Abstract
Myogenic contraction is the principal component of renal autoregulation that protects the kidney from hypertensive barotrauma. Contractions are initiated by a rise in perfusion pressure that signals a reduction in membrane potential (Em) of vascular smooth muscle cells to activate voltage-operated Ca2+ channels. Since ROS have variable effects on myogenic tone, we investigated the hypothesis that superoxide (O2·−) and H2O2 differentially impact myogenic contractions. The myogenic contractions of mouse isolated and perfused single afferent arterioles were assessed from changes in luminal diameter with increasing perfusion pressure (40–80 mmHg). O2·−, H2O2, and Em were assessed by fluorescence microscopy during incubation with paraquat to increase O2·− or with H2O2. Paraquat enhanced O2·− generation and myogenic contractions (−42 ± 4% vs. −19 ± 4%, P < 0.005) that were blocked by SOD but not by catalase and signaled via PKC. In contrast, H2O2 inhibited the effects of paraquat and reduced myogenic contractions (−10 ± 1% vs. −19 ± 2%, P < 0.005) and signaled via PKG. O2·− activated Ca2+-activated Cl− channels that reduced Em, whereas H2O2 activated Ca2+-activated and voltage-gated K+ channels that increased Em. Blockade of voltage-operated Ca2+ channels prevented the enhanced myogenic contractions with paraquat without preventing the reduction in Em. Myogenic contractions were independent of the endothelium and largely independent of nitric oxide. We conclude that O2·− and H2O2 activate different signaling pathways in vascular smooth muscle cells linked to discreet membrane channels with opposite effects on Em and voltage-operated Ca2+ channels and therefore have opposite effects on myogenic contractions.
Keywords: reactive oxygen species, renal autoregulation, chloride channels, potassium channels, protein kinase C, protein kinase G
renal autoregulation maintains a stable renal blood flow and glomerular capillary pressure during changes in blood pressure by adjustments of the tone of the afferent arterioles that are the main resistance vessel of the kidney (8). A breakdown of renal autoregulation in hypertensive chronic kidney disease (CKD) can accelerate renal damage by transmitting elevated blood pressure into sensitive glomerular capillaries (37), their podocytes (22), and the renal parenchyma (termed barotrauma) (32). Renal autoregulation is bolstered by tubuloglomerular feedback and a third mechanism but depends primarily on a myogenic contraction that entails a rapid intrinsic contraction of vascular smooth muscle cells (VSMCs) during increased perfusion pressure (6, 8).
An increase in perfusion pressure reduces the membrane potential (Em) of VSMCs of resistance vessels and opens voltage-operated Ca2+ channels (VOCCs) to raise intracellular Ca2+ that initiates myogenic contractions (8). Increases in the perfusion pressure of afferent arterioles generate ROS that have been previously variously reported to mediate (26, 27), leave unchanged (41), or impair (13, 44) autoregulation or myogenic contractions. The two primary ROS are superoxide (O2·−) and H2O2. Therefore, we tested the hypothesis that O2·− and H2O2 activate different signaling mechanisms and membrane channels that differentially change Em, VOCCs, and myogenic contractions. This has clinical impact since a defect in renal autoregulation (17), especially in the presence of hypertension (8, 17, 22), accelerates the progression of CKD and nonselective antioxidants given to models of CKD have been reported either to be successful (25), ineffective (40), or harmful (33) in protecting the kidney from further damage. The first step to therapeutic progress should be a clearer definition of the preferred target.
Paraquat (N,N′-dimethyl-4,4′-bipyridinium dichloride) is a redox-cycling quinolone. It undergoes a single electron reduction in tissues, forming a free radical that is rapidly oxidized by molecular oxygen to generate the superoxide anion radical. Therefore, paraquat was used to generate intracellular O2·− (42) to study the graded effects of O2·− on myogenic tone. PKC is activated by O2·− (19) and can mediate myogenic contractions (18) by activating Cl− channels (30) that can reduce Em of VSMCs (3) and afferent arterioles (21). Ca2+-activated Cl− channels (CaCCs) have been proposed recently to play a key role in the depolarization of Em of VSMCs during a myogenic contraction (10). However, their role needs further definition since afferent arteriolar myogenic contractions persist after nonselective blockade of Cl− channels (8, 47). We tested the hypothesis that O2·− activates PKC signaling to open CaCCs to depolarize Em that disinhibits VOCCs to increase cellular Ca2+ entry to enhance myogenic contractions.
Unlike O2·−, low concentrations of H2O2 can dilate arterioles (9), although opposite effects have also been reported in some studies (39). H2O2 can dimerize and activate PKG in VSMCs (5, 55) and activate large-conductance Ca2+-activated K+ (BKCa) channels in human coronary arterioles (49) to increase Em and cause relaxation (55). However, although BKCa channels modulate autoregulation in the cerebral circulation (20), they are usually quiescent in renal afferent arterioles (12, 38), which therefore requires further study. We tested the hypothesis that H2O2 activates PKG to hyperpolarize Em by activation of specific K+ channels to blunt myogenic contractions.
Myogenic contractions were studied directly in single afferent arterioles dissected from mouse kidneys from the measured reduction in luminal diameter during increased perfusion pressure across a physiological relevant range of 40–80 mmHg (25–27). Arteriolar O2·− was quantified from pegalated (PEG)-SOD-inhibitable ethidium-to-dihydroethidium (E:DHE) fluorescence, H2O2 from PEG-catalase-inhibitable 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (C-H2DCFDA) fluorescence, and Em from bis-(1,3-dibutylbarbituric acid)trimethine oxonol [DiBAC4(3)] fluorescence.
The results demonstrated that O2·− and H2O2 activate two distinct signaling pathways with opposite effects on VOCCs and myogenic contractions. These opposing effects of the principal ROS may underlie some of the conflicting reports of the effects of oxidative stress on renal autoregulation and myogenic contractions in disease models and, by inference, on the progression of CKD.
METHODS
Isolation and perfusion of afferent arterioles.
