Abstract
Rationale
Impulsive choice, or an inability to delay immediate gratification, has been strongly linked to the development and persistence of drug abuse. Indeed, delaying drug use itself may underlie drug addiction and relapse. Thus, employing treatments that are efficacious in reducing impulsive choice (atomoxetine; ATO) or drug-seeking behavior (progesterone; PRO) may be an effective means of treating drug addiction.
Objective
The current study assessed sex differences in the effects of PRO, ATO and their combination in a delay discounting paradigm for cocaine and for sucrose pellets.
Method
Male and female rats chose between a small-immediate or a large-delayed (0, 7.5, 15, 30, 60 sec) outcome in an impulsive choice procedure for sucrose pellets (1 vs 3 pellets) or for iv cocaine infusions (0.3 vs 0.9 mg/kg). Following baseline assessment of impulsive choice, rats received daily treatment of vehicle (VEH), PRO (0.5 mg/kg), ATO (1.5 mg/kg) or a combination (PRO + ATO) until a second assessment of impulsive choice was determined.
Results
Compared to the VEH group, females were less impulsive for cocaine following PRO or the PRO + ATO combined treatment, whereas males were less impulsive for cocaine following ATO. No treatment effects were observed on impulsive choice for sucrose pellets.
Conclusions
The present results indicate that impulsive choice for cocaine is reduced by PRO in females and by ATO in males. These findings suggest both treatments may be an effective intervention in treating cocaine abuse, but that their effectiveness differs by sex.
Keywords: Cocaine, delay discounting, female, impulsive choice, rats, recreational drug-use
Introduction
In the United States, over 1.5 million people habitually use cocaine (SAMHSA 2014), however few effective treatments are available (Somoza et al. 2013). One treatment approach advanced by Bickel et al. (2007) is to reduce maladaptive behaviors associated with drug abuse, such as impulsive choice (see reviews in Madden and Bickel 2010). Quantifying impulsive choice is usually accomplished by assessing how rapidly a delay devalues (i.e., causes one to discount) the value of a larger-later outcome (e.g. sobriety, money, family; Ainslie 1974) over a smaller-sooner one (e.g., an immediate high). Indeed, drug abusers more steeply discount (i.e., are more impulsive for) hypothetical monetary outcomes compared to non-abusing controls (e.g., Garcia-Rodriguez et al. 2013; Kirby et al. 1999; Madden et al. 1997).
Individuals who abuse drugs not only discount monetary outcomes, they also discount their preferred drug of abuse. Users of nicotine (Bickel et al. 1999), alcohol (Petry 2001), heroin (Madden et al. 1997; Giordano et al. 2002) and cocaine (Coffey et al. 2003) discount their preferred drug, and they do so at a steeper rate than they discount monetary outcomes. When abstinent (i.e., during withdrawal), drug abusers become even more impulsive and discount their preferred drug at a much steeper rate (Ashare and Hawk 2011; Field et al. 2006; Giordano et al. 2002), which could be a prominent variable underlying drug relapse. To investigate impulsive drug use in animal models, researchers could examine treatments that reduce delay discounting of drugs in nonhumans.
Delay discounting of drugs has been successfully modeled in nonhuman primates and rats. In preliminary studies, monkeys preferred larger doses of cocaine over smaller ones, and preference for the larger dose decreased (i.e., was discounted) as a function of its delay (Anderson and Woolverton 2003; Woolverton and Anderson 2006; Woolverton et al. 2007). Perry et al. (2007) reported similar findings in male and female rats using an adjusting delay procedure and found steeper discounting of cocaine as dose increased (e.g., 0.2 vs. 0.6 mg/kg; 0.4 vs 1.2 mg/kg; 0.8 vs 2.4 mg/kg cocaine). A limitation of this procedure was that preference for the large cocaine dose was not established prior to imposing delays. Thus, it is difficult to determine if larger cocaine doses were more steeply discounted or if the larger doses were more aversive and decreased large alternative preference. The present study attempts to provide a more conclusive demonstration of delay discounting of cocaine in rats by establishing preference for the large dose prior to imposing delays.
The most promising pharmacotherapies to reduce impulsive choice for cocaine are those that have reduced impulsive choice for food or have reduced the reinforcing effects of cocaine. Atomoxetine (ATO) has been shown to decrease impulsive choice for food in male rats (Bizot et al. 2011; Robinson et al. 2008); although other studies have not replicated these findings (Baarendse and Vanderschuren 2012; Broos et al. 2012b; Paterson et al. 2012; Sun et al. 2012). However, ATO has other characteristics that make it a promising candidate. In animal models of drug abuse and relapse, ATO treatment alone (Economidou et al. 2009; Economidou et al. 2011) or in combination with other treatments reduced cue- and/or cocaine-primed cocaine seeking in male (Swalve et al. 2016) and female (Zlebnik and Carroll 2014) rats, but did not reduce cocaine self-administration in humans (Economidou et al. 2011; Levin et al. 2009; Walsh et al. 2013). Taken together, these studies suggest that ATO may be an effective treatment to reduce impulsive choice for cocaine for both sexes.
Another potential treatment can be derived from studies of sex differences in drug abuse (see Anker and Carroll 2010a for a review). Across the reproductive cycle of female humans and rats, the reinforcing effects of cocaine are much weaker when PRO levels become relatively elevated compared to estrogen (e.g., Lukas et al. 1996; Lynch et al. 2000; Mello et al. 2007; Roberts et al. 1989; Sofuoglu et al. 1999). In rats, exogenously administered PRO, or its primary metabolite, allopregnanolone, attenuated stress and cocaine-primed reinstatement in females (e.g., Anker and Carroll 2010b; Anker et al. 2007; Zlebnik et al. 2014) and males (e.g, Zlebnik et al. 2014) as well as acquisition or escalation of cocaine self-administration in females (Jackson et al. 2006; Larson et al. 2007). Comparable clinical findings have been reported in both men and women with PRO reducing the physiological and pleasurable effects of cocaine (Evans and Foltin 2006, 2010; Fox et al. 2013; Sofuoglu et al. 1999; Sofuoglu 2002; Sofuoglu et al. 2004). Indeed, PRO recently reduced cocaine use in post-partum women in clinical trials (Yonkers et al. 2014). Collectively, these findings suggest that PRO may serve as an efficacious intervention for psychostimulant addiction.
