Abstract
Background
There is a paucity of research examining the impact of patient weight after heart transplant (HT) on multiple clinical outcomes.
Objectives
The purpose of this study was to compare 9 clinical outcomes in 2 groups of HT recipients (N = 347) from 2 hospitals in the midwestern and southern United States, based on their mean body mass index (BMI) during the first 3 years post-HT, and to identify risk factors for mortality.
Methods
The sample was divided into 2 groups: Group 1: 108 non-overweight patients (BMI <25; mean age 52; 29.6% females; 16.7% minorities), and Group 2: 239 overweight patients (BMI ≥25; mean age 52; 15.9% females; 13.8% minorities). Outcomes examined were: survival, re-hospitalization, rejections, infections, cardiac allograft vasculopathy (CAV), stroke, renal dysfunction, diabetes, and lymphoma.
Results
Non-overweight patients had shorter survival, were re-hospitalized more days after the HT discharge, and had more lymphoma and severe renal dysfunction. Overweight patients had more CAV, steroid-induced diabetes, and acute rejections. Mortality risk factors were: higher prednisone dose, higher cholesterol, earlier IV-treated infection, severe renal dysfunction, respiratory failure, and female patient.
Conclusions
Overweight HT patients had better survival, but more rejections, CAV, and diabetes. Non-overweight HT patients had worse survival, plus more re-hospitalization time, lymphoma, and renal dysfunction.
Keywords: Overweight heart transplant patients, Obese heart transplant patients, Heart transplant outcomes
Introduction
The deleterious effects of higher pre-transplant patient weight on several clinical outcomes after heart transplantation (HT) have been documented by HT registry data from the International Society for Heart and Lung Transplantation (ISHLT),1 as well as in studies by the research teams of Kilic,2 Guisado,3 Russo,4 Almenar,5 Lietz,6 and Grady.7,8 In addition, some researchers have investigated the negative impact of higher post-transplant patient weight on post-HT outcomes, but usually only one clinical outcome was reported in each study that related to the influence of heavier post-HT patient weight on outcomes.9–19
For example, analyses from the ISHLT9 and UNOS10 registries and research by Augustine et al11 found worse survival in heavier HT recipients. Higher post-HT BMI was also related to the development of post-transplant cardiac allograft vasculopathy (CAV)12–15 and steroid-induced diabetes,16,17 as well as graft failure. 18 In addition, Grady et al19 reported more episodes of acute rejection in heavier post-HT patients.
Therefore, this research compared 9 clinical outcomes in 2 weight groups of HT recipients (non-overweight vs overweight) during the first 3 years after HT surgery, and also identified risk factors for decreased survival.
Methods
Data source
The data for this report was derived from our 10-year prospective NIH study (1987–1997) that examined medical, physical, and psychosocial factors that can impact on multiple HT outcomes at various time points both pre-operatively and post-operatively. The study sample for this report consisted of 347 adult HT recipients (18 or older) from 2 hospitals in the midwestern and southern United States.
Data collection in parent study
In the parent study, patients were followed at standardized intervals pre-operatively while they were on the HT waiting list, and then post-operatively for up to 5 years after surgery (depending on how soon they were transplanted and how long they were in the study after surgery before funding ended).
At each of the pre-operative and post-operative time points in the parent study, comprehensive medical data was collected from patients’ charts by nurses experienced in cardiac care. In addition, at each time point patients completed a study booklet of 9 questionnaires pre-operatively and 10 questionnaires post-operatively.
The booklet questionnaires included the following physical and psychosocial factors that can influence HT outcomes: symptom distress, functional ability, work status, satisfaction with the HT outcome, compliance with the HT regimen, perceived helpfulness of HT team interventions, HT-related stressors, coping behavior, social support resources, and quality of life. (Some of the findings from the parent study are reported in Refs. 20–36.)
Before agreeing to participate in the study, patients were given the opportunity to review the pre-operative study booklet so they would know what was required of study participants. In addition, patients were paid $10 for each booklet they completed. Patients signed a consent form for study participation, and the study was approved by the Institutional Review Board at each site.
Data used for current report
The medical records data for the pre-operative period while patients were on the HT waiting list and for the first 3 years after HT were used in this analysis. Medical data was collected for the study every 3 months while patients were waiting for a heart donor, then 1, 3, 6, 9, and 12 months after surgery, and then every 6 months post-HT for years 2 and 3, and covered the entire study period.
Medical data included: baseline characteristics, pre-operative and post-operative medical and surgical history, post-HT complications, causes of death, lab test results, medications (immunosuppressant and other), hospitalizations (dates, duration, reason), and donor data. (Note: Data collection and data reliability verification procedures for this NIH study have been described in previous reports.33–36)
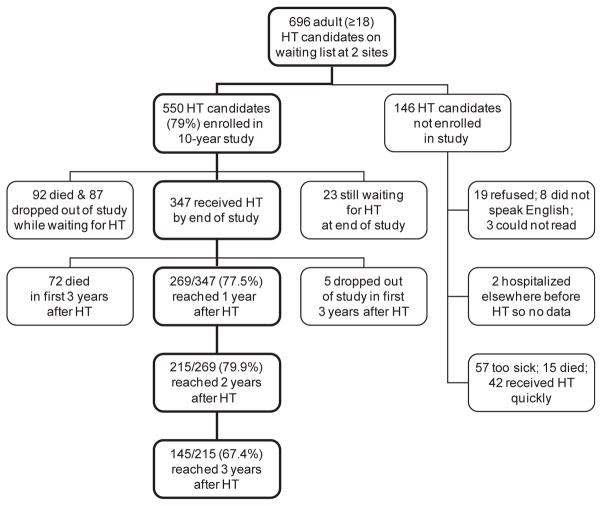
Evolution of sample size
Fig. 1 shows that, of the initial study sample of 347 patients transplanted at the 2 study sites, 72 patients died during the first 3 years after surgery (20.7%), with 72.2% of the deaths occurring in the first year after HT, 15.3% in the second year, and 12.5% in the third year. During the 3-year post-HT study period, only 5 patients dropped out of the study, stating that they were either too sick or too tired or too busy to fill out the study booklet.
