Abstract
Growing evidence indicates that drugs of abuse gain control over the individual by usurping glutamate-linked mechanisms of neuroplasticity in reward-related brain regions. Accordingly, we have shown that glutamate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) activity in the amygdala is required for the positive reinforcing effects of alcohol, which underlie the initial stages of addiction. It is unknown, however, if enhanced AMPAR activity in the amygdala facilitates alcohol self-administration, which is a kernel premise of glutamate hypotheses of addiction. Here we show that low-dose alcohol (0.6 g/kg/30-min) self-administration increases phosphorylation (activation) of AMPAR subtype GluA1 S831 (pGluA1 S831) in the central amygdala (CeA), basolateral amygdala, and nucleus accumbens core (AcbC) of selectively bred alcohol-preferring P-rats as compared to behavior-matched (non-drug) sucrose controls. The functional role of enhanced AMPAR activity was assessed via site-specific infusion of the AMPAR positive modulator, aniracetam, in the CeA and AcbC prior to alcohol self-administration. Intra-CeA aniracetam increased alcohol- but not sucrose-reinforced responding, and was ineffective following intra-AcbC infusion. Since GluA1 S831 is a Ca2+/calmodulin-dependent protein kinase II (CaMKII) substrate, we sought to determine if AMPAR regulation of enhanced alcohol self-administration is dependent on CaMKII activity. Intra-CeA infusion of the cell-permeable CaMKII peptide inhibitor m-AIP dose-dependently reduced alcohol self-administration. A sub-threshold dose of m-AIP also blocked the aniracetam-induced escalation of alcohol self-administration, demonstrating that AMPAR-mediated potentiation of alcohol reinforcement requires CaMKII activity in the amygdala. Enhanced activity of plasticity-linked AMPAR-CaMKII signaling in the amygdala may promote escalated alcohol use via increased positive reinforcement during the initial stages of addiction.
Keywords: alcohol drinking, amygdala, AMPA receptor, CaMKII, glutamate, GluA1
INTRODUCTION
The fundamental behavioral process of positive reinforcement reflects the tendency of all animals, human and non-human, to repeat responses that produce a desired outcome. Accordingly, positive reinforcement mechanisms underlie the repetitive nature of alcohol seeking-behavior during the initial binge/intoxication stages of addiction (Koob and Volkow, 2010; Wise and Koob, 2014). At the neurochemical level, evidence suggests that alcohol and other drugs of abuse gain control over behavior by usurping glutamatergic mechanisms of synaptic plasticity in reward circuits (Kalivas, 2009; Kauer and Malenka, 2007; McCool et al., 2010; Winder et al., 2002). Thus, identifying glutamatergic mechanisms of the positive reinforcing effects of alcohol is crucial for understanding the etiology of addiction.
Glutamate is the primary excitatory neurotransmitter in the mammalian brain and plays an essential role in regulating many of the biochemical, physiological, and behavioral effects of alcohol (Backstrom et al., 2004; Besheer et al., 2006; Gass and Olive, 2008; Hodge and Aiken, 1996; Hodge et al., 2006; McCool et al., 2010; Siggins et al., 2003; Weight et al., 1991; Woodward et al., 2006). Growing evidence indicates that α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptors (AMPARs) are important modulators of alcohol self-administration and relapse-like behavior (Agoglia et al., 2015; Backstrom and Hyytia, 2004; Cannady et al., 2013; Salling et al., 2014; Sanchis-Segura et al., 2006; Wang et al., 2012). AMPARs are ligand-gated ion channel receptors comprised of four subunits (GluA1-4) and mediate rapid excitatory glutamate neurotransmission and regulate synaptic strength (Bredt and Nicoll, 2003). AMPARs are involved in a variety of biological functions and dysregulation of their activity has been implicated in several neurological, psychiatric, and addictive disorders (Zhang and Abdullah, 2013), including alcoholism (Kryger and Wilce, 2010).
Repeated exposure to alcohol leads to AMPAR-mediated neuroadaptations that enhance synaptic strength in brain regions known to regulate reinforcement and reward processes (Stuber et al., 2008; Wang et al., 2012), which may promote alcohol consumption through alterations in reinforcement processes. Consistent with this concept, alcohol exposure increases extracellular glutamate levels in reward-related brain regions (Gass et al., 2011a; Griffin et al., 2015; Roberto et al., 2004). AMPAR GluA1 expression is increased in the striatum after systemically administered alcohol (Wang et al., 2012) and in nucleus accumbens by chronic intermittent consumption (Neasta et al., 2010). Surface GluA1 is also upregulated in the amygdala after chronic intermittent alcohol vapor exposure (Christian et al., 2012), and we have shown that both voluntary alcohol drinking (home-cage) and low-dose operant alcohol self-administration increase GluA1 S831 phosphorylation in the amygdala (Salling et al., 2014). Thus, AMPAR activity and expression are targets of alcohol in specific brain regions that mediate positive reinforcement.
Glutamate activity in reward-related brain regions has a well-defined role in modulating alcohol consumption, reinforcement, and seeking behaviors (Besheer et al., 2010; Rassnick et al., 1992; Schroeder et al., 2008). Systemic enhancement of glutamate activity at AMPARs increases alcohol self-administration and seeking-behavior, which shows that heightened activity at these receptors has the potential to augment alcohol reinforcement processes (Cannady et al., 2013). Further, AMPAR activity in the amygdala is required for the positive reinforcing effects of alcohol (Salling et al., 2014). However, it is not known if enhancement of AMPAR signaling in specific reward-related brain regions potentiates the positive reinforcing effects of alcohol, which would be consistent with a role for these systems in the etiology of addiction.
Recent evidence also indicates that drug use, withdrawal, and craving are associated with post-translational modification of AMPARs in reward circuits (Wolf, 2010), including phosphorylation by Ca2+/calmodulin-dependent protein kinase II (CaMKII) (Anderson et al., 2008). CaMKII is a family of Ca2+-activated Ser/Thr protein kinases that mediates many intracellular responses in the brain including glutamate release, AMPA receptor function, and synaptic plasticity (Colbran and Brown, 2004; Hudmon and Schulman, 2002; Lisman et al., 2002). A crucial function of CaMKII is to phosphorylate AMPARs at GluA1 S831 (pGluA1 S831), which leads to potentiation of AMPAR-mediated activity (Barria et al., 1997b; Mammen et al., 1997). Importantly, elevated glutamate levels after repeated alcohol use (Roberto et al., 2004) might activate CaMKII, which then phosphorylates AMPARs at GluA1 S831, promotes membrane insertion, and enhances their function (Colbran and Brown, 2004; Hudmon and Schulman, 2002; Lisman et al., 2002). We have shown that operant alcohol self-administration increases expression and phosphorylation of the alpha isoform of CaMKII (CaMKIIα) in mouse amygdala (Salling et al., 2014) and that CaMKII inhibition in the frontal cortex increases the reinforcing effects of alcohol (Faccidomo et al., 2015). However, it remains to be determined if AMPAR-mediated regulation of the positive reinforcing effects of alcohol requires CaMKII signaling.
