Abstract
Spinal cord injury (SCI) causes dramatic changes in the quality of life, including coping with bladder dysfunction which requires repeated daily and nightly catheterizations. Our laboratory has recently demonstrated in a rat SCI model that repetitive sensory information generated through task-specific stepping and/or loading can improve nonlocomotor functions, including bladder function (Ward PJ, Herrity AN, Smith RR, Willhite A, Harrison BJ, Petruska JC, Harkema SJ, Hubscher CH. J Neurotrauma 31: 819–833, 2014). To target potential underlying mechanisms, the current study included a forelimb-only exercise group to ascertain whether improvements may be attributed to general activity effects that impact target organ-neural interactions or to plasticity of the lumbosacral circuitry that receives convergent somatovisceral inputs. Male Wistar rats received a T9 contusion injury and were randomly assigned to three groups 2 wk postinjury: quadrupedal locomotion, forelimb exercise, or a nontrained group. Throughout the study (including preinjury), all animals were placed in metabolic cages once a week for 24 h to monitor water intake and urine output. Following the 10-wk period of daily 1-h treadmill training, awake cystometry data were collected and bladder and kidney tissue harvested for analysis. Metabolic cage frequency-volume measurements of voiding and cystometry reveal an impact of exercise training on multiple SCI-induced impairments related to various aspects of urinary tract function. Improvements in both the quadrupedal and forelimb-trained groups implicate underlying mechanisms beyond repetitive sensory information from the hindlimbs driving spinal network excitability of the lumbosacral urogenital neural circuitry. Furthermore, the impact of exercise training on the upper urinary tract (kidney) underscores the health benefit of activity-based training on the entire urinary system within the SCI population.
Keywords: bladder, kidney, locomotor training, contusion
improving bladder deficits is among the areas of highest priority following spinal cord injury (SCI), as urinary tract impairment has an enormous impact on the quality of life (2, 3, 26). Life-long urological care is required for SCI individuals, yet most efforts treat symptoms but do not improve intrinsic function (68, 80). Bladder management requires intermittent catheterization throughout the day/night to avoid incontinence, bladder overdistention (which can create high pressure and reflux to the kidneys), inflammation, infections, and autonomic dysreflexia.
Despite bladder dysfunction being a high priority for SCI individuals, the focus of health care professionals is on rehabilitation aimed at optimizing mobility and the remaining musculoskeletal function. Locomotor training (LT) has emerged as a safe and effective therapy for post-SCI motor deficits with many benefits (cardiovascular function, strength, mobility) (9, 23, 43, 52, 84). Recent animal studies, however, have shown that LT post-SCI also improves bladder function (46, 90), a finding consistent with a few reports from human SCI studies (42, 48, 75). For example, a recent study from our laboratory (90) has shown, using a spinal contusion model in adult male rats, functional gains of lower urinary tract function as assessed with terminal urodynamic measures after 12 wk of daily LT for a period of 60 min/day. The interaction of lower limb musculature with the bladder and its sphincter has been observed sporadically over the years, as far back as 1933, in both humans and animal studies (20, 53, 74). Flexor and extensor reflexes can be modulated by the state of bladder filling and voiding in normal humans and those with central nervous system damage (64). In humans with spasticity, the general pattern is that detrusor contractions precede limb flexor spasms (69). This vesicosomatic relationship involving lumbosacral reflex circuitries could contribute to the enhancement of bladder function with LT.
The multisystem functional gains with task-specific training have generated multiple novel hypotheses regarding potential underlying mechanisms. With respect to the improvements in bladder function with 60 min/day of stepping on a treadmill using body weight support and manual facilitation in a natural position (90), the current study was designed to expand upon our initial findings to include 1) non-weight-bearing stepping with a forelimbs-only exercise group; 2) collection of weekly metabolic cage data to monitor SCI-induced persistent polyuria (overproduction of urine); and 3) further tissue assessments that include the structural integrity of the bladder wall and the impact of training on the upper urinary tract (kidneys).
The bladder wall itself has been shown to be more compliant after spinal transection, and the extracellular matrix components largely determine its mechanical properties (36). Collagen and elastin, two major connective tissue proteins, provide tensile strength and elasticity and are implicated as being directly responsible for the mechanical changes in the bladder wall of SCI rats (83), so their quantification will give a more accurate picture of potential bladder composition remodeling.
Long-standing detrusor sphincter dyssynergia with chronic SCI, even with careful bladder management, may lead to vesicoureteral reflux due to high bladder pressures from overdistention and complications that include kidney infections, pyelonephritis, and hydronephrosis (4, 13, 59). Bladder infections from multiple daily catheterizations may also spread to the kidney. Chronic kidney disease is highly prevalent in the SCI population, with complications that include decreased glomerular filtration rate and renal plasma flow (30, 60, 73). For the current study, the expression of two proteins indicative of tissue turnover in the kidneys, transforming growth factor-β (TGF-β; a fibrogenic growth factor implicated in the pathogenesis of renal scarring) and cluster of differentiation molecule 11b (CD11b; an adhesion molecule which promotes cell-cell adhesion between leucocytes and leucocyte-endothelial cells in inflammation), were assessed to determine whether improved bladder function with LT could also reduce the kidney's susceptibility to post-SCI complications. TGF-β promotes fibrogenesis, cell apoptosis, and tissue healing and suppresses excess cellular proliferation, differentiation, and immunity (66). Although increased expression of TGF-β is considered a valuable marker in determining fibrosis with kidney disease (1, 11, 38, 94, 95), exacerbation of the immune response or autoimmunity has been reported in association with the absence or decreased expression of TGF-β (37), and the immune system is known to be impacted in persons with SCI (61). Also, damage to kidney tissue in ischemia-reperfusion injury can be mitigated by blocking CD11b (71) as it contributes to epithelial injury, inflammation, and fibrosis (32).
METHODS
All experimental procedures were conducted according to National Institutes of Health guidelines, and protocols were approved by the Institutional Animal Care and Use Committee at the University of Louisville School of Medicine.
A total of 55 adult male Wistar rats (Harlan Sprague Dawley, Indianapolis, IN), weighing initially ∼250 g, were individually housed in an animal room with a 12:12-h light-dark cycle. They had ad libitum access to water and food (Laboratory Rodent Diet).
SCI
The Infinite Horizon (IH) impactor device (Precision Systems and Instrumentation, Fairfax Station, VA) was used to make a clinically relevant contusion injury (225 kdyne) at the T9 spinal level of 48 rats (7 additional rats served as sham surgical controls). This impact produces a moderate to severe incomplete SCI. Procedures for SCI followed our previously published protocols (41, 49, 50, 91). Briefly, animals were anesthetized with a mixture of ketamine (80 mg/kg) and xylazine [10 mg/kg intraperitoneally (ip)] and 0.1-ml supplemental doses were given ip as needed to maintain a deep surgical level of anesthesia. Vital sign monitoring included heart rate with a rodent stethoscope, respiratory rate, and ventilator status with a small animal oximeter (Starr Life Sciences), body temperature with a rectal thermistor, and anesthetic depth with corneal, palpebral, pedal, and pinna reflexes as well as tail pinch.
For the spinal contusion, the cord was exposed at the T9 level via removal of the overlying vertebral lamina, and the IH impactor force-driven device (76) was used to make the contusions (no dwell time). After the injury, a piece of thrombin-soaked Gelfoam was placed into the vertebral defect, the surrounding musculature and subcutaneous tissue were sutured in layers with 4-0 monofilament, and the skin was closed with Michel clips (removed 7 days po). All rats were injected subcutaneously with 0.5 ml of dual penicillin (penicillin G coupled with Procaine, PenJect, Butler Schein, Columbus, OH) as a general prophylactic and recovered in a temperature-controlled environment. In the days immediately following SCI, all animals were also given subcutaneous injections of ketoprofen (Ketofen, 2.5 mg/kg, Fort Dodge Animal Health, Fort Dodge, IA) for analgesia (twice a day for 2 days) and 5 mg/kg gentamicin (GentaFuse, Butler Schein, Dublin, OH) to prevent bladder infections (once per day for 5 days).
