Abstract
Rationale
Some individuals are particularly responsive to reward-associated stimuli (“cues”), including the effects of these cues on craving and relapse to drug-seeking behavior. In the cases of nicotine and alcohol, cues may acquire these abilities via the incentive-enhancing properties of the drug.
Objectives
To determine the interaction between cue-responsivity and nicotine reinforcement, we studied the patterns of nicotine self-administration in rats categorized based on their tendency to approach a food predictive cue (“sign-trackers”) or a reward-delivery location (“goal-trackers”). In a second experiment, we determined whether nicotine and ethanol altered the incentive value of a food cue.
Methods
Rats were classified as sign- or goal-trackers during a Pavlovian conditioned approach paradigm. Rats then self-administered intravenous nicotine (0.03 mg/kg infusions) followed by extinction and cue induced reinstatement tests. We also tested the effects of nicotine (0.4 mg/kg base s.c.) or ethanol (0.7 g/kg i.p.) on the approach to, and reinforcing efficacy of, a food cue.
Results
Sign-trackers showed greater reinstatement in response to a nicotine cue. Further, nicotine enhanced sign-tracking but not goal-tracking to a food cue, and also enhanced responding for the food cue during the conditioned reinforcement test. Conversely, ethanol reduced sign-tracking and increased goal-tracking, but had no effect on conditioned reinforcement.
Conclusions
Our studies demonstrate that the tendency to attribute incentive value to a food cue predicts enhanced cue-induced reinstatement. Additionally, the incentive value of food cues is differentially modulated by nicotine and ethanol, which may be related to the reinforcing effects of these drugs.
Keywords: Autoshaping, acetylcholine receptor, drug addiction, alcoholism, attention, motivation, mesolimbic dopamine, behavioral pharmacology, smoking, drinking, sign-tracking, goal-tracking, Pavlovian conditioned approach
Introduction
Stimuli associated with the availability of food and abused drugs (“conditioned stimuli” or “cues”) can regulate multiple aspects of motivated behavior. In some individuals, these cues can acquire incentive value, and thus act as incentive stimuli. Incentive stimuli are defined by three distinct properties: 1) They can become attractive, eliciting approach, 2) they can reinforce new behaviors by becoming a conditioned reinforcer, and 3) they can elicit conditioned motivational states, thereby motivating reward-seeking behavior. These properties of incentive stimuli have been modelled in rodents using a variety of behavioral paradigms, including Pavlovian conditioned approach (PavCA), conditioned reinforcement, and cue induced reinstatement, respectively (Milton and Everitt 2010; Robinson et al. 2014).
However, there is significant variability among individuals in the ability of cues to acquire incentive value and become incentive stimuli. In previous work (Meyer et al. 2012a; 2012b; see also Flagel et al. 2007; 2009), we used a Pavlovian conditioned approach (PavCA) paradigm in rats to identify individual differences in sensitivity to the incentive properties of food cues. In this paradigm, brief, repeated presentations of a lever-cue predict the delivery of food pellet rewards. Rats that approach and interact with this cue, or “sign”, are called “sign-trackers”, while those that preferentially approach the reward location upon cue onset are called “goal-trackers”(Hearst and Jenkins 1974; Boakes 1977). Compared to goal-trackers, sign-trackers attribute greater motivational value to food cues, as defined by the ability of these cues to attract, reinforce behavior, and motivate food-seeking (Yager and Robinson 2010; Robinson and Flagel 2009; Meyer et al. 2014). However, sign-tracking is only one index of an underlying trait, which we refer to as the tendency to attribute incentive value to reward cues (Meyer et al. 2012a). Other manifestations of this trait include attentional deficits, action impulsivity, and behavioral flexibility (King et al. 2016; Lovic et al. 2011; Nasser et al. 2015; Tomie et al. 1998a). Consistent with this idea, sign-trackers are more likely to develop a preference for a cocaine-paired cue (Meyer et al. 2012b), and are particularly responsive to cocaine cues during self-administration (Saunders and Robinson 2010). Thus, the sign/goal-tracking distinction is a useful model for the individual differences in cue responsivity observed in drug addiction and other cue controlled disorders.
In the case of smoking behavior, nicotine may lead to addictive behavior by 1) differentially affecting individuals who are predisposed to respond to drug cues, and 2) further amplifying this tendency by enhancing the incentive value of cues through non-associative mechanisms (Caggiula et al. 2009; Chaudhri et al. 2006a; 2006b; Palmatier et al. 2006). However, it is unknown whether the response to nicotine cues is similarly divergent in sign- and goal-trackers. Sensitivity to nicotine-associated cues is critical for the maintenance of smoking behavior, and plays a central role in cue-induced craving and relapse (Rose et al. 2000; Bailey et al. 2010). For example, in addition to being a weak primary reinforcer, nicotine functions as a “reinforcement enhancer” or “incentive amplifier” with regard to cues, and it is this latter property that is responsible for the ability of nicotine cues to maintain smoking behavior and induce craving and relapse (Bevins and Palmatier 2004; Caggiula et al. 2009; 2002; Palmatier et al. 2013; 2014; Donny et al. 2003). Thus, given the importance of cues in nicotine self-administration, we hypothesized that responsivity to nicotine cues in this paradigm would be greater in sign-trackers.
In addition, the propensity to attribute incentive value to reward cues is further exacerbated by drug exposure, in that drug exposure enhances the response to a variety of Pavlovian cues (Hauser et al. 2012; Olausson et al. 2004a; 2004b). For example, previous work shows that nicotine enhances the sign-tracking response to food and water cues (Palmatier et al. 2013; Guy and Fletcher 2014). This ability of nicotine to enhance the motivational value of reward cues likely underlies its addictive properties (see Caggiula et al. 2009 for review). In the current study, we hypothesized that nicotine would enhance both approach to and the reinforcing properties of a food-associated cue. For comparison, we also tested the effects of alcohol (ethanol) injections on these measures. Previous studies have shown that rats will sign-track an ethanol-associated cue, and that the effect of ethanol on tests of impulsivity are associated with sign-tracking behavior (Tomie et al. 1998a; 1998b; Krank et al. 2008; Krank 2003). However, ethanol has potent aversive effects when administered acutely, and requires previous exposure to overcome this initial aversion (Schramm-Sapyta et al. 2010; Fidler et al. 2009; Bormann and Cunningham 1998; Asin et al. 1985; van der Kooy et al. 1983; Cunningham et al. 2002). Thus, we predicted that ethanol would decrease the incentive value of a food-associated cue.
Materials and Methods
Subjects
Adult male Sprague Dawley rats from Charles River (Wilmington, MA) and Harlan (Indianapolis, IN) were used in three experiments (64–73 days old at the beginning of testing). Each experiment used a separate group of rats (64 rats experiment 1, 91 rats experiment 2, 92 rats experiment 3). Rats were pair-housed in a temperature- and humidity-controlled room on a reverse light/dark schedule (9:30AM/PM). For experiment 1, rats were food restricted in a manner similar to Donny et al. (1998; 20 g of food chow given following conclusion of a self-administration session), because it has been shown to facilitate responding for nicotine. For experiments 2 and 3, standard laboratory chow and water were provided ad libitum except while rats were in the testing chambers (~40 min/day for experiments 2–3). All procedures were approved by the University at Buffalo Institutional Animal Care and Use Committee.
Drugs and Solutions
Nicotine hydrogen tartrate (Sigma Aldrich, St. Louis, MO) was dissolved in 0.9% saline at a concentration of 0.4 mg base/ml with a pH of 7.2–7.4 (Matta et al. 2007). For the self-administration study (experiment 1), responses were reinforced with 0.03 mg/kg infusions through the rats’ intravenous catheters. For the Pavlovian conditioning study (experiment 2), rats received subcutaneous injections (0.4 mg/kg) before being placed in the test chamber. Doses were adjusted based on body weight by adjusting the volume administered. For experiment 3, ethanol (200 Proof, Decon Laboratories) was mixed with 0.9% saline for a 20% v/v solution with a pH of 7.2–7.4. Rats received intraperitoneal injections of 0.7 g/kg.