The goal of the present preclinical studies was to examine the functional role of enhanced glutamate activity at AMPARs, specifically within limbic reward-related brain regions, in the positive reinforcing effects of alcohol. Effects of operant alcohol self-administration were first assessed on AMPAR GluA1 S831 phosphorylation and total GluA1 immunoreactivity in the nucleus accumbens and amygdala. Guided by these results, we evaluated potential mechanistic regulation of escalated alcohol self-administration via site specific infusion of the AMPAR positive modulator aniracetam. Finally, we sought to determine if aniracetam-induced increases in alcohol self-administration were dependent on CaMKII activity. These studies were conducted using selectively-bred alcohol-preferring (P-) rats, which are a genetic model of high alcohol preference (Li et al., 1979). The P-rat line has been shown to fulfill requirements of an animal model of alcoholism (Lester and Freed, 1973), including development of tolerance and dependence through voluntary drinking, and maintaining preference for ethanol over other palatable solutions (Kampov-Polevoy et al., 2000; Lankford et al., 1991); thus, providing translational relevance of the current findings to individuals with genetic risk for high alcohol preference.
MATERIALS AND METHODS
Animals
Adult male inbred alcohol-preferring (P-) rats were pair-housed in Plexiglas cages and handled daily prior to any training procedures, and subsequently single-housed following surgeries. All rats had ad libitum home-cage access to food and water. The colony room was maintained on a 12 hour light/dark cycle (lights on at 7 am) and all experiments were conducted during the light cycle. Body weights for rats in each experiment are shown in Table S1 (Supplementary Materials). All procedures were conducted in accordance with the National Institute of Health guidelines, and approved by the University of North Carolina Institutional Animal Care and Use Committee.
Experiment 1: Examination of GluA1 phosphorylation (pGluA1) after chronic alcohol self-administration
To examine the effects of operant alcohol self-administration on expression and post-translational modifications of the GluA1 subunit, P-rats were trained to self-administer either alcohol (15% v/v; n=9) or sucrose (0.4% w/v; n=9) as we describe in (Besheer et al., 2010; Cannady et al., 2013); see also Supplementary Materials and Methods). Brain tissue was collected immediately after the 28th self-administration session. Using immunohistochemistry (IHC), coronal brain sections were processed for phosphorylated GluA1 S831 (pGluA1 S831) or total GluA1. Immunoreactivity (IR) in subregions of the amygdala (lateral - LA, basolateral - BLA, and central nuclei - CeA), subregions of the nucleus accumbens (core - AcbC and shell - AcbSh; see Supplementary Methods), the prelimbic region (prefrontal cortex), and the dorsal medial striatum were evaluated as previously described (Besheer et al., 2012; Cannady et al., 2011; Spanos et al., 2012)(Supplementary Methods).
Experiment 2: Examination of site-specific positive modulation of glutamate activity at AMPA receptors on alcohol self-administration
To assess the effects of the AMPA positive modulator aniracetam, alcohol self-administering P-rats received site-specific infusion of aniracetam aimed at the CeA (0, 1, 3, µg/ 0.5 µl/side; n = 10) or AcbC (0, 1, 3, 6 µg/0.5 µl/side; n = 11) immediately prior to operant alcohol (15%, v/v) self-administration sessions as previously described (Schroeder et al., 2003); also see Surgery and Microinjection Methods in Supplementary Methods. Drug testing began on the 71st day of self-administration using a within-subjects design in which each aniracetam dose was administered in a randomized order. Following testing, rats in the amygdala group underwent a locomotor assessment to determine whether aniracetam affected general locomotor behavior (see Supplementary Methods). To assess reinforcer specificity, sucrose-trained P-rats (n = 9) received intra-CeA aniracetam (0, 1, 3, 6 µg/0.5 µl/side,) immediately before operant sucrose (0.4%, w/v) self-administration sessions beginning on the 39th day of self-administration.
Experiment 3: Examination of CaMKII involvement in aniracetam-induced potentiation of alcohol self-administration
To test if inhibition of CaMKII phosphorylation reduced alcohol self-administration, P-rats (n = 7) received intra-CeA Myristolated Autocamtide-2-related inhibitory peptide (m-AIP; 0, 5, 10, 20 µg/0.5µl/side) immediately before operant alcohol (15%, v/v) self-administration sessions. A second group of alcohol self-administration trained P-rats (n = 9) received intra-amygdala microinjections of vehicle, aniracetam (1 µg), m-AIP (10 µg), and a cocktail solution consisting of aniracetam (1 µg) + m-AIP (10 µg) to assess whether aniracetam-induced alterations in alcohol self-administration were dependent on CaMKII activity. All drug doses were administered in a randomized order using a within subjects design beginning on the 35th day of self-administration.
Drugs
Alcohol (95% (w/v); Pharmco-AAPER, Shelbyville, KY) was diluted in distilled water to 15% (v/v). The AMPAR positive modulator aniracetam (Tocris, Ellisville, Missouri), and the CaMKII peptide inhibitor myristolated autocamtide-2 -related inhibitory peptide (m-AIP; Enzo Life Sciences), were dissolved in 50% DMSO in artificial cerebral spinal fluid (aCSF). Aniracetam is an allosteric positive modulator of AMPARs that slows deactivation and desensitization of the receptor (Vyklicky et al., 1991) and has no effect on NMDA-mediated synaptic potentials (Boxall and Garthwaite, 1995). Evidence also indicates that aniracetam metabolites enhance AMPAR activity through a CaMKII-dependent mechanism (Nishizaki and Matsumura, 2002). m-AIP is a highly potent cell-permeable inhibitor that selectively inhibits the phosphorylation and activation of CaMKII and has no effect on cyclic AMP-dependent protein kinase, protein kinase C, calmodulin-dependent protein kinase IV (Ishida et al., 1995). The aniracetam and m-AIP dose ranges and were chosen based on demonstrated efficacy in altering behavior in previous studies (Masuoka et al., 2008; Rao et al., 2001; Salling et al., 2014).
Data Analysis
Alcohol- (or sucrose-) responses were analyzed by one-way repeated measures analysis of variance (RM ANOVA). Cumulative responses, and cumulative distance traveled (cm) were analyzed by between groups or RM ANOVA where appropriate. Intake (g/kg) was analyzed by one-way RM ANOVA and calculated based on rat body weight and number of reinforcers delivered. Tukey post-hoc comparisons were performed to identify differences between treatments/treatment groups. T-tests were performed to identify differences in pGluA1 S831 and total GluA1 immunoreactivity between alcohol- and sucrose self-administering rats within brain regions. Statistical significance was determined at p ≤ 0.05.
RESULTS
Experiment 1: Examination of GluA1 phosphorylation (pGluA1) after chronic alcohol self-administration
To examine chronic alcohol-induced neuroadaptations of AMPAR subunits, immunohistochemistry (IHC) was performed to assess phosphorylation and expression of the GluA1 subunit in alcohol- and sucrose self-administration trained P-rats. Mean ± S.E.M. sucrose and alcohol responses across the 28 days were 152.20 ± 28.35 and 110.90 ± 11.31 (corresponding to alcohol intake of 0.60 ± 0.06 g/kg), respectively (Fig 1A and 1B). This level of alcohol self-administration is consistent with prior studies in P-rats following 30-60 min operant sessions (Besheer et al., 2010; Cannady et al., 2013; Schroeder et al., 2005; Schroeder et al., 2008). Two-way RM-ANOVA indicated that there was no difference between baseline sucrose- and alcohol-reinforced responses analyzed over the 28 day period [F (1, 16) = 1.831, p= 0.1948]. These data indicate that operant performance was similar between alcohol and sucrose self-administering P-rats, allowing for direct comparisons of GluA1 immunoreactivity between the two self-administration groups.
