Surgery and Analysis of Hindlimb Ischemia
The hindlimb ischemia surgery was conducted according to a protocol approved by the Illinois’ Institutional Animal Care and Use Committee. The animals used were C57BL/6J SCID mice (Jackson Laboratories, ME), and they were aged 9 weeks prior to surgery. The animals were anesthetized with a xylazine (10 mg/kg) and ketamine (100 mg/kg) cocktail via intraperitoneal injection. The hair was removed from the lower half of the animal. A small incision was made on the upper thigh of the animal, and the femoral artery and vein were exposed. The femoral artery and vein were ligated, proximal and distal, with 5-0 Ethilon sutures (Johnson and Johnson, NJ), to stop blood flow to the lower limb. A 15 μL fibrin gel, in which VEGF and alginate derivatives were incorporated, was injected between the ligated ends of the artery and vein. The surgical site was closed with 5-0 Ethilon sutures, as well. The blood flow recovery was monitored with Laser Doppler Perfusion Imaging (LDPI; Perimed AB, Sweden) for the ischemic and non-ischemic limbs.
The hindlimb tissue was dissected from the animal between the two suture knots marking the surgery-induced ischemic site. The tissue was fixed in zinc-buffered formalin overnight at 4 °C. The tissues were transferred to 70% ethanol for 12 h. The tissues were processed and embedded in paraffin. The tissues were sectioned (5 μm thick) and placed on poly lysine coated slides.
The paraffin was removed from the slides with histoclear (Fisher Scientific), and the sections were rehydrated. The tissues were stained with primary antimouse CD31 antibody (1:50) (BD Pharmingen, CA), followed by a biotinylated secondary antirat mouse (1:100) (BD Pharmingen). The staining was amplified by Tyramide Signal Amplification (TSA) Biotin System (PerkinElmer Life Sciences, MA). The stain was developed with DAB chromagen substrate (BD Pharmingen). The tissues were counterstained with Mayer’s Hematoxylin (Sigma-Aldrich and blued using 0.5% sodium biocarbonate (Sigma-Aldrich).
Statistical Analysis
Error bars represent standard deviation, with n = 4 for all experiments. Statistical significance was determined using one-way ANOVA followed by Tukey’s Multiple Comparison Test (p < 0.05).
RESULTS
Chemical Modification and Characterization of Alginate with Sulfate Groups
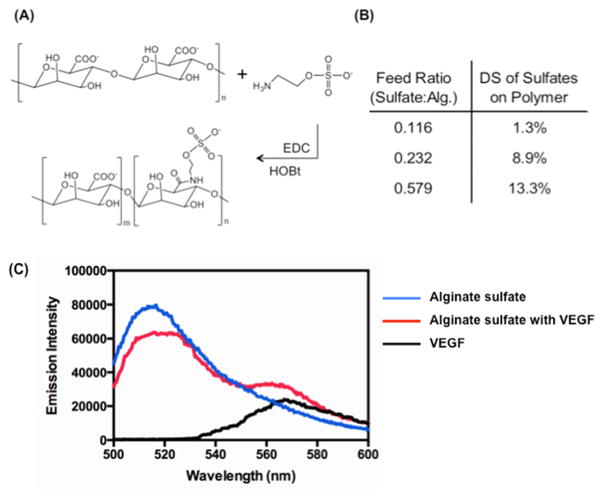
Alginate was modified with a controlled number of sulfate groups via chemical reaction between carboxylate groups of alginate and primary amine groups of 2-aminoethyl hydrogen sulfate (Figure 2A). The degree of substitution of sulfate groups linked to alginate (DSsulfate) was able to be controlled from 1.3 to 13% by increasing the molar ratio of 2-aminoethyl hydrogen sulfate to the uronic acid of alginate, as confirmed with inductively coupled plasma–mass spectrometry (Figure 2B).
Figure 2.
(A) Chemical structure of alginate and the reaction scheme for the aqueous carbodiimide chemistry used to modify alginate. (B) Amount of sulfate groups incorporated via carbodiimide chemistry to the alginate polymer chain, determined by inductively coupled plasma–mass spectrometry. The sulfate incorporation is shown in terms of the degree of substitution (DS) to the uronic acids on the alginate backbone. (C) FRET assay to evaluate the association between VEGF and alginate sulfates. The VEGF was labeled with Alexa Fluor 555 (i.e., FRET acceptor) and alginate sulfates were labeled with fluorescein (i.e., FRET donor). All three samples were excited at wavelength of 488 nm.
