Abstract
Health systems are well positioned to identify and control hypertension among their patients. However, almost one third of US adults with uncontrolled hypertension are currently receiving medical care and are unaware of being hypertensive. This study describes the development and validation of a tool that health systems can use to compare their reported hypertension prevalence with their expected prevalence. Tool users provide the number of patients aged 18 to 85 years treated annually, stratified by sex, age group, race/ethnicity, and comorbidity status. Each stratum is multiplied by stratum‐specific national prevalence estimates and the amounts are summed to calculate the number of expected hypertensive patients. The tool's validity was assessed by applying samples from cohorts with known hypertension prevalence; small differences in expected vs actual prevalence were identified (range, −3.3% to 0.6%). This tool provides clinically useful hypertension prevalence estimates that health systems can use to help inform hypertension management quality improvement efforts.
Hypertension is a significant risk factor for cardiovascular disease (CVD) and mortality.1, 2, 3, 4 Effective hypertension management, resulting in a reduction in blood pressure (BP), has been shown to greatly decrease the incidence of cardiovascular events.2, 3, 4 However, during 2003 to 2010, of the estimated 66.9 million US adults with hypertension, an estimated 35.8 million (53.5%) did not have their hypertension controlled.5 Moreover, among those with uncontrolled hypertension, 32.0 million (89.4%) reported having a usual source of health care, including 11.7 million (32.7%) who had a usual source of care but were unaware of being hypertensive.5
Health systems typically use their administrative claims data and patients' medical health records to determine their patient population's hypertension prevalence and track and report the effectiveness of their hypertension control efforts. However, these methods may not address identification of hypertensive adults who receive care within the health system but remain undiagnosed. If health systems are not identifying all of their patients in need of hypertension management, they are missing opportunities to improve their patients' health.
The purpose of this study is to describe and assess the statistical and clinical validity of a hypertension prevalence estimator tool (Estimator Tool) that was developed using data from the National Health and Nutrition Examination Survey (NHANES). This tool will allow health systems to calculate the expected hypertension prevalence among their ambulatory care patient population, based on the patients' demographic profile and prevalence of leading hypertension risk factors. Health systems could use this tool to initiate quality improvement processes that assess whether improvements in the diagnosis and management of people with hypertension are needed.
Methods
Survey and Sample Description
We developed the Estimator Tool using data from NHANES, a complex, multistage probability sample of the US civilian, noninstitutionalized population.6 To obtain statistically stable estimates, we analyzed data from seven 2‐year NHANES cycles (1999–2012) in which a total of 39,175 participants aged 18 years and older were interviewed and examined. To most closely reflect the clinical population of interest, we excluded pregnant women (ie, women at risk for gestational hypertension; n=1398) and participants with no reported visit to their primary healthcare provider within the past year (ie, people not currently in care; n=6415), for a total eligible sample size of 31,362 participants. Of these, 1136 participants were missing BP measurements or information on self‐reported current use of antihypertensive medication and 2368 participants were missing data on other covariates of interest, yielding a final analytic sample of 27,858 participants. All analyses using the NHANES data were conducted using statistical software (SUDAAN, Release 11; RTI International, Research Triangle Park, NC) to account for sampling weights and to adjust variance estimates for the multistage, clustered sample design.
BP Measurement and Hypertension Definition
A maximum of three BP readings were measured for each participant using a mercury sphygmomanometer (HgS) and auscultatory method. The mean of these recorded values was used to represent the participant's systolic BP (SBP) and diastolic BP (DBP).7 For those participants with only a single BP reading, that measurement was used. We defined participants as being hypertensive if they had an average SBP ≥140 mm Hg or an average DBP ≥90 mm Hg8 or self‐reported current use of BP‐lowering medication, defined as an answer of “yes” to both of the following questions: “Because of your high BP/hypertension, have you ever been told to take prescribed medicine?” and “Are you currently taking medication to lower your BP?”9 Participants were considered to be aware of their hypertension status if they reported receiving a hypertension diagnosis from their healthcare provider, which was defined as an answer of “yes” to the question: “Have you ever been told by a doctor or other health professional that you had hypertension, also called high blood pressure?” A similar measure of primary healthcare providers' awareness of participants' hypertension status was not available.
Logistic Regression Model Development
We developed a logistic regression model that incorporated literature‐supported hypertension risk factors and selected variables that were found to be statistically associated (P<.05) with hypertension among NHANES participants. We included the following variables in the final model: age,8, 10, 11 sex,11 race/ethnicity,1, 5, 11 income and education status,12 obesity status based on body mass index,8, 10, 13, 14 total cholesterol level status,15 prior history of CVD,8, 16 diagnosed diabetes status,8, 10 and chronic kidney disease (CKD) status8, 10 (Table 1). We used the final model to estimate the predicted marginal prevalence for each subgroup.
Table 1.
Logistic Regression Model With Significant Predictors of Hypertension Prevalence, NHANES 1999–2012 (N=27,858)
| Beta Coefficient | SE | P Value | Predicted Hypertension Prevalence | Proportion of the Population | ||
|---|---|---|---|---|---|---|
| % | SE | |||||
| Intercept | −2.0671 | 0.0622 | <.001 | |||
| Sex | ||||||
| Male | referent | 33.6 | 0.7 | 45.5 | ||
| Female | −0.6636 | 0.0761 | <.001 | 32.0 | 0.6 | 54.5 |
| Age group, y | ||||||