Apparatus
Conditioning chambers (20.5 × 24.1cm floor area, 29.2 cm high, Med-Associates Inc., St. Albans, VT) were located inside sound and light attenuating cabinets (A&B Display Systems, Bay City, MI). Fans inside the cabinets provided background noise and ventilation. A red houselight was located near the ceiling of the chamber. Each chamber had a parallel-rod floor above aspen shavings bedding. Chambers were equipped with levers, food magazines, and nose-poke ports according to the specific experimental procedures described below.
Experiment 1: Nicotine self-administration in sign- and goal-trackers
An overview of the procedures is provided in Table 1. In this experiment, rats were first identified as sign-trackers, goal-trackers or intermediates using a Pavlovian conditioned approach paradigm. Testing chamber walls were configured with a food-cup in the center and equipped with an infrared photobeam to detect snout entry. Automated food hoppers delivered 45 mg banana-flavored food pellets into the food-cup. An illuminated, retractable lever was located on either the left or right (counterbalanced) side of the food-cup. For nicotine self-administration, the food-cup and levers were removed, and replaced with two nose poke holes on either side of a blank panel. Circular stimulus lights were positioned above each nose-poke hole.
Table 1.
Overall experimental procedure and treatment groups. IVSA: intravenous self-administration. PavCA: Pavlovian conditioned approach. CRf: conditioned reinforcement. FR: fixed ratio. Note that for Experiments 2 and 3, separate paired and unpaired groups received these treatments.
| Table 1A | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PavCA | IVSA | |||||||||
| Experiment 1: Nicotine IVSA | Food-Cup Training | PavCA | Surgery/Recovery | Aquisition | Cue Removal | FR Escalation | Extinction | Reinstatement | ||
| 1 day | 5 days | 5 days | FR1 (10 days) | FR1 (2 days) | FR1 (5 days) | FR2 (2 days) | FR5 (10 days) | 8 days | 1 day | |
| Table 1B | |||||
|---|---|---|---|---|---|
| Food-Cup Training 1 day | Habituation 1 day | PavCA 5 days | Habituation 1 day | CRf 1 day | |
| Experiment 2: Nicotine PavCA | No injections | Saline | Saline | Saline | Saline |
| Nicotine | Nicotine | ||||
| Nicotine | Nicotine | Saline | Saline | ||
| Nicotine | Nicotine | ||||
| Experiment 3: Ethanol PavCA | No injections | Saline | Saline | Saline | Saline |
| Ethanol | Ethanol | ||||
| Ethanol | Ethanol | Saline | Saline | ||
| Ethanol | Ethanol | ||||
Pavlovian Conditioned Approach (PavCA)
Before training began, approximately 25 banana pellets (45 mg, Bio-Serve, Frenchtown NJ) were given to the rats in their home cages for two days before PavCA testing. Rats were then given a single food-cup training session. In this session, after being placed in the chambers for a 5-min habituation period, 25 banana-flavored food pellets were delivered into the food-cup on a variable time (VT) 30 s schedule (delivery varied between 1–60 s). Thus, the session lasted approximately 17.5 min.
PavCA training sessions occurred during each of the subsequent 5 days. After being placed in the chambers for one minute, rats were presented with 25 lever-pellet pairing trials on a VT 90s (30–150s) schedule. Each trial consisted of an 8-s lever presentation, followed by the retraction of the lever and delivery of a food pellet into the food-cup. The number of lever contacts and food-cup entries were recorded. Each session lasted approximately 37.5 min.
Rats were identified as sign- and goal trackers based on their responses to the lever-cue as described previously (Meyer et al. 2012a). Briefly, all rats were assigned a PavCA Index Score based on three measures of approach to either the lever-cue or the food-cup. This score was the average of the normalized difference in 1) probability to approach the lever minus that of the food-cup, 2) number of contacts with the lever minus the food-cup entries, 3) difference in the latency to approach the food-cup minus that of the lever. This score, which ranged from −1.0 to 1.0, was used to classify sign-trackers (scores of 0.3 to 1.0) or goal-trackers (−0.3 to −1.0). Intermediate rats (scores between −0.3 to 0.3) were not included in any analyses.
Nicotine self-administration
Sign- and goal-trackers were then implanted with jugular vein catheters as described previously (Crombag et al. 2005), and allowed to recover for at least four days. Rats were assigned to nicotine or saline self-administration groups, so that equal numbers of sign- and goal-trackers began self-administration in each group. Rats were underwent five daily sessions per week for the remainder of the study. Catheter patency was determined each week on non-test days using 0.1 mL of a 10 mg/mL solution of ketamine in 0.9% saline. Catheters were considered functional if subjects became motor ataxic within 10 s of ketamine treatment.
The nicotine self-administration procedure had several phases (see Table 1A), including FR1 acquisition (10 days), light-cue removal tests (2 days), FR escalation (FR1, FR2, FR5; 17 days), instrumental extinction (8 days), and light-cue induced reinstatement (1 day). Each session lasted 2 h. The start of each session was signaled by the illumination of the houselight. When rats completed the FR requirement (1, 2, or 5 nose-pokes in to the active port) they received an intravenous infusion of 0.03 mg/kg nicotine, concurrent with a 3 s light-cue (the illumination of a stimulus light and nose-poke port light). Each infusion was followed by a 20 s “time out” during which the houselight was extinguished and nose-pokes were recorded but not reinforced or counted toward the fixed ratio. A separate set of control animals responded for saline infusions.
To determine the impact of the light-cue on self-administration, rats were given light-cue removal probe tests on days 11 and 12, during which nose-pokes into the active hole were reinforced by either saline or nicotine infusions, but no stimulus or nose poke lights were illuminated, and the house light was not extinguished.
Next, rats underwent extinction, during which nose-pokes were measured but had no programmed consequences. Finally, during the reinstatement test, nose-pokes into the active nose-poke port were reinforced by the stimulus and nose-poke port lights on an FR1 schedule, but no infusions were given during this test.
Experiment 2: Effects of nicotine on sign-tracking, goal-tracking, and conditioned reinforcement
In this experiment, the effect of nicotine on lever-cue responsivity during PavCA was measured in a separate set of rats (n = 91). On the day following the food-cup training session, rats were assigned to four groups (saline/paired, nicotine/paired, saline/unpaired, nicotine/unpaired) and received a single injection of saline or nicotine, depending on the group assignment (see Table 1B). Rats were not tested on this day, this was intended to habituate the rats to the saline or nicotine injections. These injections continued for the PavCA phase of the experiment, which consisted of 5 daily sessions and began the next day. PavCA testing processed as described above, except that rats were injected subcutaneously with nicotine or saline 15 min before testing. In addition, an unpaired group received lever presentations which were explicitly unpaired from the food pellet delivery.
Conditioned Reinforcement
For conditioned reinforcement, the food-cup was removed and the illuminated lever was moved to the center panel between two nose-poke holes. The day after the final session of PavCA, nicotine- and saline-treated rats were further divided into four groups (see Table 1B), which did not significantly differ in terms of sign- and goal-tracking behavior [PavCA index scores ranged from −0.62 to −0.45 (± 0.08 – 0.10 SEM)]. Rats were then given another habituation injection of either nicotine or saline. Rats received the same treatment on the following day, and were placed into the chambers for the 60-min conditioned reinforcement test. Thus, there were eight groups in this 2 (nicotine treatment, saline treatment) × 2 (nicotine challenge, saline challenge) × 2 (paired lever, unpaired lever) design. There were 13–14 rats in each of the 4 paired groups and 9 rats in each of the 4 unpaired groups.
During conditioned reinforcement testing, nose-pokes into a designated “active” nose-poke port produced a 3-s presentation of the lever. Nose-pokes during the 3-s lever presentation, as well as nose-pokes into the “inactive” port, were measured but had no consequence. The number of nose-pokes into the active and inactive holes were the primary measures.
