Abstract
Background
Preclinical models reveal that stress-induced amygdala activity and impairment in fear extinction reflects reductions in anandamide driven by corticotropin-releasing hormone receptor type 1 (CRHR1) potentiation of the anandamide catabolic enzyme fatty acid amide hydrolase (FAAH).
Methods
Here we provide clinical translation for the importance of these molecular interactions using an imaging genetics strategy to examine whether interactions between genetic polymorphisms associated with differential anandamide (FAAH rs324420) and CRHR1 (CRHR1 rs110402) signaling modulate amygdala function and anxiety disorder diagnosis.
Results
Analyses revealed that individuals with a genetic background predicting relatively high anandamide and CRHR1 signaling exhibited blunted basolateral amygdala habituation, which further mediated increased risk for anxiety disorders amongst these same individuals.
Conclusions
The convergence of preclinical and clinical data suggests that interactions between anandamide and CRHR1 represent a fundamental molecular mechanism regulating amygdala function and anxiety. Our results further highlight the potential of imaging genetics to powerfully translate complex preclinical findings to clinically meaningful human phenotypes.
Keywords: anxiety, amygdala, FAAH, CRHR1, habituation, imaging genetics
INTRODUCTION
Converging evidence implicates endocannabinoid (eCB) signaling in the regulation of stress and anxiety, which may emerge, at least in part, through eCB modulation of amygdala output (1, 2). Specifically, binding of the eCB ligand anandamide (AEA) to the cannabinoid type 1 (CB1) receptor in the basolateral amygdala provides inhibitory tone through which multiple output processes, including fear extinction, and anxiety are modulated (1, 3). In turn, the magnitude of this inhibitory tone is regulated by levels of fatty acid amide hydrolase (FAAH), the primary catabolic enzyme of AEA, with relatively decreased FAAH and subsequently increased AEA facilitating fear extinction and reducing anxiety-related behavior through maintenance of inhibitory tone (1, 3, 4).
Studies in rodents have revealed that chronic and acute stress increase FAAH activity resulting in reduced AEA signaling and diminished inhibitory tone, and subsequently increased amygdala output including activation of the hypothalamic-pituitary-adrenal (HPA) axis and expression of anxiety-like behaviors (3, 5–8). A recent study suggests that stress-related reductions in AEA are driven by corticotropin-releasing hormone (CRH) release within the basolateral amygdala (9). Specifically, antagonism of the corticotropin-releasing hormone receptor type 1 (CRHR1) prevents stress-induced increases in FAAH activity; reciprocally, FAAH inhibition prevents CRH-mediated activation of the HPA axis and increased anxiety.
Here, we employ an imaging genetics strategy to model the effects of interactions between AEA and CRH signaling on human amygdala function and anxiety disorder diagnosis in 661 young adults. First, we model variability in AEA inhibitory tone through a non-synonymous single nucleotide polymorphism (SNP) within the human FAAH gene (rs324420). This C to A polymorphism (C385A) results in a proline to threonine substitution at codon 129 (Pro129Thr), with reduced FAAH expression associated with the 385A allele (10, 11). We have previously reported decreased threat-related amygdala reactivity in carriers of the 385A allele (12), reflective of relatively increased temporal habituation of amygdala activity to threat-related stimuli (4). Consistent with these data, a recent study has directly linked the 385A allele to potentiated fear extinction in humans as well as a mouse knock-in model of the FAAH C385A polymorphism (13). Secondly, we model variability in CRH signaling using a polymorphism within the human CRHR1 gene (rs110402). This intronic A to G SNP has been associated with individual differences in HPA axis function as well as stress-related risk for depression (14–17). Critical to our current study, the A allele has been associated with elevated cortisol reactivity to an acute stressor suggesting greater activation of the HPA axis in A allele homozygotes (18) but see also (19).