| 18–44 | referent | 15.0 | 0.5 | 46.1 | ||
| 45–64 | 1.1457 | 0.0736 | <.001 | 37.9 | 0.8 | 35.3 |
| 65–74 | 1.7613 | 0.0951 | <.001 | 54.8 | 1.1 | 10.7 |
| 75+ | 1.9776 | 0.0888 | <.001 | 62.1 | 1.2 | 8.0 |
| Race/ethnicity | ||||||
| Non‐Hispanic white | referent | 32.0 | 0.6 | 73.0 | ||
| Non‐Hispanic black | 0.6824 | 0.0513 | <.001 | 42.6 | 0.8 | 10.6 |
| Hispanic | −0.2553 | 0.0607 | <.001 | 28.3 | 0.8 | 11.2 |
| Other | 0.1056 | 0.0881 | .233 | 33.5 | 1.4 | 5.2 |
| Education | ||||||
| <College graduate | referent | 34.8 | 0.5 | 62.9 | ||
| College graduate | −0.2405 | 0.0483 | <.001 | 31.0 | 0.8 | 25.5 |
| <25 years of age | −1.5740 | 0.1554 | <.001 | 14.5 | 1.5 | 11.6 |
| Household income | ||||||
| <$75k | referent | 34.0 | 0.6 | 61.8 | ||
| ≥$75k | −0.1251 | 0.0659 | .060 | 32.1 | 1.0 | 15.8 |
| Missing income | −0.2679 | 0.0493 | <.001 | 30.0 | 0.7 | 22.4 |
| Obesitya | ||||||
| Yes | 0.8166 | 0.0438 | <.001 | 40.9 | 0.7 | 33.6 |
| No | referent | 28.2 | 0.6 | 66.4 | ||
| High cholesterolb | ||||||
| Yes | 0.5109 | 0.0419 | <.001 | 37.6 | 0.8 | 32.0 |
| No | referent | 29.7 | 0.5 | 68.0 | ||
| History of cardiovascular diseasec | ||||||
| Yes | 0.4500 | 0.0778 | <.001 | 39.1 | 1.2 | 7.8 |
| No | referent | 32.2 | 0.6 | 92.3 | ||
| Diabetesd | ||||||
| Yes | 0.5824 | 0.0639 | <.001 | 41.0 | 1.0 | 9.2 |
| No | referent | 31.8 | 0.5 | 90.8 | ||
| Chronic kidney diseasee | ||||||
| Yes | 0.7462 | 0.0517 | <.001 | 42.8 | 1.0 | 15.4 |
| No | referent | 30.8 | 0.5 | 84.6 | ||
| Sex and age group interaction, y | ||||||
| Male, 18–44 | referent | 19.1 | 0.7 | 21.1 | ||
| Male, 45–64 | referent | 38.5 | 1.1 | 16.2 | ||
| Male, 65–74 | referent | 51.2 | 1.7 | 4.9 | ||
| Male, 75+ | referent | 55.7 | 1.5 | 3.3 | ||
| Female, 18–44 | referent | 11.6 | 0.6 | 25.0 | ||
| Female, 45–64 | 0.6079 | 0.1038 | <.001 | 37.4 | 1.0 | 19.0 |
| Female, 65–74 | 0.9821 | 0.1279 | <.001 | 57.8 | 1.4 | 5.8 |
| Female, 75+ | 1.2508 | 0.1249 | <.001 | 67.4 | 1.5 | 4.7 |
Abbreviations: NHANES, National Health and Nutrition Examination Survey; SE, standard error. aBody mass index ≥30.0 kg/m2. bReported being told by a doctor that they had high cholesterol or classified as such according to the Third Report of the National Cholesterol Education Program Adult Treatment Panel III with a total cholesterol ≥240 mg/dL. cReported having a history of coronary heart disease, myocardial infarction, stroke, or angina. dReported being told by a doctor that they had diabetes. eHad an estimated glomerular filtration rate <60 mL/min per 1.73 m2 or albumin‐to‐creatinine ratio ≥30 mg/g.
Estimator Tool Development
We limited the number of variables included in the Estimator Tool to six to minimize the number of data elements users would have to enter into the tool. The following variables were selected because they had the strongest association with hypertension prevalence within the regression model (ie, largest beta coefficients) and the greatest likelihood of consistent collection within health systems: sex, age group (18–44, 45–64, 65–74, and ≥75 years), race/ethnicity (non‐Hispanic white, non‐Hispanic black, Hispanic, or other), and diabetes, CKD, and obesity status. Of note, prior to 2007, NHANES only reported data specific to Mexican Americans; other Hispanics were included in the “Other” race/ethnicity category and cannot be differentiated. Therefore, we considered Mexican Americans to represent all Hispanics during 1999 to 2006 within the tool. We also included sex because we found a significant statistical interaction with age in the hypertension prevalence model (P<.001). We performed trend analyses to ensure that the relationship between the hypertension and risk factor prevalence did not change during 1999 to 2012 (P≥.05).
To mimic conditions where patients may have undiagnosed diabetes, we defined NHANES participants as being “diagnosed diabetic” if they reported being told by a doctor that they had diabetes, defined as an answer of “yes” to the question “Other than during pregnancy, have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?”9 Had we used a definition based on participants' clinical measures (eg, hemoglobin A1c values), the diabetes prevalence potentially could have been around 12% greater.17 We defined CKD as participants having: (1) an estimated glomerular filtration rate <60 mL/min per 1.73 m2 using the Chronic Kidney Disease Epidemiology Collaboration equation18 or (2) albuminuria (albumin‐to‐creatinine ratio ≥30 mg/g). A similar measure of “diagnosed CKD” status is not available via NHANES to mimic the structure of the diagnosed diabetes variable. We defined obesity as a body mass index ≥30 kg/m2.7 To decrease the burden on the Estimator Tool user, we grouped the comorbidities together into one variable with the following categories: none, one, or two or three. Finally, we calculated weighted hypertension prevalence estimates and 95% confidence intervals (CIs) stratified by sex, age group, race/ethnicity, and comorbidity status.
Use of the Estimator Tool
Users of the tool are instructed to provide the number of ambulatory care patients that fall within each of the sex, age group, race/ethnicity, and comorbidity status strata, which are then multiplied by the expected prevalence for each stratum to arrive at the number of patients expected to have hypertension. We describe the suggested definitions health systems can use for each Estimator Tool element and methods for collecting BP measurements in e‐Figure 1. We added a category of “Missing” to the race/ethnicity stratum if the user has missing race/ethnicity data; the mean hypertension prevalence estimate for all race/ethnicities combined, by comorbidity status, is applied to this category and assumes that the health system has a racial/ethnic distribution similar to the nation. In addition, we included an age cap of 85 years to align with other health system reporting requirements. NHANES, however, codes all participants aged 80 years and older as being aged 80 years for confidentially reasons; therefore, some of the participants used to compute the hypertension prevalence estimates for those aged 75 to 85 years are older than 85 years.
Figure 1.
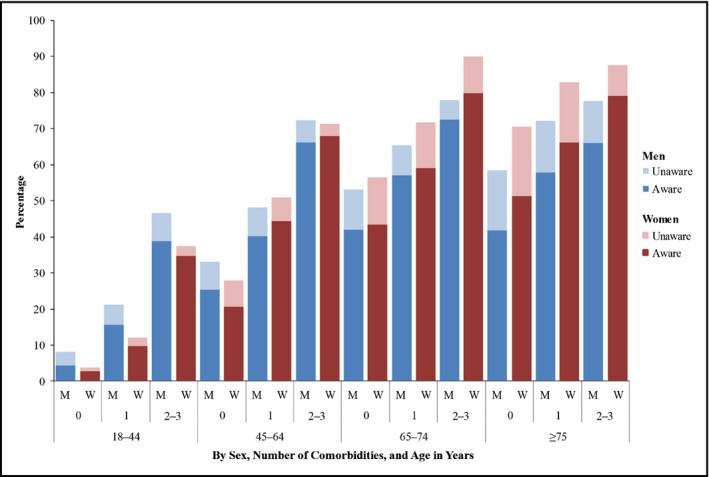
National hypertension prevalence estimates and awareness status among adults with a primary healthcare visit within the past year, by sex, age, and comorbidity status (National Health and Nutrition Examination Survey [NHANES], 1999–2012). aRepresented as the number of comorbidities, including obesity, diagnosed diabetes, and chronic kidney disease.