Fig. 1.
Flow chart showing how the analysis sample size evolved from 347 patients who received a heart transplant (HT) to the sample size at 3 years after HT surgery.
By the time the 10-year study funding ended, 269 patients had reached 1 year after HT (269/347 =77.5%), 215 patients had reached 2 years after HT (215/269 = 79.9%), and 145 patients had reached 3 years after HT (145/215 = 67.4%), and therefore had medical data available for each of those time periods.
The remainder of the patients had not yet reached either the 1-year or 2-year or 3-year post-HT time point by the time the study ended, due to waiting a long time before a compatible heart donor was found. Some patients waited as long as 4–5 years for their HT; mean waiting time was 276 days, with a maximum of 1838 days in this cohort.
BMI data and group classification
Post-HT outcomes were compared in 2 weight groups based on their mean post-transplant body mass index (BMI) for the entire length of time they were in the study after surgery for up to 3 years. Data on post-HT BMI was obtained from inpatient and outpatient medical records whenever patients came to the clinic for follow-up or came to the hospital for treatment of problems, and then a mean BMI was calculated from all the data available on a patient for the specific time period.
Body mass index is calculated as weight in kilograms divided by height in meters squared (kg/m2), and is classified by NIH into 4 main groups: underweight (BMI <18.5), normal weight (BMI 18.5–24.9), overweight (BMI 25.0–29.9), and obese (BMI ≥30).37
In this cohort, 2.3% of the patients were in the underweight group, 28.8% were in the normal weight group, 45.8% were in the overweight group, and 23.1% were in the obese group. Because of the small proportion of patients in the underweight and obese categories, some groups were combined so only 2 groups were used for this analysis: (1) Group 1: non-overweight patients (underweight and normal weight BMI groups combined) and (2) Group 2: overweight patients (overweight and obese BMI groups combined).
The non-overweight Group 1 consisted of 108 patients (31.1%) with a mean post-transplant BMI less than 25 (range = 16–24, mean = 22, SD = 1.9). The overweight Group 2 consisted of 239 patients (68.9%) with a mean post-transplant BMI of 25 or higher (range = 25–40, mean = 29, SD = 3.2). Therefore, more than two-thirds of this cohort had a mean post-transplant BMI that was higher than clinically desired.
Outcomes
The 9 clinical outcomes examined in the 2 weight groups during the first 3 years after HT were as follows: survival: the number of days survived after HT surgery; re-hospitalization: the number of days re-hospitalized after the HT surgery discharge; and 7 post-transplant complications: the number of treated acute rejection episodes, the number of IV-treated infections (infections treated with an IV antibiotic), and the incidence of the following post-HT complications: cardiac allograft vasculopathy (CAV, an accelerated form of post-HT coronary artery disease that is caused by both immunologic and non-immunologic factors, and is the leading cause of death during the first 3 years after HT38), new-onset steroid-induced diabetes, lymphoma, stroke, and severe renal dysfunction (which was defined as a serum creatinine >2.5 mg/dl or a diagnosis of renal failure or on dialysis, based on ISHLT registry data9). These outcomes were selected for analysis because reports from the international ISHLT registry consider them germane to HT patients survival.1,9,12,39–42
Analysis
Data was analyzed with SPSS (V 13). Because of the multiple variables examined in this report, a more conservative probability level of .025 (instead of .05) was used to determine significant group differences in baseline characteristics, outcomes, and mortality risk factors. Baseline characteristics of the 2 weight groups were compared with chi square tests for nominal variables and t-tests for continuous variables.
Three different tests were used to analyze outcomes: logistic regression, multivariate analysis of covariance (MANCOVA), and Kaplan–Meier survival analysis. Logistic regression was used to identify significant differences between the 2 weight groups in the incidence of 5dichotomous outcomes during the first 3 years post-HT: CAV, stroke, severe renal dysfunction, new-onset steroid-induced diabetes, and lymphoma. Outcomes were adjusted in this analysis for 3 covariates: patient gender, patient age at HT, and donor age, because female gender, older patient age, and older donor age have been found to negatively influence various HT outcomes.34,35,39–46
MANCOVA was used to identify significant group differences in 3 continuous outcomes during the first 3 years post-HT: the number of days re-hospitalized after the HT discharge, the number of treated acute rejections, and the number of IV-treated infections (adjusting for covariates of patient gender, patient age at HT, and donor age). Kaplan–Meier survival analysis was used to compare survival curves for each weight group during the first 3 years post-HT, and then Cox regression was used to identify risk factors for shorter survival. Univariate Cox regression was first used to identify potential individual variables affecting survival, which were then combined and tested in a multivariate Cox regression model to determine significant risk factors for shorter survival (using backward stepwise elimination).
For both Kaplan–Meier survival analysis and Cox regression analysis, the dependent time variable of length of survival for the censored cases was the number of days of study follow-up during the first 3 post-operative years. (Note: censored cases are those patients who did not die within the first 3 years post-HT, and those patients who did not finish the 3 years of study follow-up for reasons other than death; e.g., the study ended before the patient reached 3 years post-HT.) For Cox regression, variables that failed the crucial assumption of proportional hazards across time were converted to time-dependent variables by creating an interaction term with time.
Results
Baseline characteristics
The overall sample of 347 patients was 20.2% female (70) and 79.8% male (277), with a mean age at HT of 52 years (range = 20–71); 14.7% (51) were minorities. Significant differences in the baseline characteristics of the 2 weight groups were as follows (Table 1). The non-overweight Group 1 had a higher percentage of women. The overweight Group 2 had a higher percentage of patients with the following pre-HT characteristics: coronary artery disease as the reason for HT, diabetes, smoking history, a higher cholesterol pre-HT, and a higher BMI at transplant.
Table 1.