Figure 1. Alcohol self-administration upregulates phosphorylation of AMPAR GluA1 S831 subunit in subregions of the amygdala.
Total lever presses plotted as a function of daily 30-min self-administration sessions for (A) sucrose- or (B) alcohol exposed P-rats prior to tissue collection for immunohistochemistry (IHC). Mean (± SEM) immunoreactivity of the pGluA1 S831 positive area (pixels/1000/mm2) in the (C) basolateral amygdala (BLA), (D) central amygdala (CeA), and (D) lateral amygdala (LA) following sucrose (left) or alcohol (right) self-administration. Representative 20X photomicrographs of the BLA, CeA, and LA are of pGluA1 S831 IR after 28 days of sucrose or alcohol self-administration. Scale bar represents 50 microns. Graphed values are expressed as means ± SEM; * p < 0.05 – alcohol (ALC) vs sucrose (SUC), t-test.
Amygdala pGluA1 S831 IR was significantly increased in rats with a history of alcohol self-administration in the basolateral nucleus (BLA; t(16) = 3.671, p=0.002; Fig 1C) and the central nucleus (CeA; t(16) = 2.173, p=0.045; Fig 1D). In contrast, pGluA1 S831 IR in the lateral nucleus of the amygdala (LA) did not differ between groups (Fig 1E). Significant increases in pGluA1 S831 were observed in the nucleus accumbens core, but not the shell (Supplementary Fig 1 and Results). pGluA1 S831 IR in the dorsal medial striatum and prefrontal cortex was also examined, and no statistical differences were observed between groups (Table 1). Additionally, total GluA1 expression was measured to determine if alterations in phosphorylated GluA1 were the result of changes in GluA1 expression and no significant differences in any of the examined regions were observed (Table 2). Given a slight trend for an increase in total GluA1 protein following alcohol (Table 2), the ratio of pGluA1 to total GluA1 was compared within subject for brain regions showing a change in pGluA1. Results indicated that the changes in pGluA1 S831 IR were preserved when assessed relative to total protein: Mean±SEM pGluA1 / total GluA1 IR: BLA (sucrose=0.96±0.14 vs. alcohol=1.4±0.09; t(13)=2.6,p=0.01); CeA (sucrose=0.98±0.10 vs. alcohol=1.29±0.14; t(13)=1.8,p=0.04); and AcbC (sucrose=1.06±0.12 vs. alcohol=1.66±0.12; t(13)=3.4,p=0.002). Together, these data suggest that operant alcohol self-administration produces increases GluA1 S831 phosphorylation (e.g., activation) in the amygdala and nucleus accumbens to a greater extent that the nondrug palatable reinforcer, sucrose.
Table 1.
pGluA1 S831 immunoreactivity (pixels/1000/mm2) after alcohol and sucrose self-administration. Data represent mean ± SEM (n=9/group).
| Brain region | Alcohol Self- administration |
Sucrose Self- administration |
|---|---|---|
| Dorsal Striatum | ||
| pGluA1 S831 IR | 578.06 ± 38.09 | 512.88 ± 41.40 |
| Prefrontal Cortex | ||
| pGluA1 S831 IR | 90.76 ± 11.68 | 95.38 ± 10.78 |
Table 2.
Total GluA1 immunoreactivity (pixels/1000/mm2) after alcohol and sucrose self-administration. Data represent mean ± SEM (n=9/group).
| Brain region | Alcohol Self- administration |
Sucrose Self- administration |
|---|---|---|
| BLA | ||
| Total GluA1 IR | 278.59 ± 15.48 | 226.08 ± 26.98 |
| CeA | ||
| Total GluA1 IR | 279.89 ± 19.99 | 231.79 ± 22.23 |
| LA | ||
| Total GluA1 IR | 101.79 ± 10.20 | 78.13 ± 16.18 |
| AcbC | ||
| Total GluA1 IR | 537.87 ± 34.17 | 496.89 ± 31.82 |
| AcbSh | ||
| Total GluA1 IR | 556.85 ± 42.52 | 555.22 ± 35.62 |
Experiment 2: Examination of site-specific positive modulation of glutamate activity at AMPA receptors on alcohol self-administration
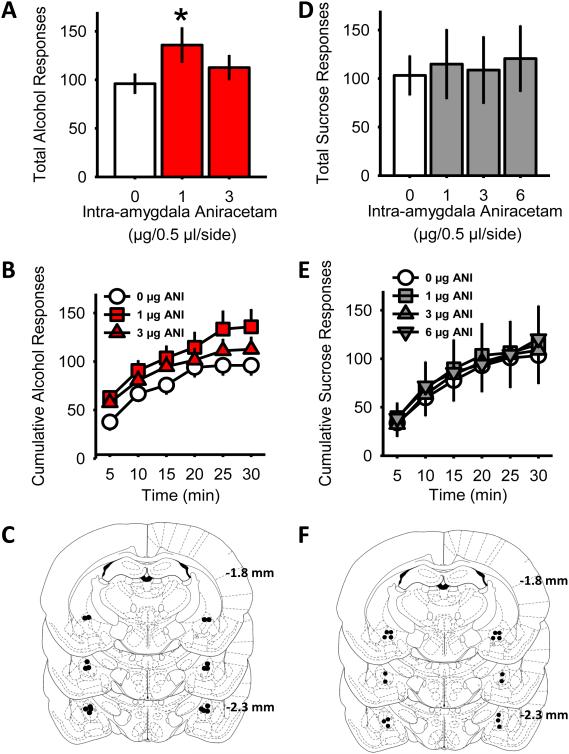
Baseline self-administration parameters are detailed in Table 3. To assess whether potentiation of AMPAR activity within limbic brain regions would alter alcohol self-administration, aniracetam (AMPAR positive modulator) was microinjected into the CeA or ACbC of P-rats trained to self-administer alcohol (15% v/v). One-way RM ANOVA indicated that intra-amygdala infusion of aniracetam significantly increased alcohol-reinforced lever responding [F(2,9) = 4.2, p<0.03; Fig 2A], with a significant increase observed following pretreatment with the lowest aniracetam dose (1 µg) relative to vehicle (p=0.03). The increase in responding resulted in an increase in alcohol intake following the aniracetam (1 µg) dose (Table 4). Analysis of cumulative alcohol-reinforced responses across the session (Fig 2B) showed a significant main effect of intra-amygdala aniracetam dose [F(2,18) = 3.78, p=0.04] and time [F(5,45) = 26.88, p<0.001] as alcohol-reinforced responding increased over the duration of the session. No significant aniracetam dose by time interaction was observed. Water-lever responses (vehicle, mean±SEM = 2.0±0.39) were unchanged by aniracetam. Accurate cannulae placements were verified for all rats in this experiment (Fig 2C). Importantly, intra-CeA aniracetam (1 µg) had no effect on locomotor activity in an open field (Supplementary Fig 2), suggesting that the increase in alcohol responses was not due to a nonspecific increase in locomotor behavior.