The tool sums the estimated number of hypertensive patients within each stratum and divides the sum by the size of the patient population to determine the health system's expected hypertension prevalence. The 95% CIs for the expected prevalence are calculated using the standard variance formula applied to this binomial mixture model.19
Estimator Tool Performance Testing
We used two criteria to test the performance of the tool's ability to estimate the expected prevalence compared with the observed prevalence. First, we used chi‐square tests to assess significant independence between the predicted and observed prevalence (P<.05). In addition, we describe the absolute difference between the predicted and observed values to better understand the clinical utility of the tool. We evaluated the tool's internal validity by applying the unweighted values from three samples each of 1500, 3000, and 6000 NHANES participants, randomly selected without replacement. These sample sizes were chosen based on a typical physician's panel size being around 1500 patients.20 To assess the tool's external validity, we pooled participants from four studies with population‐based samples: the Jackson Heart Study21 (JHS); the Arkansas Cardiovascular Health Examination Survey22 (ARCHES); the National Heart, Lung, and Blood Institute Hispanic Community Health Study/Study of Latinos23 (HCHS); and the Multi‐Ethnic Study of Atherosclerosis24 (MESA). Characterizations of participants were based on their baseline data. MESA participants were free of clinical CVD at baseline. The other three studies had no CVD‐related exclusion criteria. We chose these studies, because, unlike data from health systems, they include data on both self‐reported (ie, diagnosed) and measured hypertension status. Health systems may be missing accurate data on the measured hypertension status of their patient population. We combined the data from these studies to ascertain the tool's performance among diverse samples that include multiple race‐ethnic minority groups and likely reflect the patient populations seen within health systems. We then applied the unweighted values from three samples each of 1500, 3000, and 6000 randomly selected participants from these pooled studies. Table 2 describes the characteristics of the four pooled external cohorts compared with the weighted NHANES population.
Table 2.
Characterization of the NHANES Cohort and Cohorts Used for External Validation
| ARCHES | JHS | HCHS | MESA | Total Pooled Cohort | NHANES (1999–2012)b | ||
|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | ||||||
| Original cohort size, No. | 1383 | 5301 | 16,415 | 6814 | 29,913 | – | 68,705 |
| Effect of stepwise application of inclusion/exclusion criteria | |||||||
| Aged 18–85 y | 1369 | 5292 | 14,155 | 6814 | 27,630 | – | 39,175 |
| Not pregnant | 1369 | 5292 | 14,155 | 6814 | 27,630 | – | 37,777 |
| Receiving primary health care | 1003c | 3813d | 9960e | 6278f | 21,054 | – | 31,362g |
| Not missing race or sex | 997h | 3813i | 9960 | 6278 | 21,048 | – | 31,362 |
| Not missing hypertension status | 994 | 3758 | 9870 | 6274 | 20,896 | – | 30,226 |
| Not missing comorbidity data (final cohort)j | 906 | 3716 | 9782 | 6256 | 20,660 | – | 27,858 |
| Mean age (SD), y | 57.6 (14.4) | 56.9 (12.5) | 47.3 (14.1) | 62.2 (10.3) | 54.0 (14.4) | – | 47.1 (33.2) |
| Age category, %, y | |||||||
| 18–44 | 17.3 | 19.8 | 36.4 | 0.1 | 21.6 | – | 46.1 |
| 45–64 | 48.1 | 52.0 | 53.1 | 55.5 | 53.4 | – | 35.3 |
| 65–74 | 22.7 | 21.5 | 10.4 | 30.1 | 18.9 | – | 10.7 |
| 75–85k | 11.8 | 6.7 | 0.1 | 14.3 | 6.1 | – | 8.0 |
| Male, % | 31.9 | 31.6 | 36.0 | 46.3 | 38.2 | – | 45.5 |
| Race/ethnicity, % | |||||||
| Non‐Hispanic white | 74.0 | 0.0 | 0.0 | 40.2 | 15.4 | – | 73.0 |
| Non‐Hispanic black | 26.0 | 100.0 | 0.0 | 27.6 | 27.5 | – | 10.6 |
| Hispanic | NAh | NAi | 100.0 | 20.3 | 53.5 | – | 11.2 |
| Other | 0.0 | 0.0 | 0.0 | 12.0 | 3.6 | – | 5.2 |
| Hypertension prevalence, % | 62.4 | 66.4 | 35.2 | 49.1 | 46.2 | 39.1 | 32.9 (31.9–34.0) |
| Obesity prevalence, % | 45.9 | 54.2 | 44.0 | 32.1 | 42.3 | 39.4 | 33.6 (32.5–34.6) |
| Diabetes prevalence, % | 17.1 | 19.6 | 16.5 | 11.4 | 15.5 | 9.4 | 9.2 (8.7–9.7) |
| CKD prevalence, %j | 21.4 | 15.6 | 13.9 | 20.1 | 16.4 | 13.2 | 15.4 (14.8–16.0) |
| Comorbidities, % | |||||||
| None | 39.5 | 34.8 | 45.0 | 51.8 | 45.0 | 50.7 | 55.0 (53.9–56.1) |
| One | 40.7 | 44.8 | 38.8 | 35.1 | 38.9 | 38.0 | 34.2 (33.3–35.1) |
| Two or three | 19.8 | 20.3 | 16.2 | 13.1 | 16.2 | 11.3 | 10.9 (10.3–11.4) |
| Mean BP (SD), mm Hgl | |||||||
| Systolic BP | 131.5 (19.6) | 127.4 (18.3) | 122.0 (18.3) | 126.6 (21.4) | 126.9 (19.2) | 126.9 (17.7) | 122.4 (22.6) |
| Diastolic BP | 78.0 (11.4) | 78.5 (10.6) | 72.9 (10.9) | 71.9 (10.2) | 74.8 (10.8) | 75.8 (10.5) | 71.2 (15.7) |
Abbreviations: ARCHES, Arkansas Cardiovascular Health Examination Survey; BP, blood pressure; CKD, chronic kidney disease; HCHS, National Heart, Lung, and Blood Institute Hispanic Community Health Study/Study of Latinos; JHS, Jackson Heart Study; MESA, Multi‐Ethnic Study of Atherosclerosis; NA, not available; NHANES, National Health and Nutrition Examination Survey; SD, standard deviation. aAdjusted by age, sex, and race/ethnicity to reflect the distribution of the weighted 1999–2012 NHANES population. bWeighted values are shown for the unadjusted NHANES estimates. Confidence intervals (95%) are provided for the comorbidity prevalence estimates. cReported having a medical home. dReported having a doctor visit in the last year. eReported having one or more doctor visits in the past 12 months. fReported having a doctor's office as their main medical home. gReported visit to their primary healthcare provider within the past year. hEthnicity data were not provided. All participants were considered to be non‐Hispanic for these analyses as, per the study leaders, approximately 98% of the cohort was non‐Hispanic. iEthnicity data were not provided. jTo accommodate missing CKD‐related data within the external datasets, participants had to have values for either estimated glomerular filtration rate or albumin to creatinine ratio to be included in the CKD prevalence estimates and the pooled cohort, not necessarily both. NHANES participants had to have both values available. kAge is top coded at 80 years for NHANES participants, therefore, this age group represents those aged 75 years and older. lAmong those with recorded BP measures.
