Abstract
The critical involvement of dopamine in cognitive processes has been well established, suggesting therapies targeting dopamine metabolism may alleviate cognitive dysfunction. COMT is a catecholamine-degrading enzyme, the substrates of which include dopamine, epinephrine, and norepinephrine. The present work illustrates the potential therapeutic efficacy of COMT inhibition for alleviating cognitive impairment. A brain penetrant COMT inhibitor, tolcapone, was tested in normal and phencyclidine (PCP)-treated rats and COMT–Val transgenic mice. In a Novel Object Recognition (NOR) procedure, tolcapone counteracted a 24h-dependent forgetting of a familiar object and counteracted PCP-induced recognition deficits in the rats at doses ranging from 7.5 to 30 mg/kg. In contrast, entacapone, a COMT inhibitor which does not readily cross the blood-brain barrier failed to show efficacy at doses up to 30mg/kg. Tolcapone at a dose of 30 mg/kg also improved NOR performance in the transgenic mice, which showed clear recognition deficits. Complementing earlier studies, our results indicate that central inhibition of COMT positively impacts recognition memory processes and might constitute an appealing treatment for cognitive dysfunction related to neuropsychiatric disorders.
Keywords: COMT, tolcapone, entacapone, dopamine, novel object recognition, cognitive enhancers, rat, mouse
Introduction
The role of dopamine in cognitive processes, in normal or pathological conditions such as schizophrenia or Parkinson’s Disease, is well established (Dujardin et al., 2003, Brisch et al., 2014, Chandler et al., 2014). Cognitive dysfunction is a key, yet clinically untreated symptom of schizophrenia (Milan et al. 2010, Nuechterlein et al., 2014) and dopaminergic drugs have been proposed to ameliorate cognitive impairment associated with schizophrenia (CIAS). Stimulating post- or peri-synaptic dopaminergic receptors such as D1/5, or increasing dopamine in the synaptic cleft by inhibiting a key catabolic pathway for cortical dopamine, i.e. catechol-O-methyl transferase (COMT), may be a viable strategy for ameliorating cognitive deficits related to the fronto-temporal dysfunction observed in this disorder. However, brain penetrant D1/5 agonists or COMT inhibitors (e.g. tolcapone) advanced to human clinical trials have important side effects, such as hypotension for D1/5 agonists or idiosyncratic hepatotoxicity for tolcapone. They can be used nevertheless as tools for short-term proof of concept in animals and humans.
Tolcapone has been studied in normal volunteers stratified by the functional Val158Met polymorphism on COMT and, as predicted, tolcapone was shown to ameliorate working memory and executive dysfunctions in those persons having increased DA metabolism (Val-Val genotypes) compared to people bearing the Met-Met allele (Apud et al., 2007; Giakoumi et al., 2008; Farrell et al., 2012). Pre-clinical research with the COMT-Val transgenic mice showed that they have increased COMT activity compared to control littermates and significant deficits in novel object recognition. The novel object recognition deficit could be reversed with acute amphetamine treatment immediately following the acquisition trial (Papaleo et al., 2008). These data indicated that manipulation of the dopaminergic system is a viable strategy for modulating cognitive function in COMT-Val transgenic mice, and the current paper assessed tolcapone efficacy in this paradigm.
There is a paucity of preclinical, in particular rodent, studies using tolcapone as a pharmacological probe to evaluate the impact of increased DA function on different cognitive domains both in normal and pathological animals.
Apart from the study by Khromova et al. (1997) demonstrating dose-dependent efficacy of tolcapone (1, 3, 10 mg/kg, i.p.), with the highest dose clearly reversing scopolamine-induced deficits in an inhibitory avoidance procedure, all the subsequent published studies utilizing tolcapone in cognitive tasks used a single dose. For example, tolcapone tested at a dose of 3 mg/kg (i.p.) has been shown to slightly improve the number of correct choices in aged rats that were selected as poor performers when trained in a working memory protocol using a linear maze (Liljequist et al., 1997). Tunbridge and collaborators (2004) tested tolcapone in rats at a dose of 30 mg/kg (i.p.) and indicated beneficial effect of the compound in a set shifting protocol. Specifically, tolcapone-treated rats reached criterion faster when they had to perform an extra-dimensional shift. Laatikainen et al. (2012) showed that, in a working memory procedure, a single dose of 30 mg/kg (i.p.) improved delayed alternation performance of rats at short but not long delays. The authors also mentioned a positive impact of the same tolcapone dose in rats submitted to a novelty preference procedure in a Y-maze task. Finally, in a delayed spatial win shift procedure, 30 mg/kg tolcapone has been shown to improve performances of rats at an extended delay of 1 hour but not at 1 minute delay (Lapish et al., 2009). To our knowledge, the only published study, other than the one by Khromova (1997), in which several doses of tolcapone were used (3–30 mg/kg), reported that it was ineffective in rats performing at a sub-optimal level (reduced accuracy) in a 5-choice serial reaction time task (Paterson et al., 2011).
