Abstract
Charcot-Marie-Tooth (CMT) disease is the most common inherited peripheral neuropathy with the majority of cases involving demyelination of peripheral nerves. The pathogenic mechanisms of demyelinating CMT remain unclear, and no effective therapy currently exists for this disease. The discovery that mutations in different genes can cause a similar phenotype of demyelinating peripheral neuropathy raises the possibility that there may be convergent mechanisms leading to demyelinating CMT pathogenesis. Increasing evidence indicates that ErbB receptor-mediated signaling plays a major role in the control of Schwann cell-axon communication and myelination in the peripheral nervous system. Recent studies reveal that several demyelinating CMT-linked proteins are novel regulators of endocytic trafficking and/or phosphoinositide metabolism that may affect ErbB receptor signaling. Emerging data have begun to suggest that dysregulation of ErbB receptor trafficking and signaling in Schwann cells may represent a common pathogenic mechanism in multiple subtypes of demyelinating CMT. In this review, we focus on the roles of ErbB receptor trafficking and signaling in regulation of peripheral nerve myelination and discuss the emerging evidence supporting the potential involvement of altered ErbB receptor trafficking and signaling in demyelinating CMT pathogenesis and the possibility of modulating these trafficking and signaling processes for treating demyelinating peripheral neuropathy.
Keywords: Charcot-Marie-Tooth disease, Demyelination, Endocytic trafficking, ErbB receptor, Peripheral neuropathy
Introduction
Charcot-Marie-Tooth (CMT) disease, also known as hereditary motor and sensory neuropathy, describes a heterogeneous group of inherited peripheral nervous system disorders affecting 1 in 2500 people worldwide [1]. CMT is divided into two forms, demyelinating CMT and axonal CMT. The majority (80 %) of CMT cases belongs to the demyelinating form where the primary defect is an inability of Schwann cells to properly form or maintain myelination of axons, resulting in marked decrease in nerve conduction velocity and secondary degeneration of axons [1, 2]. Clinical presentation of CMT is dependent on the specific gene mutation, but patients typically present with weakness, sensory loss, and/or muscle wasting that usually develop during the first two decades of life [1–4]. Some patients have an infantile onset of disease with severe debilitation requiring wheelchairs for ambulation by 20 years of age [5]. Unfortunately, there is no effective treatment for CMT [6], highlighting the need for a better understanding of CMT pathogenesis in order to identify disease-modifying therapies.
Human genetic studies indicate that demyelinating CMT can be caused by autosomal dominant (CMT1), autosomal recessive (CMT4), or X-linked dominant (CMT1X) mutations [1, 2]. The most common genetic defect for demyelinating CMT is duplication of the PMP22 gene, which causes excessive production of peripheral myelin protein 22 (PMP22) [7–9]. Missense mutations in PMP22 [10] or other myelin proteins such as myelin protein zero (MPZ) and connexin 32 are also common causes of demyelinating CMT [1, 2]. These mutations can result in a toxic buildup of misfolded myelin proteins [11] and/or a loss of myelin protein function [12, 13]. The identification of demyelinating CMT-linked mutations in several non-myelin proteins suggests the existence of alternative pathogenic mechanisms for causing this disease. Recent studies of these CMT-linked proteins have revealed their role as novel regulators of endocytic trafficking and/or phosphoinositide metabolism and indicate that their mutations can lead to defects in endocytic trafficking. How these trafficking defects cause de-myelinating peripheral neuropathy is an important, unresolved question. Furthermore, how diverse mutations in different genes cause a similar phenotype of demyelinating CMT is not understood. Based on the emerging data, we propose that dysregulation of ErbB receptor trafficking and signaling in Schwann cells may represent a common pathogenic mechanism in several subtypes of demyelinating CMT.
In this review, we will first provide an overview of current knowledge on ErbB receptor signaling in the control of peripheral nerve myelination and discuss how ErbB receptor signaling may be regulated by endocytic trafficking and phosphoinositides. We will then highlight recent findings linking endocytic trafficking defects to multiple subtypes of demyelinating CMT and discuss how these trafficking defects may alter ErbB receptor trafficking and signaling in Schwann cells and thereby contribute to demyelinating CMT pathogenesis. Finally, we will discuss the potential therapeutic benefits of targeting ErbB receptor trafficking and signaling pathways for treatment of demyelinating peripheral neuropathy.
ErbB Receptor Signaling in the Control of Myelination in Peripheral Nerves
In the peripheral nervous system, myelination of axons by Schwann cells enables saltatory conduction of nerve impulses that are vital to proper motor and sensory functions [14, 15]. Schwann cell-axon communication is essential for the formation, maintenance, and function of highly organized, myelinated peripheral nerves. Neuregulin-1 (Nrg1) signaling through ErbB receptor tyrosine kinases has emerged as a major mechanism for mediating Schwann cell-axon communication in regulation of myelination (Fig. 1). Schwann cells express only two members of the ErbB family of proteins, ErbB2 and ErbB3 [16, 17]. Because ErbB2 lacks ligand-binding ability and ErbB3 lacks kinase activity, these two proteins require heterodimerization to form a functional receptor. Nrg1 binds to ErbB3 and promotes ErbB2-mediated phosphorylation of tyrosine residues in the cytoplasmic domains of both ErbB2 and ErbB3 in the ErbB2/ErbB3 heterodimer [16]. Among the six types of Nrg1 identified, the axon membrane-bound form, Nrg1 type III, is the primary ligand for activating ErbB2/ErbB3 receptor in Schwann cells in vivo to promote myelination [18–20]. In addition, Nrg1 type I, which is likely mainly produced by Schwann cells, can induce ErbB2/ErbB3 receptor activation via autocrine signaling [21, 22]. The soluble form of Nrg1 type I, which is either naturally produced or shredded from the membrane-anchored form by peptidases such as ADAM10 or ADAM17 (a disintegrin and metallopeptidase domain 10 or 17) or BACE1 (beta-secretase 1) [23–26], could also promote myelination and remyelination after nerve injury in addition to its role in maintaining Schwann cell survival [22, 27, 28].
Fig. 1.
ErbB receptor-mediated signaling in regulation of myelination. Binding of Nrg1 induces heterodimerization of ErbB2 and ErbB3 on Schwann cell surface and activation of the ErbB2/ErbB3 receptor, leading to activation of multiple downstream signaling pathways. Activated ErbB2/ErbB3 receptor stimulates class I PI3K to produce PI(3,4,5)P3 from PI(4,5)P2 (step 1), which activates Akt (step 3) signaling. This process is antagonized by PTEN which dephosphorylates PI(3,4,5)P3 back to PI(4,5)P2 (step 2). Activation of ErbB2/ErbB3 receptor also causes activation of Mek (step 4) and Erk (step 5) signaling. Endosomal PI(5)P production involves a series of reactions mediated by PI5K which produces PI(3,5)P2 from PI(3)P (step 6) and by MTMRs which convert PI(3,5)P2 to PI(5)P (step 7). The presence of PI(5)P promotes Akt signaling (step 8). In addition to the PI3K/Akt and Mek/Erk pathways, ErbB receptor also activates JNK1/c-Jun signaling (steps 9 and 10) and PLCγ/calcineurin signaling (steps 11 and 12). Together, these pathways regulate myelination and myelin maintenance
Activation of ErbB2/ErbB3 receptor by Nrg1 leads to activation of multiple downstream signaling pathways (Fig. 1). Upon stimulation by activated ErbB2/ErbB3 receptor, class I phosphoinositide 3-kinase (PI3K) [29] phosphorylates the D3 hydroxyl group of the lipid phosphoinositide PI(4,5)P2 to generate PI(3,4,5)P3 (Fig. 1, step 1). This reaction can be reversed by a lipid phosphatase known as phosphatase and tensin homolog (PTEN) [30], which removes the phosphate group added by PI3K (Fig. 1, step 2). PI(3,4,5)P3 is a key lipid signaling molecule in Schwann cells that promotes myelination, as suggested by the finding that elevated level of PI(3,4,5)P3 resulted from Schwann cell-specific inactivation of PTEN causes hypermyelination [31]. The major downstream effector of PI(3,4,5)P3 is Akt [32], which has been proposed as a key mediator of promyelination signaling in Schwann cells (Fig. 1, step 3). Activation of ErbB2/ErbB3 receptor by either Nrg1 type I or Nrg1 type III also stimulates downstream Mek (Fig. 1, step 4) and Erk (Fig. 1, step 5) signaling, as seen in cultured Schwann cells [15, 20] and peripheral nerves of mice [33]. The sustained activation of Mek-Erk signaling has been implicated in several studies to inhibit myelination [33, 34]. Recent work has shown that activated ErbB2/ErbB3 receptors can also activate PLCγ/calcineurin signaling (Fig. 1, steps 9 and 10) to cause Schwann cell differentiation and promote myelination [35]. In addition, ErbB2/ErbB3 receptor activation has been reported to stimulate JNK1/c-Jun signaling (Fig. 1, steps 11 and 12) for causing demyelination in Schwann cells [36]. While it is unclear as to how ErbB2/ErbB3 receptor activation coordinately controls these various signaling cascades in Schwann cells, the balance of these promyelination and demy-elination signaling downstream of ErbB receptors is likely to be a critical determinant in the control of myelin formation and maintenance. Future studies are needed to investigate how the signaling output of Nrg1-activated ErbB2/ErbB3 receptor is determined and modulated in Schwann cells to regulate myelination in peripheral nerves.