Baseline characteristics of 347 HT patients by 2 post-HT weight groupsa: Group 1 = non-overweight; Group 2 = overweight.
| Characteristic | Group 1 (N = 108) | Group 2 (N = 239) | χ2b | tc | Pd |
|---|---|---|---|---|---|
| Age at HT (years) | 52 ± 12 | 52 ± 9 | .06 | .951 | |
| Female gender | 29.6% | 15.9% | 8.71 | .004 | |
| Minority racee | 16.7% | 13.8% | .49 | .514 | |
| BMI (kg/m2) at HT | 21 ±3 | 27± 3 | 17.78 | .000 | |
| Diabetes | 12.0% | 21.3% | 4.28 | .025 | |
| Smoking history | 57.4% | 70.7% | 5.92 | .011 | |
| Urgent priority for HT | 47.2% | 48.5% | .05 | .908 | |
| CAD as reason for HTf | 40.7% | 58.6% | 9.50 | .002 | |
| Repeat HT | 3.7% | 4.6% | .15 | .784 | |
| VAD or TAH at HT | 11.1% | 7.9% | .91 | .416 | |
| IABP at HT | 10.2% | 6.3% | 1.64 | .270 | |
| Ventilator at HT | 4.6% | 3.3% | .33 | .552 | |
| IV inotropes at HT | 63.0% | 54.0% | 2.45 | .129 | |
| PRA >10% | 7.4% | 8.8% | .19 | .835 | |
| PVR (Wood units) | 2.6 ± 1.6 | 2.3 ± 1.3 | 2.08 | .039 | |
| Serum creatinine (mg/dl) | 1.4 ± .6 | 1.4 ± .4 | .54 | .589 | |
| Cholesterol (mg/dl) | 172 ± 41 | 185 ± 50 | 2.40 | .017 | |
| Ischemic time (min) | 171 ± 56 | 183 ± 59 | 1.75 | .081 | |
| Donor age (years) | 27 ± 11 | 26 ± 9 | .51 | .614 | |
| Weight mismatch >20%g | 25.9% | 31.8% | 1.22 | .312 | |
| CMV mismatchh | 14.8% | 11.7% | .65 | .486 |
BMI, body mass index; CAD, coronary artery disease; CMV, cytomegalovirus; HT, heart transplant; IABP, intra-aortic balloon pump; IV, intravenous; PRA, panel-reactive antibody; PVR, pulmonary vascular resistance; TAH, total artificial heart; VAD, ventricular assist device.
Weight groups based on mean post-HT BMI: Group 1 (non-overweight) = BMI <25 (range = 16–24, mean = 22, SD = 1.9); Group 2 (overweight) = BMI ≥25 (range = 25–40, mean = 29, SD = 3.2).
Chi square tests (χ2) were used for categorical variables (%).
T-tests (t) were used for continuous variables (mean ± standard deviation).
Significance determined at P ≤ .025.
Minorities included: African-Americans, Hispanics, and Asians.
Other reasons for HT included: dilated cardiomyopathy, valvular heart disease, congenital heart disease, myocarditis, and previous graft failure.
Weight mismatch >20%: percent difference between patient weight and donor weight in kilograms.
CMV mismatch: donor positive/patient negative.
Outcomes
The 9 outcomes for non-overweight patients (Group 1) as compared to overweight patients (Group 2) during the first 3 years after HT surgery were as follows.
Survival
Survival time was significantly shorter for the non-overweight Group 1 (Fig. 2). A total of 72 deaths occurred over the 3-year study period in the overall sample of 347 patients (20.7%): 31 deaths were in Group 1 (28.7% of the non-overweight group) and 41 deaths were in Group 2 (17.2% of the overweight group). The causes of death for each group are listed on Table 2; the leading causes were infection (CMV, bacterial, fungal, protozoal) and acute rejection.
Fig. 2.
Kaplan–Meier survival curves for the first 3 years after heart transplant surgery in 2 post-transplant weight groups: Group 1 = 108 non-overweight patients (body mass index <25); Group 2 = 239 overweight patients (body mass index ≥25). Log rank test was significant: P = .012. Percent survived: non-overweight patients = 71.3%; overweight patients = 82.8%. Mean number of post-transplant days survived: non-overweight patients = 825 days (SD = 42); overweight patients = 944 days (SD = 23).
Table 2.
Cause of death for 72 of 347 patients (20.7%) who died during the first 3 years after HT surgery by 2 post-HT weight groupsa: Group 1 = non-overweight; Group 2 = overweight.
| Cause of deathb | Group 1 (N = 108) | Group 2 (N = 239) | Total |
|---|---|---|---|
| CMV infection | 4 | 3 | 7 |
| Bacterial infection | 4 | 3 | 7 |
| Fungal infection | 3 | 5 | 8 |
| Protozoal infection | 0 | 1 | 1 |
| Acute rejection | 5 | 8 | 13 |
| Late graft failure | 0 | 1 | 1 |
| CAV | 3 | 1 | 4 |
| Right ventricular failure | 0 | 3 | 3 |
| Respiratory failure | 1 | 1 | 2 |
| Multi-organ failure | 0 | 3 | 3 |
| Lymphoma | 2 | 1 | 3 |
| Cerebral hemorrhage | 1 | 1 | 2 |
| Stroke | 3 | 2 | 5 |
| Dissecting aortic aneurysm | 1 | 0 | 1 |
| Myocardial infarction | 2 | 2 | 4 |
| Pulmonary embolism | 0 | 2 | 2 |
| Pancreatitis | 1 | 1 | 2 |
| Intra-operative pump failure | 1 | 2 | 3 |
| Intra-operative hemorrhage | 0 | 1 | 1 |
| Number of deaths | 31 | 41 | 72 |
| Percent of each group who died | 28.7% | 17.2% |
CAV, cardiac allograft vasculopathy; CMV, cytomegalovirus; HT, heart transplant.
Weight groups based on mean post-transplant BMI (body mass index = kg/m2): Group 1 (non-overweight) = BMI <25 (range = 16–24, mean = 22, SD = 1.9); Group 2 (overweight) = BMI ≥ 25 (range = 25–40, mean = 29, SD = 3.2).
Source of death data: autopsy report (36) or death certificate (16) or physician note on the chart (20).