Experiment 3: Effects of ethanol on sign-tracking, goal-tracking, and conditioned reinforcement
Experiment 3 was conducted similarly as experiment 2, except that nicotine injections were not given. Instead, rats (n = 92) were injected with ethanol (0.7 mg/kg; i. p.) or saline immediately before being placed into the chambers. As in Experiment 2, for the conditioned reinforcement tests, saline- and nicotine challenged groups did not significantly differ in terms of sign- and goal-tracking behavior [PavCA index scores ranged from −0.67 to −0.48 (± 0.08 – 0.10 SEM)]. There were 14 rats in each of the 4 paired groups and 9 rats in each of the 4 unpaired groups.
Statistics
PavCA data during experiment 1 were analyzed using repeated-measures analysis of variance (ANOVA) with Phenotype (sign-tracker, goal-tracker) as the between-groups measure and Day as the within-subjects measure. For self-administration data, Phenotype and Drug (saline, nicotine) were between groups factors with Day, Port (active, inactive) and Cue (light-cues present/not present) as within-subjects measures. On the reinstatement day, only the first 20 min, during which peak responding occurs, were analyzed.
Experiment 2 and 3 PavCA and conditioned reinforcement data were analyzed using repeated-measures ANOVA with PavCA Treatment (saline, drug), conditioned reinforcement Challenge (saline, drug) and Pairing (paired, unpaired) as between-subjects factors, and Day and Port as within-subjects factors. For the conditioned reinforcement tests, only the first 20 min, during which peak responding occurs, were analyzed. Fisher’s LSD post-hoc tests were used to analyze significant main effects and interactions.
Results
Experiment 1: Nicotine self-administration in sign- and goal-trackers
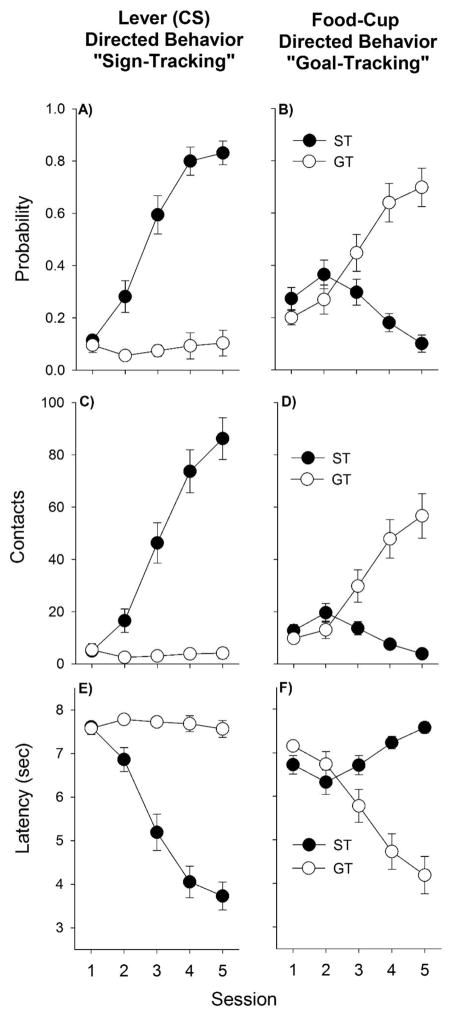
Of the 64 rats tested in PavCA, 23 sign-trackers and 15 goal-trackers were identified as described in the Methods section; the approach behaviors of these rats are shown in Fig. 1. Sign-trackers approached the lever-CS more than goal-trackers in terms of probability of approach, number of contacts, and latency to approach, while goal-trackers approached the food-cup during the CS more than sign-trackers [All Fs (1, 36) > 14.3, ps < 0.001 for the main effects of Phenotype; all Fs (4, 144) > 6.6, ps < 0.001 for the main effects of Day, all Fs (4, 144) > 31.5; ps < 0.001 for the Phenotype × Day interactions].
Figure 1.
Upon lever-cue (CS) presentation, sign-trackers approached the lever (CS) (left panels), while goal-trackers approached the food-cup (right panels). Three measures were taken: Probability (panels A–B) denotes the proportion of trials with a lever contact or a food cup contact, respectively. Panels C–D denote the total number of lever or food-cup contacts, respectively. Latency (panels E–F) depicts the time to the first contact of the lever or the food-cup, with the maximum latency being 8 s. Data are presented as means +/− SEM.
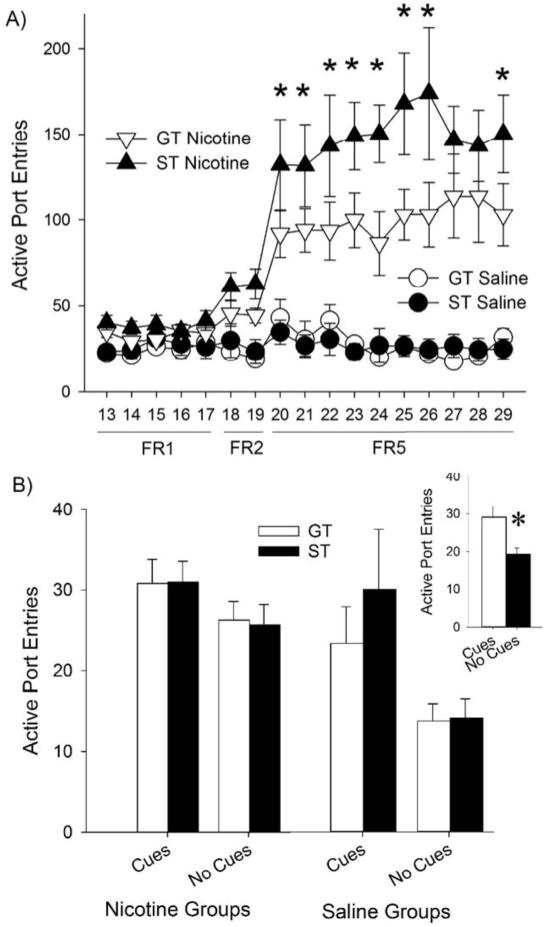
Of these rats, 15 sign-trackers and 10 goal-trackers were successfully catheterized and completed all phases of the nicotine self-administration experiment. During self-administration, there were main effects of Drug and Day [F (1, 21) = 43.1, p < 0.001 and F (16, 336) = 27.6, p < 0.001, respectively], as well as Phenotype × Day and Drug × Day interactions [F (16, 336) = 1.7, p < 0.05 and F (16, 336) = 25.8, p < 0.001, respectively]. Sign- and goal-trackers did not significantly differ under the FR1 and FR2 schedules of reinforcement, but did differ when the schedule requirement was escalated to FR5 [F (16, 336) = 2.2; p < 0.01 for the Phenotype × Drug × Day interaction). Post-hoc analyses indicated that sign-trackers responded more for nicotine than goal-trackers during the FR5 schedule (Fig. 2A). However, this did not result in significantly more nicotine infusions achieved (p > 0.05, not shown). In addition, sign- and goal-trackers did not differ during the light-cue removal test. While removal of the light-cue reduced responding in rats self-administering nicotine and saline [Fig. 2B; F (1, 21) = 11.6; p < 0.01 for the main effect of Cue], and there was a trend for rats self-administering nicotine to respond more than rats self-administering saline (p = 0.055 for the main effect of Drug), these effects did not depend on Phenotype, nor were there 2- or 3-way interactions between Cue, Phenotype, and Drug).
Fig. 2.
Sign-trackers responded more for nicotine than goal-trackers, but were equally sensitive to the removal of the light-cue. Panel A shows the number of active port entries in rats self-administering saline or nicotine. The schedule of reinforcement was increased from FR1 to FR5, as indicated on the ordinate. Asterisks denote significant differences (ps < 0.05) between sign-trackers (ST) and goal-trackers (GT) in the nicotine-treated groups. Panel B show the effect of light-cue removal on days 11–12 (“No Cues”, the average of the two days is shown), compared to responding in the presence of the light cue on day 9–10 (“Cues”). Note that rats still receive nicotine or saline infusions according to their group assignments. The inset depicts marginal means for the main effect of Cue removal; the asterisk denotes significant differences between the Cues/No Cues sessions. Data are presented as means +/− SEM.
