Abstract
The phosphatase PTP4A3 is an attractive anticancer target, but knowledge of its exact role in cells remains incomplete. A potent, structurally novel inhibitor of the PTP4A family was obtained by photooxygenation of a less active, electron-rich thienopyridone (1). Iminothienopyridinedione 13 displays increased solution stability and is readily obtained by two new synthetic routes that converge in the preparation of 1. The late-stage photooxygenation of 1 to give 13 in high yield highlights the potential of this reaction to modify the structure and properties of a biological lead compound and generate value for expanding the scope of an SAR investigation. Analog 13 should become a valuable tool for further exploration of the role of PTP4A3 in tumor progression.
Graphical Abstract
Small molecule modulators of protein function provide an indispensable tool for studying signalling pathways and disease pathology, and serve as lead structures in the design and development of new therapeutic agents.1 Phosphatases and kinases represent an essential class of proteins that govern reversible phosphorylations of the hydroxylated moieties on serine, threonine, and tyrosine residues. Phosphate group transfer is a key mechanism used by eukaryotic cells to regulate enzymatic activity, respond to extracellular signals, and sustain intracellular signal transduction.2 While the development of kinase inhibitors has resulted in numerous marketed drugs,3 phosphatases remain largely underexplored due to a perceived lack of druggability of these proteins.4,5 As a continuation of our interests in phosphatases as therapeutic targets,6 we now report on the development of new small molecule inhibitors of the protein-tyrosine phosphatase 4A3 (PTP4A3).5,7
PTP4A3, along with PTP4A1 and PTP4A2, is member of a family of prenylated dual-specificity phosphatases, also known as phosphatases of regenerating liver (PRL).5,7 Sequence identity between the PTP4A isoforms is high; notably, a C49 residue (C46 in PTP4A2), a WPD loop, a C(X)5R active site phosphatase domain, and a terminal CAAX box are conserved. This family of phosphatases was found to be overexpressed in many cancer cell lines and has been shown to play a role in regulating cell cycles and tumor cell proliferation. PTP4A3 has a higher rate of overexpression in some cancer cells than other phosphatases, including PTP4A1 and PTP4A2. Comparison of gene expression profiles in colon cancers which metastasized to the liver showed that, among the 144 upregulated genes, only PTP4A3 was overexpressed in all colon cancer metastases studied.8 Additionally, PTP4A3 is overexpressed in breast, lung, cervical, ovarian, and gastric cancers. Finally, poor patient prognosis and increased tumor invasiveness are commonly observed in many different malignancies expressing high levels of PTP4A3.9
PTP4A3 has been validated as an anticancer target in vivo, in conjunction with tumor driven angiogenesis, VEGF-dependent endothelial cell motility, and vascular permeability.10 Knockdown studies with PTP4A siRNA resulted in abrogation of tumor cell motility and ability to metastasize in a mouse model. A number of downstream signalling pathways have been suggested, including ERK1/2,11 PI3K/AKT,12 Rho GTPases,13 and Src.14 The development of a small molecule inhibitor will enable the detailed exploration of PTP4A3’s role in tumorigenesis and metastasis.15
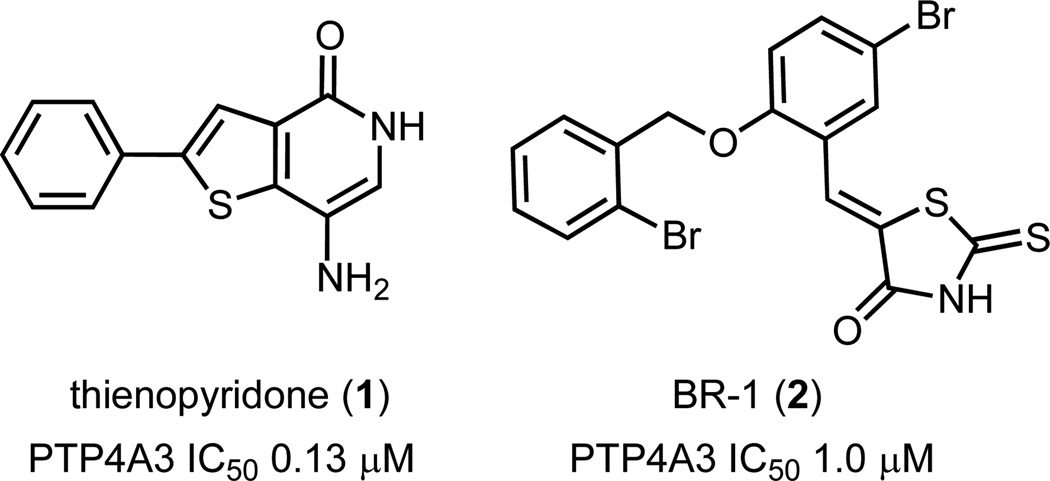
Several potential PTP4A3 inhibitors have been identified, but most suffer from structural features that may promote promiscuous bioactivity or metabolic liability.5 Two of these compounds, thienopyridone 1 and BR-1 (2), are shown in Fig 1. Structure-activity relationship (SAR) studies of rhodanine inhibitors of PTP4A3 identified 2 as a lead compound with an IC50 of about 1 µM.16 PTP4A3 was confirmed as target of 2 by the recovery of phosphorylated ezrin and cytokeratin 8, two putative PTP4A3 substrates. Selectivity of 2 towards PTP4A3 was demonstrated against 10 other phosphatases. For a mechanism of action, it was suggested that the NH in the rhodanine is deprotonated and the resulting negative charge is stabilized by the positive charge of the Arg residue in the active site. This observation was further supported by the lack of activity observed with an N-methylated derivative of 2. Furthermore, SAR studies on 2 revealed that the thione in the rhodanine ring is necessary for activity, since replacing the sulfur with an oxygen atom resulted in a significant decrease in activity. Overall, however, rhodanine-containing compounds are often associated with off-target effects.17,18
Fig 1.