Similar to NHANES, the four external surveys used protocol‐driven methods to collect BP during one clinic visit and measured participants arm circumference to ensure a properly sized BP cuff was used. We used each studies' final reported SBP and DBP values to determine participants' hypertension status; how the final BP values attributed to each participant were determined differed across the studies. The JHS used the average of two BP readings obtained using a random zero HgS and auscultatory technique. The other three external studies used an Association for the Advancement of Medical Instrumentation‐validated automated oscillometric device (AOD) to measure BP. ARCHES and HCHS used an average of three BP measures obtained using an OMRON HEM‐907 XL device (Omron Healthcare, Inc, Lake Forest, IL). MESA used the average of the second and third BP measures obtained using a Dinamap PRO 100 device (Critikon, Tampa, FL). Additional modifications were made to some of the definitions used to correspond with how the external datasets collected their data. These modifications are described in Table 2.
Results
The hypertension prevalence among NHANES participants who were in care was 32.9% (95% CI, 31.9–34.0) and among the pooled external cohort was 46.2% (unadjusted) and 39.1% (adjusted by age, sex, and race/ethnicity to the 1999–2012 weighted NHANES population) (Table 2). Among the NHANES participants, hypertension prevalence estimates were affected by each stratum of the tool (Table 3). As expected, increasing age had the strongest association with increased hypertension prevalence. Non‐Hispanic blacks tended to have the highest hypertension prevalence in the younger age groups and, while less pronounced, they also had higher rates in the older age groups. Men tended to have higher prevalence at younger ages and women at older ages.
Table 3.
Hypertension Prevalence by Sex, Age, Race/Ethnicity, and Comorbidity Status Category, Among Adults Aged 18 Years and Older With a Primary Healthcare Visit Within the Past Year—NHANES, 1999–2012
| Age, y | Race/Ethnicity | Number of Comorbidities | Hypertension Prevalence (95% CI) | |
|---|---|---|---|---|
| Men | Women | |||
| 18–44 | Non‐Hispanic white | 0 | 8.4 (7.1–9.9) | 3.5 (2.6–4.8) |
| 1 | 21.1 (17.8–24.7) | 11.6 (9.3–14.4) | ||
| 2–3 | 38.2 (26.2–51.7) | 37.3 (28.1–47.5) | ||
| Non‐Hispanic black | 0 | 10.0 (8.0–12.4) | 9.3 (7.3–11.9) | |
| 1 | 27.8 (23.1–33.1) | 17.3 (14.7–20.3) | ||
| 2–3 | 64.8 (51.5–76.1) | 52.2 (41.6–62.6) | ||
| Hispanica | 0 | 5.2 (3.6–7.4) | 1.7 (0.9–3.3) | |
| 1 | 13.9 (10.2–18.7) | 7.9 (5.3–11.8) | ||
| 2–3 | 54.6 (42.6–66.1) | 24.7 (15.8–36.4) | ||
| Other | 0 | 8.9 (5.2–14.7) | 3.4 (1.8–6.3) | |
| 1 | 25.9 (17.2–37.2) | 12.4 (6.2–23.3) | ||
| 2–3 | 43.1 (13.7–78.4) | 25.1 (8.5–54.6) | ||
| Missing/Totalb | 0 | 8.2 (7.2–9.2) | 3.8 (3.1–4.7) | |
| 1 | 21.1 (18.6–23.9) | 12.1 (10.5–14.0) | ||
| 2–3 | 46.5 (38.3–55.0) | 37.5 (31.4–43.9) | ||
| 45–64 | Non‐Hispanic white | 0 | 32.3 (28.6–36.3) | 26.8 (24.0–29.7) |
| 1 | 46.9 (42.7–51.1) | 49.2 (44.8–53.6) | ||
| 2–3 | 70.9 (65.4–75.8) | 67.3 (59.5–74.2) | ||
| Non‐Hispanic black | 0 | 46.1 (41.4–50.8) | 45.9 (40.3–51.7) | |
| 1 | 60.0 (53.6–66.1) | 66.4 (61.7–70.9) | ||
| 2–3 | 87.0 (81.1–91.3) | 86.1 (81.9–89.4) | ||
| Hispanica | 0 | 26.3 (20.8–32.7) | 23.9 (18.6–30.3) | |
| 1 | 44.0 (35.5–52.8) | 38.3 (32.7–44.2) | ||
| 2–3 | 64.5 (53.3–74.2) | 67.6 (60.0–74.4) | ||
| Other | 0 | 31.3 (21.4–43.2) | 28.0 (20.0–37.7) | |
| 1 | 50.1 (35.8–64.4) | 58.7 (45.9–70.5) | ||
| 2–3 | 68.8 (39.2–88.3) | 65.9 (45.2–81.9) | ||
| Missing/Totalb | 0 | 32.9 (29.8–36.3) | 27.9 (25.6–30.3) | |
| 1 | 48.1 (44.6–51.7) | 50.9 (47.5–54.4) | ||
| 2–3 | 72.4 (68.4–76.0) | 71.3 (66.5–75.7) | ||
| 65–74 | Non‐Hispanic white | 0 | 51.9 (46.4–57.4) | 55.3 (50.4–60.2) |
| 1 | 64.2 (58.6–69.4) | 68.8 (63.4–73.7) | ||
| 2–3 | 77.0 (69.5–83.1) | 90.0 (84.8–93.5) | ||
| Non‐Hispanic black | 0 | 71.5 (62.9–78.8) | 69.7 (57.9–79.3) | |
| 1 | 80.9 (72.9–87.0) | 88.0 (82.2–92.0) | ||
| 2–3 | 86.1 (79.6–90.7) | 92.1 (86.9–95.4) | ||
| Hispanica | 0 | 41.1 (29.8–53.3) | 65.3 (53.1–75.8) | |
| 1 | 63.8 (50.4–75.3) | 75.8 (64.2–84.5) | ||
| 2–3 | 74.5 (59.9–85.2) | 84.5 (73.9–91.3) | ||
| Other | 0 | 68.5 (47.4–84.0) | 48.5 (30.7–66.6) | |
| 1 | 62.1 (37.9–81.5) | 92.0 (76.9–97.5) | ||
| 2–3 | 80.2 (49.7–94.3) | 93.5 (64.0–99.1) | ||
| Missing/Totalb | 0 | 53.1 (48.4–57.8) | 56.3 (52.0–60.6) | |
| 1 | 65.4 (60.7–69.9) | 71.8 (67.3–75.9) | ||
| 2–3 | 77.9 (71.9–82.8) | 90.0 (86.2–92.8) | ||
| ≥75c | Non‐Hispanic white | 0 | 58.2 (53.3–63.0) | 69.3 (64.2–74.0) |
| 1 | 71.2 (67.1–75.0) | 82.5 (79.2–85.3) | ||
| 2–3 | 77.5 (71.9–82.2) | 87.5 (83.6–90.6) | ||
| Non‐Hispanic black | 0 | 68.8 (54.1–80.4) | 85.6 (70.5–93.7) | |
| 1 | 89.8 (79.3–95.3) | 84.1 (73.8–90.9) | ||
| 2–3 | 81.3 (69.3–89.3) | 93.8 (87.3–97.1) | ||
| Hispanica | 0 | 61.2 (48.1–72.9) | 74.3 (55.4–87.0) | |
| 1 | 73.0 (61.3–82.1) | 88.5 (80.6–93.4) | ||
| 2–3 | 89.0 (76.5–95.3) | 75.3 (61.5–85.4) | ||
| Other | 0 | 34.6 (11.8–67.7) | 80.6 (49.7–94.6) | |
| 1 | 68.4 (47.0–84.0) | 83.1 (62.6–93.5) | ||
| 2–3 | 58.8 (29.8–82.8) | 85.2 (62.0–95.3) | ||
| Missing/Totalb | 0 | 58.5 (54.0–62.8) | 70.6 (65.8–74.9) | |
| 1 | 72.2 (68.4–75.6) | 82.8 (79.9–85.4) | ||
| 2–3 | 77.6 (72.7–81.9) | 87.7 (84.3–90.3) | ||
Abbreviations: CI, confidence interval; NHANES, National Health and Nutrition Examination Survey. aPrior to 2007, NHANES only reported data specific to Mexican Americans; other Hispanics were included in the “Other” race/ethnicity category and are not able to be differentiated. For these purposes, Mexican Americans were considered to represent all Hispanics during 1999–2006. bAdded to accommodate those health systems that are unable to report their data by race/ethnicity or who may have some patients who lack a race/ethnicity classification. The hypertension prevalence estimate for all race‐ethnicities, by comorbidity status, is applied to this category. cAn age cap of 85 years is used in the Estimator Tool to better align with the clinical quality measures health systems are already reporting. However, NHANES codes all participants aged 80 years and older as being aged 80 years for confidentially reasons; therefore, some of the participants used to compute the hypertension prevalence estimates for those aged 75 to 85 years are older than age 85.