Regulation of ErbB Receptor Signaling by Endocytic Trafficking
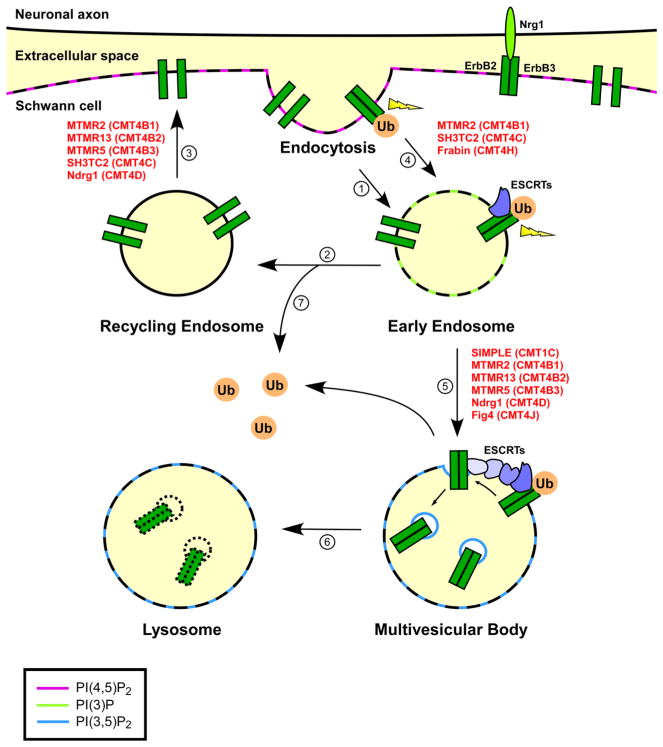
Increasing evidence indicates that ErbB receptor signaling is regulated by endocytic trafficking which controls intracellular distribution and degradation of ErbB receptors [37–40]. Our current understanding of endocytic trafficking of ErbB receptors is largely based on studies of ErbB1 (also known as epidermal growth factor receptor (EGFR)) trafficking. Relatively little is presently known about ErbB2 and ErbB3 endocytic trafficking, particularly in Schwann cells. It has become clear that ErbB receptors can undergo constitutive endocytosis in the absence of ligand (Fig. 2, step 1) followed by recycling to the cell surface (Fig. 2, steps 2 and 3). Binding of ligand such as Nrg1 to ErbB receptors not only activates downstream signaling pathways at the plasma membrane but also induces rapid endocytosis of ErbB receptors and trafficking to the early endosome (Fig. 2, step 4). The internalized ErbB receptors can still signal to downstream effector proteins at endosomes because their activated intracellular domains remain exposed to the cytoplasm. The signaling pathways transmitted at endosomes can be qualitatively different from those originated at the plasma membrane, as has been shown for EGF-activated ErbB1/EGFR [41]. Recent studies have shown that Nrg1-activated ErbB2/ErbB3 receptors could undergo en-docytosis in Schwann cells [42, 43]; however, it remains to be determined whether activated ErbB2/ErbB3 receptors trigger distinct signaling pathways at the plasma membrane and endosomes.
Fig. 2.
Proposed model of dysregulated ErbB receptor trafficking and signaling in demyelinating CMT. ErbB2 and ErbB3 proteins may undergo constitutive endocytosis in the absence of ligand (step 1) and then travel through recycling endosomes (step 2) back to the cell surface (step 3). Binding of Nrg1 triggers heterodimerization and activation of ErbB2/ErbB3 receptor, leading to activation of downstream signaling pathways at the plasma membrane (thunderbolt). Ligand binding also promotes ubiquitination (Ub) and endocytosis of ErbB receptors (step 4). The internalized ErbB receptors can still signal to their downstream effectors at the early endosome (thunderbolt). Ubiquitination of ErbB receptors may also occur at the early endosome. The ubiquitinated ErbB receptors are recognized and sorted at the early endosome by ESCRTs into intraluminal vesicles to form multivesicular bodies (step 5). Once inside the multivesicular bodies, ErbB receptors can no longer produce signaling output. As multivesicular bodies fuse with lysosomes, ErbB receptors are degraded and their signaling is completely terminated (step 6). Removal of ubiquitin signals from ErbB receptors at the early endosome by deubiquitinating enzymes (step 7) allows recycling of these receptors back to cell surface (steps 2 and 3). Mutations in proteins associated with various types of demyelinating CMT (shown in red) can affect endocytosis, endosome-to-lysosome trafficking, and endocytic recycling to cause demyelinating peripheral neuropathy. Specific phosphoinositides enriched in various membrane compartments are indicated by different colors as defined in the legend
Upon arrival at the early endosome, the internalized ErbB receptors face a sorting decision between recycling to the plasma membrane (Fig. 2, steps 2 and 3) or sorting into the intraluminal vesicles of multivesicular bodies (MVBs) for delivery to the lysosome for degradation (Fig. 2, steps 5 and 6). The endosomal sorting complex required for transport (ESCRT) machinery, composed of ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III complexes, has emerged as a central player in the control of endosome-to-lysosome trafficking of cargo proteins, such as internalized ErbB receptors [44]. As in the case of other cargo proteins, ubiquitination of ErbB receptors serves as an endosomal sorting signal for transport to the lysosomal pathway for degradation. The ubiquitinated ErbB receptors are recognized and sequestered by ESCRT-0 complex, which then recruits ESCRT-I, ESCRT-II, and ESCRT-III complexes to facilitate incorporation of ErbB receptors into endosomal invaginations and inward budding of MVB vesicles (Fig. 2, step 5). Budding of ErbB receptor-containing vesicles into the lumen of MVBs prevents ErbB receptor signaling to downstream effector proteins, thereby attenuating signal transduction. Subsequent fusion of MVBs with lysosomes delivers ErbB receptors into the lumen of lysosomes for degradation by lysosomal proteases (Fig. 2, step 6) and thus enables termination of ErbB receptor signaling.
We propose that the conceptual framework established from studies of ErbB1 receptor trafficking and signaling (Fig. 2) may also be applied to ErbB2/ErbB3 receptors in Schwann cells. Previous studies have shown that Nrg1-induced activation causes ErbB3 endocytosis and subsequent degradation by the lysosome in MCF7 breast tumor cells, although the ErbB3 degradation occurs at a slower rate than that of ErbB1 [45, 46] Furthermore, our recent work has revealed that, in response to Nrg1 binding, ErbB3 in Schwann cells can undergo endocytic degradation, which is associated with the termination of Nrg1-activated ErbB2/ErbB3 receptor signaling [43, 47]. It is unclear whether or not ErbB2 can undergo Nrg1-induced degradation, and ErbB2 has been reported to undergo rapid recycling from early endosomes to the cell surface [48, 49]. Given the importance of Nrg1-activated ErbB2/ErbB3 receptor signaling in the peripheral nervous system, it is important for future studies to elucidate the molecular mechanisms governing endocytic trafficking of Nrg1-activated ErbB2/ErbB3 receptors in Schwann cells and determine how ErbB2/ErbB3 trafficking affects the potency, duration, and functional outcomes of ErbB2/ErbB3 receptor-mediated signaling in the control of myelin formation and maintenance.
Modulation of ErbB Receptor Trafficking and Signaling by Phosphoinositides
Phosphoinositides (also known as inositol phospholipids) are increasingly recognized as important, lipid regulators of endocytic trafficking [50]. The enrichment of distinct phosphoinositides in different subcellular membranes not only helps define the identity of these membrane compartments but also serves as a mechanism for recruiting phosphoinositide-binding proteins to regulate endocytic trafficking of cargo proteins, such as ErbB receptors (Fig. 2). The phosphoinositide PI(4, 5)P2 is enriched at the plasma membrane at sites of clathrin-mediated endocytosis [51] to facilitate the formation of clathrin-coated vesicles for receptor internalization (Fig. 2, step 4). The phosphoinositide enriched in early endosomes is PI(3)P, which is generated by class III phosphoinositide 3-kinase PIKC3/Vps34 [52]. The endosomal PI(3)P is recognized by the PI(3)P-binding, FYVE (Fab1, YOTB, Vac1, and EEA1 homology) domain in ESCRT-0 subunit Hrs [52, 53] to recruit Hrs to the endosomal membrane and promote ESCRT-mediated endosomal cargo sorting and MVB vesicle formation (Fig. 2, step 5). PI(3)P can be further phosphorylated at the D5 hydroxyl group by phosphoinositide 5-kinase (PI5K) to produce PI(3,5)P2, which preferentially localizes at the limiting membranes of MVBs/late endosomes and lysosomes [50] to promote the trafficking of ErbB receptors and other cargos to the lysosomes for degradation (Fig. 2, step 5). Our current understanding of the regulatory roles of phosphoinositides in ErbB receptor trafficking is based on studies of ErbB1, and how various phosphoinositides regulate ErbB2/ErbB3 receptor trafficking in Schwann cells is an unexplored question that requires future investigation.
By regulating endocytic trafficking of ErbB receptors, phosphoinositides can impact on ErbB receptor-mediated signaling, as discussed in the preceding section. In addition, phosphoinositides can also have a direct role in regulating ErbB downstream signaling pathways. The phosphoinositide PI(3,4,5)P3 generated at the plasma membrane by class I PI3K (Fig. 1, step 1) recruits and activates the kinase Akt to enhance promyelination signaling in Schwann cells [32]. PI(5)P, a product of endosomal phosphoinositide metabolism [54, 55], also promotes Akt activation (Fig. 1, steps 6–8). In addition, the 3-phosphatase PTEN downregulates PI(3,4,5)P3 (Fig. 1, step 2) and thereby negatively regulates Akt signaling in the control of myelination [31, 56]. Future studies are needed to understand the mechanisms by which different phosphoinositides regulate endocytic trafficking and signaling of ErbB2/ErbB3 receptors in the control of the formation and function of myelinated peripheral nerves.
Dysregulation of Endocytic Trafficking as an Emerging Theme in Demyelinating CMT
Recent studies reveal that a number of demyelinating CMT-linked proteins are novel regulators of endocytic trafficking and suggest that their disease-associated mutations may lead to trafficking defects at different steps of the endocytic pathway, including endocytosis, endosomal recycling, and endosome-to-lysosome trafficking (Table 1). Emerging evidence indicates that dysregulation of endocytic trafficking processes is a common theme shared by multiple subtypes of demyelinating CMT.
Table 1.
