Abstract
We previously reported that apolipoprotein E (apoE) upregulates ATP-binding cassette transporter A1 (ABCA1) transcription through phosphatidylinositol 3-kinase (PI3K). Here we demonstrate that treatment of murine macrophages with human apoE3 enhanced Akt phosphorylation, and upregulated ABCA1 protein and mRNA expression. Inhibition of PI3K weakened apoE3-induced Akt phosphorylation, and ABCA1 protein and mRNA increase. In contrast, inhibition of Akt only diminished apoE-induced ABCA1 protein but not the mRNA level. Suppression of protein synthesis did not erase the ability of apoE3 to increase ABCA1 protein level. Further, apoE3 increased the resistance of ABCA1 protein to calpain-mediated degradation without affecting calpain activity. Treatment of macrophages with apoE3 selectively enhanced the phosphorylation of Akt1 and Akt2, but not Akt3. Knockdown of Akt1 or Akt2 increased and decreased ABCA1 protein level, respectively; while overexpression of these Akt isoenzymes caused changes in ABCA1 protein level opposite to those induced by knockdown of the corresponding Akt. These data imply that apoE3 guards against calpain-mediated ABCA1 degradation through Akt2.
Keywords: Apolipoprotein E, Akt isoenzymes, ATP-binding cassette transporter A1, calpain, protein degradation
1. Introduction
ABCA1 is a critical protein responsible for preventing excess cholesterol accumulation in cells [1]. ApoE is secreted freely by macrophages, which can aid in the removal of excess cholesterol in association with ABCA1 [2]. Recent studies from our laboratory suggest that apoE is able to upregulate ABCA1 expression via activation of a signaling pathway involving phosphatidylinositol 3-kinase (PI3K) [3,4,5,6]. Specifically, treatment of murine macrophages with apoE increased the mRNA and protein levels of ABCA1, and enhanced the phosphorylation of PI3K. Inhibition of PI3K diminished apoE-induced ABCA1 expression. Our studies also suggest that very low-density lipoprotein receptor (VLDLR), apoE receptor 2 (apoER2), and disabled-1 (dab1) are the upstream components, while PKCζ and Sp1 are the downstream components of PI3K in this pathway [3,4,5,6].
Akt is a downstream effector of PI3K. Thus, PI3K phosphorylates phosphoinositides to form phosphotidylinositol 3-phosphate and other phosphorylated phosphoinositides. These lipids bind both Akt and phosphoinositide-dependent protein kinase 1 (PDK1). This close spatial association allows PDK1 to phosphorylate Akt1, Akt2 and Akt3 on Threonine 308, 309 and 305, respectively [7]. In addition, PI3K has been reported to trigger the mammalian target of rapamycin complex 2 (mTORC2), which phosphorylates Akt at Ser-473 in Akt1, Ser-474 in Akt2, and Ser-472 in Akt3, stimulating the full enzymatic activity [8]. Despite their conserved regulatory mechanisms and sequence homology, Akt isozymes are known to differentially contribute to various cellular activities [9].
Besides regulation by transcriptional activation, ABCA1 expression is also controlled by protein degradation [10]. For example, binding of apoAI to ABCA1 has been shown to cause ABCA1 dephosphorylation at its PEST sequence (a sequence rich in proline, glutamic acid, serine, and threonine), and therefore stabilize ABCA1 from calpain-mediated degradation [11]. Data from the current report suggest that apoE3 is able to activate Akt in a PI3K-dependent manner. Akt upregulates ABCA1 expression by retardation of its protein decay but not by changing its mRNA level. The protective effect on ABCA1 protein is mediated by Akt2, but not Akt1 and Akt3, possibly by stabilizing ABCA1 against calpain-mediated proteolysis.
2. Materials and methods
2.1. Reagents
Human apoE3 (SRP4696), Calpain-1 (C6108) and cycloheximide (C7698) were purchased from Sigma-Aldrich (St. Louis, MO). Lipofectamine 2000 (#11668-027), Trizol (15596-026), siRNAs for control (#AM4611), Akt1 (#AM1670), Akt2 (#AM16708), and Akt3 (#AM16708) were purchased from Life Technologies (Carlbad, CA). Expression plasmids for Akt1 (EX-A0022-M02) and Akt2 (EX-X0037-M02) were purchased from Genecopoeia (Rockville, MD). Pepstatin A (sc-45036), lactacystin (sc-3575), ALLN (sc-221136), batimastat (sc-203833), leupeptin-HCl (sc-215242), aprotinin (sc-3595), Akt inhibitor X (sc-203811), Akt inhibitor XI (sc-221229), PI3K inhibitor LY294002, and antibodies against ABCA1 (sc-58219), β-actin (sc-47778), GAPDH (sc-32233), CD-MPR (sc-34573), p-Akt (sc-7985-R), Akt (sc-8312), and Sp1 (sc-14027) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody against p-Sp1 (T739) was purchased from Assay Biotechnology (Sunnyvale, CA). Antibodies against Akt isoforms (#9940S), pAkt1 (#9018S), and pAkt2 (#8599S) were purchased from Cell Signaling Technology (Danvers, MA). Antibody against pAkt3 (#bs-5209R) was purchased from One World Lab (San Diego, CA). Calpain activity kit (#4240-100) was purchased from Biovision (Milpitas, CA).