Table 3.
Behavioral baseline from 5 groups of P-rats showing number of responses emitted on the alcohol and water associated lever prior to infusion of the AMPAR positive modulator aniracetam (ANI) or the CaMKII inhibitor myristolated AIP (m-AIP) infusion. Alcohol intake was confirmed at the end of each session and converted to g/kg. Data represent mean ± SEM (n=7 – 11/group).
| Responses |
Alcohol Intake (g/kg) |
||
|---|---|---|---|
| Test Group | Alcohol Lever | Water Lever | |
| Alcohol Reinforcement | |||
|
|
|||
| Amygdala ANI | 117.75 ± 7.19 | 9.20 ± 1.95 | 0.69 ± 0.05 |
| Nucleus Accumbens ANI | 105.82 ± 6.49 | 8.27 ± 1.84 | 0.61 ± 0.05 |
| Amygdala AIP | 129.14 ± 15.58 | 21.79 ± 4.02 | 0.78 ± 0.09 |
| Amygdala ANI + m-AIP | 115.50 ± 10.69 | 9.78 ± 2.63 | 0.68 ± 0.05 |
|
|
|||
| Sucrose Reinforcement | |||
|
|
|||
| Amygdala ANI | 133.27 ± 25.25 | 15.89 ± 5.22 | -- |
Figure 2. Positive modulation of amygdala AMPA receptors potentiates alcohol self-administration in a reinforcer-specific manner.
Total alcohol (A) and sucrose (D) reinforced lever presses plotted as a function of intra-CeA aniracetam dose in selectively bred alcohol preferring P-rats (n = 10). Cumulative alcohol (B) and sucrose (E) reinforced responses plotted as a function of time (min) during 30-min session. Slope of the cumulative plot represents response rate. (C and F) Schematic showing injection location in the CeA for alcohol- and sucrose-trained P-rats. Graphed values are expressed as means ± SEM; *p<0.05 relative to vehicle treatment.
Table 4.
Alcohol intake (g/kg) after AMPAR positive modulator aniracetam or the CaMKII inhibitor myristolated AIP (m-AIP) infusion. Alcohol intake was confirmed at the end of each session and converted to g/kg. Data represent mean ± SEM (n=7 – 11/group).
| Aniracetam Dose (μg) | ||||
|---|---|---|---|---|
| Brain Region | 0 | 1 | 3 | 6 |
|
|
||||
| Amygdala | 0.54 ± 0.07 | 0.77 ± 0.12* | 0.65 ± 0.10 | N/A |
| N. Accumbens | 0.58 ± 0.09 | 0.54 ± 0.12 | 0.68 ± 0.11 | 0.39 ± 0.11 |
| m-AIP Dose (μg) | ||||
| Brain Region | 0 | 5 | 10 | 20 |
|
|
||||
| Amygdala | 0.81 ± 0.12 | 0.79 ± 0.12 | 0.79 ± 0.12 | 0.36 ± 0.13* |
| Aniracetam + m-AIP Dose (μg) | ||||
| Brain Region | 0 + 0 | 1 + 0 | 1 + 10 | 0 + 10 |
|
|
||||
| Amygdala | 0.52 ± 0.07 | 0.75 ± 0.09* | 0.31 ± 0.10*# | 0.56 ± 0.09 |
p < 0.05 relative to 0 or 0 + 0;
p < 0.05 relative to 1 μg aniracetam + 0 m-AIP
Examination of alcohol-reinforced responding following intra-AcbC aniracetam (1-6 µg) by one-way RM ANOVA showed a significant effect of accumbens aniracetam dose [F(3,30)=3.4, p=0.03]; however, post-hoc analyses indicated no significant differences from vehicle. Water-lever responses (vehicle, mean±SEM = 7.3±2.5) were unchanged by aniracetam. As such, this data pattern suggests that in contrast to the functional role of amygdala AMPARs in modulating alcohol self-administration, intra-accumbens aniracetam does not significantly alter alcohol self-administration (Supplementary Fig 3A-C).
To determine if the observed increase in alcohol self-administration following intra-amygdala aniracetam pretreatment (Fig 2A) was specific to alcohol, aniracetam was assessed in sucrose (0.4%) self-administering P-rats. Intra-amygdala aniracetam had no significant effect on sucrose-reinforced responses across all doses tested (Fig 2D). Cumulative sucrose-reinforced responses over time showed a main effect of time [F(5,40) = 13.53, p<0.001], but no significant main effect of intra-amygdala aniracetam dose or interaction (Fig 2E). Water-lever responses (vehicle, mean±SEM = 5.3±1.9) were unchanged by aniracetam. Accurate cannulae placements were verified for all rats included in this experiment (Fig 2F). Together, the results from this experiment show that pharmacological potentiation of intra-amygdala AMPAR activity results in an escalation in alcohol self-administration that is specific to alcohol reinforcement and is not related to a nonspecific increase in motor activity.
Experiment 3: Examination of CaMKII involvement in aniracetam-induced potentiation of alcohol self-administration
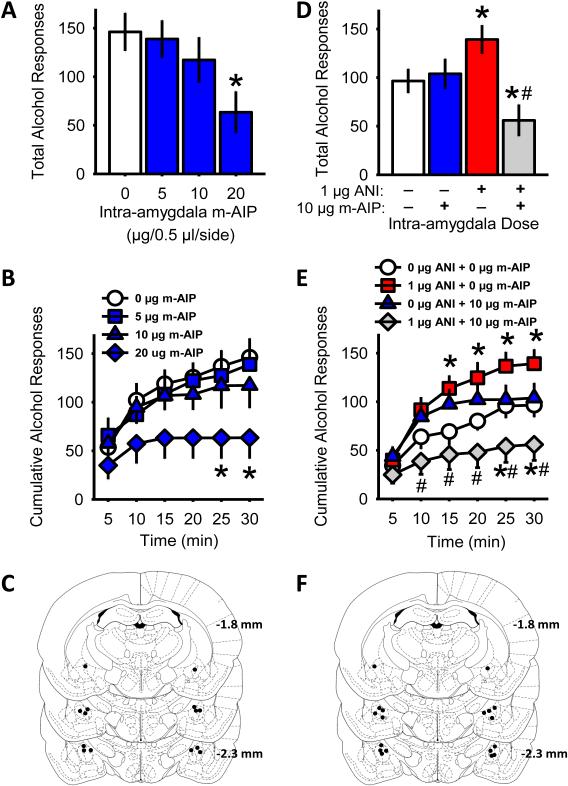
Intra-amygdala infusion of the CaMKII inhibitor m-AIP significantly reduced alcohol-reinforced lever responses [F(3,18) = 3.92, p=0.03], at the highest dose m-AIP dose (20 µg; p= 0.03; Fig 3A). Examination of cumulative alcohol responses across the session indicated a significant main effect time [F(5,30) = 21.73, p<0.001], and a significant interaction of m-AIP dose and time [F(15,90) = 2.04, p=0.02]. Specifically, alcohol-reinforced responding was significantly reduced by m-AIP (20 µg) at 25 and 30 minutes (Fig 3B). The reduction in alcohol-reinforced responding resulted in a significant decrease in alcohol intake (Table 4). Water-lever responses (vehicle, mean±SEM = 17.0±7.4) were unchanged by m-AIP. Accurate cannulae placements were verified for all rats included in this experiment (Fig 3C).