The urinary bladder was initially emptied by manual crede every 8 h until the micturition reflex occurred automatically (spontaneously triggered by contact with cage bedding or otherwise without caretaker assistance), 6–10 days after injury (29, 51). Residual urine was collected and measured three times over a 24-h period for every rat on the days immediately postinjury and recorded in the animals' surgery recovery log. Only the maximum single daily urine volume (typically the AM volume after lights on, i.e., after the active phase during which more water tends to be consumed) was used for analysis as this value is most reflective of the largest bladder capacity. Note that the amounts obtained at a 3- to 4-day time point are reflective of the initial peak bladder dysfunction immediately post-SCI with higher volumes indicative of a more severe injury (91). These data along with a 2-wk time point score of overground locomotion using the 21-point Basso-Beattie-Bresnahan (BBB) open-field locomotor test (6) (as previously described in detail) (90, 91) were set aside for end-of-study post hoc analysis to check for pretraining group differences in terms of lesion severity. Training was initiated 14 days postinjury after animals were randomly divided into three study groups (as described below). This delayed time frame was chosen because 1) rehabilitation efforts initiated too early after SCI have been shown to be detrimental as they can exacerbate secondary injury cascades (79) and 2) 2 wk are beyond the period of SCI-induced plasticity of sacral parasympathetic bladder reflex pathways (18, 19, 86) which can occur up to 10 days (29, 51).
Treadmill Training
SCI rats were randomly divided into 3 groups of 16 each 2 wk postinjury: two groups followed a step-training regimen over a treadmill belt assisted by a body weight support system (Exer-3R treadmill, Columbus Instruments) (90, 91), and a nontrained control group used no body support. All groups were harnessed with a cloth rodent vest (Robomedica, Mission Viejo, CA) with spring scales clipped onto both ends of the vest for weight support as needed. The quadrupedal-trained group (n = 16; SCI+QT) stepped on the treadmill with all four limbs, bearing weight on the hindlimbs with manual facilitation below the level of injury (90), with the spring scales self-adjusted to allow for quadrupedal stepping and complete paw placement. The forelimb-only trained group (n = 16; SCI+FT) had their hindlimbs slightly elevated from the surface of the treadmill using the spring scale (non-weight bearing; similar to arm crank exercise in human SCI) so that they only stepped with their forelimbs. The nontrained control group (n = 16; SCI) was also harnessed on the treadmill for the equivalent 1-h time frame but was not stepped. Seven additional rats, used as noninjured controls (surgical shams: spinal cord exposed like the other groups but no contusion), were handled weekly but remained in their home cages for the duration of the study.
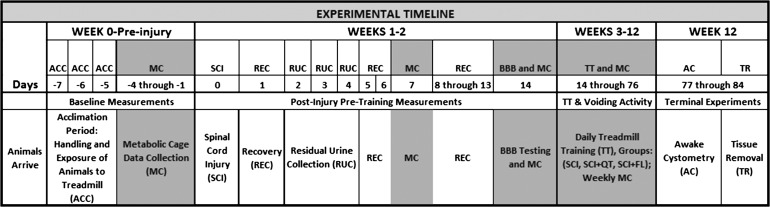
Given the time necessary for the daily step training of each rat, the current study was done in 4 groups of 12 rats each over a period of ∼2 yr. Each set of 12 comprised 4 SCI+QT, 4 SCI+FT, and 4 SCI only control rats. In a given hour session, one SCI+QT and one SCI+FT rat were on the 15-inch-wide treadmill belt with one nontrained SCI rat in a harness next to it. Thus 4 h of training were done in total per day, with 15-min breaks every hour between groups. Each set of 12 rats took part in the study for a total of ∼14 wk (acclimation period, preinjury testing, SCI, 10 wk of training/testing starting 2 wk post-SCI, awake cystometry at the end of training immediately before euthanasia and tissue removal). A time line is provided in Fig. 1.
Fig. 1.
Timeline. Experimental procedures and treadmill training are indicated relative to the time when the spinal cord injury (SCI) was made (day 0). See the text for additional definitions.
Before SCI and post-SCI/pretraining, all rats were exposed to the treadmill environment for at least two half-hour sessions. Training was initiated on schedule at 14 days after injury for all rats, given that there were no signs of infection or other complications arising from the surgery (a rare occurrence). The SCI+QT and SCI+FT groups of animals were at first placed on the treadmill in the harness vest for 20 min, beginning at a slow speed. The session time was gradually increased from 20 min to the target of 58 min over the course of the first 7 training days as described previously (90). All rats on the treadmill were monitored by an experienced investigator at all times. However, to help provide adequate afferent feedback related to stepping for the SCI+QT group (similar procedure done with locomotor training in humans), the experimenter manually assisted for plantar paw placements with full toe extension and no ankle rotation. Independent stepping was encouraged when the rats had achieved better coordination, stability, and absence of hindlimb dragging. Although sessions are terminated early for any animals showing signs of stress (for example, diarrhea, porphyrin stains in eyes, or irregular breathing pattern) or a session skipped if any abrasions from training were observed on the paw or skin (until healed, as noxious input can interfere with spinal learning) (39), there were no such instances in the current study.
Voiding Behavior
Voiding behavior was assessed weekly with a six-station Columbus Instruments Comprehensive Lab Animal Monitoring System (CLAMS). The CLAMS unit and corresponding software (Oxymax for Windows, version 4.83) was used to collect 24-h urination data and food and water intake for all groups of rats. Animals were placed in cages once a week throughout the experiment (including preinjury, the 2 wk post-SCI, and throughout the 10-wk training period) for 24-h data collection periods with food and water ad libitum. Following each 24-h testing session, each metabolic cage was disassembled, cleaned, and reassembled for the next session.
End of Study Awake Cystometry
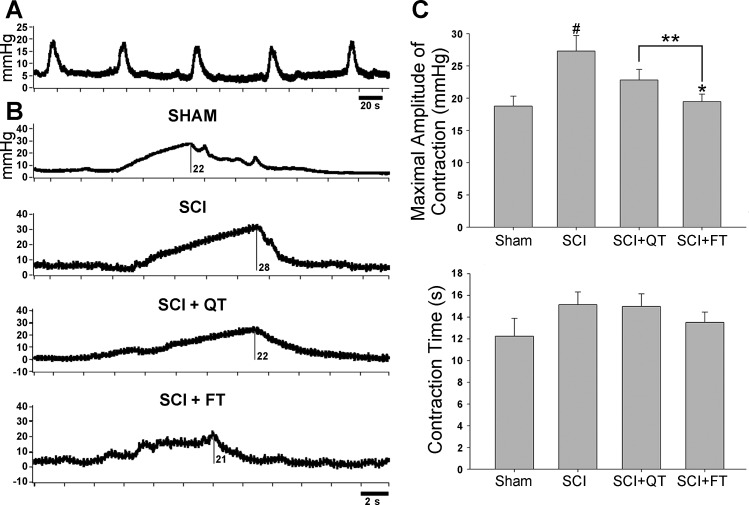
Filling cystometry (nonstop transvesical) experiments were conducted in conscious rats (see Refs. 92 and 93 for justification) after ∼10 wk of training (or equivalent time frame) for all groups. For catheter placement (47, 54, 63), a 1.5-cm midline abdominal incision was made under brief gas anesthesia (2% isoflurane). The catheter (PE-60 tubing), with previously heated tip to form a collar of ∼2 mm from the end, was inserted into the bladder through the dome. The tubing was secured to the bladder with a purse string suture (4-0 Ethilon), exteriorized, and the abdominal muscles and skin were closed with wound clips. All animals were returned to their home cages and closely monitored for the brief recovery period for signs of discomfort or stress. No animal in any group (including spinally intact shams) showed signs of stress or discomfort (e.g., irregular respiratory patterns, vocalization to handling, porphyrin staining around the eyes), and thus no analgesics were administered. Two hours after recovery from implantation, the animal was placed in the harness vest used for training to restrict movement, and the exteriorized catheter was connected to an infusion pump and pressure transducer for saline infusion at a rate of 0.25 ml/min using standard protocols (63, 92, 96). Once the voiding cycles were consistent (at least 5 consecutive voiding events with consistent time intervals in between), five consecutive cycles were recorded using a Neuralynx High Density Electrophysiology System (Lynx-8 amplifier, Neuralynx, Bozeman, MT). Various parameters, including baseline pressure, maximum amplitude of contraction, and contraction time, were retrieved for each of the five contractions and averaged for each animal as described previously (63, 90). Note that it is possible for bladder filling to indirectly induce some pressure on the abdomen and thus potentially some discomfort around the incision site, particularly in the noninjured sham group.
