Abstract
Prenatal cocaine exposure (PCE) affects neurobehavioral development, however, disentangling direct drug-related mechanisms from contextual effects (e.g., socioeconomic status) has proven challenging in humans. The effects of environmental confounds are minimal immediately after birth thus we aimed to delineate neurobehavioral correlates of PCE in a large cohort of neonates (2–6 weeks of age, N = 152) with and without drug exposure using resting state functional magnetic resonance imaging (rsfMRI) and developmental assessments at 3 months with the Bayley Scales of Infant & Toddler Development, 3rd edition. The cohort included healthy controls and neonates with similar poly-drug exposure ± cocaine. We focused on the thalamus given its critical importance in early brain development and its unique positioning in the dopamine system. Our results revealed PCE-related hyper-connectivity between the thalamus and frontal regions and a drug-common hypo-connective signature between the thalamus and motor-related regions. PCE-specific neonatal thalamo-frontal connectivity was inversely related to cognitive and fine motor scores and thalamo-motor connectivity showed a positive relationship with composite (gross plus fine) motor scores. Finally, cocaine by selective-serotonin-reuptake-inhibitor (SSRI) interactions were detected, suggesting the combined use of these drugs during pregnancy could have additional consequences on fetal development. Overall, our findings provide the first delineation of PCE-related disruptions of thalamocortical functional connectivity, neurobehavioral correlations, and drug-drug interactions during infancy.
Keywords: Thalamus, Connectivity, fMRI, Prenatal Cocaine Exposure, Neonate, Behavior
1. Introduction
Prenatal cocaine exposure (PCE) is a serious public health concern commonly associated with a number of adverse behavioral consequences throughout development (Lambert and Bauer, 2012; Ross et al., 2015). The pathophysiology of the effects of PCE are thought to operate over three different axes (Lester and Padbury, 2009); 1) neurochemical, i.e. monoaminergic interactions [dopamine (DA), norepinephrine (NE), and serotonin (5-HT)], 2) vasoconstrictive mechanisms, and 3) fetal programming. Disentangling these effects from postnatal environmental adversities, which are often co-morbid with PCE, has proven challenging in studies of later childhood and adolescence (Eiden et al., 2014; Yumoto et al., 2008). However, recent neuroimaging efforts in neonates have shown great promise, effectively allowing for more direct delineation of drug-related effects by minimizing exposures to postnatal risk factors (Grewen et al., 2014; Grewen et al., 2015; Salzwedel et al., 2015). These studies have opened a new window and thus enabled more specific investigations of PCE-related brain-behavior mechanisms starting from birth.
PCE is typified by abnormal arousal and attention regulation/reactivity to stressful conditions, as well as deficits to sustained focus, salience processing, and working memory (Mayes, 2002; Mayes et al., 1998). Imbalanced gating between different processing streams, particularly between limbic and executive regions, has been put forth as a potential mechanism (Harvey, 2004; Mayes, 2002). Indeed, neuroimaging efforts have lent credence to this theory (Grewen et al., 2014; Li et al., 2009; Li et al., 2016; Li et al., 2013; Liu et al., 2013; Rando et al., 2013; Rao et al., 2007; Roussotte et al., 2010; Salzwedel et al., 2015; Sheinkopf et al., 2009) and shown PCE-related structural and functional alterations associated with the amygdalo-frontal pathway. However the thalamus, another region that is reportedly critical for this set of PCE-affected functions, has received relatively little attention. Numerous reports support a role for the thalamus and thalamocortical connectivity in 1) arousal regulation (Paus et al., 1997) that is closely related to the performance of sustained attention (Paus et al., 1997; Sarter et al., 2001), 2) saliency detection and processing (Ding et al., 2010), especially in terms of salient error monitoring (Harsay et al., 2012; Li et al., 2008; Zhang et al., 2014), and 3) working memory through the contribution from the mediodorsal nucleus (Watanabe and Funahashi, 2012). Therefore, abnormal thalamocortical connectivity could also contribute to the reported PCE-related functional deficits and deserves further attention as a novel target for better understanding of the neural correlates of PCE. Notably, explorations of thalamocortical connectivity in infants have successfully demonstrated that both structural and functional connectivity between thalamus and limbic/frontal regions predict later cognitive abilities (Alcauter et al., 2014a; Ball et al., 2015), supporting the behavioral significance of thalamocortical connectivity during infancy. More importantly, the human thalamus, unlike other species, is richly innervated with DA-afferents (Garcia-Cabezas et al., 2007; Sanchez-Gonzalez et al., 2005) making it potentially highly susceptible to PCE-induced disruptions during in utero development (Crandall et al., 2007; Frankfurt et al., 2011; Ohtani et al., 2003; Song et al., 2002). Therefore, a systematic study of the thalamus and its functional connections may provide new insights into PCE effects and behavioral associations in infants. Lastly, poly-drug use is relatively common in women who abuse drugs during pregnancy therefore other drugs may also affect the hypothesized thalamocortical connectivity, and thus exploration of potential drug-drug interactions is also of high importance and interest (Meyer and Quenzer, 2005; Ross et al., 2015).
In this study, we systematically examined thalamocortical connectivity, its behavioral correlations, and drug-drug interactions to gain novel insights into the effects of PCE. Specifically, resting state functional magnetic resonance imaging (rsfMRI) was employed to evaluate functional connectivity in naturally sleeping neonates (2–6 weeks old at time of scan). The study cohort included neonates with in utero exposure to cocaine plus some combination of other non-cocaine drugs (PCE), exposure to similar a combination of non-cocaine drugs (NCOC), and drug-free controls (CTR). The inclusion of the NCOC drug-exposed subgroup allowed for more rigorous control of the effects due to other drugs as well as common drug-related environmental conditions. In addition, the NCOC group enabled a more thorough exploration of potential drug-drug interaction effects. We hypothesized that thalamic connectivity would show significant PCE related disruptions, particularly with frontal areas. Moreover, these neural correlates would predict later behavioral outcomes. Finally, we also expected significant interaction effects between different drug types.
2. Subjects and Methods
2.1 Subjects
The study cohort (N =152) consisted of three groups: 45 cocaine exposed infants with or without in utero exposure to marijuana, alcohol, nicotine, SSRIs, and opiates including heroin, oxycodone, oxycontin, methadone and the mixed agonist/antagonist, suboxone (PCE); 43 infants with in utero exposure to similar combination of the aforementioned drugs minus cocaine (NCOC); and 64 drug free controls (CTR). Pregnant women were recruited in the third trimester of pregnancy. Primary recruitment sites for PCE and NCOC participants were local residential and outpatient treatment programs for women with perinatal substance abuse and their children. In addition, we recruited CTR and drug-exposed mothers from Chatham, Orange, Durham, Alamance, and Wake County Health Department obstetric clinics, the University of North Carolina hospital low-income obstetrics clinic, and flyers, local advertisements, and Craigslist. At enrollment, mothers were required to be between 18 - 44 years of age and free from; 1) chronic medical or psychiatric disease, 2) untreated current clinical depression or anxiety disorder, and 3) language barrier that might prevent informed consent. A number of subjects with opiate abuse were treated with methadone or suboxone maintenance treatment for part of their pregnancies. The infants took part in the imaging experiment during the neonatal period (2–6 weeks of age) and participated in behavioral assays at approximately 3 months of age. All infants were required to be living with biological mother at time of testing. Infants were excluded for multiple reasons, including; gestational birth weight < 2500 g, delivered at < 32 weeks or > 42 weeks gestation, history of mechanical ventilation or surgery of any kind, > 24 hours in NICU, or chronic illness of any kind. Mother-infant dyads were characterized and compared on the following criteria: gestational age at birth, gestational age at scan, birth weight, pre/postnatal drug exposure, infant cognitive and motor functions, maternal education and depression at the time of scan. All participants were tested for prenatal drug use using interviews, medical record review, and postnatal urine toxicology at study visits. Prenatal drug exposure status was based on three criteria: (1) maternal self-report with Time Line Follow Back interview (Robinson et al., 2014) conducted in 3rd trimester and again at neonatal MRI visit; (2) response to a questionnaire about maternal substance use done at 3 months; and (3) medical record queries of prenatal urine toxicology. Maternal self-report or positive urine toxicology for cocaine qualified the mother–infant dyad for PCE status. Postnatal drug exposure was characterized using maternal self-reports of drug use and infant feeding method. Maternal education was determined by rank scores (Tbl 1). Maternal depression was indexed by score on the Edinburgh Postnatal Depression Scale at time of scan (Murray and Carothers, 1990). Infant behavior was assessed using the Bayley III Scales of Infant and Toddler Development (Bayley, 2006). Please see the supporting material for a detailed description of the Bayley III and the assessment procedures for the following three domains: Cognitive development; language development which includes both receptive and expressive communication as separate but combinable subtests; and motor development which also includes both fine and gross motor development as separate but combinable subtests. Research staff that conducted the Bayley assessments was blinded to specific drug-exposure status. Group means for each characteristic were compared using the analyses of variance (ANOVA) and group proportions were tested using the chi-square statistic. This study was approved by the University of North Carolina at Chapel Hill’s Biomedical Institutional Review Board.