The association between alginate sulfates and VEGF was evaluated by monitoring FRET between fluorescein (i.e., FRET donor) and Alexa Fluor 555 (i.e., FRET acceptor), each of which is coupled to the alginate sulfates and VEGF (Figure 2C). Upon excitation at wavelength of 488 nm, the aqueous mixture of fluorescently labeled alginate sulfates and VEGF displayed a decrease of the fluorescein emission intensity maximized at 520 nm as compared with the pure alginate sulfate solution. The aqueous mixture of alginate sulfates and VEGF also exhibited an increase of the Alexa Fluor 555 emission intensity maximized at 555 nm. Such decrease of the FRET donor emission intensity and increase of the FRET acceptor emission intensity mark FRET between the donor and acceptor.
Endothelial Sprouting with Alginate Sulfates
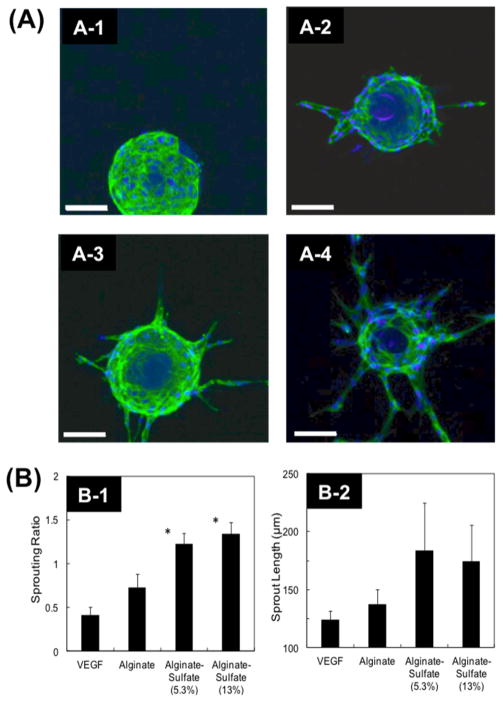
First, the effect of alginate and alginate sulfates on modulating VEGF-induced endothelial sprouting was examined using endothelial cell-coated microspheres suspended in a fibrin gel. The absence of VEGF in the media resulted in no sprout formation (Figure 3A-1,B-1). In contrast, the incorporation of VEGF at 15 ng/mL stimulated the HUVECs to form endothelial sprouts at a ratio of 0.4 sprouts per bead (Figure 3A-2,B-1). The inclusion of unmodified alginate in the fibrin matrix with VEGF resulted in a nearly 50% increase in the sprouting ratio over a fibrin gel without alginate (Figure 3A-3,B-1). More interestingly, the inclusion of alginate sulfates with DSsulfate of 8.9% molecules in the fibrin matrix at a concentration of 1 μM led to a 3-fold increase in the sprouting ratio from 0.4 to 1.2. Further increase of DSsulfate to 13% did not increase the sprouting ratio (Figure 3A-4,B-1). Alginate sulfates with DSsulfate of 8.9% or 13% also slightly increased the length of sprouts from 115 to nearly 165 μm. (Figure 3A-4,B-2).
Figure 3.
(A) Representative images of HUVECs sprouting in a fibrin gel after 8 days cultured media without VEGF (A-1), media with 15 ng/mL of VEGF (A-2), media with 15 ng/mL of VEGF and 1 μM alginate (A-3), and media with 15 ng/mL of VEGF and 1 μM alginate sulfates (DSsulfate = 5.3%) (A-4) (Scale = 100 μm). (B) Influence of VEGF, alginates, and alginate sulfates with controlled DSsulfate on the number of sprouts formed per bead (Sprouting Ratio) (B-1) and the length of sprouts (B-2) after 8 days in culture supplemented with 15 ng/mL VEGF (*p < 0.05). The alginate or alginate sulfates concentration was kept constant at 1 μM (*p < 0.05).
Cellular VEGFR-2 Activation by Alginate Sulfates
Next, the combined effect of VEGF and alginate or alginate sulfates on VEGFR-2 activation was examined to understand the mechanism by which the alginate sulfates improved VEGF-induced endothelial sprouting. The VEGFR-2 activation characterized by the phosphorylation of the receptor has been reported to modulate phenotypic activities of endothelial cells, such as proliferation, migration, and sprout formation.24 The phosphorylation level normalized to the total amount of VEGFR-2, which remained constant for all conditions, was slightly increased upon exposure of cells to VEGF only or the mixture of VEGF and alginate. Interestingly, cells exposed to the mixture of VEGF and alginate sulfates displayed nearly 2-fold increase in VEGFR-2 activation (Figure 4).