Tissue Removal
Following the cystometry recordings, rats were overdosed with the ketamine/xylazine mixture and immediately perfused with heparinized saline followed by a solution of 30% RNAlater (Ambion, Grand Island, NY) in a 1 mg/ml phosphate buffer solution for tissue retrieval (spinal cord lesion site, kidney and bladder tissue). The bladder was removed, blotted dry, and weighed. The left kidney was also removed and along with the bladder placed in a 100% solution of RNAlater for 24 h at −20°C and then flash frozen in liquid nitrogen and stored at −80°C. As described below, bladder tissue was used for measuring connective tissue proteins that provide tensile strength and elasticity (elastin and collagen) using ELISA. Western blots were done to assess the expression of two proteins in the kidney whose presence are indicative of tissue stress or damage (TGF-β and CD11b). Spinal cord tissue containing the lesion area (T8–T10) was also removed and immersed in 4% paraformaldehyde for at least 48 h, followed by a 30% sucrose/phosphate buffer solution with 1% sodium azide for at least 24 h and until the tissue was cut transversely.
After procedures for the fourth and final set of 12 rats were completed, all collected tissues (coded to maintain blindness of experimenter to group identity) were processed together (see procedures for ELISA and Western blots below). After all experiments were complete, the data were decoded and separated into groups (sham, SCI, SCI+QT, SCI+FT) and analyzed using SigmaStat. One-way ANOVA or one-way repeated measures ANOVA with a significance level of P < 0.05 was used followed by the Holm-Sidak method for pairwise multiple comparisons according to previously published protocols (90, 91).
ELISA for Elastin and Collagen
The amount of collagen and elastin present in bladder tissue was quantified using a collagen assay and elastin assay (Biocolor, Northern Ireland, UK). For collagen analysis, frozen bladder tissue (kept at −80°C) was thawed and placed in an acid-pepsin solution overnight at 4°C to make the collagen soluble. A 100-μl sample containing the tissue or three reference standards was added to a 1.5-ml microcentrifuge tube, and 1 ml of Sircol Dye reagent was added to each tube. Following 30 min on a shaker, tubes were centrifuged for 10 min and then drained leaving the collagen at the bottom of each tube. An acid-salt wash was then applied to remove any remaining dye. A 250-μl solution of alkali reagent was then added to each tube and vortexed for 5 min. Two hundred microliters of standard, blanks, and samples were then pipetted into individual wells of a 96-well plate, which was then read at 555 nm using a Spectramax Plus microplate reader (Molecular Devices, Sunnyvale, CA) to determine the tissue collagen content.
For elastin analysis, bladder tissue was placed in 0.25 M oxalic acid and heated to 100°C for 1 h. This was done twice for each sample. A 100-μl volume of sample, standard, or blank was added to a 1.5 ml microcentrifuge tube followed by 100 μl of elastin precipitating reagent. After 15 min, tubes were centrifuged and drained leaving the α-elastin at the bottom of each tube. A 1-ml volume of dye was then added to each tube and placed on a shaker for 90 min. Tubes were then centrifuged and drained again. Then, 250 μl of dye dissociation reagent was added to each tube and vortexed. Each tube was then revortexed 10 min later. An amount of 200 μl from each tube was then placed in a 96-well plate, which was read at 513 nm using the Spectramax Plus microplate reader to determine the tissue elastin content.
Western Blots for TGF-β and CD11b
Expressions of the proteins TGF-β and CD11b were analyzed with Western blotting following our previously published protocols (40). The left kidney was sectioned (to include both cortex and medulla in the sample) on an ice tray and homogenized in ice-cold lysis buffer [50 mM Tris·HCl (pH 8.0), 200 mM NaCl, 50 mM NaF, 0.3% Triton X-100, 1 M DTT, 1 M benzamidine, 100 mM Na-orthovanadate, 100 mM PMSF] and protease inhibitor (78425, Halt protease inhibitor single-use cocktail, Thermo Scientific). For the protein assay, the Bio-Rad protein assay reagent was used. Protein estimation was done in a photometer at 590-nm absorbance. Samples were loaded with 4× loading buffer (dye) by adjusting the proportion according to the value derived from the estimation. Gels were run in mini-protean gel tanks (Bio-Rad mini-protean tetra system) in 1.5-mm precast gels (456-1083, mini-protean TGX gels, Bio-Rad) at 100 V for 1.5 h. The running buffer was 1× Tris-glycine-SDS buffer. A protein ladder (161-0374, Precision plus protein standards, dual color, Bio-Rad) was used for the band-level detection. Protein was transferred overnight at 30 A on nitrocellulose membranes (162-0115, Bio-Rad) in the transfer buffer (1× Tris-glycine-methanol-SDS buffer). Membranes were then blocked in 3% nonfat dry milk, a TBST solution was applied overnight, and then the membranes were washed the next day with TBST. The membranes were then cut at the level of 75 kDa, the upper portion was incubated in rabbit anti-CD11b (75476, Abcam), and the lower in rabbit anti-TGF-β (3711, Cell Signaling). The membranes were incubated overnight in primary antibody at 1:1,000 dilution (diluted in TBST and 3% nonfat dry milk solution) on a mechanical shaker in a cold room. The following day, membranes were incubated in horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibody (7074, Cell Signaling) for 1 h at room temperature after washing in TBST buffer three times for 5 min each on a mechanical shaker. The membranes were treated with HRP antibody detection reagent (HyGlo chemiluminescent) and exposed to autoradiography film (HyBlot autoradiography film, Denville Scientific). β-Actin (4967S, Cell Signaling) was used as a loading control. After exposure for principal proteins, the membranes were stripped and reprobed with rabbit anti- β-actin antibody at 1:1,000 dilution.
All the samples were run at least three times, and the bands from all the tests were analyzed by inverse densitometry using ImageJ 1.47 (version 1.47, National Institutes of Health) according to our previous protocols (40). Each individual value was normalized by subtracting the background and dividing the value with corresponding β-actin values (minus background). The values obtained from the ImageJ program were then analyzed for the various group comparisons. For the calculation, final raw values for the proteins were obtained after subtracting the background and dividing the result with the loading control. Each value obtained from the calculation for SCI and training groups was normalized with the mean value of shams (i.e., sham controls were set to a value of 1). Statistical analysis included one-way ANOVA with Tukey's honest significant difference post hoc t-tests. All values are expressed as means ± SE. A P value of ≤0.05 was considered statistically significant.
Histology
The lesion site tissue was sectioned on a cryostat (Leica CM 1850) at 30-μm-thickness and stained with Luxol fast blue and cresyl violet (Kluver-Barrera method) per established protocols (41, 90). Spot Advanced software (Diagnostic Instruments, Sterling Heights, MI) and a Nikon E400 microscope were used to obtain measurements for quantification of the lesion epicenter (based on total lesion area) as previously described (41, 91). The percent white matter sparing was determined by dividing the white matter remaining at the epicenter by the average area of white matter present in more intact sections. The intact area of white matter for a given region is estimated by averaging measures from two sections 2 mm rostral and two 2 mm caudal to the epicenter. To compare white matter sparing with bladder function, a multiple regression analysis was performed.
RESULTS
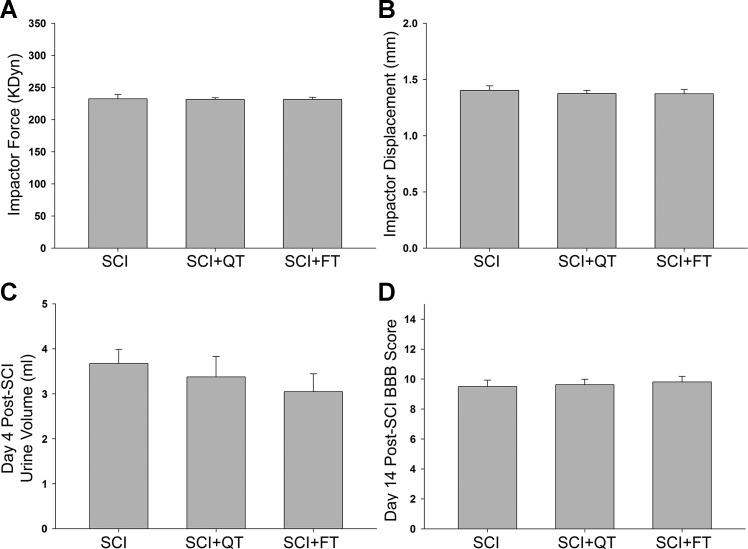
A total of 46 male rats were randomly divided into 3 groups (SCI, SCI+QT, SCI+FT) at 2 wk postinjury (2 of the initial 48 died due to complications following the injury). Post hoc analysis of the group data for contusion parameters (kilodyne force and impactor displacement), 4-day urine volume, and 2-wk BBB locomotor score reveals no significant differences with respect to the injury itself and functional outcomes between the groups before the initiation of training (Fig. 2).