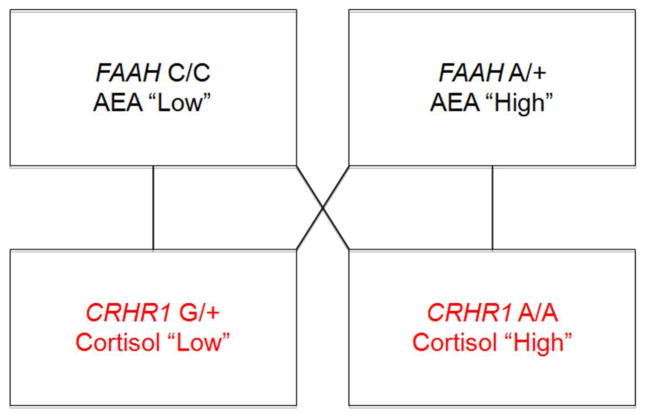
By testing the effects of interactions between FAAH rs324420 and CRHR1 rs110402 polymorphisms on amygdala activity we are able to model parallel molecular interactions between AEA and CRH signaling recently demonstrated to modulate stress-related amygdala function in rodents (Figure 1). We hypothesized that relatively increased CRH signaling would be associated with blunted habituation of the basolateral amygdala specifically in individuals with relatively high AEA-mediated inhibitory tone (i.e., FAAH 385A allele carriers who are also CRHR1 A allele homozygotes). Further, we examined if individual differences in amygdala habituation indirectly link this genetic background to the presence of an anxiety disorder. We focused on amygdala habituation, as opposed to activity, in light of evidence that reductions in amygdala activation are associated with successful fear extinction (20, 21), and that decreased amygdala habituation is associated with psychopathology characterized by anxiety and excessive fear (22–25). A focus on habituation is further consistent with knockout and pharmacologic manipulation studies in rodents suggesting that eCB signaling is critical for fear extinction, but not conditioning (1, 4, 26), and prior evidence that genetic variation in the eCB system is associated with amygdala habituation in humans (1, 27).
Figure 1. Modeling molecular interactions between AEA and CRH signaling.
Imaging genetics strategy for modeling variability in AEA inhibitory tone and CRH signaling using interactions between FAAH rs324420 and CRHR1 rs110402 genotypes.
METHODS and MATERIALS
Participants
Neuroimaging and genetic data were available from 726 participants who completed the ongoing Duke Neurogenetics Study (DNS) by January 6th, 2014. All participants provided written informed consent in accordance with Duke University guidelines and were in general good health. Study exclusion criteria included: 1) medical diagnosis of cancer, stroke, diabetes requiring insulin treatment, chronic kidney or liver disease, or lifetime psychotic symptoms; 2) use of psychotropic, glucocorticoid, or hypolipidemic medication; 3) conditions affecting cerebral blood flow and metabolism (e.g., hypertension); and 4) contraindications to MRI scanning. DSM-IV Axis I and select Axis II (i.e., antisocial personality disorder and borderline personality disorder) were assessed with the electronic Mini International Neuropsychiatric Interview (28) and Structured Clinical Interview for the DSM-IV Axis II (29). As the DNS seeks to capture broad variability in psychiatrically relevant behavioral phenotypes (e.g., depression, anxiety), participants meeting diagnostic criteria for disorders other than psychosis were included in the study (Supplementary Table S1).
Of the 726 participants 67 were excluded due to scanner-related artifacts in fMRI data (n=6), incidental structural brain abnormalities (n=2), a large number of movement outliers in fMRI data (n=21; see data quality control procedures below), scanner malfunctions (n=2), inadequate signal in our amygdala regions of interest (n=14; see coverage description below), poor behavioral performance (n=20; accuracy lower than 75%), incomplete fMRI data collection (n=1), and failed genotyping data (n=1). The final sample reported includes 661 total participants (age=19.64±1.24; 293 males; 121 with at least one DSM-IV Axis I disorder(s) including Bipolar (n=11), Generalized Anxiety (n=12), Panic (n=6), Agoraphobia (n=10), OCD (n=6), Social Anxiety (n=6), Alcohol Abuse (n=44), Alcohol Dependence (n=29), Cannabis Abuse n=14) and Cannabis dependence (n=8) ; 311 European Americans, 73 African Americans, 173 Asians, 40 Latino/as, and 64 multiracial or “other”).