2.2. Western blotting
RAW 264.7 macrophages were cultured in 10% FBS to confluence, and treated with vehicle, Akt inhibitor X (4 μM), Akt inhibitor XI (600 nM), LY294002 (50 μM), and/or cycloheximide (100 μg/ml) for 2 h. Where indicated, apoE3 (10 μg/ml) or vehicle control was added for additional 2 h incubation. The cells were lysed using M-PER mammalian protein extraction reagent containing phosphatase and protease inhibitors. Lysate supernatant was resolved on 10% SDS-PAGE gels. Proteins were transferred to a PVDF membrane. After blocking with 3% fat-free milk, the membranes were incubated sequentially with primary and second antibodies. Immunoreactivity bands were visualized using ECL-plus chemiluminescence reagent (GE Healthcare–Amersham).
2.3. Quantitative RT-PCR
Macrophages were treated as described in the legend to Fig. 1. Total RNA was extracted from RAW 264.7 cells using Trizol reagent. The sample was then subjected to reverse transcription using a high capacity cDNA reverse transcription kit (Applied Biosystems). The resulting cDNAs were subjected to quantitative PCR. The following primers were used for amplification: ABCA1, forward: 5′-GGAGCCTTTGTGGAACTCCC-3, reverse: 5′-CGCTCTCTTCAGCCACTTTAG-3′; and GAPDH, forward: 5′-GAGCCAAAAGGGTCATCATC-3′, reverse: 5′-TAAGCAGTTGGTGGTGCAGG-3′.
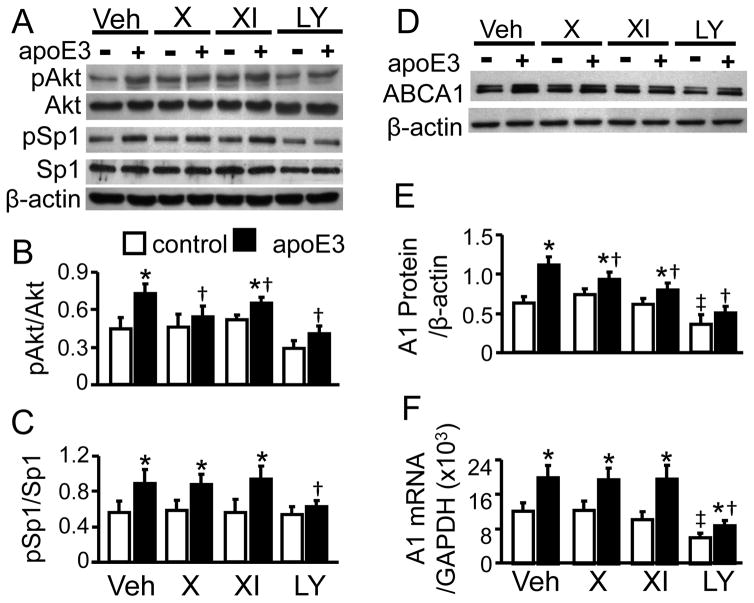
Figure 1. The Effects of Akt and PI3K Inhibitors on Akt and Sp1 Phosphorylation, and ABCA1 expression.
RAW 264. 7 macrophages were treated with 4 μM Akt inhibitor X (X), 600 nM Akt inhibitor XI (XI), 50 μM PI3K inhibitor LY294002 (LY), or vehicle medium (Veh) for 2 h, followed by 2 h treatment with 10 μg/ml apoE3 or without apoE3 (control). The proteins shown were detected by immunoblotting (A and D). (B–C) The levels of phosphorylated Akt (pAkt) and Sp1 (pSp1) were quantified relative to total Akt and total Sp1, respectively. (E) ABCA1 (A1) protein was quantified relative to β-actin. (F) ABCA1 mRNA was determined by RT-PCR, and quantified relative to GAPDH. The data represent mean ± SE of 4 independent experiments. * P< 0.05 vs. control, †P< 0.05 vs. vehicle medium in the presence of apoE3, and ‡ P<0.05 vs. vehicle medium control.
2.4. Calpain activity and calpain-1 degradation studies
Macrophages were treated as described in the legend to Fig. 2. Calpain activity was determined using a colorimetric assay kit (BioVision). Degradation by calpain-1 was performed as previously described [12], with the exception that calpain-treated or control cells were scrapped from plates after Na2EDTA was added to 20 mM, and the cells were lysed by incubation on ice for 20 minutes, with occasional mixing.
