Abstract
Social cognition enables individuals to understand others’ intentions. Social memory is a necessary component of this process, for without it, subsequent encounters are devoid of any historical information. The CA2 area of the hippocampus, particularly the vasopressin 1b receptor (Avpr1b) expressed there, is necessary for memory formation. We used optogenetics to excite vasopressin terminals, originating from the hypothalamic paraventricular nucleus, in the CA2 of mice. This markedly enhanced their social memory if the stimulation occurred during memory acquisition, but not retrieval. This effect was blocked by a Avpr1b antagonist. Finally, this enhanced memory is resistant to the social distraction of an introduced second mouse, important for socially navigating populations of individuals. Our results indicate the CA2 can increase the salience of social signals. Targeted pharmacotherapy with Avpr1b agonists or deep brain stimulation of the CA2 are potential avenues of treatment for those with declining social memory as in various dementias.
INTRODUCTION
Complex social behavior requires integration of social, spatial, and interoceptive information and often depends on individual recognition. From discriminating amongst two individuals to the loving bond that is imprinted on a mother and her child, social memories are forged, in part, as a result of encoding information by the hippocampus. These memories are a type of episodic memory whose formation depends on a complex interplay of various inputs to and circuits within the hippocampus. The signaling by these circuits, of course, relies on a rich variety of neurotransmitters and neuromodulators, including peptides1.
In particular, the neurohypophysial peptide vasopressin (Avp) affects social recognition (SR) across many mammalian species2, 3. For example, pharmacological excitation or inhibition of Avpr1a in the rat brain affects recognition of a juvenile conspecific4, 5. This work has focused on Avpr1a in the septum as a regulator of social memory6. However, burgeoning evidence is connecting a potentially distinct hippocampal Avpr1b circuit to social memory. Specifically, we have shown genetic silencing of Avpr1b expression limits normal expression of SR while not affecting spatial or non-social contextual memories7, 8. Furthermore, Avpr1b is rather restricted in its expression in the brain, prominently expressed in the CA2 region of the hippocampus9. These data have generated recent curiosity from neuroscientists about the CA2a, particularly Avpr1b function, as the mapping of social memories within the hippocampus has remained undetermined. This type of hippocampal mapping would benefit a large swath of the population, from the elderly to individuals with varying forms of dementia, hippocampal amnesia and select cognitive impairment, as these people can experience symptoms that strain their relationshipsb.
Thus, we explored the involvement of Avp signaling to the CA2, specifically to Avpr1b-expressing neurons, on the regulation of social memory. We genetically targeted excitation of the CA2 by projecting vasopressin neurons from the paraventricular nucleus of the hypothalamus (PVN) and dramatically prolonged social memory in mice from 30 minutes after a single encounter to at least 7 days. Memory enhancement occurs with optical stimulation during memory acquisition, but not during retrieval; an effect prevented by pharmacological antagonism of Avpr1b. Finally, the enhanced memory is resistant to interference by an introduced second female mouse. Our work provides new knowledge about how the CA2 is integrated into a circuit regulating social memories and suggests an avenue of exploration to treat social cognition deficits in dementia.
MATERIALS AND METHODS
Subjects
All experiments were conducted according to US National Institutes of Health guidelines for animal research and were approved by the National Institute of Mental Health Animal Care and Use Committee. Adult Avp promoter-driven Cre (Avp-Cre) males (GENSAT line QZ2730) were used for experiments conducted 5–6 weeks after viral injections. All mice were single housed during experiments and maintained on a 12-h light cycle (lights off at 1500h) with ad libitum access to food and water.
Retrograde tracing experiments used a total of 4 Avp-Cre male mice taken from 2 litters and injected with Cre-dependent herpes simplex virus (ST HSV-LS1L-WGA-CMV-GFP). Our previous research assessing the effects of genetically silencing Avpr1b on social recognition8 produced an effect size of r = 0.64. With a desired power of 0.80, an n = 8 for each treatment was the estimated sample size required for this study. Social recognition experiments used a total of 73 male mice taken from 32 litters run in six separate squads: 25 wild-type (WT) mice (Supplemental data) and 25 WT and 22 Avp-Cre mice injected with an adeno-associated virus (AAV) that expresses a Cre recombinase-activatable channelrhodopsin (ChR2) fused to mCherry (AAV2-DIO-EF1α-ChR2-mCherry). WT mice without injections were used to evaluate the time course for social recognition test. The AAV injected WT and Avp-Cre were randomly assigned to experimental conditions. All mice were between 92–139 days old at testing. A total of 41 ovariectomized BalbC female mice obtained from Jackson Laboratories (Bar Harbor, ME, USA) at 8 weeks of age were used as social stimuli.
Viruses and chemicals
The recombinant AAV vectors were serotyped with AAV2 coat proteins and packaged by the University of North Carolina Vector Core (Chapel Hill, NC, USA). The HSV vectors were packaged by the Massachusetts Institute of Technology Viral Gene Transfer Core (Cambridge, MA, USA). SSR149415 was obtained from Axon Medchem (Groningen, Netherlands).
Stereotaxic surgeries
Male mice (10–12 weeks old) were anaesthetized with tribromoethanol (Avertin®) and placed into a stereotaxic apparatus. After exposing the skull via a small incision and leveling the head position using bregma and lambda as reference points, two small holes were drilled for injections and implants. A 5 μl microliter syringe (26 g, Hamilton) with a 33 g small gauge RN needle attachment was inserted in the brain, and the virus was injected using a Micro4 microsyringe pump (World Precision Instruments). For retrograde tracing (n = 4), ST HSV-LS1L-WGA-CMV-GFP (5 × 108 transducing units per ml) was injected unilaterally in two sites of the CA2 (300nl, site 1: bregma: AP: −1.46, DV: −1.56, ML: −1.46; site 2: AP: −1.82, DV: −1.73, ML: −2.06). For behavior and Arc studies, AAV-DIO-EF1α-hChR2(H134R)-mCherry (1.5 × 1012 transducing units per ml) serotype 2 was injected bilaterally in the PVN (250nl, bregma: AP: −0.82, DV: −4.73, ML: ±0.15), and optical fibers were implanted bilaterally in the CA2 (AP: −2.18, DV: −1.67, ML: ±2.56). Pharmaco-optogenetic surgeries (WT, n = 8; Avp-Cre, n = 8) included the same targeting of AAV to the PVN plus with 26-gauge guide cannulae (Plastics One) implanted at 10° above the CA2 (AP: −2.18, DV: −1.19, ML: ±2.76). All stereotaxic injections sites were histologically verified. All ‘misses’ or ‘partial hits’ were excluded from the data analyses.
