Abstract
There has been a marked increase in the availability of synthetic drugs designed to mimic the effects of marijuana. These cannabimimetic drugs, sold illicitly as ‘Spice’ and related products, are associated with serious medical complications in some users. In vitro studies suggest that synthetic cannabinoids in these preparations are potent agonists at central cannabinoid CB1 receptors (CB1Rs), but few investigations have delineated their cellular effects, particularly in comparison with the psychoactive component of marijuana, Δ9‐tetrahydrocannabinol (Δ9‐THC). We compared the ability of three widely abused synthetic cannabinoids and Δ9‐THC to alter glutamate release and long‐term potentiation in the mouse hippocampus. JWH‐018 was the most potent inhibitor of hippocampal synaptic transmission (EC50 ~15 nM), whereas its fluoropentyl derivative, AM2201, inhibited synaptic transmission with slightly lower potency (EC50 ~60 nM). The newer synthetic cannabinoid, XLR‐11, displayed much lower potency (EC50 ~900 nM) that was similar to Δ9‐THC (EC50 ~700 nM). The effects of all compounds occurred via activation of CB1Rs, as demonstrated by reversal with the selective antagonist/inverse agonist AM251 or the neutral CB1R antagonist PIMSR1. Moreover, AM2201 was without effect in the hippocampus of transgenic mice lacking the CB1R. Hippocampal slices exposed to either synthetic cannabinoids or Δ9‐THC exhibited significantly impaired long‐term potentiation (LTP). We find that, compared with Δ9‐THC, the first‐generation cannabinoids found in Spice preparations display higher potency, whereas a recent synthetic cannabinoid is roughly equipotent with Δ9‐THC. The disruption of synaptic function by these synthetic cannabinoids is likely to lead to profound impairments in cognitive and behavioral function.
Keywords: Drug abuse, electrophysiology, marijuana
Introduction
The potential therapeutic use of marijuana and related cannabinoids has led to a strong interest in developing compounds that can selectively target cannabinoid receptors but lack abuse liability (Izzo et al. 2009; Bisogno & Di Marzo 2010). Ironically, the development of such compounds by research laboratories across the world has provided clandestine chemists the framework from which to identify and synthesize potent drugs that mimic some of the psychoactive effects of Δ9‐tetrahydrocannabinol (Δ9‐THC). Consequently, there has been a global surge in the nonmedical use of synthetic cannabimimetic substances, marketed as ‘herbal incense’ and commonly known as ‘Spice’ (Logan et al. 2012; Lewin et al. 2014). Unlike the comparatively modest psychoactive and euphoric effects of marijuana, the use of Spice and related compounds has resulted in reports of severe anxiety, tachycardia, seizures and hallucinations (Schneir et al. 2011; Harris & Brown 2013). Nearly all of the identified chemical constituents of synthetic marijuana act as agonists at cannabinoid CB1 receptors (CB1Rs), and the psychoactive compounds in these preparations are frequently modified in response to legislative control imposed upon existing chemical structures (Vardakou et al. 2010; Seely et al. 2012). The structures of three of the most popular synthetic cannabinoids and their appearance in the National Forensic Laboratory Information System database are depicted in Fig. 1. The National Forensic Laboratory Information System data reflect the prevalence of particular synthetic cannabinoids that were confiscated by local, state and federal law enforcement. The naphthoylindole JWH‐018 (Fig. 1b) appeared frequently in Spice products confiscated during the years 2010 and 2011 in the United States but was rapidly supplanted by its fluoropentyl analog AM2201 (Fig. 1c; Seely et al. 2013). In the first half of 2013, the tetramethylcyclopropyl indole XLR‐11 (Fig. 1d) became much more prevalent than JWH‐018 or AM2201 (U.S. Drug Enforcement Administration Office of Diversion 2013), and this trend continued in 2014. Given the greater use of these synthetic marijuana preparations to avoid drug screen detection for Δ9‐THC, and increasing public health concerns regarding these compounds, there is a need to more fully characterize the neurobiological actions of these synthetic cannabinoids (Seely et al. 2012; Castaneto et al. 2014).
Figure 1.
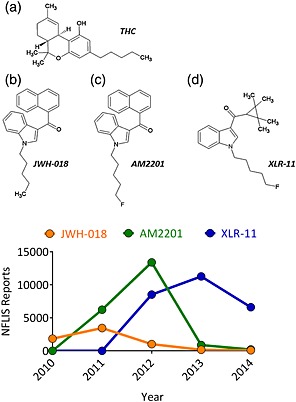
Chemical structure of the compounds tested in this study. (a) Δ9‐Tetrahydrocannabinol (THC), (b) JWH‐018, (c) AM2201 and (d) XLR‐11. Lower panel shows the total number of reports of the tested Spice compounds from the US National Forensic Laboratory Information System (NFLIS). Note the decline in reports of AM2201 and JWH‐018 and the marked rise in reports of XLR‐11 between 2012 and 2013. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Among the best characterized effects of cannabinoids is the inhibition of neurotransmitter release from axon terminals via CB1R activation (Alger 2002; Hoffman & Lupica 2013). The cognitive deficits reported following acute or repeated use of marijuana (Abel 1970; Bolla et al. 2002) may reflect the effects of Δ9‐THC at CB1Rs located on both glutamatergic and GABAergic axon terminals in the hippocampus (Hájos et al. 2000; Sullivan 2000; Hoffman et al. 2007; Puighermanal et al. 2009). Most physiological studies have utilized synthetic agonists, such as WIN55212‐2 and CP55,940, to evaluate the central actions of cannabinoids (Hoffman & Lupica 2000; Robbe et al. 2001; Holderith et al. 2011), and relatively few studies have examined the effects of Δ9‐THC at central synapses in vitro, owing to its poor solubility and penetration into brain slices (Laaris et al. 2010; Hoffman & Lupica 2013). JWH‐018 has been evaluated in cultured hippocampal neurons (Atwood et al. 2010), and other Spice compounds have only recently been studied in pre‐clinical models (Atwood et al. 2011; Basavarajappa & Subbanna 2014). In this report, we directly compare the actions of JWH‐018, AM2201 and XLR‐11 with those induced by Δ9‐THC at hippocampal glutamatergic synapses.