Figure 3. Intra-amygdala inhibition of CaMKII activity blocks aniracetam-induced increase in alcohol self-administration.
(A) Total alcohol reinforced responses plotted as a function of the CaMKII peptide inhibitor m-AIP dose in P-rats (n = 7) showing a dose-dependent reduction in alcohol reinforcement. (B) Cumulative alcohol-reinforced responses were significantly decreased by intra-amygdala m-AIP pretreatment (20 µg). (D) Intra-CeA co-administration of the CaMKII inhibitor m-AIP (10 µg) blocked the aniracetam (1 µg) induced increase in alcohol reinforced responses in P-rats (n = 9). (E) Aniracetam-induced changes in response rate (cumulative alcohol-reinforced responses) was significantly increased by aniracetam but blocked by co-infusion of m-AIP (10 µg). (C and F) Illustrations showing accurate injector cannulae placements in the CeA. Graphed values are expressed as means ± SEM; *p<0.05 relative to vehicle treatment and #p<0.05 relative to 1 μg aniracetam (ANI).
To determine if aniracetam (1 µg)-induced potentiation of alcohol self-administration (Fig 2A) depends on CaMKII activity, a cocktail of aniracetam (1 µg) and the highest m-AIP dose that did not alter alcohol self-administration (10 µg; Fig 3A) was administered in the amygdala. Significant differences in alcohol-reinforced responding were observed after intra-amygdala aniracetam+AIP cocktail infusion [F(3,24) = 12.52, p<0.001]. Intra-amygdala aniracetam (1 µg) alone significantly increased alcohol-reinforced responses (p=0.02; Fig 3D) and intake (Table 4) and m-AIP (10 µg) alone had no effect on alcohol self-administration, confirming the results of Experiment 2 (Fig 2A) and the initial assessment in this experiment 3 (Fig 3A), respectively. Interestingly, intra-amygdala infusion of the aniracetam+AIP cocktail blocked the aniracetam-induced increase in alcohol-reinforced lever responses (p<0.001; Fig 3D) with a concomitant decrease in alcohol intake (Table 4). Examination of the pattern of alcohol-reinforced responses across the sessions by a three-way ANOVA, indicated a significant main effect of time [F(5,40) = 53.22, p<0.001], an aniracetam by m-AIP interaction [F(1,8)=53.95, p<0.001], an m-AIP dose by time interaction [F(5,40)=6.66, p<0.001], and a aniracetam by m-AIP by time interaction [F(5,40) = 10.1, p<0.001]. Post-hoc analysis showed escalated alcohol-reinforced responding following intra-amygdala aniracetam (1 µg) beginning at 15 minutes and persisting for the duration of the session (Fig 3E). Moreover, this aniracetam-induced escalation in alcohol responses was significantly reduced by m-AIP (aniracetam+AIP cocktail) beginning early and lasting throughout the session (Fig 3E). Responses on the water lever (vehicle, mean±SEM = 13.4±4.1) were unchanged by drug treatment. Accurate cannulae placements were verified for all rats included in this experiment (Fig 3F). Importantly, co-administration of aniracetam and m-AIP did not alter locomotor activity in an open field (Supplementary Fig 4). Together these data indicate that aniracetam-induced increases in alcohol self-administration are dependent on amygdala CaMKII activity.
DISCUSSION
Recent evidence indicates that acute systemic enhancement of glutamate activity at AMPARs enhances the positive reinforcing effects of alcohol (Cannady et al., 2013), which is consistent with the hypothesis that heightened glutamate activity may underlie escalated alcohol use during the initial stages of addiction (Kalivas, 2009; Wise and Koob, 2014). In this study, we first sought to determine if the positive reinforcing effects of alcohol are associated with increased AMPAR phosphorylation at GluA1 S831, which is indicative of facilitated receptor activity. Operant alcohol self-administration increased pGluA1 S831 IR in subregions of the amygdala (BLA and CeA) and nucleus accumbens (AcbC) of genetically selected alcohol-preferring P-rats. These results led us to pharmacologically target AMPARs in the CeA and AcbC to assess functional involvement of enhanced AMPAR activity in potentiating alcohol reinforcement. Pharmacological enhancement of AMPAR activity, via local infusion of aniracetam in the CeA, but not AcbC, escalated alcohol self-administration. Moreover, reinforcer specificity was confirmed as intra-CeA aniracetam did not alter self-administration of the non-drug reward, sucrose. Finally, enhanced AMPAR-mediated facilitation of alcohol reinforcement was dependent on CaMKII as site-specific pretreatment with the CaMKII inhibitor m-AIP in the CeA blocked aniracetam-induced increases in alcohol self-administration. These data suggest that alcohol-induced upregulation of CaMKII-dependent AMPAR activity in the CeA may underlie the escalated positive reinforcing effects of alcohol that are required for the development of addiction (Wise and Koob, 2014).
AMPAR activity is enhanced by several molecular processes, including phosphorylation at GluA1 S831 (Diaz, 2010; Partin et al., 1996; Pei et al., 2009). Our data show that operant alcohol self-administration significantly increased GluA1 S831 phosphorylation in the BLA, CeA and AcbC relative to sucrose self-administering controls. This observation is highly significant given that GluA1 S831 phosphorylation is required for long-term potentiation (LTP; a molecular correlate of learning and memory) and facilitates trafficking of AMPARs to synaptic sites within the plasma membrane (Barria et al., 1997b; Esteban et al., 2003); suggesting that chronic alcohol self-administration may potentiate synaptic strength within these two well-characterized brain regions that regulate drug-taking and seeking behaviors. Moreover, given the prominent role of AMPAR GluA1 activity in learning, memory, and motivational processes (Crombag et al., 2008; Maren, 2005), it was important to compare potential alcohol-induced changes in GluA1 S831 phosphorylation to a behaviorally-matched control group that experienced the same complex operant conditioning and ongoing motor activity. Thus, these data show that alcohol increases AMPAR GluA1 phosphorylation (activation) to a relatively greater extent than the non-drug highly palatable reinforcer sucrose, but do not address potential sucrose-only or learning-only based effects.
Changes in phosphorylated protein may reflect altered receptor expression. This seems unlikely, however, since no significant changes were observed in total GluA1 immunoreactivity after operant alcohol self-administration (Table 2). These results contrast with studies showing upregulation of total GluA1 in the nucleus accumbens after home-cage alcohol drinking (Ary et al., 2012; Neasta et al., 2010). This difference may reflect effects of higher doses of alcohol on total GluA1 protein expression following home-cage consumption as compared to the present operant methods that resulted in lower doses. It is also possible that total GluA1 expression was upregulated in the sucrose-trained animals in our study since GluA1 subunit expression/trafficking is altered by sucrose intake (Peng et al., 2011), which could mask alcohol-induced changes in total GluA1 expression when compared to sucrose. Regardless, alcohol self-administering P-rats exhibited a greater degree of GluA1 S831 phosphorylation than parallel sucrose controls, which provides novel evidence of low-dose operant alcohol-mediated alterations in AMPAR activation in the amygdala and nucleus accumbens in a genetic model of high alcohol preference.