Figure 4.
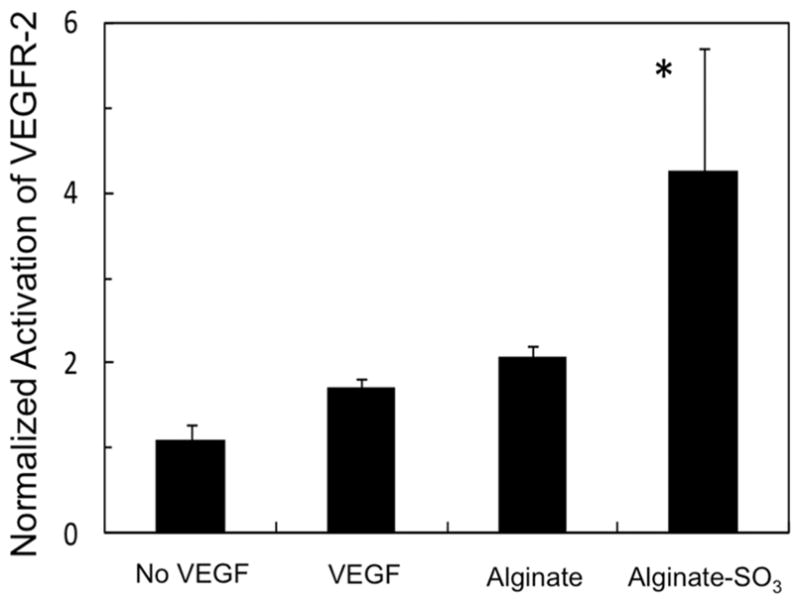
Activation level of VEGFR-2 normalized to the value for “no VEGF”. HUVECs were exposed to 20 ng/mL VEGF, except in the control condition, “No VEGF”. In addition, cells were cultured in the presence of 1 μM of alginate or alginate sulfates (DSsulfate = 5.3%) (*p < 0.05 with respect to the condition to expose HUVECs to bare VEGF).
VEGF–VEGFR Binding Kinetics Modulated by Alginate Sulfates
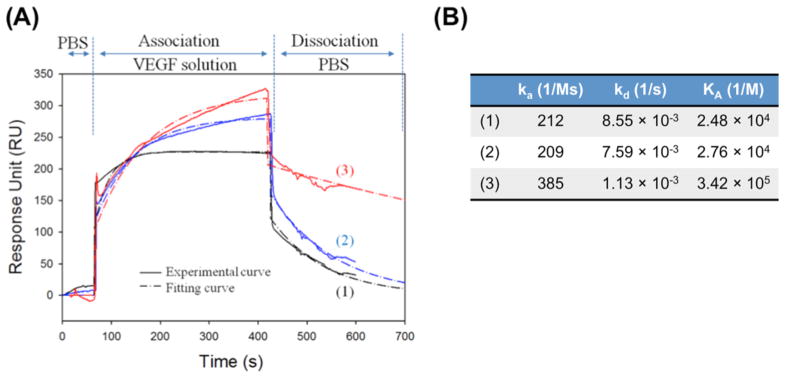
We examined whether the alginate sulfates modulate binding kinetics of VEGF–VEGFR by measuring association and dissociation rates of VEGF with VEGFR incorporated in the lipid bilayer of SPR spectroscopy. As expected, the unmodified alginate did not significantly alter the association rate (ka) and the dissociation rate (kd) of VEGF to VEGFR (Figure 5). In contrast, alginate sulfates led to almost 1.8-fold increase of ka and also 6.7 fold-decrease of kd. Therefore, alginate sulfates increased the VEGF–VEGFR binding affinity constant (KA), a ratio of ka to kd, by 1.2 times.
Figure 5.
(A) SPR sensogram for the association and disassociation of the bare VEGF (black line, 1), the mixture of VEGF and unmodified alginate (blue line, 2), and the complex of VEGF and alginate sulfates (DSsulfates = 13.3%) (red line, 3) to the VEGFR-2 immobilized on the lipid bilayer surface built on a gold chip. (B) Association (ka) and dissociation rates (kd) were quantified by fitting the RU response curve obtained in (A), to the Langmuir adsorption model. The binding affinity constant (KA) between VEGF and the VEGFR-2 was quantified by dividing ka by kd.