Re-hospitalization
The non-overweight patients (Group 1) were re-hospitalized significantly more days following the HT surgery discharge (Table 3). A total of 336 patients in the overall sample of 347 HT recipients was re-hospitalized sometime during the first 3 years after surgery (96.8%): 104 patients were in Group 1 (96.3% of the non-overweight group), and 232 patients were in Group 2 (97.1% of the overweight group). Reasons for hospital re-admissions in each group are listed on Table 4; the main reasons were acute rejection, infection, and cardiovascular problems.
Table 3.
Clinical outcomes in 347 HT recipients during the first 3 years after HT surgery by 2 post-HT weight groupsa: Group 1 = non-overweight; Group 2 = overweight.
| Outcome | Group 1 (N = 108) | Group 2 (N = 239) | χ2b | Fc | Pd |
|---|---|---|---|---|---|
| CAV | 5.6% | 18.0% | 14.84 | .002 | |
| Stroke | 10.2% | 5.4% | 2.91 | .088 | |
| Severe renal dysfunctione | 18.5% | 13.4% | 6.76 | .009 | |
| New-onset steroid-induced diabetes | 10.2% | 14.2% | 5.11 | .024 | |
| Lymphoma | 7.4% | 1.7% | 7.64 | .006 | |
| Number of days re-hospitalized after HT discharge | 36 ± 44 | 33 ± 36 | 3.37 | .010 | |
| Number of treated acute rejections | 3.1 ± 3.1 | 3.7 ± 3.6 | 4.00 | .003 | |
| Number of IV-treated infections | 2.3 ± 2.9 | 1.9 ± 2.5 | 1.68 | .155 |
CAV, cardiac allograft vasculopathy; HT, heart transplant; IV, intravenous.
Weight groups based on mean post-transplant BMI (body mass index = kg/m2): Group 1 (non-overweight) = BMI <25 (range = 16–24, mean = 22, SD = 1.9); Group 2 (overweight) = BMI ≥ 25 (range = 25–40, mean = 29, SD = 3.2).
Logistic regression (χ2) was used for dichotomous outcomes (%) and adjusted for covariates of patient gender, patient age at HT, and donor age.
MANCOVA (F) was used for continuous outcomes (mean ± standard deviation) and adjusted for covariates of patient gender, patient age at HT, and donor age.
Significance determined at P ≤ .025.
Defined as a serum creatinine >2.5 mg/dl or a diagnosis of renal failure or on dialysis.9
Table 4.
Reasons for hospital re-admissions in 347 HT recipients during the first 3 years after HT surgery by 2 post-HT weight groupsa: Group 1 = non-overweight (N = 108); Group 2 = overweight (N = 239).
| Reason for hospital re-admissionb | Group 1 n (%) | Group 2 n (%) |
|---|---|---|
| Acute rejection | 56 (51.9) | 132 (55.2) |
| CMV infections | 13 (12.0) | 37 (15.5) |
| Pneumonia | 20 (18.5) | 36 (15.1) |
| Other infections | 35 (32.4) | 85 (35.6) |
| Other pulmonary (e.g., respiratory failure, pulmonary edema, pulmonary embolism) | 10 (9.3) | 32 (13.4) |
| CAV | 69 (63.9) | 142 (59.4) |
| Cardiac arrhythmias | 8 (7.4) | 32 (13.4) |
| Syncope | 2 (1.9) | 13 (5.4) |
| Other cardiovascular (e.g., MI, HTN, mitral regurgitation, pericarditis, cardiac tamponade) | 16 (14.8) | 35 (14.6) |
| Peripheral vascular disease | 3 (2.8) | 4 (1.7) |
| GI: esophagitis, gastritis, ulcer | 15 (13.9) | 30 (12.6) |
| Other GI (e.g., gall stones, diverticulitis, pancreatitis, hepatitis, liver failure, bowel obstruction) | 13 (12.0) | 38 (15.9) |
| Diabetes | 4 (3.7) | 16 (6.7) |
| Other endocrine (e.g., hypothyroidism, parathyroidism) | 2 (1.9) | 6 (2.5) |
| Hematologic (e.g., anemia, leukopenia, thrombocytopenia) | 11 (10.2) | 16 (6.7) |
| Chronic renal insufficiency & renal failure | 3 (2.8) | 20 (8.4) |
| Other renal (e.g., kidney stones, kidney abscess) | 4 (3.7) | 7 (2.9) |
| Neurologic (e.g., stroke, encephalitis, seizures) | 9 (8.3) | 17 (7.1) |
| Orthopedic (e.g., avascular necrosis of hip, spinal fracture, back pain) | 9 (8.3) | 17 (7.1) |
| Lymphoma | 4 (3.7) | 3 (1.3) |
| Other cancers (non-skin) (e.g., renal, liver) | 0 | 2 (.8) |
| Miscellaneous reasons (e.g., EENT, urologic, dermatologic, depression) | 39 (36.1) | 75 (40.2) |
CAV, cardiac allograft vasculopathy; CMV, cytomegalovirus; EENT, eye, ear, nose and throat; GI, gastrointestinal; HT, heart transplant; HTN, hypertension; MI, myocardial infarction.
Weight groups based on mean post-transplant BMI (body mass index = kg/m2): Group 1 (non-overweight) = BMI <25 (range = 16–24, mean = 22, SD = 1.9); Group 2 (overweight) = BMI ≥ 25 (range = 25–40, mean = 29, SD = 3.2).
Reasons for re-admission are based on the discharge diagnoses recorded by the physician on the chart; multiple diagnoses could be recorded for an admission.
Post-HT complications (Table 3)
The non-overweight patients (Group 1) had a significantly higher percentage of cases with lymphoma and severe renal dysfunction. The overweight patients (Group 2) had a significantly higher number of treated acute rejections, and a significantly higher percentage of cases with CAV and new-onset steroid-induced diabetes. There were no significant differences between the 2 weight groups in the percentage of strokes or the number of IV-treated infections.
Mortality risk factors
Significant risk factors for shorter survival during the first 3 years after HT were as follows (Table 5). For the non-overweight Group 1: severe renal dysfunction, earlier IV-treated infection, respiratory failure, and female patient. For the overweight Group 2: higher prednisone dose and higher post-HT cholesterol.