2.2 Image Acquisition and Image Preprocessing
Data were collected for all neonates at 2–6 weeks of postnatal age, adjusted for prematurity, using two scanners: 1) 3T Siemens Allegra (n = 89; 14 for PCE, 34 for NCOC, and 41 for CTR) with circular polarization head coil and 2) 3T Siemens Tim Trio (n = 63; 31 for PCE, 9 for NCOC, and 23 for CTR) with 32-channel head coil. Time of day for scan was determined by infant nap schedule with the majority done between 10 am and 2 pm but was not systematically controlled. T1-weighted structural images were collected using a 3D magnetization prepared rapid gradient echo pulse sequence (repetition time (TR) = 1820 ms, echo time (TE) = 3.75 ms, inversion time (TI) = 1100 ms, flip angle = 7°, 144 slices, voxel size = 1 mm3). rsfMRI images were acquired using a T2*-weighted echo planar imaging (EPI) pulse sequence (TR = 2s, TE = 32 ms, 33 slices, voxel size = 4 mm3, number of volumes = 150). Data were preprocessed using the FMRIB’s Software Libraries (Jenkinson et al.) and AFNI (Cox, 1996). Functional preprocessing included discarding the first 10 volumes, slice-timing correction, motion correction, spatial smoothing (Gaussian kernel FWHM = 6 mm), band-pass filtering (0.01 – 0.08 Hz), data scrubbing, and regression of whole brain, white matter, cerebrospinal fluid signals and the six motion parameters. Given the higher likelihood of fine motion from naturally sleeping infants, data scrubbing was performed as an added motion correction step in addition to the standard rigid-body motion correction procedures. Specifically, volumes with global signal changes > 0.5% and/or frame-wise displacements (FD) > 0.5 mm (Power et al., 2012) were excluded (plus one before and two after). Post-scrubbing, subjects with less than 90 volumes were excluded from the study. Finally, the amount of volumes scrubbed (VS) and residual frame-wise FD (rFD) were compared across sub-groups to ensure there were no systematic differences in motion. Indeed, the parameters were indistinguishable between groups; VS (μ ± SE): PCE 7.44 ± 1.80, NCOC 8.02 ± 2.04, CTR 4.83 ± 1.21, p = 0.304; rFD: PCE 0.14 ± 0.02, NCOC, 0.13 ± 0.02, CTR 0.13 ± 0.01, p = 0.782. Severe motion during the anatomical acquisition was also grounds for exclusion. Our previous studies have demonstrated the effectiveness of this set of motion-correction practices in infant functional connectivity studies (Gao et al., 2014; Gao et al., 2014). Alignment of functional data into a common space involved two steps: (1) within-subject rigid alignment [FSL FLIRT (for FMRIB Linear Image Restoration Tool)] between functional and T1-weighted images; and (2) nonlinear [FSL FNIRT (for FMRIB Nonlinear Image Registration Tool)] registration of the T1-weighted images to a T1-weighted template image acquired from an independent subject scanned at 2 weeks of age (Gao et al., 2014). The combined transformation field (linear plus nonlinear) was used to warp the preprocessed rsfMRI data to the template space. Alignment was inspected visually for quality across all subjects.
2.3 Functional Connectivity Analyses
Thalamus functional connectivity was assessed using the seed-based temporal correlation method (Biswal et al., 1995). Seeds were defined using a previous functional parcellation obtained in healthy non-exposed infants (Alcauter et al., 2014a). Briefly, through a combined partial correlation and winner-takes-all approach Alcauter et al. detected three thalamic clusters demonstrating robust cortical functional connectivity; an anterior ventral-medial cluster that preferentially connected to frontal regions and two large hemisymmetrical posterior thalamus clusters with preferential connectivity to sensorimotor regions. For this study, we used the center-of-mass for each of the three previously defined clusters to create non-overlapping anterior (MNI: 0, −4, 0) and posterior (bilateral, MNI: −16, −24, 10 & 10, −18, 10) thalamus seeds consisting of the center voxels plus the face-connected neighboring voxels. The rationale for using the anterior and posterior seed regions rather than the entire thalamus was to 1) focus only on robust thalamocortical functional connectivity identified in typically developing neonates and 2) allow for more specific investigations of thalamo-frontal and thalamo-motor connectivity within and between groups. The use of medial anterior and lateral posterior centered seeds to dissociate thalamo-frontal from thalamo-motor connectivity is further supported by structural and functional studies in adults (O'Muircheartaigh et al., 2015; Zhang et al., 2010) and functional studies in infants (Toulmin et al., 2015), which clearly demonstrate unique neural connectivity architecture associated with the targeted sub-regions. The average time series of each seed were extracted and used to perform whole brain correlation analyses. The correlation measures were Fisher-z transformed and compared within and between groups (Chen et al., 2014). Within groups, one-sample t-tests were applied to generate group-specific functional connectivity maps. Paired t-tests were performed between the anterior and posterior seeds to highlight the segregation of the two networks. Between groups, multivariate analysis of covariance (ANCOVA) modeling was used to detect voxel-wise differences in thalamic functional connectivity while controlling for other explanatory variables (mean-centered continuous variables): gender, scanner, gestational age, birth weight, and postnatal age at scan (adjusted for prematurity). Significance was determined using a combined approach (Forman et al., 1995), which imposes a minimum p-value (i.e., p < 0.01) and cluster size (32 voxels) threshold to correct for multiple comparisons (α < 0.05) at the whole brain level.
Following cluster detection, post-hoc ANCOVA analyses were performed to confirm significance and further explore pair-wise group differences (p<0.05 after corrections for multiple comparisons using the Dunn-Sidak method) after similarly controlling for gender, scanner, gestational age, birth weight, and postnatal age at scan. To explicitly test the amount of variance explained by maternal education and depression (i.e. reflective of socioeconomic status and caregiver well-being, respectively) a separate ANOVA model was constructed using the subsample with both scores (n = 118). Here, all explanatory variables were recapitulated but none of the continuous variables were mean-centered to control for both the within- and between-group variances. Finally, we conducted a follow-up analysis to address the issue that PCE mothers are reported to engage in more prenatal drug-use compared to NCOC mothers, i.e. PCE-infants have overall more severe degree of drug-exposure. The full model, including caregiver traits, was repeated, with number of different drugs used during pregnancy included as an additional covariate.
2.4 Drug Specificity and Interactions
For each detected cluster showing group differences, additional post-hoc ANCOVAs were performed to test the specificity of cocaine effects within the drug-exposed sample. Specifically, we constructed two separate models with categorical drug-exposure status and quantitative drug use (average frequency / trimester) from each drug type as the main effects, respectively. This allowed us to test the categorical and quantitative effects of all drugs on the detected functional connectivity alterations for each cluster. Interaction terms with cocaine were also included in these models.
2.5 Brain-Behavior Analyses
Brain-behavior relationships were tested using the cluster-level functional connectivity measures and normative, age-standardized, scaled composite and subscale scores on the Bayley III Scales of Infant and Toddler Development. Models were fit using linear regression:
where Z is functional connectivity (Fisher’s Z transformation of the temporal correlation), β0 is the intercept, β1 is the slope (or regression coefficient), x is the regressor of interest, i.e. the behavioral measure, and is the error term. Findings associated with p ≤ 0.05 (uncorrected) were reported and further validated using the bootstrapping technique. The relationships after controlling for confounding variables were also explored, i.e. using the residuals from post-hoc ANCOVA analyses. Specifically, functional connectivity (Z) was replaced with the residual connectivity after for controlling for gestational age, birth weight, scan age, gender, scanner, maternal education, and maternal depression levels in the subsample with complete data (N=118).