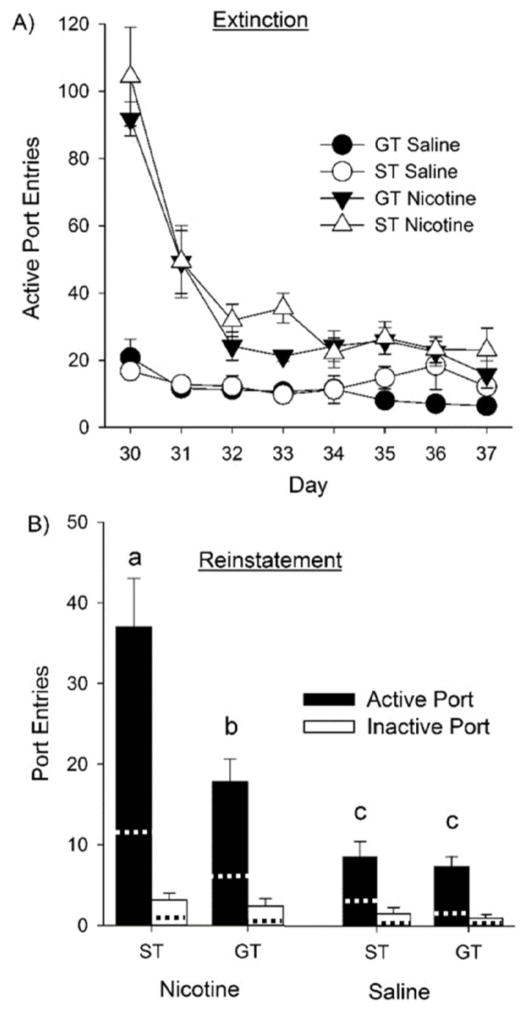
During extinction, there were main effects of Drug and Day [F (1, 21) = 50.2, p < 0.001 and F (7, 147) = 45.3, p < 0.001, respectively]. Removal of the light-cues and infusions decreased responding significantly more in rats that have been self-administering nicotine than those that had been self-administering saline [Fig 3A; F (7, 147) = 30.2; p < 0.001 for the Day × Drug interaction]. Post-hoc tests confirmed that only rats self-administering nicotine decreased responding by the last day of extinction, compared to the first day (ps < 0.001). There was no main effect or interaction with Phenotype, indicating that sign- and goal-trackers did not differ in their rates of extinction.
Fig. 3.
Sign-trackers (ST) had higher levels of cue-induced reinstatement than goal-trackers (GT). Panel A shows rats’ responding during the eight days of extinction, when the infusion and light-cue were removed. Panel B shows number of active and inactive port entries during the first 20 minutes of the reinstatement test. Dotted lines indicate port entries during the first 20 min of the last extinction test (i.e., Day 37). Bars labelled with different letters (a–c) were significantly different from each other. Thus, while both sign- and goal-trackers from the nicotine group showed significant reinstatement compared to the saline group, sign-trackers from the nicotine group had more active port entries than all other groups. Data are presented as means +/− SEM.
However, during the reinstatement test, there were main effects of Phenotype, Drug, and Port [Fs (1, 21) = 11.6, 43.4, and 8.2, respectively; all ps < 0.05], and Phenotype × Drug and Drug × Port interactions [Fs (1, 21) = 8.2; p < 0.01 and F (1, 21) = 5.6; p < 0.05, respectively]. In addition, sign-trackers that had self-administered nicotine responded more in the active port than their goal-tracker counterparts [Fig. 3B; F (1, 21) = 4.9; p < 0.05; for the Phenotype × Drug × Port interaction], which was confirmed by post-hoc analyses showing that both sign- and goal-trackers that had self-administered nicotine displayed reinstatement (when compared to saline-treated groups, ps < 0.05), and that sign-trackers responded more into the active port than any other group (ps < 0.001). This indicates that sign-trackers are more sensitive to the ability of nicotine cues to reinstate responding.
Experiment 2: Effects of nicotine on sign-tracking, goal-tracking, and conditioned reinforcement
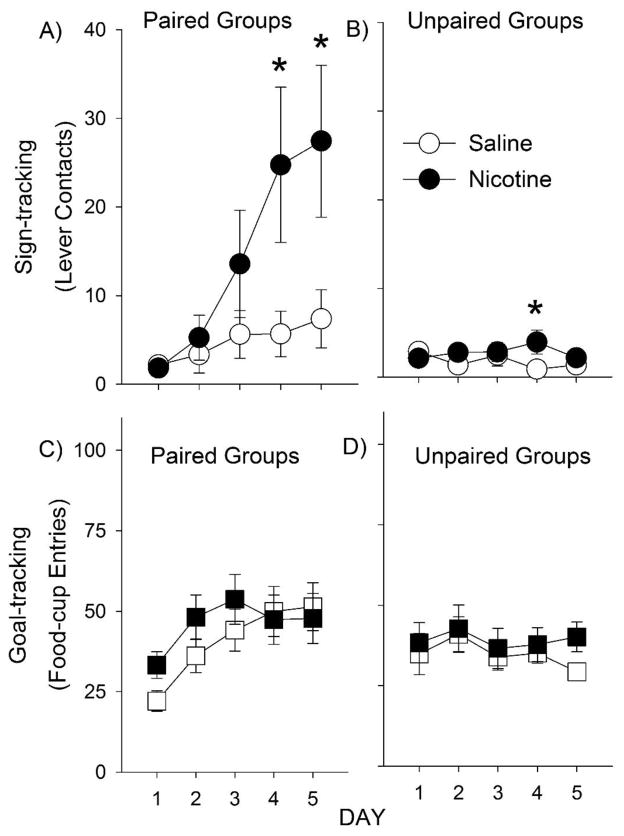
One rat from the saline treated, nicotine challenged, paired group was removed because it had an unusually high levels of responding during conditioned reinforcement (> 160 responses, which was greater than 6 standard deviations above the average response rate), and was also identified using Grubb’s test for statistical outliers. For the overall analysis of sign-tracking (i.e., lever contacts), there were main effects of Pairing and Day [F (1, 86) = 4.8, p < 0.05 and F (4, 344) = 5.0, p < 0.05, respectively], as well as Pairing × Day and Treatment × Day interactions [F (4, 344) = 5.4, p < 0.001 and F (4, 344) = 3.2, p < 0.05, respectively], but no other main effects or interactions. For goal-tracking (i.e., food-cup entries), there were main effects of Day [F (4, 344) = 5.5, p < 0.001] and a Pairing × Day interaction [F (4, 344) = 7.3, p < 0.001, respectively], but no other main effects or interactions.
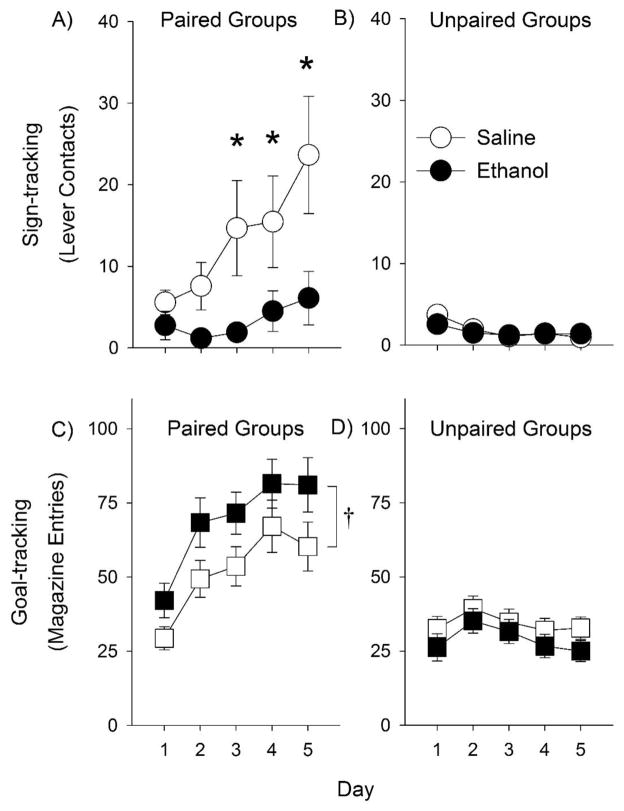
In the paired group, there was a main effect of Day and a Treatment × Day interaction for lever contacts [F (4, 212) = 8.3, p < 0.001 and F (4, 212) = 4.2, p < 0.01, respectively], but no main effect of Treatment. Post-hoc analyses indicated that nicotine significantly increased lever contacts on days 4 and 5 (Fig. 4A). There was an effect of Day and a Treatment × Day interaction for food-cup entries as well [F (4, 212) = 14.5, p < 0.05 and F (4, 212) = 2.5, p < 0.05, respectively], but no effect of Treatment. Despite the Treatment × Day interaction, post-hoc analyses did not reveal significant differences between nicotine and saline treated animals on any day of testing (Fig. 4C). In the unpaired group, there was a Treatment × Day interaction [Fig 4B; F (4, 132) = 4.9, p < 0.01], but no main effects. Post-hoc analyses indicated that nicotine significantly increased lever contacts on day 4 only (Fig. 4D). Together, these results suggest that nicotine increases sign-tracking but not goal-tracking when either paired or unpaired lever-cues are used, although this effect was more consistent across days in the paired group.