Up to now, there is no published study evaluating the impact of tolcapone in the Novel Object Recognition (NOR) paradigm or in animal models relevant for CIAS such as the chronic, low dose phencyclidine (PCP) treated rat (for review see Morris et al., 2005; Pratt et al., 2009). Interestingly, some dopaminergic D1 agonists showed efficacy in this model when animals were evaluated in a novel object recognition procedure (McLean et al., 2009; Horiguchi et al., 2013).
The objective of the present study was two-fold. The first aim was to evaluate the impact of tolcapone in a Novel Object Recognition (NOR) protocol using normal and PCP-treated rats. A range of doses covering different levels of enzyme inhibition were investigated in 24-h delay-dependent forgetting of a familiar object in rats. Entacapone, a non-CNS penetrant COMT inhibitor, was also tested in this procedure as a negative control. We then evaluated the impact of effective doses of tolcapone in rats treated with subchronic PCP (b.i.d. for 7 days followed by a 7-day washout period), a procedure used to model cognitive deficits analogous to schizophrenia. The NOR has been proposed as a valuable paradigm for CIAS (see Lyon et al., 2012 for a review), particularly in rats following sub-chronic treatment with PCP (Redrobe et al., 2010). The second aim was to evaluate the efficacy of tolcapone in COMT-Val transgenic mice which also show endogenous deficits in the novel object recognition task.
The present results indicate that tolcapone is able to counteract recognition memory deficits observed in normal rats submitted to a 24-h delay procedure, in sub-chronic PCP-treated rats submitted to a 30-min delay procedure, and in COMT-Val transgenic mice submitted to a 1-h delay procedure.
Methods
Subjects
Rats
Sprague Dawley and Long Evans rats (2–3 month old) were used; the second strain was selected for their high susceptibility to PCP treatment. They were group-housed with free access to food and water in a temperature and humidity controlled animal facility (12 h:12 h light:dark cycle). The individual rat strain used depended on the model and cognitive task, as detailed below. Behavioral experiments were carried out between 09:00 and 14:00 h, in a sound attenuated and air-regulated experimental room. All animal procedures were conducted in strict adherence to the European Union Directive 2010/63/EU and approved by UCB Ethics Committee
COMT-Val transgenic mice
The origin of the COMT-Val transgenic mice used in these experiments has been previously described in detail (Papaleo et al., 2008). Briefly, COMT-Val transgenic mice express the human Val form of the COMT gene, under the control of the NSE-tTA promoter, along with normal expression of wild-type mouse COMT. The COMT-Val mice exhibit roughly 20% more endogenous COMT enzyme activity in cortex compared to their littermate controls. Genotypes were identified by PCR analysis of tail DNA. Mice were group housed (2–4 mice/cage) in a climate-controlled animal facility and maintained on a 12 h light/dark cycle (lights on at 06,00 h) with free access to food and water. All testing was conducted in 12–24-week old male mice during the light phase. All procedures were approved by the National Institute of Mental Health Animal Care and Use Committee and followed the Guide for the Care and Use of Laboratory Animals.
Novel object recognition task in rats
The experimental arena was a square box (60×60×40 cm). The arena and the objects were cleaned between each trial in order to avoid odour trails left by the rats. The arena was placed in a dark room illuminated only by halogen lamps oriented towards the ceiling and giving a uniform, dim light in the box (around 60 lux). Animals to be tested were placed in the experimental room at least 30 min before testing. The day before the test, rats were allowed to freely explore the box for 6 min (habituation trial).
Rats were given two object exploration trials (acquisition and retention) spaced by an inter-trial interval, the duration of which is specified in Table 1. During the first trial (acquisition trial), rats explored two copies of an object (familiar object); time required to complete a fixed duration of object exploration was determined with a maximal cut-off time of 4 min. Exploration was considered to be directing the nose at a distance less than 2 cm from the object and/or touching the object. Experimenters were unaware of drug treatments to ensure blinding and avoid bias. During the retention trial, one of the two familiar objects was replaced by an unknown object (novel object), and time spent exploring each object was recorded during 4 min. Animals with naturally low levels of spontaneous exploration (total exploration lower than 6 s) were excluded. About 10–15% of rats showed a low level of exploration. A recognition index was calculated by subtracting the amount of time spent exploring the familiar object from the amount of time spent exploring the novel object divided by the total exploration time.