Genotyping
Genomic DNA was isolated from saliva collected using Oragene DNA self-collection kits (DNA Genotek, Inc.) customized for 23andMe (www.23andme.com). DNA extraction and genotyping was performed by the National Genetics Institute (NGI), a CLIA-certified clinical laboratory and subsidiary of the Laboratory Corporation of America. Custom Illumina BeadChip arrays were used to provide genome-wide data from which the following single nucleotide polymorphisms (SNPs) were extracted: FAAH rs324420 and CRHR1 rs110402. SNPs were coded according to minor allele carrier status due to low numbers of minor allele homozygotes in cells: CRHR1 G carriers (n=415; CRHR1 heterozygotes (n=277) and CRHR1 G homozygotes (n=138)), and CRHR1 A homozygotes (n=246) and FAAH A carriers (n=249; FAAH heterozygotes (n=229) and FAAH A homozygotes (20)) and FAAH C homozygotes (n=412). The genotyping rate of rs110402 was 99.9% and HWE criteria was met (all ps>.22) within each self-reported ethnicity subsample (Supplementary Table S2). The genotyping rate of rs324420 was 100% and was within HWE in each self-reported ethnicity subsample (all ps>.11). To account for differences in ancestral background, ancestrally-informative principal components were generated from eigenstrat v5.0.1 (30). K means cluster plotting and visual inspection of the top 10 components revealed that the top 5 principal components accounted for the various subgroups within our study population (Supplementary Figure S1).
fMRI paradigm
Our widely used amygdala activity paradigm consists of four face-matching task blocks interleaved with five shape-matching control blocks (31, 32). During face-matching task blocks, participants view a trio of faces expressing angry, fearful, surprised, or neutral emotions (Ekman & Friesen, 1976), and select which of two faces on the bottom matches the target face on top. Each expression-specific block (e.g., fearful facial expressions only) consists of six individual trials, with stimuli balanced for gender. Each of the six face trios is presented for four seconds with a variable inter-stimulus interval (ISI) of two to six seconds (mean=four seconds); total block length is 48 seconds. During shape-matching control blocks, participants view a trio of geometric shapes (i.e., circles, horizontal and vertical ellipses) and select which of the two shapes on the bottom matches the target shape on top. Each control block consists of 6 different shape trios, each presented for four seconds (ISI = two seconds), making a total block length of 36 seconds. All blocks are preceded by a brief instruction (“Match faces” or “Match shapes”) lasting two seconds. The total paradigm is 390 seconds in duration. Reaction times and accuracy are recorded through an MR-compatible button box.
fMRI acquisition parameters
Participants were scanned at the Duke-UNC Brain Imaging and Analysis Center using two identical GE MR750 3T scanners equipped with high-power high-duty-cycle 50-mT/m gradients at 200 T/m/s slew rate, and an eight-channel head coil for parallel imaging at high bandwidth up to 1MHz. Blood oxygen level-dependent (BOLD) fMRI data were acquired using a semiautomated high-order shimming program was used to ensure global field homogeneity. A series of 34 interleaved axial functional slices aligned with the anterior commissure-posterior commissure (AC-PC) plane were acquired for full-brain coverage using an inverse-spiral pulse sequence to reduce susceptibility artifact [TR/TE/flip angle=2000 ms/30 ms/60; FOV=240 mm; 3.75×3.75×4 mm voxels (selected to provide whole brain coverage while maintaining adequate signal-to-noise and optimizing acquisition times); interslice skip=0]. Four initial RF excitations were performed and subsequently discarded to achieve steady-state equilibrium. To allow for spatial registration of each participant’s data to a standard coordinate system, high-resolution three-dimensional structural images were acquired in 34 axial slices co-planar with the functional scans (TR/TE/flip angle=7.7 s/3.0 ms/12; voxel size=0.9×0.9×4 mm; FOV=240 mm, interslice skip=0).
fMRI processing and analysis
Whole-brain image analysis was completed using the general linear model of Statistical Parametric Mapping 8 (http://www.fil.ion.ucl.ac.uk/spm). Images for each participant were first realigned to the first volume in the time series to correct for head motion before being spatially normalized into the standard stereotactic space of the Montreal Neurological Institute (MNI) template using a 12-parameter affine model. Data were then smoothed to minimize noise and residual differences in gyral anatomy with a 6mm full-width at half-maximum (FWHM) Gaussian filter. Voxel-wise signal intensities were ratio normalized to the whole-brain global mean. The ARTifact Detection Tool (ART) (33) was used to generate regressors accounting for volumes associated with large motion (i.e., >0.6mm relative to the previous time frame) or spiking artifacts (i.e., global mean intensity 2.5 standard deviations from the entire time series). Participants for whom more than 5% of acquisition volumes were flagged by ART (n = 21) were removed from analyses. An ROI mask (AAL template) from WFU pickatlas (34) was used to ensure adequate BOLD signal across the amygdala. Participants who had less than 90% coverage of the amygdala (n=14) were excluded from analyses.
