Abstract
Background & Aim
Recent basic mechanistic studies found that proton pump inhibitors (PPIs) or histamine antagonists inhibited multiple pathways involved in non-alcoholic fatty liver disease (NAFLD) development. The aim of this study was to investigate an association between PPIs or H1/H2-receptor antagonists (H1RAs/H2RAs) use and NAFLD prevalence in the general US population.
Methods
We conducted a cross-sectional analysis of data from the National Health and Nutrition Examination Survey, 2001 – 2006. We included 10,398 adults aged 20 – 74 years who had alanine aminotransferase (ALT) data; of those, 2,058 were identified as having NAFLD and 8,340 as controls. PPIs or H1RAs/H2RAs use was defined as use of prescription medications in the preceding month. The length of use was categorized as ≤ 60 days and > 60 days. NAFLD was defined as elevated serum aminotransferases without any indication of other causes of chronic liver disease.
Results
In the multivariate unconditional logistic regression analysis, H2RAs use was inversely associated with prevalent NAFLD (odds ratio [OR] = 0.43, 95% confidence interval [CI] 0.18 – 0.99), a finding that was primarily limited to men (OR = 0.18, 95% CI 0.04 – 0.79) and those with insulin resistance (OR = 0.22, 95% CI 0.05 – 0.95). However, no significant associations were found between PPIs or H1RAs use and prevalent NAFLD.
Conclusion
These findings, from the first human study to investigate an association of PPIs or H1RAs/H2RAs use with NAFLD, suggest that H2RAs use may be associated with a lower prevalence of NAFLD, primarily among men with insulin resistance.
Keywords: histamine antagonists, proton pump inhibitor, NAFLD
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is one of the most common chronic liver diseases worldwide. It comprises of the spectrum of fatty liver disease in individuals without significant alcohol consumption, ranging from simple steatosis to steatohepatitis (NASH) and cirrhosis1. While simple steatosis is quite benign with slow histological progression, NASH is associated with hepatic injury and inflammation; which can lead to the development of fibrosis and cirrhosis1.
The molecular mechanism triggering NASH is poorly understood. Histologically, NASH is manifested by inflammatory cell infiltration. Sustained inflammation in the liver is critical in the progression of NAFLD. Compelling data indicates that inflammatory cells play a key role in the initiation and perpetuation of the inflammatory response and the progression of liver disease in NASH2-4.
A histamine antagonist consists of two common types of drugs: histamine H1-receptor antagonists (H1RAs) and histamine H2-receptor antagonists (H2RAs). H1RAs are commonly used to treat allergic reactions5. H2RAs act on the H2 histamine receptors commonly found at the parietal cells on the gastric mucosa6. Blocking this receptor reduces gastric acid secretion6. Proton pump inhibitors (PPIs) are another class of drugs with potent inhibition of gastric acid secretion7. In addition to anti-allergic and anti-acid secretory effects, these medications have been found to have anti-oxidant properties and direct effects on inflammatory cells including monocytes that might prevent inflammation7-9. The anti-inflammatory properties of these commonly used medications might influence the inflammatory cascades within and outside respiratory and gastrointestinal tracts.
There have been no reported human studies that explored the association between anti-histamines or PPIs and NAFLD. The objective of this study was to investigate an association between anti-histamines or PPIs use and the prevalence of NAFLD in the US population using the data from the National Health and Nutrition Examination Survey (NHANES) 2001-2006.
METHODS
Study population
The data were obtained from three continuous cycles of the NHANES conducted between 2001 and 2006 using a complex, multistage, stratified, clustered, probability sample design to select a representative sample of the civilian, non-institutionalized US population. The three survey cycles of 13,248 participants included data interviews, physical examinations, and laboratory tests with blood and urine samples collection. Parameters were transformed according to the provided guidelines to make the data comparable between the cycles10. A detailed description of the survey and its sampling procedures are available elsewhere. The study was approved by the National Center for Health Statistics (NCHS) Ethics Review Board.