Table 1.
Experimental parameters varying between challenges and rat strains
| Rat strain | Challenge | habituation | Exploration during acquisition | interval | Retention |
|---|---|---|---|---|---|
| Spragur Dawley | 24h delay dependent forgetting | 6 min | 20s exploration within 4 min max | 24h | 4 min |
| Long Evans | Subchronic PCP | 10 min | 8s exploration within 6 min max | 30 min | 3 min |
Novel Object Recognition task in transgenic mice
The novel object recognition protocol utilized in these experiments was similar to that used previously with COMT-Val transgenic mice (Papaleo et al, 2008). Testing was conducted in a clear, acrylic open field arena (42 × 42 × 30 cm). On day 1 of testing, mice were allowed to freely explore the empty arena for 60 minutes. On day 2, mice were placed back into the open field for a 10 min period (Acquisition phase) where they were allowed to explore two identical copies of an object. The objects were either rectangular boxes (4 × 4 × 7 cm) or Erlenmeyer flasks (4 × 7 cm). Both types of objects could be either white or black. Immediately following the acquisition phase, mice were given an injection of vehicle or tolcapone (30 mg/kg, ip). One hour later, mice were placed back into the open field (Retention phase) with a copy of the object encountered previously and a novel object, which differed from the familiar object in both color and shape. The testing sessions were videotaped and later scored by an observer blind to genotype. Time spent exploring each object was recorded and a recognition index to determine recognition memory was obtained, by subtracting the amount of time spent exploring the familiar object from the amount of time spent exploring the novel object divided by the total exploration time.
Statistical analysis
The novel object recognition test was analyzed using a two-way mixed ANOVA with Holm-Sidak post hoc testing to compare time spent exploring the novel object vs. familiar object. The factors for the ANOVA were drug treatment and prior experience with the object (familiar or novel). The index was analyzed either by one-way ANOVA followed by LSD post hoc analysis (rats) or be a t-test for comparison of COMT-Val transgenic mice and their littermate controls. Significance was set at p-values < 0.05. All graphs represent data as the mean ± SEM.
Drugs
Tolcapone (synthetized by Apichem Technology) was delivered in rats by intraperitoneal route (i.p.) as a suspension in 0.1% Tween 80 with 0.1% 1510 silicone antifoam in 1% methylcellulose to final concentration. For the COMT-Val transgenic mouse experiment, Tolcapone was purchased from AK Scientific, Inc. (Union City, CA, USA) and suspended in a vehicle of 20% (2-Hydroxypropyl)-β-cyclodextrin in saline and administered i.p. in a dose of 30 mg/kg. The injection volumes were 5 and 10 ml/kg for rats and mice, respectively.
Results
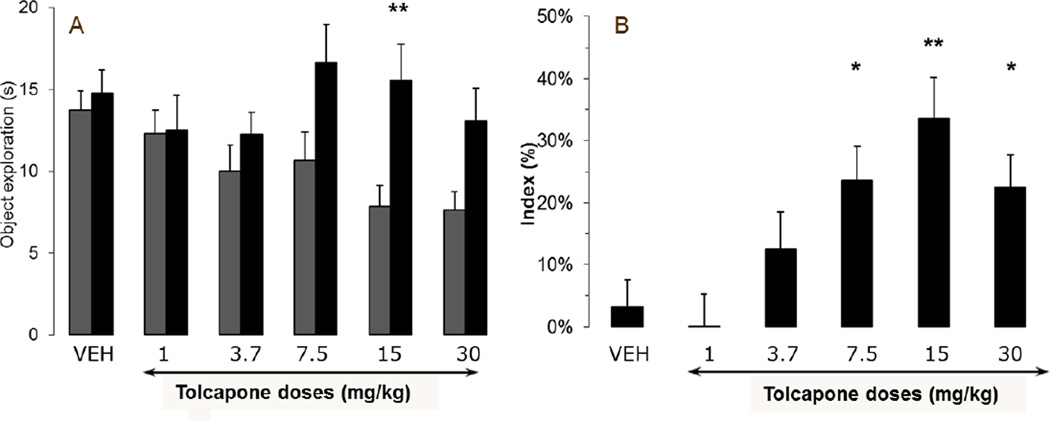
Tolcapone improved delay-dependent forgetting of NOR in rats
24h after presentation of a familiar object, rats receiving vehicle showed similar exploration of familiar and novel object. Tolcapone administered 40 min before acquisition increased time spent exploring the novel object compared to the familiar object during the retention trial [interaction effect: F(5,60)=4.27, p<0.005]. Post hoc analysis showed that the 15 mg/kg group was significantly different from the vehicle-treated group (p<0.002) (Fig. 1A). The 7.5 mg/kg and 30 mg/kg groups had effects that did not survive correction for multiple comparisons (p=0.013 and 0.024, respectively). This increase translated into a dose-dependent increase in recognition index from 7.5 to 30 mg/kg. Vehicle-treated rats showed a recognition index close to 0, indicating their inability to discriminate between the two objects when delay between acquisition trial (exploration of familiar object) and retention trial (discrimination between the familiar object and a novel one) was 24h (Fig. 1B). Tolcapone improved discrimination between the two objects as the recognition index increased from 3.3% ± 4.3 in vehicle treated rats to 33.6% ± 6.6% at 15mg/kg. This increase was statistically significant at doses ranging from 7.5 to 30 mg/kg [F(5,65)=5.10, p=0.001]. Tolcapone did not impact total object exploration [F(5,65)=1.00, NS] (data not shown).