An estimated 18.6% of NHANES participants with hypertension who were in care were unaware of being hypertensive. Awareness varied by stratum (Figure). Comorbidity status affected hypertension prevalence among both men and women in every age group, with a stepwise increase in prevalence with each increase in the number of comorbidities. The largest relative impact of comorbidities on hypertension prevalence was observed among the youngest age groups. For example, compared with men and women aged 18 to 44 years with no comorbidities (hypertension prevalence: 8.2% [95% CI, 7.2–9.2] and 3.8% [95% CI, 3.1–4.7], respectively), similar aged men and women with one comorbidity had hypertension prevalence rates 2.6 (21.1% [95% CI, 18.6–23.9]) and 3.2 (12.1% [95% CI, 10.5–14.0]) times higher, respectively, and those with two or three comorbidities had rates 5.7 (46.5% [95% CI, 38.3–55.0]) and 9.9 (37.5% [95% CI, 31.4–43.9]) times higher. Moreover, those with two or three comorbidities typically had higher hypertension prevalence rates than those with no comorbidities in the next older age group. Finally, hypertension prevalence among younger non‐Hispanic blacks appeared to be more strongly affected by comorbidity status than the other racial/ethnic groups. The relationship between hypertension prevalence and each element included in the tool did not change from 1999–2000 to 2011–2012 (Table 4).
Table 4.
Trends in Hypertension Prevalence by Risk Factor Among Adults Aged 18 Years and Older With a Primary Healthcare Visit Within the Past Year, NHANES, 1999–2012
| Prevalence During Two‐Year NHANES Cyclea | Trend | |||||||
|---|---|---|---|---|---|---|---|---|
| 99–00 | 01–02 | 03–04 | 05–06 | 07–08 | 09–10 | 11–12 | P‐Value | |
| Total | 32.2 | 30.6 | 34.8 | 33.3 | 33.6 | 33.4 | 32.6 | 0.39 |
| Men | 32.0 | 28.6 | 36.3 | 33.8 | 34.9 | 34.2 | 33.3 | 0.15 |
| Women | 32.3 | 32.4 | 33.5 | 32.8 | 32.5 | 32.7 | 32.0 | 0.83 |
| Age, y | ||||||||
| 18–44 | 10.7 | 8.4 | 11.1 | 11.1 | 11.8 | 9.7 | 10.7 | 0.59 |
| 45–64 | 39.2 | 38.2 | 46.0 | 42.5 | 42.6 | 43.9 | 42.5 | 0.16 |
| 65–74 | 66.8 | 67.5 | 69.0 | 68.6 | 66.4 | 67.8 | 61.6 | 0.20 |
| ≥75 | 78.8 | 76.1 | 76.4 | 73.1 | 75.6 | 77.8 | 76.3 | 0.73 |
| Race/ethnicity | ||||||||
| Non‐Hispanic white | 30.3 | 28.9 | 33.8 | 31.6 | 33.1 | 31.8 | 31.0 | 0.38 |
| Non‐Hispanic black | 44.6 | 47.5 | 45.4 | 46.8 | 44.5 | 46.1 | 45.9 | 0.99 |
| Hispanicb | 34.4 | 24.7 | 32.4 | 26.5 | 30.9 | 31.3 | 32.1 | 0.64 |
| Otherc | 31.7 | 36.2 | 31.9 | 36.2 | 25.2 | 37.5 | 31.0 | 0.85 |
| Comorbidities | ||||||||
| Obesity | ||||||||
| Yes | 44.8 | 40.5 | 46.3 | 43.7 | 43.5 | 45.0 | 43.0 | 0.89 |
| No | 26.3 | 25.8 | 29.0 | 27.5 | 28.2 | 26.1 | 26.7 | 0.88 |
| Diabetes | ||||||||
| Yes | 53.2 | 49.1 | 52.7 | 60.6 | 55.0 | 60.6 | 58.8 | 0.10 |
| No | 31.0 | 29.2 | 33.0 | 31.2 | 31.4 | 30.9 | 29.8 | 0.81 |
| CKD | ||||||||
| Yes | 46.1 | 47.8 | 49.7 | 51.3 | 47.2 | 48.3 | 46.4 | 0.68 |
| No | 29.3 | 28.0 | 32.0 | 30.0 | 30.7 | 31.0 | 29.6 | 0.46 |
| Number of comorbiditiesd | ||||||||
| None | 23.9 | 23.2 | 26.4 | 24.2 | 24.8 | 23.4 | 23.8 | 0.72 |
| 1 | 38.9 | 36.6 | 40.7 | 37.6 | 39.7 | 39.2 | 36.8 | 0.88 |
| 2–3 | 58.8 | 55.7 | 58.6 | 62.1 | 53.6 | 63.4 | 60.4 | 0.25 |
Abbreviations: CKD, chronic kidney disease; NHANES, National Health and Nutrition Examination Survey. aUnadjusted prevalence by NHANES cycle. bRepresents just Mexican Americans for 1999–2006 and all Hispanics from 2007–2012. cIncludes non–Mexican American Hispanics from 1999–2006. dComorbidities include obesity, diagnosed diabetes, and CKD.
Table 5 summarizes the outcomes of the validation testing. The internal validity of the Estimator Tool was strong as there were no statistically significant differences between the expected and observed hypertension prevalence among the randomly selected and unweighted NHANES participants. Clinically, the differences ranged from underestimating the prevalence by −1.9% to overestimating the prevalence by 0.6%, with the differences decreasing as the random sample sizes increased. As for the external statistical validity assessment of the tool, the differences between the expected and observed prevalence ranged from an underestimate of −2.2% to −3.3% for the random samples with 1500 participants and −1.8% to −2.2% for the samples with 6000 participants. While these represent small clinical differences, the expected vs observed estimates were all significantly different, denoting a lack of agreement.