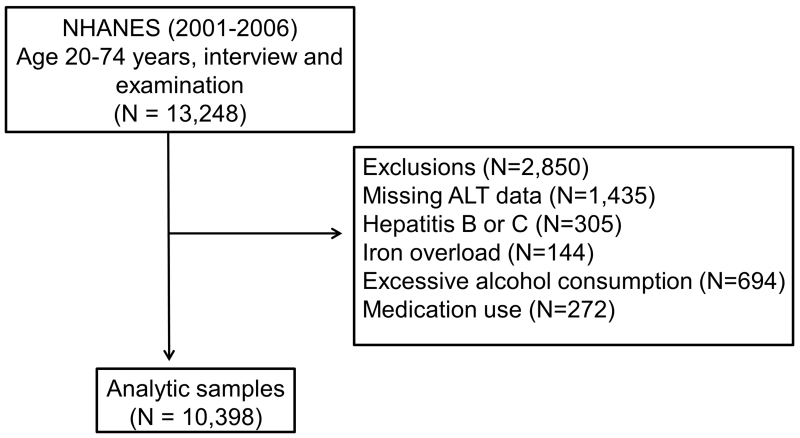
The inclusion criteria were age 20 – 74 years old and the availability of complete demographic, social, clinical, and laboratory data. We excluded 1,435 subjects without alanine aminotransferase (ALT) data and 1,415 subjects with conditions other than NAFLD that might cause serum aminotransferases elevation. These conditions included excessive alcohol consumption, viral hepatitis, iron overload, and use of medications associated with hepatotoxicity (androgens, antivirals medications, antifungals medications, nitrofuratoin, phenytoin, sulfonamides, trazadone, or tetracycline). A total of 10,398 participants were eligible for analysis (Figure 1).
Figure 1. Schematic diagram on the selection of the study participants.
Data collection
Standardized questionnaires were used to obtain self-reported data on sex, age, race or ethnicity, education, income, smoking, alcohol consumption, physical activity, medical conditions, and drug use11. Body mass index was calculated based on the standardized measurements of height and weight. Systolic and diastolic blood pressure was considered the mean of six or fewer measurements obtained at the household interview (maximum of three) and the physical examination (maximum of three).
Race or ethnicity was categorized as non-Hispanic whites, non-Hispanic blacks, Mexican-American, other Hispanics, and other, which included Aleut, Eskimo, American Indian, Asian or Pacific Islander. Education, according to completed years of schooling, was categorized as ≤ 8 years, 9 – 12 years, and ≥ 12 years. Economic status, according to the subject’s household income for the previous year, was categorized as ≤ $15,000, $15,001 – $25,000, and ≥ $25,000.
Smoking status was categorized as never, former, and current. Current smokers were defined as persons who reported having smoked > 100 cigarettes during their lifetime and who currently smoke some days or every day. Former smokers were those who reported having smoked > 100 cigarettes during their lifetime but did not smoke at the time of interview. Never smokers were those who smoked < 100 cigarettes during their lifetime12. Excessive alcohol consumption was defined as ≥1 drink/day for women and ≥2 drinks/day for men13. Participants were categorized as sedentary if they chose the option: “you sit during the day and do not walk about very much”.
Proton pump inhibitors (PPIs) or H1/H2-receptor antagonists (H1RAs/H2RAs) use was defined as the use of these medications during the month prior to the interview. The length of use was categorized as ≤ 60 days and > 60 days.
Insulin resistance was defined as a homeostasis of model assessment score (HOMA ) > 3.014; elevated serum aminotransferases were defined as ALT > 40 U/L or aspartate aminotransferase (AST) > 37 U/L in men, and ALT or AST > 31 U/ L in women; and transferrin saturation was considered as elevated when the values were ≥ 50%15.
Definitions of NAFLD and controls
NAFLD was defined as the presence of elevated serum aminotransferases without any indication of other causes of chronic liver disease such as viral hepatitis infection (defined as a positive HCV RNA or HBsAg test), iron overload, or excessive alcohol consumption. This definition is in accordance with our previous study and previous publications on NAFLD using NHANES dataset16-18. Controls were defined as participants with normal liver enzymes and no evidence of chronic liver disease.
