Abstract
Indoor smoke exposure may affect cardiovascular disease (CVD) risk via lung-mediated inflammation, oxidative stress, and endothelial inflammation. We sought to explore the association between indoor smoke exposure from burning biomass fuels and a selected group of markers for endothelial inflammation. We compared serum concentrations of amyloid A protein, E-selectin, soluble ICAM-1 and VCAM-1, von Willebrand factor (VWF), and high sensitivity C-reactive protein (hs-CRP) in 228 biomass exposed vs. 228 non-exposed participants living in Puno, Peru. Average age was 56 years (SD=13), average BMI was 26.5 kg/m2 (SD=4.4), 48% were male, 59.4% completed high school and 2% reported a physician diagnosis of CVD. In unadjusted analysis, serum levels of soluble ICAM-1 (330 vs. 302 ng/mL; p<0.001), soluble VCAM-1 (403 vs. 362 ng/mL; p<0.001), and E-selectin (54.2 vs. 52.7 ng/mL; p=0.05) were increased in biomass exposed vs. non-exposed participants, respectively; whereas serum levels of vWF (1148 vs. 1311 mU/mL; p<0.001) and hs-CRP (2.56 vs. 3.12 mg/L; p<0.001) were decreased, respectively. In adjusted analyses, chronic exposure to biomass fuels remained positively associated with serum levels of soluble ICAM-1 (p=0.03) and VCAM-1 (p=0.05) and E-selectin (p=0.05), and remained negatively associated with serum levels of vWF (p=0.02) and hs-CRP (p<0.001). Daily exposure to biomass fuel smoke was associated with important differences in specific biomarkers of endothelial inflammation and may help explain accelerated atherosclerosis among those who are chronically exposed.
Keywords: biomass fuel exposure, rural communities, endothelial inflammation biomarkers, household air pollution, cardiovascular disease, epidemiology
INTRODUCTION
Household air pollution (HAP) is the third greatest cause of disability adjusted life years lost worldwide and it is responsible for 3.3 million deaths each year (Lim et al 2015). An increasing body of evidence suggests that HAP may also be responsible for an increased risk of cardiovascular disease (CVD) (Baumgartner et al 2011; Burroughs Pena et al 2015; Clark et al 2011; Dutta et al 2011; McCracken et al 2007; McCracken et al 2011; Lee et al 2012; Painschab et al 2013). Specifically, observational studies have found higher blood pressure (Baumgartner et al 2011; Burroughs Peña et al 2015; McCracken et al 2007); a thicker carotid intima-media complex (Painschab et al 2013), and an increased prevalence of self-reported coronary heart disease, stroke and diabetes in populations chronically exposed to biomass fuel smoke (Lee et al 2012). The mechanisms proposed include lung-mediated inflammation with a systemic release of cytokines (Barregard et al 2006), oxidative stress (Banerjee et al 2012), endothelial dysfunction (Allen et al 2011; Butarak et al 2011) and thrombogenesis (Ray et al 2006), all of which could lead to atherosclerosis and adverse health outcomes (Brevetti et al 2006; Fichtlscherer et al 2004; Verma et al 2002).
A healthy endothelium is necessary for appropriate functioning of the vasculature. Endothelial inflammation as a result of noxious events or activation of the inflammatory cascade is thought to be the initial event for the development of CVD and it is likely mediated by increased expression of several substances involved in the proliferation on smooth muscle, and abnormal function of coagulation and fibrinolysis (Halcox et al 2001; Verma et al 2002; Widlansky et al 2003). Previous studies have found a relationship between exposure to particulate matter (PM) and initiation of endothelial inflammation leading to an increased risk of cardiovascular events (Barregard et al 2006; Helbing et al 2014).
Recent research has focused on identifying markers of early endothelial inflammation which could help assess CVD risk. Serum biomarkers like Von Willebrand factor (vWF), C-reactive protein (CRP), intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) are overexpressed in conditions with evidence of endothelial inflammation (Brevetti et al 2006; Hwang et al 1997; Lawson et al 2009; Ley et al 2001; Sonneveld et al 2015); however, compelling evidence for a link to CVD is lacking. We hypothesized that HAP may lead to endothelial inflammation. We compared serum levels of six selected markers of endothelial inflammation in a group of participants with and without daily exposure to biomass fuel smoke living in Puno, Peru.
METHODS
Study setting
The study population was comprised of adults aged ≥ 35 years living in Puno, Peru and surrounding rural villages (Miranda et al 2012). The Department of Puno has a population of approximately 150,000 inhabitants, and an elevation of 3,825 meters above sea level. Urban participants work primarily in commerce or education, and predominantly cook with clean fuels (liquid-propane gas, kerosene, or electricity). Typical rural participants are subsistence farmers who cook indoors almost exclusively with traditional open-fire stoves and biomass fuels (i.e., wood, animal dung, and crop residue). In a previous study, we documented that rural dwellers lived in households with a greater exposure to indoor particulate matter and carbon monoxide concentrations than did urban dwellers (Pollard et al 2014). All participants provided informed consent. The Institutional Review Boards of Johns Hopkins University in Baltimore, USA, and A.B. PRISMA and Universidad Peruana Cayetano Heredia in Lima, Peru approved the study protocol.