Combination treatments have also shown some promise in enhancing the effect of therapies in treating drug abuse (Stoops and Rush 2014), with similar findings reported in preclinical research. For example, the duel effects of wheel running, PRO and ATO when combined are typically more effective at reducing cocaine-seeking (see Swalve et al. 2016; Zlebnik and Carroll 2014; Zlebnik et al. 2014)) in male and female rats than when administered alone (see also, Comer et al. 1996). Since ATO and PRO treatments are presumed to impact impulsive choice for cocaine differently, they were presumed to be more effective in combination.
The present study examined the treatment effects of ATO and PRO alone and in combination on impulsive choice for cocaine and for sucrose pellets in male and female rats. It was hypothesized that ATO and PRO would reduce impulsive choice for cocaine and for sucrose pellets, and when combined, these treatments would produce additive effects. Since PRO treatment is biologically relevant to females, it was also hypothesized it would be more effective in females than male rats (for a review, see Carroll and Anker 2010).
Methods
Materials and Methods
Animals
Male (n = 68) and female (n = 68) Wistar rats (Harlan Inc, Indianapolis, IN) weighing 225–250g and 175–200 g, respectively, at the start of the experiment served as subjects (female endocrine status was not monitored). During lever training for sucrose pellets, rats were singly housed in polycarbonate cages with bedding. Following training, rats responding for cocaine (N = 64) were implanted with an indwelling jugular catheter and singly housed in experimental chambers with wire mesh floors. Rats had free-access to water and were fed 16 g (females) or 20 g (males) of chow (Teklad 2018, Harlan) per day to maintain body weights at ~85% of free-feeding levels. Housing rooms were maintained at 24 °C (40 – 50% humidity) under a 12/12-h light/dark cycle (lights on at 0600). The experimental protocol was approved by the University of Minnesota Institutional Animal Care and Use Committee and conducted in compliance with the Guide for the Care and Use of Animals (National Research Council, 2011).
Apparatus
Sessions were conducted in customized octagonal experimental chambers housed in wooden sound-attenuating boxes with a ventilation fan. Each experimental chamber contained two levers, each with a 3 LED light array directly above, and an overhead light (houselight) for general illumination. Chambers used to assess delay discounting of sucrose pellets included a food hopper between the levers that delivered sweetened sucrose pellets (Bio-Serv® pellets #F0021). Chambers used to assess delay discounting of cocaine included a syringe pump (PHM-100, MedAssociates, St. Albans, VT) that delivered drug through a swivel-tether (375/22PS, Instech, Plymouth Meeting, PA; C313CS-MN, PlasticsOne, Roanoke, VA) that was attached to a harness (CIH95Ab, Instech) worn by the rat. A computer running Windows XP® and Med-PC IV® software orchestrated sessions and recorded data.
Drugs
Cocaine HCl, supplied by the National Institute of Drug Abuse (Research Triangle Institute, Research Triangle Park, NC), was diluted in 0.9% NaCl (saline; SAL) to a concentration of 1.6 mg/ml and then refrigerated. Heparin (5 USP/ml) was added to the cocaine solution to aid catheter patency. The syringe pump in the experimental chamber delivered 0.025 ml/s, and the duration of the pump was set to provide 0.3 mg/kg/infusion. Progesterone (Sigma-Aldrich, St. Louis, MO) was dissolved to 0.625 mg/ml in peanut oil (USP; Sigma-Aldrich) as a vehicle (VEH) and administered subcutaneously (SC) at a 0.5 mg/kg dose. Atomoxetine (Sigma-Aldrich) was dissolved in saline (SAL; 30 mg/ml) and delivered intraperitoneally (IP) at a 1.5 mg/kg dose. PRO, ATO, and control treatments (VEH or SAL) were administered approximately 30 min prior to the start of experimental sessions. The PRO dose was based on previous work that found it was effective in reducing cocaine and cue-primed reinstatement as well as reducing breakpoints for cocaine on a progressive ratio schedule (e.g., Larson et al. 2007; Zlebnik et al. 2014). The ATO dose was selected based on previous research showing it reduced cocaine seeking (Swavle et al. 2016; Zlebnik and Carroll 2014) and impulsive choice at doses of 1–2 mg/kg (e.g., Bizot et al. 2011; Robinson et al. 2008).
Catheterization Surgery
Rats that responded for cocaine were anesthetized with a ketamine (60 mg/kg)/xylazine (10 mg/kg) mixture and an atropine supplement (0.15 ml of 0.4 mg/ml) to aid respiration. Next, a silastic catheter (Plastics One, Roanoke, VA) was implanted in the right jugular vein toward the right atrium and secured with silk sutures (see Lynch and Carroll 1999). The distal end of the catheter was subcutaneously tunneled to a medial incision 1 cm rostral to the scapulae and then connected to the harness on the rat (see apparatus). During the three-day recovery period, rats were given buprenorphine (0.05 mg/kg) every 12 hr, with heparin (10 IU/kg, intravenous, IV) and enrofloxacin (10 mg/kg, sc) provided daily and ibuprofen (50 mg/kg) continuously available in the drinking water. Catheter patency was checked weekly with a ketamine (10 mg/kg)/midazolam (0.5 mg/kg)/saline mixture, and loss of patency was assumed if a loss of righting reflex was not observed within 10 seconds. A second catheter was implanted on the other side if patency was lost on the first.
Procedure
Lever Training
Rats were initially trained to respond on a concurrent fixed-ratio (FR) 1 fixed-time (FT) 5 min schedule for food pellets. Illumination of the houselight and stimulus lights above the levers signaled the start of the session. Free pellet deliveries on the FT schedule were preceded by flashing of lights above both levers for 2 sec (i.e., an “auto-shaping” procedure). Lever training concluded once rats responded more than 25 times on each lever.