Structures of PTP4A3 inhibitors thienopyridone 1 and rhodanine BR-1 (2).
Thienopyridone 1 is the most potent known inhibitor of PTP4A3 to date and was shown to be selective for the PTP4A family over 11 other phosphatases.19 Significant inhibition of tumor cell anchorage-independent growth, induction of p130Cas cleavage, and apoptosis that is not related to increased levels of p53 were observed. The main concern with this compound is the potential for hydroquinone/quinone type redox activity resulting from the high electron density of the fused thiophene and aniline-like amino substituent on the pyridinone ring. Quinones and quinone-imines, derived from phenols and anilines, have been shown to be bioactivation metabolites responsible for idiosyncratic toxicity in marketed drugs.20 Given this compound’s potency and selectivity, however, we became interested in its further chemical and biological development.
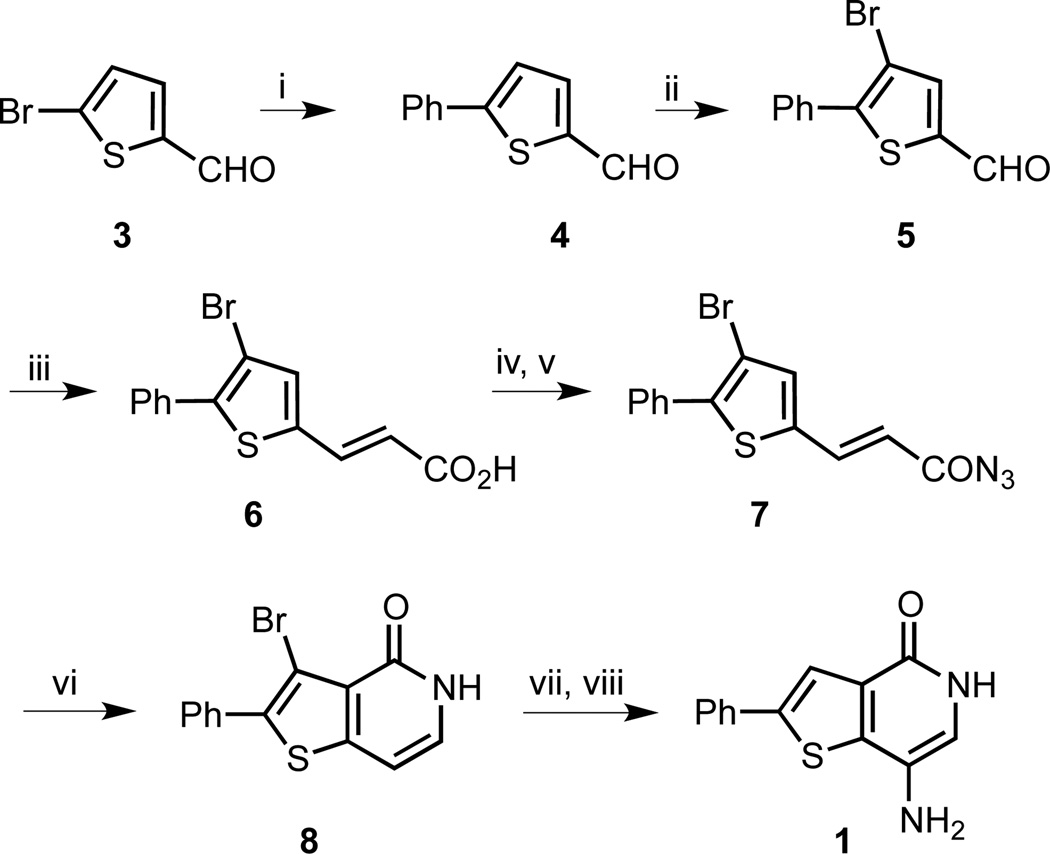
In order to establish a route suitable for SAR studies, we investigated several synthetic approaches to 1. A Suzuki cross-coupling of phenyl boronic acid with brominated thiophene 3 provided aldehyde 4 in 95% yield (Scheme 1). Regioselective bromination of 4 led to trisubstituted thiophene 5 in high yield. The bromine substituent was envisioned to offer a handle for further functionalizations. Knoevenagel condensation of 5 with malonic acid gave an 88% yield of acid 6 which was subjected to acid chloride formation and substitution with sodium azide to give acyl azide 7 in 44% yield over two steps. Curtius rearrangement and concomitant cyclization required high temperatures and produced thienopyridone 8 in 52% yield. Finally, nitration followed by hydrogenation gave 1 in 9% yield over two steps.