Male C57Bl/6 mice (Charles River, Germantown, MD), aged 3–4 mo and weighing 25–30 g, were fed regular mouse chow and euthanized. All procedures conformed with the National Institutes of Health Guide for Care and Use of Laboratory Animals. Experiments were approved by the Georgetown University Animal Care and Use Committee. Mice were euthanized, one kidney was removed from the mouse, and a single afferent arteriole with an attached glomerulus was dissected. The arteriolar lumen was perfused via a pipette containing an internal pressure pipette, and the glomerulus was stabilized by a suction pipette in an organ bath at 37°C on the stage of an inverted microscope, as previously described in detail (7, 25, 27, 52).
Measurement of arteriolar myogenic responses.
The perfusion pressure was increased from 40 to 80 mmHg while the arteriolar luminal diameter was measured directly to assess myogenic responses, as previously described (25–27). Dose-response curves for paraquat or H2O2 were performed in individual arterioles by adding drugs from the lowest to highest doses at 15-min intervals. To assess the effects of O2·−, the perfused afferent arteriole was first incubated with graded doses of paraquat (10−8–10−4 mol/l), but subsequent experiments used the ED50 dose of 10−6 mol/l. To assess the effects of H2O2, graded doses of 10−6–10−4 mol/l were used first, but subsequent experiments used the ED50 dose of 10−5 mol/l. To assess the roles of specific signaling and ion channels, perfused afferent arterioles were preincubated with 3 × 10−6 mol/l of Gö6983 to inhibit PKC, 3 × 10−5 mol/l of Rp-8-bromo-PET-cGMP to inhibit PKG, 10−5 mol/l of CaCCinh-A01 to inhibit CaCCs, 10−7 mol/l of apamin and 10−8 mol/l of charybdotoxin to inhibit BKCa and small-conductance Ca2+-activated K+ (SKCa) channels, 10−5 mol/l of linopirdine to inhibit voltage-gated K+ channel 7 (Kv7), and 10−6 mol/l of nifedipine to inhibit VOCCs. To assess the role of nitric oxide (NO), arterioles were incubated with Nω-nitro-l-arginine methyl ester (l-NAME; 10−4 mol/l), and, to assess the role of the endothelium, arterioles were perfused with saponin (0.125 mg/ml) for 10 min to remove endothelial function, which was confirmed by loss of the response to the endothelium-dependent vasodilator ACh (46).
Measurement of O2·− and H2O2.
O2·− generation in the individual perfused afferent arteriole was assessed by fluorescence microscopy from the PEG-SOD-inhibitable E:DHE fluorescence ratio (f/f0) and H2O2 from PEG-catalase-inhibitable C-H2DCFDA fluorescence, as previously described (29). Separate vessels were used to assess the changes in E:DHE and H2DCFDA fluorescence. The specificity of the signals for O2·− and for H2O2 were examined by bath addition of PEG-SOD (200 U/ml) or PEG-catalase (1,000 U/ml), respectively.
Measurement of Em.
Em was evaluated using an anionic membrane potential-sensitive fluorescent dye, DiBAC4(3) (11, 48). DiBAC4(3) partitions across cell membranes according to the Nernst equation. It enters the cytosol during reductions in Em, where its fluorescence intensity is enhanced by binding to cytosolic proteins, whereas during increases in Em, it detaches from cytosolic proteins and exits the cell, which loses fluorescence. Afferent arterioles were perfused with physiological salt solution containing 2 μM DiBAC4(3) at 36°C for 30 min in the dark before the start of the experimental protocols and throughout the experiments. Microvascular fluorescence images were observed through a fluorescence microscopy, and fluorescence intensity was quantitated with PTI Felix32.
Statistics.
Data were obtained from 5–7 mice/group and analyzed by ANOVA. Where appropriate, a post hoc Bonferroni method was used to test differences between groups. Results (mean ± SE values) were considered significant at P < 0.05.
RESULTS
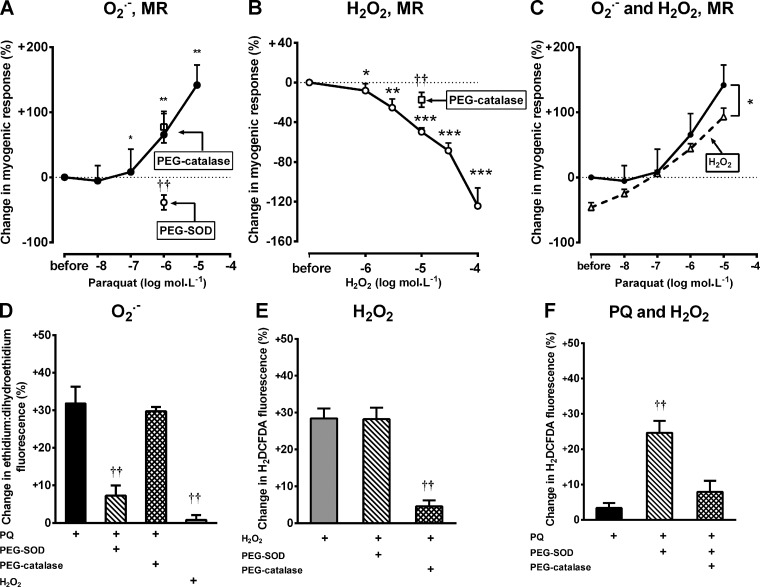
Basal afferent arteriolar diameter during perfusion at 40 mmHg was reduced by paraquat but increased by H2O2 (Table 1). An increase in perfusion pressure from 40 to 80 mmHg contracted afferent arterioles (−19 ± 4%). This myogenic contraction was increased by paraquat. This was prevented by PEG-SOD but not by PEG-catalase (Fig. 1A). Incubation with H2O2 reduced myogenic contractions, but this inhibition was reduced by PEG-catalase (Fig. 1B). H2O2 at >100 μmol/l caused variable responses, and very high concentrations caused contractions (data not shown). Incubation with 10 μmol/l H2O2 reduced the sensitivity of the myogenic contractions to paraquat (Fig. 1C).
Table 1.