Behavioral tests
To photostimulate the ChR2-positive fibers, we used low-intensity illumination from a LED light source (465-nm; PlexBright, Plexon) coupled with a LED driver (LD-1, Plexon). The light power at the specimen was 1–2 mW mm−2, measured by an optical power meter (PM100D, Thorlabs). The light output was modulated to 20-ms pulses by a programmable pulse stimulator (NI USB-6229, National Instruments) and Labview software (version 12.0.1f4, National Instruments). Tests were conduced during the dark cycle. Using Ethovision XT (version 10.0.826, Noldus Information Technology), SR was measured in a direct interaction test with or without an interference trial. For the two-trial SR test (WT, n = 8; Avp-Cre, n = 7), mice were exposed to an unfamiliar OVX female for 5 minutes and re-exposed to the original or a novel female after a 30-minute, 2-hour, 1-day, or 7-day retention interval. This protocol was adapted to study the effect of activating afferent Avp inputs to the CA2 on memory acquisition vs retrieval (WT, n = 8; Avp-Cre, n = 7) and extended retention intervals (WT, n = 9; Avp-Cre, n = 7). For the three-trial retroactive interference test (WT, n = 9; Avp-Cre, n = 7), mice were exposed twice to the same OVX female similar to the two-trial SR test. However, 5 minutes into the 30-minute retention interval, subject mice were exposed to a different female for 5 minutes as an interference trial. The SR tests were scored by the experimenter blind to the group identities.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics 19 (SPSS, Inc., an IBM Company) and were expressed as mean ± SEM.
RESULTS
Afferent Avp inputs to the CA2 originate selectively from the PVN
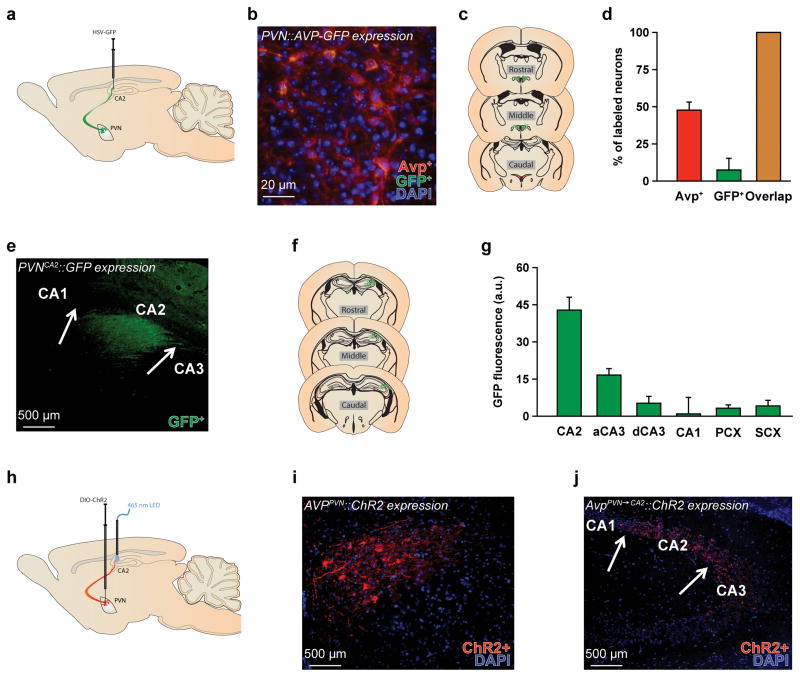
We and others recently described20, 21 Avp innervation of the CA2 arriving from the PVN (AvpPVN→CA2) (Fig. 1a–g). To understand more fully the specificity of this pathway, we studied whether AvpPVN→CA2 neurons collateralize and project to other brain regions as well as whether other populations of Avp neurons, outside of the PVN, project to the CA2. We bilaterally injected into the CA2 of Avp-Cre mice a retrogradely propagating Cre-dependent herpes simplex virus (ST HSV-LS1L-WGA-CMV-GFP) to express green fluorescent protein (GFP). Mice were perfused one week later, and brain sections were probed with antibodies to Avp and GFP (see Supplemental Experimental Procedures for more details). As expected, GFP+ neurons were detected in the PVN (Fig. 1b–d) but not in other brain regions with Avp-ir neurons, such as the supraoptic nucleus, suprachiasmatic nucleus, anterior hypothalamus, or bed nucleus of the stria terminalis. In addition to the PVN, GFP+ axonal fibers densely innervate the CA2 and immediately adjacent CA3, with sparse innervation in the surrounding structures, such as the CA1, dentate gyrus or somatosensory and parietal cortices (Fig. 1e–f). Thus, the AvpPVN→CA2 is an anatomically distinct Avp pathway that only projects to the CA2 and has no collaterals to other structures, including regions where AVPPVN neurons have been known to send afferent projections (e.g., lateral septum, ventral pallidum, medial preoptic area; Supplementary Fig. 1)22.