Scheme 1.
Synthesis of thienopyridone 1. Reagents and conditions: (i) Pd(PPh3)4, PhB(OH)2, Na2CO3, dioxane/H2O, µW 90 °C, 2 h, 95% (ii) Br2, CHCl3/AcOH, room temperature, 20 h, 98%; (iii) malonic acid, pyridine, piperidine, reflux, 5.5 h, 88%; (iv) SOCl2, DMF, toluene, reflux, 2.5 h; (v) NaN3, toluene/H2O, 0 °C to room temperature, 1.5 h, 44% (2 steps); (vi) Ph2O, µW 250 °C, 30 min, 52%; (vii) HNO3, H2SO4, 1 h, 80 °C; (viii) H-cube (1 atm), 10% Pd/C, 50 °C, 1 h, 9% (2 steps).
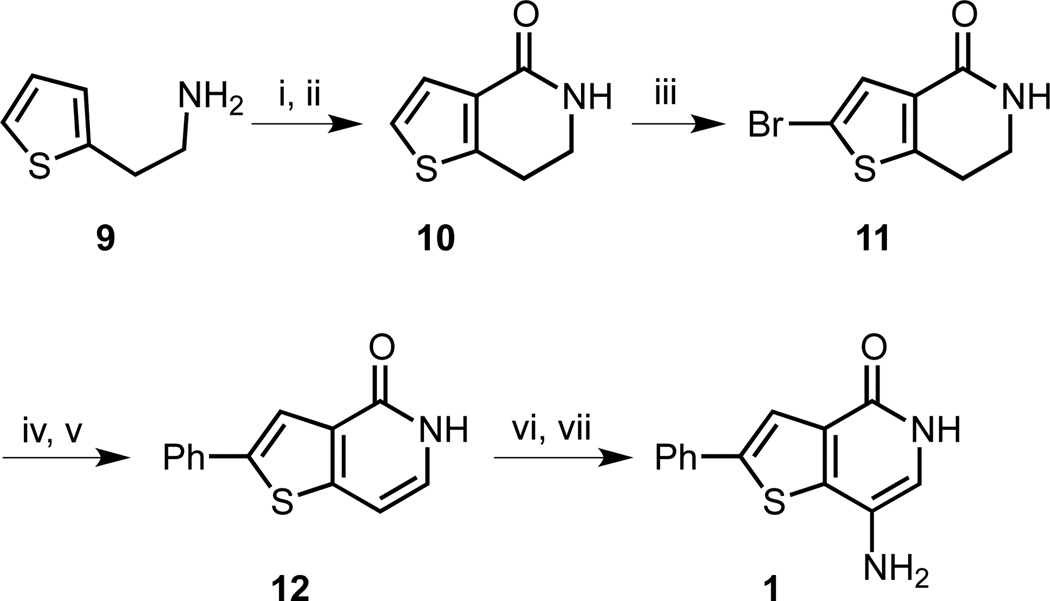
Forming the thienopyridone bicyclic system via a tandem Curtius rearrangement-cyclization is a well-known strategy,21 but this process requires high temperatures in part to isomerize the trans-double bond to allow the cyclization to take place. In agreement with literature reports, we experienced difficulties with reproducing yields and reaction scale-up was challenging, limiting the scope of our SAR studies. To overcome this bottleneck, we developed a second-generation synthesis, where we envisaged accessing a dihydro-bicyclic ring system, avoiding the alkene isomerization step (Scheme 2). Additionally, by directly introducing an isocyanate moiety, we could eliminate the need for a thermal acyl azide rearrangement. Starting with the primary amine 9, the isocyanate was formed with triphosgene. The biphasic reaction conditions were chosen to prevent side reactions of the amino group in 9 with the isocyanate. Friedel-Crafts cyclization was completed with FeCl3 acting as a Lewis acid to form lactam 10. Bromination of 10 succeeded in 75% yield and the resulting thiophene 11 was subjected to a Suzuki cross-coupling with phenyl boronic acid followed by aromatization with DDQ to yield thienopyridone 12 in 48% yield over two steps. Analogous to the previous route, nitration and then hydrogenation completed the synthesis of 1.
Scheme 2.
Second generation synthesis of thienopyridone 1. Reagents and conditions: (i) CO(OCCl3)2, NaHCO3, CH2Cl2/H2O, 0 °C, 5 h; (ii) FeCl3, CH2Cl2, 50 °C, 40 min, 71% (2 steps); (iii) Br2, AcOH, room temperature, 12 h, 75%; (iv) Pd(PPh3)4, PhB(OH)2, Na2CO3, dioxane/H2O, 90 °C, 24 h; (v) DDQ, dioxane, 101 °C, 2.5 d, 48% (2 steps); (vi) HNO3, AcOH, room temperature, 15.5 h; (vii) 10% Pd/C (17 mol%), H2 (1 atm), EtOH, room temperature, 5 h, 19% (2 steps).