Study Design
We selected a random sample of 456 participants with and without daily exposure to biomass fuels from an ongoing cohort in Puno (Miranda et al 2012). Trained field workers administered a standardized face-to-face questionnaire and comprehensive clinical evaluation as described previously (Miranda et al 2012). Certified phlebotomists collected blood for processing in a centralized testing facility. Blood stored at -70°C was later tested for serum amyloid A (SAA), E-selectin, soluble levels of ICAM-1 and sVCAM-1, vWF at the Johns Hopkins Clinical Research Unit Core Lab. Serum levels of hs-CRP were available for the entire cohort (Miranda et al 2012).
Definitions
Participants were stratified by their history of long-term household cooking with clean vs. biomass fuels. We used dwelling locale as a proxy for long-term or chronic exposure (Pollard et al 2014). We defined presence of CVD as a self-reported history of or current medication use for arrhythmias, angina, myocardial infarction, heart failure, hyperlipidemia or stroke; a history of hypertension as a self-reported history or current anti-hypertensive medication use; diabetes as a self-reported history or anti-hyperglycemic medication use; and, daily smoking as having ≥1 cigarette/day. We calculated an Alcohol Use Disorders Identification Test [AUDIT] score using questions about alcohol consumption (Daeppen et al 2000). We also calculated the homeostasis model of assessment-insulin resistance (HOMA-IR) as the product of fasting glucose (mmol/L) and fasting insulin (μU/mL)/22.5.
Assessment of endothelial function markers
The endothelial inflammation biomarkers selected were E-selectin, soluble ICAM-1 and VCAM-1, SAA, vWF, and hs-CRP. We used a commercially available multiplexed ELISA (Mesoscale Discovery, Gaithersburg, USA) to measure sICAM-1, sVCAM-1 and SAA. Electrochemiluminescent signals from the ELISA plates were detected and standard curves were calculated using a SECTOR Imager 2400 workstation (Mesoscale Discovery, Gaithersburg, USA). The sensitivities of the multiplex ELISA markers were 0.01 ng/ml, 0.07 ng/ml and 0.09 ng/ml; inter-assay coefficients of variation (CV) were 5.9%, 9.2% and 12.1%; and, intra-assay CVs were 6.48%, 3.78% and 3.21% for sICAM-1, sVCAM-1 and SAA, respectively. We measured hs-CRP using Latex (Tina-quant CRP-HS Roche/Hitachi analyzer, Indianapolis, IN, USA). We used commercial ELISA assays to measure soluble E-selectin (R&D Systems., Minneapolis, USA) and vWF (Novus Biologicals, LLC, Littleton, CO, USA). Assays for E-selectin had a sensitivity of 3 pg/ml, an intra-assay CV of 6.6% and an inter-assay CV of 6.5%, whereas that for vWF had a sensitivity of 2.5 mU/ml, an intra-assay CV of 2.57% and an inter-assay CV of 8.8%.
Biostatistical methods
Our primary objective was to compare markers of endothelial inflammation in participants with and without chronic exposure to biomass fuel smoke. In exploratory analyses, we found that several of the endothelial inflammation markers were non-normally distributed. Therefore, we used the Box-Cox Power transform to identify the best transformation for each biomarker (Table 1). We then used multivariable linear regressions to model each of the transformed biomarkers as a function of chronic exposure to biomass fuel smoke, and adjusted for age, sex, BMI, LDL/HDL, log HOMA-IR, log hemoglobin A1c, whether or not the participant completed high school, the number of people sleeping in the house, whether the participants had a self-reported history of hypertension, cardiovascular disease, or diabetes, and the AUDIT score. Finally, we calculated standardized coefficients as a means to contrast the association between chronic exposure to biomass fuel smoke and endothelial inflammation for each of the transformed biomarkers. We calculated 95% bootstrap confidence intervals for the standardized coefficients using 250 samples with replacement. We used t-tests to compare continuous variables between groups if normally distributed and Mann-Whitney U tests if non-normally distributed. We used chi-square or Fisher exact tests, when appropriate, to compare categorical variables between groups. We conducted all statistical analyses in R (www.r-project.org).
Table 1.