Statistical analyses
Sample weights were used to account for nonresponse and unequal probabilities of selection. Stratum and sampling units accounted for the complex survey design using Taylor series linearization. Demographic and clinical differences between study participants with and without prevalent NAFLD were compared using the student t-test or chi-square test, as appropriate. Unconditional multivariate logistic regression was used to assess the association between PPIs or H1RAs/H2RAs and NAFLD. Potential confounders, which included age, sex, ethnicity, socioeconomic status, BMI, physical activity, smoking status, diabetes or insulin resistance, hypertension or systolic/diastolic blood pressure, hypercholesterolemia or serum total cholesterol, triglycerides, high-density lipoprotein (HDL), and low-density lipoprotein (LDL), were assessed in stepwise regression models. The final models included for age, sex, ethnicity, socioeconomic status, waist circumference, physical activity, smoking status, insulin resistance (IR), systolic/diastolic blood pressure, and hypercholesterolemia as covariates. We also conducted analyses stratified on other risk factors, including sex, age, insulin resistance, hypertension, hypercholesterolemia, body mass index (BMI), and physical activity. All analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC, USA). A p-value ≤ 0.05 (2-sided) was considered statistically significant.
RESULTS
Demographic and clinical characteristics of the study participants
Of the 10,398 participants included for the main analyses, 2,058 were identified as having NAFLD and 8,340 as controls. Selected characteristics of the study participants, according to NAFLD status, are summarized in Table 1. On average, participants with NAFLD were nearly 1.5 years younger and had higher systolic and diastolic blood pressures; higher serum triglycerides and total and LDL-cholesterol and lower HDL-cholesterol levels; and higher circulating glycated hemoglobin, liver enzymes, and uric acid levels. Those with NAFLD were more likely to be men, obese, and Mexican-American. They had less education with higher prevalence of insulin resistance. Participants with NAFLD were less likely to take H1RAs/H2RAs and more likely to take PPIs in the prior month.
Table 1. Selected characteristics of the study participants, according to NAFLD prevalence; NHANES, 2001 – 2006*.
| Controls (n = 8,340) |
NAFLD Cases (n = 2,058) |
P- value£ |
|
|---|---|---|---|
| Age (yrs.) | 44.8 ± 0.2 | 43.2 ± 0.3 | < 0.01 |
| Men (%) | 39.8 ± 0.5 | 66.2 ± 1.0 | < 0.01 |
| Race or ethnicity (%) | |||
| Mexican-American | 21.4 ± 0.4 | 30.4 ± 1.0 | < 0.01 |
| Other Hispanic | 3.5 ± 0.2 | 5.3 ± 0.5 | |
| Non-Hispanic White | 48.6 ± 0.5 | 45.5 ± 1.1 | |
| Non-Hispanic Black | 22.4 ± 0.5 | 15.0 ± 0.8 | |
| Other | 4.1 ± 0.2 | 3.7 ± 0.4 | |
| Annual household income (%) | |||
| ≤ $15,000 | 13.9 ± 0.4 | 11.9 ± 0.7 | 0.03 |
| $15,001 – $25,000 | 15.1 ± 0.4 | 14.4 ± 0.8 | |
| > $25,000 | 71.0 ± 0.5 | 73.7 ± 1.0 | |
| Education (%) | |||
| ≤ 8 years | 11.4 ± 0.3 | 14.8 ± 0.8 | < 0.01 |
| 9 – 12 years | 15.2 ± 0.4 | 14.9 ± 0.8 | |
| > 12 years | 73.3 ± 0.5 | 70.3 ± 1.0 | |
| Body mass index (kg/m2) | 28.5 ± 0.1 | 30.3 ± 0.1 | < 0.01 |
| Waist circumference (cm) | 96.7 ± 0.2 | 102.3 ± 0.3 | < 0.01 |
| Physical activity (Sedentary) (%) | 23.3 ± 0.5 | 21.6 ± 0.9 | 0.10 |
| Smoking status (%) | |||
| Never smokers | 54.8 ± 0.5 | 53.6 ± 1.1 | 0.07 |
| Former smokers | 23.0 ± 0.5 | 25.4 ± 1.0 | |
| Current smokers | 22.1 ± 0.5 | 21.0 ± 0.9 | |
| Diabetes†(%) | 9.9 ± 0.3 | 8.6 ± 0.6 | 0.09 |