2.6 Other Methodological Considerations
The use of nuisance regression, specifically global signal regression (GSR), in the pre-processing pipeline warrants additional consideration and caution regarding the interpretation of negative correlations (Chang and Glover, 2009; Gotts et al., 2013; Murphy et al., 2009; Saad et al., 2012; Yan et al., 2013). First, to avoid confusion, we describe the results as hyper- and hypo-connectivity to describe positive or negative shifts in connectivity relative to the control (CTR) group, respectively. However, we also use the term “disrupted connectivity” to describe both types of deviations from normal. Next, in order to shed light on the potential effects of nuisance regression in this study and to gain a better understanding of relative group differences, the cluster-level post-hoc analyses were repeated using data with head-motion regression + post hoc standardization (i.e., mean subtraction; see Yan et al., 2013), but no GSR. Finally, all reported significances survive multiple comparisons correction unless explicitly labeled as marginal.
3. Results
3.1 Participant Characteristics
Summary statistics for the participant characteristics are tabulated in Table 1. Gender distributions were similar across groups. Infants with PCE were born, on average, approximately 7 days earlier and 14 ounces lighter compared to NCOC and CTR. Mothers from both the PCE and NCOC groups had lower education and higher depression levels compared with CTR but did not differ from each other. Behaviorally, PCE infants had lower cognitive and gross motor scores compared to NCOC and CTR. Non-cocaine drug use, described categorically for each drug (Yes, No) was similar between PCE and NCOC but SSRI and opiate use was marginally more prevalent in the PCE group (p = 0.05–0.06). The frequency of non-cocaine drug use was also similar between PCE and NCOC groups, however nicotine use was significantly higher in the PCE group (Tbl S1). Overall, the degree of poly-drug use was significantly higher in the PCE group: t-test, PCE vs NCOC number of drugs: t(1,86) = 6.72, p < 0.001; PCE μ = 3.33 SE = 0.16, NCOC μ = 1.98 SE = 0.12. Effective postnatal drug exposure (i.e. postnatal maternal drug use plus breast-feeding) was minimal to moderate but similar for both drug-exposed groups (Tbl S2).
Table 1.
Summary of Subject Characteristics
| Infant Characteristics | PCE (1) | NCOC (2) | CTR (3) | F or X2 | p | ηp2 | 1 vs 2 | 1 vs 3 | 2 vs 3 | |
|---|---|---|---|---|---|---|---|---|---|---|
| N Total (N Females)a | 45 (24) | 43 (24) | 64 (27) | 1.67 | 0.433 | |||||
|
Gestational Age at birth (days) |
272 ± 1.65 | 280 ± 1.20 | 278 ± 1.03 | 8.10 | < 0.001 | 0.10 | 0.001 | 0.003 | 0.871 | |
|
Gestational Age at scan (days) |
308 ± 2.92 | 305 ± 1.53 | 306 ± 1.41 | 0.80 | 0.453 | 0.01 | ||||
| Birth Weight (lbs) | 6.70 ± 0.15 | 7.51 ± 0.17 | 7.59 ± 0.12 | 11.03 | < 0.001 | 0.13 | 0.001 | <0.001 | 0.971 | |
| Bayley III Scales of Infant Development (Scaled Scores)b | ||||||||||
| Cognitive | NT(NF)a | 29 (14) | 26 (16) | 40 (18) | 1.16 | 0.561 | ||||
| Scale | 10.59 ± 0.46 | 12.19 ± 0.48 | 12.20 ± 0.38 | 4.42 | 0.015 | 0.09 | 0.048 | 0.023 | 1.000 | |
| Language Scale: NT (NF) | 29 (14) | 26 (16) | 38 (17) | 1.17 | 0.558 | |||||
| Receptive + Expressive | 21.00 ± 0.58 | 21.62 ± 0.61 | 22.42 ± 0.48 | 1.73 | 0.184 | 0.04 | ||||
| Receptive Communication | 10.41 ± 0.35 | 10.81 ± 0.37 | 11.08 ± 0.29 | 1.02 | 0.365 | 0.02 | ||||
| Expressive Communication | 10.59 ± 0.37 | 10.81 ± 0.39 | 11.30 ± 0.31 | 1.15 | 0.323 | 0.02 | ||||
| Motor Scale: NT (NF) | 28 (14) | 24 (15) | 38 (17) | 1.23 | 0.542 | |||||
| Fine + Gross | 19.50 ± 0.91 | 22.54 ± 0.96 | 22.84 ± 0.76 | 4.35 | 0.016 | 0.09 | 0.075 | 0.020 | 0.993 | |
| Fine | 10.59 ± 0.59 | 11.40 ± 0.63 | 12.00 ± 0.50 | 1.61 | 0.206 | 0.03 | ||||
| Gross | 8.75 ± 0.47 | 11.12 ± 0.49 | 10.92 ± 0.39 | 8.89 | < 0.001 | 0.17 | 0.001 | 0.001 | 0.983 | |
| Maternal Attributes | ||||||||||
|
Education (rank scores)c |
NT(NF)a | 34 (16) | 33 (19) | 56 (23) | 1.68 | 0.431 | ||||
| 4.82 ± 0.22 | 5.18 ± 0.27 | 6.20 ± 0.22 | 9.59 | <0.001 | 0.14 | 0.719 | <0.001 | 0.010 | ||
|
Depression (scale) |
NT(NF)a | 45 (24) | 41 (23) | 61 (25) | 2.02 | 0.365 | ||||
| 5.89 ± 0.72 | 5.32 ± 0.96 | 3.32 ± 0.38 | 4.59 | 0.012 | 0.06 | 0.921 | 0.016 | 0.096 | ||
| Drug Exposure (N)d | ||||||||||
| Nicotine | 40 | 37 | 0.01 | 0.938 | ||||||
| Alcohol | 14 | 17 | 0.37 | 0.546 | ||||||
| Marijuana | 22 | 20 | 0.17 | 0.992 | ||||||
| SSRIe | 14 | 5 | 3.85 | 0.050 | ||||||
| Opiatesf | 15 | 6 | 3.54 | 0.060 | ||||||
Non-sample size characteristic values shown as mean ± SE
For each set of measurements (Infant Characteristics, Bayley III Scales, and Maternal Attributes) the gender distribution across groups were tested using the Chi-square test (i.e. 2×3 contingency table).
Bayley III measures represent age-normed scaled values of raw scores. The range of possible values of scaled scores for the Cognitive and Fine Motor Scales is from 1 to 19, with a mean of 10. For the fine motor and group motor scales summed together, the possible range would be from 2 to 38, with a mean at 20.
Rank scores: Some High School=3, Graduated from High School=4, Trade School or Business College=5, Some College=6, Graduated with 4-year College Degree=7, and Post-graduate work at university = 8.
Prenatal drug exposure status was based on three criteria: (1) self-report on a Timeline Followback interview (Robinson et al. 2014) conducted at neonatal MRI visit; (2) response to a questionnaire about maternal substance use done at 3 months; and (3) medical record queries of prenatal urine toxicology. Positive prenatal status was defined as > 1 drink (Alcohol) or cigarette (NICOTINE) in any trimester, and any use of illicit drugs (Cocaine, Marijuana, Opiates) during pregnancy revealed by prenatal urine toxicology or self-report.
Antidepressant, serotonin-specific reuptake inhibitor (SSRI)
Opiates, Methadone, or Suboxone
3.2 Thalamocortical Connectivity Patterns
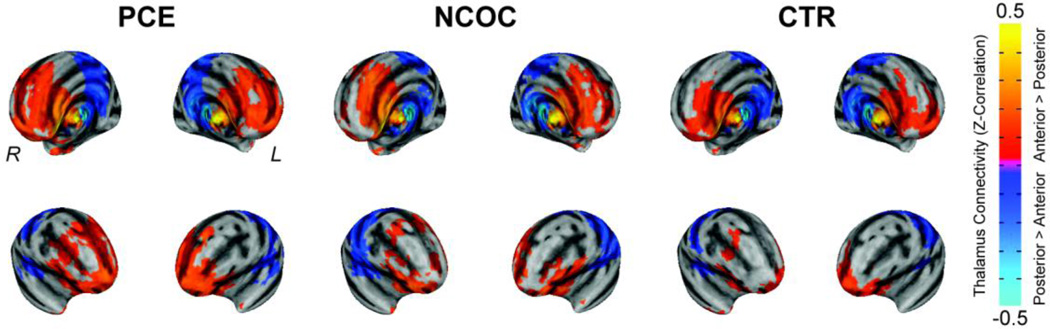
In CTR, the anterior – posterior connectivity patterns are highly consistent with our previous report (Alcauter et al., 2014b) (Fig 1); the anterior thalamus showed stronger connectivity in the frontal cortex and neighboring regions while the posterior thalamus was more correlated with the sensorimotor cortices and neighboring regions. Functional connectivity patterns in drug-exposed neonates were topologically consistent but qualitative differences were evident.