Fig. 2.
Pretraining group data. Post hoc analysis of the computer-generated IH impactor parameters [force (A); displacement (B)] indicate that there were no significant differences among the 3 groups of animals for extent of injury (as anticipated with randomization). The data in A and B are consistent with the lack of functional differences in the bladder (C; maximum residual volume collected from each rat on day 4) and overground locomotion (D) among the groups before the initiation of training, as it is known that larger residual volumes and lower Basso-Beattie-Bresnahan open-field locomotor test results are reflective of greater injury severities (91). SCI (nontrained); SCI+QT (quadrupedal trained); SCI+FT (forelimb-only trained). Values are means ± SE.
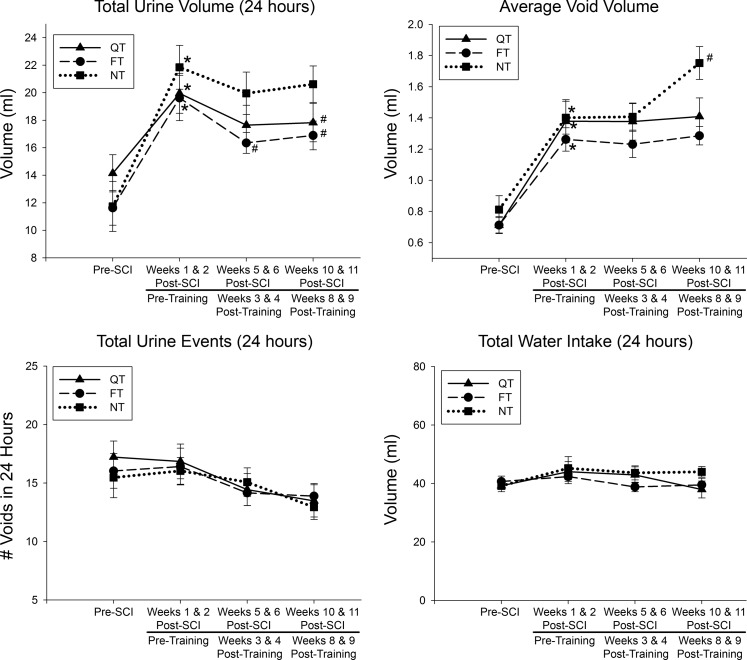
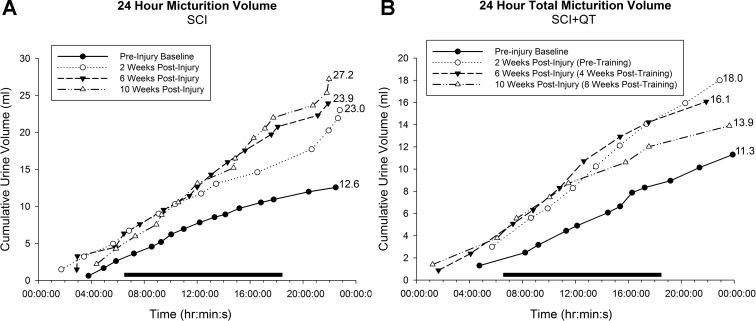
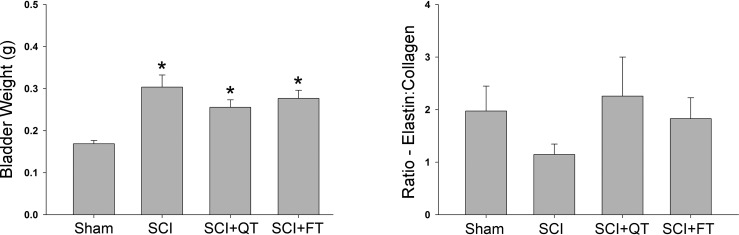
Data from the weekly metabolic cage CLAMS system was generated by Oxymax computer software and saved for post hoc analysis. To avoid potential week-by-week variability, 24-h data obtained from two separate time points were averaged for analysis, including two baseline measures preinjury, each of the 2-wk measures postinjury/pretraining, two midway training time points (weeks 3 and 4), and the last two training week time points [weeks 8 and 9-week 10 (12 post-SCI) was terminal assessment week]. The data for total 24-h urine volume, average volume per void, total number of voids in 24 h, and total 24-h water intake are presented in Fig. 3. A representative example of the 24-h micturition cycle for an SCI and an SCI+QT rat at four different time points (before injury, after injury, midtraining time point, late in training time point) is provided in Fig. 4. Note that the SCI-induced increase in production of urine after injury was not due to an increase in water intake (compare with preinjury control values for all groups in Fig. 3), as we have shown previously (91). The CLAMS data, when considered in their entirety, suggest that the higher volume per void for the SCI control group after the 9-wk training period could be a compensation for the higher 24-h production of urine (polyuria) for that group. The terminal awake cystometry data (Fig. 5) are consistent with these findings, as the maximum amplitude of contraction was significantly higher for only the SCI nontrained group relative to shams, i.e., a higher void volume requiring a larger bladder contraction to empty (#, Fig. 5). Note, however, that the trained SCI animals as a group were also significantly different from the nontrained SCI animals (**P < 0.01), but, individually, only the SCI+FT group was significantly different (*P < 0.01). No other differences were found between groups relative to shams (intercontraction interval and peak pressure; not shown). No differences were found in bladder weight and the ratio of elastin-to-collagen between SCI groups, although bladder weight was significantly higher for all groups at 12 wk post-SCI (P < 0.05; Fig. 6).
Fig. 3.
SCI-induced polyuria. Total urine volume increased significantly post-SCI (*P < 0.05) but was significantly lower after 9 wk of either SCI+QT or SCI+FT but still significantly above baseline (#P < 0.05). The average volume per void was also significantly greater after SCI (*P < 0.05), although posttraining only the nontrained (NT) SCI group had a further increase in average void volume (#P < 0.05). There were no differences found in the total number of urine events or water intake, suggesting that the higher voiding volume for the NT group after training compensated for the higher 24-h production of urine. Note that the surgical sham group was not subjected to this time-consuming testing as each animal served as its own control (i.e., pre-SCI baseline). Values are means ± SE.
Fig. 4.
Twenty-four-hour micturition cycle. Shown are representative total 24-h urine events measured with the CLAMS system for 2 rats, 1 from the SCI control group (A) and 1 from the SCI+QT group (B). Each plotted line represents a different time point of the experiment; preinjury baseline (●); 2 wk postinjury (pretraining; ○); 6 wk postinjury (4-wk posttraining time point in B; ▲); 10 wk postinjury (8-wk post-training time point in B; △). Each individual symbol represents a single urine event that is a cumulative total over the 24-h period. The horizontal bar represents the 12-h phase when the housing facility lights are off (active period). Note that the majority of urine events occur during the active phase.
Fig. 5.
Terminal awake cystometry. Shown are raw recordings of fill/void cycles from each group of animals. In A, 5 full fill/void cycles are shown for a QT animal. In B, a representative example of 1 fill/void cycle (note the scale bar) is provided for each group (different QT animal from A), and the maximum amplitude values are shown (mmHg). The group means of the averaged data is graphed in C. Significant group differences are shown relative to shams (#) and relative to nontrained SCI animals (*, **). Values are means ± SE.
Fig. 6.
Bladder tissue analysis. Bladder weight but not the ratio of collagen to elastin differed significantly at the 12-wk post-SCI time point relative to noninjured surgical shams. No differences were found between the trained and nontrained SCI groups. Values are means ± SE.