FIGURE 1.
Tolcapone ameliorated 24h delay-dependent forgetting of a familiar object when administered i.p. 40 min before acquisition in Sprague Dawley rats.
(A) Exploration of familiar (first grey column) and novel (second black column) objects during the retention trial (B) Recognition index during retention. (* = p < 0.05, ** = p < 0.01). All data presented as mean + SEM; n= 10–12 per group.
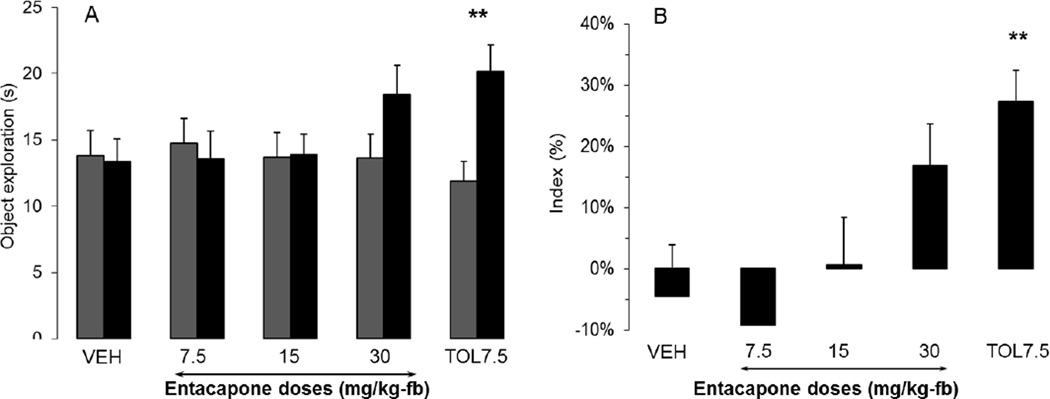
Entacapone did not showed efficacy in NOR delay-dependent forgetting in rats
The efficacy of entacapone was assessed at doses ranging from 7.5 to 30 mg/kg (Fig. 2). The exploration of the novel object during the retention trial was similar to the exploration of the familiar object at all doses of entacapone. There was a significant interaction effect [F(4,58)=4.67, p<0.005], driven by the significant effects of the tolcapone positive control group (p<0.005). The recognition indices of mice treated with entacapone (7.5, 15, and 30 mg/kg) were not statistically different those of vehicle-treated rats (p = 0.65, 0.65, and 0.054 for 7.5, 15, 30 mg/kg, respectively). It is to be noted that the highest (30mg/kg) entacapone dose showed a trend toward improvement, but it did not reach statistical significance. 7.5 mg/kg tolcapone dose was included as positive control in this experiment. Tolcapone significantly increased the recognition index (p<0.01). Total exploration time was unaffected by treatment [F(5,63)=0.52, NS].
FIGURE 2.
Entacapone did not ameliorate 24h delay-dependent forgetting of a familiar object when administered i.p. 40 min before acquisition in Sprague Dawley rats. (A) Exploration of familiar (first grey column) and novel (second black column) objects during the retention trial (B) Recognition index during retention. (** = p < 0.01). All data presented as mean + SEM; n=12–15 per group.