Given the prominent role of upregulated glutamate activity in the etiology of alcohol addiction (Gass et al., 2011b; Koob et al., 1998; Kryger and Wilce, 2010; McCool et al., 2010; Stuber et al., 2008), we hypothesized that enhanced AMPR activity in these reward-related brain regions might increase operant alcohol self-administration. Site-specific infusion of the AMPAR positive modulator aniracetam (1 μg) in the CeA increased operant alcohol self-administration indicating that heightened glutamate signaling at AMPARs functionally enhances the reinforcing effects of alcohol. Furthermore, these data suggest that enhanced AMPAR activity in the CeA might have contributed to our previous finding that systemic administration of aniracetam facilitates alcohol self-administration (Cannady et al., 2013). Interestingly, intra-CeA aniracetam produced an increase in alcohol-reinforced responding at the low dose but failed to alter performance at a single higher dose. This data pattern is consistent with our previous findings following systemically administered aniracetam (Cannady et al., 2013), and other studies showing inverted U-shaped dose-response function by aniracetam (Pizzi et al., 1993), which may indicate off-target effects of high doses. Overall, these are the first results to show that enhanced AMPAR activity in the CeA potentiates the positive reinforcing effects of alcohol.
Pharmacological studies have shown that AMPARs modulate alcohol reinforcement and seeking behavior (Backstrom and Hyytia, 2004; Sanchis-Segura et al., 2006; Stephens and Brown, 1999; Wang et al., 2012) and we recently showed that AMPAR activity in the amygdala is required for alcohol positive reinforcement in mice (Salling et al., 2014). To our knowledge, however, this is the first study to show potentiation of alcohol reinforcement via site-specific activation of the amygdala. Consistent with this result, recent evidence shows that potentiating activity of a specific amygdala-accumbens circuit via optogenetic stimulation drives motivated behavior as evidenced by increases in nose-poke-contingent self-stimulation (Stuber et al., 2011). Given this evidence, it is plausible that potentiation of amygdala AMPAR activity could alter non-drug reinforcement. However, sucrose self-administration was not altered by intra-CeA aniracetam at doses as high as 6 µg/side, which supports prior evidence that enhanced glutamate activity at AMPARs does not alter non-drug reinforcement (Cannady et al., 2013). Similarly, the effective dose of aniracetam (1 µg/side) did not alter locomotor activity, indicating the absence of nonspecific motor activation. Overall, these data suggest that activation of AMPARs in the CeA potentiates the positive reinforcing effects of alcohol, which results in escalated intake. It is important to note, however, that centrally administered drugs can diffuse to adjacent nuclei within or between brain region (Perez de la Mora et al., 2006), which may have engaged the lateral amygdala or other proximal structures.
AMPARs are widely expressed in the mammalian brain (Petralia and Wenthold, 1992) and may mediate alcohol reinforcement in striatal regions (Wang et al., 2012); however, aniracetam infusion in the AcbC had no statistically significant effect on total alcohol-reinforced responses, suggesting a relatively greater mechanistic role of the amygdala. This lack of effect in the nucleus accumbens is somewhat surprising given the well-established role for glutamate activity in this region to modulate alcohol reinforcement. Indeed, intra-accumbens blockade of NMDA and mGluR5 receptors has been shown to attenuate alcohol self-administration (Besheer et al., 2010; Gass and Olive, 2009; Rassnick et al., 1992). It is not clear if varied AMPAR expression between the amygdala and nucleus accumbens contributed to the differential effect of aniracetam on alcohol self-administration. However, this is unlikely as an intra-AcbC aniracetam dose six times higher than that of the effective dose in the amygdala was ineffective at changing behavior. That said, aniracetam infusion in the AcbC produced a dose-dependent trend for changes in alcohol-reinforced responding as evidenced by small increases and decreases both in total responding and response rate throughout the session (see Supplementary Results). Based on these trends in behavior, and the observed alcohol-induced increase in pGluA1 S831 IR in the AcbC, an alternative explanation is related to microinjection site. Several lines of evidence suggest discrete roles for each accumbens subregion (core vs shell) in mediating the rewarding properties of alcohol (Chaudhri et al., 2010). Thus, future experimentation examining potentiated AMPAR signaling in the shell may show different effects on alcohol self-administration.
A highly significant finding of this study is that AMPAR-mediated escalation of alcohol self-administration in the CeA was dependent on CaMKII. We have shown that operant alcohol self-administration increases phosphorylation of GluA1 S831 and CaMKIIα T286, and that inhibition of AMPAR or CaMKII activity in the amygdala decreases self-administration (Salling et al., 2014). It was unknown, however, if AMPAR-mediated potentiation of alcohol self-administration requires CaMKII signaling. To address this question, we first evaluated effects of amygdala infusion of the cell permeable CaMKII peptide inhibitor m-AIP on operant alcohol self-administration. In agreement with prior results in mice (Salling et al., 2014), m-AIP dose-dependently decreased alcohol self-administration in P-rats. Then, we pretreated rats with a behaviorally inactive dose of m-AIP (10 µg/side) in combination with the effective dose of aniracetam (1 µg/side) in the CeA. m-AIP blocked the aniracetam-induced increase in operant alcohol self-administration and reduced responding and intake. Although it is unclear why alcohol self-administration following the drug combination was reduced below control levels, these data provide evidence that AMPAR-mediated facilitation of alcohol reinforcement by aniracetam functions through a CaMKII-dependent mechanism within the amygdala.
Importantly, when taken together with prior data (Salling et al., 2014), these results show bidirectional effects on alcohol self-administration by AMPAR activation, or inhibition, in the amygdala, which strongly supports mechanistic regulation. We propose that alcohol self-administration increases glutamate levels in the amygdala after chronic alcohol exposure (Roberto et al., 2004) leading to phosphorylation of CaMKIIα (Salling et al., 2014) and subsequent activation of AMPARs by CaMKIIα at S831 (Barria et al., 1997a). Since CaMKII-dependent AMPAR activity underlies synaptic plasticity (Barria et al., 1997b), this suggests that moderate alcohol use may mimic plasticity-inducing events in the amygdala (Christian et al., 2012). Moreover, since CaMKII-dependent AMPAR activity is required for behavioral plasticity, such as learning and memory (Crombag et al., 2008), these plasticity-like adaptations may promote a gain in alcohol reinforcement learning and function, which supports the development of addiction (Wise and Koob, 2014). Overall, these data suggest that targeted reductions in AMPAR or CaMKII activity may be of therapeutic value for treatment of alcohol use disorders without side effects on non-drug reward processes.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported, in part, by grants R37 AA014983 and P60 AA11605 (CWH), F31 AA21063 (RC), and R01 AA019682 (JB) from the National Institute on Alcohol Abuse and Alcoholism and by the Bowles Center for Alcohol Studies at the University of North Carolina at Chapel Hill.