Linear contrasts using canonical hemodynamic response functions were then used to estimate temporal habituation as the linear decrease over successive face-matching task blocks (i.e., block 1>2>3>4) within right and left basolateral amygdala subregions defined using anatomical probability maps (35). The basolateral amygdala was selected as the region of interest due to the effects of eCB signaling documented in this region in rodent models (1, 3).
Individual contrast images (i.e., weighted sum of the beta images) were used in second-level random effects models accounting for scan-to-scan and participant-to-participant variability to determine mean habituation responses using one-sample t-tests. A voxel-level statistical threshold of p < 0.05, family-wise error (FWE) corrected for multiple comparisons across the bilateral ROIs, and a cluster-level extent threshold of 10 contiguous voxels was applied to these analyses. Parameter estimates from maximal voxels in the right and left clusters exhibiting a main effect for the habituation contrast were extracted using the VOI tool (http://www.fil.ion.ucl.ac.uk/spm) and exported for regression analyses in SPSS (v.20). Extracting parameter estimates from clusters exhibiting a main effect of condition, rather than those specifically correlated with our independent variables of interest, prevents correlation coefficient inflation that may result when an explanatory covariate is used to select a ROI. This conservative strategy has been implemented successfully in our prior studies (36–38).
Statistical analyses
Prior to analyses all habituation values from the above analyses were winsorized to maintain variability but constrain the influence of extreme outliers (i.e., following data quality control procedures, outliers more than ± 3 SDs were set at ± 3 SDs from the mean for 10 values from the left basolateral amygdala (1.5%) and five values for the right (.76%). Moderation analyses were conducted using linear regression using the PROCESS macro (39) in SPSS to test whether FAAH rs324420 x CRHR1 rs110402 genotype interactions predicted amygdala habituation after accounting for independent main effects of each genotypes as well as covariates for participant gender and ancestry using PC values. The interactions between all covariates and predictor variables (e.g., FAAH genotype x gender, CRHR1 genotype x PC 1) were included as additional covariates to better isolate the genotype interactions of specific interest (40). All predictor variables were mean centered prior to the computation of interaction terms. Significant interactions were probed using Johnson-Neyman post-hoc analyses. As an extension of these primary analyses, we examined whether amygdala habituation was predictive of any DSM-IV anxiety disorder. Any significant associations were then tested for moderated mediation to assess whether the FAAH rs324420 by CRHR1 rs110402 genotype interaction was indirectly associated with anxiety disorder diagnosis through blunted amygdala habituation. Covariates here, were identical to those included in the FAAH rs324420 by CRHR1 rs110402 genotype moderation analysis above.