Markers of endothelial inflammation and selected transformations for non-normal distributions.
| Biomarkers | Selected transformation | |
|---|---|---|
| E-selectin (ng/mL) | log(E-selectin) | |
| sICAM-1 (ng/mL) | log(sICAM-1) | |
| sVCAM-1 (ng/mL) |
|
|
| Serum amyloid A (ng/mL) | log(serum amyloid A) | |
| vWf (mU/mL) | log(vWF) | |
| hs-CRP (mg/L) | log(hs-CRP) |
RESULTS
Participant Characteristics
We measured selected markers of endothelial inflammation in a subset of 456 participants from the original cohort. Overall daily smoking prevalence was low at 1.4%. We did not find differences in age (p=0.09), sex (p=0.68), site (p=0.60), body mass index (p=0.56), systolic blood pressure (p=0.34), or level of education (p=0.64) between participants who were included in the subset for this analysis and those who were not. We summarized participant characteristics stratified by fuel exposure status in Table 2. Participants who used biomass fuels daily were slightly older, were more likely to be female, and had a lower socioeconomic status than did those who used clean fuels. They were also thinner, and had a higher blood pressure, a lower prevalence of self-reported cardiovascular disease history, a lower LDL to HDL ratio, and a lower level of insulin resistance.
Table 2.
Population Characteristics
| Biomass fuels (n=228) | Clean fuels (n=228) | P value | |
|---|---|---|---|
| Demographics | |||
| Age in years, mean (SD) | 58.1 (12.5) | 53.9 (12.2) | <0.01 |
| Sex, % male (n) | 42% (95) | 53% (121) | 0.01 |
| Completed high school, % (n) | 29.6% (67) | 89% (202) | <0.01 |
| Number of people per house, mean (SD) | 3.1 (1.9) | 4.3 (2) | <0.01 |
| Clinical Characteristics | |||
| BMI in kg/m2, mean (SD) | 25.2 (4.2) | 27.8 (4.2) | <0.01 |
| SBP in mmHg, mean (SD) | 117.7 (17.8) | 111.8 (17.5) | <0.01 |
| Self-reported history, % (n) | |||
| Hypertension | 7.0% (16) | 17.2% (39) | <0.01 |
| Diabetes | 3.1% (7) | 6.6% (15) | 0.08 |
| Cardiovascular disease | 0.4% (1) | 3.5% (8) | <0.05 |
| Daily smoking % (n) | 2.8% (6) | 0% (0) | 0.03 |
| AUDIT score, mean (SD) | 3.3 (4.9) | 3.0 (4.4) | 0.45 |
| Laboratory profile, mean (SD) | |||
| LDL/HDL | 2.8 (1.2) | 3.2 (1.3) | <0.01 |
| log(HbA1c) in % | 1.8 (0.1) | 1.8 (0.1) | 0.4 |
| log(HOMA-IR) | 0.25 (0.2) | 0.32 (0.3) | <0.01 |
Association between pairs of markers for endothelial inflammation
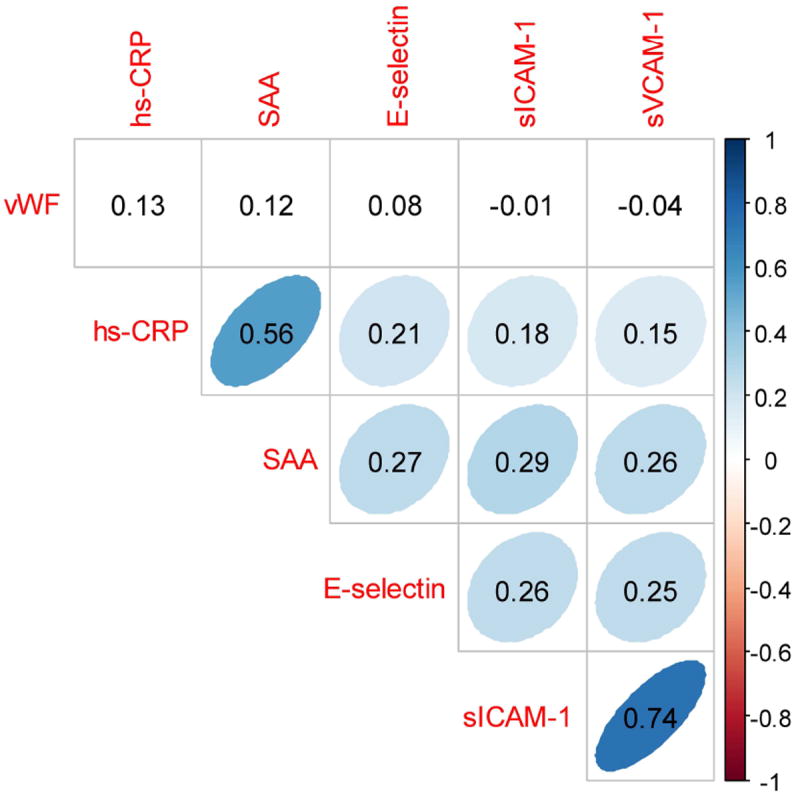
In Figure 1, we show the correlation matrix between pairs of markers of endothelial inflammation. We found positive correlation between hs-CRP and SAA (Spearman r=0.56; p<0.001) and between soluble levels of ICAM-1 and VCAM-1 (Spearman r=0.74; p<0.001). All other correlations were either weak or non-significant. In particular, vWF did not appear to be correlated with any of the other selected markers.