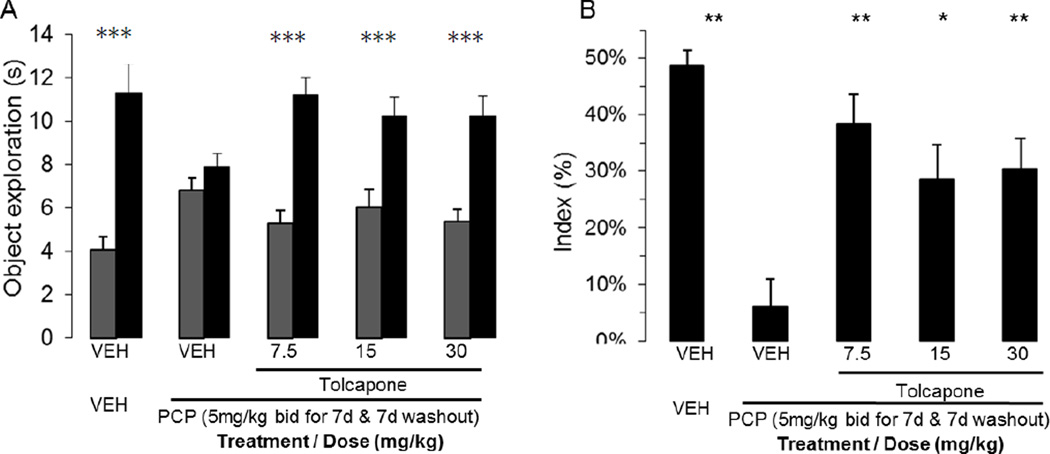
Tolcapone ameliorated subchronic phencyclidine-induced NOR deficit in rats
The efficacy of tolcapone was assessed at active doses in delay-dependent forgetting in an object recognition task following a subchronic phencyclidine challenge, using a short (30 min) delay between acquisition and retention. Rats received subchronic PCP (5mg/kg, b.i.d. for 7 days) or vehicle, followed by a 7-day washout period before the novel object recognition task. Tolcapone or vehicle were administered 40 min before acquisition. In this condition, vehicle control rats receiving vehicle for 7 days and vehicle before acquisition were able to discriminate novel from familiar objects during the retention trial. They spent more time exploring the novel than the familiar object (p<0.001). In contrast, subchronic PCP-treated rats receiving vehicle before acquisition spent an equal amount of time exploring both objects during the retention trial (p>0.05) (FIG. 3A). Tolcapone treatment before the acquisition trial increased the time spent exploring the novel object [interaction effect: F(4,85)=8.33, p<0.001]. This increase translated into a statistically significant increase in recognition index (p<0.001) at all the tested doses. The index increased from 6.0% ± 4.8 to 38.1% ± 5.3 at 7.5mg/kg dose (Fig 3B). None of the treatments significantly impacted overall exploration [F(4,89)=0.25, NS] (data not shown).
FIGURE 3.
Tolcapone alleviated the subchronic PCP-induced recognition memory deficit in Long Evan rats. (A) Exploration of novel (first grey column) and familiar (second black column) objects during the retention trial (B) Recognition index during retention. (* = p < 0.05, ** = p < 0.01). All data presented as mean + SEM (* = p<0.05, ** = p<0.01); n=17–19 per group.
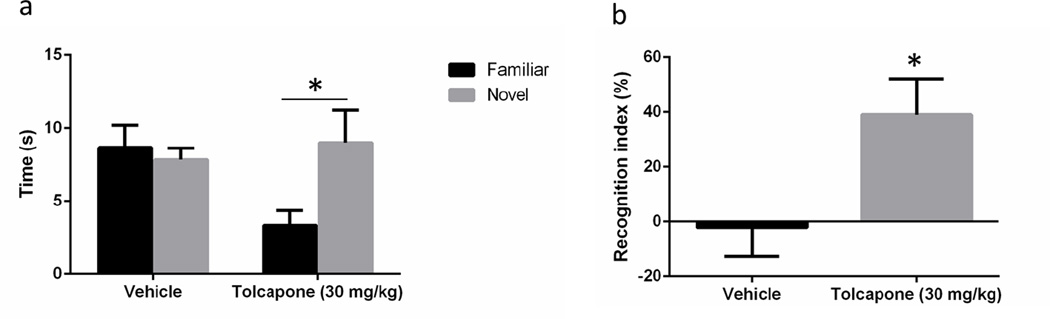
Tolcapone reversed NOR deficits observed in COMT-Val transgenic mice
In order to characterize the effects of COMT inhibition in a model characterized by high baseline COMT activity, we tested tolcapone in an NOR task in COMT-Val transgenic mice (Fig. 4). We found that acute administration of a 30 mg/kg dose immediately following the acquisition phase (see Papaleo et al. 2008) produced a significant difference between novel and familiar object exploration [interaction effect: F(1,10) = 5.51, p < 0.05]. The within-group differences in novel and familiar object exploration translated into a significant effect of tolcapone on the recognition index [t(10) = 2.46, p < 0.05]. There was no significant difference between vehicle and tolcapone-treated mice in the total exploration time during the retention trial [t(10) = 1.28, NS; data not shown].