Table 5.
Internal and External Validity Testing Results for the Hypertension Prevalence Estimator Tool, by Random Sample Size
| Hypertension Prevalence, % | Percent Difference, Estimated–Observed | P‐Valuea | ||
|---|---|---|---|---|
| Estimated (95% CI) | Observed | |||
| Internal Validity | ||||
| NHANES random samples (n=1500) | ||||
| Sample 1 | 38.7 (36.3–41.2) | 38.1 | 0.6 | 0.59 |
| Sample 2 | 38.2 (35.8–40.7) | 39.1 | −0.9 | 0.50 |
| Sample 3 | 38.1 (35.6–40.5) | 40.0 | −1.9 | 0.12 |
| NHANES random samples (n=3000) | ||||
| Sample 1 | 37.7 (35.9–39.4) | 37.5 | 0.2 | 0.86 |
| Sample 2 | 38.4 (36.6–40.1) | 39.2 | −0.8 | 0.32 |
| Sample 3 | 38.3 (36.6–40.0) | 38.6 | −0.3 | 0.73 |
| NHANES random samples (n=6000) | ||||
| Sample 1 | 38.5 (37.3–39.7) | 38.5 | 0.0 | 0.96 |
| Sample 2 | 38.8 (37.5–40.0) | 38.4 | 0.4 | 0.56 |
| Sample 3 | 38.5 (37.3–39.8) | 39.1 | −0.6 | 0.36 |
| External Validity | ||||
| Pooled random samples (n=1500) | ||||
| Sample 1 | 44.1 (42.7–45.5) | 46.3 | −2.2 | 0.080 |
| Sample 2 | 44.7 (43.3–46.1) | 48.0 | −3.3 | 0.010 |
| Sample 3 | 45.2 (43.8–46.6) | 47.7 | −2.5 | 0.054 |
| Pooled random samples (n=3000) | ||||
| Sample 1 | 44.0 (43.0–45.0) | 46.4 | −2.4 | 0.008 |
| Sample 2 | 43.2 (42.2–44.2) | 45.5 | −2.3 | 0.012 |
| Sample 3 | 44.3 (43.3–45.2) | 46.3 | −2.0 | 0.024 |
| Pooled random samples (n=6000) | ||||
| Sample 1 | 44.3 (43.6–45.0) | 46.4 | −2.1 | 0.001 |
| Sample 2 | 43.9 (43.2–44.6) | 45.7 | −1.8 | 0.005 |
| Sample 3 | 44.0 (43.3–44.7) | 46.2 | −2.2 | <0.001 |
Abbreviations: CI, confidence interval; NHANES, National Health and Nutrition Examination Survey. aChi‐square test to assess the association between the expected prevalence estimates and the observed prevalence values.
Discussion
This study found that approximately one third of the US adult population receiving healthcare services has hypertension, of which almost one in five are unaware of their hypertensive status. Moreover, we found that the Estimator Tool is a potentially useful instrument that provides hypertension prevalence estimates that differ minimally from that observed among cohorts with known hypertension prevalence. Health systems can use the tool to initiate hypertension management quality improvement efforts and help improve the identification of undiagnosed hypertension among their patient population. Health systems are uniquely positioned to identify, treat, and control hypertension among patients who are currently within their care. These systems, no matter their size, should understand the expected scope of the hypertension burden among their patient population to ascertain whether they have adequately identified all of their patients with hypertension. Without first detecting all of the patients in need of hypertension management, the implementation of evidence‐based interventions to control hypertension using strategies tailored to the health system's patient population, including standardized hypertension treatment protocols,25 team‐based care initiatives that support clinical‐community linkages,26 or self‐measured BP monitoring with clinical support,27 may not be as effective in improving CVD outcomes.
While the validation testing of the tool with the pooled external cohort data resulted in statistically significant differences in the expected vs observed hypertension prevalence values, we do not believe these differences were large enough to negate the clinical usefulness of the tool. This was especially the case for the larger samples of 6000 participants, which equates to a practice with about four full‐time physicians, where the tool only underestimated hypertension prevalence by around 2%. In general, the Estimator Tool tended to underestimate the hypertension prevalence during both the internal and external validation testing. Therefore, it likely provides a conservative estimate, especially among populations with a hypertension prevalence greater than the US prevalence.
In 2012, the U.S. Department of Health and Human Services began Million Hearts (http://millionhearts.hhs.gov), an initiative aimed at preventing 1 million heart attacks, strokes, and other related CVD events by 2017.28 The effective identification, treatment, and control of hypertension is a vital component in reaching the Million Hearts goal. To this end, Million Hearts has worked collaboratively with healthcare partners to identify strategies to detect those patients who have hypertension but are considered “hiding in plain sight”—those who are receiving care within the health system, but whose hypertension has gone undiagnosed and, therefore, unmanaged. Use of the Estimator Tool could be one of the first steps a health system takes in this process. If their expected hypertension prevalence is higher than their reported prevalence, one potential next step may be for the health system to use its medical record system to identify and follow up with patients with previous documentation of elevated BP who have not received a hypertension diagnosis and may need additional screening. Additional quality improvement steps that health systems can take across the spectrum of hypertension management are outlined in the Million Hearts Hypertension Control Change Package.29 Future evaluation of how the Estimator Tool was incorporated into and informed hypertension management quality improvement efforts and its accuracy in predicting hypertension prevalence within health systems should be conducted.
Strengths
The described tool has multiple strengths. First, it allows users to determine their estimated hypertension prevalence based on their own patients' aggregate demographic and comorbidity data that are typically available within health systems' electronic health records. In addition, the tool appears to be responsive to changes in the patient population's demographic profiles when estimating the expected hypertension prevalence. For example, although the tool was established using data that are reflective of the general US population, when data from the pooled cohort that had a higher hypertension and comorbidity prevalence were included, the tool was responsive enough to provide a clinically accurate estimate of those cohorts' hypertension prevalence. In addition, the tool incorporates comorbidity status, which was shown to be an important predictor of hypertension prevalence. Therefore, it can be used among populations with varying levels of comorbidity prevalence, including within specialty clinics (eg, endocrinology and cardiology) that often see patients with multiple health issues. Finally, flexibility has been built into the tool to accommodate health systems with limited access to the requested data elements. First, a race/ethnicity category of “Missing” was added to the standard version of the tool to accommodate health systems that are unable to report their data by race/ethnicity or who may have some patients who lack a race/ethnicity classification. Second, we developed a modified version of the tool for users who are unable to provide the comorbidity status of their patient population. The modified version applies NHANES‐estimated comorbidity prevalence estimates to the sex‐, age‐, and race/ethnicity‐specific values supplied by the user (e‐Table 1) to calculate the number of patients expected to fall into each comorbidity strata. These values are then applied to the standard version of the tool to calculate the health system's expected hypertension prevalence. We found that the modified version of the tool had similar external validity compared with the standard version among our randomly selected pooled cohort samples (e‐Table 2). However, the predictive ability of the modified tool is likely diminished if used among patient populations that have age‐, sex‐, and race/ethnicity‐stratified comorbidity prevalence that differs considerably from the general US population. Therefore, users need to consider which version is most appropriate to apply to their patient population.
