Abstract
Influenza virus infection of neonates poses a major health concern, often resulting in severe disease and hospitalization. At present, vaccines for this at-risk population are lacking. Thus, development of an effective vaccine is an urgent need. Here we have used an innovative nonhuman primate neonate challenge model to test the efficacy of a novel TLR 7/8 agonist (R848) conjugated influenza virus vaccine. The use of the intact virus represents a step forward in conjugate vaccine design as it provides multiple antigenic targets allowing for elicitation of a broad immune response. Our results show that this vaccine induces high level virus-specific antibody and cell mediated responses in neonates that result in increased virus clearance and reduced lung pathology following challenge compared to the non-adjuvanted virus vaccine. Surprisingly, the addition of a second TLR agonist (flagellin) did not enhance vaccine protection, suggesting combinations of TLR that provide increased efficacy must be determined empirically. These data support further exploration of this new conjugate influenza vaccine approach as a platform for use in the at-risk neonate population.
Introduction
Influenza virus infection is a significant cause of morbidity and mortality in young children, with young infants being at particular risk. The rate of LRTI-associated hospitalizations is >4 times higher in children less than 1 year of age compared to those between 1 and 4 years (1) and infants younger than 6 months of age are particularly vulnerable to the development of severe disease (2). Not surprisingly then, the highest risk of death occurs in the first years of life (2). Infection with this virus can promote a variety of disease states in children less than 1 year of age including otitis media, pneumonia, myositis, and laryngotracheitis.
The ability to induce protective immunity through vaccination would arguably be the most effective strategy to protect this at risk population. However, the available data show the rate of seroconversion against H1N1 strains in young infants is only 29–32% following two doses of vaccine (3, 4). Not surprisingly, a correlation was observed between age and seroconversion, with older infants converting at a higher rate than younger infants (3). These data support the critical need for approaches that will improve efficacy in the newborn/infant population.
A case can be made that an optimal vaccine against influenza should induce both cell mediated and humoral immunity. CD8+ T cells have been documented to play an important role in protection from secondary exposure to infection, e.g. following vaccination (5). A clear benefit of CD8+ T cells is their frequent specificity for highly conserved internal viral proteins which promotes the capacity to provide cross-protection (6). Data suggest generation of this response may be hampered in infants as those dying from influenza virus infection have been reported to have a paucity of CD8+ T cells in their lungs (7).
CD4+ T cells are also a critical component of viral control. The ability of CD8+ T cells to promote clearance of influenza virus in animal models is dependent on help provided by CD4 + T cells (8) and CD4+ T cells are a critical regulator of high affinity antibody generation following vaccination (9), another important contributor to protection. In this regard, Th1 cells have been reported to promote a more effective antibody response compared to Th2 cells (10–12).
The neonatal immune system presents a number of significant challenges with regard to elicitation of protective antibody and cell mediated responses. Antibody responses exhibit defects in high level, high affinity IgG production through the first year of life (13, 14). Further, there are data in mice (15–18), human cord blood (19), and nonhuman primates (our unpublished data) showing a propensity for differentiation of CD4+ T cells into Th2 cells. Finally, in human neonates there is evidence supporting a generalized defect in T cell responsiveness (14, 20–25) as well as a heightened Treg response (26, 27).
The impaired immune response in infants makes the development of effective vaccine strategies particularly challenging. Significant effort has been expended towards harnessing the power of TLR agonists for adjuvants with promising results in adults (for review see (28)). An increasing body of work supports direct conjugation of a TLR agonist to an antigen or synthesis as a fusion protein as mechanisms to improve responses following vaccination (for review see (29)). TLR agonists that have been employed in this way include Pam2Cys, Pam3Cys, MPL, flagellin, imidazoquinolines, and CpG. The proposed advantage of direct linkage lies in targeting of the immunostimulatory component and the antigen to the same cell.
While this approach results in increased immunogenicity, it is limited in that only one antigen is available for elicitation of immune responses. Here, we employed a novel approach that allows for generation of responses against an array of influenza virus antigens via conjugation of R848 to the intact virion. R848 is an imidazoquinoline compound that has potent stimulatory capabilities for both TLR7 and TLR8. In experimental settings, R848 (or its closely related analog 3M-012) has been shown to increase cell mediated immune responses when incorporated into HBsAg (30) or HIV gag (31) protein vaccines. R848 has also been reported to induce antibody production (32, 33). The finding that TLR8 agonists induce robust Th1 cytokines in neonatal APC (34) coupled with the reported ability to suppress Tregs (35) makes R848 a highly attractive candidate adjuvant for neonates.
In our studies, an amine derivative of the TLR7/8 agonist R848 was conjugated to influenza virus (IPR8-R848) to test the hypothesis that such a vaccine would result in a robust adaptive immune response in very young infants. In addition we tested the ability of addition of the TLR5 ligand flagellin to augment the response given previous studies showing that simultaneous engagement of several TLR can alter the immune response in both a qualitative and quantitative fashion (36, 37). The results from our studies show R848 conjugated inactivated influenza virus particles comprise a vaccine that induces robust antibody and cell mediated immune responses in neonates. These responses are associated with lessened pulmonary pathology and improved clearance following virus challenge.
Materials and methods
Animals
African green monkey (AGM) infants (Caribbean-origin Chlorocebus aethiops sabaeus) used in this study were housed at the Vervet Research Colony at Wake Forest School of Medicine. Infants were removed from their mothers at 1–3 days of age and moved to the nursery. Infants were initially housed in incubators and subsequently moved to caging when they were capable of thermoregulation. Animal health was assessed by monitoring body weight, temperature, respiration rate, heart rate, food intake, and activity throughout the experiment. Animals were allowed to acclimate to the nursery for 3 days prior to receiving the vaccine.
Influenza A/PR/8/34 (H1N1)
A/Puerto Rico/8/1934 [H1N1] (PR8) virus stocks were grown and titered (egg infectious dose (EID50)) in 10 day old fertilized chicken eggs (obtained from a local farm), essentially as described previously (38). Stocks were diluted in PBS, flash frozen, and stored at −80°C.
Vaccination
At 4–6 days of age, infants were vaccinated with 45μg of inactivated R848 conjugated virus (IPR8-R848) in the presence of absence of 10μg of flagellin (flg) or with inactivated IPR8 (IPR8) mixed with the same amount of the inactive 229 mutant flagellin (m229) (39). This dose of flagellin has previously been shown to be effective in young AGM (40). Control animals received PBS. Inactivation of PR8 was achieved by treating with 0.74% formaldehyde overnight at 37°C. Virus was dialyzed against PBS and tested to assure the absence of infectivity. For the IPR8-R848 conjugate vaccine, an amine derivative of R848 (heretofore referred to as R848) was linked to SM(PEG)4 (Thermo Scientific) by incubation in DMSO for 24h at 37°C (Fig. S1). R848-SM(PEG)4 was then incubated with influenza virus that had been reduced to generate free thiol groups (IPR8-R848). Unconjugated R848 was removed by extensive dialysis. This construct was then inactivated by treatment with 0.74% formaldehyde overnight at 37°C, followed by dialysis. Successful conjugation was assessed by differential stimulation of RAW264.7 cells (Wake Forest Comprehensive Cancer Center Cell and Viral Vector Core Laboratory) following incubation with similar amounts of R848 conjugated versus non-conjugated vaccine. Flagellin from Salmonella enteritidis was prepared as previously described (39). Briefly, E. coli BL21 (DE3) containing a pet29a::fliC encoding wild type flagellin or the truncated pet29a::229 encoding only the biologically inactive hypervariable region of flagellin (39, 41) were grown and lysates prepared in 8 M urea. Proteins were purified on Ni-NTA agarose (Qiagen) according to the manufacturer’s protocol. Endotoxin and nucleic acids were removed using an Acrodisc Mustang Q capsule (Pall Corporation). Purified proteins were extensively dialyzed against PBS. All injections were delivered intramuscularly in the deltoid muscle (500μl volume). Animals were boosted 21 days later. Seven infants received IPR8-R848, seven received IPR8-R848+flg, four received IPR8+m229, and three received PBS.