Figure 4.
COMT inhibition reverses novel object recognition deficit in COMT-Val transgenic mice. (a) Tolcapone administration produced a significant difference in exploration time between the novel and familiar objects. (b) Tolcapone (30 mg/kg) significantly improved the novel object recognition index compared to vehicle administration in COMT-Val transgenic mice. All data presented as mean ± SEM. n = 6/group; * p < 0.05.
Discussion
The present results show for the first time that central inhibition of COMT by tolcapone, at different doses, is able to reverse recognition memory deficits in normal rats submitted to a 24-hour delay as well as in subchronic PCP-treated rats tested at a short delay, i.e. 30 min. In addition, tolcapone at a dose of 30 mg/kg was also shown to reverse recognition deficits observed in COMT-Val transgenic mice, a transgenic line expressing the COMT-Val form of human COMT, which results, functionally, in a gain of function of the COMT enzyme (Papaleo et al., 2008). The necessity for a central effect of tolcapone was demonstrated by showing that the poorly penetrant COMT inhibitor, entacapone, was not effective in NOR with a 24-hour delay up to a dose of 30 mg/kg, the maximal dose tested. Related to this, Napolitano et al. (2003) showed that both entacapone and tolcapone produce, at similar doses, a comparable degree of peripheral COMT inhibition (liver and duodenum). Therefore, the lack of activity for entacapone cannot be explained by a smaller inhibitory effect at the COMT enzyme but rather by its poor brain penetration. This lack of effect of entacapone is in line with published data by Liljequist et al.(1997) showing that tolcapone (10 mg/kg) but not entacapone (10 and 30 mg/kg) was effective in facilitating spatial working memory in normal rats.
It is worth noting here that the minimal dose found to be statistically effective in the NOR-24h delay in normal rats, i.e. 7.5 mg/kg, was lower than the dose which is supposed to fully inhibit COMT enzyme, i.e. 30 mg/kg, as reported in the literature (Aquas et al., 1992, Tunbridge et al., 2004). However, this dose was shown by Acquas et al. (1992) to increase HVA and decrease DOPAC output in the caudate of freely moving rats. Using in vivo microdialysis, we have been able to confirm these results and showed that doses of 3.7 to 30 mg/kg (i.p.) were effective in altering HVA and DOPAC extracellular levels in the prefrontal cortex of freely moving Sprague Dawley rats (data not shown). Therefore a full inhibition of the COMT enzyme is not a necessary prerequisite for obtaining efficacy in these tasks. Indeed, the lowest statistically significant effective dose in the NOR-24h delay in normal rats was found to be fully effective in sub chronic PCP-treated rats. These results support the notion that central COMT inhibition might be of interest for cognitive deficits related to schizophrenia. Recent published findings obtained in our laboratory further indicate that tolcapone might ameliorate executive dysfunction, modeled by a set shifting procedure performed in subchronic PCP-treated rats (Troudet et al., 2014).
In the COMT-Val transgenic mice, a single dose of 30 mg/kg of tolcapone was used and found to be very effective in a NOR protocol similar to that used by Papaleo et al. (2008) to show the ability of amphetamine to partially reverse the NOR deficits observed in this line, i.e. by administering the compound after the acquisition trial. Further studies are planned to evaluate a dose-response of tolcapone in this procedure as well as in a procedure where tolcapone is administered before acquisition, in order to dissect the effect of tolcapone on acquisition versus effects on consolidation and retrieval. It should also be of interest to study the effect of tolcapone on consolidation and retrieval processes in the NOR in rats pre-treated with PCP or submitted to a delay-dependent forgetting procedure.
COMT inhibition has been proposed as a strategy to improve prefrontal cortex dependent cognitive dysfunction (Gasparini et al., 1997; Apud et al., 2007, Scheggia et al., 2012). However, recent preclinical studies by Laatikainen et al; (2012, 2013) pointed out that COMT inhibition might also ameliorate hippocampal-dependent cognitive processes. COMT is thought to be an important mechanism for the termination of dopamine signaling in the cortex, owing to the paucity of dopamine transporters in this brain region, and cortical dopaminergic activity is thought to underlie many aspects of cognition (Lewis et al., 2001; Clark et al. 2014). There is a large body of preclinical research indicating that COMT inhibition improves cognitive function across multiple domains (Khromova et al., 1997; Tunbridge et al., 2004; Lapish et al., 2009; Laatikainen et al., 2012; Risbrough et al., 2014), therefore it seems plausible that central COMT inhibition may impact on different brain areas beyond the cortex or hippocampus. Additionally, data from small clinical studies suggest that tolcapone improves cognitive function in healthy individuals and people with Parkinson’s Disease (Gasparini et al., 1997; Apud et al., 2007). Although tolcapone is an effective, brain-penetrant COMT inhibitor, concerns about long-term safety have limited its widespread use in the clinic (Kaakkola., 2010). The significant contribution of the mesocortical dopaminergic system to cognition and the experimental evidence that tolcapone can improve cognitive function in a variety of tasks indicate the usefulness of an approach targeting the design of safe, non-catechol, brain-penetrant COMT inhibitors for the symptomatic treatment of cognitive disorders in aging and in neuropsychiatric conditions such as schizophrenia or Parkinson’s disease.