Methods
Subjects
All studies utilized 4‐to 6 week‐old male wildtype C57BL6 mice or CB1+/+ and CB1−/− mice bred on a C57BL6 background. Prior to initiating experimental work, studies were approved by the NIDA IRP Animal Care and Use Committee, in accordance with NIH guidelines.
Hippocampal slice preparation
Mice were anesthetized with isoflurane and euthanized by decapitation. The brain was removed and placed in a beaker containing a modified ice‐cold artificial cerebrospinal fluid (aCSF) containing, in millimolar, N‐methyl‐d‐glucamine, 93; KCl, 2.5; NaH2PO4, 1.2; NaHCO3, 30; HEPES, 20; glucose, 25; sodium pyruvate, 3; MgCl2, 10; CaCl2, 0.5; and ascorbic acid, 5. Brain tissue was blocked and glued to the stage of a vibrating tissue slicer (Leica VT1200S, Leica Biosystems Nussloch, Germany) and submerged in modified aCSF. Transverse hippocampal slices (280 µm) were obtained and stored in standard aCSF containing, in millimolar, NaCl, 126; KCl, 3; CaCl2, 2.4; MgCl2, 1.5; NaH2PO4, 1.2; NaHCO3, 26; and glucose, 11. Slices were warmed in aCSF for 20–25 minutes at 35°C immediately after cutting and then allowed to gradually equilibrate to room temperature for at least 45 minutes prior to initiating recording. All solutions were oxygenated with 95% O2/5% CO2. We have previously found that endogenous adenosine can disrupt CB1R‐mediated inhibition of glutamate release in the hippocampus (Hoffman et al. 2010). Therefore, the selective adenosine A1 receptor antagonist, 8‐cyclopentyl‐1,3‐dipropylxanthine (DPCPX, 200 nM), was included in the aCSF throughout incubation and recordings. On average, three to four slices were used from a single animal per day. All drugs were tested in slices obtained from at least two subjects.
Electrophysiological recording
Brain slices containing the hippocampus were submerged in aCSF in a slice chamber (RC‐26, Warner Instruments, Hamden, CT, USA) and continuously perfused with aCSF (2 ml/minute) using a peristaltic pump (Cole‐Parmer Instruments, Vernon Hills, IL, USA). Bath temperature was maintained at 30–32°C using an in‐line solution heater (TC324‐C and SH27‐B, Warner Instruments). Borosilicate glass electrodes (1.5 mm o.d. × 0.86 mm i.d., Sutter Instruments, Novato, CA, USA) were fabricated using a horizontal puller (P‐97, Sutter Instruments) and filled with aCSF. Electrodes were connected to the headstage of an AC amplifier (Model 1800, A‐M Systems, Sequim, WA, USA). Under stereomicroscopic visualization, a bipolar‐stimulating electrode consisting of twisted formvar‐insulated nichrome wire (50 µm; A‐M Systems) connected to a constant current stimulus isolation unit (DS3, Digitimer) was positioned in hippocampal area CA3 to activate Schaffer collateral fibers using 0.1‐millisecond pulses. The recording electrode was manually positioned in CA1 stratum radiatum using a micromanipulator and gradually lowered while monitoring the field excitatory postsynaptic potential (fEPSP) response. Once the optimal position was determined, stimulus intensity was varied to produce an input–output curve. Baseline responses were then set by adjusting the stimulus intensity to achieve 30–50% of the maximum response. In some experiments, paired pulse stimuli were delivered at an interval of 50 milliseconds. Stimulation, data acquisition and signal analyses were performed on‐line using an A/D board (PCIe‐6321, National Instruments, Austin, TX, USA) and WinLTP software (http://www.win-ltp.com; Anderson & Collingridge 2007). Responses were obtained every 30 seconds, and drugs were applied after obtaining at least 10 minutes of stable baseline recording. High‐frequency stimulation (HFS) consisted of three consecutive 100‐Hz, 1‐second trains, delivered 10 seconds apart. A three‐way valve was used to switch between control aCSF and drug‐containing aCSF. In most cases, drugs were applied for 40–60 minutes. To maintain consistent exposure times to cannabinoid agonists in long‐term potentiation (LTP) experiments, brain slices were pre‐treated for 90 minutes in the holding chamber (Collins et al. 1994, 1995). Thereafter, recordings were performed in either standard aCSF or aCSF containing the CB1 antagonist PIMSR1 ((5‐(4‐Chlorophenyl)‐1‐(2,4‐dichlorophenyl)‐4‐methyl‐3‐[(E)‐piperidinoiminomethyl]‐1H‐pyrazole)). The perfusion apparatus was washed with ethanol for 10–15 minutes between recordings.
Chemicals and drug solutions
1‐Pentyl‐3‐(1‐naphthoyl)‐indole (JWH‐018), (1‐(5‐fluoropentyl)‐1H‐indol‐3‐yl)(naphthalen‐1‐yl)methanone (AM‐2201) and (1‐(5‐fluoropentyl)‐1H‐indol‐3‐yl)(2,2,3,3‐tetramethylcyclopropyl)methanone (XLR‐11) were supplied as dry powders by NIDA Drug Supply Program (Rockville, MD, USA). Stock solutions (1–10 mM) were prepared in dimethyl sulfoxide and frozen at −20°C until thawed for experiments. Δ9‐THC (200 mg/ml in EtOH) was provided through NIDA Drug Supply and was diluted to 10 mM in dimethyl sulfoxide. PIMSR1 was generously provided by Dr Herbert Seltzman (Research Triangle International, Research Triangle Park, NC, USA). AM251 and DPCPX were purchased from Tocris (Minneapolis, MN, USA). All drugs were dissolved to their final concentration in aCSF prior to each experiment. All other reagents were from Sigma‐Aldrich (St. Louis, MO, USA).