RESULTS
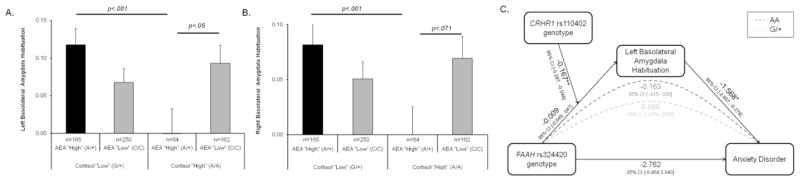
As previously reported (1), there was significant temporal habituation of left and right basolateral amygdala activation across all participants (Figure 2). A significant FAAH rs324420 x CRHR1 rs110402 genotype interaction predicted habituation in the left (F(1,639) = 7.39, p = .0067, β= −0.167, ΔR2 = 0.011) and right basolateral amygdala (F(1,639) = 7.46, p = .0065, β= −0.142, ΔR2 = 0.011; see Supplementary Table S3 for full regression model). As predicted, post-hoc tests demonstrated that individuals with a genetic background associated with relatively increased AEA inhibitory tone and increased CRH signaling (i.e., FAAH 385A allele carriers who were also CRHR1 A allele homozygotes) exhibited the least amygdala habituation (Figure 3A and B). This group of individuals exhibited significantly blunted habituation in comparison to those with relatively increased AEA but decreased CRH (i.e., FAAH 385A allele carriers who were also CRHR1 G allele carriers; left: F(1,241)=13.22, p=.0003; right: F(1,241)=11.35, p = .001) as well as those with relatively decreased AEA but increased CRH (i.e., FAAH C385 G allele homozygotes who were also CRHR1 A allele homozygotes in the left basolateral amygdala; F(1,238)=4.08, p = .045. and a trend level non-significant difference in the right basolateral amygdala; F(1,238)=3.28, p=.071). Although not significant, potentially due to reductions in power, a similar pattern is observed in a subsample of European/European-American participants only (Supplementary Figure S2; n=311; left: F(1,304) =3.22, p =.074, right: F(1,304) = 3.36, p = .068).
Figure 2. Basolateral amygdala habituation during face-matching task blocks.
Overlay illustrating clusters in the right and left basolateral amygdala exhibiting significant temporal habituation modeled as the linear decrease over successive face-matching task blocks (i.e., block 1 > block 2 > block 3 > block 4). Statistics and coordinates of peak voxel within each significant region of interest (ROI). Left basolateral amygdala: t=9.00; x= −22, y=−8, z=−16. Right basolateral amygdala: t=9.25, x=−22,y=−8,z=−16. All ps<.001, FWE < .05.
Figure 3. Genetic polymorphisms affecting anandamide and CRHR1 signaling predict basolateral amygdala function which indirectly mediates an increased risk for anxiety disorders.
A significant interaction between FAAH rs324420 x CRHR1 rs110402 genotypes predicts temporal habituation of the left (A) and right (B) basolateral amygdala. (A) and (B). Individuals with relatively increased AEA inhibitory tone and increased CRH signaling (i.e., FAAH 385A allele carriers who were also CRHR1 A homozygotes) show the least temporal habituation. Error bars indicate SEM. C). Relatively reduced amygdala habituation mediated a significant increase in rates of anxiety disorders among individuals with relatively increased AEA inhibitory tone and increased CRH signaling (i.e., FAAH 385A allele carriers who were also CRHR1 A homozygotes). Pathway coefficients represent unstandardized betas. *p<.05, **p<.01.
In our full sample, 36 participants met DSM-IV criteria for a past or present anxiety disorder (Supplementary Table S1). While there was no significant direct effect of FAAH rs324420 x CRHR1 rs110402 genotype interactions on anxiety disorder diagnosis (b=−2.76, p=0.42), there was a significant association between blunted left basolateral amygdala habituation and increased risk for an anxiety disorder (b=−1.57, p=.02). Moreover, a moderated mediation analysis indirectly linked the interaction between FAAH and CRHR1 genotypes to diagnosis of an anxiety disorder through left basolateral amygdala habituation (0.26; bootstrapped 95% Confidence Intervals (CI): Lower limit (LL): 0.029, Upper limit (UL): 0.603; Figure 3C). This moderated mediation was due to a conditional indirect effect wherein individuals with relatively increased AEA inhibitory tone and increased CRH signaling (i.e., FAAH 385A allele carriers who were also CRHR1 A homozygotes) were more likely to have an anxiety disorder as a function of reduced amygdala habituation (−0.163, bootstrapped 95% CI LL: −0.414, UL: −0.005). No such effect was found for individuals with relatively increased inhibitory tone and decreased CRH signaling (i.e., FAAH 385A allele carriers who were also CRHR1 G carriers; 0.099, bootstrapped 95% CI: LL: −0.008, UL: 0.264). Neither left amygdala habituation or interactive effects of FAAH rs324420 and CRHR1 rs110402 genotype predicted alcohol use disorders (the most prevalent form of psychopathology in the sample) or the presence of any DSM-IV Axis I psychopathology (see Supplemental Results).