Virus challenge and sampling
On d23–26 following boost, animals were sedated with 2–5% inhalant isoflurane. Animals received 1×1010 EID50 of PR8 divided equally between the intranasal (i.n.) and intratracheal (i.t.) routes, 0.25 ml i.t. and 0.25 ml i.n. (0.125 ml per nostril). For subsequent sampling, animals were sedated with isoflurane. Blood was collected in sodium heparin tubes by venipuncture on d8 and d14 post challenge, plasma obtained by centrifugation and PBMC subsequently isolated using Isolymph. Tracheal washes were performed on sedated animals on d2, 5, 8 and 14 postinfection by inserting an endotracheal tube into the trachea, instilling sterile 1.0 ml PBS and aspirating back. Due to the small volume of PBS used in the infants, 0.5mL of PBS was used to wash out the endotracheal tube. Samples were centrifuged to remove cellular material and BSA was added to a final concentration of 0.5%. Bronchial alveolar lavage (BAL) was performed at necropsy (d14) using 5 ml of PBS. Samples were centrifuged to remove cellular material and BSA added to a final concentration of 0.5%.
Assessment of lung pathology
Sections of lung were preserved in 10% neutral buffered formalin for at least 24 hours, trimmed, embedded in paraffin and routinely processed for histology. Sections were cut at 6μm and stained with hematoxylin and eosin. The slides were examined by light microscopy by an American College of Veterinary Pathologists board certified veterinary pathologist in a blinded fashion and evaluated for degree of inflammation and injury. Pathology assessment was based on interstitial and alveolar inflammatory cell infiltration and edema, pneumocyte hyperplasia, and bronchial degeneration and necrosis.
Quantitation of viral load
Viral RNA was extracted from the tracheal samples using QIAamp Viral RNA Mini Kit (Qiagen). cDNA was synthesized from mRNA by reverse transcription using Superscript III RT kit (Invitrogen) and random primers (Invitrogen). For viral quantification, RNA primer-probe sets specific for H1N1 were used (BEI Resources). RT-PCR (qRT-PCR) was performed using the Applied Biosystems 7500 real-time PCR system. EID50 values were calculated based on a standard curve generated using a stock of known EID50. The total EID50 for the sample was calculated based on the amount present in the sample volume used for the RT-PCR (140 μl) and adjusting to the total volume used for the wash.
C-reactive Protein (CRP) measurement
CRP levels in the plasma were assessed at 24h post vaccination using a C-Reactive Protein ELISA kit from ALPCO Diagnostics as per the manufacturer’s instructions. The plate was read at 450nm on a BioTek Elx800 Absorbance Microplate Reader. Amounts were calculated based on the standard curve generated using the control provided in the kit.
ELISA for the detection of influenza virus-specific antibody
Nunc MaxiSorp Elisa plates were coated with 1 μg/well PR8 or 0.2 μg/well of recombinant HA (BEI Resources) in sodium carbonate/bicarbonate coating buffer (pH 9.5). Plates were blocked with 1x Blocking Buffer (10x Blocking Buffer, Sigma) plus 2% goat serum (Lampire Biologicals) and washed. The wash buffer used throughout the assay was PBS with 0.1% Tween 20. Plasma or respiratory samples were serially diluted in 1x Blocking Buffer. Wells without virus served as a negative control. HRP-conjugated antibody specific for monkey IgG (Fitzgerald) or IgM (LifeSpan Bioscience) was used to detect bound antibody. Plates were developed with 3,3′,5,5′-Tetramethylbenzidine dihydrochloride (Sigma) and read at 450nm on a BioTek Elx800 Absorbance Microplate Reader. Absorbance for each dilution was calculated by subtracting the OD value obtained for the corresponding non-virus coated wells. Threshold titer was defined as the value that reached 3 times the assay background, i.e. wells that received only sample diluent.
Neutralization assay
Heat-inactivated (56°C for 1 hour) samples were serially diluted in a sterile 96-well flat bottom plate. 7.5×106 EID50 of PR8-GFP (kindly provided by Dr. Adolfo Garcia-Sastre (42)) was added to each well and incubated for 2 hours at 37°C and 5% CO2 to allow for antibody binding. 2×105 U937 cells (Wake Forest Comprehensive Cancer Center Cell and Viral Vector Core Laboratory) were then added to each well and incubated overnight at 37°C. The next morning, samples were acquired on a BD FACSCalibur and analyzed with CellQuest Pro software (Becton Dickinson) to determine the percentage of U937 cells that were positive for GFP. Controls for each experiment consisted of U937 cells alone and U937 cells infected with the PR8-GFP virus in the absence of plasma. Maximal %GFP was calculated for each experiment and nonlinear regression (Graphpad Prism) used to determine the dilution at which the 50% maximum PR8-GFP infected U937 cells was achieved.
T cell ELISPOT
Dendritic cells were generated from bone marrow isolated from the animals in the study by culture in the presence of GM-CSF (40 ng/ml) and IL-4 (40 ng/ml) for six days. Bone marrow was collected under an approved protocol. Differentiation was assessed by flow cytometric staining for CD11c. DC were infected with GFP-PR8 virus (42) and successful infection determined by flow cytometric analysis. Infected or mock-infected DC were co-cultured in triplicate with autologous cells for 48 hours in ELISPOT plates coated with anti-IFNγ (GZ-4) or anti-IL-4 (IL-4-I) capture antibody (MABTECH). Following incubation, spots were detected using biotin-conjugated anti-IFNγ (7-B6) or anti-IL-4 (IL-4-II) detection antibody (MABTECH), streptavidin-HRP conjugated antibody, and TrueBlue substrate solution. Spots were analyzed by ImmunoSpot Analyzer (Cellular Technology Ltd) and ImmunoSpot (version 3.2) software. The number of cytokine secreting cells per tissue was calculated based on the total number of cells recovered.
Statistical analysis
For continuous outcomes, groups were compared using 2-sample t-tests (if the treatment group had two levels) or analysis of variance (ANOVA) models (if there were 3 or more levels for the treatment group), with specific contrasts defined to compare pairs of groups when appropriate. If outcome data were not normally distributed logarithmic transformations were used prior analyses. For analyses that included repeated measures (i.e. for IgG) a two way repeated measures mixed model (2-way repeated measures analysis of variance (ANOVA)) was fit with the primate considered as a random effect in the model and treatment group and day considered as fixed effects. The treatment group by day interaction was examined first in these models and if found to be non-significant then that term would be removed. Comparisons between pairs of groups or on particular days were performed within these mixed models if the overall group or day effects were found to be significant. Data were analyzed using Prism 5 software (GraphPad) or SAS Version 9.3.
Animal Approval
All animal protocols were approved by the Institutional Animal Care and Use Committee at Wake Forest School of Medicine. The WFSM animal care and use protocol adhered to the U.S. Animal Welfare Act and Regulations.
Results
Infant vaccination
To assess candidate vaccines for the ability to elicit robust immune responses in young infants, we developed an African green monkey model of neonate vaccination (43). This serves as a tractable model for the human infant as it provides a period of infancy of adequate length to effectively evaluate prime boost strategies. In addition, this model is advantageous given the similarity of NHP and humans in TLR distribution and function (44). Our experimental vaccine is comprised of formalin inactivated influenza PR8 virus (IPR8) adjuvanted through direct conjugation to a synthetic amine derivative of R848, a potent TLR7/8 ligand (Fig S1). In some cases we also employed a combination adjuvant approach by including the soluble TLR5 agonist flagellin (flg), which was shown in our previous studies to possess some potential to enhance both T and B cell recall responses, i.e. the effect of flagellin was restricted to boosting (43). Control animals received inactivated PR8 together with an inactive flagellin construct (m229, non-adjuvanted control) or PBS (non-vaccinated control).