In order to decrease the electron-density and potential redox-liability in the heterocyclic scaffold of 1, we studied photooxygenation conditions and the possibility to selectively introduce oxygen atoms on the thienopyridone (Table 1). Photooxygenation is a useful synthetic strategy to diversify electron-rich heterocycles and arenes. While currently still underutilized, it offers a convenient method to introduce novel structural motifs.22 For example, Cossy and Belotti used a photooxygenation for the preparation of quinoline-5,8-quinones,23 and Prisinzano et al. generated salvidivin A from salvinorin A by exposure to sun light.24 The reaction of 1 under ambient laboratory light generated the novel 7-iminothieno[3,2-c]pyridine-4,6(5H,7H)-dione 13, but the conversion was incomplete with a significant amount of 1 left after 2 days (entry 1). The photooxygenation was also attempted in a halogenated solvent by replacing MeOH with hexafluoroisopropanol (HFIP), but only a trace conversion of 1 to 13 was observed (entry 2). Conversely, placing a 23-W compact fluorescent lamp (CFL) at a distance of 15 cm away from the reaction mixture in a borosilicate or Pyrex flask completed this transformation in 18 h (entry 3), and a yield of 85% was obtained after 23 h (entry 4).
Table 1.
Photooxygenation of 1 to 13.
| Entry | Solvent | Light Source | Conversion Timea (isolated yield of 13) |
|---|---|---|---|
| 1 | MeOHb | ambient light | > 2 d (nd) |
| 2 | HFIPb | ambient light | 1 d (trace) |
| 3 | MeOHb | 23 W CFLc | 18 h (>80%) |
| 4 | MeOHd | 23 W CFLc | 23 h (85%) |
| 5 | MeOHe | 23 W CFLc | 2.5 d (77%) |
Monitored by HR LC/MS for disappearance of 1;
On a 7–8 mg scale of 1 at 1–1.1 mg/mL conc.;
Compact fluorescent lamp at a distance of 15 cm;
6.0 mg of 1 in 5.5 mL of MeOH;
32.5 mg of 1 in 30 mL of MeOH;
nd = not determined
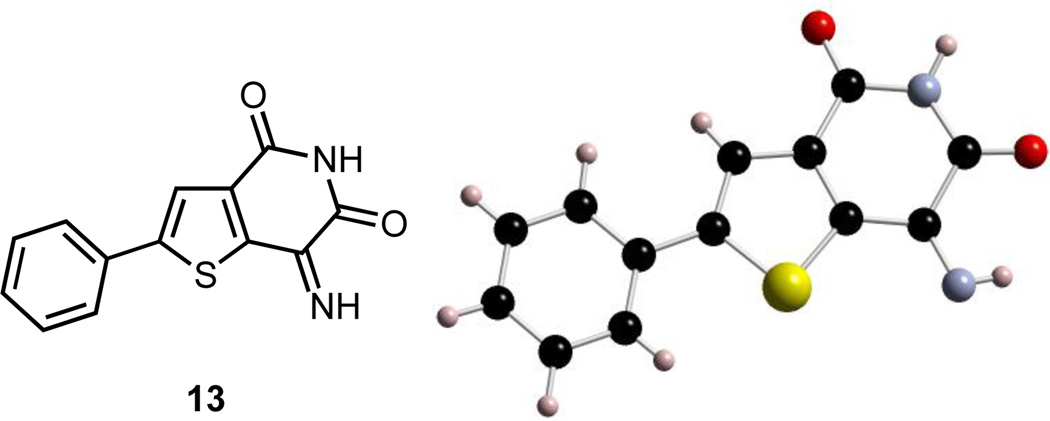
Due to its poor solubility in MeOH, 13 started to precipitate out of solution within a few hours. It is worth noting that this transformation was accomplished without the use of any additives, and the product isolation required a simple filtration for purification. Compound 13 was acquired as an amorphous brown powder from MeOH in an average yield of 81% (Table 1, entries 4–5). Larger scale synthesis required longer reaction times, presumably due to poor light penetration of the larger solvent volume. We are currently also exploring alternate methods for this photochemical process.25 Crystalline 13 was obtained by slow evaporation from a solution in acetonitrile, and the structural assignment was confirmed by X-ray crystallography (Fig. 2).
Fig 2.