Basal afferent arteriolar diameter at 40 mmHg of perfusion pressure
| Arteriolar Diameter, μm |
|||
|---|---|---|---|
| Drug | Before drug addition | After drug | Change, % |
| Paraquat (1 μM) | 8.7 ± 0.5 | 5.7 ± 0.4 | −35.3 ± 1.3** |
| H2O2 (10 μM) | 9.3 ± 0.8 | 10.0 ± 0.7 | +9.1 ± 3.3* |
| Gö-6983 (3 μM) | 10.1 ± 0.7 | 10.1 ± 0.8 | −0.3 ± 1.2 |
| Rp-8-bromo-PET-cGMP (30 μM) | 9.0 ± 0.6 | 9.0 ± 0.6 | −0.2 ± 1.0 |
| CaCCinh-A01 (10 μM) | 11.3 ± 0.8 | 11.8 ± 0.7 | +4.6 ± 1.1* |
| Apamin (100 nM) + charybdotoxin (10 nM) | 11.0 ± 0.4 | 10.9 ± 0.4 | −1.0 ± 0.5 |
| Linopirdine (10 μM) | 9.4 ± 0.3 | 9.3 ± 0.3 | −0.7 ± 0.7 |
| Apamin + charybdotoxin + linopirdine | 9.3 ± 0.4 | 9.1 ± 0.3 | −2.5 ± 1.1 |
| TRAM34 (1 μM) | 11.1 ± 0.5 | 11.0 ± 0.5 | −0.4 ± 0.3 |
| Nifedipine (1 μM) | 11.1 ± 0.5 | 11.1 ± 0.5 | +0.1 ± 0.9 |
| Nω-nitro-l-arginine methyl ester (100 μM) | 10.4 ± 0.4 | 9.7 ± 0.4 | −7.0 ± 0.6*** |
Values are means ± SE; n = 5–7 per group. Shown are values before and 15–30 min after bath addition of the drug.
P < 0.05,
P < 0.01, and
P < 0.005 compared with before drug addition.
Fig. 1.
Paraquat (PQ) enhances afferent arteriolar contractions during increases in perfusion pressure (PP) from 40 to 80 mmHg, whereas H2O2 has opposite effects. A: effects of PQ (10−6 mol/l) alone or with pegalated (PEG)-SOD (200 U/ml) or with PEG-catalase (1,000 U/ml) on myogenic contractions. B: effects of H2O2 (10−5 mol/l) alone or with PEG-SOD (200 U/ml) or with PEG-catalase (1,000 U/ml) on myogenic contractions. C: effects of PQ (10−6 mol/l) alone or with H2O2 (10−5 mol/l). D: changes in the ethidium-to-dihydroethidium (E:DHE) fluorescence ratio of afferent arterioles with PQ (10−6 mol/l) alone or with PEG-SOD (200 U/ml) or with PEG-catalase (1,000 U/ml). E: changes in 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) fluorescence of afferent arterioles by H2O2 (10−5 mol/l) alone or with PEG-SOD (200 U/ml) or with PEG-catalase (1,000 U/ml). F: changes in H2DCFDA fluorescence of afferent arterioles by PQ (10−6 mol/l) alone or with PEG-SOD (200 U/ml) or with PEG-catalase (1,000 U/ml). MR, myogenic response. *P < 0.05, **P < 0.01, and ***P < 0.005 compared with vehicle; ††P < 0.01 compared with PQ or H2O2 alone.
The E:DHE fluorescence signal for O2·− was enhanced during increased perfusion pressure, as previously described (27), and was enhanced further by paraquat (Fig. 1D). This was >85% prevented by PEG-SOD but was unaffected by PEG-catalase and was unchanged by 10−5 mol/l H2O2 (Fig. 1D). The C-H2DCFDA fluorescence signal for H2O2 was enhanced by H2O2 but was >85% prevented by PEG-catalase but not by PEG-SOD (Fig. 1E) and was little changed by 10−6 mol/l paraquat alone. However, paraquat with PEG-SOD increased H2DCFDA fluorescence, whereas this was reduced by coincubation with PEG-catalase (Fig. 1F).
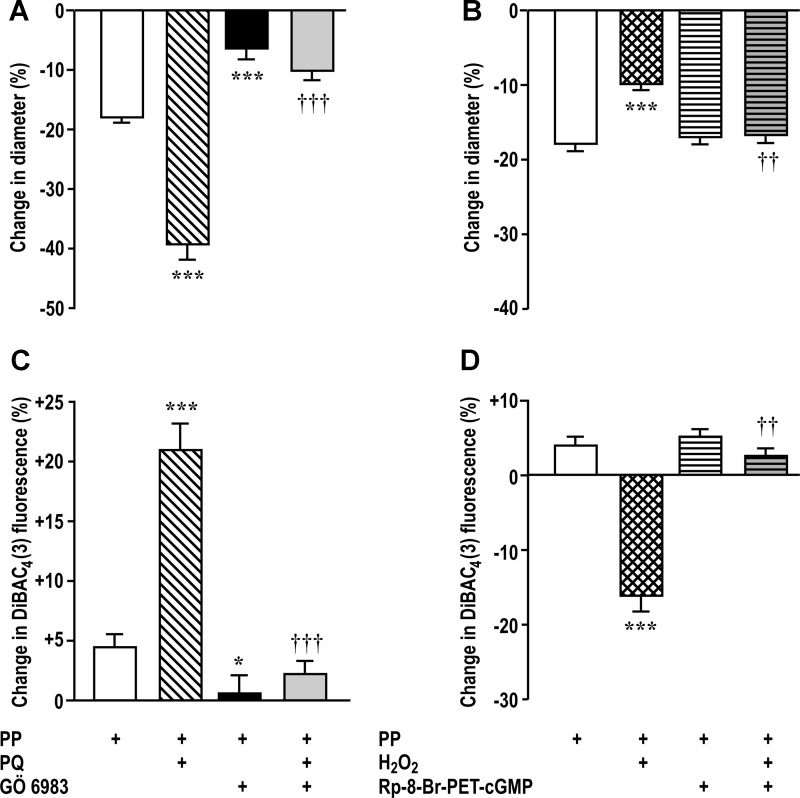
Incubation of afferent arterioles with 3 × 10−6 mol/l Gö6983 to block PKC inhibited basal myogenic contraction and blocked >80% of its augmentation by paraquat (Fig. 2A). Incubation with 3 × 10−5 mol/l Rp-8-bromo-PET-cGMP to block PKG had no effect on basal myogenic contraction but blocked >90% of its inhibition by H2O2 (Fig. 2B).
Fig. 2.