DISCUSSION
The present results uniquely extend recent observations in rodents that stress-induced interactions between AEA and CRH signaling modulate amygdala function associated with anxiety and fear extinction (9). In rodents, stress-induced CRH signaling via CRHR1 in the basolateral amygdala results in increased activity of FAAH. The increased activity of this catabolic enzyme subsequently results in decreased AEA and a loss of inhibitory tone necessary for reducing anxiety and maintaining fear extinction. Here, we demonstrate parallel effects in humans using two functional genetic polymorphisms to model variability in AEA inhibitory tone and CRH signaling. Specifically, we find the least temporal habituation of the basolateral amygdala, a neuroimaging correlate of fear extinction, in individuals who have relatively high AEA inhibitory tone (i.e., FAAH 385A allele carriers) and relatively high CRH signaling (i.e., CRHR1 A allele homozygotes). Moreover, the blunted habituation of the left amygdala in these individuals mediated a significantly increased risk for anxiety disorders. Although speculative, the laterality of this mediated risk may reflect the preferential contributions of the left amygdala to sustained evaluation of threat (41), which is a distinguishing feature of anxiety disorders (42).
Our study is not without limitations. Modeling variability in signaling pathways using functional genetic polymorphisms does not provide direct evidence for these interactions in humans; establishing functional correlates of our target polymorphisms through assays of circulating cortisol or AEA concentrations would underscore the accuracy of our model (43). The amygdala habituation phenotype we examined may be associated with fear extinction; for instance, during early extinction trials the amygdala is activated by stimuli previously conditioned to aversive outcomes, while in late extinction trials it is not (20, 21). However, our task did not condition individuals to specific stimuli. Instead, it relied upon stimuli that have presumably been conditioned in everyday experience (e.g., facial expressions of fear). As such, amygdala habituation as presently measured is not a direct analogue of traditional fear extinction (e.g., to a stimulus previously conditioned within the laboratory; e.g., (25). Further, we did not collect behavioral measures of fear extinction (e.g., skin conductance response; (1, 13), which may, speculatively mediate associations between amygdala habituation and anxiety disorder risk. The concurrent examination of neural and behavioral phenotypes in the context of imaging genetics research may provide clues to these relationships in future research.
The cross-sectional nature of our study is a further limitation regarding the contribution of the observed pathways to risk for anxiety disorders. Our moderated mediational model makes specific directional predictions regarding the link between amygdala habituation and psychopathology. While there is a robust but primarily non-human animal literature that is consistent with these directional assumptions, it is possible that alterations in habituation follow rather than precede the development of an anxiety disorder. Notably, however, blunted amygdala habituation has been observed in at risk children prior to the development of an anxiety disorder (24). Lastly, while the FAAH polymorphism investigated has been well characterized with regard to FAAH expression (10, 11) the functional characterization of the CRHR1 polymorphism is based upon previously reported associations with cortisol, a downstream consequence of CRHR1 activation (18), of which null reports exist (19) and which evidence suggests that childhood adversity may moderate (14).
Despite these limitations, our findings suggest conserved effects of interactions between AEA and CRH on amygdala function in rodents and humans and highlight the value of imaging genetics for translating preclinical findings to clinical phenotypes. These data are further useful for understanding how pharmacologic manipulation of the endocannabinoid system may be harnessed to treat anxiety and serve as a cautionary note on the potential importance of individual differences (44, 45). Our data suggest that relatively increased AEA inhibitory tone moderates the anxiogenic effects of increased CRH signaling through attenuated amygdala habituation. As such, targeted facilitation of AEA inhibitory tone (through FAAH inhibition) may decrease anxiety and promote fear extinction in the absence of high CRH signaling, but may have paradoxical effects in the presence of increased CRH signaling by pharmacologically increasing the dependence of amygdala regulation on AEA-mediated inhibitory tone. Alternatively, however, it is possible that enhanced anxiogenic effects of CRH in the context of high AEA may not simply be explained by overall levels, but by the increased ability of CRH against this background to compromise AEA signaling by increasing FAAH activity. Thus, FAAH inhibition may be even more effective in the context of both high CRHR1 and AEA signaling, as it may prevent the anxiogenic effects of CRH by blocking the ability of CRHR1-stimulated increases in FAAH to inhibit AEA. Research in rodents showing that stress-induced anxiety and related phenotypes are prevented by pharmacologically inhibiting FAAH is consistent with this interpretation (3, 5, 9). Further pharmacogenetic research is necessary, however, to dissociate these proposed models and validate the efficacy of FAAH inhibitors as a potential therapeutic treatment approach.