Footnotes
DISCLOSURE/CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Agoglia AE, Holstein SE, Reid G, Hodge CW. CaMKIIalpha-GluA1 Activity Underlies Vulnerability to Adolescent Binge Alcohol Drinking. Alcoholism, clinical and experimental research. 2015;39:1680–1690. doi: 10.1111/acer.12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- Ary AW, Cozzoli DK, Finn DA, Crabbe JC, Dehoff MH, Worley PF, Szumlinski KK. Ethanol up-regulates nucleus accumbens neuronal activity dependent pentraxin (Narp): implications for alcohol-induced behavioral plasticity. Alcohol. 2012 doi: 10.1016/j.alcohol.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Ionotropic glutamate receptor antagonists modulate cue-induced reinstatement of ethanol-seeking behavior. Alcohol Clin Exp Res. 2004;28:558–565. doi: 10.1097/01.alc.0000122101.13164.21. [DOI] [PubMed] [Google Scholar]
- Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J Biol Chem. 1997a;272:32727–32730. doi: 10.1074/jbc.272.52.32727. [DOI] [PubMed] [Google Scholar]
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997b;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Cannady R, Grondin JJ, Hodge CW. Intra-amygdala inhibition of ERK(1/2) potentiates the discriminative stimulus effects of alcohol. Behav Brain Res. 2012;228:398–405. doi: 10.1016/j.bbr.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Grondin JJ, Cannady R, Sharko AC, Faccidomo S, Hodge CW. Metabotropic glutamate receptor 5 activity in the nucleus accumbens is required for the maintenance of ethanol self-administration in a rat genetic model of high alcohol intake. Biol Psychiatry. 2010;67:812–822. doi: 10.1016/j.biopsych.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Stevenson RA, Hodge CW. mGlu5 receptors are involved in the discriminative stimulus effects of self-administered ethanol in rats. European journal of pharmacology. 2006;551:71–75. doi: 10.1016/j.ejphar.2006.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxall AR, Garthwaite J. Synaptic excitation mediated by AMPA receptors in rat cerebellar slices is selectively enhanced by aniracetam and cyclothiazide. Neurochem Res. 1995;20:605–609. doi: 10.1007/BF01694543. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Cannady R, Fisher KR, Durant B, Besheer J, Hodge CW. Enhanced AMPA receptor activity increases operant alcohol self-administration and cue-induced reinstatement. Addict Biol. 2013;18:54–65. doi: 10.1111/adb.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannady R, Grondin JJ, Fisher KR, Hodge CW, Besheer J. Activation of group II metabotropic glutamate receptors inhibits the discriminative stimulus effects of alcohol via selective activity within the amygdala. Neuropsychopharmacology. 2011;36:2328–2338. doi: 10.1038/npp.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Schairer WW, Janak PH. Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:783–791. doi: 10.1038/npp.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian DT, Alexander NJ, Diaz MR, Robinson S, McCool BA. Chronic intermittent ethanol and withdrawal differentially modulate basolateral amygdala AMPA-type glutamate receptor function and trafficking. Neuropharmacology. 2012;62:2430–2439. doi: 10.1016/j.neuropharm.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbran RJ, Brown AM. Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr Opin Neurobiol. 2004;14:318–327. doi: 10.1016/j.conb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Sutton JM, Takamiya K, Lee HK, Holland PC, Gallagher M, Huganir RL. A necessary role for GluR1 serine 831 phosphorylation in appetitive incentive learning. Behav Brain Res. 2008;191:178–183. doi: 10.1016/j.bbr.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz E. Regulation of AMPA receptors by transmembrane accessory proteins. Eur J Neurosci. 2010;32:261–268. doi: 10.1111/j.1460-9568.2010.07357.x. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Faccidomo S, Reid G, Agoglia A, Ademola S, Hodge C. CaMKII inhibition in the prefrontal cortex specifically increases the positive reinforcing effects of sweetened alcohol in C57BL/6J mice. Behav Brain Res under revision. 2015 doi: 10.1016/j.bbr.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Role of protein kinase C epsilon (PKCvarepsilon) in the reduction of ethanol reinforcement due to mGluR5 antagonism in the nucleus accumbens shell. Psychopharmacology (Berl) 2009;204:587–597. doi: 10.1007/s00213-009-1490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Sinclair CM, Cleva RM, Widholm JJ, Olive MF. Alcohol-seeking behavior is associated with increased glutamate transmission in basolateral amygdala and nucleus accumbens as measured by glutamate-oxidase-coated biosensors. Addiction biology. 2011a;16:215–228. doi: 10.1111/j.1369-1600.2010.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Sinclair CM, Cleva RM, Widholm JJ, Olive MF. Alcohol-seeking behavior is associated with increased glutamate transmission in basolateral amygdala and nucleus accumbens as measured by glutamate-oxidase-coated biosensors. Addict Biol. 2011b;16:215–228. doi: 10.1111/j.1369-1600.2010.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, Ramachandra VS, Knackstedt LA, Becker HC. Repeated cycles of chronic intermittent ethanol exposure increases basal glutamate in the nucleus accumbens of mice without affecting glutamate transport. Front Pharmacol. 2015;6:27. doi: 10.3389/fphar.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Aiken AS. Discriminative stimulus function of ethanol: role of GABAA receptors in the nucleus accumbens. Alcohol Clin Exp Res. 1996;20:1221–1228. doi: 10.1111/j.1530-0277.1996.tb01116.x. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, Besheer J, Schroeder JP. The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology (Berl) 2006;183:429–438. doi: 10.1007/s00213-005-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudmon A, Schulman H. Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem. 2002;71:473–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H. A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochem Biophys Res Commun. 1995;212:806–812. doi: 10.1006/bbrc.1995.2040. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Matthews DB, Gause L, Morrow AL, Overstreet DH. P rats develop physical dependence on alcohol via voluntary drinking: changes in seizure thresholds, anxiety, and patterns of alcohol drinking. Alcohol Clin Exp Res. 2000;24:278–284. [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, Merlo-Pich E, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryger R, Wilce PA. The effects of alcoholism on the human basolateral amygdala. Neuroscience. 2010;167:361–371. doi: 10.1016/j.neuroscience.2010.01.061. [DOI] [PubMed] [Google Scholar]
- Lankford MF, Roscoe AK, Pennington SN, Myers RD. Drinking of high concentrations of ethanol versus palatable fluids in alcohol-preferring (P) rats: valid animal model of alcoholism. Alcohol. 1991;8:293–299. doi: 10.1016/0741-8329(91)90417-u. [DOI] [PubMed] [Google Scholar]