Acknowledgements
Research funds were provided by the Lieber Institute for Brain Development, the Intramural Research Program of NIMH, and UCB Biopharma
REFERENCES
- Apud JA, Weinberger DR. Treatment of cognitive deficits associated with schizophrenia: potential role of catechol-O-methyltransferase inhibitors. CNS Drugs. 2007;21(7):535–557. doi: 10.2165/00023210-200721070-00002. [DOI] [PubMed] [Google Scholar]
- Apud JA, Mattay V, Chen J, Kolachana BS, Callicott JH, Rasetti R, et al. Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology. 2007;32(5):1011–1020. doi: 10.1038/sj.npp.1301227. [DOI] [PubMed] [Google Scholar]
- Aquas E, Carboni E, de Ree RH, Da Prada M, Di Chiara G. Extracellular concentrations of dopamine and metabolites in the rat caudate after oral administration of a novel catechol-o-methyltransferase inhibitor Ro-40-7592. J Neurochem. 1992;59(1):326–330. doi: 10.1111/j.1471-4159.1992.tb08907.x. [DOI] [PubMed] [Google Scholar]
- Brisch R, Saniotis A, Wolf R, Bielau H, Bernstein HG, Steiner J, et al. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Front Psychiatry. 2014;5:47. doi: 10.3389/fpsyt.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler DJ, Waterhouse BD, Gao WJ. New perspectives on catecholaminergic regulation of executive circuits: evidence for independent modulation of prefrontal functions by midbrain dopaminergic and noradrenergic neurons. Front Neural Circuits. 2014;8:53. doi: 10.3389/fncir.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KL, Noudoost B. The role of prefrontal catecholamines in attention and working memory. Front Neural Circuits. 2014;8:33. doi: 10.3389/fncir.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin K, Laurent B. Dysfunction of the human memory systems: role of the dopaminergic transmission. Curr Opin Neurol. 2003;(Suppl 2):S11–S16. doi: 10.1097/00019052-200312002-00003. [DOI] [PubMed] [Google Scholar]
- Farrell SM, Tunbridge EM, Braeutigam S, Harrison PJ. COMT Val(158)Met genotype determines the direction of cognitive effects produced by catechol-O-methyltransferase inhibition. Biol Psychiatry. 2012;71(6):538–544. doi: 10.1016/j.biopsych.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31(2):297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Gasparini M, Fabrizio E, Bonifati V, Meco G. Cognitive improvement during Tolcapone treatment in Parkinson's disease. J Neural Transm. 1997;104(8–9):887–894. doi: 10.1007/BF01285556. [DOI] [PubMed] [Google Scholar]
- Horiguchi M, Hannaway KE, Adelekun AE, Huang M, Jayathilake K, Meltzer HY. D(1) receptor agonists reverse the subchronic phencyclidine (PCP)-induced novel object recognition (NOR) deficit in female rats. Behav Brain Res. 2013;238:36–43. doi: 10.1016/j.bbr.2012.09.030. [DOI] [PubMed] [Google Scholar]
- Kaakkola S. Problems with the present inhibitors and a relevance of new and improved COMT inhibitors in Parkinson's disease. Int Rev Neurobiol. 2010;95:207–225. doi: 10.1016/B978-0-12-381326-8.00009-0. [DOI] [PubMed] [Google Scholar]
- Khromova I, Voronina T, Kraineva VA, Zolotov N, Männistö PT. Effects of selective catechol-O-methyltransferase inhibitors on single-trial passive avoidance retention in male rats. Behav Brain Res. 1997;86(1):49–57. doi: 10.1016/s0166-4328(96)02242-5. [DOI] [PubMed] [Google Scholar]
- Laatikainen LM, Sharp T, Bannerman DM, Harrison PJ, Tunbridge EM. Modulation of hippocampal dopamine metabolism and hippocampal-dependent cognitive function by catechol-O-methyltransferase inhibition. J Psychopharmacol. 2012;26(12):1561–1568. doi: 10.1177/0269881112454228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laatikainen LM, Sharp T, Harrison PJ, Tunbridge EM. Sexually dimorphic effects of catechol-(o)-methyltransferase (COMT) inhibition on dopamine metabolism in multiple brain regions. Plos One. 2013;8(4):e61839. doi: 10.1371/journal.pone.0061839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapish CC, Ahn S, Evangelista LM, So K, Seamans JK, Phillips AG. Tolcapone enhances food-evoked dopamine efflux and executive memory processes mediated by the rat prefrontal cortex. Psychopharmacology (Berl) 2009;202(1–3):521–530. doi: 10.1007/s00213-008-1342-1. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A. Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J Comp Neurol. 2001;432(1):119–136. doi: 10.1002/cne.1092. [DOI] [PubMed] [Google Scholar]