Figure 1. Visualization of thalamocortical functional connectivity segregation in neonates: cocaine + poly-drug (PCE), non-cocaine + poly-drug (NCOC), and healthy controls (CTR).
Functional connectivity is depicted on the surface model and pseudo-colored based on the Fisher Z-transformation of the temporal correlation (Z-Correlation, see color bar) with the seed regions (anterior – posterior thalamus). Thresholds were set using a combined approach (α < 0.05): voxel-wise p ≤ 0.01, minimum number of voxels = 32, third-nearest neighbor clustering (i.e. voxels cluster together if faces, or edges, or corners touch).
3.3 Drug-Related Thalamocortical Functional Connectivity Alterations
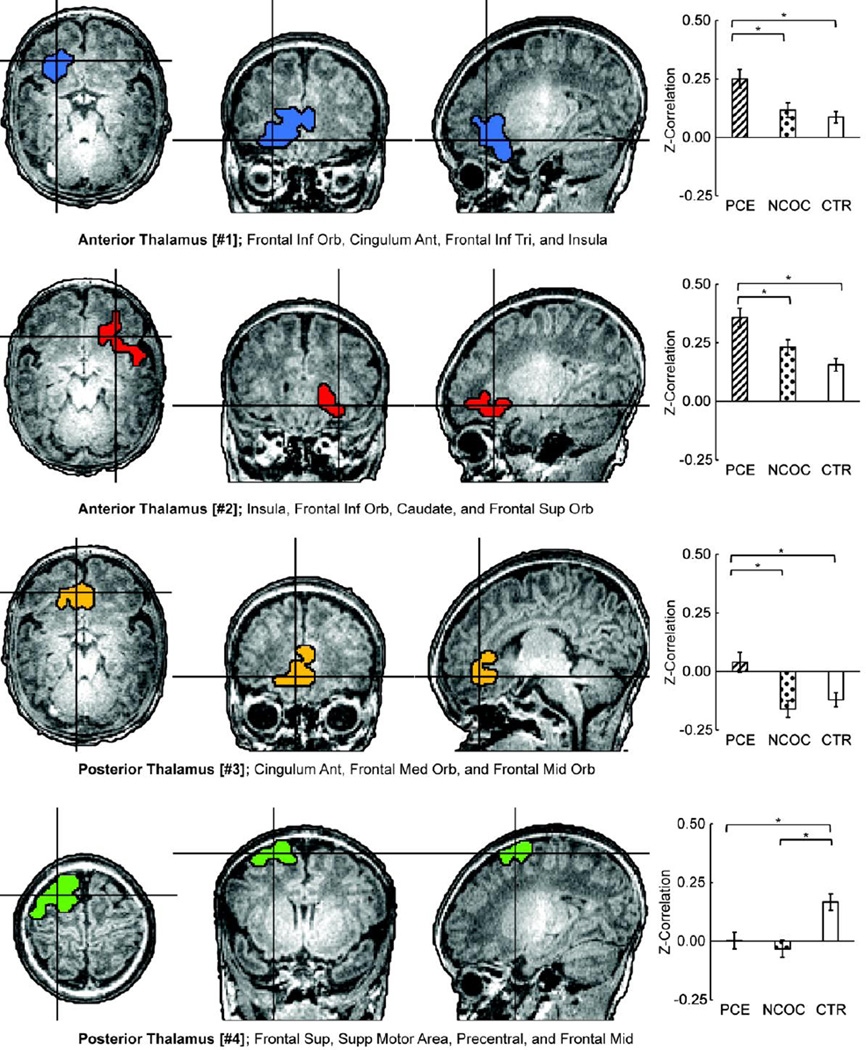
Significant functional connectivity differences across groups were detected in four clusters (Fig 2; anterior thalamus #1–2 and posterior thalamus #3–4). Significant group main-effects after correcting for multiple comparisons were verified for all four clusters (p < 0.001) based on post-hoc ANCOVA analysis after controlling for scanner, gender, gestational age, postnatal age, and birth weight (Tbl S3). Pair-wise comparisons revealed the PCE group to be statistically different (p ≤ 0.009) and hyper-connective compared to NCOC and CTR for clusters [#1–3]; PCE and NCOC neonates were hypo-connective compared with CTR for cluster [#4] (p ≤ 0.002, Tbl S3). Scanner type was significant for cluster [#1] but further examination of the group trends within the two scanners separately revealed highly consistent relative patterns across the two scanners (although with overall magnitude differences) that were similar to the overall trend in Figure 2, suggesting preserved relative group difference patterns across the two scanners (Fig S1). No significant main effects of maternal education or depression were detected, and the group main-effects and pair-wise differences remained largely consistent with those reported above after controlling for maternal education and depression (Tbl S4). Similarly, group main-effects and pair-wise differences were comparable after controlling for overall number of drugs used during pregnancy (Tbl. S5). The effects of nuisance signal regression were further tested in clusters [#3] and [#4], i.e. clusters with putative negative connectivity, using data with motion regression plus post-hoc standardization (mean subtraction, (Yan et al., 2013)). Group trends and main effects were maintained (Fig. S2), however shifts in negative connectivity towards more positive values were noted.
Figure 2. Localization of group-wise (PCE, NCOC, CTR) functional connectivity differences at the cluster-level and post-hoc comparisons by group.
(Left) Clusters depicted on the high-resolution anatomical reference image. Two clusters were detected for each seed region (anterior and posterior thalamus, four total; #1–4). Clusters were detected using the combined threshold approach controlling for gestational age, gestational weight, scan age, gender, and scanner (α < 0.05): multivariate group-wise difference (PCE, NCOC, and CTR) p ≤ 0.01, minimum number of voxels = 32, third-nearest neighbor clustering (i.e. voxels cluster together if faces, or edges, or corners touch). List of areas include AAL regions with approximately 10% or greater cluster-overlap. (Right) Comparisons of functional connectivity within cluster by subgroup. (*) indicate significant (p ≤ 0.05 dunn-sidak corrected) pair-wise differences between groups while accounting for participant characteristics mentioned above. Data plotted as mean ± s.e.m.