X-ray structure of 13 resulting from photooxygenation of 1 (CCDC 1476250)
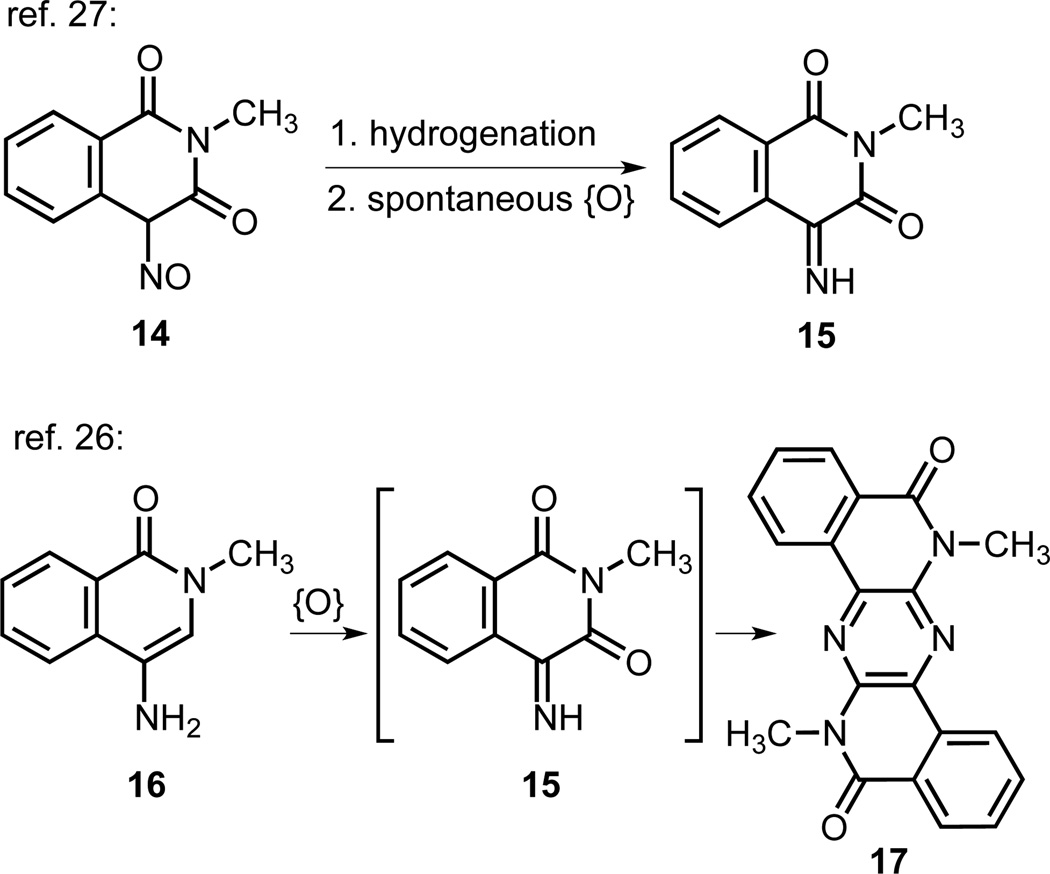
To the best of our knowledge, the oxidized thiophene-containing scaffold of 13 has not previously been reported. Two literature studies of related imino-isoquinolinediones are shown in Fig. 3.26,27,28 The Otomasu group prepared compound 15 by a hydrogenation of nitrosodione 14 to the amine, which underwent a spontaneous oxidation to 15.27 The Henry group found that 4-amino-2-methyl-1-isoquinolone (16), in contrast to an analogous 5- or 7- amino derivative, was converted in situ to pyridazine 17, which they postulated to result from a condensation of 16 and air-oxidized 15.26
Fig 3.
Previously reported oxidations of structurally related compounds, analogous to the transformation of 1 to 13.
In a side-by-side LCMS/UV stability study of 1 and 13 at a concentration of 0.1 mg/mL in DMSO/H2O (5:1) with an internal standard (0.01 mg/mL vanillin), we observed significant (>80%) decomposition of 1 within 24 h whereas 13 remained >95% unchanged after 3 days; thus suggesting a considerable structural stabilization in the photooxygenation product 13 that should translate into improved biological tractability.
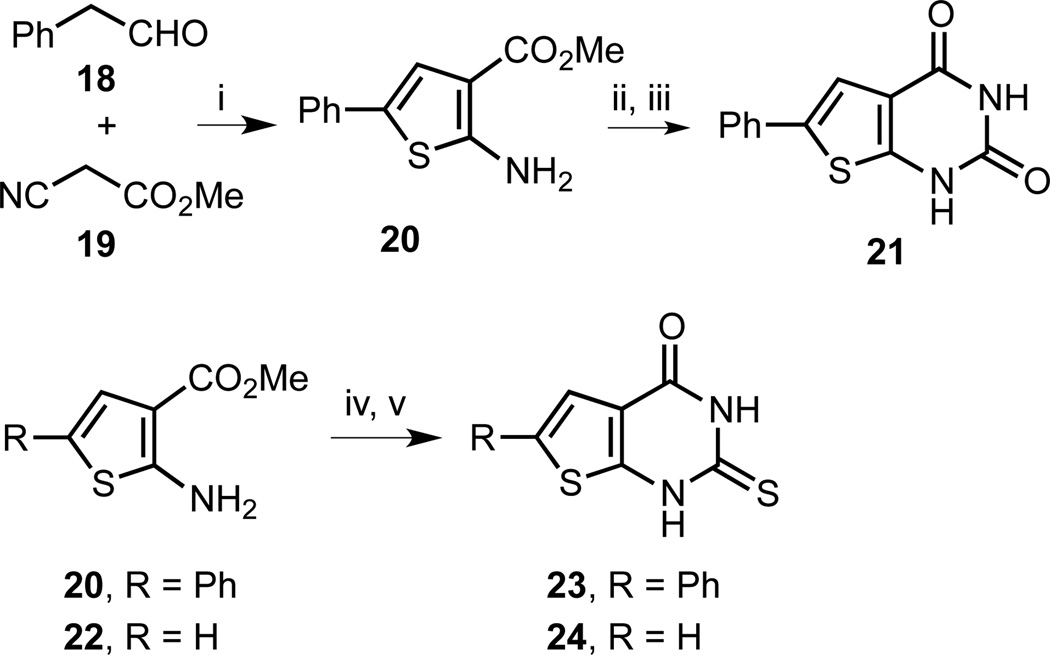
For a further expansion of the SAR of 1 and 13, we envisioned pyrimidinediones as a related, synthetically readily accessible heterocyclic ring system (Scheme 3). Pyrimidinediones resemble a hybrid construct of 1, 2, and 13. The pyrimidinedione 21 mimics the carbonyl functionalities in 13 as well as the rhodanine ring in 2. For an even closer structural match with 2, we introduced a thione functionality in 23 and 24, similar to the thione group in the rhodanine. Analogs 21 and 23 also incorporate the 2-phenylthiophene moiety of 1 and 13.