Fig. 4.
Nicotine enhances sign-tracking but not goal-tracking during Pavlovian conditioned approach training. Panels A–B show the number of lever contacts (sign-tracking) in paired and unpaired groups; nicotine or saline was injected 15 min before each session. Panels C–D show the number of food-cup entries (goal-tracking). Asterisks indicate significant differences between nicotine- and saline-treated rats. Data are presented as means +/− SEM.
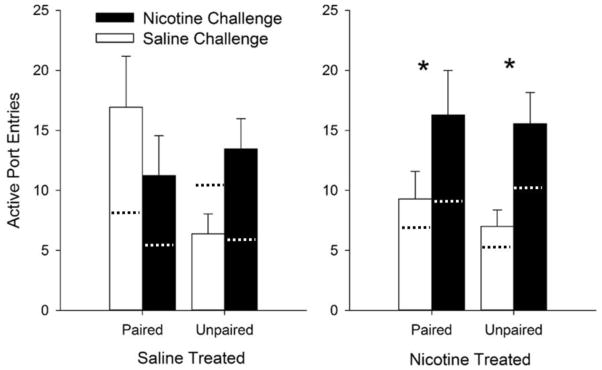
During the conditioned reinforcement test, the effect of nicotine on responding for the lever-CS depended on previous treatment with nicotine [Fig. 5; F (1, 82) = 5.4, p < 0.05 for the interaction between Treatment, Challenge, Pairing, and Port]. In addition, there was a main effect of Port [F (1, 82) = 19.4, p < 0.001, F (1, 82) = 4.1, p < 0.05; F (1, 82) = 5.0, p < 0.05], but no other main effects or interactions. Post-hoc analysis indicated that nicotine-challenged rats made more nose-pokes into the active port than saline-treated rats, but only in rats that had previously received nicotine (ps < 0.05).
Fig. 5.
Nicotine challenge increases the reinforcing efficacy of the lever-cue in paired and unpaired groups. Active port entries are compared in rats previously treated with nicotine or saline, subdivided into rats receiving a nicotine or saline challenge. Dotted lines indicate mean inactive port responses. Asterisks denote significant increases in nicotine- vs. saline-challenged groups (p < 0.05). Data are presented as means +/− SEM.
Experiment 3: Effects of ethanol on sign-tracking, goal-tracking, and conditioned reinforcement
For the overall analysis of lever contacts (i.e., sign-tracking), there were main effects of Pairing and Day [F (1, 88) = 5.5, p < 0.05 and F (4, 352) = 3.1, p < 0.05, respectively], and a Pairing × Day interaction [F (4, 352) = 5.1, p < 0.05], but no other main effects or interactions. For goal-tracking (i.e., food-cup entries), there were main effects of Pairing and Day [F (1, 88) = 27.1, p < 0.001 and F (4, 352) = 10.7, p < 0.001, respectively] and Treatment × Pairing and Pairing × Day interactions [F (1, 88) = 4.1, p < 0.05 and F (4, 352) = 11.2, p < 0.001, respectively], but no other main effects or interactions.
In the paired group, there were main effects of Treatment, Day, and a Treatment × Day interaction for lever contacts [F (1, 54) = 5.1, p < 0.05; F (4, 216) = 6.3, p < 0.001, and F (2, 216) = 2.6, p < 0.05, respectively]. Post-hoc analysis indicated that ethanol significantly decreased lever contacts on days 3–5 of conditioning in the paired group (Fig. 6A; ps < 0.05). For food-cup entries, there were main effects of Day and Treatment [F (4, 216) = 19.6, p < 0.001 and F (1, 54) = 4.0, p = 0.05, respectively], but there was no Day × Treatment interaction, indicating a general enhancing effect of ethanol on goal-tracking (Fig. 6B). In the unpaired group, there was an effect of Day for lever contacts [F (4, 136) = 5.3, p < 0.001] but not for food-cup entries. There were no main effects of Treatment or Treatment × Day interactions for either measure. Together, these results indicate that ethanol decreases sign-tracking and increases goal-tracking, but not when an unpaired stimulus is presented.
Fig. 6.
Ethanol decreases sign-tracking during Pavlovian conditioned approach. Panels A–B show the number of lever contacts (sign-tracking) in paired and unpaired groups; ethanol or saline was injected immediately before each session. Panels C–D show the number of food-cup entries (goal-tracking). Asterisks indicate significant differences between ethanol- and saline-treated rats (ps < 0.05). Dagger (†) denotes the main effect of Treatment (p = 0.05). Data are presented as means +/− SEM.
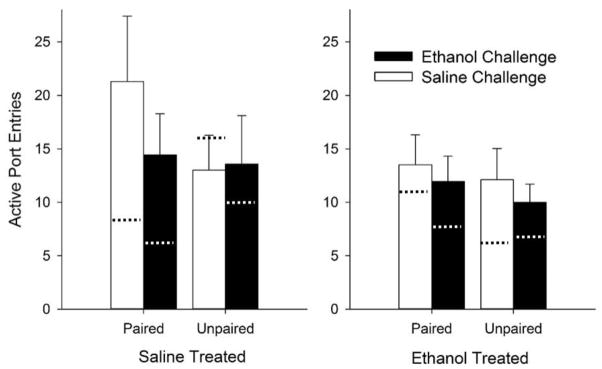
During the conditioned reinforcement test, there was a main effect of Port [F (1, 84) = 9.2, p < 0.01], demonstrating that the lever-cue was an effective reinforcer (Fig. 7). There were no other main effects or interactions, although previous treatment with ethanol tended to reduce responding during conditioned reinforcement in unpaired and paired groups, but this effect was not statistically significant (p = 0.06 for the Pretreatment × Pairing × Port interaction). Further, ethanol challenge did not alter responding (p > 0.05 for the main effects and interactions involving Challenge). These results suggest that ethanol does not substantially alter conditioned responding for a food cue.
Fig. 7.
Ethanol does not have significant effects on the reinforcing efficacy of the lever-cue. Active port entries are compared in rats previously treated with ethanol or saline, subdivided into rats receiving an ethanol or saline challenge on test day. Dotted lines indicate mean inactive port responses. Data are presented as means +/− SEM.
Discussion
In these experiments, we sought to determine whether the pattern of nicotine self-administration is related to cue-responsivity (i.e., in sign-trackers and goal-trackers), and whether nicotine and ethanol alter the incentive properties of cues. Our major findings show that sign-trackers are more sensitive to nicotine paired light cues during the cue-induced reinstatement model of relapse, and that nicotine enhances signtracking and conditioned reinforcing properties of a food cue. These experiments are consistent with the idea that nicotine acts as a reinforcement-enhancer or, more specifically, an incentive-enhancer, in that cues are more attractive, reinforcing, and motivate instrumental responding in rats that have been given nicotine (Yager and Robinson 2015; Palmatier et al. 2013; Bevins and Palmatier 2004). Here we show this effect in instrumental and Pavlovian paradigms, using nicotine and food cues, and in nicotine-exposed and nicotine free states. In addition, we show that ethanol does not have this reinforcement-enhancing effect during Pavlovian conditioning and conditioned reinforcement tests.