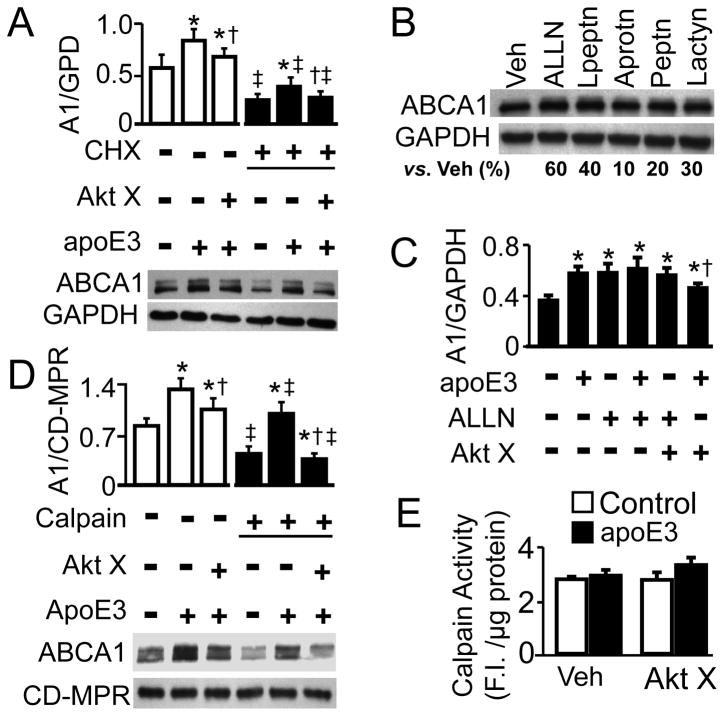
Figure 2. Role of Calpain in ApoE-Induced ABCA1 Protein.
(A) Macrophages were treated with 100 μg/ml cycloheximide (CHX) or vehicle medium in the presence or absence of 4 μM Akt inhibitor X (Akt X) for 2 h, followed by 2 h incubation with 10 μg/ml apoE3 or medium alone. (B) Macrophages were treated with vehicle medium (Veh), 50 μM ALLN, 1 mM leupeptin (Lpeptn), 10 μM aprotinin (Aprotn), 20μM pepstatin (Peptn), or 5 μM lactacystin (Lactyn) for 2 h. (C) Macrophages were treated with 50 μM ALLN or vehicle medium in the presence or absence of 4 μM Akt inhibitor X (Akt X) for 2 h, followed by 2 h incubation with 10 μg/ml apoE3 or control medium. (D) Macrophages were treated with or without 4 μM Akt inhibitor X (Akt X) for 2 h, followed by 2 h treatment with 10 μg/ml apoE3 or control medium alone. The cells were then treated with 3 μg/ml of calpain-1 or vehicle medium. ABCA1 (A1) protein was detected by immunoblotting, and quantified relative to GAPDH (A–C) or CD-MPR (D). (E) Macrophages were treated with 4 μM Akt X or vehicle medium for 2 h, followed by 2 h treatment with 10 μg/ml apoE3 or control medium. Calpain activity was measured with a calpain activity kit. Data represent mean ± SE of 3–4 independent experiments. (A) * P< 0.05 vs. cells without apoE3 treatment in the vehicle or CHX treatment group, respectively; † P< 0.05 vs. cells treated with apoE3 but without Akt X in the vehicle or CHX group, respectively; and ‡P< 0.05 vs. similarly treated cells without CHX. (C) * P< 0.05 vs. cells treated with medium alone; and †P< 0.05 vs. cells treated with apoE3 alone. (D) * P< 0.05 vs. cells without apoE3 treatment in the vehicle or calpain treatment group; †P< 0.05 vs. cells treated with apoE3 but without Akt X in the vehicle or calpain group, respectively; and ‡ P< 0.05 vs. similarly treated cells without calpain.
2.5. Knockdown and overexpression of Akt Isoenzymes
For knockdown of Akt, macrophages were transfected with 300 pM negative control siRNA or siRNAs specific to Akt1, Akt2, or Akt3 by modification of the electroporation procedure previously described [13]. Specifically, siRNAs in water were mixed with equal volume of buffer: (100 mM KH2P04, pH 7.5; 100 mM HEPES, pH 7.5; 500 mM sucrose; 20 mM EGTA, pH 8.8; 20 mM MgCl2, and 20 mM glutathione). After transfection, cells were incubated in 10% FBS for 48 h. For overexpression of Akt, macrophages cells were transfected with 7.5 μg of empty vector, Akt1, Akt2, or Akt3 expression plasmid as done under knockdown conditions.