3.4 Drug Specificity and Interactions
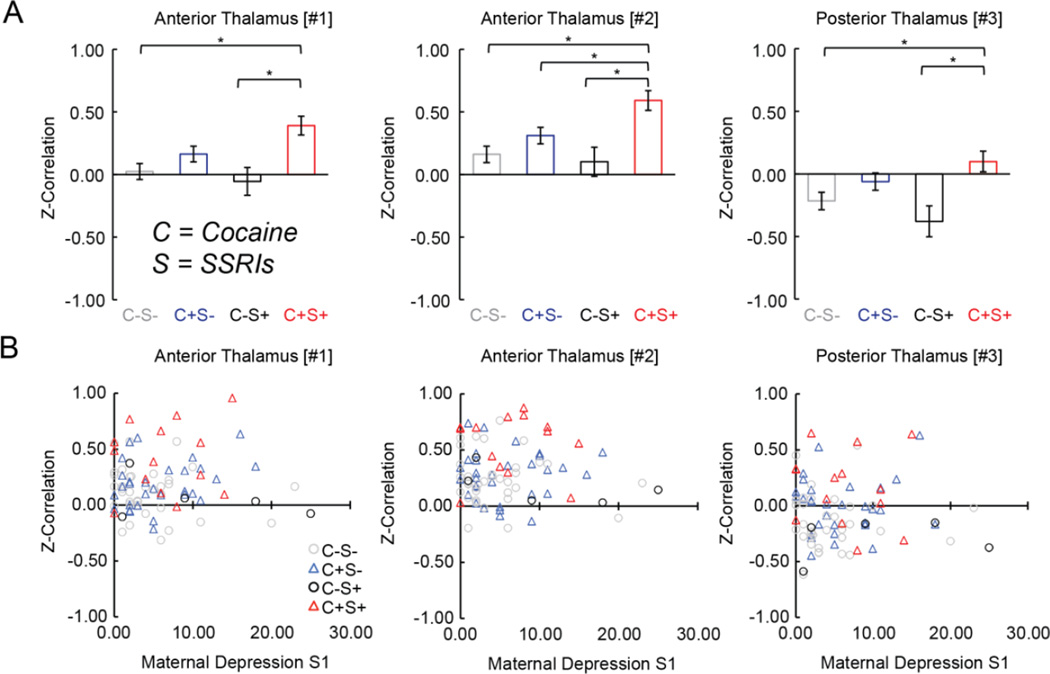
Drug specificity and interactions were tested categorically and quantitatively. Cocaine was the only significant (p ≤ 0.003) main-effect in the categorical model for the three PCE-specific clusters (Tbl S6). Furthermore, marginal categorical cocaine*SSRI interaction effects (p ≤ 0.03 uncorrected) were consistently detected for all three clusters (Tbl S6): neonates with combined cocaine and SSRI exposure (C+S+) were found to be uniquely hyper-connective amongst the drug-exposed groups (C±S± Fig 3A). Notably, the cocaine*SSRI interaction remained significant after controlling for maternal depression (N = 86; clusters #1–3, F(3,85) = 5.93–7.25, p ≤ 0.001) and the relationship between connectivity and maternal depression was insignificant for each group and cluster (Fig 3B). A parallel examination of behavioral outcomes also showed a significant reduction of the cognitive score in the C+S+ group (Fig S3). Marginal categorical effects were observed for Marijuana ([#1] and [#3]) and Opiates ([#1]), however there were no interactions with Cocaine for these and the remaining non-SSRI drugs (Tbl S6). In the continuous model (Tbl S7), there were again three marginal effects detected for Nicotine and Opiates in cluster [#1] and Nicotine in cluster [#3], with no significant interactions.
Figure 3. Functional connectivity in drug-exposed neonates grouped by cocaine and selective-serotonin-reuptake inhibitors (SSRIs) exposure and visualization as a function of maternal depression.
(A) Functional connectivity plotted by group (C = Cocaine, S= SSRIs) for each cluster showing an interaction effect (see Tbl S5). *’s indicate significant (p ≤ 0.05, dunn-sidak corrected) pair-wise differences. Data plotted as population marginal mean ± sem. (B) Functional connectivity plotted as a function of maternal depression. Linear fits (all non-significant) omitted for sake of clarity. Data points correspond to individual subjects.
3.5 Brain-behavior Relationships
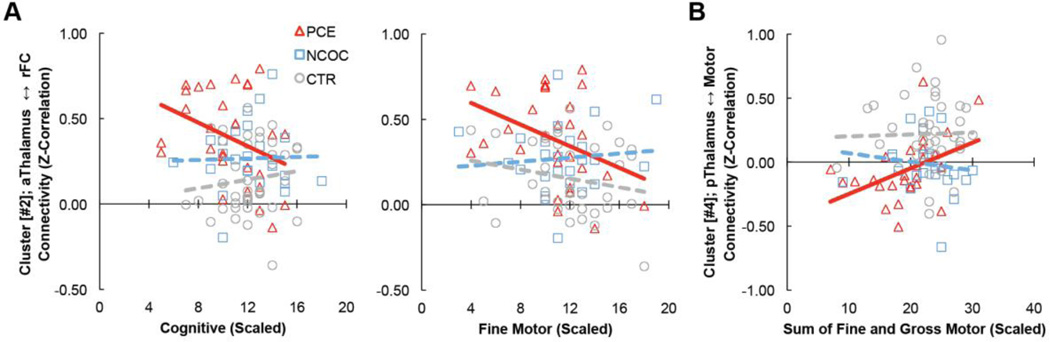
Connectivity between the anterior thalamus and right frontal cluster was marginally negatively correlated with cognitive and fine motor scores in the PCE group (‘Cognitive’: r = −0.36, p = 0.058, boot-strapping confidence interval (rci) = [−0.01 −0.63]; ‘Fine Motor’: r = −0.37, p = 0.043 rci: [−0.03 −0.63]; Fig 4A). Connectivity between the posterior thalamus and motor-related regions positively correlated with the composite (sum of fine and gross) motor-scaled score measured in the PCE group (r = 0.43, p = 0.022, rci: [0.12 0.72], Fig 4B). Similar trends were observed after controlling for gestational age, birth weight, scan age, gender, scanner, maternal education, and maternal depression levels in the subsample with complete data (N=118): anterior thalamus [#2] ‘Cognitive’, r = −0.25, p = 0.188; anterior thalamus [#2] ‘Fine Motor’, r = −0.26, p = 0.181; posterior thalamus [#4] ‘Motor’, r = +0.41, p = 0.028. All other brain-behavior relationships were statistically insignificant.
Figure 4. Brain-behavior relationships.
Cluster-level functional connectivity (Z-Correlation) versus selected infant behavioral measures assessed using the Bayley III Scales of Infant and Toddler Development (Bayley-III; Cognitive, Fine Motor and overall Motor [Fine+Gross] Scales). (A) Cluster [#2]; anterior thalamus ↔ right frontal cortex connectivity vs cognition (left) and fine motor (right). (B) Cluster [#4]; posterior thalamus ↔ motor connectivity vs sum of fine and gross motor (combined motor). Bayley-III scores represent age-normed scaled values. A scaled score of 10 represents the mean of the distribution for the Cognitive and the Fine Motor scores. A scaled score of 20 would be the mean value for fine and gross motor summed. Data points correspond to individual subjects color coded by sub-group; Δ PCE, □ NCOC, ○ CTR. Solid lines represent marginally significant (p ≤ 0.05, uncorrected) linear fit.
4. Discussion
This study sought to characterize alterations in thalamic connectivity associated with prenatal cocaine exposure using a large sample of typical and drug-exposed newborns. Our study revealed cocaine-specific disruptions in functional connectivity between the anterior thalamus and frontal regions in addition to a drug-common effect between the posterior thalamus and motor-related areas. Thalamocortical connectivity marginally correlated with later behavioral outcomes in the PCE group. Moreover, an interaction between cocaine and selective-serotonin-reuptake-inhibitors (SSRIs) was detected; suggesting the combination of these two drugs could be additionally disruptive to fetal development.
PCE exerts its effects on the developing infant brain through at least three mechanisms that include biochemical, vascular, and fetal programming components, in addition to potential contributions from other factors including caregiver status, quality of life or socioeconomic status, and drug-drug interactions (Ross et al., 2015). Biochemically, carefully controlled animal experiments have demonstrated that cocaine exposure compromises dopaminergic signaling (DA) in the developing brain and leads to permanent structural, functional, and behavioral change (Harvey, 2004; Stanwood et al., 2001). Specifically, DA modulates multiple aspects of neural development, including proliferation (Ohtani et al., 2003), migration (Crandall et al., 2007), and dendrite growth (Song et al., 2002), thus PCE is thought to alter neural circuit orchestration and ultimately result in abnormal neurophysiological signatures. In particular, mesocortical disruption is thought to mediate arousal dysregulation, a hallmark of the PCE-effect, which ultimately could lead to cognitive deficits later in life (Mayes, 2002). In earlier work, we demonstrated the earliest neuroimaging-based evidence of PCE-related morphological [i.e. reduced prefrontal cortical grey matter (Grewen et al., 2014)], and functional [i.e. hyper amygdalo-frontal connectivity (Salzwedel et al., 2015)] alterations in neonates with PCE. These findings parallel a growing body of work, which have demonstrated amygdala and/or frontal abnormalities in humans with PCE (Li et al., 2009; Li et al., 2016; Li et al., 2013; Liu et al., 2013; Rando et al., 2013; Rao et al., 2007; Roussotte et al., 2010; Sheinkopf et al., 2009), however no definitive brain-behavior relationships have been established. In this study, we have shown significant PCE-related disruptions and brain-behavioral relationships associated with the thalamus in exposed neonates, suggesting that thalamocortical pathways are also part of the PCE-effect cascade during early brain development.