Data analysis and statistics
Data are presented as mean ± standard error of the mean. Comparisons were made using t‐tests or ANOVA where appropriate, with a critical value for statistical significance set at P < 0.05. A Holm–Sidak's multiple comparisons test was used to measure the mean level of LTP between 55 and 60 minutes following HFS. Drug responses were defined as the change in the fEPSP slope at the time of the peak drug effect, typically 40 or 60 minutes after drug application. Prior experiments with cannabinoids have established that the relatively long drug onset time reflects partitioning of these highly lipophilic molecules into the brain slice. Responses were normalized to the baseline recording period. Dose–response curves were generated using a three‐parameter, global curve fitting nonlinear regression algorithm in Prism (GraphPad Scientific, San Diego, CA, USA):
where Bottom and Top represent the plateaus and EC50 represents the agonist concentration that produces a response halfway between the top and bottom of the curve. The bottom was constrained to a value of 0. A global curve fit was first used to determine whether the top plateau differed among the family of curves (JWH‐018, AM2201, Δ9‐THC and XLR‐11). An additional sum of squares F‐test revealed that the maximum plateaus did not significantly differ (F 3,82 = 1.203, P = 0.3139). In addition, a one‐way ANOVA revealed no significant differences in the maximal inhibition produced by each compound (F 3,17 = 0.7656, P = 0.5289). Thus, the Top was allowed to be shared as a single value for all data sets, and the EC50 values were calculated.
Results
Inhibition of glutamate release by Δ9‐tetrahydrocannabinol
The cannabinoids found in synthetic marijuana preparations are presumably used as substitutes for the phytocannabinoid Δ9‐THC because they may mimic some of its pharmacological properties in the brain. Therefore, we first defined the effects of Δ9‐THC on excitatory synaptic transmission in the hippocampus in vitro. Glutamatergic fEPSPs, elicited by stimulation of Schaffer collateral axons, were recorded in area CA1 in transverse mouse hippocampal slices. Bath application of Δ9‐THC caused a concentration‐dependent reduction of fEPSP rising slope (Fig. 2a), and this was completely reversed by the neutral CB1 antagonist PIMSR1 (Hurst et al. 2006; Fig. 2b; n = 7, 99 ± 4% of control, P = 0.9534, paired two‐tailed t‐test). The maximal inhibition of fEPSPs was 39 ± 6% (n = 7), with an EC50 of 707 nM (95% CI = 333–1053 nM). These effects of Δ9‐THC are comparable with those obtained at GABAergic synapses in mouse hippocampus in our lab (EC50 1.2 μM, 43 ± 2% maximal inhibition; Laaris et al. 2010).
Figure 2.
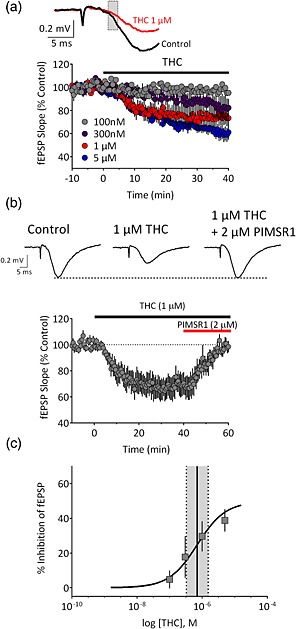
Concentration‐dependent inhibition of hippocampal glutamate release by Δ9‐tetrahydrocannabinol (THC). (a) Time course of the inhibition of the initial slope of fEPSPs (indicated by shaded box in upper traces) by Δ9‐THC across concentrations, applied at the time indicated by the horizontal bar. (b) Upper, representative averaged traces (n = 5–7 sweeps) demonstrating the effect of Δ9‐THC and reversal of its effects by the neutral CB1 antagonist PIMSR1. Lower, summary time course of recordings (n = 7 recordings) demonstrating the reversal of Δ9‐THC effects by PIMSR1. (c) Concentration–response curve for Δ9‐THC (n = 6–7 slices per concentration). The EC50 (solid line) was calculated to be 707 nM. Dashed lines indicate the 95% confidence interval. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Inhibition of glutamate release by designer cannabinoids
Among the first generation of synthetic cannabinoid compounds isolated from confiscated synthetic marijuana products was JWH‐018 (Uchiyama et al. 2010; Wiley et al. 2014b). This compound was previously shown to inhibit glutamate release at hippocampal synapses in culture (Atwood et al. 2010). Consistent with these results, JWH‐018 inhibited fEPSPs in mouse brain slices (Fig. 3a), and its EC50 (Fig. 3b; 14.0 nM; 95% CI = 6–35 nM) was remarkably similar to that observed in cultured hippocampal neurons (14.9 nM; Atwood et al. 2010). The maximal inhibition was 46 ± 7% (n = 5) at 100 nM, and this was fully reversed by PIMSR1 (Fig. 4c; n = 3, 97 ± 6% of control, P = 0.184, two‐tailed paired t‐test).
Figure 3.