- Lester D, Freed EX. Criteria for an animal model of alcoholism. Pharmacology, biochemistry, and behavior. 1973;1:103–107. doi: 10.1016/0091-3057(73)90062-2. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L, McBride WJ, Waller MB. Progress toward a voluntary oral consumption model of alcoholism. Drug Alcohol Depend. 1979;4:45–60. doi: 10.1016/0376-8716(79)90040-1. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem. 1997;272:32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Masuoka T, Saito S, Kamei C. Participation of hippocampal ionotropic glutamate receptors in histamine H(1) antagonist-induced memory deficit in rats. Psychopharmacology (Berl) 2008;197:107–114. doi: 10.1007/s00213-007-1013-7. [DOI] [PubMed] [Google Scholar]
- McCool BA, Christian DT, Diaz MR, Lack AK. Glutamate plasticity in the drunken amygdala: the making of an anxious synapse. Int Rev Neurobiol. 2010;91:205–233. doi: 10.1016/S0074-7742(10)91007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neasta J, Ben Hamida S, Yowell Q, Carnicella S, Ron D. Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders. Proc Natl Acad Sci U S A. 2010;107:20093–20098. doi: 10.1073/pnas.1005554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizaki T, Matsumura T. The aniracetam metabolite 2-pyrrolidinone induces a long-term enhancement in AMPA receptor responses via a CaMKII pathway. Brain Res Mol Brain Res. 2002;98:130–134. doi: 10.1016/s0169-328x(01)00331-x. [DOI] [PubMed] [Google Scholar]
- Partin KM, Fleck MW, Mayer ML. AMPA receptor flip/flop mutants affecting deactivation, desensitization, and modulation by cyclothiazide, aniracetam, and thiocyanate. J Neurosci. 1996;16:6634–6647. doi: 10.1523/JNEUROSCI.16-21-06634.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei W, Huang Z, Wang C, Han Y, Park JS, Niu L. Flip and flop: a molecular determinant for AMPA receptor channel opening. Biochemistry. 2009;48:3767–3777. doi: 10.1021/bi8015907. [DOI] [PubMed] [Google Scholar]
- Peng XX, Ziff EB, Carr KD. Effects of food restriction and sucrose intake on synaptic delivery of AMPA receptors in nucleus accumbens. Synapse. 2011 doi: 10.1002/syn.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez de la Mora M, Lara-Garcia D, Jacobsen KX, Vazquez-Garcia M, Crespo-Ramirez M, Flores-Gracia C, Escamilla-Marvan E, Fuxe K. Anxiolytic-like effects of the selective metabotropic glutamate receptor 5 antagonist MPEP after its intra-amygdaloid microinjection in three different non-conditioned rat models of anxiety. Eur J Neurosci. 2006;23:2749–2759. doi: 10.1111/j.1460-9568.2006.04798.x. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992;318:329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Pizzi M, Fallacara C, Arrighi V, Memo M, Spano PF. Attenuation of excitatory amino acid toxicity by metabotropic glutamate receptor agonists and aniracetam in primary cultures of cerebellar granule cells. J Neurochem. 1993;61:683–689. doi: 10.1111/j.1471-4159.1993.tb02173.x. [DOI] [PubMed] [Google Scholar]
- Rao Y, Xiao P, Xu S. Effects of intrahippocampal aniracetam treatment on Y-maze avoidance learning performance and behavioral long-term potentiation in dentate gyrus in rat. Neurosci Lett. 2001;298:183–186. doi: 10.1016/s0304-3940(00)01744-4. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Pulvirenti L, Koob GF. Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacology (Berl) 1992;109:92–98. doi: 10.1007/BF02245485. [DOI] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J Neurosci. 2004;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salling MC, Faccidomo SP, Li C, Psilos K, Galunas C, Spanos M, Agoglia AE, Kash TL, Hodge CW. Moderate Alcohol Drinking and the Amygdala Proteome: Identification and Validation of Calcium/Calmodulin Dependent Kinase II and AMPA Receptor Activity as Novel Molecular Mechanisms of the Positive Reinforcing Effects of Alcohol. Biological psychiatry. 2014 doi: 10.1016/j.biopsych.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C, Borchardt T, Vengeliene V, Zghoul T, Bachteler D, Gass P, Sprengel R, Spanagel R. Involvement of the AMPA receptor GluR-C subunit in alcohol-seeking behavior and relapse. J Neurosci. 2006;26:1231–1238. doi: 10.1523/JNEUROSCI.4237-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Olive F, Koenig H, Hodge CW. Intra-amygdala infusion of the NPY Y1 receptor antagonist BIBP 3226 attenuates operant ethanol self-administration. Alcohol Clin Exp Res. 2003;27:1884–1891. doi: 10.1097/01.ALC.0000098875.95923.69. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Overstreet DH, Hodge CW. The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology. 2005;179:262–270. doi: 10.1007/s00213-005-2175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Spanos M, Stevenson JR, Besheer J, Salling M, Hodge CW. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP. Neuropharmacology. 2008;55:546–554. doi: 10.1016/j.neuropharm.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggins GR, Martin G, Roberto M, Nie Z, Madamba S, De Lecea L. Glutamatergic transmission in opiate and alcohol dependence. Ann N Y Acad Sci. 2003;1003:196–211. doi: 10.1196/annals.1300.012. [DOI] [PubMed] [Google Scholar]
- Spanos M, Besheer J, Hodge CW. Increased sensitivity to alcohol induced changes in ERK Map kinase phosphorylation and memory disruption in adolescent as compared to adult C57BL/6J mice. Behav Brain Res. 2012;230:158–166. doi: 10.1016/j.bbr.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DN, Brown G. Disruption of operant oral self-administration of ethanol, sucrose, and saccharin by the AMPA/kainate antagonist, NBQX, but not the AMPA antagonist, GYKI 52466. Alcohol Clin Exp Res. 1999;23:1914–1920. [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Hahn J, Cho SL, Guillory A, Bonci A. Voluntary ethanol intake enhances excitatory synaptic strength in the ventral tegmental area. Alcohol Clin Exp Res. 2008;32:1714–1720. doi: 10.1111/j.1530-0277.2008.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyklicky L, Jr., Patneau DK, Mayer ML. Modulation of excitatory synaptic transmission by drugs that reduce desensitization at AMPA/kainate receptors. Neuron. 1991;7:971–984. doi: 10.1016/0896-6273(91)90342-w. [DOI] [PubMed] [Google Scholar]
- Wang J, Ben Hamida S, Darcq E, Zhu W, Gibb SL, Lanfranco MF, Carnicella S, Ron D. Ethanol-mediated facilitation of AMPA receptor function in the dorsomedial striatum: implications for alcohol drinking behavior. J Neurosci. 2012;32:15124–15132. doi: 10.1523/JNEUROSCI.2783-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weight FF, Lovinger DM, White G. Alcohol inhibition of NMDA channel function. Alcohol Alcohol Suppl. 1991;1:163–169. [PubMed] [Google Scholar]
- Winder DG, Egli RE, Schramm NL, Matthews RT. Synaptic plasticity in drug reward circuitry. Curr Mol Med. 2002;2:667–676. doi: 10.2174/1566524023361961. [DOI] [PubMed] [Google Scholar]
- Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:254–262. doi: 10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. Regulation of AMPA receptor trafficking in the nucleus accumbens by dopamine and cocaine. Neurotox Res. 2010;18:393–409. doi: 10.1007/s12640-010-9176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward JJ, Ron D, Winder D, Roberto M. From blue states to up states: a regional view of NMDA-ethanol interactions. Alcohol Clin Exp Res. 2006;30:359–367. doi: 10.1111/j.1530-0277.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Abdullah JM. The role of GluA1 in central nervous system disorders. Rev Neurosci. 2013;24:499–505. doi: 10.1515/revneuro-2013-0021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.