2.6. Statistical analysis
Data are reported as the mean ± SE. Differences among control and treatment groups were analyzed by Student’s unpaired t-test (for two groups) and one-way or multiple factor analysis of variance (for more than two groups) followed by Tukey’s post-hoc test. Statistical significance was considered when P was less than 0.05.
3. Results
3.1. Akt suppression inhibits apoE3-induced ABCA1 protein but not mRNA
Fig. 1A–B show that apoE is able to activate Akt, i.e., stimulation of macrophages with apoE3 increased Akt phosphorylation by ~62%, compared to untreated control cells. Pretreatment of the cells with PI3K inhibitor LY294002 blocked apoE3-induced Akt phosphorylation. These results, together with our previous findings that apoE3 enhanced PI3K phosphorylation [5], suggest that Akt is downstream of PI3K in apoE-induced signal transduction.
It has been reported that phosphorylation of Sp1 at Thre739 enhances its transcriptional activity [14]. The Sp1 antibody used in this study specifically determines the phosphorylation of Sp1 Thr739. Data in Fig. 1A and 1C show that apoE3 treatment enhanced Sp1 phosphorylation by ~45%. Inhibition of PI3K blocked apoE-induced Sp1 phosphorylation. These results are consistent with our previous report that activation of Sp1 is a mechanism by which PI3K regulates apoE-induced ABCA1 expression [3,5]. Here, we tested the causative role of Akt in apoE-induced Sp1 phosphorylation, using two Akt inhibitors. It is known that Akt inhibitor X suppresses the kinase activity of Akt by inhibiting its phosphorylation [15], while Akt inhibitor XI inhibits Akt activity by interaction with its kinase and pleckstrin homology (PH) domains [16]. As Fig. 1A and 1B show, Akt inhibitors X and XI lessened apoE-induced Akt phosphorylation by ~71 and 58%, respectively. However, none of the Akt inhibitors significantly affected the phosphorylation level of Sp1.
Fig. 1D–E show that ABCA1 protein and mRNA levels are increased by ~77 and 64%, respectively, due to apoE3. Inhibition of PI3K significantly lowered both the mRNA and protein levels of ABCA1 under control and apoE3 treatment conditions. In contrast, the effect of Akt inhibitors on ABCA1 mRNA and protein varied. Specifically, inhibition of Akt significantly diminished apoE3-induced ABCA1 protein, but did not alter its mRNA level. As the data in Fig. 1D–E show, the apoE3-induced ABCA1 protein level dropped by ~26 and 30% in cells treated with Akt inhibitors X and XI, respectively. In contrast, the ABCA1 mRNA induced by apoE3 stayed the same in the presence or absence of Akt inhibitors. Treatment with Akt inhibitors X and XI did not significantly change the steady-state level of ABCA1 mRNA and protein.
3.2. ApoE3 increases the resistance of ABCA1 protein to protease-mediated degradation
Having ruled out a regulatory role of Akt in apoE3-induced ABCA1 mRNA expression, we then studied the effect of apoE3 and Akt inhibitor on ABCA1 protein expression in the presence of translational inhibitor cycloheximide (CHX). Under this condition, protein synthesis is inhibited by ~98% [17], and the level of cellular proteins is regulated primarily by proteolytic degradation. The data in Fig. 2A show that treatment of macrophages with CHX significantly reduced ABCA1 protein, as compared to vehicle treatment. However, CHX did not prevent the ability of apoE3 to increase ABCA1 expression, i.e., apoE3 significantly increased ABCA1 protein level in CHX-treated cells. Akt inhibitor X blocked apoE3-induced ABCA1 protein in these cells.
Next we studied the involvement of proteases in ABCA1 degradation by treating cells with various protease inhibitors, including cysteine protease inhibitor ALLN, serine and cysteine protease inhibitor leupeptin, serine protease inhibitor aprotinin, aspartyl protease inhibitor pepstatin A, and proteasome protease inhibitor lactacystin. Data in Fig. 2B show that all the protease inhibitors tested more or less increased ABCA1 protein level in RAW 246.7 cells. The highest increases resulted from ALLN and leupeptin, i.e., the ABCA1 protein level was ~60 and 40% higher in cells treated with ALLN or leupeptin, respectively, than in vehicle medium-treated cells. Both ALLN and leupeptin are able to inhibit calpain activity. Fig. 2C shows that treatment of cells with both ALLN and apoE3 failed to induce greater increase in ABCA1 protein level as compared to the treatment with either apoE3 or ALLN alone. Akt inhibitor X did not significantly affect ALLN-increased ABCA1 protein level, despite significantly inhibiting the upregulatory effect of apoE3 on ABCA1 protein.