Figure 1. Correlations among markers for endothelial inflammation.
Chronic exposure to biomass fuel and markers of endothelial inflammation
In Table 3, we summarized biomarkers of endothelial inflammation by fuel exposure status. Serum levels of soluble ICAM-1 and VCAM-1, and E-selectin, were significantly higher in participants who used biomass fuels daily compared to those who used clean fuels, whereas vWF and hs-CRP were significantly lower. On the other hand, serum levels of SAA were similar. In Figure 2, we summarized mean serum levels by age and fuel exposure categories to explore for age by fuel exposure status interactions. In general, most markers appeared to increase with age regardless of fuel exposure status with the exception of serum levels of vWF among the group of participants who used biomass fuels daily. Soluble levels of ICAM-1 and VCAM-1 were consistently elevated among participants who used biomass fuel daily vs. clean fuel regardless of age category, whereas there appeared to be more variability in levels of E-selectin, SAA, vWF and hs-CRP by fuel exposure status and age.
Table 3.
Serum levels of specific markers of endothelial inflammation.
| Biomarker, mean (SD) | Biomass fuel group (n=228) |
Clean fuel group (n=228) |
p value |
|---|---|---|---|
| E-selectin | |||
| E-selectin (ng/mL) | 54.2 (16.8) | 52.7 (22.1) | |
| log(E-selectin) | 3.95 (0.29) | 3.89 (0.39) | 0.05 |
| Soluble ICAM-1 | |||
| sICAM-1 (ng/mL) | 330.9 (99.5) | 302.3 (88.7) | |
| log(sICAM-1) | 5.76 (0.28) | 5.67 (0.28) | <0.001 |
| Soluble VCAM-1 | |||
| sVCAM-1 (ng/mL) | 403.3 (141.7) | 361.8 (121.0) | |
| 0.051 (0.007) | 0.054 (0.008) | <0.001 | |
| Serum amyloid A (SAA) | |||
| SAA (ng/mL) | 6031 (22774) | 4295 (13825) | |
| log(SAA) | 7.50 (1.24) | 7.44 (1.07) | 0.57 |
| Von Willebrand factor (vWF) | |||
| vWF (mU/mL) | 1148 (974) | 1311 (888) | |
| log(vMF) | 6.83 (0.62) | 7.02 (0.53) | <0.001 |
| High-sensitivity C-reactive protein | |||
| hs-CRP (mg/L) | 2.56 (7.05) | 3.12 (9.01) | |
| log(hs-CRP) | -0.14 (1.17) | 0.45 (1.06) | <0.001 |
Figure 2. Relationship between biomarkers and age for exposed and non-exposed groups.
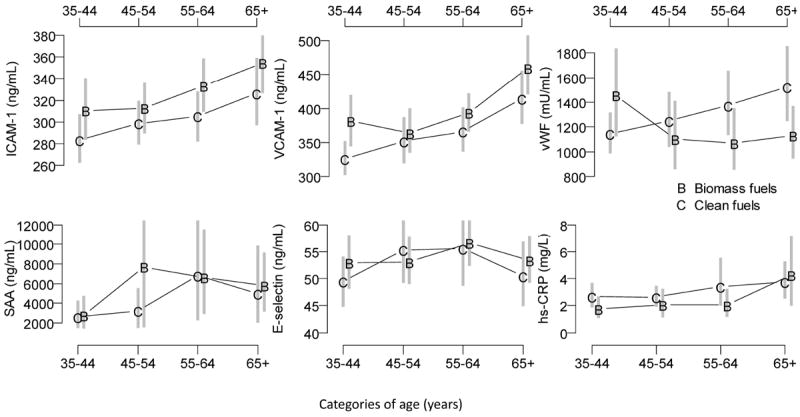
Mean and 95% confidence interval for serum biomarker concentrations in the biomass fuel (B) and clean fuel (C) groups. Vertical grey segments represent 95% bootstrap percentile confidence intervals.