Summary of endocytic trafficking defects implicated in different subtypes of demyelinating CMT
| Trafficking defect | CMT type | Inheritance | Mutant protein | References |
|---|---|---|---|---|
| Endocytosis dysfunction | CMT4B1 | AR | MTMR2 | [67] |
| CMT4C | AR | SH3TC2 | [42] | |
| CMT4H | AR | Frabin | [72] | |
| Endosomal recycling inhibition | CMT4B1 | AR | MTMR2 | [73] |
| CMT4B2 | AR | MTMR13 | [73] | |
| CMT4B3 | AR | MTMR5 | [73] | |
| CMT4C | AR | SH3TC2 | [77, 78] | |
| CMT4D | AR | Ndrg1 | [80] | |
| Endosome-to-lysosome trafficking impairment | CMT1C | AD | SIMPLE | [43] |
| CMT4B1 | AR | MTMR2 | [91] | |
| CMT4B2 | AR | MTMR13 | [128] | |
| CMT4B3 | AR | MTMR5 | [62] | |
| CMT4D | AR | Ndrg1 | [81] | |
| CMT4J | AR | Fig 4 | [97] |
AD autosomal dominant, AR autosomal recessive
Endocytosis Impairment
Endocytosis dysfunction has recently been implicated in the pathogenesis of several recessively inherited types of demye-linating CMT, including CMT4B, CMT4C, and CMT4H (Fig. 2, steps 1 and 4). CMT4B has three subtypes, CMT4B1, CMT4B2, and CMT4B3, which are caused by mutations in MTMR2, MTMR13, and MTMR5, respectively [57–63]. These MTMR genes encode ubiquitously expressed, myotubularin-related protein (MTMR) family of phosphoinositide 3-phosphatases [64, 65]. MTMR2 is a catalytically active 3-phosphatase that dephosphorylates PI(3)P and PI(3,5)P2 to PI and PI(5)P, respectively [64], whereas MTMR13 and MTMR5 are catalytically inactive phosphatases that associate with MTMR2 to enhance its 3-phosphatase activity [64, 66]. The cellular functions of these MTMR proteins remain poorly understood. A recent study showed that short hairpin RNA (shRNA)-mediated depletion of endogenous MTMR2 in cultured hippocampal neurons causes enhanced endocytosis of AMPA receptor subunit GluR2 and suggested that MTMR2 may negatively regulate endocytosis through its interaction with PSD95 [67]. Furthermore, studies in Drosophila reveal that Mtm phosphatase, the single homologue of both human MTM1 and MTMR2, may regulate endocytosis and cortical remodeling by acting on a pool of PI(3)P generated by class II phosphoinositide 3-kinase at the plasma membrane [68, 69]. These findings support a potential link between dysregulated endocytosis and CMT4B pathogenesis.
CMT4C-causing mutations are found in the SH3TC2 gene that encodes a 1288 amino acid protein of unknown function with two Src homology 3 (SH3) domains and multiple tetratricopeptide repeats (TPRs) [70]. SH3TC2 is expressed in Schwann cells as well as in other cell types, where a population of SH3TC2 is localized at the plasma membrane [71]. A recent study reported that SH3TC2 regulates endocytosis of Nrg1-activated ErbB2 receptors in Schwann cells [42]. Although the mechanism by which SH3TC2 regulates endocytosis remains elusive, it is possible that SH3TC2 may facilitate endocytosis via the interaction of its SH3 domains with proline-rich regions of proteins involved in controlling clathrin-mediated vesicle endocytosis. CMT4C-linked SH3TC2 mutations were found to impair the localization of SH3TC2 at the plasma membrane [71] and disrupt the ability of SH3TC2 to facilitate ErbB2 receptor endocytosis [42], supporting the involvement of dysregulated endocytosis in CMT4C pathogenesis.
CMT4H is an early-onset peripheral neuropathy caused by mutations in the Fdg4 gene that encodes FGD1-related actin filament-binding protein (Frabin), a guanine nucleotide exchange factor for the Rho GTPase Cdc42 [72]. Frabin is expressed in a wide variety of tissues, and its cellular function remains poorly understood. A recent study has shown that shRNA-mediated depletion of endogenous Frabin leads to inhibition of transferrin receptor internalization in rat RT4 Schwann cells [72], suggesting a role of Frabin in regulation of endocytosis. Because CMT4H is a recessively inherited disease caused by loss-of-function mutations in Frabin, these results implicate endocytosis impairment as a potential pathogenic mechanism in CMT4H neuropathy.
Endosomal Recycling Dysfunction
Emerging evidence points to a link between dysregulation of endosomal recycling and the pathogenesis of demyelinating CMT types CMT4B, CMT4C, and CMT4D (Fig. 2, step 3). Recent findings in Drosophila suggest a role for CMT4B-associated proteins MTMR2, MTMR13, and MTMR5 in regulation of endosomal recycling, as Drosophila Sbf, the single homologue of human MTMR5 and MTMR13, was found to function together with the MTM1/MTMR2 homologue Mtm and GTPases Rab21 and Rab11 in regulating endosomal recycling to the plasma membrane for macrophage protrusion formation [73]. Interestingly, a proteomic analysis has identified receptor-mediated endocytosis 8 (RME-8) as a MTMR2-regulated, PI(3)P-binding protein [74]. Although RME-8 was initially identified as a protein required for endocytosis in C. elegans [75], recent evidence indicates that RME-8 is primarily involved in regulation of postendocytic trafficking, such as Notch recycling from endosome to the plasma membrane [76] These results implicate a role for altered endosomal recycling in CMT4B pathogenesis.
Studies of CMT4C-associated protein SH3TC2 have shown that, in addition to its localization at the plasma membrane, SH3TC2 also localizes to the recycling endosomes [71, 77]. Two groups have shown that SH3TC2 preferentially binds the GTP-bound form of small GTPase Rab11 and promotes recycling of internalized transferrin receptors to the cell surface in HeLa cells [77, 78], supporting a function of SH3TC2 as a novel Rab11 effector in the regulation of endosomal recycling. Furthermore, these groups reported that CMT4C-associated mutations abolish the interaction of SH3TC2 with Rab11, causing SH3TC2 mislocalization from recycling endosomes to the cytosol and impaired transferrin receptor recycling in HeLa and HEK293 cells [77, 78]. These findings provide evidence supporting the potential involvement of endosomal recycling dysfunction in the pathogenesis of CMT4C neuropathy.
CMT4D is an autosomal recessive demyelinating neuropathy caused by mutations in the NDRG1 gene encoding N-myc downstream regulated 1 (Ndrg1), a ubiquitously expressed protein which is primarily localized to the cytoplasm and endosomes [79, 80]. Although the function of Ndrg1 remains unclear, recent studies have shown that Ndrg1 has a role in regulating recycling of internalized proteins such as low-density lipoprotein (LDL) receptor in A431 cells and E-cadherin in HEK293 cells through recycling endosomes to the cell surface [80, 81]. A truncation mutation responsible for most CMT4D cases produces a non-functional form of Ndrg1 that is unable to facilitate endosomal recycling [81]. Although it remains to be determined whether Ndrg1 has a role in regulating recycling of LDL receptor, E-cadherin, or other cargos in peripheral nerves, these results from studies in cell lines raise the possibility that impaired endosomal recycling may be involved in CMT4D neuropathy.
Defective Endosome-to-Lysosome Trafficking
Dysregulation of endosome-to-lysosome trafficking has emerged as a common theme for several types of demyelinating CMT that include the autosomal dominant form CMT1C and autosomal recessive forms CMT4B, CMT4D, and CMT4J (Fig. 2, step 5). CMT1C is caused by missense mutations in the gene encoding small integral membrane protein of lysosome/late endosome (SIMPLE; also known as lipopolysaccharide-induced TNF factor (LITAF)), a ubiquitously expressed protein of unknown function [82–85]. Our group has recently generated highly specific anti-SIMPLE antibodies and found that endogenous SIMPLE is an early endosomal membrane protein [43, 47] rather than a nuclear protein [86] or a lysosomal/late endosomal membrane protein [87] as previously suggested. Our studies reveal that SIMPLE is a novel regulator of endosome-to-lysosome trafficking, acting with the ESCRT machinery in controlling endosomal sorting of cargo proteins (e.g., EGFR and ErbB3 receptors) to the lysosomal pathway for degradation [43, 47]. We found that SIMPLE is a post-translationally inserted, C-tail-anchored endosomal membrane protein and its membrane insertion is impaired by CMT1C-linked mutations, resulting in SIMPLE mislocalization from the endosomal membrane to the cytosol [43, 47]. We further showed that the disease-causing SIMPLE mutant proteins are loss-of-function mutants that exert dominant pathogenic effects to disrupt endosome-to-lysosome trafficking by interfering with ESCRT recruitment to the endosomes [43]. These findings provide strong evidence linking dysfunction of ESCRT-regulated endosome-to-lysosome trafficking to demyelinating CMT pathogenesis.
A recent study of CMT4D-associated protein Ndrg1 [81] reported that siRNA-mediated depletion of endogenous Ndrg1 not only impairs endosomal recycling of LDL receptors to the cell surface but also causes a reduction in ESCRT protein levels and impaired endosome-to-lysosome trafficking and degradation of LDL receptors in A431 cells. Although similar experiments have not yet been performed in Schwann cells, the findings from the study of Ndrg1 in A431 cells [81], together with the autosomal recessive inheritance of CMT4D-linked mutations, suggest the possible involvement of impaired ESCRT-regulated endosome-to-lysosome trafficking in CMT4D pathogenesis. Interestingly, genetic studies of teetering mice have identified a spontaneous missense mutation in the ESCRT component Hrs as the genetic defect for causing the peripheral neuropathy phenotype of teetering mice [88, 89]. These emerging data provide additional support for a role of defective ESCRT-regulated endosome-to-lysosome trafficking in demyelinating peripheral neuropathy.
In addition to ESCRT dysfunction, impairment of phosphoinositide-mediated regulation of endosome-to-lysosome trafficking has also been implicated demyelinating CMT pathogenesis. Studies of CMT4B-linked proteins MTMR2, MTMR13, and MTMR5 have shown that these proteins primarily act at endosomes to regulate levels of PI(3)P and PI(3,5)P2 by promoting their dephosphorylation [64–66, 90]. Depletion of endogenous MTMR2 by siRNAs leads to abnormal accumulation of PI(3)P and PI(3,5)P2 on endosomes and impaired endosome-to-lysosome trafficking of EGFR in human squamous cell carcinoma SCC-12F and A431 cells [91]. Furthermore, MTMR2 has been shown to regulate PI(3)P-dependent endosomal targeting of RME-8 [74], a protein known to be involved in regulation of EGFR trafficking from endosomes to lysosomes for degradation [92–94]. These findings suggest that altered phosphoinositide metabolism caused by CMT4B-linked, loss-of-function mutations could affect endosome-to-lysosome trafficking, thereby contributing to the pathogenesis of demyelinating CMT.
CMT4J is a demyelinating neuropathy caused by mutations in fat-induced gene 4 (FIG 4) encoding Fig 4, an ubiquitously expressed, phosphoinositide 5-phosphatase that dephosphorylates PI(3,5)P2 to PI(3)P [95]. Fig 4 also binds and activates PI5K that generates PI(3,5)P2 from PI(3)P at the endosome [96]. As a result, loss of Fig 4 causes a decrease rather than an increase of PI(3,5)P2 levels [96, 97]. Furthermore, the endosomes and lysosomes are enlarged in Fig 4-deficient cells [96, 97], suggesting an involvement of impaired PI(3,5)P2-regulated endolysosomal trafficking in CMT4J pathogenesis.