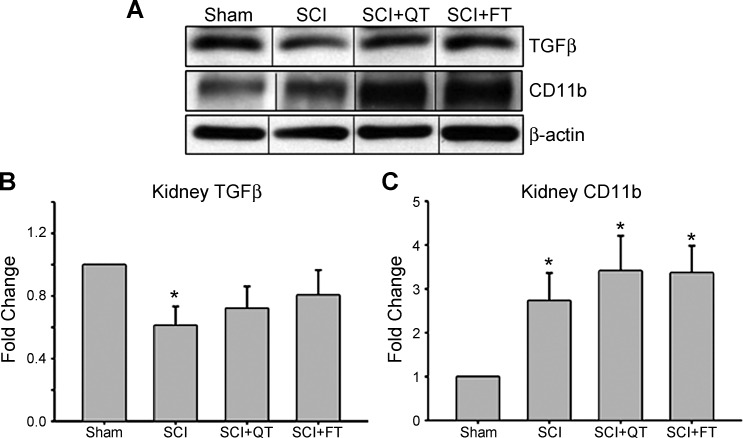
Expression of the proteins TGF-β and CD11b were analyzed with Western blots using the left kidneys from shams and the three SCI groups. For TGF-β, two different bands were detected: one protein band located at 25 kDa and the other at ∼50 kDa. These bands represent mature TGF-β and TGF-β precursors (known to produce a band between 45 and 65 kDa), respectively, per the manufacturer's datasheet. The 25-kDa bands (mature) of TGF-β were analyzed, and a comparison between the expressions in sham vs. SCI (nontrained) animals revealed a significant decrease in kidney TGF-β levels following chronic SCI (Fig. 7B). For the SCI+QT and SCI+FT trained groups, the protein levels were not significantly different from shams (P > 0.05). In contrast, the kidney CD11b levels (location at ∼160 kDa per the manufacturer's datasheet) increased in density at the 12-wk postinjury time point (SCI) and remained significantly higher, relative to shams, in the SCI+QT/SCI+FT training groups (Fig. 7C).
Fig. 7.
Kidney transforming growth factor (TGF)-β and CD11b levels. A: representative examples of kidney TGF-β and CD11b expression levels. B: both SCI+QT and SCI+FT groups had similar expression of TGF-β relative to surgical sham controls. Note that although the SCI trained group levels showed a trend toward sham, they were not significantly different from the nontrained SCI group. C: expression of CD11b was significantly higher relative to shams for all SCI groups, regardless of training. Values are means ± SE. *Significant difference (P < 0.05).
Histological analysis of white matter sparing at the lesion epicenter as well as estimates of the total lesion volume revealed no significant differences between the three groups of SCI rats. A summary of the mean data is presented in Fig. 8. Total lesion volume ranges for the three groups were 9.1–15.6 mm3 (SCI), 9.6–14.7 mm3 (SCI+QT), and 9.0–14.9 mm3 (SCI+FT). Epicenter percent white matter sparing ranges for the three groups were 4.5–32.4% (SCI), 6.1–42.2% (SCI+QT), and 5.9–44.1% (SCI+FT). To further explore the possibility of relationships between injury severity and bladder function, a multiple regression analysis was done, and no relationship was found (P > 0.05) between epicenter white matter sparing and bladder outcomes (including voided volume) within each training group, and the training effect is independent of lesion variability.
Fig. 8.
Lesion histology. No differences were found between the trained and nontrained groups of injured animals at the 12-wk post-SCI time point. Values are means ± SE.
DISCUSSION
The results of the present study demonstrate an impact of exercise training on multiple post-SCI-induced impairments related to various aspects of urinary tract function using metabolic cage frequency-volume measurements of voiding and awake cystometry. Improvements in both the SCI+QT as well as the SCI+FT groups implicate underlying mechanisms beyond repetitive sensory information from the hindlimbs driving spinal network excitability of the lumbosacral urogenital neural circuitry. Furthermore, preliminary evidence for the potential impact of exercise training on the upper urinary tract in addition to the lower urinary tract underscores overall health benefits of activity-based training on the entire urinary system within the SCI population.
SCI-Induced Polyuria
Metabolic cage data indicate that following SCI, mean 24-h urine volume and the average volume per void increased in all groups of animals, a finding consistent with our previous study demonstrating SCI-induced polyuria for a wide range of spinal lesion severities (mild to severe) (91). After 10 wk of activity-based training, total urine volume and average volume per void were significantly lower in both the SCI+QT and SCI+FT groups relative to the SCI group, although the trained group values were still significantly above preinjury baseline. The further increase in average void volume at the later time points could be reflecting better efficiency (i.e., lower residual volumes), which would be consistent with the significantly higher maximum contraction amplitudes for the SCI rats relative to the sham animals as revealed with terminal cystometry. Note that the metabolic cage procedure that was done weekly does not give a measure of residual volume. Future studies would need to involve a time course for awake cystometry with a chronically implanted bladder catheter to further address these novel findings. The benefits regarding the duration of intense physical activity with 60 consecutive min of training (current and previous data on locomotor and nonlocomotor systems including the bladder) (90) but not 30 min of stepping as we have shown previously for SCI-induced polyuria (91), are consistent with a study on locomotion using a rat spinal transection model showing dependence of functional recovery on the number of repetitions of the weight-bearing stepping activity (15).
Potential mechanisms for improving SCI-induced bladder dysfunction with activity-based training include general exercise effects that impact target organ-neural interactions or potential plasticity of the spinal bladder reflex circuitry (such as changes in the properties of afferent neurons in the bladder receptors and/or cell bodies and/or terminals within superficial laminae of the dorsal horn). For example, one likely contributor to the observed SCI-induced polyuria and subsequent alterations in bladder function is hyponatremia (a decrease in the level of serum sodium), which can often occur due to high water content in the blood (55). Hyponatremia is often associated with hyposmolality (a decrease in blood osmolality). One of the easiest ways for the body to increase the level of sodium and increase plasma osmolality is to increase water excretion through urination. Several studies have shown that hyponatremia develops following SCI (10, 33, 70). The trigger that the body uses to increase water excretion in response to hyponatremia and/or hyposmolality is to decrease the release of antidiuretic hormone (ADH; also known as vasopressin) from the posterior pituitary gland (neurohypophysis). When plasma ADH levels are low, the body excretes more water. Importantly, a number of studies involving able-bodied human subjects indicates that exercise can increase plasma ADH levels (45, 87, 88). Given that exercise and LT are rehabilitative therapies that many SCI individuals receive, there is potential for improvement in urological function as long as sufficient amounts are provided [recall that our animal data indicate improvements with 60 but not 30 min (91) per day of intense training]. Our initial data with human SCI research participants (at least 2 yr postinjury) indicate significant improvements in bladder function (as assessed with cystometry) following 80 sessions of LT or LT plus stand training for 60 min/day 5 days/wk (48). Note that if polyuria following SCI is triggered by hyponatremia and/or hyposmolality, then there should be a decrease in the plasma ADH levels post-SCI. Recent data from our laboratory using male rats with a T9 contusion indicate that serum ADH levels decrease significantly 2 wk after severe SCI (unpublished observations), which is consistent with our metabolic cage data showing significantly elevated total 24-h urine volume at 2 wk (Fig. 3). Further studies are in progress. Anecdotally, SCI individuals often report limiting fluid intake to decrease the number of catheterizations over a 24-h period. However, this lack of fluid intake can lead to other metabolic consequences (77). Although polyuria is common after SCI (56), few studies have investigated the mechanisms underlying this condition.
An additional potential mechanism for the observed changes in urological function with LT could involve somato-pudendal reflex interactions such as the flexor and extensor reflex circuitry interactions with external urethral sphincter reflexes as described previously by Tai et al. (81). Motor and autonomic output of the spinal cord is driven in large part by afferent input and local or propriospinal circuitry emphasized after SCI conditions (7, 12, 17, 34, 44), which create the potential for interactions and the triggering of some plasticity within the lumbosacral circuitry to the bladder. For example, multiple sensory inputs from the periphery during locomotion, particularly limb loading (28) and stepping rate (31), provide information to these networks to improve stepping (16, 21, 22, 24, 25). These interactions, however, would not explain the improvement observed with the forelimb exercise group of rats receiving 60 min of daily training. It is conceivable, however, that intense repetitive forelimb exercise with sufficient spinal network excitability via residual supraspinal input induces a net improvement in functional reorganization of the lumbosacral neural circuitry (14, 58, 67, 78). In individuals with an incomplete spinal cord injury, there is some facilitation of the lower extremity muscles in those subjects when walking with reciprocal arm swing vs. without (walking while holding on to parallel bars) (8, 85). Specifically, the presence of some residual intact long propriospinal interenlargement pathways that mediate interlimb coordination for locomotor function in the rat (72) could induce adaptive changes to neural networks within the lumbosacral spinal cord, such as those controlling the bladder. However, given the severity of injury (22.0 ± 2.7% white matter sparing at the epicenter for the forelimb-trained SCI group) and the fact that there was little or no hindlimb activity in the FT group during training and no air-stepping, the probability for the occurrence of such interactions is likely low. Also note that the animals with the most spared white matter did not have the best recovery of bladder function. Thus, although load-bearing on the hindlimb has been shown to be of critical importance for stepping (82), other systemic factors may be vital contributors for improving bladder function with task-specific training based rehabilitation.