The overall experimental design is shown in Fig. 1A. Infant AGM received the initial vaccination at 4–6 days of age. Animals were boosted 21 days later and challenged with live influenza virus 23–26 days following boost. Infants were monitored for virus load and recall responses following challenge and necropsied at 14 days post-challenge (pc). To assess safety, infants were monitored for changes in temperature, respiration, heart rate, and overall health every 4 hours after receiving the vaccine for 24 hours. No consistent change in any of these health indicators was observed (data not shown). Further, there were no local site reactions following vaccination.
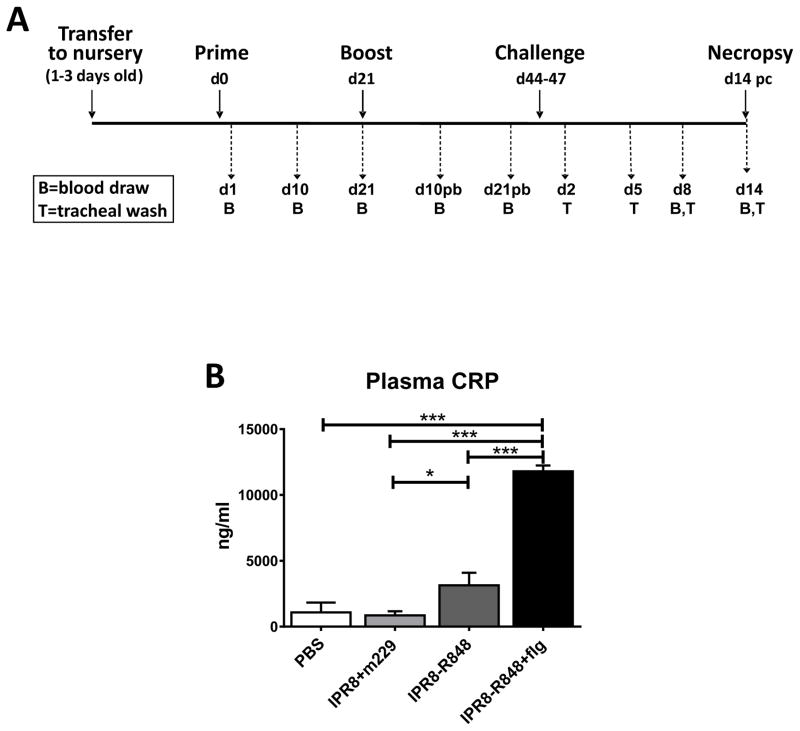
Figure 1. Experimental study design and CRP levels following vaccination.
(A) Neonate African green monkeys (4–6 days old) were vaccinated (d0) and boosted 21 days later with inactivated PR8 influenza virus (IPR8) together with the inactive form of flagellin (m229) (n=4) or R848 conjugated IPR8 in the presence or absence of flagellin (n=7). Non-vaccinated ontrol animals received PBS (n=3). Blood (B) was drawn on days 1, 10, and 21 following initial vaccination and days 10 and 21 following boost. Twenty-three to twenty-six days following boost, animals were challenged with live PR8 virus (1010 EID50). On d14 post challenge (pc) animals were necropsied. Tracheal wash (T) samples were acquired on d2, 5, 8, and 14 pc and blood (B) on d8 and d14 pc. (B) Twenty-four hours following initial vaccination, plasma CRP levels were measured. Significance was determined by one way ANOVA. *p<0.05, ***p<0.001.
As a measure of systemic immune activation/inflammation, C-reactive protein (CRP) levels were assessed at 24h post-vaccination. We fit a one way analysis of variance (ANOVA) model to compare the four groups, followed by pairwise comparisons of groups using t tests based on contrasts within the ANOVA model. We observed a highly significant (p<0.001) increase in CRP levels at 24h post-vaccination in animals receiving the vaccine with the combination of R848 and flagellin adjuvants (Fig. 1B). This level was not higher than that observed in our previous study of vaccination with flagellin as the sole adjuvant (43). Thus, the increase in CRP appears to be mediated predominantly by flagellin. In agreement with this, we observed only a modest increase in CRP level in IPR8-R848 vaccinated infants compared to those vaccinated with IPR8+m229. The increases in CRP (2.9–10.8 fold) are in line with those reported in other vaccine studies (45, 46). There were no significant increases in IL-6 or TNFα in the vaccinated infants (data not shown), suggesting the absence of a systemic proinflammatory response.
R848 is a robust adjuvant for generation of an influenza virus-specific antibody response
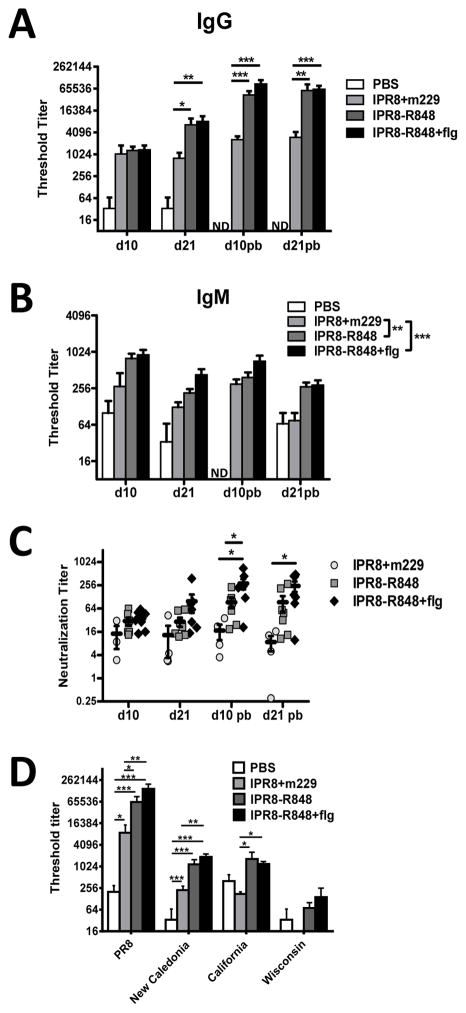
Circulating levels of influenza-specific IgG and IgM were measured at 10 and 21 days following primary and boost vaccinations. The 2-way repeated measures ANOVA model showed a significant group by day interaction suggesting that the rate of change in IgG levels were different depending on the group. Based on this significant interaction, we compared the groups at each specific day. The level of anti-influenza virus IgG in vaccinated versus PBS treated infants was significantly increased in all cases at all times assessed (p<0.001). This is expected and as such significance is not indicated on the figure for increased ease of viewing. Inclusion of conjugated R848 resulted in a significant increase in virus-specific IgG at d21 post-primary vaccination as well as at d10 and 21 post-boost (pb) compared to animals that received m229 (Fig. 2A). The addition of flagellin to IPR8-R848 did not result in a significant increase compared to IPR8-R848 alone (Fig. 2A).
Figure 2. Conjugation of R848 to IPR8 results in an increase in total and neutralizing influenza virus-specific antibody as well as antibody capable of recognizing alternative H1 molecules.
Levels of PR8-specific IgG (A) and IgM (B) and neutralizing antibody (C) in plasma were measured 10 and 21 days following prime and boost. Averaged data are shown. Significantly increased influenza-specific IgG and IgM responses were observed when R848 was conjugated to inactivated virus. The addition of flagellin did not significantly improve systemic responses. (D) Plasma obtained from infants at d21 post-boost was assessed by ELISA for the presence of IgG antibodies capable of recognizing the HA molecule from A/New Caledonia/20/1999 (H1N1), A/California/07/2009 (H1N1) pdm09, and A/Wisconsin/67/2005 (H3N2). Significance was determined using a repeated measures mixed model fit. *p<0.05, **p<0.01, ***p<0.001. ND, not detected.
Influenza-specific IgM levels were also assessed. We found no evidence for a day by group interaction (p=0.92). Therefore, overall group comparisons could be made across the four time points by adjusting for day rather than by comparing groups separately on each day. The presence of R848 significantly increased the influenza specific IgM response compared to m229 control animals (Fig. 2B). As with IgG, there was no significant difference between IPR8-R848 and IPR8-R848+flg. Further, as with IgG, all vaccinated animals had a highly significant increase compared to animals that received PBS (p<0.001). No virus-specific IgA was detected as would be expected following intramuscular delivery (data not shown). Together, these results show the presence of R848 conjugated to the virus particle had a robust adjuvant effect for elicitation of antibody in infants following vaccination. The addition of flagellin to the IPR8-R848 vaccine did not further increase systemic levels of vaccine induced, virus-specific antibody.