| Hypertension†(%) | 28.2 ± 0.5 | 28.6 ± 1.0 | 0.78 |
| Hypercholesterolaemia†(%) | 39.3 ± 0.7 | 44.9 ± 1.4 | < 0.01 |
| Insulin resistance ¥ (%) | 32.0 ± 0.7 | 56.2 ± 1.6 | < 0.01 |
| Glycated hemoglobin (%) | 5.5 ± 0.01 | 5.7 ± 0.02 | < 0.01 |
| Systolic BP (mm Hg) | 122.0 ± 0.2 | 123.3 ± 0.4 | < 0.01 |
| Diastolic BP (mm Hg) | 70.0 ± 0.1 | 72.9 ± 0.3 | < 0.01 |
| Serum triglycerides (mmol/L) | 1.6 ± 0.02 | 2.1 ± 0.06 | < 0.01 |
| Serum total cholesterol (mmol/L) | 5.2 ± 0.01 | 5.4 ± 0.03 | < 0.01 |
| Serum LDL-cholesterol (mmol/L) | 3.0 ± 0.01 | 3.2 ± 0.04 | < 0.01 |
| Serum HDL cholesterol (mmol/L) | 1.4 ± 0.005 | 1.2 ± 0.008 | < 0.01 |
| Serum alanine aminotransferase (U/L) |
19.4 ± 0.1 | 47.0 ± 1.0 | < 0.01 |
| Serum aspartate aminotransferase (U/L) |
21.4 ± 0.1 | 35.9 ± 0.5 | < 0.01 |
| Serum γ-glutamyltransferase (U/L) | 21.8 ± 0.2 | 48.4 ± 1.5 | < 0.01 |
| Serum uric acid (μmol/L) | 303.1 ± 0.9 | 345.7 ± 1.9 | < 0.01 |
| PPIs use (%) | 6.1 ± 0.3 | 6.4 ± 0.5 | 0.59 |
| H2RAs use (%) | 1.8 ± 0.1 | 1.7 ± 0.3 | 0.91 |
| H1RAs use (%) | 3.4 ± 0.2 | 2.7 ± 0.4 | 0.14 |
Values are percentages ± standard errors (SE) for categorical variables and means ± SE for continuous variables
From Student t-test for continuous variables and chi square test for categorical variables
Self-reported doctor diagnosis and medication use
Insulin resistance was defined as a homeostasis of model assessment score, or HOMA, > 3.0
Associations of H1RAs/H2RAs and PPIs and the prevalence of NAFLD
The results are summarized in Tables 2 and 3. After adjustment for potential confounders, the use of H2RAs was significantly associated with lower prevalence of NAFLD by 57% regardless of the length of use, when compared to those who did not take H2RAs. The decreasing in the prevalence of NAFLD among H2RA users was only observed in men (OR 0.18, 95%CI 0.04-0.79) and those with insulin resistance (OR 0.22, 95%CI 0.05-0.95). There was no difference in the prevalent NAFLD among H1RA and PPI users (Tables 2, 4 and 5).
Table 2. Association of proton-pump inhibitors (PPIs) or H1/H2-receptor antagonists (H1RAs/H2RAs) use with prevalent NAFLD; NHANES, 2001 – 2006.
| Crude OR (95% CI) | Adjusted¥ OR (95% CI) |
|
|---|---|---|
| PPIs use | ||
| No | 1.00 | 1.00 |
| Yes | 1.06 (0.87 – 1.29) | 0.97 (0.67 – 1.39) |
|
Length of
use |
||
| Never | 1.00 | 1.00 |
| ≤ 60 days | 1.31 (0.77 – 2.23) | 0.86 (0.24 – 3.08) |
| > 60 days | 1.03 (0.83 – 1.27) | 0.98 (0.67 – 1.43) |
| Ptrend* = 0.70 | ||
| H2RAs use | ||
| No | 1.00 | 1.00 |
| Yes | 0.98 (0.68 – 1.41) | 0.43 (0.18 – 0.99) |
|
Length of
use |
||
| Never | 1.00 | 1.00 |
| ≤ 60 days | 1.74 (0.79 – 3.80) | 0.47 (0.05 – 3.97) |
| > 60 days | 0.85 (0.56 – 1.30) | 0.42 (0.16 – 1.09) |
| Ptrend* = 0.67 | ||
| H1RAs use | ||
| No | 1.00 | 1.00 |
| Yes | 0.80 (0.60 – 1.07) | 0.78 (0.46 – 1.31) |
|
Length of
use |
||
| Never | 1.00 | 1.00 |
| ≤ 60 days | 1.07 (0.57 – 2.03) | 0.44 (0.05 – 3.61) |
| > 60 days | 0.75 (0.54 – 1.04) | 0.81 (0.47 – 1.40) |
| Ptrend* = 0.10 | ||
Abbreviations: NAFLD, non-alcoholic fatty liver disease; NHANES, National Health and Nutrition Examination Survey; OR, odds ratio; CI, confidence interval; IR, insulin resistance
Cochran-Armitage trend test
Unconditional logistic regression model, adjusted for age, sex, ethnicity, socioeconomic status, waist circumference, physical activity, smoking status, insulin resistance (IR), systolic/diastolic blood pressure, and hypercholesterolemia
Table 3. Association of H2-receptor antagonists (H2RAs) use with prevalent NAFLD according to selected risk factors; NHANES, 2001 – 2006.