Kidney Findings
The results from the present study demonstrate that after a clinically relevant spinal contusion injury, there is a significantly lower level of TGF-β expression in the kidney (NT group) relative to shams. Since TGF-β controls T cell activation and abolition of TGF-β causes gradual infiltration of leucocytes into multiple organs (37, 62, 65), our finding may indicate the presence of an altered immune response in the kidneys during the chronic phase after SCI. In addition to lower TGF-β levels, there was a corresponding rise of CD11b expression in the kidney after chronic SCI (SCI group relative to shams). TGF-β has a known inhibitory effect on CD11b expression (5), which may explain the post-SCI increase in CD11b activity. Note that activation of macrophages, which play a role in both injury and repair in kidney tissue (27), is required for the synthesis of TGF-β, so a rise in CD11b expression may also indicate activation of endogenous macrophages for tissue homeostasis.
In both SCI training groups, the level of TGF-β expression was not significantly different from the levels in surgical sham animals, indicating the possibility of immune homeostasis and maintenance of renal health (normalized glomerular filtration rate and kidney function) and decreased susceptibility to infection, which would impact the quality of life for the SCI population. Exercise has been shown to reverse negative immune alterations (35), including after SCI (61), but the time since injury and the ideal starting point of training as well as the duration and intensity (57, 89) need further consideration to optimize the most effective strategy not just for urinary tract function but functional recovery in general.
Perspectives and Significance
Bladder dysfunction after SCI is rarely studied in experimental animals yet is overwhelmingly the most significant concern for those suffering from SCI, and urological complications result in significant morbidity and mortality. Importantly, even small improvements in bladder function can have a tremendous impact on these individuals' continual health and quality of life. Results from this animal study provide some initial clues about potential underlying mechanisms regarding our findings on the effects of activity-dependent plasticity induced by LT after chronic SCI on nonlocomotor systems (i.e., bladder function).
Conclusions
Our studies to date indicate that activity-based training can influence urological outcomes, which are of great importance to persons with SCI. The positive benefits of exercise on bladder function post-SCI are likely indirect. These novel findings suggest that physical activity after SCI could translate to significant quality of life gains.
GRANTS
This study was supported by Department of Defense Grant W81XWH-11-1-0668. This project utilized KSCIRC Neuroscience Core facilities supported by National Institutes of Health/National Center for Research Resources Grant P30 8P30GM103507.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.H.H. and S.J.H. contributed conception and design of research; C.H.H., L.R.M., J.D.F., J.E.A., P.P., and A.N.H. analyzed data; C.H.H., L.R.M., P.P., A.N.H., and S.J.H. interpreted results of experiments; C.H.H., L.R.M., J.D.F., J.E.A., and P.P. prepared figures; C.H.H. and P.P. drafted manuscript; C.H.H., L.R.M., P.P., A.N.H., and S.J.H. edited and revised manuscript; C.H.H., L.R.M., J.D.F., J.E.A., P.P., A.N.H., and S.J.H. approved final version of manuscript; L.R.M., J.D.F., J.E.A., P.P., and A.N.H. performed experiments.
ACKNOWLEDGMENTS
The authors thank Christine Yarberry and Darlene Burke for assistance.
REFERENCES
- 1.Abbate M, Zoja C, Morigi M, Rottoli D, Angioletti S, Tomasoni S, Zanchi C, Longaretti L, Donadelli R, Remuzzi G. Transforming growth factor-beta1 is up-regulated by podocytes in response to excess intraglomerular passage of proteins: a central pathway in progressive glomerulosclerosis. Am J Pathol 161: 2179–2193, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma 21: 1371–1383, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Anderson KD, Borisoff JF, Johnson RD, Stiens SA, Elliott SL. The impact of spinal cord injury on sexual function: concerns of the general population. Spinal Cord 45: 328–337, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Balsara ZR, Ross SS, Dolber PC, Wiener JS, Tang Y, Seed PC. Enhanced susceptibility to urinary tract infection in the spinal cord-injured host with neurogenic bladder. Infect Immun 81: 3018–3026, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basoni C, Nobles M, Grimshaw A, Desgranges C, Davies D, Perretti M, Kramer IM, Genot E. Inhibitory control of TGF-beta1 on the activation of Rap1, CD11b, and transendothelial migration of leukocytes. FASEB J 19: 822–824, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12: 1–21, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Beaumont E, Kaloustian S, Rousseau G, Cormery B. Training improves the electrophysiological properties of lumbar neurons and locomotion after thoracic spinal cord injury in rats. Neurosci Res 62: 147–154, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Behrman AL, Harkema SJ. Locomotor training after human spinal cord injury: a series of case studies. Phys Ther 80: 688–700, 2000. [PubMed] [Google Scholar]
- 9.Behrman AL, Lawless-Dixon AR, Davis SB, Bowden MG, Nair P, Phadke C, Hannold EM, Plummer P, Harkema SJ. Locomotor training progression and outcomes after incomplete spinal cord injury. Phys Ther 85: 1356–1371, 2005. [PubMed] [Google Scholar]
- 10.Biyani A, Inman CG, el Masry WS. Hyponatraemia after acute spinal injury. Injury 24: 671–673, 1993. [DOI] [PubMed] [Google Scholar]
- 11.Border WA, Okuda S, Languino LR, Ruoslahti E. Transforming growth factor-beta regulates production of proteoglycans by mesangial cells. Kidney Int 37: 689–695, 1990. [DOI] [PubMed] [Google Scholar]
- 12.Button DC, Kalmar JM, Gardiner K, Marqueste T, Zhong H, Roy RR, Edgerton VR, Gardiner PF. Does elimination of afferent input modify the changes in rat motoneurone properties that occur following chronic spinal cord transection? J Physiol 586: 529–544, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardenas DD, Hooton TM. Urinary tract infection in persons with spinal cord injury. Arch Phys Med Rehabil 76: 272–280, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Carp JS, Wolpaw JR. Motoneuron plasticity underlying operantly conditioned decrease in primate H-reflex. J Neurophysiol 72: 431–442, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Cha J, Heng C, Reinkensmeyer DJ, Roy RR, Edgerton VR, De Leon RD. Locomotor ability in spinal rats is dependent on the amount of activity imposed on the hindlimbs during treadmill training. J Neurotrauma 24: 1000–1012, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Colombo G, Wirz M, Dietz V. Effect of locomotor training related to clinical and electrophysiological examinations in spinal cord injured humans. Ann NY Acad Sci 860: 536–538, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Cope TC, Bodine SC, Fournier M, Edgerton VR. Soleus motor units in chronic spinal transected cats: physiological and morphological alterations. J Neurophysiol 55: 1202–1220, 1986. [DOI] [PubMed] [Google Scholar]
- 18.de Groat WC, Araki I, Vizzard MA, Yoshiyama M, Yoshimura N, Sugaya K, Tai C, Roppolo JR. Developmental and injury induced plasticity in the micturition reflex pathway. Behav Brain Res 92: 127–140, 1998. [DOI] [PubMed] [Google Scholar]
- 19.de Groat WC, Kruse MN, Vizzard MA, Cheng CL, Araki I, Yoshimura N. Modification of urinary bladder function after spinal cord injury. Adv Neurol 72: 347–364, 1997. [PubMed] [Google Scholar]
- 20.Denny-Brown D, Robertson E. The state of the bladder and its sphincter in complete transverse lesions of the spinal cord and cauda equina. Brain 56: 397–469, 1933. [Google Scholar]