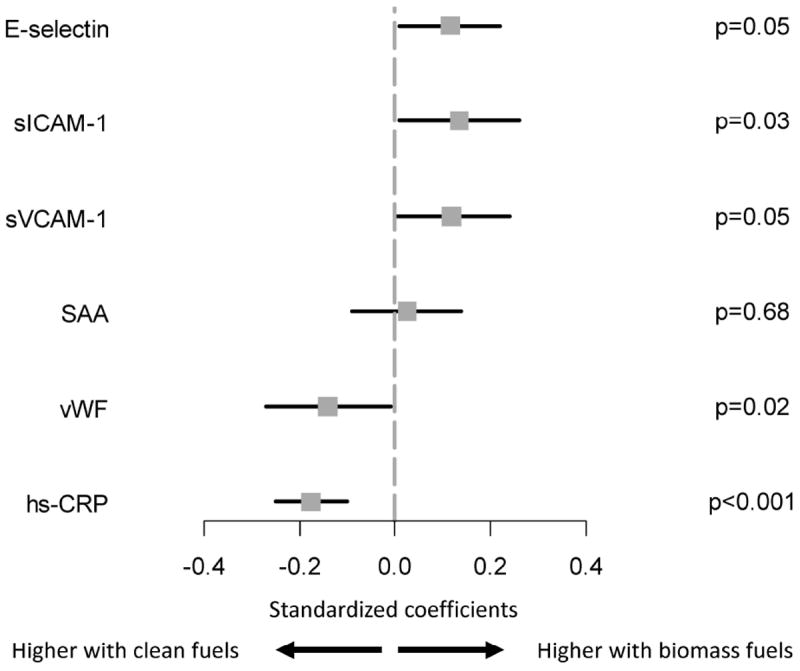
In Figure 3, we summarized standardized coefficients for the adjusted relationship between chronic exposure to biomass fuels and transformed values of selected markers of endothelial inflammation. Specifically, in multivariable analyses, we found that serum levels of soluble ICAM-1, soluble VCAM-1, and E-selectin remained significantly higher in participants who used biomass fuels daily, and vWF and hs-CRP remained significantly lower. We did not find age by fuel exposure category interactions for E-selectin (p=0.38), soluble ICAM-1 (p=0.95) or VCAM-1 (p=0.52), SAA (p=0.92), vWF (p=0.15), or hs-CRP (p=0.33). We also did not find sex by fuel exposure category interactions for E-selectin (p=0.06), soluble ICAM-1 (p=0.65) or VCAM-1 (p=0.49), SAA (p=0.88), vWF (p=0.17), or hs-CRP (p=0.42).
Figure 3. Standardized coefficients obtained multivariable regression to measure the association between chronic exposure to biomass fuel use and selected endothelial function markers.
DISCUSSION
We measured a select group of serum biomarkers as a proxy of endothelial health. While none of these markers have been proven to be a direct expression of endothelial function, they may provide some insights into local inflammation that occurs at the level of endothelial cells (Felmeden and Lip 2005). In this study, we compared endothelial inflammation biomarkers in participants with and without daily exposure to biomass fuels. Specifically, we found that serum levels of soluble ICAM-1 and VCAM-1, and E-selectin were elevated in participants with daily biomass fuel use vs. those who used clean fuels, whereas vWF and hs-CRP were lower. These relationships were unaffected even after adjusting for well-recognized factors that contribute to endothelial dysfunction such as age, sex, body mass index, HDL, and self-reported history of hypertension, cardiovascular disease, and diabetes, and alcohol use.
While there is strong evidence that higher levels of ambient air pollution are linked to an increased risk of CVD, epidemiological evidence for a link between biomass fuel smoke exposure and CVD risk is still emerging. For example, Dutta et al found that both fine (PM2.5) and coarse (PM10) concentrations were positively related to increase CVD risk in rural India (Dutta et al 2011). Exposure to biomass fuel smoke has been linked to higher blood pressure in China (Baumgartner et al 2011), Guatemala (McCracken et al 2007), and Peru (Burroughs Peña et al 2015). Our group also reported that daily exposure to biomass fuel was associated with a thicker carotid intima media and a higher prevalence of plaques (Painschab et al 2013).
Our biomarker results are consistent with previous studies that address the relationship between ambient air pollution and endothelial health. Alexeeff et al found that levels of ICAM-1 were positively correlated with higher concentrations of black carbon exposure (Alexeeff et al 2011). Bind et al found higher serum levels of ICAM and VCAM were associated with higher exposures to traffic related pollutants (Bind et al 2012). Few studies, however, have examined the relationship between household air pollution from burning biomass fuels and endothelial function markers. One recent study by Buturak et al found that flow mediated dilation and endothelial-independent vasodilation were reduced in individuals with biomass fuel exposure (Butarak et al 2011).
The negative relationship found between either vWF or hs-CRP and biomass exposure status does not match with our expectations. Indeed, we would have anticipated finding positive associations between these markers and daily exposure to biomass fuel smoke. Nonetheless, other studies have reported similar findings. In the MESA study, investigators reported a lack of association between CRP and long-term exposure to traffic related pollutants (Hajat et al 2015). A double blind randomized study involving healthy individuals vs. those with metabolic syndrome who were exposed to either filtered air or diesel air residuals showed non-significant increase in cytokines, ICAM or VCAM (Krishnan et al 2013; Davel et al 2012). Other investigators like Hildebrant et al found that decreased serum levels of vWF were association with higher levels of almost all measured pollutants. These investigators suggested that vWF was a non-specific biomarker that can change in response to several other factors (Hildebrandt et al 2009). A similar phenomenon may be occurring with CRP. Specifically, hs-CRP is an inflammatory marker that responds to acute exposures. In contrast, to biomass fuel smoke was chronic exposure among our participants, which could lead to compensatory pathways and thus lowering values of serum hs-CRP. Finally another plausible explanation the observed lower hs-CRP levels could be that rural participants who used biomass fuels daily have higher levels of physical activity due to farming activities compared to the urban participants (Plaisance et al., 2006).