Altered ErbB Receptor Trafficking and Signaling Implicated in Demyelinating CMT
The emergence of defects in endocytic trafficking as a common theme in a number of demyelinating CMT diseases raises an important question as to how these trafficking defects produce demyelinating neuropathy that specifically affects the peripheral nervous system. The findings that mutations in ubiquitously expressed regulators of endocytic trafficking cause demyelinating peripheral neuropathy suggest that Schwann cells are more vulnerable than most of other cells to defect in endocytic trafficking. Recent evidence has begun to suggest that CMT mutation-induced endocytic trafficking dysregulation could alter ErbB2/ErbB3 receptor trafficking and signaling in Schwann cells (Table 2). We propose that dysregulation of ErbB receptor trafficking and signaling in Schwann cells may represent a common pathogenic mechanism contributing to the pathogenesis of multiple subtypes of demyelinating CMT.
Table 2.
Effects of demyelinating CMT-linked mutations on ErbB receptor level, trafficking, and/or downstream signaling
| CMT type | Mutant protein | Altered ErbB level | ErbB receptor trafficking defect | ErbB downstream signaling dysregulation | References |
|---|---|---|---|---|---|
| CMT1C | SIMPLE | ↑ | Impaired endosome-to-lysosome trafficking | ↑ Erk signaling | [43] |
| CMT4C | SH3TC2 | ? | Endocytosis dysfunction; may inhibit endosomal recycling | ↓ JNK1 signaling | [42] |
| CMT1A | PMP22 | ↑ | Unknown | ↑ Erk signaling ↑ Akt signaling |
[27, 106, 107] |
| CMT1X | Cx32 | ↑ | Unknown | ↑ Erk signaling | [106, 112] |
| CMT4B1 | MTMR2 | ? | Unknown, may alter endocytosis, recycling and/or | ↓ Akt signaling | [54, 62, 67, 73, 113] |
| CMT4B2 | MTMR13 | endosome-to-lysosome trafficking | |||
| CMT4B3 | MTMR5 | ||||
| CMT4J | Fig 4 | ? | Unknown, may impair endolysosomal trafficking | ↓ Akt signaling? | [96, 97] |
| CMT4D | Ndrg1 | ? | Unknown, may inhibit endosomal recycling and/or endosome-to-lysosome trafficking | ↓ PTEN ↑ Erk signaling ↑ PI3K/Akt signaling |
[81, 116] |
| CMT4H | Frabin | ? | Unknown, may affect endocytosis | ↓ Phosphoinositide signaling | [72, 117–119] |
Specific changes in ErbB level and ErbB downstream signaling cascades are as follows: increased (↑), decreased (↓), or unknown (?)
Impaired ErbB Receptor Endosome-to-Lysosome Trafficking and Signaling Attenuation in CMT1C
Studies from our laboratory have identified the CMT1C-associated protein SIMPLE as a positive regulator of endosome-to-lysosome trafficking required for controlling Nrg1-induced ErbB3 downregulation in Schwann cells [43, 47]. We found that expression of CMT1C-linked SIMPLE mutant proteins impairs endosome-to-lysosome trafficking and degradation of ErbB3 protein in Schwann cells [43] and induces a peripheral neuropathy phenotype in transgenic mice [98]. Consistent with a critical role of endosome to-lysosome trafficking in signaling attenuation [99], our results indicate that SIMPLE mutant-induced trafficking impairment causes reduced attenuation of Nrg1-activated ErbB2/ErbB3 receptor signaling, leading to prolonged downstream Erk signaling [43]. ErbB receptor and Erk signaling hyper-activation has been shown to cause Schwann cell dedifferentiation and demyelination [100, 101]. Together, these findings suggests a pathogenic pathway by which CMT1C-linked SIMPLE mutation causes dysregulation of ErbB receptor trafficking and signaling in Schwann cells and thereby triggers demyelination and peripheral neuropathy.
Defective ErbB Receptor Endocytosis and JNK1/c-Jun Signaling in CMT4C
CMT4C-associated protein SH3TC2 has been proposed to function as a positive regulator of endocytosis [42] or of endosomal recycling to the plasma membrane [77, 78]. There is evidence that SH3TC2 is required for controlling Nrg1-induced ErbB2 endocytosis in Schwann cells [42], but whether SH3TC2 has a role in regulating ErbB recycling to the cell surface remains to be determined. CMT4C-linked mutations were found to impair the ability of SH3TC2 to promote ErbB2 receptor endocytosis in Schwann cells [42]. The signaling consequence of SH3TC2 mutant-induced ErbB trafficking impairment is unclear, but it has been suggested that the pathogenic SH3TC2 loss-of-function mutations may cause a reduction in downstream JNK1/c-Jun signaling based on immunoblot analysis of sciatic nerve extracts from the SH3TC2 knockout mice [42]. However, the proposed role of reduced JNK1/c-Jun signaling in causing peripheral nerve hypomyelination in the SH3TC2 knockout mice [102] seems to be in conflict with the reported function of JNK1/c-Jun signaling in the inhibition of myelination [36]. Further studies are warranted to define the ErbB receptor trafficking and signaling defects caused by CMT4C-linked SH3TC2 mutations and their roles in demyelinating peripheral neuropathy.
Elevated ErbB Receptor Levels and Altered Erk and Akt Signaling in CMT1A and CMT1X
CMT1A, an autosomal dominant form of demyelinating CMT, is the most common hereditary peripheral neuropathy, which accounts for over 50 % of all CMT cases. CMT1A is caused by duplication of the PMP22 gene that encodes peripheral myelin protein 22 (PMP22), leading to PMP22 overexpression and aggregation in Schwann cells [103, 104]. The molecular mechanism underlying CMT1A pathogenesis remains unclear, but overexpressed and aggregated PMP22 protein has been proposed to disrupt Schwann cell function and cause demyelinating neuropathy via gain-of-function mechanisms [105]. Analysis of peripheral nerves from CMT1A patients reveals increased levels of ErbB2 and ErbB3 proteins in Schwann cells compared to the controls [106], which could potentially result from impaired endocytic trafficking and degradation of ErbB receptors. The CMT1A-associated increases in ErbB2 and ErbB3 levels would be predicted to cause over-activation of ErbB receptor signaling, leading to abnormal downstream signaling in Schwann cells. In support of this possibility, increased Erk signaling was found in CMT1A mouse [107] and rat [27] models, and reduced Akt signaling was observed in CMT1A rat model [27]. Together, these findings raise an intriguing possibility that dysregulated ErbB2/ErbB3 receptor trafficking and signaling may be involved in CMT1A pathogenesis, a hypothesis which requires investigation by future experiments.
CMT1X, an X-chromosome-linked, dominant form of de-myelinating CMT, is the second most common hereditary peripheral neuropathy, which accounts for about 10 % of all CMT cases. CMT1X is caused by mutations in the GJB1 gene that encodes the gap junction protein connexin32 (Cx32) [108–110]. Studies have shown that CMT1X-linked Cx32 mutations impair gap junction formation through a combination of loss-of-function and gain-of-function effects [111], but how these mutations cause demyelinating CMT neuropathy is incompletely understood. Elevated ErbB3 protein levels were found in the peripheral nerves of CMT1X patients [106], suggesting that abnormal ErbB receptor signaling could contribute to CMT1X pathogenesis. This idea is supported by a recent study showing increased Erk signaling in Schwann cells of Cx32-deficient mice [112], suggesting that dysregulated ErbB receptor signaling may also be involved in CMT1X neuropathy.
Impaired Phosphoinositide-Mediated Regulation of ErbB Receptor Trafficking and Signaling Implicated in CMT4B, CMT4D, CMT4H, and CMT4J
MTMR2, MTMR13, and MTMR5 are members of the MTMR family of phosphoinositide 3-phosphatases that are mutated in CMT4B1, CMT4B2, and CMT4B3, respectively. The findings that CMT4B-linked mutations cause accumulation of excess PI(3)P and PI(3,5)P2 [64, 65] and dysregulation of ErbB1/EGFR endosome-to-lysosome trafficking in carcinoma cells [91] raise an experimentally testable hypothesis that CMT4B-linked mutations may alter ErbB2/ErbB3 receptor endosome-to-lysosome trafficking in Schwann cells and thereby cause dysregulation of ErbB receptor downstream signaling contributing to demyelinating CMT pathogenesis. In addition, CMT4B-linked mutations may also impair Akt signaling by inhibiting the production of PI(5)P, a lipid activator of Akt signaling [54, 55]. In support of these possibilities, reduced Akt signaling was observed in peripheral nerves of MTMR2/MTMR13 double knockout mice, a mouse model of CMT4B1 and CMT4B2 [113]. Future studies are needed to further test this hypothesis and determine the effects of CMT4B-linked mutations on ErbB receptor trafficking and signaling in Schwann cells and their roles in CMT4B pathogenesis.
CMT4J-linked protein Fig 4 is a phosphoinositide 5-phosphatase with a critical role in regulation of intracellular PI(3,5)P2 level and endolysosomal trafficking [96, 97, 114]. Interestingly, a recent study reported a genetic interaction between Fig 4 and MTMR2 in the control of phosphoinositide metabolism and myelin homeostasis [115]. Based on the finding of abnormally enlarged endosomes and lysosomes induced by loss of Fig 4 [96, 97], we hypothesize that CMT4J-linked loss-of-function mutations in Fig 4 may cause altered endolysosomal trafficking and signaling of ErbB receptors in Schwann cells and thereby contribute to CMT4J pathogenesis.
CMT4D-associated protein Ndrg1 has been proposed to function as a positive regulator of endosome-to-plasma membrane recycling [80, 81] and endosome-to-lysosome trafficking [81]. Based on these proposed functions of Ndrg1, it is possible that CMT4D-linked Ndrg1 mutations may cause dysregulation of ErbB2/ErbB3 receptor trafficking and signaling in Schwann cells to trigger CMT4D pathogenesis. A recent study reported a role for Ndrg1 in upregulating PTEN expression and inhibiting the PI3K/Akt and Erk signaling pathways [116], raising the possibility that the loss-of-function mutations in Ndrg1 found in CMT4D may cause reduced PTEN expression and enhanced PI3K/Akt and Erk signaling in Schwann cells.