Impulsive Choice for Sucrose Pellets Training
Following lever training, all rats were put in an impulsive choice procedure (see Fox et al. 2008) that presented trials whereby a response on the right lever delivered 3 sucrose pellets following a delay of T seconds, and a response on the left lever delivered 1 sucrose pellet immediately. Each session, rats were presented with 3 blocks of 12 trials. Each block began with two sample trials that required the rat to experience each alternative separately prior to 10 choice trials during which both alternatives were available. Once preference for the large alternative was established under a 0-s delay (>80% large choices averaged across two consecutive sessions), delays imposed following a response to the large alternative during which the left stimulus light flashed at 2 Hz. The delay to the large alternative increased each session until the largest delay was reached (7.5, 15, 30, 60 sec), upon which the delay was reduced back to 0 sec (i.e., a delay gradient). All trials were separated by a compensating 60-sec intertrial interval (ITI) that was adjusted to ensure all trials were equivalently spaced regardless of the delay experienced on the previous trial (e.g., a 15 sec delay resulted in a 45 sec ITI blackout of the overhead light before the next trial). Sessions concluded after 30 choice trials or 60 min, whichever occurred first. The proportion of large alternative choices was calculated each session. Stability was determined visually and mathematically by assessing trend and variability (< 0.1 S.E.M on average across delay values), respectively, in the proportion of large choices at each delay value across the last three delay gradients. Once stable, the proportion of large choices at each delay was averaged across the three gradients to represent the baseline level of impulsive choice. Subsequently, rats assigned to the impulsive choice for cocaine procedure underwent surgery, while the remaining rats were put on drug treatment to assess the impact on impulsive choice for sucrose pellets.
Treatment Assessments for Impulsive Choice for Sucrose Pellets
Following baseline stability, female (n = 36) and male (n = 36) rats were assigned to balance baseline delay gradient shape across treatment groups: VEH (VEH + SAL), PRO (PRO + SAL), ATO (ATO + VEH) or the combination treatment (PRO + ATO). Rats received daily injections 30 min prior to experimental sessions until one more delay gradient was captured. To assess changes in impulsive choice for sucrose pellets across treatment conditions, the proportion of large alternative choices was assessed as a function of delay.
Treatment Assessments for Impulsive Choice for Cocaine
Once stable baseline delay gradients were established under the impulsive choice for sucrose pellets procedure, female (n = 32) and male rats (n = 32) underwent IV catheter implantation and recovered in the drug self-administration chamber for three days. On the fourth day, the first impulsive choice for cocaine session began at 0900 hr. Sessions were conduced in a similar manner to the impulsive choice for sucrose pellets procedure except for a few differences: 1) Rats chose between 1 immediate or 3 delayed infusions of cocaine (0.3 mg/kg/inf) on the left and right levers, respectively, 2) To reduce the maximum dose of cocaine available per hr, the ITI (i.e., time between trials) was increased to 120 sec and the session duration was increased to 3 hr, 3) Rats were housed in the experimental chambers to enhance the duration of catheter patency, 4) Baseline impulsive choice was assessed until the proportion of large choices at 3 of 4 delays was below the average proportion at the 0 sec delay, and then drug treatment was provided until a second gradient was completed. Although rare, if a rat did not complete more than 10 choice trials in a session then the delay value was presented again the following session. Female (n = 32) and male rats (n = 32) were assigned to one of four treatment groups to balance baseline delay gradient shape across groups. All rats received daily injections (0830 hr) of a drug treatment (e.g., VEH, PRO, etc.) 30 min before every session until a drug treatment gradient was completed. To assess changes in impulsive choice for cocaine across treatment conditions, the proportion of large alternative choices was assessed as a function of delay. Additionally, the average latency to make a choice response was calculated at each delay value and compared within-subjects across treatment conditions (latency data for two female subjects could not be calculated due to a program malfunction).
Data Analysis
Between- and within-group differences in delay discounting for cocaine, delay discounting for sucrose pellets, cocaine intake, and choice latencies were each assessed separately in females and males using a linear mixed-model with a 3-way analysis of variance (ANOVA) structure for the fixed effects (Group X Treatment Outcome X Delay); to accommodate potential non-sphericity in the correlation structure, random effects for rats and delay-specific residual variances were used. To provide a unitary measure of impulsive choice, an area-under-the-curve (AUC; Myerson et al. 2001) analysis was conducted on individual baseline and treatment gradients of every rat. This AUC measure was used to compare baseline delay gradients within the sucrose pellet and cocaine groups using a 2-way ANOVA (Sex X Group). Additionally, sex differences in treatment effects were assessed using 3-way ANOVA (Sex X Group X Treatment Outcome) on the AUC measures from the impulsive choice for cocaine and for sucrose pellet conditions. For all analyses, “Group” refers to treatment group, while “Treatment Outcome” refers to pre-treatment baseline vs. post-treatment. For all ANOVAs, a Holm (Holm, 1979) type I error adjustment was applied to all post-hoc tests of pre-treatment baseline vs. post-treatment (e.g., for each delay tested within sex and group) and to all post-hoc tests to compare AUC across treatment groups (e.g., within treatment outcome and sex).
Results
Treatment Assessments for Impulsive Choice for Sucrose Pellets
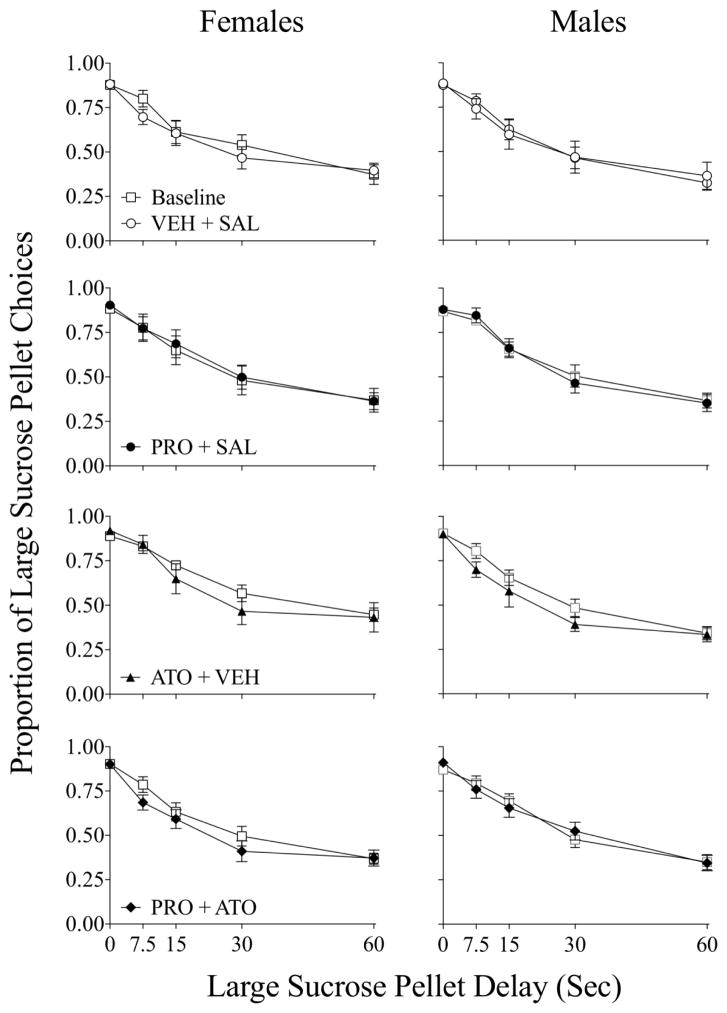
Figure 1 shows the proportion of large sucrose pellet choices across the delay to the large alternative during baseline (open squares) and following different treatments in female (left column) and male (right column) rats. Both female and male rats discounted the large sucrose pellet alternative, as preference for the large alternative was high at the 0-sec delay and significantly decreased as the delay was increased across sessions in female (F4, 288 = 212.54, p < 0.01) and male rats (F4, 288 = 439.94, p < 0.001). There were no significant differences in AUC from baseline during any treatment condition in either sex.