Table 5.
Significant risk factors for shorter 3-year survival in 347 HT recipients by 2 post-HT weight groupsa: Group 1 = non-overweight; Group 2 = overweight.
| Risk factorb | Group 1 (N = 108) | Group 2 (N = 239) | Wald statistic | Pc | Relative riskd |
|---|---|---|---|---|---|
| Higher prednisone dose (mg) | 21.6 ± 11.8 | 24.6 ± 14.5 | 54.57 | .000 | |
| Severe renal dysfunctione | 18.5% | 13.4% | 14.71 | .000 | 3.24 |
| Earlier IV-treated infection (days) | 256 ± 368 | 335 ± 385 | 9.92 | .002 | |
| Female patient | 29.6% | 15.9% | 7.65 | .006 | 2.30 |
| Higher cholesterol level (mg/dl) | 209 ± 42 | 221 ± 46 | 7.28 | .007 | |
| Respiratory failure | 31.5% | 18.8% | 6.72 | .010 | 2.12 |
HT, heart transplant; IV, intravenous.
Weight groups based on mean post-transplant BMI (body mass index = kg/m2): Group 1 (non-overweight) = BMI <25 (range = 16–24, mean = 22, SD = 1.9); Group 2 (overweight) = BMI ≥ 25 (range = 25–40, mean = 29, SD = 3.2).
From multivariate Cox regression. Nonsignificant variables in regression model: patient age at HT, patient race, repeat HT, cardiac allograft vasculopathy, diabetes, lymphoma, liver failure, stroke, time to first treated acute rejection.
Significance determined at P ≤ .025.
Relative risk applies to dichotomous risk factors only.
Defined as a serum creatinine >2.5 mg/dl or a diagnosis of renal failure or on dialysis.9
Discussion
Seven of the 9 outcomes examined in this analysis were significantly different between overweight and non-overweight HT recipients during the first 3 years after surgery. The overweight patients (Group 2) survived longer during the first 3 years after HT, but had more treated acute rejections, CAV, and new-onset steroid-induced diabetes. In addition to surviving a shorter time, the non-overweight patients (Group 1) were also re-hospitalized more days after the HT surgery discharge during the first 3 years post-HT, and had more lymphoma and severe renal dysfunction.
Therefore, contrary to expectations, it was the overweight HT recipients in this cohort (Group 2) who survived significantly longer during the first 3 years after HT, in spite of having 2 of the 6 significant mortality risk factors identified in this analysis: higher cholesterol level after transplant and higher prednisone dose during this time period. Research by Augustine et al11 had shown that HT recipients who were heavier after surgery had shorter survival time during the first post-operative year of their study follow-up, as also supported by registry data from both ISHLT9 and UNOS.10 However, our study found the opposite outcome when examined over a longer time span. In concert with our result, Clark et al47 (using data on 1900 international HT patients) also reported better survival after HT with a higher post-transplant BMI.
Therefore, it was the non-overweight HT recipients in our cohort (Group 1) who had shorter survival during the first 3 years after HT. This can partially be explained by the fact that the non-overweight patients had a significantly higher percentage of cases with lymphoma (a serious complication of immunosuppressant drugs48) and severe renal dysfunction, also due to the adverse effects of immunosuppressants. Severe renal dysfunction was a significant risk factor in our analysis that contributed to shorter survival in the non-overweight patients in Group 1. Previous ISHLT registry analyses also support severe renal dysfunction as a risk factor for HT mortality.39,42
In addition, the non-overweight Group 1 had the mortality risk factors of a higher percentage of cases with respiratory failure, and they experienced an IV-treated infection sooner after HT than Group 2. Registry analyses from the ISHLT identified earlier IV-treated infections as a risk factor for decreased survival in HT patients.39 Also, the non-overweight patients spent significantly more time in the hospital following discharge after the HT during the first 3 post-transplant years than Group 2. Re-admissions were mainly for cardiovascular problems, infection, and acute rejection.
Moreover, the non-overweight patients (Group 1) also had a significantly higher percentage of women than Group 2, which proved to be a significant demographic risk factor in our analysis that contributed to the shorter survival in Group 1. In fact, Group 1 (non-overweight) had almost doubled the percentage of female patients as compared to Group 2 (29.6% vs 15.9%). Previous ISHLT registry reports,1,9,39,40,42 as well as numerous other studies,43–46,49–52 have shown worse outcomes in female HT recipients in such areas as mortality, re-hospitalization, rejection, and renal dysfunction.
Therefore, the non-overweight patients in this cohort (Group 1) had 4 of the 6 significant mortality risk factors identified in this analysis, and they also had significantly worse outcomes in 4 of the 9 outcomes examined in this report. This explains why the non-overweight patients in this cohort had shorter survival during the first 3 years after HT surgery, instead of the overweight patients, as has been found in other studies on HT mortality based on post-HT patient weight.9–11,39–41
However, heavier post-transplant body weight did adversely affect 3 outcomes in the overweight HT recipients in this cohort (Group 2). The overweight patients had more episodes of treated acute rejection during the first 3 years post-transplant. Grady et al19 also found that HT recipients with a higher post-transplant BMI were at greater risk for acute rejection over the 5-year follow-up period of their study, as also supported by ISHLT registry data.39
In addition, the overweight patients in our study (Group 2) had a higher percentage of cases with CAV during the first 3 years of post-transplant follow-up.
Registry data from ISHLT,12 as well as research by Milaniak et al,13 Winters et al,14 and Valantine,15 support our finding on the relationship between heavier post-HT body weight and the development of post-transplant CAV.
Also, the overweight patients (Group 2) had a higher percentage of cases with new-onset steroid-induced diabetes during the first 3 years after surgery. Several studies53–56 have documented the relationship between heavier pre-HT patient weight and the development of post-HT diabetes, but only 2 studies16,17 were found that reported an association between higher post-HT BMI and new-onset diabetes, due to the higher prednisone intake early after transplant, thus supporting our finding.