CMT4H-associated protein Frabin has been shown to regulate endocytosis of transferrin receptors in Schwann cells [72]; thus, it is possible that Frabin also participates in regulation of endocytosis of ErbB2/ErbB3 receptors in Schwann cells. Although the effects of CMT4H-linked Frabin mutations on ErbB receptor trafficking have not been examined, a number of disease-causing Frabin mutations were found in its phosphoinositide-recognition domains, namely, the two PH domains with binding specificity to PI(3,4,5)P3, PI(4,5)P2, and PI(3,4)P2 and one FYVE domain with binding specificity to PI(3)P [72, 117–119]. As described earlier, these phosphoinositides play important roles in regulation of ErbB2/ErbB3 receptor trafficking and signaling (Figs. 1 and 2); thus, it is possible that CMT4H-linked Frabin mutations may impair phosphoinositide-mediated regulation of ErbB receptor trafficking and signaling, thereby contributing to CMT4H pathogenesis.
Targeting ErbB Receptor Trafficking and Signaling as Potential Therapeutic Intervention in Demyelinating CMT
The finding of dysregulated ErbB receptor trafficking and signaling in multiple forms of demyelinating CMT suggests the possibility of modulating these processes as novel approaches for treating demyelinating CMT. One way to modulate ErbB receptor signaling can be achieved at the ligand level through Nrg1 supplementation, as studies have shown that both Nrg1 type III and Nrg1 type I can regulate Schwann cell differentiation and myelination in a concentration-dependent manner [22, 27, 100, 120]. A recent work demonstrated that supplementing recombinant human Nrg1 type I reduced the increased Erk signaling and enhanced the decreased Akt signaling in a transgenic rat model of CMT1A and ameliorated its demyelinating peripheral neuropathy phenotype [27], supporting the therapeutic potential of Nrg1 supplementation for treating CMT1A and possibly other forms of demyelinating CMT that involve impaired ErbB receptor signaling.
Targeting other steps of the ErbB receptor trafficking and signaling pathways may also provide therapeutic benefit to combat demyelinating peripheral neuropathy. For example, ErbB receptor kinase inhibitor PKI 166 is able to abrogate Mycobacterium leprae-induced ErbB2 and Erk signaling over-activation and demyelination in mice [33]. Mek inhibitor, such as CI-1040 and U0126, has been shown to reduce the augmented Erk signaling and demyelination phenotype in rodent models of CMT1A [27, 107] and CMT1X [112]. In addition, agents such as geldanamycin [121] and ErbB receptor-specific monoclonal antibodies [122–124] that facilitate ErbB receptor endocytosis and degradation could be explored in preclinical studies for treating certain forms of CMT with elevated ErbB levels and augmented Erk signaling (Table 2). Furthermore, given the critical role of phosphoinositides in modulation of ErbB receptor trafficking and signaling and their involvement in CMT pathogenesis, phosphoinositide kinase and/or phosphatase inhibitors [125–127] could be tested for potential therapeutic effects to reduce ErbB receptor trafficking and signaling defects and ameliorate demyelinating CMT neuropathy.
Concluding Remarks
Progress made in human genetics has led to the identification of about 20 different genes whose mutations cause demyelinating forms of CMT disease. The fact that these different CMT forms share a similar phenotype of demyelinating peripheral neuropathy raises the possibility that there may be convergent mechanisms and pathways leading to demyelinating CMT pathogenesis. Based on emerging evidence, we propose that dysregulation of ErbB2/ErbB3 receptor trafficking and signaling could be a unifying pathogenic mechanism for various forms of de-myelinating CMT, including the most common form CMT1A. Future studies are needed to further test this hypothesis and define the molecular mechanisms governing ErbB2/ErbB3 receptor trafficking and signaling in Schwann cells and their impairment by various CMT-linked mutations. Recent studies indicate that a number of demyelinating CMT-linked proteins are novel regulators of endocytic trafficking; it is important to elucidate their mechanisms of action and determine how endocytic trafficking regulates ErbB2/ErbB3 receptor-mediated downstream signaling cascades in the control of myelination and peripheral nerve function. A better understanding of ErbB receptor trafficking and signaling network in health and CMT disease will facilitate the development of new therapeutic strategies for treating demyelinating peripheral neuropathy.
Acknowledgments
The authors’ research is supported by National Institutions of Health (NIH) grants NS063501 (S.M.L.), NS093550 (L.S.C.), GM103613, and NS092343 (L.L.).
Footnotes
Compliance with Ethical Standard
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Patzko A, Shy ME. Update on Charcot-Marie-Tooth disease. Curr Neurol Neurosci Rep. 2011;11(1):78–88. doi: 10.1007/s11910-010-0158-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pareyson D, Marchesi C. Diagnosis, natural history, and management of Charcot-Marie-Tooth disease. Lancet Neurol. 2009;8(7):654–667. doi: 10.1016/S1474-4422(09)70110-3. [DOI] [PubMed] [Google Scholar]
- 3.Jani-Acsadi A, Krajewski K, Shy ME. Charcot-Marie-Tooth neuropathies: diagnosis and management. Semin Neurol. 2008;28(2):185–194. doi: 10.1055/s-2008-1062264. [DOI] [PubMed] [Google Scholar]
- 4.Reilly MM, Shy ME. Diagnosis and new treatments in genetic neuropathies. J Neurol Neurosurg Psychiatry. 2009;80(12):1304–1314. doi: 10.1136/jnnp.2008.158295. [DOI] [PubMed] [Google Scholar]
- 5.Patzko A, Shy ME. Charcot-Marie-Tooth disease and related genetic neuropathies. Continuum (Minneap Minn) 2012;18(1):39–59. doi: 10.1212/01.CON.0000411567.34085.da. [DOI] [PubMed] [Google Scholar]
- 6.Ekins S, Litterman NK, Arnold RJ, Burgess RW, Freundlich JS, Gray SJ, Higgins JJ, Langley B, Willis DE, Notterpek L, Pleasure D, Sereda MW, Moore A. A brief review of recent Charcot-Marie-Tooth research and priorities. F1000Research. 2015;4:53. doi: 10.12688/f1000research.6160.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshikawa H, Nishimura T, Nakatsuji Y, Fujimura H, Himoro M, Hayasaka K, Sakoda S, Yanagihara T. Elevated expression of messenger RNA for peripheral myelin protein 22 in biopsied peripheral nerves of patients with Charcot-Marie-Tooth disease type 1A. Ann Neurol. 1994;35(4):445–450. doi: 10.1002/ana.410350412. [DOI] [PubMed] [Google Scholar]
- 8.Hanemann CO, Stoll G, D'Urso D, Fricke W, Martin JJ, Van Broeckhoven C, Mancardi GL, Bartke I, et al. Peripheral myelin protein-22 expression in Charcot-Marie-Tooth disease type 1a sural nerve biopsies. J Neurosci Res. 1994;37(5):654–659. doi: 10.1002/jnr.490370513. [DOI] [PubMed] [Google Scholar]
- 9.Patel PI, Roa BB, Welcher AA, Schoener-Scott R, Trask BJ, Pentao L, Snipes GJ, Garcia CA, et al. The gene for the peripheral myelin protein PMP-22 is a candidate for Charcot-Marie-Tooth disease type 1A. Nat Genet. 1992;1(3):159–165. doi: 10.1038/ng0692-159. [DOI] [PubMed] [Google Scholar]
- 10.Russo M, Laura M, Polke JM, Davis MB, Blake J, Brandner S, Hughes RA, Houlden H, et al. Variable phenotypes are associated with PMP22 missense mutations. Neuromuscul Disord. 2011;21(2):106–114. doi: 10.1016/j.nmd.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Lee SM, Chin LS, Li L. Protein misfolding and clearance in demyelinating peripheral neuropathies: therapeutic implications. Commun Integr Biol. 2012;5(1):107–110. doi: 10.4161/cib.18638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warner LE, Roa BB, Lupski JR. Absence of PMP22 coding region mutations in CMT1A duplication patients: further evidence supporting gene dosage as a mechanism for Charcot-Marie-Tooth disease type 1A. Hum Mutat. 1996;8(4):362–365. doi: 10.1002/(SICI)1098-1004(1996)8:4<362::AID-HUMU10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Pennuto M, Tinelli E, Malaguti M, Del Carro U, D’Antonio M, Ron D, Quattrini A, Feltri ML, et al. Ablation of the UPR-mediator CHOP restores motor function and reduces demyelination in Charcot-Marie-Tooth 1B mice. Neuron. 2008;57(3):393–405. doi: 10.1016/j.neuron.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seidl AH. Regulation of conduction time along axons. Neuroscience. 2014;276:126–134. doi: 10.1016/j.neuroscience.2013.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nave KA, Werner HB. Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Biol. 2014;30:503–533. doi: 10.1146/annurev-cellbio-100913-013101. [DOI] [PubMed] [Google Scholar]
- 16.Newbern J, Birchmeier C. Nrg1/ErbB signaling networks in Schwann cell development and myelination. Semin Cell Dev Biol. 2010;21(9):922–928. doi: 10.1016/j.semcdb.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J. Molecular regulators of nerve conduction—lessons from inherited neuropathies and rodent genetic models. Exp Neurol. 2015;267:209–218. doi: 10.1016/j.expneurol.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu X, Fan Q, Hou H, Yan R. Neurological dysfunctions associated with altered BACE1-dependent Neuregulin-1 signaling. J Neurochem. 2015 doi: 10.1111/jnc.13395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47(5):681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304(5671):700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 21.Hu X, Hu J, Dai L, Trapp B, Yan R. Axonal and Schwann cell BACE1 is equally required for remyelination of peripheral nerves. J Neurosci. 2015;9:3806–3814. doi: 10.1523/JNEUROSCI.5207-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stassart RM, Fledrich R, Velanac V, Brinkmann BG, Schwab MH, Meijer D, Sereda MW, Nave KA. A role for Schwann cell-derived neuregulin-1 in remyelination. Nat Neurosci. 2013;16(1):48–54. doi: 10.1038/nn.3281. [DOI] [PubMed] [Google Scholar]