- 21.Dietz V. Locomotor recovery after spinal cord injury. Trends Neurosci 20: 346–347, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Dietz V, Colombo G, Jensen L. Locomotor activity in spinal man. Lancet 344: 1260–1263, 1994. [DOI] [PubMed] [Google Scholar]
- 23.Dietz V, Harkema SJ. Locomotor activity in spinal cord-injured persons. J Appl Physiol 96: 1954–1960, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Dietz V, Quintern J, Sillem M. Stumbling reactions in man: significance of proprioceptive and pre-programmed mechanisms. J Physiol 386: 149–163, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dietz V, Wirz M, Curt A, Colombo G. Locomotor pattern in paraplegic patients: training effects and recovery of spinal cord function. Spinal Cord 36: 380–390, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Ditunno PL, Patrick M, Stineman M, Ditunno JF. Who wants to walk? Preferences for recovery after SCI: a longitudinal and cross-sectional study. Spinal Cord 46: 500–506, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Duffield JS. Macrophages and immunologic inflammation of the kidney. Semin Nephrol 30: 234–254, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edgerton VR, Roy RR, Hodgson JA, Prober RJ, de Guzman CP, de Leon R. A physiological basis for the development of rehabilitative strategies for spinally injured patients. J Am Paraplegia Soc 14: 150–157, 1991. [DOI] [PubMed] [Google Scholar]
- 29.Ferrero SL, Brady TD, Dugan VP, Armstrong JE, Hubscher CH, Johnson RD. Effects of lateral funiculus sparing, spinal lesion level, and gender on recovery of bladder voiding reflexes and hematuria in rats. J Neurotrauma 32: 200–208, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischer MJ, Krishnamoorthi VR, Smith BM, Evans CT, St Andre JR, Ganesh S, Huo Z, Stroupe KT. Prevalence of chronic kidney disease in patients with spinal cord injuries/disorders. Am J Nephrol 36: 542–548, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Forssberg H, Grillner S, Halbertsma J. The locomotion of the low spinal cat. I. Coordination within a hindlimb. Acta Physiol Scand 108: 269–281, 1980. [DOI] [PubMed] [Google Scholar]
- 32.Fujiu K, Manabe I, Nagai R. Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J Clin Invest 121: 3425–3441, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furlan JC, Fehlings MG. Hyponatremia in the acute stage after traumatic cervical spinal cord injury: clinical and neuroanatomic evidence for autonomic dysfunction. Spine 34: 501–511, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Gardiner PF. Changes in alpha-motoneuron properties with altered physical activity levels. Exerc Sport Sci Rev 34: 54–58, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 11: 607–615, 2011. [DOI] [PubMed] [Google Scholar]
- 36.Gloeckner DC, Sacks MS, Fraser MO, Somogyi GT, de Groat WC, Chancellor MB. Passive biaxial mechanical properties of the rat bladder wall after spinal cord injury. J Urol 167: 2247–2252, 2002. [PubMed] [Google Scholar]
- 37.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 12: 171–181, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Goumenos DS, Tsakas S, El Nahas AM, Alexandri S, Oldroyd S, Kalliakmani P, Vlachojannis JG. Transforming growth factor-beta(1) in the kidney and urine of patients with glomerular disease and proteinuria. Nephrol Dial Transplant 17: 2145–2152, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Grau JW, Washburn SN, Hook MA, Ferguson AR, Crown ED, Garcia G, Bolding KA, Miranda RC. Uncontrollable stimulation undermines recovery after spinal cord injury. J Neurotrauma 21: 1795–1817, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Gupta DS, Hubscher CH. Estradiol treatment prevents injury induced enhancement in spinal cord dynorphin expression. Front Physiol 3: 28, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hall BJ, Lally JE, Vukmanic EV, Armstrong JE, Fell JD, Gupta DS, Hubscher CH. Spinal cord injuries containing asymmetrical damage in the ventrolateral funiculus is associated with a higher incidence of at-level allodynia. J Pain 11: 864–875, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377: 1938–1947, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harkema SJ, Hillyer J, Schmidt-Read M, Ardolino E, Sisto SA, Behrman AL. Locomotor training: as a treatment of spinal cord injury and in the progression of neurologic rehabilitation. Arch Phys Med Rehabil 93: 1588–1597, 2012. [DOI] [PubMed] [Google Scholar]
- 44.Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol 77: 797–811, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Hew-Butler T, Noakes TD, Soldin SJ, Verbalis JG. Acute changes in arginine vasopressin, sweat, urine and serum sodium concentrations in exercising humans: does a coordinated homeostatic relationship exist? Br J Sports Med 44: 710–715, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horst M, Heutschi J, van den Brand R, Andersson KE, Gobet R, Sulser T, Courtine G, Eberli D. Multisystem neuroprosthetic training improves bladder function after severe spinal cord injury. J Urol 189: 747–753, 2013. [DOI] [PubMed] [Google Scholar]
- 47.Hubscher CH, Gupta DS, Brink TS. Convergence and cross talk in urogenital neural circuitries. J Neurophysiol 110: 1997–2005, 2013. [DOI] [PubMed] [Google Scholar]
- 48.Hubscher CH, Herrity AN, Montgomery LR, Willhite AM, Angeli CA, Harkema SJ. Task-specific training-based rehabilitation improves bladder outcomes following human spinal cord injury. In: 2015 Abstract Viewer/Itinerary Planner, Society for Neuroscience. Chicago, IL: Society for Neuroscience, 2015. [Google Scholar]
- 49.Hubscher CH, Johnson RD. Chronic spinal cord injury induced changes in the responses of thalamic neurons. Exp Neurol 197: 177–188, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Hubscher CH, Johnson RD. Effects of acute and chronic midthoracic spinal cord injury on neural circuits for male sexual function. I. Ascending pathways. J Neurophysiol 82: 1381–1389, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Hubscher CH, Johnson RD. Effects of acute and chronic midthoracic spinal cord injury on neural circuits for male sexual function. II. Descending pathways. J Neurophysiol 83: 2508–2518, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Jayaraman A, Shah P, Gregory C, Bowden M, Stevens J, Bishop M, Walter G, Behrman A, Vandenborne K. Locomotor training and muscle function after incomplete spinal cord injury: case series. J Spinal Cord Med 31: 185–193, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jolesz FA, Cheng-Tao X, Ruenzel PW, Henneman E. Flexor reflex control of the external sphincter of the urethra in paraplegia. Science 216: 1243–1245, 1982. [DOI] [PubMed] [Google Scholar]
- 54.Kaddumi EG, Hubscher CH. Convergence of multiple pelvic organ inputs in the rat rostral medulla. J Physiol 572: 393–405, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khokhar AM, Ramage CM, Slater JD. Radioimmunoassay of arginine-vasopressin in human urine and its use in physiological and pathological states. J Endocrinol 79: 375–389, 1978. [DOI] [PubMed] [Google Scholar]
- 56.Kilinc S, Akman MN, Levendoglu F, Ozker R. Diurnal variation of antidiuretic hormone and urinary output in spinal cord injury. Spinal Cord 37: 332–335, 1999. [DOI] [PubMed] [Google Scholar]
- 57.Kliesch WF, Cruse JM, Lewis RE, Bishop GR, Brackin B, Lampton JA. Restoration of depressed immune function in spinal cord injury patients receiving rehabilitation therapy. Paraplegia 34: 82–90, 1996. [DOI] [PubMed] [Google Scholar]
- 58.Knikou M, Angeli CA, Ferreira CK, Harkema SJ. Soleus H-reflex gain, threshold, and amplitude as function of body posture and load in spinal cord intact and injured subjects. Int J Neurosci 119: 2056–2073, 2009. [DOI] [PubMed] [Google Scholar]