PKC mediates the effects of PQ on myogenic contractions and membrane depolarization and PKG mediates the effects of H2O2. A and C: effects of PQ (10−6 mol/l) and/or Gö6983 (3 × 10−6 mol/l). B and D: effects of H2O2 (10−5 mol/l) and/or Rp-cGMPs (3 × 10−5 mol/l). DiBAC4(3), bis-(1,3-dibutylbarbituric acid)trimethine oxonol. ***P < 0.005 compared with vehicle; ††P < 0.01 and †††P < 0.005 compared with PQ or H2O2 alone.
An increase in DiBAC4(3) fluorescence implies a reduced Em. An increase in perfusion pressure increased fluorescence modestly (Fig. 2C), which was increased further by paraquat and was prevented by blockade of PKC (Fig. 2C). The increase in fluorescence with perfusion pressure was reversed by incubation with H2O2, and this was prevented by blockade of PKG (Fig. 2D).
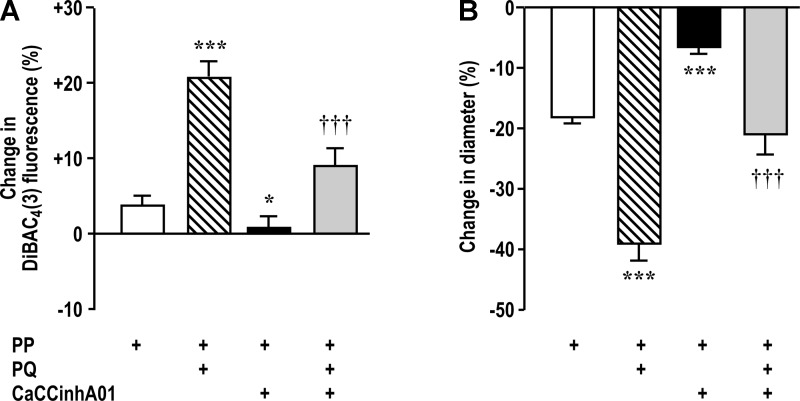
Blockade of CaCCs with 10−5 mol/l CaCCinh-A01 increased basal afferent arteriolar diameter (Table 1), blocked the reduction in Em with perfusion pressure, and blocked 65% of the augmentation of Em with paraquat (Fig. 3A). Likewise, CaCCinh-A01 blunted myogenic contractions and blocked 40% of its augmentation by paraquat (Fig. 3B).
Fig. 3.
Membrane potential (Em) is reduced by activation of Ca2+-activated Cl− channels (CaCCs) with PP or PQ. DiBAC4(3) fluorescence (A) was increased with PP and PQ (10−6 mol/l) (implying depolarization), but this was blunted by inhibition of CaCCs with CaCCinh-A01 (10−5 mol/l). Luminal diameter (B) was reduced by PP, and this was enhanced by PQ but blunted by CaCCinh-A01. *P < 0.05 and ***P < 0.005 compared with vehicle; †††P < 0.005 compared with PQ alone.
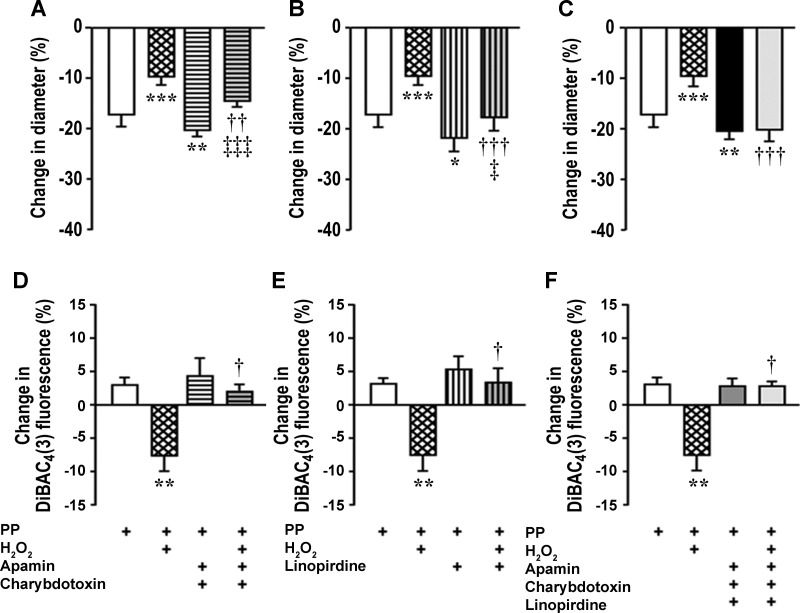
Pilot experiments were undertaken to determine the effects of antagonists of different K+ channels on myogenic contractions since variable responses have been previously reported (8). Responses were reduced or unchanged after blockade of ATP-sensitive K+ (KATP) channels, intermediate-conductance Ca2+-activated K+ channels, and inward rectifier K+ (Kir2) channels with glibenclamide, TRAM-34, and ML-133, respectively (data not shown). Therefore, these channels were not further investigated as mechanisms for reduced myogenic contraction with H2O2. However, blockade of SKCa and BKCa channels with apamin (10−7 mol/l) and charybdotoxin (10−8 mol/l) or blockade of Kv7 with 10−5 mol/l linopirdine enhanced myogenic contractions and therefore were selected for further study. These antagonists individually reduced, but did not prevent, the blunting of myogenic contractions by H2O2 (Fig. 4, A and B). However, incubation of arterioles with all of these three K+ channel blockers together prevented the effects of H2O2 to reduce myogenic contractions (Fig. 4C) or to hyperpolarize Em (Fig. 4, D–F).
Fig. 4.
H2O2 activates small-conductance Ca2+-activated K+ (SKCa) channels, large-conductance Ca2+-activated K+ (BKCa) channels, and voltage-activated K+ channel 7 (Kv7) to blunt myogenic contractions. The reduction in luminal diameter with PP was inhibited by H2O2, but the effect of H2O2 was lessened by blockade of SKCa and BKCa channels with apamin (10−7 mol/l) and charybdotoxin (10−8 mol/l) (A) or blockade of Kv7 by linopirdine (10−5 mol/l; B) and was prevented by blockade of all channels by a combination of all three blockers (C). The increase in Em by H2O2 was prevented by blockade of K+ channels. DiBAC4(3) fluorescence was decreased with H2O2 (10−5 mol/l) (implying hyperpolarization), but this effect of H2O2 was lessened by apamin (10−7 mol/l) and charybdotoxin (10−8 mol/l; D) or linopirdine (10−5 mol/l; E) and prevented by a combination of all three blockers (F). *P < 0.05, **P < 0.01, and ***P < 0.005 compared with vehicle; †P < 0.05, ††P < 0.01, and †††P < 0.005 compared with H2O2 alone; ‡P < 0.05 and ‡‡‡P < 0.005 compared with antagonists alone.