Limitations
There are potential limitations to the methods used to develop and validate the Estimator Tool. First, NHANES surveys only the noninstitutionalized US population and does not include those on active duty with the military or persons residing in nursing homes and other institutions; therefore, there may be some limitations to the generalizability of the findings. Second, 14 years of data were used to calculate the stratum‐specific prevalence estimates; however, because consistent trends in hypertension prevalence occurred during this time and there were no observed changes in the association between the tool elements and hypertension risk, use of this historical data should still be able to accurately and precisely predict current hypertension prevalence estimates. Third, due to data availability, results for Mexican Americans were used to represent all Hispanics during 1999 to 2006. However, no change occurred among Hispanics' hypertension prevalence during the entire period 1999 to 2012 (Table 4); therefore, this change in categorization likely did not affect the overall findings. Fourth, NHANES estimates calculated for those aged 75 years and older are applied to health systems' data for patients aged 75 to 85 years. While this may slightly overestimate the hypertension prevalence in this age group, it makes the tool more functional for health systems as they are often asked to report on their hypertension management outcomes for only those adults aged 18 to 85 years, such as reporting on measures related to the National Quality Forum's Controlling High Blood Pressure 0018 measure.30 Fifth, modifications were made to the definitions used among the external datasets used for validation to most closely align with the definitions used among the NHANES participants, potentially allowing for misclassification. Sixth, while the Estimator Tool includes the strongest predictors of hypertension prevalence according to our NHANES model, these factors and other factors not included in the tool may affect hypertension prevalence differently across health systems. Moreover, we assume users of the tool have adequately identified and counted the number of people with comorbidities within their patient population, which may not be accurate. Therefore, the tool's accuracy in estimating hypertension prevalence will likely vary across users.
In addition, there was variation in how the datasets used to develop and validate the tool collected their BP measurements—either via HgS and auscultatory technique or AOD—and summarized their average BP values, which may limit the generalizability of the findings. While HgS devices are still currently used in some clinical practices to collect BP, use of AODs is now common practice, mainly to eliminate potential exposure to mercury by patients and staff.31, 32, 33 However, AODs cannot exactly reproduce HgS readings and subsequent BP classifications, because each device type uses different approaches to obtain BP readings.34, 35 In addition, studies have noted differences in BP values and hypertension classification when results are compared using different AODs.36 Despite this limitation, use of data from studies that collected and summarize BPs using different devices and methods allowed for a “real‐world” assessment of how the Estimator Tool may be used by health systems. There are several other factors that may affect the tool user's results, including the time and frequency of BP measurement, technical issues, and patient‐specific factors.37, 38, 39, 40 Technical guidelines on how to obtain BP accurately regardless of device used are available at http://www.measureuppressuredown.com/HCProf/toolkit.pdf.
Another key component in accurately estimating a population's hypertension prevalence is that a common hypertension definition is used. Most notably, a formal hypertension diagnosis is recommended to rely “on the average of two or more properly measured, seated BP readings on each of two or more office visits,” conditions that cannot be completely met during the one NHANES visit8; therefore, the BP measure–based criteria used here may overestimate hypertension prevalence and the prevalence of undiagnosed hypertension. While this potential limitation likely did not affect the results of the validation assessment, as the external studies used for validation also collected BP at only one visit, it may affect the results obtained by health systems who use the tool as they likely require more than one visit to diagnose hypertension. In addition, the debate continues over what population‐level thresholds should be used to identify BP treatment goals.41 The model supporting this tool uses the thresholds recommended for the general population by the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7), as these recommendations remain endorsed by organizations including the American College of Cardiology and American Heart Association and the National Institutes of Health.8 Further, they align with Healthy People 2020 measures and NHANES follows the JNC 7 guidelines to obtain and classify BP measures. If health systems are following other guidance, eg, the thresholds recommended by James and coworkers,42 the hypertension prevalence estimates of the Estimator Tool may not be directly comparable to their measured hypertension prevalence, especially among health systems with large elderly populations.43 Moreover, health systems may use slightly different clinical and/or administrative definitions for the comorbidities included in the Estimator Tool. The clinical definitions outlined in e‐Figure 1 follow the most current national recommendations and align with the definitions applied to the NHANES participants to develop this tool. Finally, while we obtained informal input on the usability of the tool from a diverse collection of Million Hearts partners that helped inform its development, including adding modified versions to assist lower‐resourced clinics, we did not formally evaluate the usability of the tool across a broad array of health systems. Therefore, there may be some unforeseen limitations in the tool's usefulness among certain types of health systems, especially ones without a fully operational electronic health record system.
Conclusions
Many steps are necessary to optimally manage hypertension among a patient population. Identification of all of the patients with hypertension is one of the important first steps in this process. The Estimator Tool described here appears to be a clinically useful instrument that can be used to compare reported vs expected hypertension prevalence in health systems with diverse patient demographic and comorbidity profiles. Improvements in the identification of patients with hypertension followed by the use of evidence‐based strategies to effectively manage their BP should lead to better BP control and decrease the number of heart attack, stroke, and other negative CVD events.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Disclosures
The authors report no specific funding in relation to this research and no conflicts of interest to disclose.
Supporting information
e‐Figure 1. Clinical definitions that health systems are encouraged to use to best align with currently endorsed guidelines and the methods used to develop the Hypertension Prevalence Estimator Tool.
e‐Table I. Comorbidity prevalence, by sex, age, and race‐ethnicity (National Health and Nutrition Examination Survey, 2005–2012).
e‐Table II. External validity testing results for the modified Hypertension Prevalence Estimator Tool that does not include comorbidity status, by random sample size.
Acknowledgments
We thank David Meyers, MD, Chief Medical Officer, Agency for Healthcare Research and Quality, Paul Muntner, PhD, Professor, Department of Epidemiology, University of Alabama at Birmingham, and Daniel Lackland, DrPH, Professor, Medical College of South Carolina, for their review of the manuscript and the helpful recommendations they provided. We thank Namvar Zohoori, MD, MPH, PhD, Director, Center for Health Advancement and Chronic Disease Director, Arkansas Department of Health, for his expertise in guiding our assessment of the Arkansas Cardiovascular Health Examination Survey data. We thank Adolfo Correa, MD, MPH, PhD, Director, Jackson Heart Study (JHS), for his expertise in guiding our assessment of the JHS data. The JHS is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, and HHSN268201300050C from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute on Minority Health and Health Disparities. This manuscript was prepared in part using Hispanic Community Health Study/Study of Latinos (HCHS) and Multi‐Ethnic Study of Atherosclerosis (MESA) research materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center. This manuscript does not necessarily reflect the opinions or views of the ARCHES, JHS, HCHS, MESA, or NHLBI.
J Clin Hypertens (Greenwich). 2016;18:750–761. DOI: 10.1111/jch.12746. Published 2016. This article is a U.S. Government work and is in the public domain in the USA.