It has been suggested that helical apolipoproteins stabilize ABCA1 against calpain-mediated degradation [12]. The data in Fig. 2D show that apoE3 reduced the susceptibility of ABCA1 to exogenous calpain, and Akt inhibitor attenuated the protective role of apoE3 against calpain-mediated ABCA1 degradation. Specifically, incubation with 4 nM calpain for 20 min resulted in ~27, 67, and 49% decrease in ABCA1 protein level, respectively, in cells treated with apoE3, Akt inhibitor X plus apoE3, or control medium alone. On the other hand, neither apoE3 nor Akt inhibitor X significantly changed endogenous calpain activity (Fig. 2E).
3.3. Akt isoform-dependent regulation of apoE3-induced ABCA1 protein expression
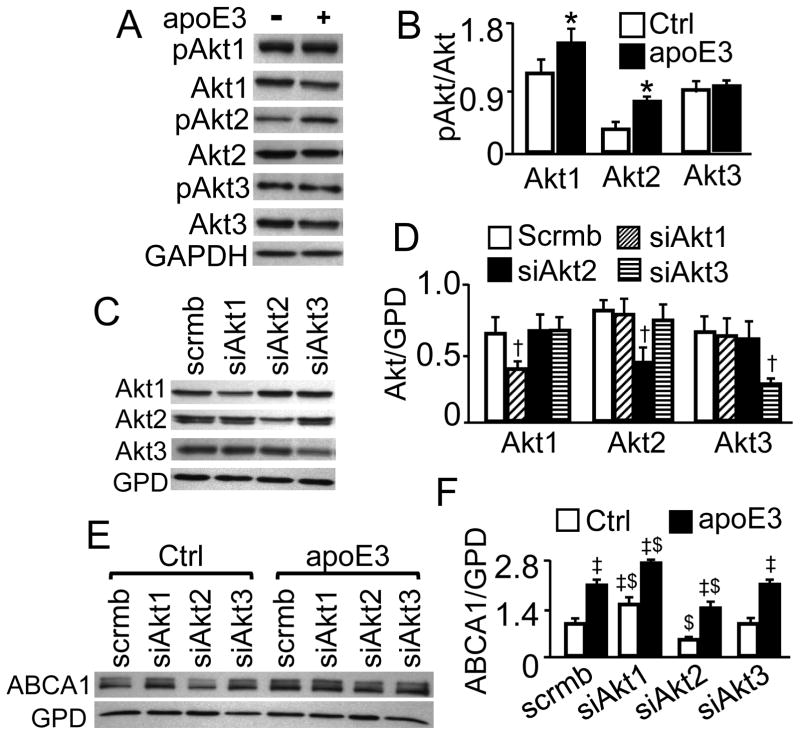
As shown in Fig. 3A–B, murine macrophages express all the three isozymes of Akt. Treatment with apoE3 for 2 h enhanced the phosphorylation of Akt1 and Akt2 by 45 and 80%, respectively. However, the same treatment did not alter the phosphorylation level of Akt3.
Figure 3. Effect of ApoE3 on Akt Isoform Phosphorylation, and effect of Akt Isoform Knockdown on ABCA1 Protein.
(A–B) Macrophages were treated with apoE3 or medium alone as a control (Ctrl) for 2 h. (C–F) Macrophages were transfected with Akt1, Akt2 or Akt3 siRNA (respectively designated as siAkt1, siAkt2 and siAkt3), or scrambled siRNAs (scrmb), and treated with apoE3 or culture medium alone (Ctrl) for 2 h. Phosphorylated Akt (pAkt), total Akt and ABCA1 were detected by immunoblotting, and quantified relative to total Akt (B) and GAPDH (GPD) (D and F), respectively. The data represent mean ± SE of 4–5 independent experiments. * P< 0.05 vs. control, † P<0.05 vs. cells transfected with scrambled siRNA, ‡ P<0.05 vs. control, and $ P<0.05 vs. scrambled siRNA-transfected cells treated with control medium or apoE3.
To address the involvement of Akt isoforms in ABCA1 expression, RAW 264.7 macrophages were transfected with Akt1, Akt2 or Akt3 siRNA. Data in Fig. 3C–D show that these isoform-specific siRNAs reduced the protein level of Akt1, Akt2 and Akt3 by ~40, 56 and 40%, respectively, compared to transfection with scrambled siRNA. Fig. 3E–F show that the ABCA1 protein level in Akt1 siRNA-transfected cells was ~62 and 31% higher than in scrambled siRNA-transfected cells under control and apoE3 treatment conditions, respectively. In contrast, the ABCA1protein level in cells transfected with Akt2 siRNAs was ~49 and 33% lower than in those transfected with scrambled siRNAs in the absence and presence of apoE3, respectively. Knockdown of Akt3 did not result in significant change in ABCA1 protein levels in apoE3-treated nor untreated cells.