- 23.Hu X, He W, Diaconu C, Tang X, Kidd GJ, Macklin WB, Trapp BD, Yan R. Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves. FASEB J. 2008;22(8):2970–2980. doi: 10.1096/fj.08-106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo X, Prior M, He W, Hu X, Tang X, Shen W, Yadav S, Kiryu-Seo S, et al. Cleavage of neuregulin-1 by BACE1 or ADAM10 protein produces differential effects on myelination. J Biol Chem. 2011;286(27):23967–23974. doi: 10.1074/jbc.M111.251538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.La Marca R, Cerri F, Horiuchi K, Bachi A, Feltri ML, Wrabetz L, Blobel CP, Quattrini A, et al. TACE (ADAM17) inhibits Schwann cell myelination. Nat Neurosci. 2011;14(7):857–865. doi: 10.1038/nn.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleck D, van Bebber F, Colombo A, Galante C, Schwenk BM, Rabe L, Hampel H, Novak B, et al. Dual cleavage of neuregulin 1 type III by BACE1 and ADAM17 liberates its EGF-like domain and allows paracrine signaling. J Neurosci. 2013;33(18):7856–7869. doi: 10.1523/JNEUROSCI.3372-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fledrich R, Stassart RM, Klink A, Rasch LM, Prukop T, Haag L, Czesnik D, Kungl T, et al. Soluble neuregulin-1 modulates disease pathogenesis in rodent models of Charcot-Marie-Tooth disease 1A. Nat Med. 2014;20(9):1055–1061. doi: 10.1038/nm.3664. [DOI] [PubMed] [Google Scholar]
- 28.Fricker FR, Lago N, Balarajah S, Tsantoulas C, Tanna S, Zhu N, Fageiry SK, Jenkins M, et al. Axonally derived neuregulin-1 is required for remyelination and regeneration after nerve injury in adulthood. J Neurosci. 2011;31(9):3225–3233. doi: 10.1523/JNEUROSCI.2568-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Tennekoon GI, Birnbaum M, Marchionni MA, Rutkowski JL. Neuregulin signaling through a PI3K/Akt/Bad pathway in Schwann cell survival. Mol Cell Neurosci. 2001;17(4):761–767. doi: 10.1006/mcne.2000.0967. [DOI] [PubMed] [Google Scholar]
- 30.Wishart MJ, Taylor GS, Slama JT, Dixon JE. PTEN and myotubularin phosphoinositide phosphatases: bringing bioinformatics to the lab bench. Curr Opin Cell Biol. 2001;13(2):172–181. doi: 10.1016/s0955-0674(00)00195-2. [DOI] [PubMed] [Google Scholar]
- 31.Goebbels S, Oltrogge JH, Wolfer S, Wieser GL, Nientiedt T, Pieper A, Ruhwedel T, Groszer M, et al. Genetic disruption of PTEN in a novel mouse model of tomaculous neuropathy. EMBO Mol Med. 2012;4(6):486–499. doi: 10.1002/emmm.201200227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogata T, Iijima S, Hoshikawa S, Miura T, Yamamoto S, Oda H, Nakamura K, Tanaka S. Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. J Neurosci. 2004;24(30):6724–6732. doi: 10.1523/JNEUROSCI.5520-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tapinos N, Ohnishi M, Rambukkana A. ErbB2 receptor tyrosine kinase signaling mediates early demyelination induced by leprosy bacilli. Nat Med. 2006;12(8):961–966. doi: 10.1038/nm1433. [DOI] [PubMed] [Google Scholar]
- 34.Napoli I, Noon LA, Ribeiro S, Kerai AP, Parrinello S, Rosenberg LH, Collins MJ, Harrisingh MC, et al. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron. 2012;73(4):729–742. doi: 10.1016/j.neuron.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 35.Kao SC, Wu H, Xie J, Chang CP, Ranish JA, Graef IA, Crabtree GR. Calcineurin/NFAT signaling is required for neuregulin-regulated Schwann cell differentiation. Science. 2009;323(5914):651–654. doi: 10.1126/science.1166562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parkinson DB, Bhaskaran A, Arthur-Farraj P, Noon LA, Woodhoo A, Lloyd AC, Feltri ML, Wrabetz L, et al. c-Jun is a negative regulator of myelination. J Cell Biol. 2008;181(4):625–637. doi: 10.1083/jcb.200803013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2008;314(17):3093–3106. doi: 10.1016/j.yexcr.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miaczynska M, Pelkmans L, Zerial M. Not just a sink: endosomes in control of signal transduction. Curr Opin Cell Biol. 2004;16(4):400–406. doi: 10.1016/j.ceb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Tomas A, Futter CE, Eden ER. EGF receptor trafficking: consequences for signaling and cancer. Trends Cell Biol. 2014;24(1):26–34. doi: 10.1016/j.tcb.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roepstorff K, Grovdal L, Grandal M, Lerdrup M, van Deurs B. Endocytic downregulation of ErbB receptors: mechanisms and relevance in cancer. Histochem Cell Biol. 2008;129(5):563–578. doi: 10.1007/s00418-008-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polo S, Di Fiore PP. Endocytosis conducts the cell signaling orchestra. Cell. 2006;124(5):897–900. doi: 10.1016/j.cell.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 42.Gouttenoire EA, Lupo V, Calpena E, Bartesaghi L, Schupfer F, Medard JJ, Maurer F, Beckmann JS, et al. Sh3tc2 deficiency affects neuregulin-1/ErbB signaling. Glia. 2013;61(7):1041–1051. doi: 10.1002/glia.22493. [DOI] [PubMed] [Google Scholar]
- 43.Lee SM, Chin LS, Li L. Charcot-Marie-Tooth disease-linked protein SIMPLE functions with the ESCRT machinery in endosomal trafficking. J Cell Biol. 2012;199(5):799–816. doi: 10.1083/jcb.201204137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shields SB, Piper RC. How ubiquitin functions with ESCRTs. Traffic. 2011;12(10):1306–1317. doi: 10.1111/j.1600-0854.2011.01242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2009;315(4):683–696. doi: 10.1016/j.yexcr.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 46.Cao Z, Wu X, Yen L, Sweeney C, Carraway KL., 3rd Neuregulin-induced ErbB3 downregulation is mediated by a protein stability cascade involving the E3 ubiquitin ligase Nrdp1. Mol Cell Biol. 2007;27(6):2180–2188. doi: 10.1128/MCB.01245-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chin LS, Lee SM, Li L. SIMPLE: a new regulator of endosomal trafficking and signaling in health and disease. Commun Integr Biol. 2013;6(3):e24214. doi: 10.4161/cib.24214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene. 2000;19(53):6102–6114. doi: 10.1038/sj.onc.1203973. [DOI] [PubMed] [Google Scholar]
- 49.Austin CD, De Maziere AM, Pisacane PI, van Dijk SM, Eigenbrot C, Sliwkowski MX, Klumperman J, Scheller RH. Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin. Mol Biol Cell. 2004;15(12):5268–5282. doi: 10.1091/mbc.E04-07-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cullen PJ, Carlton JG. Phosphoinositides in the mammalian endolysosomal network. Sub-cell Biochem. 2012;59:65–110. doi: 10.1007/978-94-007-3015-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haucke V. Phosphoinositide regulation of clathrin-mediated endocytosis. Biochem Soc Trans. 2005;33(Pt 6):1285–1289. doi: 10.1042/BST20051285. [DOI] [PubMed] [Google Scholar]
- 52.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11(5):329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 53.Williams RL, Urbe S. The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol. 2007;8(5):355–368. doi: 10.1038/nrm2162. [DOI] [PubMed] [Google Scholar]
- 54.Carricaburu V, Lamia KA, Lo E, Favereaux L, Payrastre B, Cantley LC, Rameh LE. The phosphatidylinositol (PI)-5-phosphate 4-kinase type II enzyme controls insulin signaling by regulating PI-3,4,5-trisphosphate degradation. Proc Natl Acad Sci U S A. 2003;100(17):9867–9872. doi: 10.1073/pnas.1734038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coronas S, Ramel D, Pendaries C, Gaits-Iacovoni F, Tronchere H, Payrastre B. PtdIns5P: a little phosphoinositide with big functions? Biochem Soc Sympos. 2007;74:117–128. doi: 10.1042/BSS0740117. [DOI] [PubMed] [Google Scholar]
- 56.Macklin WB. The myelin brake: when enough is enough. Sci Signal. 2010;3(140):pe32. doi: 10.1126/scisignal.3140pe32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bolino A, Levy ER, Muglia M, Conforti FL, LeGuern E, Salih MA, Georgiou DM, Christodoulou RK, et al. Genetic refinement and physical mapping of the CMT4B gene on chromosome 11q22. Genomics. 2000;63(2):271–278. doi: 10.1006/geno.1999.6088. [DOI] [PubMed] [Google Scholar]
- 58.Senderek J, Bergmann C, Weber S, Ketelsen UP, Schorle H, Rudnik-Schoneborn S, Buttner R, Buchheim E, et al. Mutation of the SBF2 gene, encoding a novel member of the myotubularin family, in Charcot-Marie-Tooth neuropathy type 4B2/11p15. Hum Mol Genet. 2003;12(3):349–356. doi: 10.1093/hmg/ddg030. [DOI] [PubMed] [Google Scholar]
- 59.Azzedine H, Bolino A, Taieb T, Birouk N, Di Duca M, Bouhouche A, Benamou S, Mrabet A, et al. Mutations in MTMR13, a new pseudophosphatase homologue of MTMR2 and Sbf1, in two families with an autosomal recessive demyelinating form of Charcot-Marie-Tooth disease associated with early-onset glaucoma. Am J Hum Genet. 2003;72(5):1141–1153. doi: 10.1086/375034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hirano R, Takashima H, Umehara F, Arimura H, Michizono K, Okamoto Y, Nakagawa M, Boerkoel CF, et al. SET binding factor 2 (SBF2) mutation causes CMT4B with juvenile onset glaucoma. Neurology. 2004;63(3):577–580. doi: 10.1212/01.wnl.0000133211.40288.9a. [DOI] [PubMed] [Google Scholar]