Figure 1. The AvpPVN→CA2 pathway is anatomically distinct.
a, The diagram illustrating ST HSV-LS1L-WGA-CMV-GFP viral injections in the hippocampal CA2 and subsequent expression in the CA2 (fibers) and hypothalamic paraventricular nucleus (PVN, soma and fibers). b–c, Image and diagram of coronal sections show the expression of green fluorescent protein (GFP+, green) following a Cre-inducible HSV-GFP injection and vasopressin (AVP+, red) immunostaining in the PVN of AVP promoter-driven Cre (Avp-Cre) mice. Cell nuclei are stained with 4′,6-diamidino-2-phenylindole (DAPI, blue). d, Quantification of Avp+, GFP+, and GFP+ neurons that are Avp+ in the PVN reveal that a subset of Avp+ neurons express the viral GFP+ and therefore project to the CA2. Further, all GFP+ neurons co-express Avp+, indicating that the HSV-GFP selectively labeled Avp+ neurons (n = 3 sections from 4 mice). e–f, Image and diagram of coronal sections show that Avp fibers (labeled by expression of GFP+) projecting from the PVN innervate the CA2 and immediately adjacent CA3. Arrows are used to identify anatomical boundaries for the CA areas. g, Specifically, GFP+ fluorescence intensity, reported in arbitrary units (a.u.), is highest in the CA2, and to a lesser extent immediately adjacent CA3 (aCA3), compared to the surrounding regions, which include the distal CA3 (dCA3), CA1, parietal cortex (PCX), and somatosensory cortex (SCX). h, The schematic illustrates AAV2-DIO-EF1α-hChR2(H134R)-mCherry viral injections and optical stimulation, emitting at a 465-nm wavelength from a LED light, of AvpPVN→CA2 fibers. i–j, These are images of coronal sections showing expression of ChR2 fused to mCherry (ChR2+, red) in the PVN and CA2 of Avp-Cre mice. Arrows are used to identify anatomical boundaries for the CA areas. Cell nuclei are stained with DAPI (blue). Scale bars are labeled for all images.
Targeted activation of the AvpPVN→CA2 pathway enhances social memory
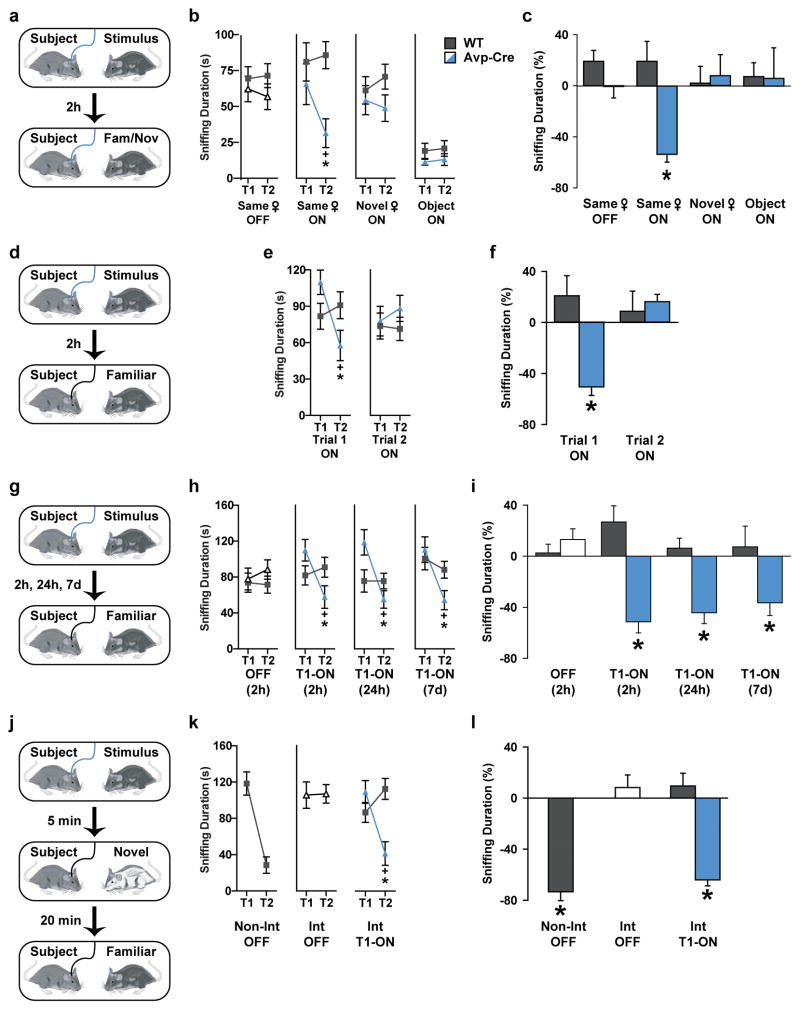
We next studied directly the relevance of the AvpPVN→CA2 pathway to SR. We stimulated fibers in the CA2 after injection of an adeno-associated virus (AAV) that expresses a Cre recombinase-activatable channelrhodopsin (ChR2) fused to mCherry (AAV2-DIO-EF1α-ChR2-mCherry) into the PVN of Avp-Cre mice (Fig. 1h–j). Optical fibers were also implanted bilaterally in the CA2. We then waited five to six weeks for the ChR2 to be transported to the CA2. When we examined the brains after the behavioral testing, we observed robust ChR2-mCherry expression in the PVN, CA2 and immediately adjacent CA3. Both WT and Avp-Cre mice were tested in a two-trial SR test, in which the subject mouse is exposed to an unfamiliar ovariectomized (OVX) female. After a specific retention interval, the subject mouse is either exposed to the same female or a novel female. SR is defined by the normal decline or habituation of olfactory investigation (e.g., close nasal contact with the stimulus mouse, as in this study) in response to repeated exposure to the same OVX female. WT mice recognize familiar females within a limited retention interval, from 30 minutes to 1 hour, and display little to no recognition response after 2 hours23 (Supplementary Fig. 2). Like WT mice, Avp-Cre mice are unable to recognize familiar females after a 2-hour retention period (Fig. 2a–c). However, low-intensity illumination (using 20-ms pulses of 465-nm light at 1.5mW mm−2, Fig. 1h) focused on the CA2 during both social exposures enhances SR (Fig. 2a–c) and neuronal activity in the PVN and CA2 (Supplementary Fig. 3). In contrast, mice sociability is unaffected by this optical stimulation as the durations of investigation are similar in both trials if the subject mice encounter two different unfamiliar females in the two trials (Fig. 2b). In addition, no change in investigation of an inanimate object is observed during a recognition test.