Figure 1.
Proportion of large (vs. small) sucrose pellet choices across large alternative delay during baseline (open squares) and treatment conditions in female and male rats.
Treatment Assessments for Impulsive Choice for Cocaine
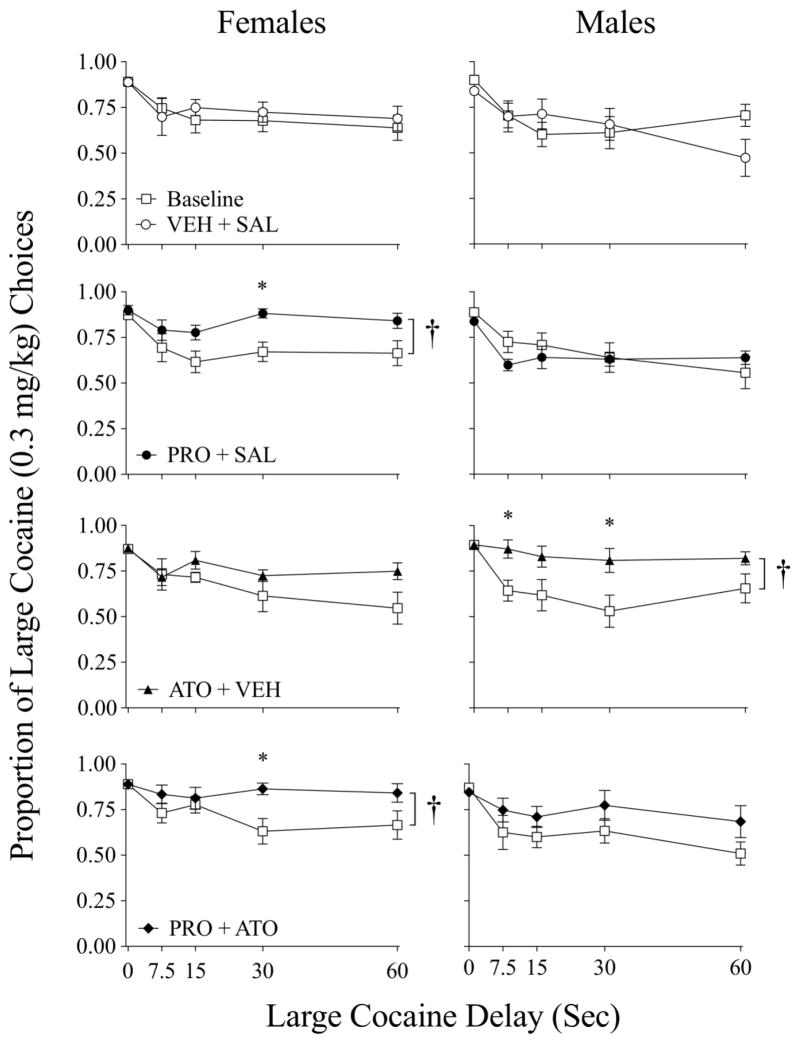
Figure 2 displays the proportion of large cocaine choice across delay to the large alternative during baseline (open squares) and following the different treatments in female (left column) and male (right column) rats. Rats discounted the large cocaine dose as preference for it was high at the 0-sec delay and significantly decreased as a function of delay in both female (F4, 252 = 40.56, p < 0.01) and male rats (F4, 252 = 56.22, p < 0.01). In females, a 3-way (Group X Treatment Outcome X Delay) ANOVA revealed a main effect of treatment outcome (F1, 252 = 25.09, p < 0.01) and a significant treatment outcome x delay interaction (F4, 252 = 5.56, p < 0.01). A post-hoc comparison of treatment outcome yielded significant increases in the proportion of large alternative choices following PRO (30 sec delay) and PRO + ATO (30 sec delay). In Males, a 3-way (Group X Treatment Outcome X Delay) ANOVA revealed a main effect of treatment (F1, 252 = 7.58, p < 0.01), and interactions of treatment X delay (F4, 252 = 4.28, p < 0.01) and of treatment X group (F3, 252 = 6.73, p < 0.001). Post-hoc assessment of treatment outcome showed significant increases in the proportion of large cocaine choices follow ATO treatment at the 7.5 and 30 sec delays.
Figure 2.
Proportion of large (vs. small) cocaine dose choices as a function of large cocaine delay across baseline (open squares) treatment conditions in female and male rats. † - significant change in AUC baseline to treatment (p < 0.05; see Table 1). * - significant post-hoc difference at delay (p < 0.05).