The combination of R848 and flagellin results in increased neutralizing antibody compared to R848 alone
The presence of neutralizing antibody was measured by the ability to inhibit infection of tissue culture cells by a GFP-expressing influenza PR8 virus. The neutralization titer was defined as the dilution of plasma that inhibited infectivity by 50% compared to infection in the absence of plasma. This approach was chosen as it is a direct measure of the ability of the antibody to prevent infection. The assay was validated against the standard HAI assay, finding that it provided similar relative results (43). The 2-way repeated measures ANOVA model found a significant day by group interaction and as such pairwise comparisons among groups were performed separately on each day. Statistically significant increases in neutralizing titer were observed at both days following boost in infants that received the IPR8-R848+flg vs. m229 adjuvanted vaccine (Fig. 2C, circles versus diamonds). Comparison of IPR8-R848+flg to IPR8-R848 vaccinated infants revealed a significant increase at d10 pb but this was lost at d21 pb. While it did not reach statistical significance, an increase in the average neutralization titer was observed for animals vaccinated with IPR8-R848 versus IPR8+m229. The failure to obtain significance may be a result of heterogeneity among infants in these two groups. These data suggest R848 induces increases in neutralizing titer and show the combination of R848 and flagellin can enhance neutralizing antibody at some time points compared to R848 alone.
The higher antibody levels generated in response to R848 conjugated vaccines result in increased levels of antibodies that can recognize homologous, but not heterologous subtype HA molecules
To determine whether the presence of the adjuvants could impact the ability of antibody to recognize additional strains of influenza virus, we assessed recognition of HA molecules derived from the vaccine PR8 strain, A/New Caledonia/20/1999 (H1), A/California/07/2009 (H1), and A/Wisconsin/67/2005 (H3). Plasma from animals that were 21 days post boost was used as this was the time at which maximal antibody levels were detected. As expected from the above analyses, all vaccines induced antibody that could recognize PR8 HA (Fig. 2D). None of the vaccines elicited antibodies capable of recognizing the heterologous H3 molecule from the Wisconsin strain (Fig. 2D). Analysis of the recognition of additional H1 molecules showed vaccination with IPR8 in the absence of adjuvant (IPR8+m229) resulted in detectable antibodies to the New Caledonia, but not the California H1 molecule (Fig. 2D). The presence of R848 or R848+flg could promote recognition of the HA molecule from New Caledonia in addition to California. Thus, the presence of R848 or R848+flg was capable of generating an antibody response with expanded capability to recognize HA molecules from other strains (Fig. 2D). These data support inclusion of R848 as a mechanism to promote the presence of antibodies with broader cross strain recognition. This could occur through higher total virus-specific antibody and/or increased capacity for cross-recognition.
R848 promotes a greatly augmented systemic antibody response following virus challenge
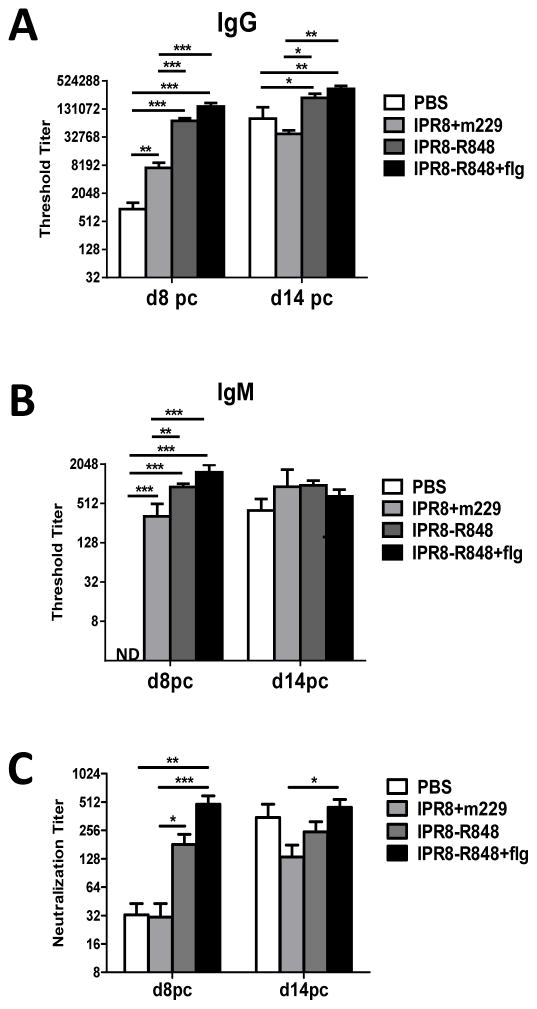
An important goal of vaccination is to generate adaptive immune cells that can respond rapidly and effectively following pathogen challenge. To assess the recall potential of the immune response generated in the vaccinated infants, influenza virus was delivered by the combined intratracheal and intranasal routes at 23–26 days post boost. On day 8 and 14 pc, the circulating level of virus-specific antibody was measured. At day 8 pc, animals that had received R848-conjugated vaccine exhibited greatly increased levels of virus specific IgG antibody compared to animals that received the negative control (m229) adjuvanted vaccine (Fig. 3A). Infants vaccinated with IPR8-R848 had, on average, a 10.2 fold increase and those vaccinated with IPR8-R848+flg a 20.6 fold increase compared to infants that received IPR8+m229. While antibody was increased, the R848+flg group was not significantly different from R848 alone. Virus-specific IgM levels were also increased at this timepoint (Fig. 3B). At d14 pc, virus-specific IgG antibody in infants vaccinated with adjuvanted vaccines remained significantly higher than those receiving IPR8+m229 (Fig. 3A). Interestingly at d14 pc, infants vaccinated with IPR8+m229 and non-vaccinated infants exhibited similar levels of influenza-specific IgG (Fig. 3A), suggesting the non-adjuvanted vaccine did not induce a recall response following challenge that was superior to that of a naïve infant.
Figure 3. IPR8 conjugation with R848 results in an increase in virus-specific total and neutralizing antibody at early times following challenge.
The amount of influenza-specific IgG (A) and IgM (B) antibody as well as neutralizing titer (C) was measured in plasma at d8 and d14 pc. Significance was determined using a repeated measures mixed model fit. *p<0.05, **p<0.01, ***p<0.001. ND, not detected.
Analysis of the neutralizing potential of virus-specific antibody in plasma revealed a significantly higher titer at d8 pc in infants that were vaccinated with the two R848 containing vaccines compared to animals vaccinated with IPR8+m229 (Fig. 3C). Those receiving the vaccine that included both R848 and flagellin had a higher average neutralizing titer, although the difference was not statistically significant compared to R848 alone (p=0.08) (Fig. 3C). Together these data show the presence of R848 during vaccination of young infants resulted in an increase in total virus-specific antibody as well as neutralizing antibody at early times following challenge, a timepoint that is likely critical in determining the outcome of infection, e.g. disease severity.