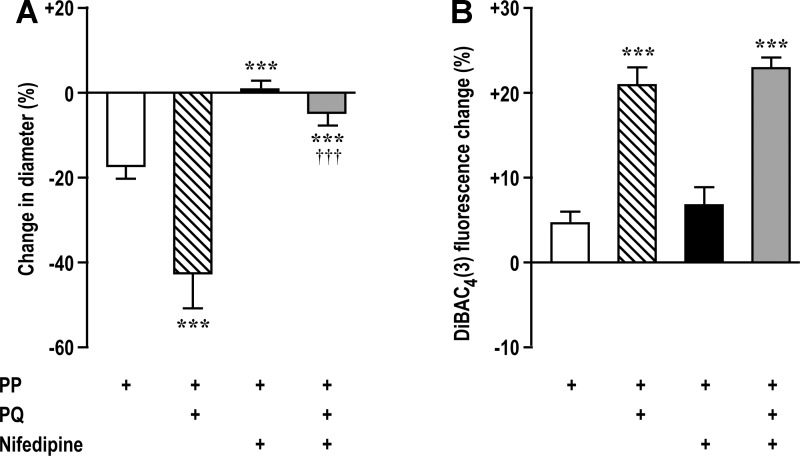
Blockade of VOCCs with 10−6 mol/l nifedipine prevented myogenic contractions and blocked >85% of the enhancing effect of paraquat (Fig. 5A) yet left intact the reduction in Em with paraquat (Fig. 5B).
Fig. 5.
Changes in Em are upstream from activation of voltage-operated Ca2+ channels. Luminal diameter (A) was reduced by PP and reduced further by PQ (10−6 mol/l), but this was largely prevented by nifedipine (10−6 mol/l). Em (B) was reduced by PP or PQ (10−6 mol/l) (implying depolarization), but these effects were unchanged by nifedipine (10−6 mol/l). ***P < 0.005 compared with vehicle; †††P < 0.005 compared with PQ alone.
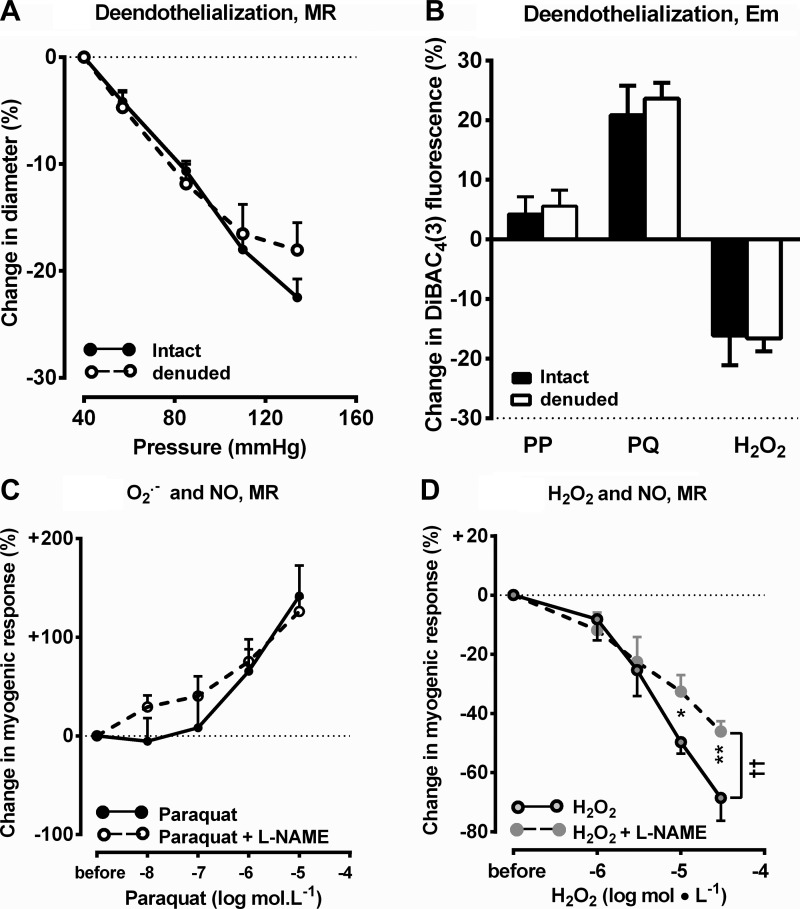
Functional removal of the endothelium by intraluminal perfusion of saponin did not affect myogenic contractions (Fig. 6A) or Em (Fig. 6B). Blockade of NO generation with l-NAME (10−4 mol/l) reduced basal afferent arteriolar diameter (Table 1) but did not prevent the enhanced myogenic contractions with paraquat (Fig. 6C) and did not modify the reduction in myogenic contractions with low concentration of H2O2 but did enhance the effects of higher concentrations (Fig. 6D).
Fig. 6.
Changes in DiBAC4(3) fluorescence by PP alone or with saponin (0.125 mg/ml; A), changes in luminal diameter by by PP alone or with saponin (0.125 mg/ml; B), by PQ (10−6 mol/l) alone or with Nω-nitro-l-arginine methyl ester (l-NAME; 10−6 mol/l; C), or by H2O2 (10−5 mol/l) alone or with l-NAME (10−6 mol/l; D) during increasing PP from 40 to 80 mmHg. *P < 0.05 and **P < 0.01 compared with vehicle (by two-way ANOVA); ††P < 0.01.