Table 1 presents mean AUC of rats during baseline and treatment in the impulsive choice for cocaine procedure (see Figure 2). From the 3-way (Sex X Group X Treatment Outcome) ANOVA, baseline levels of impulsive choice were not significantly different between sexes. However, sex and treatment differences were observed as revealed by a main effect of Treatment Outcome (F1, 56 = 24.92, p < 0.001) and Sex (F1, 56 = 5.84, p < 0.05). Post-hoc assessments revealed a significant increase in AUC (a reduction in impulsive choice for cocaine) following the PRO and PRO + ATO treatments in females and following the ATO treatment in males. Changes in impulsive choice for cocaine, however, did not alter the latency to make a choice. In both females and males, there were no significant differences following treatment for any group at any delay.
Table 1.
Mean Area-Under-the-Curve (± S.E.M.) during baseline and treatment across treatment 1 groups in female and male rats.
| VEH + SAL
|
PRO + SAL
|
ATO + VEH
|
PRO + ATO
|
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Tx | Baseline | Tx | Baseline | Tx | Baseline | Tx | |
| Females | 0.782 (0.041) | 0.817 (0.041) | 0.775 (0.040) | 0.939* (0.020) | 0.742 (0.053) | 0.863 (0.028) | 0.783 (0.051) | 0.957* (0.042) |
| Males | 0.737 (0.059) | 0.760 (0.076) | 0.736 (0.067) | 0.770 (0.044) | 0.683 (0.060) | 0.923* (0.039) | 0.702 (0.034) | 0.876 (0.037) |
p < 0.05 significant difference between baseline and treatment
Cocaine Intake
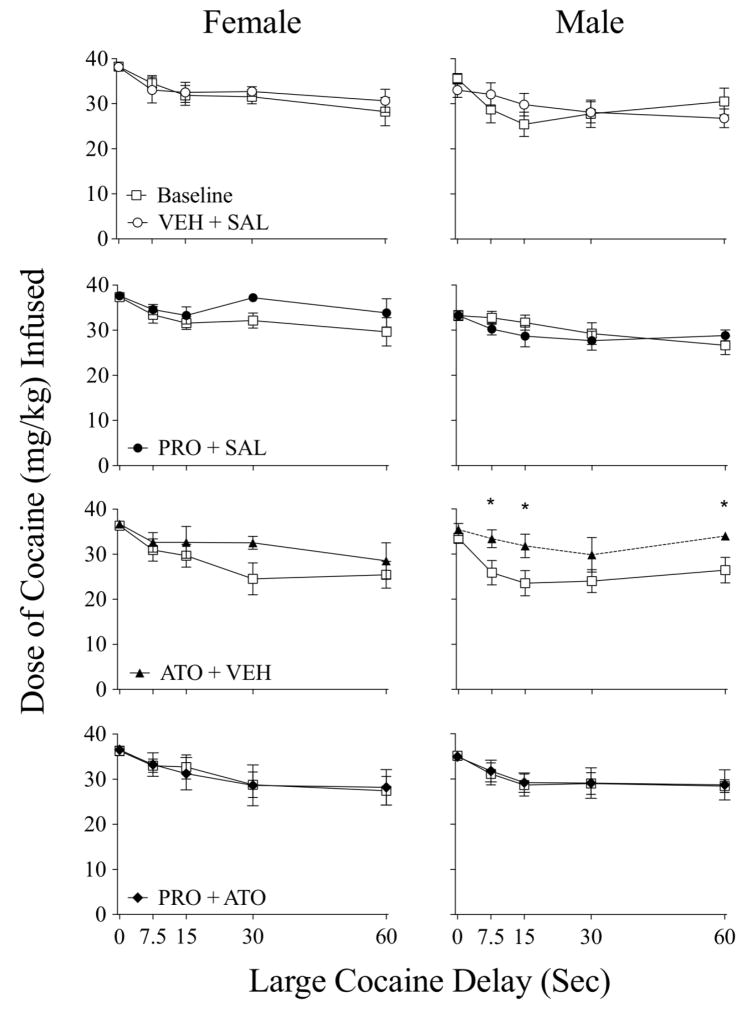
Figure 3 presents average cocaine intake (mg/kg) of female (left column) and male (right column) rats as a function of delay during baseline and during treatment conditions for each of the four treatment groups. The amount of cocaine infused decreased slightly as the large alternative delay increased as evidenced by a main effect of delay in both females (F4, 252 = 31.54, p < .01) and males (F4, 252 = 26.11, p < 0.001). The amount of cocaine infused by females increased slightly under the ATO treatment condition. In females, the ANOVA returned a main effect of treatment outcome (F1, 252 = 4.12, p < .05) and group (F3, 252 = 3.14, p < .05). In males, the ANOVA revealed a significant main effect of treatment outcome (F1, 252 = 5.06, p < .05) and an interaction of treatment outcome X group (F3, 252 = 6.15, p < 0.001), with significant increases following ATO at the 7.5, 15 and 60 sec delays.
Figure 3.
Cocaine intake (mg/kg) as a function of the delay to the large cocaine dose during baseline (open squares) and treatment in female and male rats. Due to the slight decrease in trials completed during treatment conditions, there was no significant increase in cocaine intake with the increase in large cocaine choices during treatment conditions. * - significant post-hoc difference at delay (p < 0.05).
Sex Differences in Impulsive Choice
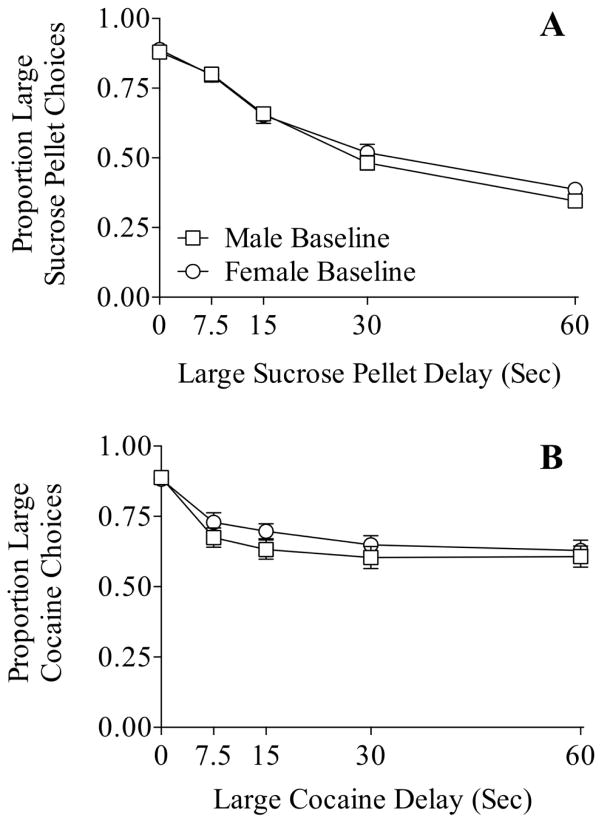
Figure 4 displays the mean baseline proportion of large alternative choices as a function of the delay to the large alternative in male and female rats for the impulsive choice for sucrose pellets (Panel A) and for cocaine (Panel B) procedures. In general, there were no sex differences in delay discounting for sucrose pellets or for cocaine.