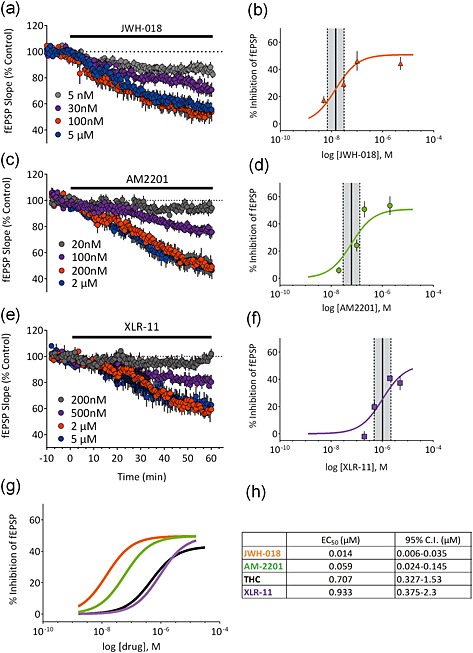
Concentration–response curves at hippocampal CA1 synapses for the Spice constituents JWH‐018, AM2201 and XLR‐11. Left panels (a,c,e) show the time course of each compound at several concentrations; right panels (b,d,f) show concentration–response curves (n = 3–8 slices per concentration). The EC50 (solid lines) and 95% confidence intervals (dashed lines) are indicated for each compound. (g) shows the curves replotted on the same graph to highlight the potency differences. Note that JWH‐018 (orange trace) is the most potent compound in the series relative to Δ9‐tetrahydrocannabinol (THC) (black trace) and that XLR‐11 is similar in potency to Δ9‐THC. The table in (h) summarizes the mean calculated EC50 values and 95% confidence intervals for each of the tested compounds. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.
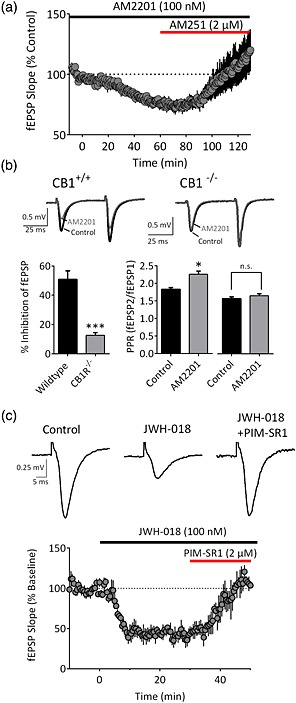
AM2201 and JWH‐018 act via CB1 receptors to inhibit glutamate release. (a) Time course of AM2201 (100 nM) and reversal by the CB1 antagonist/inverse agonist AM251 (n = 4). (b) Representative traces demonstrating the effects of AM2201 (200 nM) in slices from a wildtype (CB1+/+) and knockout (CB1−/−) mouse. The summary demonstrates the significant difference (***P < 0.001, n = 6, two‐tailed unpaired t‐test) in the effects between wildtype and CB1−/− mice. In wildtype mice, AM2201 significantly enhanced the paired‐pulse ratio (*P < 0.05, two‐tailed paired t‐test), whereas this effect was absent in CB1−/− mice (P = 0.1302, two‐tailed paired t‐test). (c) Traces and mean time course (n = 3) demonstrating fEPSP inhibition by JWH‐018 (100 nM) and its reversal by PIMSR1 (2 μM). [Colour figure can be viewed at http://wileyonlinelibrary.com]
Following the placement of JWH‐018 on the US Drug Enforcement Agency Schedule I list in March 2011 (Drug Enforcement Administration, USA, Department of Justice 2011), a second generation of synthetic cannabinoids began to appear in confiscated products. Among these, a fluoropentyl derivative of JWH‐018 known as AM2201 that binds to the CB1R with low nanomolar affinity (Deng & Makriyannis 2005) was detected in increasing amounts (Fig. 1). We observed that AM2201 also potently inhibited fEPSPs, with an EC50 slightly higher than JWH‐018 (Fig. 3c,g; 59 nM; 95% CI = 24–145 nM), and generated a maximal inhibition of 53 ± 7% at 200 nM (n = 6; Fig. 3d). Consistent with a presynaptic site of action, AM2201 significantly enhanced the paired pulse ratio in response to paired stimuli (Fig. 4b; n = 6, P = 0.01, two‐tailed paired t‐test). The effect of AM2201 was reversed by bath application of the CB1R antagonist/inverse agonist AM251 (Fig. 4a; n = 4, 118 ± 18% of control, P = 0.3825, two‐tailed paired t‐test), and it had no effect on glutamate release in slices obtained from CB1R knockout mice (Fig. 4b; n = 6, P < 0.001, t = 6.246, d.f. = 10, unpaired t‐test versus control).
By the end of the year 2012, a new designer cannabinoid‐like compound was increasingly isolated from confiscated synthetic marijuana products. This synthetic compound was structurally novel and referred to as XLR‐11 [(1‐(5‐fluoropentyl)‐1H‐indol‐3‐yl)(2,2,3,3‐tetramethylcyclopropyl)methanone] (Seely et al. 2013; U.S. Drug Enforcement Administration Office of Diversion 2013). When the effect of XLR‐11 on glutamatergic fEPSPs was examined, we found that it inhibited these responses with a lower potency than JWH‐018 or AM‐2201 (Fig. 3e,f; EC50 = 933 nM; 95% CI = 0.38–2.3 μM). Moreover, XLR‐11 exhibited a trend toward a lower degree of maximal inhibition of fEPSPs compared with these other synthetic cannabinoids (Fig. 3f,g, maximal inhibition = 41 ± 2% at 2 μM; n = 4). The inhibition of the response produced by XLR‐11 was also blocked by pre‐treatment of slices with 5 μM PIMSR1 indicating a role for CB1Rs in this response (n = 9, 95 ± 3% of control, P = 0.02, t = 2.348, d.f. = 20 versus 5 μM XLR‐11 alone).