DISCUSSION
The details of the myogenic mechanism remain controversial despite its importance in renal, cerebral, and cardiac protection against damage from hypertension (6, 8, 22, 32). We confirmed that an increase in perfusion pressure induces strong myogenic contractions. O2·− is generated in afferent arterioles from normal mice or mice with genetic deletion of SOD and mediates much of the myogenic contractions (26, 27, 29). The main new findings are that the generation of O2·− with increased perfusion pressure is enhanced dose dependently by paraquat, which also enhances myogenic contractions. In contrast, almost no generation of H2O2 by perfusion pressure could be detected in normal afferent arterioles. This is consistent with the very low endogenous vascular H2O2 concentration of perhaps <10−6 mol/l (31). This study showed that such low concentration of H2O2 had no effects on basal diameter, Em, or myogenic contractions of afferent arterioles. However, incubation with >10−6 mol/l H2O2 impaired myogenic contractions as in a previous study (24) of a mouse model of CKD. Em of afferent arterioles is depolarized by increased perfusion pressure and is enhanced by paraquat via activation of PKC, but this is transformed into hyperpolarization with H2O2 via activation of PKG. Blockade of CaCCs blunts the reduction in Em and the enhanced myogenic contractions with paraquat. A combined blockade of SKCa, BKCa, and Kv7 channels blocks the effects of H2O2 to hyperpolarize Em and to blunt myogenic contractions. Blockade of VOCCs prevents myogenic contractions and the enhancing effects of paraquat but leaves intact the changes in Em. The vascular endothelium is not involved in myogenic contractions. NO synthase (NOS) is not required for the blunting of the myogenic response by modest concentrations of H2O2.
E:DHE fluorescence in afferent arterioles was a relatively specific signal for O2·− since its fluorescence was increased by perfusion pressure or by paraquat but not by H2O2, and the effects of paraquat were greatly diminished by incubation with PEG-SOD but not by PEG-catalase. DHE is oxidized by O2·− to oxyethidium, which is detected with ethidium using our fluorescence system. Precise quantitation of O2·− requires HLPC separation of oxyethidium from ethidium (15), but this is not possible with a single afferent arteriole. Nevertheless, our results suggest that the majority of the fluorescence signal in arterioles loaded with DHE can be ascribed to O2·− since it is prevented by PEG-SOD but not by PEG-catalase. C-H2DCFDA fluorescence was a relatively specific signal for H2O2 since its fluorescence was increased by exogenous H2O2 or H2O2 generated from paraquat-induced O2·− by PEG-SOD but not by paraquat alone. The increased H2O2 signals were prevented by PEG-catalase but not by PEG-SOD.
Most of the enhanced myogenic contractions with paraquat could be ascribed to O2·− since the enhanced E:DHE signal and enhanced myogenic contractions were reduced or prevented by metabolism of O2·− by PEG-SOD but were unaffected by PEG-catalase. Paraquat also enhances afferent arteriolar contractions during increased ROS generation by thromboxane (50). Recently, Vogel et al. (50) reported that paraquat enhances Ca2+ entry into arterioles via l-type VOCCs but leaves unchanged cellular Ca2+ derived from store-operated Ca2+ channels and Ca2+-induced Ca2+ release from the sarcoplasmic reticulum. This is quite consistent with our finding that paraquat reduced Em since this should disinhibit L-type channels, raise intracellular Ca2+, and enhance myogenic contractions. Indeed, our data demonstrated that the reduction in Em with paraquat is upstream from activation of VOCCs since blockade of VOCCs with nifedipine disabled myogenic contractions without perturbing the reduction in Em. This extends findings from a previous report (27) showing that myogenic contractions were prevented by incubation in Ca2+-free solution without perturbing the increase in O2·−.
The myogenic response of the afferent arteriole is initiated by stretch or deformation of VSMCs, which is transduced into intracellular signals. The activated signals lead to a reduction in Em that disinhibits VOCCs to increase intracellular Ca2+ and thereby to activate myosin light chain kinase, which mediates the contraction (8, 50). O2·− generated from NADPH oxidase is required for mouse afferent arteriolar myogenic contractions since these are blocked by apocynin (27). Although these arterioles express neutrophil oxidases (NOX)-2 and NOX-4 as well as p47phox and p22phox (2, 51), the NOX-2/p22phox/p47phox pathway is implicated since an increase in perfusion pressure of afferent arterioles from p47phox gene-deleted mice fails to generate O2·− or contractions (26). Step increases in perfusion pressure elicit step increases in vascular inositol 1,4,5-triphosphate and diacylglycerol, which can activate PKC (34). Our results confirm that PKC is required for myogenic contractions and further show that it mediates the membrane depolarization by perfusion pressure or paraquat. This is consistent with activation of PKC by O2·− in renal tubules (19, 53), Moreover, the reduction in Em and enhanced myogenic contraction with paraquat depend in part on Cl− channels (CaCCs). The relationship between O2·− and activation of Cl− channels is complex. O2·− may traverse membranes via Cl− channels in activated neutrophils, and O2·− can open Cl− channels in the phagocytic vacuole (43) and cell membrane (45). CaCCs mediate vascular contractions to thromboxane (1). Moreover, Cl− channels in endothelial cells mediate changes in cell membrane current with O2·− (4), but this is the first documentation of the role of CaCCs in mediating myogenic responses to O2·−. Overall, the data indicate that O2·− generated from paraquat or p47phox/NOX-2 activates PKC signaling that subsequently activates CaCCs, depolarizes Em of VSMCs, disinhibits VOCCs, raises intracellular Ca2+, and initiates a myogenic contraction.