The incentive value of a reward stimulus can be measured by three related but dissociable properties: whether it elicits approach, reinforces new behaviors, or invigorates motivated responding. Each of these properties is operationalized using different experimental measures, respectively: 1) sign-tracking during PavCA, 2) active-port responding during a conditioned reinforcement test, and 3) active port responding during a cue-induced reinstatement test (Milton and Everitt 2010; Robinson et al. 2014; Cardinal et al. 2003). In the current study, we demonstrate that nicotine enhances the incentive value in all three of these tests. Caggiulla and others (Caggiula et al. 2009; Palmatier et al. 2007; Palmatier et al. 2006; Bevins and Palmatier 2004; Palmatier et al. 2013) have described three major effects of nicotine in terms of incentive value: 1) it is a weak primary reinforcer, in that rats will self-administer it at low rates in the absence of cues, 2) it can enhance the incentive value of cues via Pavlovian associations (i.e., conditioned reinforcement), and 3) it can enhance the incentive value of cues via non-associative mechanisms. In the current self-administration experiment, the nicotine-associated light cues acquired incentive value through Pavlovian associations, which then supported responding during the reinstatement test. We note that this test is used as a model for relapse to drug-seeking behavior (Gamaleddin et al. 2012; Liu et al. 2008; Epstein et al. 2006), but it also is a test of conditioned reinforcement, in which responding is maintained by the incentive value of the nicotine acquired through Pavlovian associations. However, nicotine can enhance incentive value through non-associative mechanisms as well, as discussed below.
Cue-maintained nicotine self-administration
In previous studies examining food and cocaine reinstatement, sign- and goal-trackers responded similarly during food and cocaine self-administration, although sign-trackers received slightly more food reinforcers (Yager and Robinson 2010). In our current nicotine self-administration study, sign- and goal-trackers did not differ during the FR1 schedule of reinforcement, but responded more for the nicotine and its associated light-cue during the FR5 schedule. This is likely due to the incentive-enhancing effect of nicotine that has been demonstrated in several papers showing that nicotine self-administration is reduced in the absence of cues, and that cues paired with nicotine can acquire incentive value (Caggiula et al. 2009; Palmatier et al. 2006; Yager and Robinson 2015). We confirmed this in the current study by including a cue removal test, during which responding for nicotine was reduced. However, unlike previous findings using a cocaine cue, sign- and goal-trackers did not differ during the cue removal test. This may be because this test was conducted during the FR1 phase of the experiment, during which responding was relatively low compared to the FR5 phase. Thus, it may be that sign/goal-tracker differences would have been evident if the cue-removal test was conducted during the FR5 schedule.
Cue-induced reinstatement
The difference between sign- and goal-trackers during the cue induced reinstatement test is consistent with other studies showing that sign-trackers are more sensitive to reinstatement induced by food and cocaine cues (Yager and Robinson 2010; Saunders and Robinson 2010). A recent study (Yager et al. 2015) also found that sign-trackers were also more sensitive to a light-cue predictive of a fast-acting opioid (remifentanil), although that study involved Pavlovian and conditioned reinforcement paradigms only, instead of the instrumental self-administration/reinstatement paradigm. Thus, sign-trackers are more sensitive to a broad class of non-drug and drug cues, including drugs acting directly on dopamine, opioid, and cholinergic systems. As discussed previously (Meyer et al. 2012a; Robinson et al. 2014), we propose that this is due to trait differences in the tendency to attribute incentive motivational value to reward cues, such that sign-trackers have the most extreme tendency compared to intermediate and goal-tracking individuals. The differences in this behavioral trait likely involve differences in the ability of food and drug cues to activate the dopamine system, because pharmacological manipulation of this system has been shown to alter the sign-tracking response to food cues, cocaine cues, and remifentanil cues (Flagel et al. 2011; Saunders and Robinson 2012; Yager et al. 2015), and in several forms of reinstatement (Lu et al. 2004; Shaham and Stewart 1996). It is possible that nicotine activates this system differentially in sign- and goal-trackers as well. In addition, one study has reported that cholinergic projections from the basal forebrain to the prefrontal cortex are differentially engaged in sign- and goal-trackers during tests of sustained attention (Paolone et al. 2013). Thus, the basal forebrain projections to the prefrontal cortex are a potential substrate for the incentive amplifying effects of nicotine.
Pavlovian conditioned approach
As described above, cues paired with nicotine during self-administration of the drug acquired incentive value via Pavlovian mechanisms. This leads to a relatively long-lasting enhancement of incentive value that can be observed during reinstatement testing. In contrast, the enhancement of sign-tracking by nicotine could not have been due to associations between the lever-cue and nicotine, because nicotine was given as a single injection before being placed in the chamber, and never explicitly paired with the lever-cue. Further, the effect of nicotine was not due to a general enhancement of some aspect of learning such as attention, otherwise nicotine would have enhanced goal-tracking behavior as well. Instead, the effect of nicotine was due either to a facilitation of incentive value attribution to the lever-cue, or to an acute effect of nicotine on incentive value. The increase in incentive value (as measured by sign-tracking) observed during the Pavlovian conditioning test did not result in an enhancement of conditioned reinforcement, in that nicotine pre-exposed animals did not show enhanced conditioned reinforcement, unless they were challenged with nicotine during the test. This suggests that the effect of nicotine is acute, in that the food cue’s incentive value is only enhanced in the presence of nicotine. Unexpectedly, nicotine did not enhance conditioned reinforcement in rats that had no history of nicotine treatment. Is it possible that nicotine is aversive initially, and then tolerance develops to the aversive effect concurrently with sensitization to its incentive-amplifying effect (Fowler and Kenny 2014; Guy and Fletcher 2013; Goldberg and Spealman 1983; see also Podlesnik et al. 2010 for discussion). We tried to ameliorate this by giving a single nicotine injection the day before the test, but perhaps this was insufficient to overcome the aversive effects of nicotine. That said, nicotine also enhanced the responding for an unpaired stimulus, which reflects the possibility that nicotine enhances the reinforcing properties of stimuli that have not been paired with reward as well, such as has been shown by others (Palmatier et al. 2013). The initial reinforcing properties of these stimuli are likely to be important for this effect, because nicotine and other stimulants may disrupt the habituation to the sensory reinforcement maintained by a variety of stimuli (Lloyd et al. 2012; 2014a; 2014b).
We also demonstrate that ethanol does not have the incentive-enhancing effect, because it reduced sign-tracking and did not alter conditioned reinforcement. The route of administration may be important in determining this effect. For example, non-ethanol-dependent rats typically do not readily self-administer ethanol or develop ethanol-induced place preference, unless the ethanol is administered intragastrically (Ciccocioppo et al. 1999), intracranially (Rodd et al. 2005), or the rats have had a long history of ethanol exposure (Sciascia et al. 2014; Carnicella et al. 2014; Simms et al. 2008). Further, ethanol reduced sign-tracking but increased goal-tracking, suggesting that the effect of ethanol was not due to a reduction in learning, rather a specific reduction in incentive attribution to the lever-cue. It is unknown whether a procedure that induces ethanol dependence, such as forced intake or vapor inhalation, renders rats sensitive to the incentive-enhancing effects of ethanol. Indeed, studies that have demonstrated sign-tracking in response to an ethanol cue have done so only after the rats had been extensively exposed to ethanol (Srey et al. 2015; Krank 2003). Thus, the incentive-decreasing effect of ethanol in the current study is not surprising given that this ethanol treatment is not reinforcing in rats. Thus, drugs that have opposite reinforcing effects (nicotine vs ethanol), also have contrasting effects on the incentive value of a food cue.