Figure 4.
Mean baseline proportion of large alternative choices in male (squares) and female (circles) rats as a function of large alternative delay during the impulsive choice for sucrose pellets (Panel A) and for cocaine (Panel B) conditions. No significant differences between sexes were observed.
Discussion
The present study provides the first demonstration that a pharmacological intervention can be employed to reduce impulsivity for any drug, including cocaine. Compared to the VEH control group, both ATO and PRO treatments reduced impulsive choice for cocaine as indicated by the increase in AUC (Table 1). The effects, however, differed by sex, with PRO reducing impulsivity in females and ATO reducing impulsivity in males (Figure 2; Table 1). These treatments, however, did not alter impulsive choice for sucrose pellets (see Figure 1), which suggests that these drug treatments, at their present doses, selectively interacted with the cocaine impulsive choice procedure and the subject sex in some fashion.
The sex difference in the reduction of impulsive choice for cocaine by PRO is likely related to two factors: 1) PRO reduces cocaine-seeking behavior relatively more in females than males (see a review by Quinones-Jenab and Jenab, 2010) and 2) PRO differentially reduces the reinforcing efficacy of smaller doses of cocaine more than larger ones in females. The sex difference in the effect of PRO is likely related to its capacity to blunt the reinforcement enhancement effect of estrogen, a female gonadal hormone, on cocaine (Roberts et al. 1989). Indeed, Anker et al. (2009) reported allopregnanalone, the primary metabolite of PRO, reduced cocaine-primed reinstatement in female, but not male rats (see also Larson et al. 2007). Regarding the dose dependent effects of PRO on cocaine, Mello et al. (2007) reported high PRO levels during the luteal menstrual phase reduced progressive ratio breakpoints (e.g., reinforcing effects) in female monkeys at low (0.0032 mg/kg), but not higher doses of cocaine (0.032 mg/kg). Thus, the reduction in impulsive choice for cocaine observed with PRO may have resulted from a relatively greater reduction in the rewarding effects of the smaller vs. larger cocaine dose, shifting preference to the large alternative. Collectively, these two factors could have combined to reduce impulsive choice for cocaine in females following PRO treatment, although a more complete examination of the dose-response effects in males in warranted.
The reduction of impulsive choice for cocaine by ATO in males is, perhaps, more complex since little sex difference work has been done on this topic. The dose of ATO (1.5 mg/kg) employed is within the dose range (1–2 mg/kg) that has reduced impulsive choice in male rats, presumably by reducing sensitivity to the large alternative delay (Bizot et al. 2011; Robinson et al. 2008). However, like other studies employing this dose range, here it did not alter impulsive choice for sucrose pellets (Baarendse and Vanderschuren 2012; Paterson et al. 2012; Sun et al. 2012). This disparity in the effect of ATO on sucrose pellets and cocaine suggests that it is interacting with some aspect of the impulsive choice for cocaine procedure. Indeed, recent work by Swavle et al. (2016) showed males (vs. females) had a relatively greater reduction in cocaine-seeking behavior during reinstatement when treated with the same dose of ATO (1.5 mg/kg) used in the present study. Given the present results and the lack of studies examining sex differences in the effects of ATO on cocaine self-administration, additional research in this area is warranted.
The combined effects of PRO + ATO were similar to PRO alone in females and ATO alone in males, either suggesting these drugs solely drove the effect or that there were ceiling effect in the impulsive choice for cocaine measure. Specifically, the baseline preference for the large cocaine alternative was significantly higher than what is typical for food (see Figure 4), which resulted in less room for an increase when assessing the combination treatments. Thus, further research is warranted to determine if longer delay lengths and different cocaine dose ranges produce steeper cocaine gradients that would allow for combination treatment effects to emerge.
More broadly, the present study demonstrates delay discounting of cocaine in male and female rats as preference for a large dose of cocaine decreased as the delay to the large cocaine delivery was increased (see Figure 4B). These findings extend previous work showing delay discounting of cocaine in rats (Perry et al. 2007) and nonhuman primates (Anderson and Woolverton 2003; Woolverton and Anderson 2006; Woolverton et al. 2007). No sex differences were observed between male and female rats in impulsive choice for sucrose pellets or for cocaine (Figure 4); baseline AUC measures of impulsive choice were similar between sexes. The absence of sex differences in impulsive choice for either reinforcer is somewhat consistent to previous findings by Perry et al. (2007). In this study, they reported no sex differences in impulsive choice for cocaine or food pellets except in one condition where low-saccharin preferring females were more impulsive for food pellets.
The present study sought to model the increased impulsivity for drugs that occurs in abstinence (Giordano et al. 2002) and to explore possible treatments. However, one difficulty in modeling a treatment for the increased discounting of (i.e., impulsivity for) hypothetical drugs observed in humans during withdrawal (e.g., a hypothetical choice between two amounts of heroin) is that in rats, actual drug outcomes must be delivered as choice outcomes, which introduces several interpretational issues. One issue is that treatments that reduced impulsivity led to an increased preference for the large cocaine alternative, which typically would result in increased drug intake, which was observed in males in the present study (see Figure 3). Another difficulty is that the reduction in impulsive choice observed here was due to the drug treatments interacting with the behaviorally disruptive (Smethells and Carroll 2015) or impulsivity-increasing effects of acute and chronic cocaine exposure (e.g., Broos et al. 2012a; Dandy and Gatch 2009; Mitchell et al. 2014; Simon et al. 2007; but see Winstanley et al. 2007). Regardless of these issues, the reduction in impulsive choice for cocaine provides support for employing the present treatments given the strong relationship between drug abuse and impulsive choice (see Madden and Bickel, 2010, for a review).