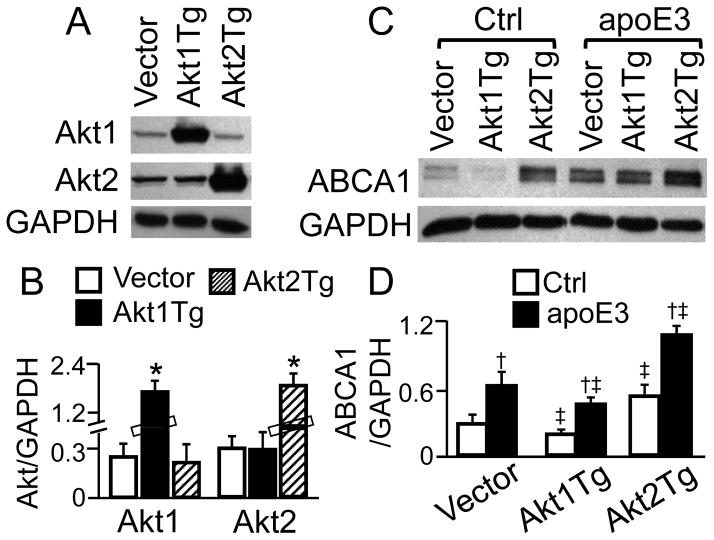
We also determined the effect of Akt isozyme overexpression on ABCA1 protein expression. As can be seen in Fig. 4A–B, transfection of macrophages with Akt1 and Akt2 expression plasmids elevated the protein level of Akt1 and Akt2 by ~5.2 and 4.7 fold, respectively, compared to transfection with empty vector. The data in Fig. 4C–D show that the ABCA1 protein level in macrophages transfected Akt1 expression plasmid was ~33 and 27% lower than in those transfected with empty vectors under control and apoE treatment conditions, respectively. On the other hand, the ABCA1 protein level in Akt2 overexpression cells was ~89 and 80% higher than in apoE3-treated and untreated cells, respectively.
Figure 4. Effect of Akt Isoform Overexpression on ABCA1 Protein.
Macrophages were transfected with Akt1 or Akt2 expression vectors (respectively designated as Akt1Tg and Akt2Tg) or empty vectors (A–B). The plasmid-transfected cells were treated with apoE3 or control medium (Ctrl) for 2 h (C–D). Akt1/2 and ABCA1 were detected by immunoblotting, quantified relative to GAPDH. Data represent the mean ± SEM of 3–4 independent experiments. * P<0.05 vs. cells transfected with empty vector; †P<0.05 vs. control; and ‡ P<0.05 vs. empty vector-transfected cells treated with control medium or apoE3.
4. Discussion
We have previously reported that apoE acts through PI3K to activate Sp1, which in turn accelerates ABCA1 transcription [4,5,6]. Here we demonstrated that treatment of RAW 264.7 macrophages with human apoE3 enhanced Akt and Sp1 phosphorylation, and raised ABCA1 mRNA and protein levels. Inhibition of PI3K diminished apoE3-induced Akt and Sp1 phosphorylation, and lowered ABCA1 mRNA and protein levels. In contrast, inhibition of Akt did not alter Sp1 phosphorylation or ABCA1 mRNA expression induced by apoE3. These observations suggest that apoE3 activates Akt through a pathway involving PI3K; however, activation of Akt is not required for apoE3 to trigger Sp1 and upregulate ABCA1 mRNA expression. Data from the present report also demonstrated that apoE3 is able to raise ABCA1 protein level in the presence of translational inhibitor cycloheximide. Inhibition of Akt diminished apoE3-induced ABCA1 protein expression in the presence or absence of cycloheximide. Thus, apoE3 elevates ABCA1 protein not only by upregulation of ABCA1 mRNA expression but also by retardation of its protein degradation. Activation of Akt is a step in the signaling cascade through which apoE3 retards ABCA1 protein degradation.
Calpain has previously been implicated in degradation of ABCA1 [11,18]. Consistent with this, our data demonstrate that ALLN and leupeptin induced greater increase in the basal steady-state ABCA1 protein level than other protease inhibitors. As a thiol protease, calpain is inhibited by both ALLN and leupeptin. ApoE3 did not further increase ABCA1 protein level in ALLN-pretreated cells. The failure of the combined apoE3 and ALLN to increase ABCA1 beyond either one of them alone suggests that the two act through a common intermediate. Namely, apoE3 upregulates ABCA1 expression likely by inhibiting calpain-mediated protein degradation. Indeed, our studies show that apoE3 reduced ABCA1 degradation by calpain, i.e., ABCA1 degradation induced by exogenous calpain was significantly less in apoE3-treated cells than in untreated control cells. Inhibition of Akt weakened the protective effect of apoE3 against calpain-mediated degradation. However, apoE3 did not alter endogenous calpain activity. Taken together, we suggest that activation of Akt by apoE3 protects against calpain-mediated ABCA1 degradation by increasing its stability but not by inhibiting calpain activity.