| Crude OR (95% CI) | Adjusted¥ OR (95% CI) | |
|---|---|---|
| Sex | ||
| Male | 0.88 (0.55 – 1.42) | 0.18 (0.04 – 0.79) |
| Female | 1.04 (0.57 – 1.92) | 0.78 (0.26 – 2.35) |
| Age | ||
| ≤ 60 yrs. | 1.03 (0.62 – 1.69) | 0.39 (0.11 – 1.38) |
| > 60 yrs. | 1.25 (0.72 – 2.17) | 0.46 (0.13 – 1.57) |
| BMI | ||
| < 25 | 2.20 (0.94 – 5.12) | 2.15 (0.53 – 8.75) |
| 25-30 | 0.93 (0.51 – 1.69) | 0.31 (0.07 – 1.37) |
| ≥ 30 | 0.66 (0.37 – 1.15) | 0.13 (0.02 – 1.05) |
| IR | ||
| No | 0.80 (0.34 – 1.88) | 0.72 (0.25 – 2.11) |
| Yes | 0.33 (0.13 – 0.86) | 0.22 (0.05 – 0.95) |
OR, odds ratio; CI, confidence interval; IR, insulin resistance
Unconditional logistic regression model, adjusted for PPIs and H1RAs use, age, sex, ethnicity, socioeconomic status, waist circumference, physical activity, smoking status, insulin resistance (IR), systolic/diastolic blood pressure, and hypercholesterolemia
Table 4. Association of H1-receptor antagonists (H1RAs) use with prevalent NAFLD according to selected risk factors; NHANES, 2001 – 2006.
| Crude OR (95% CI) | Adjusted¥ OR (95% CI) |
|
|---|---|---|
| Sex | ||
| Male | 1.12 (0.76 – 1.65) | 1.20 (0.62 – 2.32) |
| Female | 0.65 (0.40 – 1.06) | 0.34 (0.11 – 1.13) |
| Age | ||
| ≤ 60 yrs. | 0.74 (0.53 – 1.02) | 0.74 (0.39 – 1.40) |
| > 60 yrs. | 1.16 (0.63 – 2.13) | 1.05 (0.39 – 2.82) |
| BMI | ||
| < 25 | 0.48 (0.19 – 1.19) | 0.79 (0.17 – 3.62) |
| 25-30 | 0.94 (0.60 – 1.48) | 1.01 (0.46 – 2.23) |
| ≥ 30 | 0.78 (0.50 – 1.21) | 0.54 (0.24 – 1.24) |
| IR | ||
| No | 0.57 (0.28 – 1.13) | 0.83 (0.38 – 1.78) |
| Yes | 0.96 (0.55 – 1.69) | 0.77 (0.37 – 1.62) |
OR, odds ratio; CI, confidence interval; IR, insulin resistance
Unconditional logistic regression model, adjusted for PPIs and H2RAs use, age, sex, ethnicity, socioeconomic status, waist circumference, physical activity, smoking status, insulin resistance (IR), systolic/diastolic blood pressure, and hypercholesterolemia
Table 5. Association of proton-pump inhibitors (PPIs) use with prevalent NAFLD according to selected risk factors; NHANES, 2001 – 2006.