The present study employed a single dose of ATO and PRO that were previously shown to be effective treatments for reducing impulsive choice and/or reinstatement of cocaine seeking in rats (e.g., Bizot et al. 2011; Zlebnik et al. 2014). The use of a single dose, however, is a limitation of the present study. Additional examination of the effects of the presently employed treatments on impulsive choice for cocaine is necessary to determine if sex differences are observed across a wider range of doses. Additionally, it might be possible that a stronger therapeutic effect could occur when treatments are provided both singly and in combination.
In summary, the present study demonstrated that impulsive choice for cocaine was reduced by PRO in females and ATO in males. However, these treatments did not alter impulsive choice for sucrose pellets, which suggests ATO and PRO may specifically target impulse choice for cocaine. Since progesterone is an endogenous hormone in women that counteracts the cocaine-enhancing effects of estrogen (Roberts et al. 1989), this treatment may be differentially more effective in treating cocaine abuse in women than men (see preliminary work by Younkers et al. 2014). Indeed, future work could examine the interaction of endocrine status with these treatments, since it was not monitored in the present study. Overall, the present results suggest a sex difference in the effects of ATO and PRO to reduce impulsive choice for cocaine.
Acknowledgments
We would like to thank Dr. Natalie Zlebnik for her thoughtful contributions, Seth Johnson for 2 technical assistance, and lastly Jared Mitchell and Heather Veghlan for assisting in conducting 3 this research. This study was supported by NIH/NIDA P50 DA033942 (MEC [PI] and LEE) and 4 a NIDA training grant T32 DA007097 (JRS; Thomas Molitor - PI).
References
- Ainslie GW. Impulse control in pigeons. J Exp Anal Behav. 1974;21:485–489. doi: 10.1901/jeab.1974.21-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KG, Woolverton WL. Effects of dose and infusion delay on cocaine self-administration choice in rhesus monkeys. Psychopharmacology. 2003;167:424–430. doi: 10.1007/s00213-003-1435-9. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and role of ovarian hormones. In: Neill JC, Kulkarni J, editors. Biological Basis of Sex Differences in Psychopharmacology: Current Topics in Behavioral Neurosciences. London, UK: Springer; 2010a. pp. 73–96. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend. 2010b;107:264. doi: 10.1016/j.drugalcdep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Holtz NA, Zlebnik N, Carroll ME. Effects of allopregnanolone on the reinstatement of cocaine-seeking behavior in male and female rats. Psychopharmacology. 2009;203:63–72. doi: 10.1007/s00213-008-1371-9. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Larson EB, Gliddon LA, Carroll ME. Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Exp and Clin Psychopharmacol. 2007;15:472–480. doi: 10.1037/1064-1297.15.5.472. [DOI] [PubMed] [Google Scholar]
- Ashare RL, Hawk LW. Effects of smoking abstinence on impulsive behavior among smokers high and low in ADHD-like symptoms. Psychopharmacology. 2011;219:537–547. doi: 10.1007/s00213-011-2324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarendse PJJ, Vanderschuren LJMJ. Dissociable effects of monoamine reuptake inhibitors on distinct forms of impulsive behavior in rats. Psychopharmacology. 2012;219:313–326. doi: 10.1007/s00213-011-2576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: Competing neural systems and temporal discounting processes. Drug and Alcohol Dependence. 2007;90(Supplement 1):S85–S91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Bizot J-C, David S, Trovero F. Effects of atomoxetine, desipramine, d-amphetamine and methylphenidate on impulsivity in juvenile rats, measured in a T-maze procedure. Neurosci Lett. 2011;489:20–24. doi: 10.1016/j.neulet.2010.11.058. [DOI] [PubMed] [Google Scholar]
- Broos N, Diergaarde L, Schoffelmeer AN, et al. Trait impulsive choice predicts resistance to extinction and propensity to relapse to cocaine seeking: A bidirectional investigation. Neuropsychopharmacol. 2012a;37:1377–1386. doi: 10.1038/npp.2011.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broos N, Schmaal L, Wiskerke J, et al. The relationship between impulsive choice and impulsive action: A cross-species translational study. PLoS ONE. 2012b;7:e36781. doi: 10.1371/journal.pone.0036781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian steroid hormones in animal models of drug dependence. Horm Behav. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Comer SD, Lac ST, Wyvell CL, Carroll ME. Combined effects of buprenorphine and a nondrug alternative reinforcer on IV cocaine self-administration in rats maintained under FR schedules. Psychopharmacology. 1996;125:355–360. doi: 10.1007/BF02246018. [DOI] [PubMed] [Google Scholar]
- Dandy KL, Gatch M. The effects of chronic cocaine exposure on impulsivity in rats. Behav Pharmacol. 2009;20 doi: 10.1097/FBP.0b013e328330ad89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Dalley JW, Everitt BJ. Selective norepinephrine reuptake inhibition by atomoxetine prevents cue-induced heroin and cocaine seeking. Biol Psychiat. 2011;69:266–274. doi: 10.1016/j.biopsych.2010.09.040. [DOI] [PubMed] [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, et al. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry. 2009;65:851–856. doi: 10.1016/j.biopsych.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacol. 2006;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Does the response to cocaine differ as a function of sex or hormonal status in human and non-human primates? Horm Behav. 2010;58:13–21. doi: 10.1016/j.yhbeh.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Santarcangelo M, Sumnall H, et al. Delay discounting and the behavioural economics of cigarette purchases in smokers: the effects of nicotine deprivation. Psychopharmacology. 2006;186:255–263. doi: 10.1007/s00213-006-0385-4. [DOI] [PubMed] [Google Scholar]
- Fox AT, Hand DJ, Reilly MP. Impulsive choice in a rodent model of attention-deficit/hyperactivity disorder. Behav Brain Res. 2008;187:146–152. doi: 10.1016/j.bbr.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Fox HC, Sofuoglu M, Morgan PT, et al. The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: Impact of gender and cue type. Psychoneuroendocrinol. 2013;38:1532–1544. doi: 10.1016/j.psyneuen.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Rodríguez O, Secades-Villa R, Weidberg S, Yoon JH. A systematic assessment of delay discounting in relation to cocaine and nicotine dependence. Behav Process. 2013;99:100–105. doi: 10.1016/j.beproc.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Giordano LA, Bickel WK, Loewenstein G, et al. Mild opioid deprivation increases the degree that opioid-dependent outpatients discount delayed heroin and money. Psychopharmacology. 2002;163:174–182. doi: 10.1007/s00213-002-1159-2. [DOI] [PubMed] [Google Scholar]