Supplementary Material
Acknowledgments
The Duke Neurogenetics Study is supported by Duke University and the National Institutes of Health (R01-DA033369). CHD receives support from NIH (T32-DA007313). ARH receives additional support from the National Institutes of Health (R01-DA031579 & R01-AG049789). RB was supported by the Klingenstein Third Generation Foundation; he receives additional support from the National Institutes of Health (R01-AG045231).
Footnotes
FINANCIAL DISCLOSURES
All authors report no biomedical financial interests or potential conflict of interest.
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gunduz-Cinar O, MacPherson KP, Cinar R, Gamble-George J, Sugden K, Williams B, et al. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Molecular psychiatry. 2013;18:813–823. doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TTY, Gray JM, et al. Endogenous cannabinoid signaling is essential for stress adaptation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9406–9411. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ, et al. Suppression of Amygdalar Endocannabinoid Signaling by Stress Contributes to Activation of the Hypothalamic-Pituitary-Adrenal Axis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:2733–2745. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunduz-Cinar O, Hill MN, McEwen BS, Holmes A. Amygdala FAAH and anandamide: mediating protection and recovery from stress. Trends in pharmacological sciences. 2013;34:637–644. doi: 10.1016/j.tips.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haller J, Barna I, Barsvari B, Gyimesi Pelczer K, Yasar S, Panlilio LV, et al. Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmacology. 2009;204:607–616. doi: 10.1007/s00213-009-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreira FA, Kaiser N, Monory K, Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2008;54:141–150. doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Rademacher DJ, Meier SE, Shi LY, Ho WSV, Jarrahian A, Hillard CJ. Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology. 2008;54:108–116. doi: 10.1016/j.neuropharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. The European journal of neuroscience. 2005;21:1057–1069. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- 9.Gray JM, Vecchiarelli HA, Morena M, Lee TT, Hermanson DJ, Kim AB, et al. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:3879–3892. doi: 10.1523/JNEUROSCI.2737-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8394–8399. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiang KP, Gerber AL, Sipe JC, Cravatt BF. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Human molecular genetics. 2004;13:2113–2119. doi: 10.1093/hmg/ddh216. [DOI] [PubMed] [Google Scholar]
- 12.Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F, et al. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biological psychiatry. 2009;66:9–16. doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dincheva I, Drysdale AT, Hartley CA, Johnson DC, Jing D, King EC, et al. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat Commun. 2015;6:6395. doi: 10.1038/ncomms7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: effects on hypothalamic-pituitary-adrenal axis reactivity. Biological psychiatry. 2009;66:681–685. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heim C, Bradley B, Mletzko TC, Deveau TC, Musselman DL, Nemeroff CB, et al. Effect of Childhood Trauma on Adult Depression and Neuroendocrine Function: Sex-Specific Moderation by CRH Receptor 1 Gene. Frontiers in behavioral neuroscience. 2009;3:41. doi: 10.3389/neuro.08.041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Archives of general psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cicchetti D, Rogosch FA, Oshri A. Interactive effects of corticotropin releasing hormone receptor 1, serotonin transporter linked polymorphic region, and child maltreatment on diurnal cortisol regulation and internalizing symptomatology. Development and psychopathology. 2011;23:1125–1138. doi: 10.1017/S0954579411000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sumner JA, McLaughlin KA, Walsh K, Sheridan MA, Koenen KC. CRHR1 genotype and history of maltreatment predict cortisol reactivity to stress in adolescents. Psychoneuroendocrinology. 2014;43:71–80. doi: 10.1016/j.psyneuen.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahon PB, Zandi PP, Potash JB, Nestadt G, Wand GS. Genetic association of FKBP5 and CRHR1 with cortisol response to acute psychosocial stress in healthy adults. Psychopharmacology. 2013;227:231–241. doi: 10.1007/s00213-012-2956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahs F, Kragel PA, Zielinski DJ, Brady R, LaBar KS. Medial prefrontal pathways for the contextual regulation of extinguished fear in humans. NeuroImage. 