Conclusion
During the first 3 years after surgery, overweight HT recipients had better survival, but had more CAV, diabetes, and acute rejection. Non-overweight HT recipients had worse 3-year survival, plus more re-hospitalization time, lymphoma, and renal dysfunction. Therefore, examining multiple outcomes demonstrated that both weight groups of HT patients in this cohort suffered different types of poor outcomes during the first 3 years after HT surgery.
Study limitations and strengths
Limitations of this study are: (1) Medical data for the third year of post-HT study follow-up was only available on 145 patients of the original 347 study patients transplanted due to several reasons: deaths during the 3 years, the often long wait for a heart donor, some patients not yet reaching the 3-year time point by the time the 10-year study funding ended, and a few patients dropping out of the study for health or work reasons; and (2)Weight groups had to be combined for analysis into only 2 groups (non-overweight vs overweight) because of the small number of patients in the underweight and obese BMI groups.
Strengths of this study are: (1) Multiple HT outcomes were examined longitudinally; (2) Comprehensive medical data was collected for the study at frequent intervals both pre-operatively and post-operatively for the 3-year study period; (3) Medical data was retrieved from patients charts by nurses experienced in cardiac care; and (4) Several methods were used to assess the reliability of the retrieval, recording, coding, and computer entry of the medical records data. The main method was a comparison of the original data from the medical records to a sample of 25% of the study data. Study records were systematically chosen for reliability review so that the data on many of the patients would be verified for some of both the pre-operative and post-operative study periods.
Implications for clinical practice and research
The fact that both the overweight and the non-overweight HT patients in this cohort experienced several poor outcomes during the first 3 years after surgery that were serious and required the investment of considerable health care resources provides important information for clinical practice. Clinicians tend to focus more on excessive body weight as having negative consequences for HT outcomes; however, this study demonstrated that patients who are at normal weight or only mildly underweight can also suffer adverse outcomes after HT.
This study needs to be replicated with a larger sample of patients from multiple sites who are followed for a longer period of time after HT, in order to verify the generalizability of our findings over an extended post-transplant time and over clinical sites.
Acknowledgments
Funding: National Institutes of Health (NINR: #NR01693, #NR01693/S, #5R01-NR01693; NHLBI: #HL49336), Sandoz Pharmaceuticals Corporation, Earl Bane Estate, American Association of Critical Care Nurses, Sigma Theta Tau, Loyola University Research Committee, Loyola University School of Nursing, Loyola University Medical Center. Study PI: Dr Anne Jalowiec.
Abbreviations
- BMI
body mass index
- CAD
coronary artery disease
- CAV
cardiac allograft vasculopathy
- CMV
cytomegalovirus
- dl
deciliters
- EENT
eye, ear, nose, and throat
- GI
gastrointestinal
- HT
heart transplant
- HTN
hypertension
- IABP
intra-aortic balloon pump
- ISHLT
International Society for Heart and Lung Transplantation
- IV
intravenous
- kg
kilograms
- MANCOVA
multivariate analysis of covariance
- m
meters
- mg
milligrams
- MI
myocardial infarction
- NIH
National Institutes of Health
- PRA
panel-reactive antibody
- PVR
pulmonary vascular resistance
- SD
standard deviation
- TAH
total artificial heart
- UNOS
United Network for Organ Sharing
- VAD
ventricular assist device
References
- 1.International Society for Heart and Lung Transplantation. Registry Data. 2010 Online slides www.ishlt.org/registries/
- 2.Kilic A, Conte JV, Shah AS, Yuh DD. Orthotopic heart transplantation in patients with metabolic risk factors. Ann Thorac Surg. 2012;93:718–724. doi: 10.1016/j.athoracsur.2011.11.054. [DOI] [PubMed] [Google Scholar]
- 3.Guisado Rasco A, Sobrino Marquez JM, Nevado Portero J, Romero Rodriguez N, Ballasteros Prada S, et al. Impact of overweight on survival and primary graft failure after heart transplantation. Transplant Proc. 2010;42:3178–3180. doi: 10.1016/j.transproceed.2010.05.139. [DOI] [PubMed] [Google Scholar]
- 4.Russo MJ, Hong KN, Davies RR, Chen JM, Mancini DM, et al. The effect of body mass index on survival following heart transplantation: do outcomes support consensus guidelines? Ann Surg. 2010;25:144–152. doi: 10.1097/SLA.0b013e3181b5db3c. [DOI] [PubMed] [Google Scholar]
- 5.Almenar L, Cardo ML, Martinez-Dolz L, Garcia-Palomar C, Rueda J, et al. Risk factors affecting survival in heart transplant patients. Transplant Proc. 2005;9:4011–4013. doi: 10.1016/j.transproceed.2005.09.160. [DOI] [PubMed] [Google Scholar]
- 6.Lietz K, John R, Burke EA, Ankersmit JH, McCue JD, et al. Pretransplant cachexia and morbid obesity are predictors of increased mortality after heart transplantation. Transplantation. 2001;72:277–283. doi: 10.1097/00007890-200107270-00020. [DOI] [PubMed] [Google Scholar]
- 7.Grady KL, White-Williams C, Naftel D, Costanzo MR, Pitts D, et al. Are preoperative obesity and cachexia risk factors for post heart transplant morbidity and mortality: a multi-institutional study of weight-height indices. Cardiac Transplant Research Database (CTRD) Group. J Heart Lung Transplant. 1999;18:750–763. doi: 10.1016/s1053-2498(99)00035-2. [DOI] [PubMed] [Google Scholar]