Our study has several strengths. First, this is the largest study of markers of endothelial inflammation and chronic exposure to biomass fuel smoke. Second, we were able to measure several biomarkers thought to be related to endothelial inflammation that could help us elucidate specific mechanisms involved in endothelial damage. Third, our study derived its samples from a population-based study in a high altitude setting in two disparate socioeconomic settings, allowing for increased environmental, dietary, and lifestyle variability. Our study also has some potential shortcomings. First, we used dwelling location as a proxy for chronic biomass exposure which could increase the risk of confounding bias. However, in a previous study we measured household particulate matter and carbon monoxide of a representative subset of homes and confirmed that rural households in our study area had significantly higher levels of indoor environmental exposures than did urban households (Pollard et al 2014). Second, although endothelial inflammatory biomarkers are positively associated with cardiovascular disease, it is unclear how high altitude may affect many of these biomarkers. Third, socioeconomic status can be related to endothelial inflammation and adverse cardiovascular outcomes (Hong et al 2006), and there may be unmeasured confounders that are defined by dwelling location including physical activity and time spent indoors. Fourth, while we did not find interactions between age or sex by fuel exposure, biomass exposed and non-exposed groups differed both in age and sex which could affect our overall findings. Finally, even though inflammatory biomarkers have been related to endothelial damage and dysfunction, direct methods to measure endothelial function like flow-mediation dilation are still needed to establish a direct link.
CONCLUSION
In summary, we found that specific biomarkers related to endothelial inflammation — namely serum levels of soluble ICAM-1, VCAM-1, and E-selectin — were positively associated with daily exposure to biomass fuels in our study population. We postulate that these markers may be useful surrogates to measure improvements in endothelial health in relation to reductions in household air pollution levels. Nonetheless, longitudinal studies measuring endothelial inflammatory biomarkers with pollutant concentrations, or assessment of biomarkers in field intervention trials that use improved cookstoves or clean fuels are necessary to further determine the applicability of these biomarkers.
PRACTICAL IMPLICATIONS.
Specific biomarkers related to endothelial inflammation (soluble ICAM-1, VCAM-1, and E-selectin) were positively associated with daily exposure to biomass fuels in our study population. These markers may be useful surrogates to measure improvements in endothelial health in response to reductions in household air pollution levels.
Acknowledgments
Sources of funding: This project was funded in part by Federal Funds of the National Heart, Lung and Blood Institute, United States National Institutes of Health, Department of Health and Human Services under contract number HHSN268200900033C. William Checkley was further supported by a Pathway to Independence Award (R00HL096955) from the National Heart, Lung and Blood Institute, National Institutes of Health. Phabiola Herrera was further supported by a Seed Grant from the United States National Heart, Lung and Blood Institute, National Institutes of Health, Department of Health and Human Services under contract number.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
Other members of the CRONICAS Cohort Study group: Antonio Bernabé-Ortiz, Juan P. Casas, George Davey Smith, Shah Ebrahim, Héctor H. García, Germán Málaga, Víctor M. Montori, Liam Smeeth; Gregory B. Diette; Robert Wise.