The combined presence of R848 and flagellin results in the highest level of virus-specific respiratory IgG following challenge
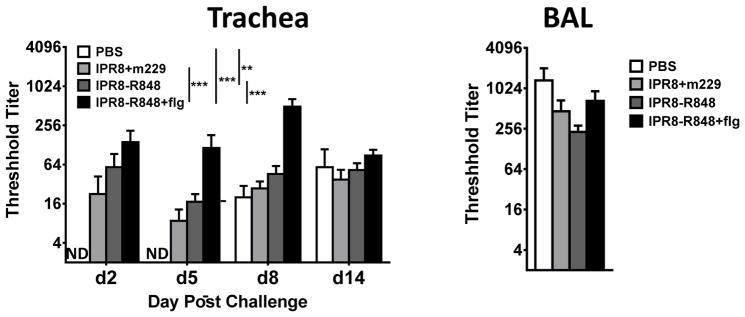
We also assessed the presence of antibody in the trachea (d8 and d14) and BAL (d14) following challenge. Two way repeated measures ANOVA analysis of the dataset revealed evidence of non-significant day by group interaction (p=0.23). Therefore, for the analysis of tracheal antibody we compared groups across all four timepoints by adjusting for day rather than comparing groups on each day. Compared to the IPR8+m229 vaccine group, only the IPR8-R848+flg group resulted in a significantly increased virus-specific IgG response in the trachea (Fig. 4), which was unexpected given the significant increase in systemic virus-specific IgG in the IPR8-R848 vaccinated infants. No significant differences across the groups were detected in the BAL at d14. Together these data suggest that the combination of flagellin and conjugated R848 may promote greater virus-specific IgG antibody in the respiratory tract.
Figure 4. The combination of flagellin and conjugated R848 results in the highest level of virus-specific IgG antibody in the respiratory tract at early times post challenge.
Influenza virus–specific IgG in the trachea was measured at d2, 5, 8, and 14 pc and in the lung (BAL) at d14 pc. Significance was determined using a repeated measures mixed model fit. **p<0.01, ***p<0.001. ND, not detected.
Vaccination with R848-conjugated IPR8 results in an increase in IFNγ-producing T cells following challenge
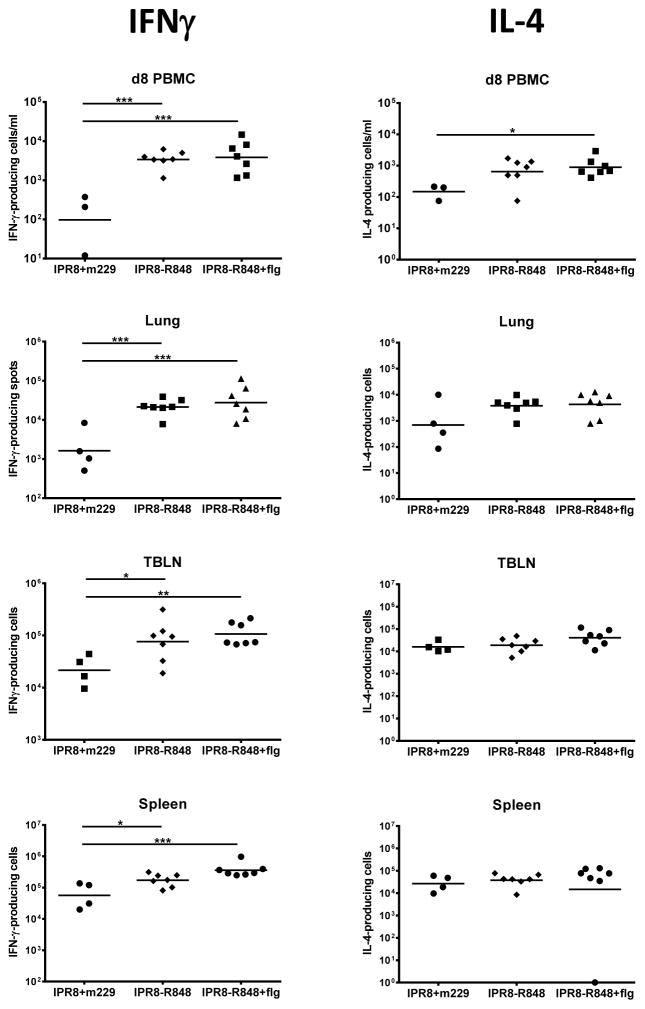
We next evaluated the T cell response present in vaccinated animals following challenge. IFNγ and IL-4 producing cells were assessed at d8 pc in the blood and at d14 pc in the lung, spleen, and lung-draining tracheobronchial lymph node (TBLN) (Fig. 5). Infants that received either of the R848-conjugated vaccines showed significantly enhanced numbers of IFNγ-producing cells in the blood at d8 pc and in all tissues evaluated at d14 pc. IL-4 producing cells were significantly increased only in d8 PBMC of infants vaccinated with the dual adjuvant vaccine; however, it is noteworthy that the R848-mediated increase in IL-4 producing cells was modest compared to that observed for the IFNγ-producing population (Fig. 5). Thus, conjugation of R848 to inactivated influenza virus results in a population of cells that exhibits a selective and robust induction of IFNγ-producing, influenza-specific T cells during the recall response elicited following virus challenge. This enhancement was not further increased by the addition of flagellin.
Figure 5. Infants vaccinated with IPR8-R848 have an increased number of IFNγ+ influenza-specific T cells after challenge.
IFNγ and IL-4-producing influenza-specific T cells were quantified by ELISPOT in the blood at d8 pc and in the lung, TBLN, and spleen at d14 pc following stimulation in the presence of autologous DC infected with influenza virus. Individual animals and the geometric mean are shown. Of note, one IPR8+m229 PBMC blood draw at d8 did not yield an adequate number of cells for analysis. Significance was assessed using one way ANOVA. *p<0.05, **p<0.01,***p<0.001.
The presence of R848 results in improved clearance and reduced pathology following challenge
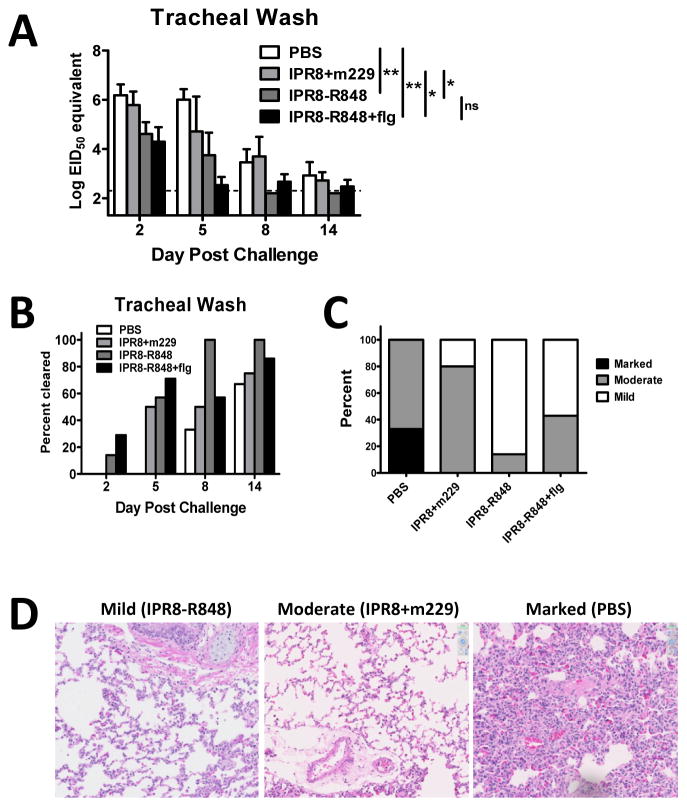
A protective vaccine should result in decreased viral burden and reduction in disease following challenge. To assess the protective capacity of the candidate vaccines, viral load in the trachea was measured over time by qRT-PCR. Viral load is presented as EID50 equivalents based on a standard curve generated using a stock of known EID50, with the acknowledgement that there may be differences in the ratio of infectious virions:RNA in virus obtained from the infants and that grown in vitro. As the two-way repeated measures ANOVA analysis suggested no evidence of a day by group interaction (p=0.25), we compared groups across all four timepoints by adjusting for day rather than comparing groups on each day. Animals vaccinated with the either IPR8-R848 or IPR8-R848+flg exhibited significantly decreased viral load in the trachea (Fig. 6A) and cleared virus earlier compared to the other constructs (Fig. 6B). The addition of flagellin to the IPR8-R848 vaccine did not significantly enhance clearance. Viral RNA in the lung at d14 pc was below detection in all groups (data not shown).