2015;122:262–271. doi: 10.1016/j.neuroimage.2015.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 22.Green SA, Hernandez L, Tottenham N, Krasileva K, Bookheimer SY, Dapretto M. Neurobiology of Sensory Overresponsivity in Youth With Autism Spectrum Disorders. JAMA psychiatry. 2015;72:778–786. doi: 10.1001/jamapsychiatry.2015.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swartz JR, Wiggins JL, Carrasco M, Lord C, Monk CS. Amygdala habituation and prefrontal functional connectivity in youth with autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:84–93. doi: 10.1016/j.jaac.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blackford JU, Allen AH, Cowan RL, Avery SN. Amygdala and hippocampus fail to habituate to faces in individuals with an inhibited temperament. Soc Cogn Affect Neurosci. 2013;8:143–150. doi: 10.1093/scan/nsr078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunduz-Cinar O, Flynn S, Brockway E, Kaugars K, Baldi R, Ramikie TS, et al. Fluoxetine Facilitates Fear Extinction Through Amygdala Endocannabinoids. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carey CE, Agrawal A, Zhang B, Conley ED, Degenhardt L, Heath AC, et al. Monoacylglycerol lipase (MGLL) polymorphism rs604300 interacts with childhood adversity to predict cannabis dependence symptoms and amygdala habituation: Evidence from an endocannabinoid system-level analysis. Journal of abnormal psychology. 2015;124:860–877. doi: 10.1037/abn0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheehan DV, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clincial Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 29.First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS American Psychiatric Press, Inc. Structured Clinical Interview for DSM-IV Axis II Personality Disorders, (SCID-II) Washington D.C: American Psychiatric Press, Inc; 1997. [Google Scholar]
- 30.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nature genetics. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 31.Ahs F, Davis CF, Gorka AX, Hariri AR. Feature-based representations of emotional facial expressions in the human amygdala. Social Cognitive and Affective Neuroscience. 2013 doi: 10.1093/scan/nst112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carre JM, Fisher PM, Manuck SB, Hariri AR. Interaction between trait anxiety and trait anger predict amygdala reactivity to angry facial expressions in men but not women. Soc Cogn Affect Neurosci. 2012;7:213–221. doi: 10.1093/scan/nsq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitfield-Gabrieli S. Artifact and QA Manual. MIT; 2009. [Google Scholar]
- 34.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 35.Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and embryology. 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- 36.Bogdan R, Williamson DE, Hariri AR. Mineralocorticoid receptor Iso/Val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. The American journal of psychiatry. 2012;169:515–522. doi: 10.1176/appi.ajp.2011.11060855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nikolova YS, Koenen KC, Galea S, Wang CM, Seney ML, Sibille E, et al. Beyond genotype: serotonin transporter epigenetic modification predicts human brain function. Nature neuroscience. 2014;17:1153–1155. doi: 10.1038/nn.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swartz JR, Knodt AR, Radtke SR, Hariri AR. A neural biomarker of psychological vulnerability to future life stress. Neuron. 2015;85:505–511. doi: 10.1016/j.neuron.2014.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, New York: Guilford Press; 2013. [Google Scholar]
- 40.Keller MC. Gene x environment interaction studies have not properly controlled for potential confounders: the problem and the (simple) solution. Biological psychiatry. 2014;75:18–24. doi: 10.1016/j.biopsych.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain research Brain research reviews. 2004;45:96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. The American journal of psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 43.Hill MN, Bierer LM, Makotkine I, Golier JA, Galea S, McEwen BS, et al. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology. 2013;38:2952–2961. doi: 10.1016/j.psyneuen.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaetani S, Dipasquale P, Romano A, Righetti L, Cassano T, Piomelli D, et al. The endocannabinoid system as a target for novel anxiolytic and antidepressant drugs. International review of neurobiology. 2009;85:57–72. doi: 10.1016/S0074-7742(09)85005-8. [DOI] [PubMed] [Google Scholar]
- 45.Hill MN, Gorzalka BB. The endocannabinoid system and the treatment of mood and anxiety disorders. CNS & neurological disorders drug targets. 2009;8:451–458. doi: 10.2174/187152709789824624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.