- Liljequist R, Haapalinna A, Ahlander M, Li YH, Männistö PT. Catechol O-methyltransferase inhibitor tolcapone has minor influence on performance in experimental memory models in rats. Behav Brain Res. 1997;82(2):195–202. doi: 10.1016/s0166-4328(97)80989-8. [DOI] [PubMed] [Google Scholar]
- Lyon L, Saksida LM, Bussey TJ. Spontaneous object recognition and its relevance to schizophrenia: a review of findings from pharmacological, genetic, lesion and developmental rodent models. Psychopharmacology (Berl) 2012;220(4):647–672. doi: 10.1007/s00213-011-2536-5. [DOI] [PubMed] [Google Scholar]
- McLean SL, Idris NF, Woolley ML, Neill JC. D(1)-like receptor activation improves PCP-induced cognitive deficits in animal models: Implications for mechanisms of improved cognitive function in schizophrenia. Eur Neuropsychopharmacol. 2009;19(6):440–450. doi: 10.1016/j.euroneuro.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Agid Y, Brüne M, Bullmore ET, Carter CS, Clayton NS, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2010;11(2):141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Cochran SM, Pratt JA. PCP: from pharmacology to modelling schizophrenia. Curr Opin Pharmacol. 2005;5(1):101–106. doi: 10.1016/j.coph.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Napolitano A, Bellini G, Boroni E, Zürcher G, Bonuccelli U. Effect of peripheral and central catechol-o- methyltransferase inhibition on striatal extracellular levels of dopamine: a microdialysis study in freely moving rats. Parkinsonism And Related Disorders. 2003;9:145–150. doi: 10.1016/s1353-8020(02)00016-0. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Ventura J, Subotnik KL, Bartzokis G. The early longitudinal course of cognitive deficits in schizophrenia. J Clin Psychiatry. 2014;75(Suppl 2):25–29. doi: 10.4088/JCP.13065.su1.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, Chen J. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J Neurosci. 2008;28:8709–8723. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Ricciardi J, Wetzler C, Hanania T. Sub-optimal performance in the 5-choice serial reaction time task in rats was sensitive to methylphenidate, atomoxetine and d-amphetamine, but unaffected by the COMT inhibitor tolcapone. Neurosci Res. 2011;69(1):41–50. doi: 10.1016/j.neures.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Pratt JA, Winchester C, Egerton A, Cochran SM, Morris BJ. Modelling prefrontal cortex deficits in schizophrenia: implications for treatment. Br J Pharmacol. 2008;153(Suppl 1):S465–S470. doi: 10.1038/bjp.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redrobe JP, Bull S, Plath N. Translational Aspects of the Novel Object Recognition Task in Rats Abstinent Following Sub-Chronic Treatment with Phencyclidine (PCP): Effects of Modafinil and Relevance to Cognitive Deficits in Schizophrenia. Front Psychiatry. 2010;1:146. doi: 10.3389/fpsyt.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough V, Ji B, Hauger R, Zhou X. Generation and characterization of humanized mice carrying COMT158 Met/Val alleles. Neuropsychopharmacology. 2014;39(8):1823–1832. doi: 10.1038/npp.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheggia D, Sannino S, Scattoni ML, Papaleo F. COMT as a drug target for cognitive functions and dysfunctions. CNS Neurol Disord Drug Targets. 2012;11(3):209–221. doi: 10.2174/187152712800672481. [DOI] [PubMed] [Google Scholar]
- Troudet R, Michaux A, Hanon E, Lamberty Y, Detrait ER. Optimization and pharmacological validation of a set shifting procedure for assessing executive function in rats. Proceedings of measuring behavior. 2014 doi: 10.1016/j.jneumeth.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24(23):5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]