Figure 2. Activation of AvpPVN→CA2 neurons enhances social memory.
a, The two-trial social recognition test included two five-minute exposures to social or object stimuli with concurrent optical stimulation (20-ms pulses of 465-nm light at 1.5mW mm−2). The retention interval was established at 2 hr. b, Behavioral data are from wild-type (WT, grey) and AVP promoter-driven Cre (Avp-Cre, white or blue) mice injected in the PVN with an adeno-associated virus containing a Cre-inducible ChR2-mCherry (AAV2-DIO-EF1α-hChR2(H134R)-mCherry) and implanted with optical fibers bilaterally in the CA2. Mice were exposed to an unfamiliar OVX female for 5 minutes and re-exposed to the original (Same ♀) or a novel (Novel ♀) female after a 2-hr retention interval. This same protocol was used for object recognition (Object). The optical stimulation was present (ON) or absent (OFF) for both trials. During the two-trial social recognition test, Avp-Cre mice (n = 7) with optical stimulation displayed decreased investigation during trial 2 of a mouse encountered in trial 1 (ANOVA: genotype X trial F(1,13) = 6.08, P < 0.05); no recognition was observed by WT (n = 8) or Avp-Cre under any other condition (ANOVA: genotype X trial F(1,13) < 1.21, P > 0.29). Mice did not habituate to multiple sessions of social recognition, observable by the similarity in Trial 1 sniffing behavior in the three social recognition conditions. Mice did display a social preference, sniffing social stimuli more during the acquisition trial then object stimuli. c, The percent change score for investigation was significantly decreased in optically stimulated Avp-Cre mice compared to WT mice (t(13) = 4.10, P < 0.001). No genotypic differences occurred in the change of investigation in any other condition (t(13) < 1.58, P > 0.13). d, The two-trial recognition test was repeated in Avp-Cre and WT mice as previously described under the Same ♀ condition; however, the optical stimulation was only present during the acquisition (Trial 1 ON) or retrieval (Trial 2 ON) trial. e, Avp-Cre (n = 7), but not WT (n = 9), decreased investigation behavior from trial 1 to trial 2 only when optical stimulation occurred during trial 1 (ANOVA: genotype X trial F(1,14) = 22.74, P < 0.0005); no effect occurred with trial 2 optical stimulation (ANOVA: genotype X trial F(1,14) = 1.24, P = 0.28). f, The investigation change score decreased in Avp-Cre mice receiving optical stimulation in trial 1 compared to WT mice (t(14) = 3.78, P < 0.005). No effect occurred from trial 2 optical stimulation (t(14) = 0.40, P = 0.69). g, The two-trial recognition test was repeated in Avp-Cre and WT mice as previously described under the Same ♀ condition with optical stimulation absent (OFF) or presented only during the acquisition trial (T1-ON). However, the retention interval was adjusted to 2 hr, 1 day, or 7 days. h, In this Extended retention recognition test, WT (n = 9) and Avp-Cre (n = 7) mice displayed similar investigation response in both trials without optical stimulation (ANOVA: genotype X trial F(1,14) = 1.24, P = 0.28). Avp-Cre mice receiving optical stimulation in trial 1 decreased investigation from trial 1 to trial 2 compared to WT mice, regardless of retention interval (ANOVA: genotype X trial F(1,14) > 14.00, P < 0.005). i, Avp-Cre mice receiving optical stimulation had a pronounced change in investigation behavior compared WT, regardless of retention interval (t(14) > 3.05, P < 0.01. No genotypic differences were observed in the absence of optical stimulation (t(14) = 1.65, P = 0.12). j, For the three-trial retroactive interference test, WT and Avp-Cre mice were exposed twice to the same OVX female similar to the two-trial SR test. However, 5 minutes into the 30-minute retention interval, subject mice were either undisturbed (non-interference condition, Non-Int) or exposed to a different female for 5 minutes as an interference trial (Int). k, WT mice (n = 9) decrease investigation behavior to a mouse encountered 30 min previously without optical stimulation (Non-Int, OFF) (ANOVA: genotype X trial F(1,14) > 37.62, P < 0.00005). Avp-Cre mice (n = 7) do not change investigation behavior to a mouse encountered in trial 1, if a second mouse is introduced during the retention period and in the absence of optical stimulation (Int, OFF). However, Avp-Cre, but not WT, mice receiving optical stimulation decrease investigation behavior when encountering a mouse for the second time, even with an interference trial (Int, T1-ON). l, The change in investigation occurs in WT mice without interference (Non-Int, OFF) and Avp-Cre mice receiving optical stimulation even after an interference trial (Int, T1-ON) (t(14) > 6.96, P < 0.00001). Interference trials limit change in investigation behavior in WT mice and Avp-Cre mice without optical stimulation (Int, OFF) (P > 0.23).
Previous pharmacological studies demonstrated Avp signaling in specific brain regions could affect either the acquisition and formation of new memories5 or retention and retrieval of stored memories2. Therefore, to learn when the Avp terminal stimulation strengthened social memory, we restricted the period of stimulation to either the initial encounter (acquisition trial) or subsequent encounter (retrieval trial). We found that optical stimulation to release Avp within the CA2 during the acquisition trial is sufficient to enhance SR. Stimulation only during the retrieval trial has no affect on SR (Fig. 2d–f). Interestingly, SR is lengthened for at least 7 days after acquisition trial stimulation (Fig. 2g–i). This represents at least an 80-fold increase in the duration of this social memory.