Disruption of LTP by designer cannabinoids
The deleterious effects of cannabinoids on learning and memory are well established in humans (Abel 1970) and in animal models (Wise et al. 2009; Han et al. 2012). It is hypothesized that the ability of cannabinoid agonists to impair hippocampal long‐term potentiation may play a role in the cognitive impairments produced by these drugs (Misner & Sullivan 1999; Hoffman et al. 2007; Abush & Akirav 2010; Basavarajappa & Subbanna 2014; Navakkode & Korte 2014). We therefore evaluated the effects of the synthetic cannabinoids on hippocampal LTP and compared these effects with those of Δ9‐THC. In untreated, control slices (n = 16), delivery of HFS (3 × 100‐Hz trains) resulted in reliable, stable potentiation of fEPSP slopes (Fig. 5a and b; 143 ± 7% of baseline). In contrast, LTP was absent in slices incubated for 90 minutes in 200 nM JWH‐018 (Fig. 5a and b; n = 11, 90 ± 7% of baseline, P = 0.0003 versus control). This ability of JWH‐018 to block LTP was prevented in another group of slices when the agonist treatment was followed by application of the CB1R antagonist PIMSR1 (2 μM), beginning 30 minutes prior to HFS (Fig. 5a and b; n = 5, 127 ± 19% of baseline, P = 0.3320 versus control). As shown in Fig. 5b, slices incubated for 90 minutes with AM‐2201 (200 nM; n = 11), XLR‐11 (1 μM; n = 14) or Δ9‐THC (1 μM; n = 13) also showed significantly reduced LTP (AM2201, 105 ± 12% of baseline, P = 0.0076 versus control; XLR‐11, 103 ± 8% of baseline, P = 0.0035 versus control; Δ9‐THC, 118 ± 7% of baseline, P = 0.0416 versus control). Together, these results suggest that, in addition to their ability to acutely depress glutamatergic transmission, the synthetic cannabinoids present in ‘Spice’ preparations also limit synaptic plasticity in the hippocampus.
Figure 5.
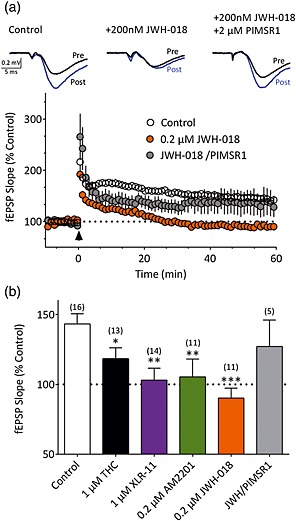
Disruption of hippocampal LTP by synthetic cannabinoids. (a) Representative traces from a control (untreated) slice, a slice pre‐treated for 90 minutes with JWH‐018 (0.2 μM) and a slice pre‐treated with JWH‐018, followed by 2 μM PIMSR1. Traces are averaged from the period immediately prior to (pre) and 60 minutes following (post) high‐frequency stimulation. Time course for all control slices (n = 16) and slices pre‐treated with JWH‐018 (n = 11) or JWH‐018 with subsequent PIMSR1 application (n = 5). LTP was elicited by high‐frequency stimulation delivered at the time indicated by the arrowhead. (b) Average LTP observed in control slices and slices pre‐treated with agonists at the indicated concentrations. The numbers of slices are indicated in parentheses. A one‐way ANOVA detected significant differences (F (5,64) = 4.51, P = 0.001) among the treatment groups. *P < 0.05, **P < 0.01, ***P < 0.001 versus control; Holm–Sidak multiple comparisons test. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Discussion
The present study compares the effects of several synthetic cannabinoids recently identified in psychoactive Spice formulations, using a functional synaptic response in a brain area relevant to the cognitive effects of marijuana. Importantly, our studies involved direct comparisons of these synthetic cannabinoids with Δ9‐THC, the primary psychoactive active cannabinoid in marijuana. All of the cannabinoid molecules dose‐dependently suppressed hippocampal synaptic glutamate release, with the following relative order of potency: JWH‐018 > AM2201 >> Δ9‐THC > XLR‐11, and the effects of all compounds were blocked by the selective CB1R antagonists AM251 or PIMSR1, consistent with the expression of CB1Rs on hippocampal glutamate axon terminals (Kawamura et al. 2006). The potency of JWH‐018 is also in agreement with a previous report obtained in cultured hippocampal neurons (Atwood et al. 2010). The high potency of JWH‐018 and AM2201 in inhibiting the synaptic response is consistent with the high affinity of these compounds at CB1Rs previously reported in receptor binding assays (Ki = 9 and 1 nM, respectively; Carroll et al. 2012), as well as with the ability of low doses of these compounds to substitute for Δ9‐THC in drug discrimination assays (Baumann et al. 2014; Järbe & Gifford 2014; Wiley et al. 2014a). XLR‐11 is structurally distinct from JWH‐018 and AM2201, having been derived from a series of cyclopropylindoles synthesized for potential use as cannabinoid 2 receptor agonists (Frost et al. 2010). This compound, which is emerging as a major component of Spice‐related substances (U.S. Drug Enforcement Administration Office of Diversion 2013), has been reported to bind to CB1Rs, with slightly greater affinity than Δ9‐THC, and to also substitute for Δ9‐THC in a mouse drug discrimination paradigm (Wiley et al. 2013). Our results suggest that XLR‐11 is nearly equivalent to Δ9‐THC at inhibiting glutamate release via CB1Rs but that both compounds are far less potent than the synthetic constituents found in earlier synthetic marijuana formulations. Based on these observations, it is tempting to speculate that the adverse physiological and psychological effects often associated with use of synthetic marijuana formulated with higher‐affinity CB1R agonists (Winstock & Barratt 2013) may be driving the demand for a synthetic drug with properties closer to those of Δ9‐THC. Correspondingly, the incidence of adverse reactions may be smaller with lower‐affinity synthetic cannabinoids.