- 59.Ku JH, Choi WJ, Lee KY, Jung TY, Lee JK, Park WH, Shim HB. Complications of the upper urinary tract in patients with spinal cord injury: a long-term follow-up study. Urol Res 33: 435–439, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Kuhlemeier KV, McEachran AB, Lloyd LK, Stover SL, Tauxe WN, Dubovsky EV, Fine PR. Renal function after acute and chronic spinal cord injury. J Urol 131: 439–445, 1984. [DOI] [PubMed] [Google Scholar]
- 61.Leicht CA, Goosey-Tolfrey VL, Bishop NC. Spinal cord injury: known and possible influences on the immune response to exercise. Exerc Immunol Rev 19: 144–163, 2013. [PubMed] [Google Scholar]
- 62.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25: 455–471, 2006. [DOI] [PubMed] [Google Scholar]
- 63.Maggi CA, Santicioli P, Meli A. The nonstop transvesical cystometrogram in urethane-anesthetized rats: a simple procedure for quantitative studies on the various phases of urinary bladder voiding cycle. J Pharmacol Methods 15: 157–167, 1986. [DOI] [PubMed] [Google Scholar]
- 64.Mai J, Pedersen E. Clonus depression by propranolol. Acta Neurol Scand 53: 395–398, 1976. [DOI] [PubMed] [Google Scholar]
- 65.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity 25: 441–454, 2006. [DOI] [PubMed] [Google Scholar]
- 66.Moustakas A, Pardali K, Gaal A, Heldin CH. Mechanisms of TGF-beta signaling in regulation of cell growth and differentiation. Immunol Lett 82: 85–91, 2002. [DOI] [PubMed] [Google Scholar]
- 67.Nielsen J, Petersen N, Ballegaard M, Biering-Sorensen F, Kiehn O. H-reflexes are less depressed following muscle stretch in spastic spinal cord injured patients than in healthy subjects. Exp Brain Res 97: 173–176, 1993. [DOI] [PubMed] [Google Scholar]
- 68.Pagliacci MC, Franceschini M, Di Clemente B, Agosti M, Spizzichino L. A multicentre follow-up of clinical aspects of traumatic spinal cord injury. Spinal Cord 45: 404–410, 2007. [DOI] [PubMed] [Google Scholar]
- 69.Pedersen E, Petersen T, Schroder HD. Relation between flexor spasms, uninhibited detrusor contractions and anal sphincter activity. J Neurol Neurosurg Psychiatry 49: 273–277, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peruzzi WT, Shapiro BA, Meyer PR Jr, Krumlovsky F, Seo BW. Hyponatremia in acute spinal cord injury. Crit Care Med 22: 252–258, 1994. [DOI] [PubMed] [Google Scholar]
- 71.Rabb H, Mendiola CC, Dietz J, Saba SR, Issekutz TB, Abanilla F, Bonventre JV, Ramirez G. Role of CD11a and CD11b in ischemic acute renal failure in rats. Am J Physiol Renal Fluid Electrolyte Physiol 267: F1052–F1058, 1994. [DOI] [PubMed] [Google Scholar]
- 72.Reed WR, Shum-Siu A, Whelan A, Onifer SM, Magnuson DS. Anterograde labeling of ventrolateral funiculus pathways with spinal enlargement connections in the adult rat spinal cord. Brain Res 1302: 76–84, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodriguez-Romero V, Cruz-Antonio L, Franco-Bourland RE, Guizar-Sahagun G, Castaneda-Hernandez G. Changes in renal function during acute spinal cord injury: implications for pharmacotherapy. Spinal Cord 51: 528–531, 2013. [DOI] [PubMed] [Google Scholar]
- 74.Sato A, Sato Y, Sugimoto H, Tervi N. Reflex changes in the urinary bladder after mechanical and thermal stimulation of the skin at various segmental levels in cats. Neuroscience 2: 111–117, 1977. [DOI] [PubMed] [Google Scholar]
- 75.Schalow G. Cure of urinary bladder functions in severe (95%) motoric complete cervical spinal cord injury in human. Electromyogr Clin Neurophysiol 50: 155–179, 2010. [PubMed] [Google Scholar]
- 76.Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE Jr. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma 20: 179–193, 2003. [DOI] [PubMed] [Google Scholar]
- 77.Shirreffs SM, Merson SJ, Fraser SM, Archer DT. The effects of fluid restriction on hydration status and subjective feelings in man. Br J Nutr 91: 951–958, 2004. [DOI] [PubMed] [Google Scholar]
- 78.Skinner RD, Houle JD, Reese NB, Berry CL, Garcia-Rill E. Effects of exercise and fetal spinal cord implants on the H-reflex in chronically spinalized adult rats. Brain Res 729: 127–131, 1996. [PubMed] [Google Scholar]
- 79.Smith RR, Brown EH, Shum-Siu A, Whelan A, Burke DA, Benton RL, Magnuson DS. Swim training initiated acutely after spinal cord injury is ineffective and induces extravasation in and around the epicenter. J Neurotrauma 26: 1017–1027, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stover SL, DeVivo MJ, Go BK. History, implementation, and current status of the National Spinal Cord Injury Database. Arch Phys Med Rehabil 80: 1365–1371, 1999. [DOI] [PubMed] [Google Scholar]
- 81.Tai C, Roppolo JR, de Groat WC. Spinal reflex control of micturition after spinal cord injury. Restor Neurol Neurosci 24: 69–78, 2006. [PMC free article] [PubMed] [Google Scholar]
- 82.Timoszyk WK, Nessler JA, Acosta C, Roy RR, Edgerton VR, Reinkensmeyer DJ, de Leon R. Hindlimb loading determines stepping quantity and quality following spinal cord transection. Brain Res 1050: 180–189, 2005. [DOI] [PubMed] [Google Scholar]
- 83.Toosi KK, Nagatomi J, Chancellor MB, Sacks MS. The effects of long-term spinal cord injury on mechanical properties of the rat urinary bladder. Ann Biomed Eng 36: 1470–1480, 2008. [DOI] [PubMed] [Google Scholar]
- 84.Trimble MH, Behrman AL, Flynn SM, Thigpen MT, Thompson FJ. Acute effects of locomotor training on overground walking speed and H-reflex modulation in individuals with incomplete spinal cord injury. J Spinal Cord Med 24: 74–80, 2001. [DOI] [PubMed] [Google Scholar]
- 85.Visintin M, Barbeau H. The effects of parallel bars, body weight support and speed on the modulation of the locomotor pattern of spastic paretic gait. A preliminary communication. Paraplegia 32: 540–553, 1994. [DOI] [PubMed] [Google Scholar]
- 86.Vizzard MA. Neurochemical plasticity and the role of neurotrophic factors in bladder reflex pathways after spinal cord injury. Prog Brain Res 152: 97–115, 2006. [DOI] [PubMed] [Google Scholar]
- 87.Wade CE. Response, regulation, and actions of vasopressin during exercise: a review. Med Sci Sports Exerc 16: 506–511, 1984. [DOI] [PubMed] [Google Scholar]
- 88.Wade CE, Claybaugh JR. Plasma renin activity, vasopressin concentration, and urinary excretory responses to exercise in men. J Appl Physiol Respir Environ Exercise Physiol 49: 930–936, 1980. [DOI] [PubMed] [Google Scholar]
- 89.Wang J, Song H, Tang X, Yang Y, Vieira VJ, Niu Y, Ma Y. Effect of exercise training intensity on murine T-regulatory cells and vaccination response. Scand J Med Sci Sports 22: 643–652, 2012. [DOI] [PubMed] [Google Scholar]
- 90.Ward PJ, Herrity AN, Smith RR, Willhite A, Harrison BJ, Petruska JC, Harkema SJ, Hubscher CH. Novel multi-system functional gains via task specific training in spinal cord injured male rats. J Neurotrauma 31: 819–833, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ward PJ, Hubscher CH. Persistent polyuria in a rat spinal contusion model. J Neurotrauma 29: 2490–2498, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yoshiyama M, Nezu FM, Yokoyama O, de Groat WC, Chancellor MB. Changes in micturition after spinal cord injury in conscious rats. Urology 54: 929–933, 1999. [DOI] [PubMed] [Google Scholar]
- 93.Yoshiyama M, Roppolo JR, Takeda M, de Groat WC. Effects of urethane on reflex activity of lower urinary tract in decerebrate unanesthetized rats. Am J Physiol Renal Physiol 304: F390–F396, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu L, Border WA, Huang Y, Noble NA. TGF-beta isoforms in renal fibrogenesis. Kidney Int 64: 844–856, 2003. [DOI] [PubMed] [Google Scholar]
- 95.Zhou TB, Qin YH, Lei FY, Zhao YJ, Huang WF. Association of PAX2 with cell apoptosis in unilateral ureteral obstruction rats. Ren Fail 34: 194–202, 2012. [DOI] [PubMed] [Google Scholar]
- 96.Zinck ND, Rafuse VF, Downie JW. Sprouting of CGRP primary afferents in lumbosacral spinal cord precedes emergence of bladder activity after spinal injury. Exp Neurol 204: 777–790, 2007. [DOI] [PubMed] [Google Scholar]