Figure 6. Vaccination with R848 conjugated IPR8 results in recall responses that are more protective.
(A) Virus load in the trachea following virus challenge of vaccinated animals was assessed by RT-PCR. Virus in the trachea of IPR8-R848 vaccinated infants was reduced following challenge compared to m229 vaccinated and PBS animals. Data shown are EID50 equivalents calculated based on known concentrations of influenza virus. Significance was determined using a repeated measures mixed model fit. *p<0.05, **p<0.01. (B) Data represent the percentage of animals in which virus was below the limit of detection in the trachea at each indicated time following challenge. IPR8-R848 vaccinated animals show earlier clearance compared to other vaccine groups. (C) The severity of lung pathology was determined in a blinded fashion by a board certified veterinary pathologist. Pathology was assigned as mild, moderate, or marked for each animal. Infants with mild pathology exhibited alveoli that were open and regularly contained few inflammatory cells that were mostly macrophages with few neutrophils. Alveolar septa were mildly thickened in some areas. Connective tissue fibers around vessels were separated by clear space, consistent with edema. Moderate changes were characterized by slight thickening of the alveolar walls due to fibrin and prominent type 2 pneumocytes (type 2 pneumocyte hyperplasia), more prominent perivascular edema and regular intra-alveolar macrophages. Severe interstitial pneumonia was characterized by diffuse hypercellularity, obscuring the small airways. Whereas the majority of animals vaccinated with IPR8+m229 had moderate lung pathology, the vast majority of animals receiving IPR8-R848 exhibited mild disease. (D) Representative sections for mild, moderate, and marked changes in the lungs of challenged animals are shown.
We also assessed pathology in the lungs of vaccinated infants at 14 days post challenge. Lung sections were scored in a blinded fashion by a board certified veterinary pathologist into three categories; marked, moderate or mild. Three of the four infants vaccinated with IPR8+m229 had moderate changes following challenge compared to 1 of 7 IPR8-R848 vaccinated infants (Fig. 6C and D). The remainder of infants that received IPR8-R848 showed mild pathology. Unexpectedly, 3 of 7 IPR8-R848+flg vaccinated infants had moderate changes. Thus, while the antibody response in the respiratory tract of infants that received the combination adjuvant vaccine was increased, the dual adjuvanted vaccine was not associated with less pulmonary pathology or increased viral clearance compared to R848 alone.
Discussion
Impaired immune responsiveness significantly hampers effective vaccination of neonates. Generation of vaccine approaches that can overcome these barriers is critical given the severe disease that often accompanies respiratory virus infection in this population together with the limited number of available therapeutics. To test immunogenicity and efficacy of candidate vaccines against influenza in neonates, we have established an NHP model that is arguably the most relevant system to probe these questions. With this model, we assessed a novel vaccine construct, generated using a methodology developed in our laboratory, which allows conjugation of the TLR7/8 agonist R848 directly to the influenza virus particle. The selection of R848 was made based on the potential to stimulate DC, B cells, and T cells (34, 47–50) as well as to suppress T regulatory cells (through TLR8) (35). The last is of particular interest given reports showing increased function and/or differentiation of T regulatory cells in neonates (26, 27, 51). Further, while TLR responsiveness is impaired in neonates, in myeloid neonate derived DC there is evidence that TLR8 responsiveness is less diminished than that of TLR7 (34). Thus, the dual TLR7/8 targeting by R848 makes it particularly attractive for neonate vaccines. Finally, while previous studies have reported the increased potency of vaccines when TLR agonists are covalently coupled to the antigen (for review see Fujita et al (29)), these constructs have the limitation that they employ a single antigenic target. Thus, the immune response is relatively narrow. In contrast, the vaccine used in our study contains all viral proteins allowing for multiple antigenic targets.
Our results demonstrate conjugation of R848 to the influenza virion results in a highly augmented virus-specific T cell response as well as IgG antibody (both total and neutralizing) compared to non adjuvanted inactivated virus. Importantly, augmentation of the T cell response is primarily within the IFNγ-producing population. At present, a limitation of our experimental design is the inability to determine whether the IFNγ production is from Th1 versus CD8+ T cells, an area of future interest for assessment. In addition, the extent to which any influenza-specific CD8+ T cells that may be present possess cytolytic activity is not known. Nonetheless, our data show that this vaccine approach promotes generation of the most beneficial immune responses (IFNγ-producing T cells and antibody) with regard to protection from influenza virus infection.
The increases observed with R848 are significantly greater than those observed in our previous study in which the utility of flagellin alone was assessed (43). Thus, the novel approach of conjugation of R848 to the virion studied here results in a superior vaccine. The ability to markedly enhance IgG responses in these very young infants is extremely promising as antibody production is known to be generally weak for the first year of life (22). Critically, the enhanced immune response in the infants vaccinated with the R848-conjugated virus particle was clinically beneficial as these individuals exhibited increased virus clearance and reduced disease. The relative contribution of antibody versus T cell responses to the increased clearance is presently unknown. Given that the challenge virus was the same as that used in the vaccine, it is possible that antibody is a primary mediator.
We note that in our model, virus-specific IgG is nearly undetectable in the infants at the time of vaccination as the adult animals do not have apprecialbe virus-specific antibody. Admittedly, this is in contrast to what would be observed in human infants, who would have mother-derived antibody. The goal of our study was to develop vaccines that could overcome the impaired neonate immune response and induce robust adaptive immunity. It will be critical in future studies to understand the impact of pre-existing antibody on our approach and to identify strategies that can allow for immune response induction in the face of maternal antibody.
The success of R848 is likely the result of both the TLRs targeted as well as the linkage to the virus particle. TLR activation via R848 has been shown to be a potent activator of neonatal DC (34), which are generally suboptimally responsive to TLR ligation (52–54). In addition, a recent study in mice reported the arrangement of R848 is a profound determinant of its in vivo activity (55). In that study, the authors tested the stimulatory capacity of HPMA-based polymers to which R848 was bound. When conditions where used in which these structures formed particles, R848 was found to possess much greater activity with regard to stimulation of innate immune responses in vivo, leading ultimately to increased CD8+ and Th1 responses. The basis for the increased activity was found to be enhanced uptake by APC in the draining lymph node. Immunostimulatory particulate forms of the R848-polymer were approximately 700 nm in diameter compared to the poorly stimulatory unimolecular polymer coils of 10–20 nm (55). It is tempting to speculate that attachment of R848 to the influenza virion, which is approximately 100 nm, mimics the structure and function of the particulate forms of the R848-spolymer.
An additional strategy evaluated here was the delivery of multiple TLR ligands. A number of studies support the increased stimulatory capacity of combined TLR (30, 36, 37, 56, 57). As an example, T cells derived from human cord blood that were stimulated with the combination of TLR2 and TLR5 agonists exhibited greater proliferation and cytokine production compared to cells stimulated in the presence of either agonist alone (58). We chose flagellin for the dual adjuvant approach given our previous studies in this NHP infant model revealed a flagellin-mediated increase in antibody and T cell recall responses (43), although these were less robust than those we have obtained with the R848-conjugated vaccine. Surprisingly, the addition of flagellin to IPR8-R848 had limited benefit with regard to antibody generation and T cell number, with the exception of the trachea where animals vaccinated with the combined adjuvants showed higher levels of influenza-specific IgG following challenge. However, this enhanced level of antibody did not appear to provide greater protection from pulmonary damage or increased clearance. In fact, this response may have been less protective with regard to tissue damage in our model. Thus, at least in the context of our inactivated R848-congugated influenza virus vaccine, there is limited evidence of benefit in the neonate from the combination of R848 and flagellin. Whether an alternative TLR ligand would result in a superior immune response and protection from pulmonary damage is a question that merits further investigation.
In summary, these data show vaccination of NHP neonates with R848 conjugated inactivated influenza virus results in an exceptionally robust antibody response following primary vaccination, boost, and challenge as well as a potent IFNγ-producing recall T cell response. We saw no signs of adverse reactions in infants receiving this construct; thus, there appear to be no safety concerns that would obviate further study. These data strongly support the utility of R848 conjugated inactivated influenza virus as an effective vaccine in this vulnerable population.