- 61.Conforti FL, Muglia M, Mazzei R, Patitucci A, Valentino P, Magariello A, Sprovieri T, Bono F, et al. A new SBF2 mutation in a family with recessive demyelinating Charcot-Marie-Tooth (CMT4B2) Neurology. 2004;63(7):1327–1328. doi: 10.1212/01.wnl.0000140617.02312.80. [DOI] [PubMed] [Google Scholar]
- 62.Nakhro K, Park JM, Hong YB, Park JH, Nam SH, Yoon BR, Yoo JH, Koo H, et al. SET binding factor 1 (SBF1) mutation causes Charcot-Marie-Tooth disease type 4B3. Neurology. 2013;81(2):165–173. doi: 10.1212/WNL.0b013e31829a3421. [DOI] [PubMed] [Google Scholar]
- 63.Bolino A, Bolis A, Previtali SC, Dina G, Bussini S, Dati G, Amadio S, Del Carro U, et al. Disruption of Mtmr2 produces CMT4B1-like neuropathy with myelin outfolding and impaired spermatogenesis. J Cell Biol. 2004;167(4):711–721. doi: 10.1083/jcb.200407010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robinson FL, Dixon JE. The phosphoinositide-3-phosphatase MTMR2 associates with MTMR13, a membrane-associated pseudophosphatase also mutated in type 4B Charcot-Marie-Tooth disease. J Biol Chem. 2005;280(36):31699–31707. doi: 10.1074/jbc.M505159200. [DOI] [PubMed] [Google Scholar]
- 65.Berger P, Bonneick S, Willi S, Wymann M, Suter U. Loss of phosphatase activity in myotubularin-related protein 2 is associated with Charcot-Marie-Tooth disease type 4B1. Hum Mol Genet. 2002;11(13):1569–1579. doi: 10.1093/hmg/11.13.1569. [DOI] [PubMed] [Google Scholar]
- 66.Kim SA, Vacratsis PO, Firestein R, Cleary ML, Dixon JE. Regulation of myotubularin-related (MTMR)2 phosphatidylinositol phosphatase by MTMR5, a catalytically inactive phosphatase. Proc Natl Acad Sci U S A. 2003;100(8):4492–4497. doi: 10.1073/pnas.0431052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee HW, Kim Y, Han K, Kim H, Kim E. The phosphoinositide 3-phosphatase MTMR2 interacts with PSD-95 and maintains excitatory synapses by modulating endosomal traffic. J Neurosci. 2010;30(16):5508–5518. doi: 10.1523/JNEUROSCI.4283-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Velichkova M, Juan J, Kadandale P, Jean S, Ribeiro I, Raman V, Stefan C, Kiger AA. Drosophila Mtm and class II PI3K coregulate a PI(3)P pool with cortical and endolysosomal functions. J Cell Biol. 2010;190(3):407–425. doi: 10.1083/jcb.200911020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ribeiro I, Yuan L, Tanentzapf G, Dowling JJ, Kiger A. Phosphoinositide regulation of integrin trafficking required for muscle attachment and maintenance. PLoS Genet. 2011;7(2):e1001295. doi: 10.1371/journal.pgen.1001295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Senderek J, Bergmann C, Stendel C, Kirfel J, Verpoorten N, De Jonghe P, Timmerman V, Chrast R, et al. Mutations in a gene encoding a novel SH3/TPR domain protein cause autosomal recessive Charcot-Marie-Tooth type 4C neuropathy. Am J Hum Genet. 2003;73(5):1106–1119. doi: 10.1086/379525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lupo V, Galindo MI, Martinez-Rubio D, Sevilla T, Vilchez JJ, Palau F, Espinos C. Missense mutations in the SH3TC2 protein causing Charcot-Marie-Tooth disease type 4C affect its localization in the plasma membrane and endocytic pathway. Hum Mol Genet. 2009;18(23):4603–4614. doi: 10.1093/hmg/ddp427. [DOI] [PubMed] [Google Scholar]
- 72.Horn M, Baumann R, Pereira JA, Sidiropoulos PN, Somandin C, Welzl H, Stendel C, Luhmann T, et al. Myelin is dependent on the Charcot-Marie-Tooth Type 4H disease culprit protein FRABIN/FGD4 in Schwann cells. Brain. 2012;135(Pt 12):3567–3583. doi: 10.1093/brain/aws275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jean S, Cox S, Schmidt EJ, Robinson FL, Kiger A. Sbf/MTMR13 coordinates PI(3)P and Rab21 regulation in endocytic control of cellular remodeling. Mol Biol Cell. 2012;23(14):2723–2740. doi: 10.1091/mbc.E12-05-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xhabija B, Taylor GS, Fujibayashi A, Sekiguchi K, Vacratsis PO. Receptor mediated endocytosis 8 is a novel PI(3)P binding protein regulated by myotubularin-related 2. FEBS Lett. 2011;585(12):1722–1728. doi: 10.1016/j.febslet.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y, Grant B, Hirsh D. RME-8, a conserved J-domain protein, is required for endocytosis in Caenorhabditis elegans. Mol Biol Cell. 2001;12(7):2011–2021. doi: 10.1091/mbc.12.7.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gomez-Lamarca M, Snowdon LA, Seib E, Klein T, Bray S. Rme-8 depletion perturbs Notch recycling and predisposes to pathogenic signaling. J Cell Biol. 2015;210(3):517. doi: 10.1083/jcb.20141100107172015c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roberts RC, Peden AA, Buss F, Bright NA, Latouche M, Reilly MM, Kendrick-Jones J, Luzio JP. Mistargeting of SH3TC2 away from the recycling endosome causes Charcot-Marie-Tooth disease type 4C. Hum Mol Genet. 2010;19(6):1009–1018. doi: 10.1093/hmg/ddp565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stendel C, Roos A, Kleine H, Arnaud E, Ozcelik M, Sidiropoulos PN, Zenker J, Schupfer F, et al. SH3TC2, a protein mutant in Charcot-Marie-Tooth neuropathy, links peripheral nerve myelination to endosomal recycling. Brain. 2010;133(Pt 8):2462–2474. doi: 10.1093/brain/awq168. [DOI] [PubMed] [Google Scholar]
- 79.Lachat P, Shaw P, Gebhard S, van Belzen N, Chaubert P, Bosman FT. Expression of NDRG1, a differentiation-related gene, in human tissues. Histochem Cell Biol. 2002;118(5):399–408. doi: 10.1007/s00418-002-0460-9. [DOI] [PubMed] [Google Scholar]
- 80.Kachhap SK, Faith D, Qian DZ, Shabbeer S, Galloway NL, Pili R, Denmeade SR, DeMarzo AM, et al. The N-Myc down regulated gene1 (NDRG1) is a Rab4a effector involved in vesicular recycling of E-cadherin. PLoS ONE. 2007;2(9):e844. doi: 10.1371/journal.pone.0000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pietiainen V, Vassilev B, Blom T, Wang W, Nelson J, Bittman R, Back N, Zelcer N, et al. NDRG1 functions in LDL receptor trafficking by regulating endosomal recycling and degradation. J Cell Sci. 2013;126(Pt 17):3961–3971. doi: 10.1242/jcs.128132. [DOI] [PubMed] [Google Scholar]
- 82.Street VA, Bennett CL, Goldy JD, Shirk AJ, Kleopa KA, Tempel BL, Lipe HP, Scherer SS, et al. Mutation of a putative protein degradation gene LITAF/SIMPLE in Charcot-Marie-Tooth disease 1C. Neurology. 2003;60(1):22–26. doi: 10.1212/wnl.60.1.22. [DOI] [PubMed] [Google Scholar]
- 83.Bennett CL, Shirk AJ, Huynh HM, Street VA, Nelis E, Van Maldergem L, De Jonghe P, Jordanova A, et al. SIMPLE mutation in demyelinating neuropathy and distribution in sciatic nerve. Ann Neurol. 2004;55(5):713–720. doi: 10.1002/ana.20094. [DOI] [PubMed] [Google Scholar]
- 84.Saifi GM, Szigeti K, Wiszniewski W, Shy ME, Krajewski K, Hausmanowa-Petrusewicz I, Kochanski A, Reeser S, et al. SIMPLE mutations in Charcot-Marie-Tooth disease and the potential role of its protein product in protein degradation. Hum Mutat. 2005;25(4):372–383. doi: 10.1002/humu.20153. [DOI] [PubMed] [Google Scholar]
- 85.Street VA, Goldy JD, Golden AS, Tempel BL, Bird TD, Chance PF. Mapping of Charcot-Marie-Tooth disease type 1C to chromosome 16p identifies a novel locus for demyelinating neuropathies. Am J Hum Genet. 2002;70(1):244–250. doi: 10.1086/337943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Myokai F, Takashiba S, Lebo R, Amar S. A novel lipopolysaccharide-induced transcription factor regulating tumor necrosis factor alpha gene expression: molecular cloning, sequencing, characterization, and chromosomal assignment. Proc Natl Acad Sci U S A. 1999;96(8):4518–4523. doi: 10.1073/pnas.96.8.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moriwaki Y, Begum NA, Kobayashi M, Matsumoto M, Toyoshima K, Seya T. Mycobacterium bovis Bacillus Calmette-Guerin and its cell wall complex induce a novel lysosomal membrane protein, SIMPLE, that bridges the missing link between lipopolysaccharide and p53-inducible gene, LITAF(PIG7), and estrogen-inducible gene, EET-1. J Biol Chem. 2001;276(25):23065–23076. doi: 10.1074/jbc.M011660200. [DOI] [PubMed] [Google Scholar]
- 88.Watson JA, Bhattacharyya BJ, Vaden JH, Wilson JA, Icyuz M, Howard AD, Phillips E, DeSilva TM, et al. Motor and sensory deficits in the teetering mice result from mutation of the ESCRT component HGS. PLoS Genet. 2015;11(6):e1005290. doi: 10.1371/journal.pgen.1005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meier H. The neuropathy of teetering, a neurological mutation in the mouse. Arch Neurol. 1967;16(1):59–66. doi: 10.1001/archneur.1967.00470190063008. [DOI] [PubMed] [Google Scholar]
- 90.Franklin NE, Taylor GS, Vacratsis PO. Endosomal targeting of the phosphoinositide 3-phosphatase MTMR2 is regulated by an N-terminal phosphorylation site. J Biol Chem. 2011;286(18):15841–15853. doi: 10.1074/jbc.M110.209122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cao C, Backer JM, Laporte J, Bedrick EJ, Wandinger-Ness A. Sequential actions of myotubularin lipid phosphatases regulate endosomal PI(3)P and growth factor receptor trafficking. Mol Biol Cell. 2008;19(8):3334–3346. doi: 10.1091/mbc.E08-04-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fujibayashi A, Taguchi T, Misaki R, Ohtani M, Dohmae N, Takio K, Yamada M, Gu J, et al. Human RME-8 is involved in membrane trafficking through early endosomes. Cell Struct Funct. 2008;33(1):35–50. doi: 10.1247/csf.07045. [DOI] [PubMed] [Google Scholar]