References
- Alexeeff SE, Coull BA, Gryparis A, Suh H, Sparrow D, Vokonas PS, Schwartz J. Medium-term exposure to traffic-related air pollution and markers of inflammation and endothelial function. Environ Health Perspect. 2011;119:481–6. doi: 10.1289/ehp.1002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RW, Carlsten C, Karlen B, Leckie S, Van Eeden S, Vedal S, et al. An air filter intervention study of endothelial function among healthy adults in a woodsmoke-impacted community. Am J Respir Crit Care Med. 2011;183:1222–30. doi: 10.1164/rccm.201010-1572OC. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Mondal NK, Das D, Ray MR. Neutrophilic inflammatory response and oxidative stress in premenopausal women chronically exposed to indoor air pollution from biomass burning. Inflammation. 2012;35:671–83. doi: 10.1007/s10753-011-9360-2. [DOI] [PubMed] [Google Scholar]
- Barregard L, Sällsten G, Gustafson P, Andersson L, Johansson L, Basu S, et al. Experimental exposure to wood-smoke particles in healthy humans: effects on markers of inflammation, coagulation, and lipid peroxidation. Inhal Toxicol. 2006;18:845–53. doi: 10.1080/08958370600685798. [DOI] [PubMed] [Google Scholar]
- Baumgartner J, Schauer JJ, Ezzati M, Lu L, Cheng C, Patz JA, et al. Indoor air pollution and blood pressure in adult women living in rural China. Environ Health Perspect. 2011;119:1390–5. doi: 10.1289/ehp.1003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bind MA, Baccarelli A, Zanobetti A. Air pollution and markers of coagulation, inflammation and endothelial function: Associations and epigene-environment interactions in an elderly cohort. Epidemiology. 2012;23:332–340. doi: 10.1097/EDE.0b013e31824523f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevetti G, Schiano V, Chiariello M. Cellular adhesion molecules and peripheral arterial disease. Vasc Med. 2006;11:39–47. doi: 10.1191/1358863x06vm645ra. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–78. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Burroughs Peña M, Romero KM, Velazquez EJ, Davila-Roman VG, Gilman RH, Wise RA, et al. Relationship between daily exposure to biomass fuel smoke and blood pressure in high-altitude Peru. Hypertension. 2015;65:1134–40. doi: 10.1161/HYPERTENSIONAHA.114.04840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buturak A, Genç A, Ulus OS, Duygu E, Okmen AS, Uyarel H. Evaluation of the effects of chronic biomass fuel smoke exposure on peripheral endothelial functions: an observational study. Anadolu Kardiyol Derg. 2011;11:492–7. doi: 10.5152/akd.2011.132. [DOI] [PubMed] [Google Scholar]
- Clark ML, Bazemore H, Reynolds SJ, Heiderscheidt JM, Conway S, Bachand AM, et al. A Baseline Evaluation of Traditional Cook Stove Smoke Exposures and Indicators of Cardiovascular and Respiratory Health among Nicaraguan Women. Int J Occup Environ Health. 2011;17:113–21. doi: 10.1179/107735211799030942. [DOI] [PubMed] [Google Scholar]
- Daeppen JB, Yersin B, Landry U, Pecoud A, Decrey H. Reliability and validity of the Alcohol Use Disorders Identification Test (AUDIT) imbedded within a general health risk screening questionnaire: results of a survey in 332 primary care patients. Alcohol Clin Exp Res. 2000;24:659–65. [PubMed] [Google Scholar]
- Davel AP, Lemos M, Pastro LM, Pedro SC, de André PA, Hebeda C, et al. Endothelial dysfunction in the pulmonary artery induced by concentrated fine particulate matter exposure is associated with local but not systemic inflammation. Toxicology. 2012;295:39–46. doi: 10.1016/j.tox.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Dutta A, Mukherjee B, Das D, Banerjee A, Ray MR. Hypertension with elevated levels of oxidized low-density lipoprotein and anticardiolipin antibody in the circulation of premenopausal Indian women chronically exposed to biomass smoke during cooking. Indoor Air. 2011;21:165–76. doi: 10.1111/j.1600-0668.2010.00694.x. [DOI] [PubMed] [Google Scholar]
- Felmeden DC, Lip GY. Endothelial function and its assessment. Expert Opin Investig Drugs. 2005;14:1319–36. doi: 10.1517/13543784.14.11.1319. [DOI] [PubMed] [Google Scholar]
- Fichtlscherer S, Breuer S, Zeiher AM. Prognostic value of systemic endothelial dysfunction in patients with acute coronary syndromes: Further evidence for the existence of the “vulnerable” patient. Circulation. 2004;110:1926–32. doi: 10.1161/01.CIR.0000143378.58099.8C. [DOI] [PubMed] [Google Scholar]
- Hajat A, Allison M, Diez-Roux AV, Jenny NS. Long-term Exposure to Air Pollution and Markers of Inflammation, Coagulation, and Endothelial Activation: A Repeat-measures Analysis in the Multi-Ethnic Study of Atherosclerosis (MESA) Epidemiology. 2015;26:310–20. doi: 10.1097/EDE.0000000000000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halcox JP, Quyyumi AA. Coronary vascular endothelial function and myocardial ischemia: why should we worry about endothelial dysfunction? Coron Artery Dis. 2001;12:475–84. doi: 10.1097/00019501-200109000-00006. [DOI] [PubMed] [Google Scholar]