| Crude OR (95% CI) | Adjusted¥ OR (95% CI) | |
|---|---|---|
| Sex | ||
| Male | 0.85 (0.65 – 1.12) | 0.98 (0.59 – 1.62) |
| Female | 1.44 (1.08 – 1.93) | 0.98 (0.56 – 1.70) |
| Age | ||
| ≤ 60 yrs. | 1.34 (1.06 – 1.70) | 1.09 (0.67 – 1.77) |
| > 60 yrs. | 0.86 (0.59 – 1.26) | 0.75 (0.42 – 1.35) |
| BMI | ||
| < 25 | 1.30 (0.77 – 2.19) | 1.17 (0.48 – 2.90) |
| 25-30 | 0.80 (0.56 – 1.14) | 0.92 (0.49 – 1.75) |
| ≥ 30 | 1.07 (0.81 – 1.40) | 1.05 (0.62 – 1.78) |
| IR | ||
| No | 0.72 (0.44 – 1.17) | 0.76 (0.41 – 1.40) |
| Yes | 1.07 (0.75 – 1.53) | 1.10 (0.69 – 1.76) |
OR, odds ratio; CI, confidence interval; IR, insulin resistance
Unconditional logistic regression model, adjusted for H1RAs and H2RAs use, age, sex, ethnicity, socioeconomic status, waist circumference, physical activity, smoking status, insulin resistance (IR), systolic/diastolic blood pressure, and hypercholesterolemia
DISCUSSION
Our cross-sectional study results to investigate an association of H1RAs/H2RAs or PPIs use with NAFLD suggest that only the use of H2RAs was associated with a lower prevalence of NAFLD in the general US population, primarily among men and those with insulin resistance.
The pathogenesis of NAFLD and the progression to NASH are complex, involving a combination of lipid oxidation, oxidative stress, inflammatory cells as well as proinflammatory cytokines1, 16. Alterations in lipid metabolism drive the polarization of the Kupffer cells into the proinflammatory phenotype; which trigger the recruitment of inflammatory cells and the progression of underlying NAFLD 2. Several signaling cascades are also affected. Among them are the NFκB and the c-Jun-N terminal kinases (JNK). Their activation contribute to worsening steatosis and hepatic inflammation2.
Histamine antagonists and PPIs are two medication classes which are most commonly used in the United States19. There are several proposed molecular mechanisms underlying the possible effectiveness of histamine antagonists and PPIs against NAFLD. Histamine can influence numerous functions of the cells involved in the regulation of immune response including macrophages20. In fact, macrophages express histamine receptors and also secrete histamine, which can selectively recruit the major effector cells into tissue leading to chronic inflammation20. Because of these reasons, it is speculated that modulation of histamine’s function through the use of antagonists might interfere with its inflammatory effects. In fact, several studies have shown that both H1RAs and H2RAs suppress inflammatory responses via inhibition of NFκB, p38 MAP, and JNK8, 21, 22. PPIs are potent blockers of gastric acid secretion and have been found to have anti-oxidant properties through their effects on inflammatory cells thus mitigating inflammation7, 23.
We found that only H2RAs were significantly associated with lower prevalence of NAFLD. The explanation of our observations is unclear. . Though H1RAs and H2RA2 have been shown to inhibit inflammation9, their role in the pathogenesis of NAFLD is still elusive. In fact, a previous study found that H1RAs exacerbate high fat diet-induced hepatic steatosis in mice24. While NAFLD with hepatic triglyceride accumulation was observed in H2RA null mice25, the study by Wake et al., on the other hand, found that H2RAs inhibit the expression and the production of inflammatory cytokines in human peripheral blood mononuclear cells26. Additionally, though some of the anti-inflammatory actions that have been observed by PPIs, it is not clear that oral PPIs dosing can achieve the high drug concentrations in plasma and tissue to reproduce the effect observed in vitro studies7.
The gender difference on the effect of H2RAs and the prevalence of NAFLD deserves further discussion. Flores et al conducted a study to determine if differences exist in the pharmacokinetic (PK) parameters of oral ranitidine caused by gender. Twenty subjects (10 men and 10 women) were enrolled when subjects received a tablet dose of 300 mg ranitidine (H2RA) and blood samples were drawn at several times after its ingestion for the PK analysis. It is interesting that the clearance of the medication was higher in women27. In another ranitidine PK study of 16 healthy volunteers (8 men and 8 women), the oral clearance is ~10.5% higher in women than that in men28. It is plausible that our findings reflect the gender differences in the PK of H2RAs.
It is unclear on why the effect of H2RAs is primarily observed in patients with insulin resistance. Gentile et al. evaluated the role of ranitidine on glucose, insulin levels in 9 healthy volunteers. Interestingly they found that ranitidine infusion influences hepatic clearance of glucose and insulin29. The effect of H2RAs on insulin/glucose hemostasis and its effect on NAFLD might need to be investigated further.