Supplementary Material
Acknowledgments
We thank Dr. Steve Mizel for the provision of flagellin, BEI Resources for providing recombinant HA molecules, and Dr. Adolfo Garcia-Sastre for provision of the GFP-PR8 virus. We acknowledge with deep gratitude the efforts of the dedicated nursery staff and Dr. Matthew Novak for consultation on nursery procedures. We thank Dr. Karen Haas for helpful suggestions related to the manuscript.
Abbrevations
- AGM
African green monkey
- p.c
post challenge
References
- 1.Foote EM, Singleton RJ, Holman RC, Seeman SM, Steiner CA, Bartholomew M, Hennessy TW. Lower respiratory tract infection hospitalizations among American Indian/Alaska Native children and the general United States child population. Int J Circumpolar Health. 2015;74:29256. doi: 10.3402/ijch.v74.29256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esposito S, Principi N. The rational use of influenza vaccines in healthy children and children with underlying conditions. Curr Opin Infect Dis. 2009;22:244–249. doi: 10.1097/QCO.0b013e32832a58e4. [DOI] [PubMed] [Google Scholar]
- 3.Groothuis JR, Levin MJ, Rabalais GP, Meiklejohn G, Lauer BA. Immunization of high-risk infants younger than 18 months of age with split-product influenza vaccine. Pediatrics. 1991;87:823–828. [PubMed] [Google Scholar]
- 4.Halasa NB, Gerber MA, Chen Q, Wright PF, Edwards KM. Safety and immunogenicity of trivalent inactivated influenza vaccine in infants. J Infect Dis. 2008;197:1448–1454. doi: 10.1086/587643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin JP, Somanathan S, Roy S, Calcedo R, Wilson JM. Lung homing CTLs and their proliferation ability are important correlates of vaccine protection against influenza. Vaccine. 2010;28:5669–5675. doi: 10.1016/j.vaccine.2010.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamura S, Tanimoto T, Kurata T. Mechanisms of broad cross-protection provided by influenza virus infection and their application to vaccines. Jpn J Infect Dis. 2005;58:195–207. [PubMed] [Google Scholar]
- 7.Welliver TP, Garofalo RP, Hosakote Y, Hintz KH, Avendano L, Sanchez K, Velozo L, Jafri H, Chavez-Bueno S, Ogra PL, McKinney L, Reed JL, Welliver RC., Sr Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis. 2007;195:1126–1136. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eichelberger MC, Wang ML, Allan W, Webster RG, Doherty PC. Influenza virus RNA in the lung and lymphoid tissue of immunologically intact and CD4-depleted mice. J Gen Virol. 1991;72(Pt 7):1695–1698. doi: 10.1099/0022-1317-72-7-1695. [DOI] [PubMed] [Google Scholar]
- 9.Eichelberger M, Golding H, Hess M, Weir J, Subbarao K, Luke CJ, Friede M, Wood D. Vaccine; FDA/NIH/WHO public workshop on immune correlates of protection against influenza A viruses in support of pandemic vaccine development; Bethesda, Maryland, US. December 10–11, 2007; 2008. pp. 4299–4303. [DOI] [PubMed] [Google Scholar]
- 10.Bungener L, Geeraedts F, Ter Veer W, Medema J, Wilschut J, Huckriede A. Alum boosts TH2-type antibody responses to whole-inactivated virus influenza vaccine in mice but does not confer superior protection. Vaccine. 2008;26:2350–2359. doi: 10.1016/j.vaccine.2008.02.063. [DOI] [PubMed] [Google Scholar]
- 11.Moran TM, Park H, Fernandez-Sesma A, Schulman JL. Th2 responses to inactivated influenza virus can be converted to Th1 responses and facilitate recovery from heterosubtypic virus infection. J Infect Dis. 1999;180:579–585. doi: 10.1086/314952. [DOI] [PubMed] [Google Scholar]
- 12.Weldon WC, Wang BZ, Martin MP, Koutsonanos DG, Skountzou I, Compans RW. Enhanced immunogenicity of stabilized trimeric soluble influenza hemagglutinin. PLoS One. 2010;5:e12466. doi: 10.1371/journal.pone.0012466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegrist CA. The challenges of vaccine responses in early life: selected examples. J Comp Pathol. 2007;137(Suppl 1):S4–9. doi: 10.1016/j.jcpa.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 15.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30:585–591. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Lee HH, Bell JJ, Gregg RK, Ellis JS, Gessner A, Zaghouani H. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity. 2004;20:429–440. doi: 10.1016/s1074-7613(04)00072-x. [DOI] [PubMed] [Google Scholar]
- 17.Lee HH, Hoeman CM, Hardaway JC, Guloglu FB, Ellis JS, Jain R, Divekar R, Tartar DM, Haymaker CL, Zaghouani H. Delayed maturation of an IL-12-producing dendritic cell subset explains the early Th2 bias in neonatal immunity. J Exp Med. 2008;205:2269–2280. doi: 10.1084/jem.20071371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roux X, Remot A, Petit-Camurdan A, Nahori MA, Kiefer-Biasizzo H, Marchal G, Lagranderie M, Riffault S. Neonatal lung immune responses show a shift of cytokines and transcription factors toward Th2 and a deficit in conventional and plasmacytoid dendritic cells. Eur J Immunol. 2011;41:2852–2861. doi: 10.1002/eji.201041224. [DOI] [PubMed] [Google Scholar]
- 19.Naderi N, Pourfathollah AA, Alimoghaddam K, Moazzeni SM. Cord blood dendritic cells prevent the differentiation of naive T-helper cells towards Th1 irrespective of their subtype. Clin Exp Med. 2009;9:29–36. doi: 10.1007/s10238-008-0020-2. [DOI] [PubMed] [Google Scholar]
- 20.Winkler S, Willheim M, Baier K, Schmid D, Aichelburg A, Graninger W, Kremsner PG. Frequency of cytokine-producing T cells in patients of different age groups with Plasmodium falciparum malaria. J Infect Dis. 1999;179:209–216. doi: 10.1086/314571. [DOI] [PubMed] [Google Scholar]
- 21.Xainli J, Baisor M, Kastens W, Bockarie M, Adams JH, King CL. Age-dependent cellular immune responses to Plasmodium vivax Duffy binding protein in humans. J Immunol. 2002;169:3200–3207. doi: 10.4049/jimmunol.169.6.3200. [DOI] [PubMed] [Google Scholar]
- 22.Randolph DA. The neonatal adaptive immune system. NeoReviews. 2005;6:e454–e462. [Google Scholar]
- 23.Zhao Y, Dai ZP, Lv P, Gao XM. Phenotypic and functional analysis of human T lymphocytes in early second- and third-trimester fetuses. Clin Exp Immunol. 2002;129:302–308. doi: 10.1046/j.1365-2249.2002.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clerici M, DePalma L, Roilides E, Baker R, Shearer GM. Analysis of T helper and antigen-presenting cell functions in cord blood and peripheral blood leukocytes from healthy children of different ages. J Clin Invest. 1993;91:2829–2836. doi: 10.1172/JCI116526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miscia S, Di Baldassarre A, Sabatino G, Bonvini E, Rana RA, Vitale M, Di Valerio V, Manzoli FA. Inefficient phospholipase C activation and reduced Lck expression characterize the signaling defect of umbilical cord T lymphocytes. J Immunol. 1999;163:2416–2424. [PubMed] [Google Scholar]
- 26.Fernandez MA, Puttur FK, Wang YM, Howden W, Alexander SI, Jones CA. T regulatory cells contribute to the attenuated primary CD8+ and CD4+ T cell responses to herpes simplex virus type 2 in neonatal mice. J Immunol. 2008;180:1556–1564. doi: 10.4049/jimmunol.180.3.1556. [DOI] [PubMed] [Google Scholar]