Despite the differences in potency among the compounds tested, we did not observe statistically significant differences in the maximum inhibition (efficacy) of glutamatergic synaptic transmission. This was surprising for Δ9‐THC, because previous studies have suggested that it is a partial agonist at CB1Rs in G‐protein activation assays (Sim et al. 1996; Burkey et al. 1997) and at inhibition of synaptic responses in cultured hippocampal neurons (Shen & Thayer 1999; Straiker & Mackie 2005). Interestingly, the study by Shen and Thayer reported that Δ9‐THC suppressed excitatory transmission by ~60% (Shen & Thayer 1999), which is typically the maximum inhibition seen by cannabinoid agonists in vitro and is greater than the effect observed in the present study. We have previously demonstrated that Δ9‐THC displays full agonist properties at CB1Rs on hippocampal GABAergic terminals (Laaris et al. 2010). However, because tonic adenosine levels limit Δ9‐THC effects at hippocampal glutamatergic, but not GABAergic synapses (Hoffman et al. 2010), all of the present studies were performed in the presence of the adenosine A1 receptor antagonist DPCPX. It is therefore possible that these conditions permitted us to observe more robust and reliable inhibition of excitatory transmission by Δ9‐THC. Together, our results suggest that these compounds primarily differ from Δ9‐THC in terms of their relative potency at CB1Rs expressed on hippocampal glutamatergic terminals, rather than in their maximum efficacy.
The ability of synthetic cannabinoids to inhibit neurotransmitter release in the hippocampus likely has consequences for cognitive function. Prior studies have demonstrated that JWH‐018 (Compton et al. 2012) and the related naphthoylindole JWH‐081 (Basavarajappa & Subbanna 2014) disrupt spatial learning in rodents. In the present study, all of the drugs tested, including Δ9‐THC, suppressed glutamatergic transmission to a similar extent and significantly reduced hippocampal LTP. This is consistent with prior work demonstrating cannabinoid‐mediated inhibition of synaptic plasticity both in vivo (Abush & Akirav 2010) and in vitro (Basavarajappa & Subbanna 2014; Collins et al. 1994; Misner & Sullivan 1999; Navakkode & Korte 2014). In our studies, slices were exposed to each agonist for 90 minutes, followed by evaluation of LTP in agonist‐free aCSF. This was performed in order to standardize the exposure time to the agonists, similar to previous studies (Collins et al. 1994, 1995). The slow‐onset time of these agonists to inhibit glutamatergic transmission, coupled with the limited washout of these lipophilic compounds, suggests that they were present in the tissue during the recordings. Consistent with this, PIMSR1 prevented the effect of JWH‐018 on LTP when applied immediately following the 90‐minute pre‐treatment period and 30 minutes prior to HFS. Thus, the most parsimonious explanation of our data is that reduced LTP reflects ongoing suppression of glutamatergic transmission by actions of these compounds at CB1Rs. Indeed, this is the general mechanism through which other CB1 agonists have been demonstrated to block LTP (Collins et al. 1994, 1995; Misner & Sullivan 1999; Sullivan 2000). The ability of these and other Spice compounds to disrupt hippocampal LTP is likely to have important consequences for learning and memory (Pastalkova et al. 2006; Whitlock et al. 2006; Compton et al. 2012; Basavarajappa & Subbanna 2014). We have previously demonstrated that repeated exposure to Δ9‐THC disrupts hippocampal LTP and alters signaling at both glutamatergic and GABAergic synapses (Hoffman et al. 2007). Although we have not yet examined the effects of these compounds on GABAergic axon terminals in the hippocampus, where CB1Rs are also widely expressed (Katona et al. 1999; Hoffman & Lupica 2000; Dudok et al. 2014), the expected inhibition of GABAergic function likely also contributes to disrupted network activity in the hippocampus, leading to deficits in cognitive function (Hájos et al. 2000; Puighermanal et al. 2009). In addition, it is possible that the in vivo effects of these compounds will reflect the activity of both the parent compound and its metabolites, several of which retain strong biological activity at CB1Rs (Brents et al. 2011; Fantegrossi et al. 2014). Overall, the present findings demonstrate that synthetic cannabinoids most often found in psychoactive synthetic marijuana products have profound effects on hippocampal neurotransmission and are much more potent than Δ9‐THC at activating CB1Rs. This much higher affinity for CB1Rs by synthetic cannabinoids, as compared with Δ9‐THC, is likely to prolong the period in which this receptor is activated, thereby extending the duration of behavioral and psychological effects of these drugs. As CB1Rs are ubiquitously expressed in the human brain and extensively engaged by these agonists, the notion that synthetic cannabinoids are benign versions of naturally occurring marijuana is not supported. Given the continued widespread use of these marijuana‐like products, especially by young people, future studies are warranted to elucidate the acute and chronic effects of synthetic cannabinoids on mood, learning and memory, and other physiological processes across development.
Acknowledgements
This research was supported by the National Institute on Drug Abuse Intramural Research Program. The views expressed herein are those of the authors and do not necessarily represent the views of the Drug Enforcement Administration, the US Department of Justice or any officer or entity of the United States of America.
Disclosure/Conflict of Interest
The authors declare no conflict of interest.
Author contributions
CRL and AFH designed the experiments. MDL, JRK and CES performed the experiments and analyzed data. AFH, MHB and CRL wrote the manuscript. All authors critically reviewed the content and approved the final version of the manuscript.
Hoffman, A. F. , Lycas, M. D. , Kaczmarzyk, J. R. , Spivak, C. E. , Baumann, M. H. , and Lupica, C. R. (2017) Disruption of hippocampal synaptic transmission and long‐term potentiation by psychoactive synthetic cannabinoid ‘Spice’ compounds: comparison with Δ9‐tetrahydrocannabinol. Addiction Biology, 22: 390–399. doi: 10.1111/adb.12334.