Another important finding in this report is that the regulatory effect of Akt on ABCA1 expression is isoform dependent. Specifically, we observed that apoE3 enhanced the phosphorylation of Akt1 and Akt2, but not Akt3. The antibodies used in this report specifically detect the phosphorylation of serine 473, 474 and 472 of Akt1, Akt2 and Ak3, respectively. These serine residues are phosphorylated by mTORC2 [8], a protein complex localized primarily to the endoplasmic reticulum (ER) [19]. It has been suggested that phosphorylation by PDK1 is a prerequisite for Akt to translocate to the ER [20]. It is possible that apoE3 enhances the phosphorylation of Akt1/2 by PDK1, and thus increases the translocation to the ER, with subsequent phosphorylation by mTORC2. Our data also demonstrated that knockdown of Akt2 reduced ABCA1 protein level, while overexpression of Akt2 elevated ABCA1 protein regardless of the absence or presence of apoE3. Knockdown or overexpression of Akt1 resulted in ABCA1 levels opposite to that of Akt2. These findings suggest that Akt1 downregulates and Ak2 upregulates ABCA1 protein expression in both basal and apoE3-induced states. Previous studies have shown Akt isoform-specific regulation on a variety of cellular activities, such as glucose metabolism and tumorigenesis. Several potential models have been proposed to describe the functional specificity of Akt isoforms, e.g., tissue-specific distribution and distinct substrates of the Akt isoforms [9]. Over 100 substrate candidates have been reported so far, but for most of them, no isoform specificity has been reported. Data from this report show that RAW 264.7 cells express all the three Akt isozymes. Thus, tissue-specific isoform expression could not account for the Akt isoform-dependent regulation on ABCA1 protein expression.
Distinct subcellular location of Akt isoforms has been suggested to be a reason for their functional specificity. Namely, different isoforms of fully activated Akt distribute to distinct subcellular compartments, where they trigger diverse downstream signal pathways and result in isoform-specific regulation. For example, preferential accumulation of Akt2, relative to Akt1, to the plasma membrane has been suggested to be primary in determining Akt2-specific control of insulin-induced translocation of GLUT4 from the cytoplasm to the plasma membrane [21], although insulin is able to activate both Akt1 and Akt2 [22]. The current study did not determine the subcellular location of Akt isoforms. However, our data suggest that apoE3 is also able to enhance the phosphorylation level of both Akt1 and Akt2, and activation of Akt2 is primary for the increased ABCA1 protein level by apoE3.
In summary, data from the present report suggest that apoE3 upregulates ABCA1 expression via two pathways, i.e., acceleration of ABCA1 transcription via an Akt-independent pathway, and retardation of ABCA1 proteolytic degradation through an Akt2-dependent pathway.
Apolipoprotein E3 (apoE3) enhances Akt1 and Akt2 phosphorylation
Inhibition of Akt diminishes apE3-induced ABCA1 protein but not mRNA
ApE3-increased ABCA1 protein is reduced by Akt2 but not by Akt1 knockdown
Acknowledgments
Funding: This study was supported by NIH grants SC1HL101431, G12 MD007586, the Vanderbilt CTSA grant UL1 TR000445 from NCATS/NIH; and Meharry MeTRC grant U54MD0007593.
Footnotes
Conflicts
The authors have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Serfaty-Lacrosniere C, Civeira F, Lanzberg A, Isaia P, Berg J, Janus ED, Smith MP, Jr, Pritchard PH, Frohlich J, Lees RS, et al. Homozygous Tangier disease and cardiovascular disease. Atherosclerosis. 1994;107:85–98. doi: 10.1016/0021-9150(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 2.Zhang WY, Gaynor PM, Kruth HS. Apolipoprotein E produced by human monocyte-derived macrophages mediates cholesterol efflux that occurs in the absence of added cholesterol acceptors. J Biol Chem. 1996;271:28641–28646. doi: 10.1074/jbc.271.45.28641. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Zhao Y, Guo Z, Zhou L, Okoro EU, Yang H. Transcriptional regulation of ATP-binding cassette transporter A1 expression by a novel signaling pathway. J Biol Chem. 2011;286:8917–8923. doi: 10.1074/jbc.M110.214429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X, Guo Z, Okoro EU, Zhang H, Zhou L, Lin X, Rollins AT, Yang H. Up-regulation of ATP binding cassette transporter A1 expression by very low density lipoprotein receptor and apolipoprotein E receptor 2. J Biol Chem. 2012;287:3751–3759. doi: 10.1074/jbc.M111.310888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okoro EU, Zhao Y, Guo Z, Zhou L, Lin X, Yang H. Apolipoprotein E4 is deficient in inducing macrophage ABCA1 expression and stimulating the Sp1 signaling pathway. PLoS One. 2012;7:e44430. doi: 10.1371/journal.pone.0044430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Y, Chen X, Yang H, Zhou L, Okoro EU, Guo Z. A novel function of apolipoprotein E: upregulation of ATP-binding cassette transporter A1 expression. PLoS One. 2011;6:e21453. doi: 10.1371/journal.pone.0021453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leevers SJ, Vanhaesebroeck B, Waterfield MD. Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr Opin Cell Biol. 1999;11:219–225. doi: 10.1016/s0955-0674(99)80029-5. [DOI] [PubMed] [Google Scholar]
- 8.Razmara M, Heldin CH, Lennartsson J. Platelet-derived growth factor-induced Akt phosphorylation requires mTOR/Rictor and phospholipase C-gamma1, whereas S6 phosphorylation depends on mTOR/Raptor and phospholipase D. Cell Commun Signal. 2013;11:3. doi: 10.1186/1478-811X-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez E, McGraw TE. The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle. 2009;8:2502–2508. doi: 10.4161/cc.8.16.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang N, Tall AR. Regulation and mechanisms of ATP-binding cassette transporter A1-mediated cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003;23:1178–1184. doi: 10.1161/01.ATV.0000075912.83860.26. [DOI] [PubMed] [Google Scholar]
- 11.Martinez LO, Agerholm-Larsen B, Wang N, Chen W, Tall AR. Phosphorylation of a pest sequence in ABCA1 promotes calpain degradation and is reversed by ApoA-I. J Biol Chem. 2003;278:37368–37374. doi: 10.1074/jbc.M307161200. [DOI] [PubMed] [Google Scholar]
- 12.Wang N, Chen W, Linsel-Nitschke P, Martinez LO, Agerholm-Larsen B, Silver DL, Tall AR. A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoA-I. J Clin Invest. 2003;111:99–107. doi: 10.1172/JCI16808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okoro EU, Zhang H, Guo Z, Yang F, Smith C, Jr, Yang H. A Subregion of Reelin Suppresses Lipoprotein-Induced Cholesterol Accumulation in Macrophages. PLoS One. 2015;10:e0136895. doi: 10.1371/journal.pone.0136895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milanini-Mongiat J, Pouyssegur J, Pages G. Identification of two Sp1 phosphorylation sites for p42/p44 mitogen-activated protein kinases: their implication in vascular endothelial growth factor gene transcription. J Biol Chem. 2002;277:20631–20639. doi: 10.1074/jbc.M201753200. [DOI] [PubMed] [Google Scholar]
- 15.Thimmaiah KN, Easton JB, Germain GS, Morton CL, Kamath S, Buolamwini JK, Houghton PJ. Identification of N10-substituted phenoxazines as potent and specific inhibitors of Akt signaling. J Biol Chem. 2005;280:31924–31935. doi: 10.1074/jbc.M507057200. [DOI] [PubMed] [Google Scholar]
- 16.Barve V, Ahmed F, Adsule S, Banerjee S, Kulkarni S, Katiyar P, Anson CE, Powell AK, Padhye S, Sarkar FH. Synthesis, molecular characterization, and biological activity of novel synthetic derivatives of chromen-4-one in human cancer cells. J Med Chem. 2006;49:3800–3808. doi: 10.1021/jm051068y. [DOI] [PubMed] [Google Scholar]
- 17.Chow SC, Peters I, Orrenius S. Reevaluation of the role of de novo protein synthesis in rat thymocyte apoptosis. Exp Cell Res. 1995;216:149–159. doi: 10.1006/excr.1995.1019. [DOI] [PubMed] [Google Scholar]
- 18.Arakawa R, Yokoyama S. Helical apolipoproteins stabilize ATP-binding cassette transporter A1 by protecting it from thiol protease-mediated degradation. J Biol Chem. 2002;277:22426–22429. doi: 10.1074/jbc.M202996200. [DOI] [PubMed] [Google Scholar]
- 19.Boulbes DR, Shaiken T, Sarbassov dD. Endoplasmic reticulum is a main localization site of mTORC2. Biochem Biophys Res Commun. 2011;413:46–52. doi: 10.1016/j.bbrc.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Betz C, Hall MN. Where is mTOR and what is it doing there? J Cell Biol. 2013;203:563–574. doi: 10.1083/jcb.201306041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez E, McGraw TE. Insulin-modulated Akt subcellular localization determines Akt isoform-specific signaling. Proc Natl Acad Sci USA. 2009;106:7004–7009. doi: 10.1073/pnas.0901933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang ZY, Zhou QL, Coleman KA, Chouinard M, Boese Q, Czech MP. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc Natl Acad Sci USA. 2003;100:7569–7574. doi: 10.1073/pnas.1332633100. [DOI] [PMC free article] [PubMed] [Google Scholar]