Treatment of Ischemic Hindlimb
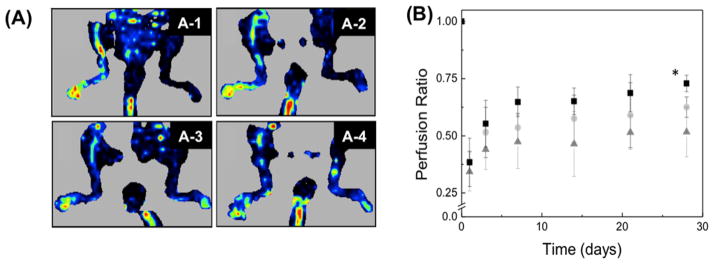
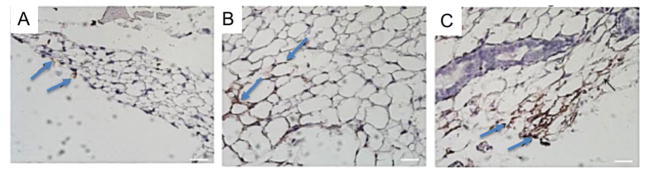
Finally, the effect of alginate and alginate sulfates on VEGF-mediated angiogenesis was evaluated in vivo by injecting the fibrin gel encapsulated with VEGF and alginate derivatives into an ischemic hindlimb. According to the LDPI image, the local injection of VEGF loaded in a fibrin gel increased the perfusion ratio, defined as a ratio of perfusion in ischemic leg to nonischemic leg, from 0.30 to 0.50 through 28 days, (Figure 6A-2,B). Injection of fibrin gel loaded with the mixture of VEGF and unmodified alginate increased the perfusion ratio at Day 28 to 0.62 (Figure 6A-3,B). The perfusion ratio was further increased to 0.75 with the use of alginate sulfates mixed with VEGF (Figure 6A-4,B). The improved blood perfusion was further related to the density of blood vessels that formed in the hindlimb tissues. According to immunostained images of blood vessels, incorporation of alginate sulfate into the VEGF-encapsulated fibrin gel resulted in the increase of density of CD31-positive blood vessels compared to conditions to treat muscle with fibrin gel loaded with VEGF (Figure 7).
Figure 6.
(A) LDPI images of mice after the ischemic hindlimb surgery. Ischemia was introduced by ligating the femoral artery in the right leg. The red color intensity represents the relative intensity of perfusion. The ischemic leg imaged after 24 h (A-1) was treated for 28 days with 2.5 μg of VEGF in a fibrin gel (A-2), 2.5 μg of VEGF + unmodified alginate gel in a fibrin gel (A-3), and 2.5 μg of VEGF + alginate sulfates in a fibrin gel (A-4). (B) Quantification of the mean perfusion in the legs of mice with ischemic hindlimbs. The ischemic leg was treated with 2.5 μg of VEGF in a fibrin gel (▲), fibrin gel + alginate (●), and fibrin gel + alginate sulfates (■). The perfusion in the ischemic limb was normalized to the perfusion in the nonischemic limb (n = 4 mice, p < 0.05).
Figure 7.
Representative images of histological section stained for CD31. Tissues were stained with CD31 antibody marked by a DAB chromagen solution, shown in brown. The tissues were counterstained with hematoxylin, shown in blue. The ischemic leg was treated with 2.5 μg of VEGF in a fibrin gel (A), fibrin gel + alginate (B), and fibrin gel + alginate-sulfate (C). Blue arrows indicate CD31-positve blood vessels (scale = 25 μm).
DISCUSSION
The results of this study demonstrate that the use of a sulfated alginate molecule could improve efficacy of VEGF-induced angiogenesis by modulating binding affinity between VEGF and VEGFR, and phosphorylation of VEGFR2. Specifically, the complex of VEGF and alginate sulfates significantly increased the number of endothelial lumen sprouts. In addition, the alginate sulfates could increase the phosphorylation level of VEGFR2 in endothelial cells and also the binding affinity as compared with the unmodified alginate. Furthermore, the alginate sulfates incorporated in the fibrin gel together with VEGF were able to increase the level of blood perfusion recovery in an ischemic hindlimb as compared to a fibrin gel encapsulated only with VEGF.