Thalamocortical connectivity is central to a myriad of brain functions involved in both primary and higher order domains (Jones, 2007; Sherman, 2007). In humans, unlike other species, the thalamus receives strong DA input (Garcia-Cabezas et al., 2007; Sanchez-Gonzalez et al., 2005) thus making it potentially susceptible to dopaminergic-based insults. Indeed, thalamic abnormalities are commonly observed in brain disorders linked to DA deficits (Nair et al., 2013; Woodward et al., 2012). We detected PCE-specific hyper-connectivity between the thalamus and frontal regions, including the insula, as well as the anterior cingulate and other prefrontal areas, which collectively are critical hubs in the salience network (Seeley et al., 2007). The salience network has been reported to have a causal role in the switching between fronto-parietal executive control and default-mode networks across task paradigms and stimulus modalities, which is thought to facilitate flexible behavioral performance (Gao and Lin, 2012; Menon and Uddin, 2010). Interestingly, cortically driven action selection and attentional shift with the appearance of salient stimuli has also been reported to be closely related to the thalamus’s “gating” role (Ding et al., 2010). Specifically, when salient stimuli appear, ongoing motor behavior typically ceases and the subject orients to the stimulus. Mechanistically, salient stimuli produce intralaminar thalamic neuron activation which subsequently enhances the action suppression role of related striatocortical connections and leads to cessation of ongoing motor activity (Ding et al., 2010). Therefore, the hyper-thalamo-salience network connectivity reported here may imply aberrant thalamic interaction with the salience network which subsequently could impede proper salience processing and/or executive control in down-stream areas (Cho et al., 2013). Importantly, infants’ early learning experience depends on their attention allocation (i.e., infants will learn the most from what they attend to), which is largely driven by the “bottom-up” process of salience processing rather than “top-down” attention control at this early age (Jasso and Triesch, 2008). Therefore, the potential improper salience processing associated with the observed hyper-thalamo-salience connectivity may impede normal early learning and subsequent development of various behaviors, including cognition and motor functions. Indeed, our results showed that higher thalamo-salience network connectivity in the PCE group is marginally related to lower cognitive and fine motor scores at 3 months of age, supporting the behavioral significance of the detected thalamic functional connectivity alterations. Moreover, the posterior thalamus-motor connectivity positively predicted combined motor scores. Given our finding of PCE-specific hyper-connectivity for anterior thalamus-salience network connection and hypo-connectivity for posterior thalamus-motor connectivity, our results indicate that greater disruptions in functional connectivity could lead to worse behavioral outcomes. Note, there is also a high level of functional specificity in these brain-behavioral relationships: thalamo-frontal cluster connectivity is associated with cognitive composite scores while thalamo-motor connectivity relates to combined motor scores. Overall, these results are highly consistent with previous work in infants showing that thalamocortical functional (Alcauter et al., 2014a) and structural connectivity measures (Ball et al., 2015) predict later behavior.
A major strength of the current study is the inclusion of a dedicated non-cocaine poly-drug exposed control group, which allowed us to more rigorously explore potential contributions from other drugs and their interactions with cocaine. As expected, we detected a marginally significant but consistent interaction between SSRI and cocaine for all three PCE-specific clusters indicating that the combination of SSRIs and cocaine in pregnant women may lead to elevated neonatal functional connectivity abnormality. Examination of subsequent behaviors at 3 months also showed greater impairment in cognitive outcomes for infants with combined prenatal cocaine and SSRI exposure (Figure S3). Moreover, this interaction was significant after controlling for maternal depression levels, suggesting it may reflect a true drug-drug interaction. Mechanistically, although the neurobehavioral effects of cocaine are thought to be chiefly driven by blockade of DA reuptake, cocaine also non-selectively blocks serotonin (5-HT) and norepinephrine (NE) reuptake transporters (Koe, 1976). Therefore, pending further validation, potential interplay between cocaine-affected serotonin signaling and that from SSRIs may underlie the observed interaction. Consistent with our findings, antidepressant drugs have been shown to enhance cocaine-induced toxicity (Macedo et al., 2004; O'Dell et al., 2000) and the manipulation of the serotonin system has been shown to alter, and often enhance, the behavioral effects of cocaine in animal models (Muller et al., 2003; Walsh and Cunningham, 1997). Given the common practice of SSRI prescription for depressed pregnant women with drug dependence (Yamamoto et al., 2014), the discovered interaction may have significant clinical implications.
The effects of categorical drug use and dose were not as significant for other drugs. The amount of nicotine use was higher in the PCE group however there were no categorical effects of nicotine or nicotine-cocaine interactions on functional connectivity. Moreover, despite poly-drug use being more pronounced in the PCE-cohort, our results were largely unaltered after controlling for the number of drugs used during pregnancy. This finding is consistent with our previous finding that PCE-related structural differences were also unaffected by the overall number of drugs used (Grewen et al., 2014). However, there was a marginal effect of mean cigarettes smoked per day on functional connectivity in two clusters, thus future studies specifically designed for nicotine exposure are warranted. Moreover, marginal effects were also observed for other non-cocaine drugs (e.g., Marijuana, Nicotine, Opiates) further justifying additional studies targeting each drug and their respective pathways [e.g., Marijuana (Grewen et al., 2015)].
Similar to our previous studies (Grewen et al., 2014; Grewen et al., 2015; Salzwedel et al., 2015), we detected a drug-common effect in the motor pathway. This finding is consistent with behavioral reports demonstrating motor function abnormalities within a broad spectrum of prenatal drug exposures (Connor et al., 2006; Fallone et al., 2014; Kronstadt, 1991).
4.1 Limitations and other considerations
Socioeconomic status (SES) and maternal depression levels can also affect brain development (Rifkin-Graboi et al., 2013) and behavior (Sandman and Davis, 2012), therefore we used maternal education as a proxy of SES and included both maternal education and depression levels in our statistical models. Importantly, neither of these variables produced significant main effects and statistical control for these variables did not alter our main conclusions, suggesting that our results likely reflect drug-dependent mechanisms. However, longitudinal studies are needed to examine the long-term effects on functional connectivity and behavioral development.
In previous work we have explored the potential effects arising from global signal regression (GSR) and, in general, have found that our conclusions on relative group differences are largely invariant with or without this preprocessing step (Gao et al., 2013; Grewen et al., 2015; Salzwedel et al., 2015). Here, for clusters with putative negative connectivity, we again found similar group trends and main effects using an alternative standardization technique (post-hoc mean subtraction). These results suggest that although GSR can shift the distribution of correlation values, it does not appear to alter relative group differences (Yan et al., 2013). However, the negative signs of the related functional connectivity values should be interpreted accordingly given the application of GSR.
Beyond seed-based analysis, network or system-level approaches [i.e. independent component analysis (Gao et al., 2014), graph theory (Gao et al., 2011)] could elucidate additional PCE-specific effects. For proof of concept, we retroactively analyzed the relationship between amygdalo- and insula-frontal (Salzwedel et al., 2015) and thalamo-frontal functional connectivity in this cohort. Interestingly, we discovered significant positive correlations (Fig. S4) among the related functional connectivity alterations, suggesting that PCE-related dysconnectivity with frontal regions could be mediated by a common mechanism. Therefore, future studies employing system-level approaches combining multiple networks are warranted in order to gain additional insights. Ideally, the effects of PCE should be studied in cocaine-exposed infants without the influence of other drugs. However, due to the prevalence of poly-drug use in cocaine using mothers it is currently not feasible to generate sufficient sample sizes for such analyses in this study.
4.2 Conclusions
In summary, we have delineated a detailed profile of PCE-related thalamocortical functional connectivity disruptions in neonates. Our results revealed both PCE-specific and drug-common functional connectivity alterations which subsequently marginally predicted behavioral outcomes. Finally, our finding of enhanced abnormalities in connectivity associated with the combined use of cocaine and SSRIs deserve further independent validation and attention given the potential clinical implications.
Supplementary Material
Highlights.
Large cohort of neonates (N = 152) to study effects of prenatal drug exposure.
fMRI reveals cocaine specific disruptions in thalamocortical functional connectivity.
Connectivity correlates with behavioral performance in cocaine-exposed group.
Cocaine by selective-serotonin-reuptake-inhibitor (SSRI) interactions detected.