As Avpr1b KO mice have impairments in temporal order memory7, we also examined whether the effect of optical stimulation could survive a social distraction through the use of a retroactive interference paradigm. Briefly, mice were exposed to a SR test with a 30-minute retention interval; however, during the retention period, mice were presented a second unfamiliar OVX female, known as an “interference trial.” We found that WT mice are able to recognize OVX females after a 30-minute interval, but recognition for the original female is impaired after an interference trial (Fig. 2j–l). This retroactive interference is eliminated when the AvpPVN→CA2 pathway is stimulated during the acquisition trial with the original female, thus, preserving temporal order memory.
Memory enhancement in the CA2 depends on local Avpr1b action
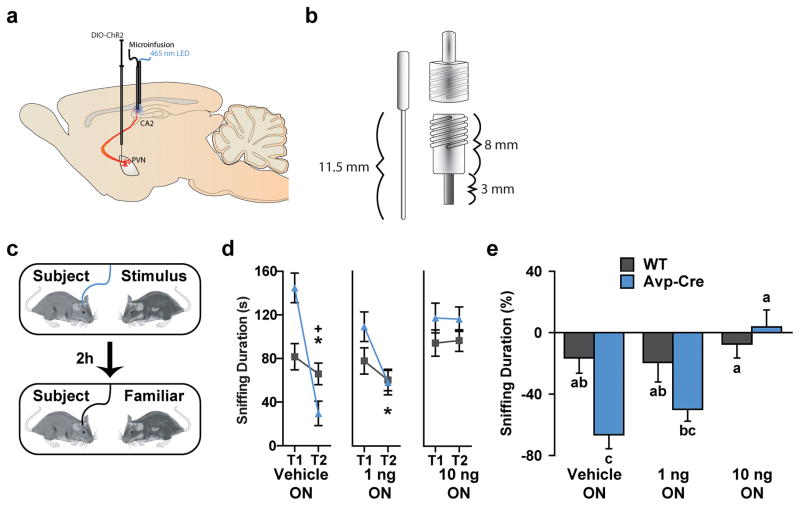
Finally, we wanted to verify the pharmacological specificity of the optical stimulation of the Avp terminals in CA2. Previous studies have implicated the dorsal and ventral hippocampus, dorsal and lateral septum, olfactory bulb, and medial amygdala as sites at which the related Avpr1a can influence SR24. We do not find this receptor expressed in the mouse dorsal hippocampus (Supplementary Fig. 4). However, as mentioned above, the Avpr1b is prominently expressed in the CA2 region of the hippocampus9. Therefore, we examined whether pharmacological antagonism of Avpr1b in the CA2 hindered Avp-enhancement of social memory. Intra-CA2 microinjections of the Avpr1b antagonist (SSR149415), through guide cannulas designed to accommodate micro-injector needles for drug infusion as well as optical fibers, blocked SR enhancement (Fig. 3). Thus, the PVN, through an anatomically distinct AvpPVN→CA2 pathway that is dependent on Avpr1b signaling, potentiates acquisition of new memories, enhancing and extending social memory.
Figure 3. Pharmacological antagonism of Avpr1b in the CA2 blocks optogenetic memory enhancement.
a, The schematic illustrates AAV-ChR2-mCherry expression in AVP promoter-driven Cre (Avp-Cre) mice with intra-CA2 optical stimulation (emitting at a 465-nm wavelength from a LED light) and microinjections of the Avpr1b antagonist (SSR149415). b, Specifically, mice were injected with an adeno-associated virus containing a Cre-inducible ChR2-mCherry (AAV2-DIO-EF1α-hChR2(H134R)-mCherry) in the PVN and implanted with guide cannulae bilaterally in the CA2. The diagram illustrates the optical fiber (left; 11.5 mm shaft), 21-gauge guide cannula (bottom right; 8 mm pedestal, 3 mm metal cannula), and a dust cap outfitted with a ceramic sleeve threaded through and anchored with epoxy to the dust cap (top right). These modified dust caps and guide cannulae were designed to accommodate micro-injector needles for drug infusion as well as optical fibers. c, Before the acquisition trial, wild-type (WT) and Avp-Cre mice received 200 nl microinjections of a vehicle or an Avpr1b antagonist (SSR149415, 1 ng or 10 ng). Mice were then exposed to an unfamiliar OVX female for 5 minutes with optical stimulation (T1-ON) and re-exposed to the original female (Same ♀ condition) after a 2-hour retention interval. d, Avp-cre mice (blue, n = 8) receiving optical stimulation decreased investigation of a mouse encountered in trial 1 during trial 2 when concurrently treated with a drug vehicle and, to a lesser extent, 1 ng of SSR149415 but not a higher dose (10 ng) of this Avpr1b antagonist (ANOVA: genotype X drug X trial F(2, 42) = 10.98, P < 0.0005). Avp-Cre mice investigated the stimulus mouse during trial 2 significantly less than WT mice (grey, n = 8) but only when receiving the drug vehicle. e, Avp-Cre mice receiving optical stimulation displayed greater change in investigation compared to WT mice when concurrently receiving vehicle infusions, no change occurred when an Avpr1b antagonist (1 ng or 10 ng) was injected (ANOVA: genotype X drug F(2, 28) = 4.08, P < 0.05).
DISCUSSION
Memories for people, places, and events are a fundamental facet of the concept of self. The hippocampus is a critical region for declarative memory25. O’Keefe and Nadel26 suggested that the hippocampus provides a neural basis of cognitive mapping. This original proposal has served as the starting point of many studies that demonstrated CA1 place cells encode a map of an individual’s spatial location27. Although these findings are highly important, they present a narrow approach to the original proposal, limiting the hippocampus to spatial organization. However, recently, an effort has been made to re-evaluate the literature gap as it pertains to other potential functions of the hippocampus28, 29, and even more recent data suggest its crucial role in social cognition30. Still, the social computing paths remain unclear - more specifically, the mapping of social memories within the hippocampus.