However, Tomie et al. (1998b) found that ethanol enhanced sign-tracking, as measured by approach probability. In that study, rats received daily 10% (v/v) ethanol injections (0, 0.25, 0.5, 0.7, or 1.0 g/kg) for five consecutive sessions (11–15). The lowest dose (0.25 g/kg) decreased sign-tracking, the while 0.5 and 0.7 g/kg doses increased sign-tracking. That study also included an unpaired group that received 0.5 g/kg ethanol while the lever was presented randomly with respect to the food. In this unpaired group, there was less sign-tracking compared to the 0.5 g/kg paired group, although sign-tracking was similar compared to the saline paired group. The source of the discrepancy between Tomie (1998b) and our study is unclear, but may be due to the rat strain used (Long-Evans vs. Sprague-Dawley), or the use of food-restricted vs. free-feeding rats, the number of ethanol exposures, and the measure of sign-tracking (total responses vs. probability). Further, in Tomie (1998b) the increase in sign tracking was relative to saline-injected rats, but was not evident when these doses of ethanol were compared to a ‘no-injection’ control. Thus, the injection procedure itself may have reduced sign-tracking probability, while the effect of ethanol may have been due to an anxiolytic effect, restoring sign-tracking levels to that of non-injected controls. Finally, it may be that, after extended testing, our ethanol-treated rats would have switched from goal-tracking to sign-tracking, as has been shown for ethanol cues (Srey et al. 2015). Thus, it may be that the food-restricted Long-Evans rats from Tomie et al. (1998b) had switched from goal- to sign-tracking more rapidly than our free-feeding Sprague-Dawley rats. However, Tomie et al. (1998b) did not report the levels of goal-tracking in their rats, so future studies are needed to investigate this possibility.
In conclusion, we demonstrate that nicotine enhances the incentive value of cues, and that some rats are particularly sensitive to the reinstatement-inducing effects of a nicotine cue. In this manner, nicotine may lead to addictive behavior by 1) capitalizing on the predisposition to respond to reward cues in general, and 2) further amplifying this tendency by enhancing the incentive value of cues through non-associative mechanisms. It is likely these effects of nicotine play important roles throughout the addictive process: first by enhancing nicotine-taking behavior, then by facilitating continued drug-taking in the presence of drug cues, and finally, by potentiating “incentive habits” that increase relapse. (Tiffany 1999; 1990; Everitt and Robbins 2016; Belin et al. 2013; Meyer et al. 2015; Piazza and Deroche-Gamonet 2013).
Acknowledgments
This research was supported by a grant from the Office of the Director, National Institutes of Health, and the National Institute on Alcohol Abuse and Alcoholism (AA024112).
The authors would like to thank Michael Demyan and Adam Rutz for their excellent technical support, and Dr. Carrie Ferrario for her comments on an earlier version of the manuscript.
Footnotes
The authors have no financial conflicts of interest to declare.
References
- Asin KE, Wirtshafter D, Tabakoff B. Failure to establish a conditioned place preference with ethanol in rats. Pharmacol Biochem Behav. 1985;22:169–73. doi: 10.1016/0091-3057(85)90372-7. [DOI] [PubMed] [Google Scholar]
- Bailey SR, Goedeker KC, Tiffany ST. The impact of cigarette deprivation and cigarette availability on cue-reactivity in smokers. Addiction. 2010;105:364–72. doi: 10.1111/j.1360-0443.2009.02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Belin-Rauscent A, Murray JE, Everitt BJ. Addiction: failure of control over maladaptive incentive habits. Curr Opin Neurobiol. 2013;23:564–72. doi: 10.1016/j.conb.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Palmatier MI. Extending the role of associative learning processes in nicotine addiction. Behav Cogn Neurosci Rev. 2004;3:143–58. doi: 10.1177/1534582304272005. [DOI] [PubMed] [Google Scholar]
- Boakes R. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz H, editors. Operant-Pavlovian interactions. Lawrence Erlbaum Associates; Hillsdale, NJ: 1977. pp. 67–97. [Google Scholar]
- Bormann NM, Cunningham CL. Ethanol-induced conditioned place aversion in rats: effect of interstimulus interval. Pharmacol Biochem Behav. 1998;59:427–32. doi: 10.1016/s0091-3057(97)00455-3. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 2002;163:230–7. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Marbini HD, Toner AJ, Bussey TJ, Robbins TW, Everitt BJ. Role of the anterior cingulate cortex in the control over behavior by Pavlovian conditioned stimuli in rats. Behav Neurosci. 2003;117:566–87. doi: 10.1037/0735-7044.117.3.566. [DOI] [PubMed] [Google Scholar]
- Carnicella S, Ron D, Barak S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol. 2014;48:243–52. doi: 10.1016/j.alcohol.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology (Berl) 2006a;189:27–36. doi: 10.1007/s00213-006-0522-0. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006b;184:353–66. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Froldi R, Quitadamo E, Massi M. Ethanol induces conditioned place preference in genetically selected alcohol-preferring rats. Psychopharmacology (Berl) 1999;141:235–41. doi: 10.1007/s002130050830. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Gorny G, Li Y, Kolb B, Robinson TE. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cereb Cortex. 2005;15:341–8. doi: 10.1093/cercor/bhh136. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Tull LE, Rindal KE, Meyer PJ. Distal and proximal pre-exposure to ethanol in the place conditioning task: tolerance to aversive effect, sensitization to activating effect, but no change in rewarding effect. Psychopharmacology (Berl) 2002;160:414–24. doi: 10.1007/s00213-001-0990-1. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology (Berl) 1998;136:83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annu Rev Psychol. 2016;67:23–50. doi: 10.1146/annurev-psych-122414-033457. [DOI] [PubMed] [Google Scholar]
- Fidler TL, Oberlin BG, Struthers AM, Cunningham CL. Schedule of passive ethanol exposure affects subsequent intragastric ethanol self-infusion. Alcohol Clin Exp Res. 2009;33:1909–23. doi: 10.1111/j.1530-0277.2009.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology 56 Suppl. 2009;1:139–48. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–7. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 2007;191:599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ. Nicotine aversion: Neurobiological mechanisms and relevance to tobacco dependence vulnerability. Neuropharmacology. 2014;76(Pt B):533–44. doi: 10.1016/j.neuropharm.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamaleddin I, Wertheim C, Zhu AZ, Coen KM, Vemuri K, Makryannis A, Goldberg SR, Le Foll B. Cannabinoid receptor stimulation increases motivation for nicotine and nicotine seeking. Addict Biol. 2012;17:47–61. doi: 10.1111/j.1369-1600.2011.00314.x. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD. Suppression of behavior by intravenous injections of nicotine or by electric shocks in squirrel monkeys: effects of chlordiazepoxide and mecamylamine. J Pharmacol Exp Ther. 1983;224:334–40. [PubMed] [Google Scholar]
- Guy EG, Fletcher PJ. Nicotine-induced enhancement of responding for conditioned reinforcement in rats: role of prior nicotine exposure and alpha4beta2 nicotinic receptors. Psychopharmacology (Berl) 2013;225:429–40. doi: 10.1007/s00213-012-2832-8. [DOI] [PubMed] [Google Scholar]
- Guy EG, Fletcher PJ. The effects of nicotine exposure during Pavlovian conditioning in rats on several measures of incentive motivation for a conditioned stimulus paired with water. Psychopharmacology (Berl) 2014;231:2261–71. doi: 10.1007/s00213-013-3375-3. [DOI] [PubMed] [Google Scholar]
- Hauser SR, Getachew B, Oster SM, Dhaher R, Ding ZM, Bell RL, McBride WJ, Rodd ZA. Nicotine modulates alcohol-seeking and relapse by alcohol-preferring (P) rats in a time-dependent manner. Alcohol Clin Exp Res. 2012;36:43–54. doi: 10.1111/j.1530-0277.2011.01579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearst E, Jenkins HM. Sign tracking: The stimulus-reinforcer relation and directed action. Proceedings of the Psychonomic Society; Austin, TX. 1974. [Google Scholar]