This study has several limitations. First, the cross sectional study design of NHANES prohibits the assessment for the causality between the use of H2RAs and NAFLD association. It is also important to note that the NHANES dataset only reports prescription medication use. Several H1RAs, H2RAs and PPIs are available without a prescription. It is possible that there were participants taking these medications from the over the counter sources which were not captured in the dataset. Additionally, the information on the exact doses of these medications and the duration of administration are also lacking. The study design also disallows us to determine the compliance with the medications in these subjects. Second, the data on inflammatory markers such as TNF alpha are not available. Hence, we cannot directly test the differences in their levels in these subjects, stratified by anti-histamine or PPI use. Lastly, the diagnosis of NAFLD was based on serum aminotransferases, but not confirmed by ultrasonography or liver biopsy; therefore, some study participants may have been misclassified as having NAFLD or not. We and others have used the definition of abnormal ALT in subjects without excessive alcohol use and other chronic liver disease etiologies, as the indirect marker for the presence of NAFLD18, 30, 31. In fact, when compared to those with normal ALT, these subjects were more obese and had several features of metabolic syndrome mimicking those with NAFLD18. Despite these weaknesses, our study is strengthened by the sample size and the study cohort representing the US population.
In conclusion, we found that the use of H2RAs may be associated with a lower prevalence of NAFLD, primarily among men with insulin resistance. Further studies are needed to confirm our observation.
Acknowledgments
Sources of funding: This study is supported by K08 AA016570 from the NIH/NIAAA, 1I01CX000361-01 from the Veterans Affairs Research and Administration, Indiana University Research Support Fund Grant, and W81XWH-12-1-0497 from United States Department of Defense (All to S.L)
Glossary
List of Abbreviations
- BP
Blood pressure
- H1RA
histamine H1-receptor antagonist
- H2RA
histamine H2-receptor antagonist
- NAFLD
non-alcoholic fatty liver disease
- NHANES
National Health and Nutrition Examination Survey
- PPI
proton pump inhibitor
Footnotes
Conflicts of Interest: Both authors report no conflicts of interest relevant to this manuscript
Part of this work was presented at the Digestive Disease Week 2015, Washington, DC
Reference List
- 1.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 2.Marra F, Lotersztajn S. Pathophysiology of NASH: perspectives for a targeted treatment. Curr Pharm Des. 2013;19:5250–5269. doi: 10.2174/13816128113199990344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miura K, Yang L, van RN, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1310–G1321. doi: 10.1152/ajpgi.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tosello-Trampont AC, Landes SG, Nguyen V, Novobrantseva TI, Hahn YS. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-alpha production. J Biol Chem. 2012;287:40161–40172. doi: 10.1074/jbc.M112.417014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simons FE, Simons KJ. Histamine and H1-antihistamines: celebrating a century of progress. J Allergy Clin Immunol. 2011;128:1139–1150. doi: 10.1016/j.jaci.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Moayyedi P, Soo S, Deeks J, Delaney B, Innes M, Forman D. Pharmacological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006:CD001960. doi: 10.1002/14651858.CD001960.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci. 2009;54:2312–2317. doi: 10.1007/s10620-009-0951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho EJ, An HJ, Shin JS, et al. Roxatidine suppresses inflammatory responses via inhibition of NF-kappaB and p38 MAPK activation in LPS-induced RAW 264.7 macrophages. J Cell Biochem. 2011;112:3648–3659. doi: 10.1002/jcb.23294. [DOI] [PubMed] [Google Scholar]
- 9.Ciz M, Lojek A. Modulation of neutrophil oxidative burst via histamine receptors. Br J Pharmacol. 2013;170:17–22. doi: 10.1111/bph.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Analytic and Reporting Guidelines: The National Health and Nutrition Examination Survey (NHANES) 2015. [Google Scholar]
- 11.National Center for Health Statistics . Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-1994. 2015. [Google Scholar]
- 12.Cigarette smoking-attributable morbidity---United States, 2000. MMWR Morb Mortal Wkly Rep. 2003;52:842–844. [PubMed] [Google Scholar]