- Holm S. A Simple Sequentially Rejective Bonferroni Test Procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacol. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psych. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Larson EB, Anker JJ, Gliddon LA, et al. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol. 2007;15:461–471. doi: 10.1037/1064-1297.15.5.461. [DOI] [PubMed] [Google Scholar]
- Levin FR, Mariani J, Secora A, et al. Atomoxetine Treatment for cocaine abuse and adult attention deficit/hyperactivity disorder (ADHD): A preliminary open trial. J Dual Diag. 2009;5:41–56. doi: 10.1080/15504260802628767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas SE, Sholar M, Lundahl LH, et al. Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology. 1996;125:346–354. doi: 10.1007/BF02246017. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology. 2000;152:132–139. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Bickle WK. Impulsivity: The behavioral and neurological science of discounting. APA; Washington, DC: 2010. [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control patients: Drug and monetary rewards. Exp Clin Psychopharmacol. 1997;5:256. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Mello NK, Knudson IM, Mendelson JH. Sex and menstrual cycle effects on progressive ratio measures of cocaine self-administration in cynomolgus monkeys. Neuropsychopharmacol. 2007;32:1956–1966. doi: 10.1038/sj.npp.1301314. [DOI] [PubMed] [Google Scholar]
- Mitchell MR, Weiss VG, Ouimet DJ, et al. Intake-dependent effects of cocaine self-administration on impulsive choice in a delay discounting task. Behav Neurosci. 2014;128:419–429. doi: 10.1037/a0036742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the Care and Use of Laboratory Animals. 8. The National Academic Press; Washington, DC: 2011. [Google Scholar]
- Paterson NE, Wetzler C, Hackett A, Hanania T. Impulsive action and impulsive choice are mediated by distinct neuropharmacological substrates in rat. Int J Neuropsychopharmacol. 2012;15:1473–1487. doi: 10.1017/S1461145711001635. [DOI] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Anderson MM, Morgan AD, Carroll ME. Impulsivity (delay discounting) for food and cocaine in male and female rats selectively bred for high and low saccharin intake. Pharmacol Biochem Behav. 2007;86:822–837. doi: 10.1016/j.pbb.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Quinones-Jenab V, Jenab S. Progesterone attenuates cocaine-induced responses. Hormones and Behavior. 2010;58:22–32. doi: 10.1016/j.yhbeh.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Bennett SL, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Robinson ESJ, Eagle DM, Mar AC, et al. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacol. 2008;33:1028–1037. doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B. Cocaine exposure causes long-term increases in impulsive choice. Behav Neurosci. 2007;121:543–549. doi: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smethells JR, Carroll ME. Discrepant effects of acute cocaine on impulsive choice (delay discounting) in female rats during an increasing- and adjusting-delay procedure. Psychopharmacology. 2015;232:2455–2462. doi: 10.1007/s00213-015-3874-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav. 2002;72:431–435. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, et al. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol Biochem Behav. 2004;78:699–705. doi: 10.1016/j.pbb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Somoza EC, Winship D, Gorodetzky CW, Lewis D, Ciraulo DA, Galloway GP, et al. A multisite, double-blind, placebo-controlled clinical trial to evaluate the safety and efficacy of vigabatrin for treating cocaine dependence. JAMA Psychiat. 2013;70:630–637. doi: 10.1001/jamapsychiatry.2013.872. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Rush CR. Combination pharmacotherapies for stimulant use disorder: a review of clinical findings and recommendations for future research. Expert Rev Clin Pharmacol. 2014;7(3):363–374. doi: 10.1586/17512433.2014.909283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. [Google Scholar]
- Sun H, Cocker PJ, Zeeb FD, Winstanley CA. Chronic atomoxetine treatment during adolescence decreases impulsive choice, but not impulsive action, in adult rats and alters markers of synaptic plasticity in the orbitofrontal cortex. Psychopharmacology. 2012;219:285–301. doi: 10.1007/s00213-011-2419-9. [DOI] [PubMed] [Google Scholar]
- Swalve N, Smethells JR, Zlebnik NE, Carroll ME. Sex differences in reinstatement of cocaine-seeking with combination treatments of progesterone and atomoxetine. Pharmacology Biochemistry and Behavior. 2016;145:17–23. doi: 10.1016/j.pbb.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Middleton LS, Wong CJ, et al. Atomoxetine does not alter cocaine use in cocaine dependent individuals: A double blind randomized trial. Drug Alcohol Depend. 2013;130:150–157. doi: 10.1016/j.drugalcdep.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, LaPlant Q, Theobald DEH, et al. FosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J Neurosci. 2007;27:10497–10507. doi: 10.1523/JNEUROSCI.2566-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Anderson KG. Effects of delay to reinforcement on the choice between cocaine and food in rhesus monkeys. Psychopharmacology. 2006;186:99–106. doi: 10.1007/s00213-006-0355-x. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Myerson J, Green L. Delay discounting of cocaine by rhesus monkeys. Exp Clin Psychopharmacol. 2007;15:238. doi: 10.1037/1064-1297.15.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA, Forray A, Nich C, et al. Progesterone for the reduction of cocaine use in post-partum women with a cocaine use disorder: a randomized, double-blind, placebo-controlled, pilot study. Lancet Psychiat. 2014;1:360–367. doi: 10.1016/S2215-0366(14)70333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Carroll ME. Effects of the combination of wheel running and atomoxetine on cue- and cocaine-primed reinstatement in rats selected for high or low impulsivity. Psychopharmacology. 2014;232:1049–1059. doi: 10.1007/s00213-014-3744-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Saykao AT, Carroll ME. Effects of combined exercise and progesterone treatments on cocaine seeking in male and female rats. Psychopharmacology. 2014;231:3787–3798. doi: 10.1007/s00213-014-3513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]