If the CA1 region encodes spatial memory, where is social memory processed in the hippocampus? And why might some memories be stronger than others? We have previously shown that adult mouse brain Avpr1b is confined to the CA2, immediately adjacent CA3, and fasciola cinerium9 (and unpublished observations) and is essential for normal social memory8, 31. Recently, others have shown that inhibition of the CA2 selectively impairs SR14, 16. It is possible that the CA2 processes the declarative social information from the environment and reforms or strengthens hippocampal representations while leaving the hippocampal mapping system fundamentally intact. Indeed, the CA2 forms strong excitatory synapses with the CA1 allowing for cortical inputs to influence CA1 neuronal output via a disynaptic circuit32, and a conspecific in the environment decreases the intensity and precision of, but does not eliminate, spatial firing of CA1 place cells33. Our data expand upon these findings and demonstrate that direct excitation of PVN Avp neuron terminals that innervate the CA2 enhances memory formation and extends social memories. Mice sociability is unaffected by this optical stimulation as the durations of investigation are similar in both trials if the subject mice encounter two different unfamiliar females in the two trials. As Avp signaling induces significant potentiation of excitatory synaptic responses in the CA215, this could prime, or raise the probability, that these neurons will be recruited or allocated into a memory trace34. This action also fits nicely with the roles of the PVN as a key integrator of, and responder to, signals from external and internal milieus. Many current theories of hippocampal-dependent learning and memory processes may be challenged by the potential impact of the CA2 region, including the influence of Avp signaling.
WT mice living in single housing retain the memory of briefly encountered social stimuli for about 30 minutes to 1 hour, as demonstrated in this study and previous research23, 35. Social memory can be extended by retroactive facilitation (i.e., repeated exposures to the original social stimulus) or pharmacologically by systemic Avp treatments36. Our results demonstrate a circuit through which Avp can act to generate extended social memories. The fact that this new memory endures for at least 7 days suggests that Avp signaling in the CA2 during memory acquisition strengthens the salience of social information and allows this information to be consolidated into a long-term memory, dissociating decay of the memory trace from the passage of time. Further, the memory trace of a conspecific is sensitive to disruption after acquisition by newly learned information or subsequent interactions with other social stimuli. This is called retroactive interference and can occur up to 15 hours after the original memory trace in rodents37. Avp signaling appears to modulate the impact that new information has on a lingering memory trace. For example, Avpr1b knockout mice have deficits in SR and struggle to create temporal associations or to remember the temporal order in which objects are presented to them7. In addition, peripheral administration of a non-specific Avpr1 antagonist during memory consolidation can interfere with SR36. Our data demonstrate that Avp signaling in the CA2 during acquisition can block retroactive interference and facilitate temporal order memory. The fact that these effects occur in a matter of minutes provides insight into how mice, and potentially other social species, are able to navigate a dynamic social environment while still being able to retain pertinent information about conspecifics to generate familiarity and build relationships.
Our demonstration that excitation of the AvpPVN→CA2 pathway during acquisition, but not retrieval, enhances social memory suggests that the mechanisms regulating social memory acquisition and retrieval in the hippocampus are distinct. At a cellular level, a selective Avpr1b agonist induces significant potentiation of excitatory synaptic responses in CA2, but not in CA1 or in slices from Avpr1b knockout mice15. Furthermore, this synaptic potentiation relies on NMDA (N-methyl-D-aspartic acid) receptor activation, calcium and calcium/calmodulin-dependent protein kinase II activity (PKA); but not on cAMP-dependent protein kinase activity or presynaptic mechanisms. Interestingly, NMDA receptors and PKA in the hippocampus are critical for acquisition of spatial and fear memories, but retrieval of these memories appears independent of such activity38. Thus, it is possible that social memory acquisition occurs through similar cellular mechanisms as spatial and fear memories in the hippocampus, with the distinction that NMDA receptor-dependent function in the CA2 is sensitive to Avp signaling.
It has become apparent that more than one Avp circuit is involved in social memory. Avp is present in the dorsal hippocampus39, and local administration of anti-Avp serum impairs SR in rats40. Recently, the PVN has been identified as the source of Avp innervation to the CA2 in mice21 and rats20. Here, we document that the AvpPVN→CA2 pathway enhances SR and is anatomically distinct as a subset of Avp neurons in the PVN that project to the CA2. Further, we observed that these neurons have no collaterals to other structures. Still, the impact of Avp on social memory is not limited to the hippocampus24. The lateral septum, among other regions in the mouse and rat brain, has been associated with Avp regulation of SR. Pharmacological activation or antagonism of Avpr1a in the rat lateral septum enhances or impairs SR, respectively5. The Avp fibers in the lateral septum originate from the bed nucleus of the stria terminalis and medial amygdala41, 42, not the PVN as those in the CA2. This would suggest that multiple discrete Avp circuits are involved in regulating social memory.
In the first decade of the 20th century, Richard Semon, first described engrams as the ensemble of neurons serving as the physical representation of memory in the brain43. While many have attempted to identify the biological locus for memory, most notably in a series of lesion studies by Karl Lashley44, technology has only now advanced to a state to allow neuroscientists to identify specific populations of cells activated during learning and evoked in recall of the original memory. It is now possible to label neuronal populations active during learning to either inhibit or reactivate memory-related neuronal populations45. Using a method that permits optogenetic labeling and manipulation of specific neuronal populations, fear memory recall can be impaired by limiting the activity of neuronal populations in the hippocampal CA3, CA1, and dentate gyrus which were active during the acquisition of the fear memory46, 47. This same method has been applied to reactivate populations of neurons in the hippocampus to mimic cue-induced behavioral recall. This has allowed the reactivation of genuine memories48, creation of false memories49, and switching memory valence50. The results of these studies, promote the understanding of the biological basis of memory, or engrams, in the hippocampus. Nevertheless, the exploration of the network-level processes specific for the acquisition of engrams is fundamental for a comprehensive understanding of memory. The first study to enhance memory by stimulating a hippocampal-dependent circuit was published last year. In that work, excitation of a midbrain to CA1 dopaminergic circuit during spatial exploration and learning improved spatial memory performance51. Our work presents the first evidence of a specific neural circuit that directly regulates hippocampal-dependent social memory, and that its stimulation is sufficient to enhance long-term SR and overcome interference. As technology advances, we expect to expand our understanding of the ‘social memory engrams’ and gain more insights into the pathologies involving social memory deficits.