Scheme 3.
Synthesis of thieno[2,3-d]pyrimidine-2,4(1H,3H)-dione and 2-thioxo-2,3-dihydrothieno[2,3-d]pyrimidin-4(1H)-one analogs. Reagents and conditions: (i) S8, Et3N, DMF, room temperature, 21 h, 90%; (ii) ClSO2NCO, CH2Cl2, −78 °C to room temperature, 40 min; (iii) [a] dioxane/H2O, room temperature to 85 °C, 45 min; [b] aqueous NaOH, 85 °C, 30 min; [c] aqueous HCl, room temperature, 57% (2 steps); (iv) BzCl, NH4NCS, CH3CN, reflux, 6 h, 69% (R = Ph), 67% (R = H); (v) [a] KOH, EtOH, reflux, 14–19 h; [b] aqueous HCl, room temperature, 49% (23), 57% (24).
The synthesis of pyrimidinediones started with a Gewald reaction of phenyl acetaldehyde (18) and cyano malonate 19 in the presence of elemental sulphur (Scheme 3). Aminothiophene 20 was obtained in excellent yield and subjected to chlorosulfonyl isocyanate to generate the urea. A one-pot sequence of cyclization, hydrolysis, and acid-quench yielded the desired thienopyrimidinedione 21 in 57% yield over two steps. Analogously, the thiourea derivatives were synthesized from 20 and 22 with ammonium thiocyanate in the presence of benzoyl chloride followed by a one-pot cyclization, hydrolysis, and an acid-quench to yield analogs 23 and 24 in good yields.
The in vitro biochemical evaluation of all compounds was carried out using recombinant human PTP4A3, overexpressed as a His6-tag fusion protein in E. coli and purified on a metal affinity column. Assays were performed using 6,8-difluoro-4-methylumbelliferyl phosphate (DiFMUP) as an artificial substrate at 25 °C for 30 min in 40 mM Tris-HCl (pH 7.0), 75 mM NaCl, 2 mM EDTA, and 4 mM DTT buffer. The reaction was carried out in 45 µL total volume per well of a black 384-well plate and initiated upon addition of DiFMUP at a final concentration of 12 µM (3× the Km of PTP4A3 for DiFMUP, to ensure that the reaction velocity remained constant throughout the assay) to each well containing 1 µg of full-length protein. The fluorescence was measured using a SpectraMax M5 plate reader at 358 nm excitation and 455 nm emission. Fluorescence values were used to calculate the percent inhibition of enzyme activity relative to maximal activity, PTP4A3 in the absence of inhibitor, and maximal inhibition, PTP4A3 in the presence of 2 mM Na3VO4.
Consistent with previous reports,19 1 had an IC50 of 132 nM against PTP4A3. The desaminothienopyridone 12 was remarkably less active than 1, highlighting the essential role of the 7-amino substitution. The novel imino-pyridinedione 13, in contrast, exhibited a greatly improved IC50 of 18 nM, making it the most potent PTP4A3 inhibitor reported to date. Moreover, 13 was ca. 3× more potent against PTP4A3 vs PTP4A1 and PTP4A2, which showed IC50 values of 50 and 52 nM, respectively, in our assay. We observed no significant inhibition of the enzymatic activity of another protein tyrosine phosphatase, PTP1B, with concentrations of 13 as high as 80 µM. Importantly, under the assay conditions (90 min, in the presence or absence of DTT), we did not detect quantifiable (>5%) spontaneous conversion of 1 to 13. The importance of the 7-nitrogen substitution in 13 was supported by the complete lack of PTP4A3 inhibition with analogs 21, 23 and 24. However, these compounds should be useful inactive controls for further pharmacological studies.
Conclusions
This work establishes the first literature record of scalable routes to thienopyridone 1, a moderately potent inhibitor of PTP4A3. Furthermore, we employed a facile photooxygenation reaction to generate a novel 7-iminothieno[3,2-c]pyridine-4,6(5H,7H)-dione, 13. Interestingly, 13 proved to be almost 10-fold more potent than 1 as well as much more stable in solution. While photoxygenation is currently still significantly less popular than the late-stage fluorination of bioactive scaffolds,29 we suggest that this strikingly straightforward “reagent-free” method to modify the structure and properties of heterocyclic building blocks has great value for expanding the scope of SAR investigations. Our synthesis enables access to a low-nanomolar small molecule inhibitor that should prove to be a valuable tool for a further exploration of PTP4A3’s role in tumor progression. Since thienopyridone 1 does not have any short-term cytotoxicity in cells, the higher potency of 13 might overcome this limitation.7b Compound 13 is also noteworthy in comparison to 1 by the improvement in the drug-score of the lead structure to 0.87 from 0.79.30 The unique scaffold of 13 and the SAR information obtained with 12, 21, 23 and 24 will guide our future medicinal chemistry studies and the development of clinically useful potent and selective inhibitors of PTP4A3.