References
- 1. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang Q, Cogswell ME, Flanders WD, et al. Trends in cardiovascular health metrics and associations with all‐cause and CVD mortality among US adults. JAMA. 2012;307:1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. He J, Ogden LG, Bazzano LA, et al. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow‐up study. Arch Intern Med. 2001;161:996–1002. [DOI] [PubMed] [Google Scholar]
- 4. Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention . Vital signs: awareness and treatment of uncontrolled hypertension among adults—United States, 2003‐2010. MMWR Morb Mortal Wkly Rep. 2012;61:703–709. [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention . National Health and Nutrition Examination Survey: surveys and data collection systems (2010 data) http://www.cdc.gov/nchs/nhanes.htm. Accessed August 4, 2015.
- 7. Zipf G, Chiappa M, Porter KS, et al. National health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat. 2013;56:1–37. [PubMed] [Google Scholar]
- 8. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention . National Health and Nutrition Examination Survey (NHANES) questionnaire and exam protocol. http://www.cdc.gov/nchs/about/major/nhanes/questexam. Accessed August 4, 2015.
- 10. Selassie A, Wagner CS, Laken ML, et al. Progression is accelerated from prehypertension to hypertension in blacks. Hypertension. 2011;58:579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011‐2012. NCHS Data Brief. 2013;133:1–8. [PubMed] [Google Scholar]
- 12. Gillespie CD, Hurvitz KA. Prevalence of hypertension and controlled hypertension—United States, 2007‐2010. MMWR Surveill Summ. 2013;62:144–148. [PubMed] [Google Scholar]
- 13. Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA. 2009;302:401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Banda JA, Clouston K, Sui X, et al. Protective health factors and incident hypertension in men. Am J Hypertens. 2010;23:599–605. [DOI] [PubMed] [Google Scholar]
- 15. Sakurai M, Stamler J, Miura K, et al. Relationship of dietary cholesterol to blood pressure: the INTERMAP study. J Hypertens. 2011;29:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ostchega Y, Hughes JP, Terry A, et al. Abdominal obesity, body mass index, and hypertension in US adults: NHANES 2007–2010. Am J Hypertens. 2012;25:1271–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med. 2014;160:517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murphy K. Machine Learning: A Probabilistic Perspective. Cambridge, MA: Massachusetts Institute of Technology; 2012. [Google Scholar]
- 20. Murray M, Davies M, Boushon B. Panel size: how many patients can one doctor manage? Fam Pract Manag. 2007;14:44–51. [PubMed] [Google Scholar]
- 21. Taylor HA Jr, Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;4(suppl 6):S6‐4‐17. [PubMed] [Google Scholar]
- 22. Zohoori N, Pulley L, Jones C, et al. Conducting a statewide health examination survey: the Arkansas Cardiovascular Health Examination Survey (ARCHES). Prev Chronic Dis. 2011;8:A67. [PMC free article] [PubMed] [Google Scholar]
- 23. Sorlie PD, Aviles‐Santa LM, Wassertheil‐Smoller S, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bild DE, Bluemke DA, Burke GL, et al. Multi‐ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 25. Frieden TR, King SM, Wright JS. Protocol‐based treatment of hypertension: a critical step on the pathway to progress. JAMA. 2014;311:21–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guide to Community Preventive Services . Cardiovascular disease prevention and control: team‐based care to improve blood pressure control. http://www.thecommunityguide.org/cvd/teambasedcare.html. Accessed August 4, 2015.
- 27. Centers for Disease Control and Prevention . Self‐measured blood pressure monitoring: action steps for public health practitioners http://millionhearts.hhs.gov/Docs/MH_SMBP.pdf. Accessed on August 4, 2015.
- 28. Frieden TR, Berwick DM. The “Million Hearts” initiative—preventing heart attacks and strokes. N Engl J Med. 2011;365:e27. [DOI] [PubMed] [Google Scholar]
- 29. Centers for Disease Control and Prevention . Hypertension control: change package for clinicians. http://millionhearts.hhs.gov/Docs/HTN_Change_Package.pdf. Accessed August 4, 2015.
- 30. Patel MM, Datu B, Roman D, et al. Progress of health plans toward meeting the million hearts clinical target for high blood pressure control—United States, 2010‐2012. MMWR Morb Mortal Wkly Rep. 2014;63:127–130. [PMC free article] [PubMed] [Google Scholar]
- 31. Dasgupta K, Quinn RR, Zarnke KB, et al. The 2014 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2014;30:485–501. [DOI] [PubMed] [Google Scholar]
- 32. Campbell NR, Berbari AE, Cloutier L, et al. Policy statement of the World Hypertension League on noninvasive blood pressure measurement devices and blood pressure measurement in the clinical or community setting. J Clin Hypertens (Greenwich). 2014;16:320–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Emergency Care Research Institute . Replacing mercury‐column sphygmomanometers. Health Devices. 2003;32:109–117. [PubMed] [Google Scholar]
- 34. Alpert BS, Quinn D, Gallick D. Oscillometric blood pressure: a review for clinicians. J Am Soc Hypertens. 2014;8:930–938. [DOI] [PubMed] [Google Scholar]
- 35. Ostchega Y, Nwankwo T, Sorlie PD, et al. Assessing the validity of the Omron HEM‐907XL oscillometric blood pressure measurement device in a national survey environment. J Clin Hypertens (Greenwich). 2010;12:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Adler C, Ellert U, Neuhauser HK. Disagreement of the two oscillometric blood pressure measurement devices, Datascope Accutorr Plus and Omron HEM‐705CP II, and bidirectional conversion of blood pressure values. Blood Press Monit. 2014;19:109–117. [DOI] [PubMed] [Google Scholar]
- 37. Fishman PA, Anderson ML, Cook AJ, et al. Accuracy of blood pressure measurements reported in an electronic medical record during routine primary care visits. J Clin Hypertens (Greenwich). 2011;13:821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. [DOI] [PubMed] [Google Scholar]
- 39. Ali S, Rouse A. Practice audits: reliability of sphygmomanometers and blood pressure recording bias. J Hum Hypertens. 2002;16:359–361. [DOI] [PubMed] [Google Scholar]
- 40. Myers MG, McInnis NH, Fodor GJ, Leenen FH. Comparison between an automated and manual sphygmomanometer in a population survey. J Hum Hypertens. 2008;21:280–283. [DOI] [PubMed] [Google Scholar]
- 41. Wright JT Jr, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR . Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499–503. [DOI] [PubMed] [Google Scholar]
- 42. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 43. Navar‐Boggan AM, Pencina MJ, Williams K, et al. Proportion of US adults potentially affected by the 2014 hypertension guideline. JAMA. 2014;311:1424–1429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
e‐Figure 1. Clinical definitions that health systems are encouraged to use to best align with currently endorsed guidelines and the methods used to develop the Hypertension Prevalence Estimator Tool.
e‐Table I. Comorbidity prevalence, by sex, age, and race‐ethnicity (National Health and Nutrition Examination Survey, 2005–2012).
e‐Table II. External validity testing results for the modified Hypertension Prevalence Estimator Tool that does not include comorbidity status, by random sample size.