Supplementary Material
Acknowledgments
We thank Emily Shepard for assistance with mouse colony maintenance and genotyping. We are grateful for the gifts of the vasopressin antibody from Dr. Harold Gainer and HSV from Dr. Ted Usdin. Alex Avram kindly wrote the software we used to control the optogenetic stimulations. We also thank Drs. Stafford Lightman and Michael Brownstein for their comments on a previous version of this manuscript. This research was supported by the intramural research program of the NIMH (ZIA-MH-002498-24).
Footnotes
The CA2 was first described over 80 years ago10 and is distinctive from the CA3 and CA1 in terms of cytoarchitecture, connectivity, physiology, and gene expression patterns10–13. Behavioral studies support a unique functional role for CA2 in social memory and aggression toward intruders14–16.
For example, individuals with hippocampal amnesia have trouble consciously recalling shared experiences, remembering names of people they meet, and consolidating new information into mental representations of their current relationships17–19. This likely contributes in self-reporting of only a few close friendships and limited community involvement.
Conflict of Interest: All authors declare that there are no competing financial interests in relation to the work described.
References
- 1.Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Alescio-Lautier B, Rao H, Paban V, Devigne C, Soumireu-Mourat B. Inhibition of the vasopressin-enhancing effect on memory retrieval and relearning by a vasopressin V1 receptor antagonist in mice. Eur J Pharmacol. 1995;294:763–770. doi: 10.1016/0014-2999(95)00632-x. [DOI] [PubMed] [Google Scholar]
- 3.Dluzen DE, Muraoka S, Engelmann M, Landgraf R. The effects of infusion of arginine vasopressin, oxytocin, or their antagonists into the olfactory bulb upon social recognition responses in male rats. Peptides. 1998;19:999–1005. doi: 10.1016/s0196-9781(98)00047-3. [DOI] [PubMed] [Google Scholar]
- 4.Le Moal M, Dantzer R, Michaud B, Koob GF. Centrally injected arginine vasopressin (AVP) facilitates social memory in rats. Neurosci Lett. 1987;77:353, 335–359. doi: 10.1016/0304-3940(87)90527-1. [DOI] [PubMed] [Google Scholar]
- 5.Dantzer R, Koob GF, Bluthe RM, Le Moal M. Septal vasopressin modulates social memory in male rats. Brain Res. 1988;457:143–147. doi: 10.1016/0006-8993(88)90066-2. [DOI] [PubMed] [Google Scholar]
- 6.Engelmann M. Vasopressin in the septum: Not important versus causally involved in learning and memory--two faces of the same coin? Prog Brain Res. 2008;170:389–395. doi: 10.1016/S0079-6123(08)00432-9. [DOI] [PubMed] [Google Scholar]
- 7.DeVito LM, Konigsberg R, Lykken C, Sauvage M, Young WS, 3rd, Eichenbaum H. Vasopressin 1b receptor knock-out impairs memory for temporal order. J Neurosci. 2009;29:2676–2683. doi: 10.1523/JNEUROSCI.5488-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WS., 3rd Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- 9.Young WS, Li J, Wersinger SR, Palkovits M. The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience. 2006;143:1031–1039. doi: 10.1016/j.neuroscience.2006.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de No RL. Studies on the structure of the cerebral cortex. II. Continuation of the study of ammonic system. J Psychol Neurol. 1934;46:113–177. [Google Scholar]
- 11.Lein ES, Callaway EM, Albright TD, Gage FH. Redefining the boundaries of the hippocampal CA2 subfield in the mouse using gene expression and 3-dimensional reconstruction. J Comp Neurol. 2005;485:1–10. doi: 10.1002/cne.20426. [DOI] [PubMed] [Google Scholar]
- 12.Woodhams PL, Celio MR, Ulfig N, Witter MP. Morphological and functional correlates of borders in the entorhinal cortex and hippocampus. Hippocampus. 1993;3:303–311. [PubMed] [Google Scholar]
- 13.Zhao X, Lein ES, He A, Smith SC, Aston C, Gage FH. Transcriptional profiling reveals strict boundaries between hippocampal subregions. J Comp Neurol. 2001;441:187–196. doi: 10.1002/cne.1406. [DOI] [PubMed] [Google Scholar]
- 14.Hitti FL, Siegelbaum SA. The hippocampal CA2 region is essential for social memory. Nature. 2014;508:88–92. doi: 10.1038/nature13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pagani JH, Zhao M, Cui Z, Williams Avram SK, Caruana DA, Dudek SM, et al. Role of the vasopressin 1b receptor in rodent aggressive behavior and synaptic plasticity in hippocampal area CA2. Mol Psychiatry. 2014;20:490–499. doi: 10.1038/mp.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevenson EL, Caldwell HK. Lesions to the CA2 region of the hippocampus impair social memory in mice. Eur J Neurosci. 2014;40:3294–3301. doi: 10.1111/ejn.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davidson PS, Drouin H, Kwan D, Moscovitch M, Rosenbaum RS. Memory as social glue: Close interpersonal relationships in amnesic patients. Frontiers in psychology. 2012;3:531. doi: 10.3389/fpsyg.2012.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warren DE, Duff MC, Magnotta V, Capizzano AA, Cassell MD, Tranel D. Long-term neuropsychological, neuroanatomical, and life outcome in hippocampal amnesia. Clin Neuropsychol. 2012;26:335–369. doi: 10.1080/13854046.2012.655781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duff MC, Wszalek T, Tranel D, Cohen NJ. Successful life outcome and management of real-world memory demands despite profound anterograde amnesia. J Clin Exp Neuropsychol. 2008;30:931–945. doi: 10.1080/13803390801894681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Hernandez VS. Synaptic innervation to rat hippocampus by vasopressin-immuno-positive fibres from the hypothalamic supraoptic and paraventricular nuclei. Neuroscience. 2013;228:139–162. doi: 10.1016/j.neuroscience.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Cui Z, Gerfen CR, Young WS., 3rd Hypothalamic and other connections with dorsal CA2 area of the mouse hippocampus. J Comp Neurol. 2013;521:1844–1866. doi: 10.1002/cne.23263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rood BD, Stott RT, You S, Smith CJ, Woodbury ME, De Vries GJ. Site of origin of and sex differences in the vasopressin innervation of the mouse (Mus musculus) brain. J Comp Neurol. 2013;521:2321–2358. doi: 10.1002/cne.23288. [DOI] [PubMed] [Google Scholar]