References
- Abel EL (1970) Marijuana and memory. Nature 227:1151–1152. [DOI] [PubMed] [Google Scholar]
- Abush H, Akirav I (2010) Cannabinoids modulate hippocampal memory and plasticity. Hippocampus 20:1126–1138. [DOI] [PubMed] [Google Scholar]
- Alger BE (2002) Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol 68:247–286. [DOI] [PubMed] [Google Scholar]
- Anderson WW, Collingridge GL (2007) Capabilities of the WinLTP data acquisition program extending beyond basic LTP experimental functions. J Neurosci Methods 162:346–356. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Huffman J, Straiker A, Mackie K (2010) JWH018, a common constituent of “Spice” herbal blends, is a potent and efficacious cannabinoid CB receptor agonist. Br J Pharmacol 160:585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Lee D, Straiker A, Widlanski TS, Mackie K (2011) CP47,497‐C8 and JWH073, commonly found in “Spice” herbal blends, are potent and efficacious CB(1) cannabinoid receptor agonists. Eur J Pharmacol 659:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS, Subbanna S (2014) CB1 receptor‐mediated signaling underlies the hippocampal synaptic, learning, and memory deficits following treatment with JWH‐081, a new component of spice/K2 preparations. Hippocampus 24:178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Solis E, Watterson LR, Marusich J, Fantegrossi WE, Wiley JL (2014) Baths salts, spice, and related designer drugs: the science behind the headlines. J Neurosci 34:15150–15158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Di Marzo V (2010) Cannabinoid receptors and endocannabinoids: role in neuroinflammatory and neurodegenerative disorders. CNS Neurol Disord ‐ Dr 9:564–573. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL (2002) Dose‐related neurocognitive effects of marijuana use. Neurology 59:1337–1343. [DOI] [PubMed] [Google Scholar]
- Brents LK, Reichard EE, Zimmerman SM, Moran JH, Fantegrossi WE, Prather PL (2011) Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH‐018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS One 6:e21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkey TH, Quock RM, Consroe P, Ehlert FJ, Hosohata Y, Roeske WR, Yamamura HI (1997) Relative efficacies of cannabinoid CB1 receptor agonists in the mouse brain. Eur J Pharmacol 336:295–298. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Lewin AH, Mascarella SW, Seltzman HH, Reddy PA (2012) Designer drugs: a medicinal chemistry perspective. Ann N Y Acad Sci 1248:18–38. [DOI] [PubMed] [Google Scholar]
- Castaneto MS, Gorelick D, Desrosiers N, Hartman RL, Pirard S, Huestis M (2014) Synthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend 144C:12–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DR, Pertwee RG, Davies SN (1994) The action of synthetic cannabinoids on the induction of long‐term potentiation in the rat hippocampal slice. Eur J Pharmacol 259:R7–R8. [DOI] [PubMed] [Google Scholar]
- Collins DR, Pertwee RG, Davies SN (1995) Prevention by the cannabinoid antagonist, SR141716A, of cannabinoid‐mediated blockade of long‐term potentiation in the rat hippocampal slice. Br J Pharmacol 115:869–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton DM, Seeds M, Pottash G, Gradwohl B, Welton C, Davids R (2012) Adolescent exposure of JWH‐018 “Spice” produces subtle effects on learning and memory performance in adulthood. J Behav Brain Sci 02:146–155. [Google Scholar]
- Deng H, Makriyannis A (2005) Cannabimimetic indole derivatives. US Pat 6:900,236. [Google Scholar]
- Drug Enforcement Administration D of J (2011) Schedules of controlled substances: temporary placement of five synthetic cannabinoids into schedule I. Fed Regist 76 FR 1107:11075–11078. [PubMed] [Google Scholar]
- Dudok B et al. (2014) Cell‐specific STORM super‐resolution imaging reveals nanoscale organization of cannabinoid signaling. Nat Neurosci 18:75–86. doi: 10.1038/nn.3892. [Epub 8 Dec 2014 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Moran JH, Radominska‐Pandya A, Prather PL (2014) Distinct pharmacology and metabolism of K2 synthetic cannabinoids compared to Δ(9)‐THC: mechanism underlying greater toxicity? Life Sci 97:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, El‐Kouhen OF, Yao BB, Hsieh GC, Pai M, Zhu CZ, Chandran P, Meyer MD (2010) Indol‐3‐ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity. J Med Chem 53:295–315. [DOI] [PubMed] [Google Scholar]
- Hájos N, Katona I, Naiem SS, MacKie K, Ledent C, Mody I, Freund TF (2000) Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci 12:3239–3249. [DOI] [PubMed] [Google Scholar]
- Han J, Kesner P, Metna‐Laurent M, Duan T, Xu L, Georges F, Koehl M, Abrous DN, Mendizabal‐Zubiaga J, Grandes P, Liu Q, Bai G, Wang W, Xiong L, Ren W, Marsicano G, Zhang X (2012) Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 148:1039–1050. [DOI] [PubMed] [Google Scholar]
- Harris CR, Brown A (2013) Synthetic cannabinoid intoxication: a case series and review. J Emerg Med 44:360–366. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Laaris N, Kawamura M, Masino S, Lupica CR (2010) Control of cannabinoid CB1 receptor function on glutamate axon terminals by endogenous adenosine acting at A1 receptors. J Neurosci 30:545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR (2000) Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J Neurosci 20:2470–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR (2013) Synaptic targets of Δ9‐tetrahydrocannabinol in the central nervous system. Cold Spring Harb Perspect Med 3(8): a012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Oz M, Yang R, Lichtman AH, Lupica CR (2007) Opposing actions of chronic Delta9‐tetrahydrocannabinol and cannabinoid antagonists on hippocampal long‐term potentiation. Learn Mem 14:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holderith N, Németh B, Papp OI, Veres JM, Nagy GA, Hájos N (2011) Cannabinoids attenuate hippocampal γ oscillations by suppressing excitatory synaptic input onto CA3 pyramidal neurons and fast spiking basket cells. J Physiol 589:4921–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst D, Umejiego U, Lynch D, Seltzman H, Hyatt S, Roche M, McAllister S, Fleischer D, Kapur A, Abood M, Shi S, Jones J, Lewis D, Reggio P (2006) Biarylpyrazole inverse agonists at the cannabinoid CB1 receptor: importance of the C‐3 carboxamide oxygen/lysine3.28(192) interaction. J Med Chem 49:5969–5987. [DOI] [PubMed] [Google Scholar]