Acknowledgments
This study was supported by NIH R03 grant (R03 DA036645-01A1) to Gao, W and Grewen, K.M., R21 grant (1R21NS088975-01A1) to Gao, W, P01DA022446 to Grewen, K.M., and Cedars-Sinai Institutional Support to Gao, W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: The authors declare no competing financial interests.
References
- Alcauter S, Lin W, Smith JK, Short SJ, Goldman BD, Reznick JS, Gilmore JH, Gao W. Development of Thalamocortical Connectivity during Infancy and Its Cognitive Correlations. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014a;34:9067–9075. doi: 10.1523/JNEUROSCI.0796-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcauter S, Lin W, Smith JK, Short SJ, Goldman BD, Reznick JS, Gilmore JH, Gao W. Development of Thalamocortical Connectivity during Infancy and Its Cognitive Correlations. J Neurosci. 2014b;34:9067–9075. doi: 10.1523/JNEUROSCI.0796-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G, Pazderova L, Chew A, Tusor N, Merchant N, Arichi T, Allsop JM, Cowan FM, Edwards AD, Counsell SJ. Thalamocortical Connectivity Predicts Cognition in Children Born Preterm. Cerebral cortex. 2015 doi: 10.1093/cercor/bhu331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of Infant and Toddler Development. 3rd Edition. San Antonio, TX: Harcourt Assessment/Psychological Corporation; 2006. [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Chang C, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. NeuroImage. 2009;47:1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Adleman NE, Saad ZS, Leibenluft E, Cox RW. Applications of multivariate modeling to neuroimaging group analysis: a comprehensive alternative to univariate general linear model. Neuroimage. 2014;99:571–588. doi: 10.1016/j.neuroimage.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YT, Fromm S, Guyer AE, Detloff A, Pine DS, Fudge JL, Ernst M. Nucleus accumbens, thalamus and insula connectivity during incentive anticipation in typical adults and adolescents. NeuroImage. 2013;66:508–521. doi: 10.1016/j.neuroimage.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor PD, Sampson PD, Streissguth AP, Bookstein FL, Barr HM. Effects of prenatal alcohol exposure on fine motor coordination and balance: A study of two adult samples. Neuropsychologia. 2006;44:744–751. doi: 10.1016/j.neuropsychologia.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and biomedical research, an international journal. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crandall JE, McCarthy DM, Araki KY, Sims JR, Ren JQ, Bhide PG. Dopamine receptor activation modulates GABA neuron migration from the basal forebrain to the cerebral cortex. J Neurosci. 2007;27:3813–3822. doi: 10.1523/JNEUROSCI.5124-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding JB, Guzman JN, Peterson JD, Goldberg JA, Surmeier DJ. Thalamic Gating of Corticostriatal Signaling by Cholinergic Interneurons. Neuron. 2010;67:294–307. doi: 10.1016/j.neuron.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden RD, Godleski S, Colder CR, Schuetze P. Prenatal cocaine exposure: the role of cumulative environmental risk and maternal harshness in the development of child internalizing behavior problems in kindergarten. Neurotoxicology and teratology. 2014;44:1–10. doi: 10.1016/j.ntt.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallone MD, LaGasse LL, Lester BM, Shankaran S, Bada HS, Bauer CR. Reactivity and regulation of motor responses in cocaine-exposed infants. Neurotoxicology and teratology. 2014;43:25–32. doi: 10.1016/j.ntt.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a clustersize threshold. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Frankfurt M, Salas-Ramirez K, Friedman E, Luine V. Cocaine alters dendritic spine density in cortical and subcortical brain regions of the postpartum and virgin female rat. Synapse (New York, NY) 2011;65:955–961. doi: 10.1002/syn.20918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Alcauter S, Elton A, Hernandez-Castillo CR, Smith JK, Ramirez J, Lin W. Functional Network Development During the First Year: Relative Sequence and Socioeconomic Correlations. Cerebral cortex (New York, NY : 1991) 2014:1–10. doi: 10.1093/cercor/bhu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Alcauter S, Smith J, Gilmore J, W L. Development of Human Brain Cortical Network Architecture during Infancy. Brain Structure and Function. 2014 doi: 10.1007/s00429-014-0710-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Giovanello KS, Smith JK, Shen D, Zhu H, Lin W. Temporal and spatial evolution of brain network topology during the first two years of life. PloS one. 2011;6:e25278–e25278. doi: 10.1371/journal.pone.0025278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Shen D, Smith JK, Zhu H, Lin W. The synchronization within and interaction between the default and dorsal attention networks in early infancy. Cerebral cortex (New York, NY : 1991) 2013;23:594–603. doi: 10.1093/cercor/bhs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Lin W. Frontal parietal control network regulates the anti-correlated default and dorsal attention networks. Hum Brain Mapp. 2012;33:192–202. doi: 10.1002/hbm.21204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cabezas MA, Rico B, Sanchez-Gonzalez MA, Cavada C. Distribution of the dopamine innervation in the macaque and human thalamus. Neuroimage. 2007;34:965–984. doi: 10.1016/j.neuroimage.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Saad ZS, Jo HJ, Wallace GL, Cox RW, Martin A. The perils of global signal regression for group comparisons: a case study of Autism Spectrum Disorders. Frontiers in human neuroscience. 2013;7:356–356. doi: 10.3389/fnhum.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewen K, Burchinal M, Vachet C, Gouttard S, Gilmore JH, Lin W, Johns J, Elam M, Gerig G. Prenatal cocaine effects on brain structure in early infancy. Neuroimage. 2014;101:114–123. doi: 10.1016/j.neuroimage.2014.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewen K, Salzwedel A, Gao W. Functional connectivity disruption in neonates with prenatal marijuana exposure. Frontiers in Human Neuroscience. 2015;9 doi: 10.3389/fnhum.2015.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsay HA, Spaan M, Wijnen JG, Ridderinkhof KR. Error Awareness and Salience Processing in the Oddball Task: Shared Neural Mechanisms. Frontiers in Human Neuroscience. 2012;6:246. doi: 10.3389/fnhum.2012.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey Ja. Cocaine effects on the developing brain: current status. Neuroscience and biobehavioral reviews. 2004;27:751–764. doi: 10.1016/j.neubiorev.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Jasso H, Triesch J. Learning to Attend -- From Bottom-Up to Top-Down. In: Lucas P, Erich R, editors. In Attention in Cognitive Systems Theories and Systems from an Interdisciplinary Viewpoint. Springer-Verlag; 2008. pp. 106–122. [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. Fsl. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. 2nd edn. Cambridge University Press; 2007. [Google Scholar]
- Koe BK. Molecular geometry of inhibitors of the uptake of catecholamines and serotonin in synaptosomal preparations of rat brain. The Journal of pharmacology and experimental therapeutics. 1976;199:649–661. [PubMed] [Google Scholar]
- Kronstadt D. Complex Developmental Issues of Prenatal Drug Exposure. Drug-Exposed Infants. 1991;1 [Google Scholar]
- Lambert BL, Bauer CR. Developmental and behavioral consequences of prenatal cocaine exposure: a review. Journal of perinatology : official journal of the California Perinatal Association. 2012;32:819–828. doi: 10.1038/jp.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BM, Padbury JF. Third Pathophysiology of Prenatal Cocaine Exposure. Developmental Neuroscience. 2009;31:23–35. doi: 10.1159/000207491. [DOI] [PubMed] [Google Scholar]