- 23.Bluthe RM, Gheusi G, Dantzer R. Gonadal steroids influence the involvement of arginine vasopressin in social recognition in mice. Psychoneuroendocrinology. 1993;18:323–335. doi: 10.1016/0306-4530(93)90028-j. [DOI] [PubMed] [Google Scholar]
- 24.Gabor CS, Phan A, Clipperton-Allen AE, Kavaliers M, Choleris E. Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behavioral Neuroscience. 2012;126:97–109. doi: 10.1037/a0026464. [DOI] [PubMed] [Google Scholar]
- 25.Cohen NJ, Eichenbaum H. Memory, amnesia, and the hippocampal system. MIT Press; Cambridge, MA: 1993. [Google Scholar]
- 26.O’Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford University Press; New York: 1978. [Google Scholar]
- 27.Griffin AL, Hallock HL. Hippocampal signatures of episodic memory: Evidence from single-unit recording studies. Front Behav Neurosci. 2013;7:54. doi: 10.3389/fnbeh.2013.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eichenbaum H, Cohen NJ. Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron. 2014;83:764–770. doi: 10.1016/j.neuron.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartley T, Lever C, Burgess N, O’Keefe J. Space in the brain: How the hippocampal formation supports spatial cognition. Philos Trans R Soc Lond B Biol Sci. 2014;369:20120510. doi: 10.1098/rstb.2012.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tavares RM, Mendelsohn A, Grossman Y, Williams CH, Shapiro M, Trope Y, et al. A map for social navigation in the human brain. Neuron. 2015;87:231–243. doi: 10.1016/j.neuron.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wersinger SR, Temple JL, Caldwell HK, Young WS., 3rd Inactivation of the oxytocin and the vasopressin (Avp) 1b receptor genes, but not the Avp 1a receptor gene, differentially impairs the Bruce effect in laboratory mice (Mus musculus) Endocrinology. 2008;149:116–121. doi: 10.1210/en.2007-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chevaleyre V, Siegelbaum SA. Strong CA2 pyramidal neuron synapses define a powerful disynaptic cortico-hippocampal loop. Neuron. 2010;66:560–572. doi: 10.1016/j.neuron.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zynyuk L, Huxter J, Muller RU, Fox SE. The presence of a second rat has only subtle effects on the location-specific firing of hippocampal place cells. Hippocampus. 2012;22:1405–14016. doi: 10.1002/hipo.20977. [DOI] [PubMed] [Google Scholar]
- 34.Yiu AP, Mercaldo V, Yan C, Richards B, Rashid AJ, Hsiang HL, et al. Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron. 2014;83:722–735. doi: 10.1016/j.neuron.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10:47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 36.Dantzer R, Bluthe RM, Koob GF, Le Moal M. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology (Berl) 1987;91(3):363–368. doi: 10.1007/BF00518192. [DOI] [PubMed] [Google Scholar]
- 37.Engelmann M. Competition between two memory traces for long-term recognition memory. Neurobiol Learn Mem. 2009;91:58–65. doi: 10.1016/j.nlm.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11:180–187. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- 39.Landgraf R, Neumann I, Pittman QJ. Septal and hippocampal release of vasopressin and oxytocin during late pregnancy and parturition in the rat. Neuroendocrinology. 1991;54:378–383. doi: 10.1159/000125917. [DOI] [PubMed] [Google Scholar]
- 40.van Wimersma Greidanus TB, Maigret C. The role of limbic vasopressin and oxytocin in social recognition. Brain Res. 1996;713(1–2):153–159. doi: 10.1016/0006-8993(95)01505-1. [DOI] [PubMed] [Google Scholar]
- 41.de Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- 42.Caffé AR, van Leeuwen FW, Luiten PG. Vasopressin cells in the medial amygdala of the rat project to the lateral septum and ventral hippocampus. J Comp Neurol. 1987;261:237–252. doi: 10.1002/cne.902610206. [DOI] [PubMed] [Google Scholar]
- 43.Semon RW. The mneme. George Allen & Unwin; London: 1921. p. 304. [Google Scholar]
- 44.Lashley KS. In search of the engram. Symp Soc Exp Biol. 1950;4:454–482. [Google Scholar]
- 45.Reijmers L, Mayford M. Genetic control of active neural circuits. Front Mol Neurosci. 2009;2:27. doi: 10.3389/neuro.02.027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Denny CA, Kheirbek MA, Alba EL, Tanaka KF, Brachman RA, Laughman KB, et al. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron. 2014;83:189–201. doi: 10.1016/j.neuron.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka KZ, Pevzner A, Hamidi AB, Nakazawa Y, Graham J, Wiltgen BJ. Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron. 2014;84:347–354. doi: 10.1016/j.neuron.2014.09.037. [DOI] [PubMed] [Google Scholar]
- 48.Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL, et al. Creating a false memory in the hippocampus. Science. 2013;341:387–391. doi: 10.1126/science.1239073. [DOI] [PubMed] [Google Scholar]
- 50.Redondo RL, Kim J, Arons AL, Ramirez S, Liu X, Tonegawa S. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature. 2014;513:426–430. doi: 10.1038/nature13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McNamara CG, Tejero-Cantero A, Trouche S, Campo-Urriza N, Dupret D. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat Neurosci. 2014;17:1658, 1616–1660. doi: 10.1038/nn.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.