- 93.Girard M, McPherson PS. RME-8 regulates trafficking of the epidermal growth factor receptor. FEBS Lett. 2008;582(6):961–966. doi: 10.1016/j.febslet.2008.02.042. [DOI] [PubMed] [Google Scholar]
- 94.Girard M, Poupon V, Blondeau F, McPherson PS. The DnaJ-domain protein RME-8 functions in endosomal trafficking. J Biol Chem. 2005;280(48):40135–40143. doi: 10.1074/jbc.M505036200. [DOI] [PubMed] [Google Scholar]
- 95.Nicholson G, Lenk GM, Reddel SW, Grant AE, Towne CF, Ferguson CJ, Simpson E, Scheuerle A, et al. Distinctive genetic and clinical features of CMT4J: a severe neuropathy caused by mutations in the PI(3,5)P(2) phosphatase FIG 4. Brain. 2011;134(Pt 7):1959–1971. doi: 10.1093/brain/awr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lenk GM, Ferguson CJ, Chow CY, Jin N, Jones JM, Grant AE, Zolov SN, Winters JJ, et al. Pathogenic mechanism of the FIG 4 mutation responsible for Charcot-Marie-Tooth disease CMT4J. PLoS Genet. 2011;7(6):e1002104. doi: 10.1371/journal.pgen.1002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chow CY, Zhang Y, Dowling JJ, Jin N, Adamska M, Shiga K, Szigeti K, Shy ME, et al. Mutation of FIG 4 causes neuro-degeneration in the pale tremor mouse and patients with CMT4J. Nature. 2007;448(7149):68–72. doi: 10.1038/nature05876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee SM, Sha D, Mohammed AA, Asress S, Glass JD, Chin LS, Li L. Motor and sensory neuropathy due to myelin infolding and paranodal damage in a transgenic mouse model of Charcot-Marie-Tooth disease type 1C. Hum Mol Genet. 2013;22(9):1755–1770. doi: 10.1093/hmg/ddt022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seto ES, Bellen HJ, Lloyd TE. When cell biology meets development: endocytic regulation of signaling pathways. Genes Dev. 2002;16(11):1314–1336. doi: 10.1101/gad.989602. [DOI] [PubMed] [Google Scholar]
- 100.Syed N, Reddy K, Yang DP, Taveggia C, Salzer JL, Maurel P, Kim HA. Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination. J Neurosci. 2010;30(17):6122–6131. doi: 10.1523/JNEUROSCI.1681-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Quintes S, Goebbels S, Saher G, Schwab MH, Nave KA. Neuronglia signaling and the protection of axon function by Schwann cells. J Peripher Nerv Syst. 2010;15(1):10–16. doi: 10.1111/j.1529-8027.2010.00247.x. [DOI] [PubMed] [Google Scholar]
- 102.Arnaud E, Zenker J, de Preux Charles AS, Stendel C, Roos A, Medard JJ, Tricaud N, Kleine H, et al. SH3TC2/KIAA1985 protein is required for proper myelination and the integrity of the node of Ranvier in the peripheral nervous system. Proc Natl Acad Sci U S A. 2009;106(41):17528–17533. doi: 10.1073/pnas.0905523106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ionasescu VV, Ionasescu R, Searby C, Barker DF. Charcot-Marie-Tooth neuropathy type 1A with both duplication and non-duplication. Hum Mol Genet. 1993;2(4):405–410. doi: 10.1093/hmg/2.4.405. [DOI] [PubMed] [Google Scholar]
- 104.Wise CA, Garcia CA, Davis SN, Heju Z, Pentao L, Patel PI, Lupski JR. Molecular analyses of unrelated Charcot-Marie-Tooth (CMT) disease patients suggest a high frequency of the CMTIA duplication. Am J Hum Genet. 1993;53(4):853–863. [PMC free article] [PubMed] [Google Scholar]
- 105.Li J, Parker B, Martyn C, Natarajan C, Guo J. The PMP22 gene and its related diseases. Mol Neurobiol. 2013;47(2):673–698. doi: 10.1007/s12035-012-8370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Massa R, Palumbo C, Cavallaro T, Panico MB, Bei R, Terracciano C, Rizzuto N, Bernardi G, et al. Overexpression of ErbB2 and ErbB3 receptors in Schwann cells of patients with Charcot-Marie-tooth disease type 1A. Muscle Nerve. 2006;33(3):342–349. doi: 10.1002/mus.20460. [DOI] [PubMed] [Google Scholar]
- 107.Kohl B, Fischer S, Groh J, Wessig C, Martini R. MCP-1/CCL2 modifies axon properties in a PMP22-overexpressing mouse model for Charcot-Marie-tooth 1A neuropathy. Am J Pathol. 2010;176(3):1390–1399. doi: 10.2353/ajpath.2010.090694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Murphy SM, Polke J, Manji H, Blake J, Reiniger L, Sweeney M, Houlden H, Brandner S, et al. A novel mutation in the nerve-specific 5'UTR of the GJB1 gene causes X-linked Charcot-Marie-Tooth disease. J Peripher Nerv Syst. 2011;16(1):65–70. doi: 10.1111/j.1529-8027.2011.00321.x. [DOI] [PubMed] [Google Scholar]
- 109.Kleopa KA, Zamba-Papanicolaou E, Alevra X, Nicolaou P, Georgiou DM, Hadjisavvas A, Kyriakides T, Christodoulou K. Phenotypic and cellular expression of two novel connexin32 mutations causing CMT1X. Neurology. 2006;66(3):396–402. doi: 10.1212/01.wnl.0000196479.93722.59. [DOI] [PubMed] [Google Scholar]
- 110.Kleopa KA. The role of gap junctions in Charcot-Marie-Tooth disease. J Neurosci. 2011;31(49):17753–17760. doi: 10.1523/JNEUROSCI.4824-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kleopa KA, Abrams CK, Scherer SS. How do mutations in GJB1 cause X-linked Charcot-Marie-Tooth disease? Brain Res. 2012;1487:198–205. doi: 10.1016/j.brainres.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Groh J, Heinl K, Kohl B, Wessig C, Greeske J, Fischer S, Martini R. Attenuation of MCP-1/CCL2 expression ameliorates neuropathy in a mouse model for Charcot-Marie-Tooth 1X. Hum Mol Genet. 2010;19(18):3530–3543. doi: 10.1093/hmg/ddq269. [DOI] [PubMed] [Google Scholar]
- 113.Berger P, Tersar K, Ballmer-Hofer K, Suter U. The CMT4B disease-causing proteins MTMR2 and MTMR13/SBF2 regulate AKT signalling. J Cell Mol Med. 2011;15(2):307–315. doi: 10.1111/j.1582-4934.2009.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443(7112):651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 115.Vaccari I, Dina G, Tronchere H, Kaufman E, Chicanne G, Cerri F, Wrabetz L, Payrastre B, et al. Genetic interaction between MTMR2 and FIG 4 phospholipid phosphatases involved in Charcot-Marie-Tooth neuropathies. PLoS Genet. 2011;7(10):e1002319. doi: 10.1371/journal.pgen.1002319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kovacevic Z, Chikhani S, Lui GY, Sivagurunathan S, Richardson DR. The iron-regulated metastasis suppressor NDRG1 targets NEDD4L, PTEN, and SMAD4 and inhibits the PI3K and Ras signaling pathways. Antioxid Redox Signal. 2013;18(8):874–887. doi: 10.1089/ars.2011.4273. [DOI] [PubMed] [Google Scholar]
- 117.Delague V, Jacquier A, Hamadouche T, Poitelon Y, Baudot C, Boccaccio I, Chouery E, Chaouch M, et al. Mutations in FGD4 encoding the Rho GDP/GTP exchange factor FRABIN cause autosomal recessive Charcot-Marie-Tooth type 4H. Am J Hum Genet. 2007;81(1):1–16. doi: 10.1086/518428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baudot C, Esteve C, Castro C, Poitelon Y, Mas C, Hamadouche T, El-Rajab M, Levy N, et al. Two novel missense mutations in FGD4/FRABIN cause Charcot-Marie-Tooth type 4H (CMT4H) J Peripher Nerv Syst. 2012;17(2):141–146. doi: 10.1111/j.1529-8027.2012.00405.x. [DOI] [PubMed] [Google Scholar]
- 119.Boubaker C, Hsairi-Guidara I, Castro C, Ayadi I, Boyer A, Kerkeni E, Courageot J, Abid I, et al. A novel mutation in FGD4/FRABIN causes Charcot Marie Tooth disease type 4H in patients from a consanguineous Tunisian family. Ann Hum Genet. 2013;77(4):336–343. doi: 10.1111/ahg.12017. [DOI] [PubMed] [Google Scholar]
- 120.Mei L, Nave KA. Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron. 2014;83(1):27–49. doi: 10.1016/j.neuron.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hartmann F, Horak EM, Cho C, Lupu R, Bolen JB, Stetler-Stevenson MA, Pfreundschuh M, Waldmann TA, et al. Effects of the tyrosine-kinase inhibitor geldanamycin on ligand-induced Her-2/Neu activation, receptor expression and proliferation of Her-2-positive malignant cell lines. Int J Cancer. 1997;70(2):221–229. doi: 10.1002/(sici)1097-0215(19970117)70:2<221::aid-ijc14>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 122.Belleudi F, Marra E, Mazzetta F, Fattore L, Giovagnoli MR, Mancini R, Aurisicchio L, Torrisi MR, et al. Monoclonal antibody-induced ErbB3 receptor internalization and degradation inhibits growth and migration of human melanoma cells. Cell Cycle. 2012;11(7):1455–1467. doi: 10.4161/cc.19861. [DOI] [PubMed] [Google Scholar]
- 123.Sak MM, Szymanska M, Bertelsen V, Hasmann M, Madshus IH, Stang E. Pertuzumab counteracts the inhibitory effect of ErbB2 on degradation of ErbB3. Carcinogenesis. 2013;34(9):2031–2038. doi: 10.1093/carcin/bgt173. [DOI] [PubMed] [Google Scholar]
- 124.Aurisicchio L, Marra E, Roscilli G, Mancini R, Ciliberto G. The promise of anti-ErbB3 monoclonals as new cancer therapeutics. Oncotarget. 2012;3(8):744–758. doi: 10.18632/oncotarget.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nature reviews Drug Discov. 2009;8(8):627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liao J, Marumoto T, Yamaguchi S, Okano S, Takeda N, Sakamoto C, Kawano H, Nii T, et al. Inhibition of PTEN tumor suppressor promotes the generation of induced pluripotent stem cells. Mol Ther. 2013;21(6):1242–1250. doi: 10.1038/mt.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dillon LM, Miller TW. Therapeutic targeting of cancers with loss of PTEN function. Curr Drug Targets. 2014;15(1):65–79. doi: 10.2174/1389450114666140106100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Robinson FL, Niesman IR, Beiswenger KK, Dixon JE. Loss of the inactive myotubularin-related phosphatase Mtmr13 leads to a Charcot-Marie-Tooth 4B2-like peripheral neuropathy in mice. Proc Natl Acad Sci U S A. 2008;105(12):4916–4921. doi: 10.1073/pnas.0800742105. [DOI] [PMC free article] [PubMed] [Google Scholar]