- Izzo A, Borrelli F, Capasso R, Di Marzo V, Mechoulam R (2009) Non‐psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci 30:515–527. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Gifford RS (2014) “Herbal incense”: designer drug blends as cannabimimetics and their assessment by drug discrimination and other in vivo bioassays. Life Sci 97:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlágh B, Sík A, Käfalvi A, Vizi ES, Mackie K, Freund TF (1999) Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci 19:4544–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno‐Shosaku T, Kano M (2006) The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci 26:2991–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laaris N, Good CH, Lupica CR (2010) Delta9‐tetrahydrocannabinol is a full agonist at CB1 receptors on GABA neuron axon terminals in the hippocampus. Neuropharmacology 59:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin AH, Seltzman HH, Carroll FI, Mascarella SW, Reddy PA (2014) Emergence and properties of spice and bath salts: a medicinal chemistry perspective. Life Sci 97:9–19. [DOI] [PubMed] [Google Scholar]
- Logan BK, Reinhold LE, Xu A, Diamond FX (2012) Identification of synthetic cannabinoids in herbal incense blends in the United States. J Forensic Sci 57:1168–1180. [DOI] [PubMed] [Google Scholar]
- Misner DL, Sullivan JM (1999) Mechanism of cannabinoid effects on long‐term potentiation and depression in hippocampal CA1 neurons. J Neurosci 19:6795–6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navakkode S, Korte M (2014) Pharmacological activation of CB1 receptor modulates long term potentiation by interfering with protein synthesis. Neuropharmacology 79:525–533. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC (2006) Storage of spatial information by the maintenance mechanism of LTP. Science 313:1141–1144. [DOI] [PubMed] [Google Scholar]
- Puighermanal E, Marsicano G, Busquets‐Garcia A, Lutz B, Maldonado R, Ozaita A (2009) Cannabinoid modulation of hippocampal long‐term memory is mediated by mTOR signaling. Nat Neurosci 12:1152–1158. [DOI] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ (2001) Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J Neurosci 21:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneir AB, Cullen J, Ly BT (2011) “Spice” girls: synthetic cannabinoid intoxication. J Emerg Med 40:296–299. [DOI] [PubMed] [Google Scholar]
- Seely K, Lapoint J, Moran JH, Fattore L (2012) Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry 39:234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely K, Patton AL, Moran CL, Womack ML, Prather PL, Fantegrossi WE, Radominska‐Pandya A, Endres GW, Channell KB, Smith NH, McCain KR, James LP, Moran JH (2013) Forensic investigation of K2, Spice, and “bath salt” commercial preparations: a three‐year study of new designer drug products containing synthetic cannabinoid, stimulant, and hallucinogenic compounds. Forensic Sci Int 233:416–422. [DOI] [PubMed] [Google Scholar]
- Shen M, Thayer S (1999) Delta9‐tetrahydrocannabinol acts as a partial agonist to modulate glutamatergic synaptic transmission between rat hippocampal neurons in culture. Mol Pharmacol 55:8–13. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Hampson RE, Deadwyler S, Childers SR (1996) Effects of chronic treatment with delta9‐tetrahydrocannabinol on cannabinoid‐stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci 16:8057–8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker A, Mackie K (2005) Depolarization‐induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol 569:501–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM (2000) Cellular and molecular mechanisms underlying learning and memory impairments produced by cannabinoids. Learn Mem 7:132–139. [DOI] [PubMed] [Google Scholar]
- U.S. Drug Enforcement Administration Office of Diversion (2013) National Forensic Laboratory Information System: Midyear Report 2013. Springfield, VA: U.S. Drug Enforcement Administration. [Google Scholar]
- Uchiyama N, Kikura‐Hanajiri R, Ogata J, Goda Y (2010) Chemical analysis of synthetic cannabinoids as designer drugs in herbal products. Forensic Sci Int 198:31–38. [DOI] [PubMed] [Google Scholar]
- Vardakou I, Pistos C, Spiliopoulou C (2010) Spice drugs as a new trend: mode of action, identification and legislation. Toxicol Lett 197:157–162. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF (2006) Learning induces long‐term potentiation in the hippocampus. Science 313:1093–1097. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Lefever TW, Cortes R, Marusich J (2014a) Cross‐substitution of Δ9‐tetrahydrocannabinol and JWH‐018 in drug discrimination in rats. Pharmacol Biochem Behav 124:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Marusich J, Huffman JW (2014b) Moving around the molecule: relationship between chemical structure and in vivo activity of synthetic cannabinoids. Life Sci 97:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Marusich J, Lefever TW, Grabenauer M, Moore KN, Thomas BF (2013) Cannabinoids in disguise: Δ9‐tetrahydrocannabinol‐like effects of tetramethylcyclopropyl ketone indoles. Neuropharmacology 75:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstock AR, Barratt MJ (2013) Synthetic cannabis: a comparison of patterns of use and effect profile with natural cannabis in a large global sample. Drug Alcohol Depend 131:106–111. [DOI] [PubMed] [Google Scholar]
- Wise LE, Thorpe AJ, Lichtman AH (2009) Hippocampal CB(1) receptors mediate the memory impairing effects of Delta(9)‐tetrahydrocannabinol. Neuropsychopharmacology 34:2072–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]