- 13.Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178:38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 15.Stepanova M, Rafiq N, Younossi ZM. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: a population-based study. Gut. 2010;59:1410–1415. doi: 10.1136/gut.2010.213553. [DOI] [PubMed] [Google Scholar]
- 16.Shen H, Shahzad G, Jawairia M, Bostick RM, Mustacchia P. Association between aspirin use and the prevalence of nonalcoholic fatty liver disease: a cross-sectional study from the Third National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2014;40:1066–1073. doi: 10.1111/apt.12944. [DOI] [PubMed] [Google Scholar]
- 17.Liangpunsakul S, Chalasani N. Serum vitamin D concentrations and unexplained elevation in ALT among US adults. Dig Dis Sci. 2011;56:2124–2129. doi: 10.1007/s10620-011-1707-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liangpunsakul S, Chalasani N. Unexplained elevations in alanine aminotransferase in individuals with the metabolic syndrome: results from the third National Health and Nutrition Survey (NHANES III) Am J Med Sci. 2005;329:111–116. doi: 10.1097/00000441-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Gawron AJ, Feinglass J, Pandolfino JE, Tan BK, Bove MJ, Shintani-Smith S. Brand name and generic proton pump inhibitor prescriptions in the United States: insights from the national ambulatory medical care survey (2006-2010) Gastroenterol Res Pract. 2015;2015:689531. doi: 10.1155/2015/689531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jutel M, Akdis M, Akdis CA. Histamine, histamine receptors and their role in immune pathology. Clin Exp Allergy. 2009;39:1786–1800. doi: 10.1111/j.1365-2222.2009.03374.x. [DOI] [PubMed] [Google Scholar]
- 21.Park HJ, Kim HJ, Park HK, Chung JH. Protective effect of histamine H2 receptor antagonist ranitidine against rotenone-induced apoptosis. Neurotoxicology. 2009;30:1114–1119. doi: 10.1016/j.neuro.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Flamand N, Plante H, Picard S, Laviolette M, Borgeat P. Histamine-induced inhibition of leukotriene biosynthesis in human neutrophils: involvement of the H2 receptor and cAMP. Br J Pharmacol. 2004;141:552–561. doi: 10.1038/sj.bjp.0705654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashioka S, Klegeris A, McGeer PL. Proton pump inhibitors exert anti-inflammatory effects and decrease human microglial and monocytic THP-1 cell neurotoxicity. Exp Neurol. 2009;217:177–183. doi: 10.1016/j.expneurol.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Raveendran VV, Kassel KM, Smith DD, et al. H1-antihistamines exacerbate high-fat diet-induced hepatic steatosis in wild-type but not in apolipoprotein E knockout mice. Am J Physiol Gastrointest Liver Physiol. 2014;307:G219–G228. doi: 10.1152/ajpgi.00027.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang KY, Tanimoto A, Yamada S, et al. Histamine regulation in glucose and lipid metabolism via histamine receptors: model for nonalcoholic steatohepatitis in mice. Am J Pathol. 2010;177:713–723. doi: 10.2353/ajpath.2010.091198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wake H, Takahashi HK, Mori S, Liu K, Yoshino T, Nishibori M. Histamine inhibits advanced glycation end products-induced adhesion molecule expression on human monocytes. J Pharmacol Exp Ther. 2009;330:826–833. doi: 10.1124/jpet.109.155960. [DOI] [PubMed] [Google Scholar]
- 27.Flores PJ, Juarez OH, Flores PC, et al. Effects of gender and phase of the menstrual cycle on the kinetics of ranitidine in healthy volunteers. Chronobiol Int. 2003;20:485–494. [PubMed] [Google Scholar]
- 28.Abad-Santos F, Carcas AJ, Guerra P, et al. Evaluation of sex differences in the pharmacokinetics of ranitidine in humans. J Clin Pharmacol. 1996;36:748–751. doi: 10.1002/j.1552-4604.1996.tb04245.x. [DOI] [PubMed] [Google Scholar]
- 29.Gentile S, Marmo R, Costume A, et al. The role of ranitidine infusion on glucose, insulin and C-peptide serum levels induced by oral glucose tolerance test in healthy subjects. Acta Diabetol Lat. 1986;23:165–170. doi: 10.1007/BF02624676. [DOI] [PubMed] [Google Scholar]
- 30.Ruhl CE, Everhart JE. Joint effects of body weight and alcohol on elevated serum alanine aminotransferase in the United States population. Clin Gastroenterol Hepatol. 2005;3:1260–1268. doi: 10.1016/s1542-3565(05)00743-3. [DOI] [PubMed] [Google Scholar]
- 31.Ruhl CE, Everhart JE. Coffee and caffeine consumption reduce the risk of elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2005;128:24–32. doi: 10.1053/j.gastro.2004.09.075. [DOI] [PubMed] [Google Scholar]