We suggest that the increase in the number of endothelial sprouts demonstrated with the in vitro 3D sprouting assay is caused by the improved association between VEGF and VEGFR in the presence of alginate sulfates, as demonstrated with the SPR assay. It is proposed that alginate substituted with polar sulfate groups electrostatically associate with VEGF as confirmed with the FRET assay. The resulting bulky VEGF-alginate sulfates complex would reduce the entropy for dissociation from VEGFR, as marked with 7-fold decrease of the dissociation rate, compared with the bare VEGF and the mixture of VEGF and unmodified alginate.26 Overall, the VEGF and VEGFR could associate more favorably in the presence of alginate sulfates, thus resulting in the increase of the total number of VEGF associated with cellular receptors.
We also propose that the increased number of VEGF bound to VEGFR per cell lead to the elevated VEGFR-2 phosphorylation level attained with alginate sulfates. The enhanced VEGF signaling should stimulate endothelial cells adhered to a microsphere to grow, migrate, and finally form endothelial sprouts, as marked with the significant increase of the number of endothelial sprouts. The smaller increase of the length of endothelial sprouts may be attributed to the uniform distribution of VEGF around endothelial cells on a dextran microsphere embedded in the fibrin gel. It is known that the concentration gradient of the VEGF is an important factor in extending endothelial lumen.27
The in vivo evaluation of the mouse hindlimb ischemia model reconfirmed the significant role of alginate sulfates on stimulating vascularization in defective tissue, despite the complexity of the in vivo environment. Coupled with immunostained images of capillaries, the enhanced blood perfusion recovery observed with the LDPI analysis indicates that alginate sulfates attribute to the increase in the number of capillary blood vessels newly formed in tissues surrounding the ischemic site. Based on results of in vitro studies, we suggest that the complex of VEGF and alginate sulfates stably bound with host blood vessel-forming endothelial precursor cells and further amplify VEGFR-2 activation. In addition, alginate sulfates may sequester VEGF in the fibrin gel and serve to improve bioavailability of VEGF in the ischemic site, which should be further examined in future studies.
Previously, there have been some studies to study biological effects of sulfated polymer on growth factor-mediated neovascularization. 25 However, to the best of our knowledge, the role of alginate sulfates in orchestrating neovascularization and improving perfusion recovery treatment of ischemic tissues have not been examined to date. Therefore, the results of this study will be greatly useful in better understanding the biological role of alginate sulfates and also defining the extent by which the polymer modulates VEGF-induced angiogenic signals. We also propose that the results of this study will be broadly useful to enhancing therapeutic efficacy of growth factors, even at reduced dosage.
CONCLUSIONS
In conclusion, the results of this study demonstrate that alginate sulfates can associate with VEGF and improve the efficacy of VEGF in stimulating neovascularization and subsequently enhancing recovery of perfusion in the ischemic hindlimb. This enhanced neovascularization attained with alginate sulfates was attributed to the enhanced binding affinity of VEGF to VEGFR and the elevated VEGFR-2 activation level. Therefore, as confirmed with in vitro sprouting assay, the VEGF-alginate sulfates complex could stimulate endothelial sprouting more significantly than the bare VEGF or the mixture of VEGF and unmodified alginate. Overall, the findings from this study are broadly applicable to a wide variety of growth factor- and cytokine-dependent therapies and could help to expedite use of these molecular therapies in various clinical settings for wound healing and tissue regeneration.
Acknowledgments
This work was supported by American Heart Association (Scientist Development Grant 0830468Z), National Science Foundation (STC-EBICS Grant CBET-0939511), and the National Institute of Health (1R01 HL109192).
Footnotes
Notes
The authors declare no competing financial interest.