- 27.Holbrook BC, Hayward SL, Blevins LK, Kock N, Aycock T, Parks GD, Alexander-Miller MA. Nonhuman primate infants have an impaired respiratory but not systemic IgG antibody response following influenza virus infection. Virology. 2015;476:124–133. doi: 10.1016/j.virol.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maisonneuve C, Bertholet S, Philpott DJ, De Gregorio E. Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants. Proc Natl Acad Sci USA. 2014;111:12294–12299. doi: 10.1073/pnas.1400478111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujita Y, Taguchi H. Overview and outlook of Toll-like receptor ligand-antigen conjugate vaccines. Ther Deliv. 2012;3:749–760. doi: 10.4155/tde.12.52. [DOI] [PubMed] [Google Scholar]
- 30.Ma R, Du JL, Huang J, Wu CY. Additive effects of CpG ODN and R-848 as adjuvants on augmenting immune responses to HBsAg vaccination. Biochem Biophys Res Commun. 2007;361:537–542. doi: 10.1016/j.bbrc.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 31.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci U S A. 2005;102:15190–15194. doi: 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomai MA, Miller RL, Lipson KE, Kieper WC, Zarraga IE, Vasilakos JP. Resiquimod and other immune response modifiers as vaccine adjuvants. Expert Rev Vaccines. 2007;6:835–847. doi: 10.1586/14760584.6.5.835. [DOI] [PubMed] [Google Scholar]
- 33.Vasilakos JP, Tomai MA. The use of Toll-like receptor 7/8 agonists as vaccine adjuvants. Expert Rev Vaccines. 2013;12:809–819. doi: 10.1586/14760584.2013.811208. [DOI] [PubMed] [Google Scholar]
- 34.Levy O, Suter EE, Miller RL, Wessels MR. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood. 2006;108:1284–1290. doi: 10.1182/blood-2005-12-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang RF. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 36.Warger T, Osterloh P, Rechtsteiner G, Fassbender M, Heib V, Schmid B, Schmitt E, Schild H, Radsak MP. Synergistic activation of dendritic cells by combined Toll-like receptor ligation induces superior CTL responses in vivo. Blood. 2006;108:544–550. doi: 10.1182/blood-2005-10-4015. [DOI] [PubMed] [Google Scholar]
- 37.Krumbiegel D, Zepp F, Meyer CU. Combined Toll-like receptor agonists synergistically increase production of inflammatory cytokines in human neonatal dendritic cells. Hum Immunol. 2007;68:813–822. doi: 10.1016/j.humimm.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Klimov A, Balish A, Veguilla V, Sun H, Schiffer J, Lu X, Katz JM, Hancock K. Influenza virus titration, antigenic characterization, and serological methods for antibody detection. Method Mol Biol. 2012;865:25–51. doi: 10.1007/978-1-61779-621-0_3. [DOI] [PubMed] [Google Scholar]
- 39.McDermott PF, Ciacci-Woolwine F, Snipes JA, Mizel SB. High-affinity interaction between gram-negative flagellin and a cell surface polypeptide results in human monocyte activation. Infect Immun. 2000;68:5525–5529. doi: 10.1128/iai.68.10.5525-5529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weimer ET, Ervin SE, Wozniak DJ, Mizel SB. Immunization of young African green monkeys with OprF epitope 8-OprI-type A- and B-flagellin fusion proteins promotes the production of protective antibodies against nonmucoid Pseudomonas aeruginosa. Vaccine. 2009;27:6762–6769. doi: 10.1016/j.vaccine.2009.08.080. [DOI] [PubMed] [Google Scholar]
- 41.Honko AN, Mizel SB. Mucosal administration of flagellin induces innate immunity in the mouse lung. Infect Immun. 2004;72:6676–6679. doi: 10.1128/IAI.72.11.6676-6679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manicassamy B, Manicassamy S, Belicha-Villanueva A, Pisanelli G, Pulendran B, Garcia-Sastre A. Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus. Proc Natl Acad Sci U S A. 2010;107:11531–11536. doi: 10.1073/pnas.0914994107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim JR, Holbrook BC, Hayward SL, Blevins LK, Jorgensen MJ, Kock ND, De Paris K, D’Agostino RB, Jr, Aycock ST, Mizel SB, Parks GD, Alexander-Miller MA. Inclusion of flagellin during vaccination against influenza enhances recall responses in nonhuman primate neonates. J Virol. 2015;89:7291–7303. doi: 10.1128/JVI.00549-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ketloy C, Engering A, Srichairatanakul U, Limsalakpetch A, Yongvanitchit K, Pichyangkul S, Ruxrungtham K. Expression and function of Toll-like receptors on dendritic cells and other antigen presenting cells from non-human primates. Vet Immunol Immunopathol. 2008;125:18–30. doi: 10.1016/j.vetimm.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Turley CB, Rupp RE, Johnson C, Taylor DN, Wolfson J, Tussey L, Kavita U, Stanberry L, Shaw A. Safety and immunogenicity of a recombinant M2e-flagellin influenza vaccine (STF2.4xM2e) in healthy adults. Vaccine. 2011;29:5145–5152. doi: 10.1016/j.vaccine.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 46.Taylor DN, Treanor JJ, Strout C, Johnson C, Fitzgerald T, Kavita U, Ozer K, Tussey L, Shaw A. Induction of a potent immune response in the elderly using the TLR-5 agonist, flagellin, with a recombinant hemagglutinin influenza-flagellin fusion vaccine (VAX125, STF2.HA1 SI) Vaccine. 2011;29:4897–4902. doi: 10.1016/j.vaccine.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Caron G, Duluc D, Fremaux I, Jeannin P, David C, Gascan H, Delneste Y. Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J Immunol. 2005;175:1551–1557. doi: 10.4049/jimmunol.175.3.1551. [DOI] [PubMed] [Google Scholar]
- 48.Bekeredjian-Ding IB, Wagner M, Hornung V, Giese T, Schnurr M, Endres S, Hartmann G. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J Immunol. 2005;174:4043–4050. doi: 10.4049/jimmunol.174.7.4043. [DOI] [PubMed] [Google Scholar]
- 49.Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A. The toll-like receptor repertoire of human B lymphocytes: inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood. 2003;102:956–963. doi: 10.1182/blood-2002-11-3355. [DOI] [PubMed] [Google Scholar]
- 50.Kabelitz D. Expression and function of Toll-like receptors in T lymphocytes. Curr Opin Immunol. 2007;19:39–45. doi: 10.1016/j.coi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 51.Wang G, Miyahara Y, Guo Z, Khattar M, Stepkowski SM, Chen W. “Default” generation of neonatal regulatory T cells. J Immunol. 2010;185:71–78. doi: 10.4049/jimmunol.0903806. [DOI] [PubMed] [Google Scholar]
- 52.De Wit D, Tonon S, Olislagers V, Goriely S, Boutriaux M, Goldman M, Willems F. Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J Autoimmun. 2003;21:277–281. doi: 10.1016/j.jaut.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol. 2006;177:1956–1966. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES, Hajjar AM, Hawkins NR, Self SG, Wilson CB. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lynn GM, Laga R, Darrah PA, Ishizuka AS, Balaci AJ, Dulcey AE, Pechar M, Pola R, Gerner MY, Yamamoto A, Buechler CR, Quinn KM, Smelkinson MG, Vanek O, Cawood R, Hills T, Vasalatiy O, Kastenmuller K, Francica JR, Stutts L, Tom JK, Ryu KA, Esser-Kahn AP, Etrych T, Fisher KD, Seymour LW, Seder RA. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat Biotechnol. 2015;33:1201–1210. doi: 10.1038/nbt.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 57.Zhu Q, Egelston C, Gagnon S, Sui YJ, Belyakov IM, Klinman DM, Berzofsky JA. Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice. J Clin Invest. 2010;120:607–616. doi: 10.1172/JCI39293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCarron M, Reen DJ. Activated human neonatal CD8+ T cells are subject to immunomodulation by direct TLR2 or TLR5 stimulation. J Immunol. 2009;182:55–62. doi: 10.4049/jimmunol.182.1.55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.