- 8.Grady KL, Costanzo MR, Fisher S, Koch D. Preoperative obesity is associated with decreased survival after heart transplantation. J Heart Lung Transplant. 1996;15:863–871. [PubMed] [Google Scholar]
- 9.International Society for Heart and Lung Transplantation. Registry Data. 2015 Online slides www.ishlt.org/registries/
- 10.Weber DJ, Hashmi ZA, Gracon AS, Hellman YM, Patel AJ, et al. Recipient body mass index and age interact to impact survival after heart transplantation. Clin Transplant. 2014;11:1279–1286. doi: 10.1111/ctr.12460. [DOI] [PubMed] [Google Scholar]
- 11.Augustine SM, Baumgartner WA, Kasper EK. Obesity and hypercholesterolemia following heart transplantation. J Transpl Coord. 1998;8:164–169. doi: 10.7182/prtr.1.8.3.eg2p05773u818q54. [DOI] [PubMed] [Google Scholar]
- 12.Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, et al. The registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report 2012. J Heart Lung Transplant. 2012;31:1052–1064. doi: 10.1016/j.healun.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Milaniak I, Przybylowski P, Wierzbicki K, Sadowski J. Post-transplantation body mass index in heart transplant recipients: determinants and consequences. Transplant Proc. 2014;8:2844–2847. doi: 10.1016/j.transproceed.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 14.Winters GL, Kendall TJ, Radio SJ, Wilson JE, Costanzo-Nordin MR, et al. Post-transplant obesity and hyperlipidemia: major predictors of severity of coronary arteriopathy in failed human heart allografts. J Heart Lung Transplant. 1990;9:364–371. [PubMed] [Google Scholar]
- 15.Valantine H. Cardiac allograft vasculopathy after heart transplantation: risk factors and management. J Heart Lung Transplant. 2004;23(suppl 5):S187–S193. doi: 10.1016/j.healun.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Mogollon Jimenez MV, Sobrino Marquez JM, Arizon Munoz JM, Sanchez Brotons JA, Guisado Rasco A, et al. Incidence and importance of de novo diabetes mellitus after heart transplantation. Transplant Proc. 2008;9:3053–3055. doi: 10.1016/j.transproceed.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Dolz L, Almenar L, Martinez-Ortiz L, Arnau MA, Chamorro C, et al. Predictive factors for development of diabetes mellitus post-heart transplant. Transplant Proc. 2005;37:4064–4066. doi: 10.1016/j.transproceed.2005.09.161. [DOI] [PubMed] [Google Scholar]
- 18.Kobashigawa JA, Starling RC, Mehra MR, Kormos RL, Bhat G, et al. Multicenter retrospective analysis of cardiovascular risk factors affecting long-term outcome of de novo cardiac transplant recipients. J Heart Lung Transplant. 2006;25:1063–1069. doi: 10.1016/j.healun.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Grady KL, Naftel D, Pamboukian SV, Frazier OH, Hauptman P, et al. Postoperative obesity and cachexia are risk factors for morbidity and mortality after heart transplant: multi-institutional study of post-operative weight change. J Heart Lung Transplant. 2005;24:1424–1430. doi: 10.1016/j.healun.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Grady KL, Jalowiec A, Grusk B, White-Williams C, Robinson JA. Symptom distress in cardiac transplant candidates. Heart Lung. 1992;21:434–439. [PubMed] [Google Scholar]
- 21.Jalowiec A, Grady KL, White-Williams C, Fazekas S, Laff M, et al. Symptom distress three months after heart transplantation. J Heart Lung Transplant. 1997;16:604–614. [PubMed] [Google Scholar]
- 22.Jalowiec A, Grady KL, White-Williams C. Functional status one heart after heart transplant. J Cardiopulm Rehabil Prev. 2007;27:24–32. doi: 10.1097/01.hcr.0000265029.25392.6e. [DOI] [PubMed] [Google Scholar]
- 23.White-Williams C, Jalowiec A, Grady K. Who returns to work after heart transplantation? J Heart Lung Transplant. 2005;24:2255–2261. doi: 10.1016/j.healun.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Jalowiec A, Grady KL, White-Williams C. Satisfaction with heart transplantation. J Cardiovasc Nurs. 2006;21:134–139. doi: 10.1111/j.0889-7204.2006.05565.x. [DOI] [PubMed] [Google Scholar]
- 25.Grady KL, Jalowiec A, White-Williams C. Patient compliance at one year and two years after heart transplantation. J Heart Lung Transplant. 1998;17:383–394. [PubMed] [Google Scholar]
- 26.Grady KL, Jalowiec A, White-Williams C, Hetfleisch M, Penicook J, et al. Heart transplant candidates’ perception of helpfulness of health care provider interventions. Cardiovasc Nurs. 1993;29:33–37. [PubMed] [Google Scholar]
- 27.Jalowiec A, Grady KL, White-Williams C. Stressors in patients awaiting a heart transplant. Behav Med. 1994;19:145–154. doi: 10.1080/08964289.1994.9935185. [DOI] [PubMed] [Google Scholar]
- 28.Jalowiec A, Grady KL, White-Williams C. Predictors of perceived coping effectiveness in patients awaiting a heart transplant. Nurs Res. 2007;56:260–268. doi: 10.1097/01.NNR.0000280612.83071.61. [DOI] [PubMed] [Google Scholar]
- 29.Grady KL, Jalowiec A, White-Williams C, Pifarre R, Kirklin JK, et al. Predictors of quality of life in patients with advanced heart failure awaiting transplantation. J Heart Lung Transplant. 1995;14:2–10. [PubMed] [Google Scholar]
- 30.Grady KL, Jalowiec A, White-Williams C. Improvement in quality of life in heart failure patients who undergo transplantation. J Heart Lung Transplant. 1996;15:749–757. [PubMed] [Google Scholar]
- 31.Grady KL, Jalowiec A, White-Williams C. Quality of life 6 months after heart transplantation compared with indicators of illness severity before transplantation. Am J Crit Care. 1998;7:106–116. [PubMed] [Google Scholar]