References
- 1.Frangogiannis NG. Chemokines in ischemia and reperfusion. Thromb Haemostasis. 2007;97(5):738–47. [PubMed] [Google Scholar]
- 2.Portaluppi F, Lemmer B. Chronobiology and chronotherapy of ischemic heart disease. Adv Drug Delivery Rev. 2007;59:952–65. doi: 10.1016/j.addr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 3.Brevetti G, Giugliano G, Brevetti L, Hiatt WR. Inflammation in Peripheral Artery Disease. Circulation. 2010;122(18):1862–75. doi: 10.1161/CIRCULATIONAHA.109.918417. [DOI] [PubMed] [Google Scholar]
- 4.Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006;47(5):921–9. doi: 10.1016/j.jacc.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 5.http://www.mayoclinic.com
- 6.Muir RL. Peripheral arterial disease: Pathophysiology, risk factors, diagnosis, treatment, and prevention. J Vasc Nurs. 2009;27(2):26–30. doi: 10.1016/j.jvn.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Germani A, Di Campli C, Pompilio G, Biglioli P, Capogrossi MC. Regenerative Therapy in Peripheral Artery Disease. Cardiovasc Ther. 2009;27(4):289–304. doi: 10.1111/j.1755-5922.2009.00105.x. [DOI] [PubMed] [Google Scholar]
- 8.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999;5(12):1359–64. doi: 10.1038/70928. [DOI] [PubMed] [Google Scholar]
- 10.Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005;65:550–63. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–71. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 12.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125(9):1591–8. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 13.Grosskreutz CL, Anand-Apte B, Duplaa C, Quinn TP, Terman BI, Zetter B, D’Amore PA. Vascular endothelial growth factor-induced migration of vascular smooth muscle cells in vitro. Microvasc Res. 1999;58(2):128–36. doi: 10.1006/mvre.1999.2171. [DOI] [PubMed] [Google Scholar]
- 14.Elcin YM, Dixit V, Gitnick G. Extensive in vivo angiogenesis following controlled release of human vascular endothelial cell growth factor: implications for tissue engineering and wound healing. Artif Organs. 2001;25(7):558–65. doi: 10.1046/j.1525-1594.2001.025007558.x. [DOI] [PubMed] [Google Scholar]
- 15.Kong HJ, Kim ES, Huang YC, Mooney DJ. Design of biodegradable hydrogel for the local and sustained delivery of angiogenic plasmid DNA. Pharm Res. 2008;25(5):1230–8. doi: 10.1007/s11095-007-9526-7. [DOI] [PubMed] [Google Scholar]
- 16.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9(6):685–93. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 17.DeVolder RJ, Bae H, Lee J, Kong H. Directed Blood Vessel Growth Using an Angiogenic Microfiber/Microparticle Composite Patch. Adv Mater. 2011;23(28):3139. doi: 10.1002/adma.201100823. [DOI] [PubMed] [Google Scholar]
- 18.Jay SM, Saltzman WM. Controlled delivery of VEGF via modulation of alginate microparticle ionic crosslinking. J Controlled Release. 2009;134(1):26–34. doi: 10.1016/j.jconrel.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel ZS, Young S, Tabata Y, Jansen JA, Wong MEK, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43(5):931–40. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laschke MW, Harder Y, Amon M, Martin I, Farhadi J, Ring A, Torio-Padron N, Schramm R, Rucker M, Junker D, Haufel JM, Carvalho C, Heberer M, Germann G, Vollmar B, Menger MD. Angiogenesis in tissue engineering: Breathing life into constructed tissue substitutes. Tissue Eng. 2006;12(8):2093–104. doi: 10.1089/ten.2006.12.2093. [DOI] [PubMed] [Google Scholar]
- 21.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20(1):45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 22.Cha C, Kohmon RE, Kong H. Biodegradable Polymer Crosslinker: Independent Control of Stiffness, Toughness, and Hydrogel Degradation Rate. Adv Funct Mater. 2009;19(19):3056–62. [Google Scholar]
- 23.Karlsson R, Michaelsson A, Mattsson L. Kinetic analysis of monoclonal antibody-antigen interactions with a new biosensor based analytical system. J Immunol Methods. 1991;145(1–2):229–40. doi: 10.1016/0022-1759(91)90331-9. [DOI] [PubMed] [Google Scholar]
- 24.Cao L, Arany PR, Wang Y-S, Mooney DJ. Promoting angiogenesis via manipulation of VEGF responsiveness with notch signaling. Biomaterials. 2009;30(25):4085–93. doi: 10.1016/j.biomaterials.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freeman I, Kedem A, Cohen S. The effect of sulfation of alginate hydrogels on the specific binding and controlled release of heparin-binding proteins. Biomaterials. 2008;29(22):3260–8. doi: 10.1016/j.biomaterials.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 26.Ashikari-Hada S, Habuchi H, Kariya Y, Kimata K. Heparin regulates vascular endothelial growth factor(165)-dependent mitogenic activity, tube formation, and its receptor phosphorylation of human endothelial cells - Comparison of the effects of heparin and modified heparins. J Biol Chem. 2005;280(36):31508–15. doi: 10.1074/jbc.M414581200. [DOI] [PubMed] [Google Scholar]
- 27.Silva EA, Mooney DJ. Effects of VEGF temporal and spatial presentation on angiogenesis. Biomaterials. 2010;31(6):1235–41. doi: 10.1016/j.biomaterials.2009.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]