- King CP, Palmer AA, Woods LC, Hawk LW, Richards JB, Meyer PJ. Premature responding is associated with approach to a food cue in male and female heterogeneous stock rats. Psychopharmacology (Berl) 2016 doi: 10.1007/s00213-016-4306-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krank MD. Pavlovian conditioning with ethanol: sign-tracking (autoshaping), conditioned incentive, and ethanol self-administration. Alcohol Clin Exp Res. 2003;27:1592–8. doi: 10.1097/01.ALC.0000092060.09228.DE. [DOI] [PubMed] [Google Scholar]
- Krank MD, O’Neill S, Squarey K, Jacob J. Goal- and signal-directed incentive: conditioned approach, seeking, and consumption established with unsweetened alcohol in rats. Psychopharmacology (Berl) 2008;196:397–405. doi: 10.1007/s00213-007-0971-0. [DOI] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Palmatier MI, Donny EC, Sved AF. Cue-induced reinstatement of nicotine-seeking behavior in rats: effect of bupropion, persistence over repeated tests, and its dependence on training dose. Psychopharmacology (Berl) 2008;196:365–75. doi: 10.1007/s00213-007-0967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DR, Gancarz AM, Ashrafioun L, Kausch MA, Richards JB. Habituation and the reinforcing effectiveness of visual stimuli. Behav Processes. 2012;91:184–91. doi: 10.1016/j.beproc.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DR, Hausknecht KA, Richards JB. Nicotine and methamphetamine disrupt habituation of sensory reinforcer effectiveness in male rats. Exp Clin Psychopharmacol. 2014a;22:166–75. doi: 10.1037/a0034741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DR, Medina DJ, Hawk LW, Fosco WD, Richards JB. Habituation of reinforcer effectiveness. Front Integr Neurosci. 2014b;7:107. doi: 10.3389/fnint.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovic V, Saunders BT, Yager LM, Robinson TE. Rats prone to attribute incentive salience to reward cues are also prone to impulsive action. Behav Brain Res. 2011;223:255–61. doi: 10.1016/j.bbr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(Suppl 1):214–26. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Cogan ES, Robinson TE. The form of a conditioned stimulus can influence the degree to which it acquires incentive motivational properties. PLoS One. 2014;9:e98163. doi: 10.1371/journal.pone.0098163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, King CP, Ferrario CR. Motivational Processes Underlying Substance Abuse Disorder. Current topics in behavioral neurosciences. 2015 doi: 10.1007/7854_2015_391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS One. 2012a;7:e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Ma ST, Robinson TE. A cocaine cue is more preferred and evokes more frequency-modulated 50-kHz ultrasonic vocalizations in rats prone to attribute incentive salience to a food cue. Psychopharmacology (Berl) 2012b;219:999–1009. doi: 10.1007/s00213-011-2429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Everitt BJ. The psychological and neurochemical mechanisms of drug memory reconsolidation: implications for the treatment of addiction. Eur J Neurosci. 2010;31:2308–19. doi: 10.1111/j.1460-9568.2010.07249.x. [DOI] [PubMed] [Google Scholar]
- Nasser HM, Chen Y-w, Fiscella K, Calu DJ. Individual variability in behavioral flexibility predicts sign-tracking tendency. Frontiers in Behavioral Neuroscience. 2015:9. doi: 10.3389/fnbeh.2015.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 2004a;171:173–8. doi: 10.1007/s00213-003-1575-y. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 2004b;173:98–104. doi: 10.1007/s00213-003-1702-9. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl) 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Kellicut MR, Brianna Sheppard A, Brown RW, Robinson DL. The incentive amplifying effects of nicotine are reduced by selective and non-selective dopamine antagonists in rats. Pharmacol Biochem Behav. 2014;126:50–62. doi: 10.1016/j.pbb.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Marks KR, Jones SA, Freeman KS, Wissman KM, Sheppard AB. The effect of nicotine on sign-tracking and goal-tracking in a Pavlovian conditioned approach paradigm in rats. Psychopharmacology (Berl) 2013;226:247–59. doi: 10.1007/s00213-012-2892-9. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Matteson GL, Black JJ, Liu X, Caggiula AR, Craven L, Donny EC, Sved AF. The reinforcement enhancing effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated drug injections. Drug Alcohol Depend. 2007;89:52–9. doi: 10.1016/j.drugalcdep.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolone G, Angelakos CC, Meyer PJ, Robinson TE, Sarter M. Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. J Neurosci. 2013;33:8321–35. doi: 10.1523/JNEUROSCI.0709-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonet V. A multistep general theory of transition to addiction. Psychopharmacology (Berl) 2013;229:387–413. doi: 10.1007/s00213-013-3224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlesnik CA, Jimenez-Gomez C, Woods JH. A choice procedure to assess the aversive effects of drugs in rodents. J Exp Anal Behav. 2010;93:203–23. doi: 10.1901/jeab.2010.93-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009;65:869–73. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: Individual differences. Neuropharmacology. 2014;76(Part B):450–459. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, Murphy JM, Li TK, Lumeng L, McBride WJ. Chronic ethanol drinking by alcohol-preferring rats increases the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol. Alcohol Clin Exp Res. 2005;29:358–66. doi: 10.1097/01.alc.0000156127.30983.9d. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Johnson M. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacol Biochem Behav. 2000;67:71–81. doi: 10.1016/s0091-3057(00)00301-4. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biol Psychiatry. 2010;67:730–6. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci. 2012;36:2521–32. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, DiFeliceantonio AG, Foscue E, Glowacz S, Haseeb N, Wang N, Zhou C, Kuhn CM. Aversive effects of ethanol in adolescent versus adult rats: potential causes and implication for future drinking. Alcohol Clin Exp Res. 2010;34:2061–9. doi: 10.1111/j.1530-0277.2010.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciascia JM, Mendoza J, Chaudhri N. Blocking dopamine d1-like receptors attenuates context-induced renewal of pavlovian-conditioned alcohol-seeking in rats. Alcohol Clin Exp Res. 2014;38:418–27. doi: 10.1111/acer.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Effects of opioid and dopamine receptor antagonists on relapse induced by stress and re-exposure to heroin in rats. Psychopharmacology (Berl) 1996;125:385–91. doi: 10.1007/BF02246022. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–23. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srey CS, Maddux JM, Chaudhri N. The attribution of incentive salience to Pavlovian alcohol cues: a shift from goal-tracking to sign-tracking. Front Behav Neurosci. 2015;9:54. doi: 10.3389/fnbeh.2015.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. Cognitive concepts of craving. Alcohol Res Health. 1999;23:215–24. [PMC free article] [PubMed] [Google Scholar]
- Tomie A, Aguado AS, Pohorecky LA, Benjamin D. Ethanol induces impulsive-like responding in a delay-of-reward operant choice procedure: impulsivity predicts autoshaping. Psychopharmacology (Berl) 1998a;139:376–82. doi: 10.1007/s002130050728. [DOI] [PubMed] [Google Scholar]
- Tomie A, Cunha C, Mosakowski EM, Quartarolo NM, Pohorecky LA, Benjamin D. Effects of ethanol on Pavlovian autoshaping in rats. Psychopharmacology (Berl) 1998b;139:154–9. doi: 10.1007/s002130050700. [DOI] [PubMed] [Google Scholar]
- van der Kooy D, O’Shaughnessy M, Mucha RF, Kalant H. Motivational properties of ethanol in naive rats as studied by place conditioning. Pharmacol Biochem Behav. 1983;19:441–5. doi: 10.1016/0091-3057(83)90117-x. [DOI] [PubMed] [Google Scholar]
- Yager LM, Pitchers KK, Flagel SB, Robinson TE. Individual variation in the motivational and neurobiological effects of an opioid cue. Neuropsychopharmacology. 2015;40:1269–77. doi: 10.1038/npp.2014.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Robinson TE. Cue-induced reinstatement of food seeking in rats that differ in their propensity to attribute incentive salience to food cues. Behav Brain Res. 2010;214:30–4. doi: 10.1016/j.bbr.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Robinson TE. Individual variation in the motivational properties of a nicotine cue: sign-trackers vs. goal-trackers. Psychopharmacology (Berl) 2015;232:3149–60. doi: 10.1007/s00213-015-3962-6. [DOI] [PMC free article] [PubMed] [Google Scholar]