- 32.Grady KL, Jalowiec A, White-Williams C. Preoperative psychosocial predictors of hospital length of stay after heart transplantation. J Cardiovasc Nurs. 1999;14:12–26. doi: 10.1097/00005082-199910000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Jalowiec A, Grady KL, White-Williams C. First-year clinical outcomes in gender-mismatched heart transplant recipients. J Cardiovasc Nurs. 2012;27:519–527. doi: 10.1097/JCN.0b013e31822ce6c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jalowiec A, Grady KL, White-Williams C. Gender and age differences in symptom distress and functional disability one year after heart transplant surgery. Heart Lung. 2011;40:21–30. doi: 10.1016/j.hrtlng.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jalowiec A, Grady KL, White-Williams C. Predictors of rehospitalization time during the first year after heart transplant. Heart Lung. 2008;37:344–355. doi: 10.1016/j.hrtlng.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grady KL, Jalowiec A, White-Williams C. Predictors of quality of life in patients at one year after heart transplantation. J Heart Lung Transplant. 1999;18:202–210. doi: 10.1016/s1053-2498(98)00048-5. [DOI] [PubMed] [Google Scholar]
- 37.NIH, NHLBI. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Washington, DC: HHS, PHS; 1998. [PubMed] [Google Scholar]
- 38.Ramzy D, Vivek R, Brahm J, Santiago M, Delgado D, et al. Cardiac allogaft vasculopathy: a review. Can J Surg. 2005;48:319–327. [PMC free article] [PubMed] [Google Scholar]
- 39.Lund LH, Edwards LB, Kucherayavaya AY, Benden C, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: thirty-second official adult heart transplantation report 2015; focus theme: early graft failure. J Heart Lung Transplant. 2015;34:1244–1254. doi: 10.1016/j.healun.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-eighth adult heart transplant report 2011. J Heart Lung Transplant. 2011;30:1078–1094. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Lund LH, Edwards LB, Kucheryavaya AY, Dipchand AI, Benden C, et al. The Registry of the International Society for Heart and Lung Transplantation: thirtieth official adult heart transplant report 2013; focus theme: age. J Heart Lung Transplant. 2013;32:951–964. doi: 10.1016/j.healun.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Stehlik J, Edwards LB, Kucheryavaya AY, Aurora P, Christie JD, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult heart transplant report 2010. J Heart Lung Transplant. 2010;29:1089–1103. doi: 10.1016/j.healun.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Lietz K, John R, Kocher A, Schuster M, Mancini DM, et al. Increased prevalence of autoimmune phenomena and greater risk for alloreactivity in female heart transplant recipients. Circulation. 2001;104(suppl 1):I177–I183. doi: 10.1161/hc37t1.094704. [DOI] [PubMed] [Google Scholar]
- 44.Wechsler ME, Giardina EG, Sciacca RR, Rose EA, Barr ML. Increased early mortality in women undergoing cardiac transplantation. Circulation. 1995;91:1029–1035. doi: 10.1161/01.cir.91.4.1029. [DOI] [PubMed] [Google Scholar]
- 45.Fabbri A, Bryan AJ, Sharples LD, Dunning J, Caine N, et al. Influence of recipient and donor gender on outcome after heart transplantation. J Heart Lung Transplant. 1992;11:701–707. [PubMed] [Google Scholar]
- 46.Esmore D, Keogh A, Spratt P, Jones B, Chang V. Heart transplantation in females. J Heart Lung Transplant. 1991;10:335–341. [PubMed] [Google Scholar]
- 47.Clark AL, Knosalla C, Birks E, Loebe M, Davos CH, et al. Heart transplantation in heart failure: the prognostic importance of body mass index at time of surgery and subsequent weight changes. Eur J Heart Fail. 2007;8:839–844. doi: 10.1016/j.ejheart.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 48.Opelz G, Henderson R. Incidence of non-Hodgkin lymphoma in kidney and heart transplant recipients. Lancet. 1993;342:1514–1516. doi: 10.1016/s0140-6736(05)80084-4. [DOI] [PubMed] [Google Scholar]
- 49.Thomas HL, Banner NR, Murphy CL, Steenkamp R, Birch R, et al. Incidence, determinants, and outcome of chronic kidney disease after adult heart transplantation in the United Kingdom. Transplantation. 2012;93:1151–1157. doi: 10.1097/TP.0b013e31824e7620. [DOI] [PubMed] [Google Scholar]
- 50.Mastrobuoni S, DellAquilla AM, Azcarate PM, Rabago G, Herreros J. Long-term survival (>20 years) following heart transplantation. J Cardiovasc Surg (Torino) 2012;53:677–684. [PubMed] [Google Scholar]
- 51.Delgado JF, Crespo-Leiro MG, Gomez-Sanchez MA, Paniagua MJ, Gonzalez-Vilchez F, et al. Risk factors associated with moderate-to-severe renal dysfunction among heart transplant patients: results from the CAPRI study. Clin Transplant. 2010;24:E194–E200. doi: 10.1111/j.1399-0012.2010.01249.x. [DOI] [PubMed] [Google Scholar]
- 52.Hamour IM, Omar F, Lyster HS, Palmer A, Banner NR. Chronic kidney disease after heart transplantation. Nephrol Dial Transplant. 2009;24:1655–1662. doi: 10.1093/ndt/gfn759. [DOI] [PubMed] [Google Scholar]
- 53.Bedanova H, Ondrasek J, Cerny J, Orban M, Spinarova L, et al. Impact of diabetes mellitus on survival rates after heart transplantation. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009;153:283–287. doi: 10.5507/bp.2009.047. [DOI] [PubMed] [Google Scholar]
- 54.Nieuwenhuis MG, Kirkels JH. Predictability and other aspects of post-transplant diabetes mellitus in heart transplant recipients. J Heart Lung Transplant. 2001;20:703–708. doi: 10.1016/s1053-2498(01)00257-1. [DOI] [PubMed] [Google Scholar]
- 55.Ye X, Kuo HT, Sampaio MS, Jiang Y, Reddy R, et al. Risk factors for development of new-onset diabetes mellitus in adult heart transplant recipients. Transplantation. 2010;89:1526–1532. doi: 10.1097/TP.0b013e3181dd6bd9. [DOI] [PubMed] [Google Scholar]
- 56.Kahn J, Rehak P, Schweiger M, Wasler A, Wascher T, et al. The impact of overweight on the development of diabetes after heart transplantation. Clin Transplant. 2006;20:62–66. doi: 10.1111/j.1399-0012.2005.00441.x. [DOI] [PubMed] [Google Scholar]