Supplementary Material
Table 2.
In vitro evaluation of inhibition of PTP4A3 activity.
| Entry | Compound | IC50 [µM±S.D.]a |
|---|---|---|
| 1 | 1 | 0.132 ± 0.003 |
| 2 | 12 | >80 |
| 3 | 13 | 0.018 ± 0.014 |
| 4 | 21 | >240 |
| 5 | 23 | >100 |
| 6 | 24 | >100 |
n=3; for inhibition curves see ESI.
Acknowledgments
The authors would like to acknowledge Enamine Ltd. (Kyiv, Ukraine) for the scale up of thienopyridone 1, as well as financial support from the National Institutes of Health (R21 CA191944 and F31 CA196062), the Fiske Drug Discovery Fund, Boehringer Ingelheim Pharmaceuticals, Inc. (Ridgefield, CT) for generous discretionary support, and the Ivy Foundation Biochemical grant program.
Footnotes
Electronic Supplementary Information (ESI) available: Experimental procedures for chemical synthesis, compound characterizations, copies of NMR spectra for previously unknown compounds, and biological data. See DOI: 10.1039/x0xx00000x
Contributor Information
Elizabeth R. Sharlow, Email: ers7g@virginia.edu.
John S. Lazo, Email: lazo@virginia.edu.
Peter Wipf, Email: pwipf@pitt.edu.
Notes and references
- 1.(a) Schreiber SL, et al. Cell. 2015;161:1252. doi: 10.1016/j.cell.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Huryn DM, Resnick LO, Wipf P. J. Med. Chem. 2013;56:7161. doi: 10.1021/jm400132d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Sacco F, Perfetto L, Castagnoli L, Cesareni G. FEBS Lett. 2012;586:2732. doi: 10.1016/j.febslet.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jin J, Pawson T. Philos. Trans. R. Soc. Lond. D. Biol. Sci. 2012;367:2540. doi: 10.1098/rstb.2012.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roskoski R. Pharmacol. Res. 2015;100:1. doi: 10.1016/j.phrs.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 4.(a) Bialy L, Waldmann H. Angew. Chem. Int. Ed. 2005;44:3814. doi: 10.1002/anie.200461517. [DOI] [PubMed] [Google Scholar]; (b) Lazo JS, Wipf P. Curr. Opin. Invest. Drugs. 2009;10:1297. [PubMed] [Google Scholar]; (c) De Munter S, Köhn M, Bollen M. ACS Chem. Biol. 2013;8:36. doi: 10.1021/cb300597g. [DOI] [PubMed] [Google Scholar]; (d) Lazo JS, Sharlow ER. Annu. Rev. Pharmacol. Toxicol. 2016;56:23. doi: 10.1146/annurev-pharmtox-010715-103440. [DOI] [PubMed] [Google Scholar]
- 5.Sharlow ER, Wipf P, McQueeney KE, Bakan A, Lazo JS. Expert Opin. Investig. Drugs. 2014;23:661. doi: 10.1517/13543784.2014.892579. [DOI] [PubMed] [Google Scholar]
- 6.See, for example: Lazo JS, Aslan DC, Southwick EC, Cooley KA, Ducruet AP, Joo B, Vogt A, Wipf P. J. Med. Chem. 2001;44:4042. doi: 10.1021/jm0102046. Brisson M, Nguyen T, Vogt A, Yalowich J, Giorgianni A, Tobi D, Bahar I, Stephenson CRJ, Wipf P, Lazo JS. Mol. Pharmacol. 2004;66:824. doi: 10.1124/mol.104.001784. Rosenker KMG, Paquette WD, Johnston PA, Sharlow ER, Vogt A, Bakan A, Lazo JS, Wipf P. Bioorg. Med. Chem. 2015;23:2810. doi: 10.1016/j.bmc.2015.01.043.