- Li CSR, Yan P, Chao HHA, Sinha R, Paliwal P, Constable RT, Zhang S, Lee TW. Error-specific medial cortical and subcortical activity during the stop signal task: A functional magnetic resonance imaging study. Neuroscience. 2008;155:1142–1151. doi: 10.1016/j.neuroscience.2008.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Coles CD, Lynch ME, Hamann S, Peltier S, LaConte S, Hu X. Prenatal cocaine exposure alters emotional arousal regulation and its effects on working memory. Neurotoxicology and teratology. 2009;31:342–348. doi: 10.1016/j.ntt.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Coles CD, Lynch ME, Luo Y, Hu X. Longitudinal changes of amygdala and default mode activation in adolescents prenatally exposed to cocaine. Neurotoxicology and Teratology. 2016;53:24–32. doi: 10.1016/j.ntt.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Santhanam P, Coles CD, Ellen Lynch M, Hamann S, Peltier S, Hu X. Prenatal cocaine exposure alters functional activation in the ventral prefrontal cortex and its structural connectivity with the amygdala. Psychiatry research. 2013;213:47–55. doi: 10.1016/j.pscychresns.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lester BM, Neyzi N, et al. REgional brain morphometry and impulsivity in adolescents following prenatal exposure to cocaine and tobacco. JAMA Pediatrics. 2013;167:348–354. doi: 10.1001/jamapediatrics.2013.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo DS, Santos RS, Belchior LD, Neto MA, Vasconcelos SM, Lima VT, Fonteles MM, Viana GS, de Sousa FC. Effect of anxiolytic, antidepressant, and antipsychotic drugs on cocaine-induced seizures and mortality. Epilepsy & behavior : E&B. 2004;5:852–856. doi: 10.1016/j.yebeh.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Mayes LC. A behavioral teratogenic model of the impact of prenatal cocaine exposure on arousal regulatory systems. Neurotoxicology and teratology. 2002;24:385–395. doi: 10.1016/s0892-0362(02)00200-3. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Grillon C, Granger R, Schottenfeld R. Regulation of Arousal and Attention in Preschool Children Exposed to Cocaine Prenatally. Annals of the New York Academy of Sciences. 1998;846:126–143. [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain structure & function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Quenzer LF. Psychopharmacology: Drugs, the Brain, and Behavior . Sinauer Associates,Publishers; 2005. [Google Scholar]
- Muller CP, Carey RJ, Huston JP. Serotonin as an important mediator of cocaine's behavioral effects. Drugs of today. 2003;39:497–511. doi: 10.1358/dot.2003.39.7.799442. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L, Carothers AD. The validation of the Edinburgh Post-natal Depression Scale on a community sample. Br J Psychiatry. 1990;157:288–290. doi: 10.1192/bjp.157.2.288. [DOI] [PubMed] [Google Scholar]
- Nair A, Treiber JM, Shukla DK, Shih P, Müller R-A. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain. 2013;136:1942–1955. doi: 10.1093/brain/awt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell LE, George FR, Ritz MC. Antidepressant drugs appear to enhance cocaineinduced toxicity. Experimental and clinical psychopharmacology. 2000;8:133–141. doi: 10.1037//1064-1297.8.1.133. [DOI] [PubMed] [Google Scholar]
- O'Muircheartaigh J, Keller SS, Barker GJ, Richardson MP. White Matter Connectivity of the Thalamus Delineates the Functional Architecture of Competing Thalamocortical Systems. Cerebral Cortex (New York, NY) 2015;25:4477–4489. doi: 10.1093/cercor/bhv063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani N, Goto T, Waeber C, Bhide PG. Dopamine modulates cell cycle in the lateral ganglionic eminence. J Neurosci. 2003;23:2840–2850. doi: 10.1523/JNEUROSCI.23-07-02840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Zatorre RJ, Hofle N, Caramanos Z, Gotman J, Petrides M, Evans AC. Timerelated changes in neural systems underlying attention and arousal during the performance of an auditory vigilance task. J Cogn Neurosci. 1997;9:392–408. doi: 10.1162/jocn.1997.9.3.392. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes Ka, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando K, Chaplin TM, Potenza MN, Mayes L, Sinha R. Prenatal cocaine exposure and gray matter volume in adolescent boys and girls: relationship to substance use initiation. Biological psychiatry. 2013;74:482–489. doi: 10.1016/j.biopsych.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H, Wang J, Giannetta J, Korczykowski M, Shera D, Avants BB, Gee J, Detre Ja, Hurt H. Altered resting cerebral blood flow in adolescents with in utero cocaine exposure revealed by perfusion functional MRI. Pediatrics. 2007;120:e1245–e1254. doi: 10.1542/peds.2006-2596. [DOI] [PubMed] [Google Scholar]
- Rifkin-Graboi A, Bai J, Chen H, Hameed WB, Sim LW, Tint MT, Leutscher-Broekman B, Chong YS, Gluckman PD, Fortier MV, et al. Prenatal maternal depression associates with microstructure of right amygdala in neonates at birth. Biol Psychiatry. 2013;74:837–844. doi: 10.1016/j.biopsych.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav. 2014;28:154–162. doi: 10.1037/a0030992. [DOI] [PubMed] [Google Scholar]
- Ross EJ, Graham DL, Money KM, Stanwood GD. Developmental consequences of fetal exposure to drugs: what we know and what we still must learn. Neuropsychopharmacology. 2015;40:61–87. doi: 10.1038/npp.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussotte F, Soderberg L, Sowell E. Structural, metabolic, and functional brain abnormalities as a result of prenatal exposure to drugs of abuse: evidence from neuroimaging. Neuropsychology review. 2010;20:376–397. doi: 10.1007/s11065-010-9150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2:25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzwedel AP, Grewen KM, Vachet C, Gerig G, Lin W, Gao W. Prenatal Drug Exposure Affects Neonatal Brain Functional Connectivity. The Journal of Neuroscience. 2015;35:5860–5869. doi: 10.1523/JNEUROSCI.4333-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gonzalez MA, Garcia-Cabezas MA, Rico B, Cavada C. The primate thalamus is a key target for brain dopamine. J Neurosci. 2005;25:6076–6083. doi: 10.1523/JNEUROSCI.0968-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Davis EP. Neurobehavioral risk is associated with gestational exposure to stress hormones. Expert review of endocrinology & metabolism. 2012;7:445–459. doi: 10.1586/eem.12.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Research Reviews. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinkopf SJ, Lester BM, Sanes JN, Eliassen JC, Hutchison ER, Seifer R, Lagasse LL, Durston S, Casey BJ. Functional MRI and response inhibition in children exposed to cocaine in utero. Preliminary findings. Developmental neuroscience. 2009;31:159–166. doi: 10.1159/000207503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM. The thalamus is more than just a relay. Curr Opin Neurobiol. 2007;17:417–422. doi: 10.1016/j.conb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song ZM, Undie AS, Koh PO, Fang YY, Zhang L, Dracheva S, Sealfon SC, Lidow MS. D1 dopamine receptor regulation of microtubule-associated protein-2 phosphorylation in developing cerebral cortical neurons. J Neurosci. 2002;22:6092–6105. doi: 10.1523/JNEUROSCI.22-14-06092.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanwood GD, Washington RA, Shumsky JS, Levitt P. Prenatal cocaine exposure produces consistent developmental alterations in dopamine-rich regions of the cerebral cortex. Neuroscience. 2001;106:5–14. doi: 10.1016/s0306-4522(01)00256-1. [DOI] [PubMed] [Google Scholar]
- Toulmin H, Beckmann CF, O'Muircheartaigh J, Ball G, Nongena P, Makropoulos A, Ederies A, Counsell SJ, Kennea N, Arichi T, et al. Specialization and integration of functional thalamocortical connectivity in the human infant. Proceedings of the National Academy of Sciences. 2015;112:6485–6490. doi: 10.1073/pnas.1422638112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Cunningham KA. Serotonergic mechanisms involved in the discriminative stimulus, reinforcing and subjective effects of cocaine. Psychopharmacology. 1997;130:41–58. doi: 10.1007/s002130050210. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Funahashi S. Thalamic mediodorsal nucleus and working memory. Neuroscience & Biobehavioral Reviews. 2012;36:134–142. doi: 10.1016/j.neubiorev.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical Dysconnectivity in Schizophrenia. American Journal of Psychiatry. 2012;169:1092–1099. doi: 10.1176/appi.ajp.2012.12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, McCormick MC, Burris HH. Disparities in antidepressant use in pregnancy. J Perinatol. 2014 doi: 10.1038/jp.2014.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan CG, Craddock RC, Zuo XN, Zang YF, Milham MP. Standardizing the intrinsic brain: towards robust measurement of inter-individual variation in 1000 functional connectomes. Neuroimage. 2013;80:246–262. doi: 10.1016/j.neuroimage.2013.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumoto C, Jacobson SW, Jacobson JL. Fetal substance exposure and cumulative environmental risk in an African American cohort. Child development. 2008;79:1761–1776. doi: 10.1111/j.1467-8624.2008.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cerebral cortex. 2010;20:1187–1194. doi: 10.1093/cercor/bhp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Hu S, Bednarski SR, Erdman E, Li C-sR. Error-related functional connectivity of the thalamus in cocaine dependence. NeuroImage: Clinical. 2014;4:585–592. doi: 10.1016/j.nicl.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.