- Helbing T, Olivier C, Bode C, Moser M, Diehl P. Role of microparticles in endothelial dysfunction and arterial hypertension. World J Cardiol. 2014;6:1135–9. doi: 10.4330/wjc.v6.i11.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt K, Rückerl R, Koenig W, Schneider A, Pitz M, Heinrich J, et al. Short-term effects of air pollution: a panel study of blood markers in patients with chronic pulmonary disease. Part Fibre Toxicol. 2009;6:25. doi: 10.1186/1743-8977-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Nelesen RA, Krohn PL, Mills PJ, Dimsdale JE. The association of social status and blood pressure with markers of vascular inflammation. Psychosom Med. 2006;68:517–23. doi: 10.1097/01.psy.0000227684.81684.07. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM, et al. Circulating Adhesion Molecules VCAM-1, ICAM-1, and E-selectin in Carotid Atherosclerosis and Incident Coronary Heart Disease Cases: The Atherosclerosis Risk In Communities (ARIC) Study. Circulation. 1997;96:4219–25. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- Krishnan RM, Sullivan JH, Carlsten C, Wilkerson HW, Beyer RP, Bammler T, et al. A randomized cross-over study of inhalation of diesel exhaust, hematological indices, and endothelial markers in humans. Part Fibre Toxicol. 2013;10:7. doi: 10.1186/1743-8977-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Reports. 2009;61:22–32. doi: 10.1016/s1734-1140(09)70004-0. [DOI] [PubMed] [Google Scholar]
- Lee BJ, Kim B, Lee K. Air pollution exposure and cardiovascular disease. Toxicol Res. 2014;30:71–5. doi: 10.5487/TR.2014.30.2.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Hang J, Zhang F, Dai H, Su L, Christiani DC. In-home solid fuel use and cardiovascular disease: a cross-sectional analysis of the Shanghai Putuo study. Environ Health. 2012;11:18. doi: 10.1186/1476-069X-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Huo Y. VCAM-1 is critical in atherosclerosis. J Clin Invest. 2001;107:1209–10. doi: 10.1172/JCI13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2015;380:2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Xu D, Cheng Y. Systematic review and meta-analysis of the adverse health effects of ambient PM2.5 and PM10 pollution in the Chinese population. Environ Res. 2015;136:196–204. doi: 10.1016/j.envres.2014.06.029. [DOI] [PubMed] [Google Scholar]
- Mccracken J, Smith KR, Stone P, Díaz A, Arana B, Schwartz J. Intervention to lower household wood smoke exposure in Guatemala reduces ST-segment depression on electrocardiograms. Environ Health Perspect. 2011;119:1562–8. doi: 10.1289/ehp.1002834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken JP, Smith KR, Díaz A, Mittleman MA, Schwartz J. Chimney stove intervention to reduce long-term wood smoke exposure lowers blood pressure among Guatemalan women. Environ Health Perspect. 2007;115:996–1001. doi: 10.1289/ehp.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda JJ, Bernabe-Ortiz A, Smeeth L, Gilman RH, Checkley W. Addressing geographical variation in the progression of non-communicable diseases in Peru: the CRONICAS cohort study protocol. BMJ Open. 2012;2:e000610–e000610. doi: 10.1136/bmjopen-2011-000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painschab MS, Davila-Roman VG, Gilman RH, Vasquez-Villar AD, Pollard SL, Wise RA, et al. Chronic exposure to biomass fuel is associated with increased carotid artery intima-media thickness and a higher prevalence of atherosclerotic plaque. Heart. 2013;99:984–91. doi: 10.1136/heartjnl-2012-303440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaisance EP, Grandjean PW. Physical activity and high-sensitivity C-reactive protein. Sports Med. 2006;36:443–58. doi: 10.2165/00007256-200636050-00006. [DOI] [PubMed] [Google Scholar]
- Pollard SL, Williams DL, Breysse PN, Baron PA, Grajeda LM, Gilman RH, et al. A cross-sectional study of determinants of indoor environmental exposures in households with and without chronic exposure to biomass fuel smoke. Environ Health. 2014;13:21. doi: 10.1186/1476-069X-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray MR, Mukherjee S, Roychoudhury S, Bhattacharya P, Banerjee M, Siddique S, et al. Platelet activation, upregulation of CD11b/ CD18 expression on leukocytes and increase in circulating leukocyte-platelet aggregates in Indian women chronically exposed to biomass smoke. Hum Exp Toxicol. 2006;25:627–35. doi: 10.1177/0960327106074603. [DOI] [PubMed] [Google Scholar]
- Shah AS, Lee KK, McAllister DA. Short term exposure to air pollution and stroke: systematic review and meta-analysis. BMJ. 2015;350:1295. doi: 10.1136/bmj.h1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneveld MA, Cheng JM, Oemrawsingh RM, de Maat MP, Kardys I, Garcia-Garcia HM, van Geuns RJ, et al. Von Willebrand factor in relation to coronary plaque characteristics and cardiovascular outcome. Results of the ATHEROREMO-IVUS study. Thromb Haemost. 2015;113:577–84. doi: 10.1160/TH14-07-0589. [DOI] [PubMed] [Google Scholar]
- Verma S, Anderson TJ. Fundamentals of endothelial function for the clinical cardiologist. Circulation. 2002;105:546–9. doi: 10.1161/hc0502.104540. [DOI] [PubMed] [Google Scholar]
- Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–60. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]