- 7.(a) Stephens BJ, Han H, Gokhale V, Von Hoff DD. Mol. Cancer Ther. 2005;4:1653. doi: 10.1158/1535-7163.MCT-05-0248. [DOI] [PubMed] [Google Scholar]; (b) Hoeger, B B, Diether M, Ballester PJ, Köhn M. Eur. J. Med. Chem. 2014;88:89. doi: 10.1016/j.ejmech.2014.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saha S, Bardelli A, Buckhaults P, Velculescu VE, Rago C, St. Croix B, Romans KE, Choti MA, Lengauer C, Kinzler KW, Vogelstein B. Science. 2001;294:1343. doi: 10.1126/science.1065817. [DOI] [PubMed] [Google Scholar]
- 9.Guzińska-Ustymowicz K, Pryczynicz A. Anticancer Agents Med. Chem. 2011;11:99. doi: 10.2174/187152011794941145. [DOI] [PubMed] [Google Scholar]
- 10.(a) Zimmerman MW, Homanics GE, Lazo JS. PLoS ONE. 2013;8:e58300. doi: 10.1371/journal.pone.0058300. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zimmerman MW, McQueeney KE, Isenberg JS, Pitt BR, Wasserloos KA, Homanics GE, Lazo JS. J. Biol. Chem. 2014;289:5904. doi: 10.1074/jbc.M113.480038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ming J, Liu N, Gu Y, Qui X, Wang EH. Pathology. 2009;41:118. doi: 10.1080/00313020802579268. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Quah SY, Dong JM, Manser E, Tang JP, Zeng Q. Cancer Res. 2007;67:2922. doi: 10.1158/0008-5472.CAN-06-3598. [DOI] [PubMed] [Google Scholar]
- 13.Fiordalisi JJ, Keller PJ, Cox AD. Cancer Res. 2006;66:3153. doi: 10.1158/0008-5472.CAN-05-3116. [DOI] [PubMed] [Google Scholar]
- 14.Liang F, Liang J, Wang W-Q, Sun J-P, Udho E, Zhang Z-Y. J. Biol. Chem. 2007;282:5413. doi: 10.1074/jbc.M608940200. [DOI] [PubMed] [Google Scholar]
- 15.Lazo JS, Wipf P. Oncol. Res. 2003;13:347. doi: 10.3727/096504003108748555. [DOI] [PubMed] [Google Scholar]
- 16.(a) Ahn JH, Kim SJ, Park WS, Cho SY, Ha JD, Kim SS, Kang SK, Jeong DG, Jung S-K, Lee S-H, Kim HM, Park SK, Lee KH, Lee CW, Ryu SE, Choi J-K. Bioorg. Med. Chem. Lett. 2006;16:2996. doi: 10.1016/j.bmcl.2006.02.060. [DOI] [PubMed] [Google Scholar]; (b) Min G, Lee S-K, Kim H-N, Han Y-M, Lee R-H, Jeong DG, Han DC, Kwon B-M. Bioorg. Med. Chem. Lett. 2013;23:3769. doi: 10.1016/j.bmcl.2013.04.092. [DOI] [PubMed] [Google Scholar]
- 17.Mendgen T, Steuer C, Klein CD. J. Med. Chem. 2012;55:743. doi: 10.1021/jm201243p. [DOI] [PubMed] [Google Scholar]
- 18.Tang SQ, Lee YYI, Packiaraj DS, Ho HK, Chai CLL. Chem. Res. Toxicol. 2015;28:2019. doi: 10.1021/acs.chemrestox.5b00247. [DOI] [PubMed] [Google Scholar]
- 19.Daouti S, et al. Cancer Res. 2008;68:1162. doi: 10.1158/0008-5472.CAN-07-2349. [DOI] [PubMed] [Google Scholar]
- 20.Stepan AF, Walker DP, Bauman J, Price DA, Baillie TA, Kalgutkar AS, Aleo MD. Chem. Res. Toxicol. 2011;24:1345. doi: 10.1021/tx200168d. [DOI] [PubMed] [Google Scholar]
- 21.See, for example: New JS, Christopher WL, Yevich JP, Butler R, Schlemmer RF, VanderMaelen CP, Cipollina JA. J. Med. Chem. 1989;32:1147. doi: 10.1021/jm00126a002. Gentile G, Bernasconi G, Pozzan A, Merlo G, Marzorati P, Bamborough P, Bax B, Bridges A, Brough C, Carter P, Cutler G, Neu M, Takada M. Bioorg. Med. Chem. Lett. 2011;21:4823. doi: 10.1016/j.bmcl.2011.06.050. Li L, Degardin M, Lavergne T, Malyshev DA, Dhami K, Ordoukhanian P, Romesberg FE. J. Am. Chem. Soc. 2014;136:826. doi: 10.1021/ja408814g.
- 22.George MV, Bhat V. Chem. Rev. 1979;79:447. [Google Scholar]
- 23.Cossy J, Belotti D. Tetrahedron Lett. 2001;42:4329. [Google Scholar]
- 24.Simpson DS, Katavic PL, Lozama A, Harding WW, Parrish D, Deschamps JR, Dersch CM, Partilla JS, Rothman RB, Navarro H, Prisinzano TE. J. Med. Chem. 2007;50:3596. doi: 10.1021/jm070393d. [DOI] [PubMed] [Google Scholar]
- 25.Schuster EM, Wipf P. Isr. J. Chem. 2014;54:361. [Google Scholar]
- 26.Henry RA, Heller CA, Moore DW. J. Org. Chem. 1975;40:1760. [Google Scholar]
- 27.Tahara S, Shigetsuna M, Otomasu H. Chem. Pharm. Bull. 1982;30:3133. [Google Scholar]
- 28.Borisova KL, Mel’man GI, Denisenko VA, Glazunov VP, Anufriev VF. Russ. Chem. Bull. Int. Ed. 2012;61:616. [Google Scholar]
- 29.(a) Iesce MR, Cermola F, Temussi F. Curr. Org. Chem. 2005;9:109. [Google Scholar]; (b) Neumann CN, Ritter T. Angew. Chem., Int. Ed. 2015;54:3216. doi: 10.1002/anie.201410288. [DOI] [PubMed] [Google Scholar]
- 30.Drug-relevant properties were calculated with OSIRIS Property Explorer. ( http://www.organic-chemistry.org/prog/peo/) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.