A novel finding was that exogenous H2O2 blunted myogenic contractions of afferent arterioles, but H2O2 apparently was not generated with increased perfusion pressure in normal mouse afferent arterioles since myogenic contractions of normal vessels were unaffected by metabolizing H2O2 with PEG-catalase. However, micromolar concentrations of exogenous H2O2 inhibited myogenic contractions and antagonized the effects of O2·− in a dose-dependent manner. We used the ED50 dose of H2O2 (10−5 mol/l) that blunted about half of myogenic contraction and increased Em as indicated by a reduction of intracellular DiBAC4(3) fluorescence. H2O2 can alter vascular contractions by Ca2+-dependent and -independent manners (55). The finding that H2O2 did not completely prevent myogenic contractions while reversing the changes in Em suggests a Ca2+-independent component of action. An increase in H2O2 can enhance K+ conductance in VSMCs (5) and in human coronary arterioles (55). However, H2O2 can block mitochondrial KATP channels (56), which might underlie the apparently paradoxical reduction in myogenic contractions observed after inhibition of KATP channels with glibenclamide (data not shown). In contrast, Kv channels are activated by H2O2 in coronary arteries (35) and mesenteric arterioles (36), where they mediate vasodilation. H2O2 activates BKCa channels in fibroblasts (14) and human coronary arteries (31, 55) and Kv7 channels in neurons (16). These reports are consistent with our conclusion that H2O2 activates BKCa, Kv, and Kv7 channels to inhibit myogenic contractions. Although rat afferent arteriolar K+ channels are normally quiescent, they can be activated by ROS in models of diabetes, where they mediate the vasodilation that contributes to diabetic hyperfiltration (49). Blockade of SKCa, BKCa, or Kv7 channels was required to prevent the responses to H2O2. This indicates that H2O2 blunts myogenic contractions by activation of specific K+ channels but suggests significant redundancy amongst them. Basal levels of H2O2 were not implicated in the modulation of K+ channels or contractions with perfusion pressure since there were no effects of PEG-catalase. However, exogenous H2O2 activated specific K+ channels, hyperpolarized Em, and prevented myogenic contraction via PKG. The regulation of K+ channels by ROS has been closely studied in the heart, where they are implicated in ischemic dysrhythmias (54). H2O2 activates PKG (5) and BKCa channels in coronary arterioles (49, 55), as in afferent arterioles in the present study.
H2O2 can activate aortic endothelial NOS3 (28). However, myogenic contractions were unaltered in NOS3 gene-deleted mice (27) and usually do not depend on the endothelium (8), as shown in this study. Although blockade of NOS in this study reduced basal arteriolar diameter and enhanced myogenic contractions moderately, it did not prevent the blunting of the response by H2O2, except at the highest concentrations. This suggests that NO determines basal vascular diameter in afferent arteriole but does not normally mediate the effects of physiological concentrations of H2O2 on myogenic tone.
This study has some limitations. First, PKC has more than 10 isoforms. The specific isoform that mediates the effects of O2·− needs further study. Second, patch-clamp techniques would be required to precisely confirm the ion channels involved in changes in Em, but these are hard to perform on vessels that are stretching and contracting. Third, this study did not separate effects of pressure and flow. Flow normally induces an endothelium-dependent relaxation in preconstricted coronary arterioles (55).
In conclusion, O2·− and H2O2 modulate myogenic contractions differentially. O2·− acts via PKC-induced activation of channels, including CaCCs, which depolarizes Em to disinhibit VOCCs and enhance myogenic tone, whereas H2O2 acts via PKG-induced activation of SKCa, BKCa, and Kv7 channels to hyperpolarize Em and reduce myogenic tone.
Perspectives
These findings may help to explain previous conflicting reports of the effects of ROS on autoregulation and myogenic contractions. Mice with SOD gene deletions generate excessive vascular O2·− and develop very strong myogenic contractions (7, 29). Surprisingly, mice with the reduced renal mass model of CKD that excrete sixfold more 8-isoprostane F2α (produced by the reaction of O2·− with arachidonate) (23) and also generate excessive afferent arteriolar O2·− have severely blunted myogenic contractions (25). However, mice with reduced renal mass also excreted threefold more H2O2, which our present study suggests could have antagonized the effects of O2·− to enhance myogenic contractions. Thus, overproduction of H2O2 in the afferent arteriole may be the cause of the reduced myogenic contractions in some settings despite increased O2·−, but this remains to be investigated. Generation of H2O2 from metabolism of O2·− by SOD in the afferent arteriole during prolonged oxidative stress also may prevent excessive and prolonged contractions leading to renal ischemia and might thereby be beneficial in maintaining some renal blood flow.
GRANTS
This work was supported by National Institutes of Health Grants DK-49870, DK-36079, and HL-68086, by funds from the George E. Schreiner Chair of Nephrology, the Smith-Kogod Family Foundation, and the Georgetown University Hypertension, Kidney and Vascular Research Center, and by National Nature Science Foundation of China Grant 31471100 (to E. Y. Lai).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.L. and E.Y.L. conception and design of research; L.L. and E.Y.L. performed experiments; L.L. and E.Y.L. analyzed data; L.L., E.Y.L., A.W., and W.J.W. interpreted results of experiments; L.L. and E.Y.L. prepared figures; L.L. and E.Y.L. drafted manuscript; C.S.W. edited and revised manuscript; C.S.W. approved final version of manuscript.
REFERENCES
- 1.Alapati VR, McKenzie C, Blair A, Kenny D, MacDonald A, Shaw AM. Mechanisms of U46619- and 5-HT-induced contraction of bovine pulmonary arteries: role of chloride ions. Br J Pharmacol 151: 1224–1234, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araujo M, Wilcox CS. Oxidative stress in hypertension: role of the kidney. Antioxid Redox Signal 20: 74–101, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgarten CM, Clemo HF. Swelling-activated chloride channels in cardiac physiology and pathophysiology. Progr Biophys Mol Biol 82: 25–42, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Brzezinska AK, Lohr N, Chilian WM. Electrophysiological effects of O2·− on the plasma membrane in vascular endothelial cells. Am J Physiol Heart Circ Physiol 289: H2379–H2386, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Burgoyne JR, Oka S, Ale-Agha N, Eaton P. Hydrogen peroxide sensing and signaling by protein kinases in the cardiovascular system. Antioxid Redox Signal 18: 1042–1052, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke M, Pabbidi MR, Farley J, Roman RJ. Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol 12: 845–858, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlstrom M, Lai EY, Ma Z, Steege A, Patzak A, Eriksson UJ, Lundberg JO, Wilcox CS, Persson AE. Superoxide dismutase 1 limits renal microvascular remodeling and attenuates arteriole and blood pressure responses to angiotensin II via modulation of nitric oxide bioavailability. Hypertension 56: 907–913, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlstrom M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev 95: 405–511, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Pearlman A, Luo Z, Wilcox CS. Hydrogen peroxide mediates a transient vasorelaxation with tempol during oxidative stress. Am J Physiol Heart Circ Physiol 293: H2085–H2092, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Edwards A, Layton AT. Calcium dynamics underlying the myogenic response of the renal afferent arteriole. Am J Physiol Renal Physiol 306: F34–F48, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epps DE, Wolfe ML, Groppi V. Characterization of the steady-state and dynamic fluorescence properties of the potential-sensitive dye bis-(1,3-dibutylbarbituric acid)trimethine oxonol [Dibac4(3)] in model systems and cells. Chem Phys Lipids 69: 137–150, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Fallet RW, Bast JP, Fujiwara K, Ishii N, Sansom SC, Carmines PK. Influence of Ca2+-activated K+ channels on rat renal arteriolar responses to depolarizing agonists. Am J Physiol Renal Physiol 280: F583–F591, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fellner RC, Cook AK, O'Connor PM, Zhang S, Pollock DM, Inscho EW. High-salt diet blunts renal autoregulation by a reactive oxygen species-dependent mechanism. Am J Physiol Renal Physiol 307: F33–F40, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng B, Ye WL, Ma LJ, Fang Y, Mei YA, Wei SM. Hydrogen peroxide enhanced Ca2+-activated BK currents and promoted cell injury in human dermal fibroblasts. Life Sci 90: 424–431, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol 287: C895–C902, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Gamper N, Zaika O, Li Y, Martin P, Hernandez CC, Perez MR, Wang AY, Jaffe DB, Shapiro MS. Oxidative modification of M-type K+ channels as a mechanism of cytoprotective neuronal silencing. EMBO J 25: 4996–5004, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin KA, Picken MM, Bidani AK. Blood pressure lability and glomerulosclerosis after normotensive 5/6 renal mass reduction in the rat. Kidney Int 65: 209–218, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Harder DR, Narayanan J, Gebremedhin D, Roman RJ. Transduction of physical force by the vascular wall. Role of phospholipase C and cytochrome P450 metabolites of arachidonic acid. Trends Cardiovasc Med 5: 7–14, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Hong NJ, Garvin JL. Endogenous flow-induced superoxide stimulates Na/H exchange activity via PKC in thick ascending limbs. Am J Physiol Renal Physiol 307: F800–F805, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaggar JH, Wellman GC, Heppner TJ, Porter VA, Perez GJ, Gollasch M, Kleppisch T, Rubart M, Stevenson AS, Lederer WJ, Knot HJ, Bonev AD, Nelson MT. Ca2+ channels, ryanodine receptors and Ca2+-activated K+ channels: a functional unit for regulating arterial tone. Acta Physiol Scand 164: 577–587, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Jensen BL, Ellekvist P, Skott O. Chloride is essential for contraction of afferent arterioles after agonists and potassium. Am J Physiol Renal Physiol 272: F389–F396, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Kriz W, Lemley KV. A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J Am Soc Nephrol 26: 258–269, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai EY, Luo Z, Onozato ML, Rudolph EH, Solis G, Jose PA, Wellstein A, Aslam S, Quinn MT, Griendling K, Le T, Li P, Palm F, Welch WJ, Wilcox CS. Effects of the antioxidant drug tempol on renal oxygenation in mice with reduced renal mass. Am J Physiol Renal Physiol 303: F64–F74, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai EY, Martinka P, Fahling M, Mrowka R, Steege A, Gericke A, Sendeski M, Persson PB, Persson AE, Patzak A. Adenosine restores angiotensin II-induced contractions by receptor-independent enhancement of calcium sensitivity in renal arterioles. Circ Res 99: 1117–1124, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Lai EY, Onozato ML, Solis G, Aslam S, Welch WJ, Wilcox CS. Myogenic responses of mouse isolated perfused renal afferent arterioles: effects of salt intake and reduced renal mass. Hypertension 55: 983–989, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai EY, Solis G, Luo Z, Carlstrom M, Sandberg K, Holland S, Wellstein A, Welch WJ, Wilcox CS. p47phox is required for afferent arteriolar contractile responses to angiotensin II and perfusion pressure in mice. Hypertension 59: 415–420, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai EY, Wellstein A, Welch WJ, Wilcox CS. Superoxide modulates myogenic contractions of mouse afferent arterioles. Hypertension 58: 650–656, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laude K, Cai H, Fink B, Hoch N, Weber DS, McCann L, Kojda G, Fukai T, Schmidt HH, Dikalov S, Ramasamy S, Gamez G, Griendling KK, Harrison DG. Hemodynamic and biochemical adaptations to vascular smooth muscle overexpression of p22phox in mice. Am J Physiol Heart Circ Physiol 288: H7–H12, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Feng D, Luo Z, Welch WJ, Wilcox CS, Lai EY. Remodeling of afferent arterioles from mice with oxidative stress does not account for increased contractility but does limit excessive wall stress. Hypertension 66: 550–556, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Du X. Effects of α1-adrenoceptor agonist phenylephrine on swelling-activated chloride currents in human atrial myocytes. J Membr Biol 248: 7–18, 2015. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Bubolz AH, Mendoza S, Zhang DX, Gutterman DD. H2O2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles. Circ Res 108: 566–573, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol 290: R1153–R1167, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu H, Zhen J, Wu T, Peng A, Ye T, Wang T, Yu X, Vaziri ND, Mohan C, Zhou XJ. Superoxide dismutase mimetic drug tempol aggravates anti-GBM antibody-induced glomerulonephritis in mice. Am J Physiol Renal Physiol 299: F445–F452, 2010. [DOI] [PubMed] [Google Scholar]
- 34.Narayanan J, Imig M, Roman RJ, Harder DR. Pressurization of isolated renal arteries increases inositol trisphosphate and diacylglycerol. Am J Physiol Heart Circ Physiol 266: H1840–H1845, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Ohanyan V, Yin L, Bardakjian R, Kolz C, Enrick M, Hakobyan T, Kmetz J, Bratz I, Luli J, Nagane M, Khan N, Hou H, Kuppusamy P, Graham J, Fu FK, Janota D, Oyewumi MO, Logan S, Lindner JR, Chilian WM. Requisite role of Kv1.5 channels in coronary metabolic dilation. Circ Res 117: 612–621, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park SW, Noh HJ, Sung DJ, Kim JG, Kim JM, Ryu SY, Kang K, Kim B, Bae YM, Cho H. Hydrogen peroxide induces vasorelaxation by enhancing 4-aminopyridine-sensitive Kv currents through S-glutathionylation. Pflügers Arch 467: 285–297, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelayo JC, Westcott JY. Impaired autoregulation of glomerular capillary hydrostatic pressure in the rat remnant nephron. J Clin Invest 88: 101–105, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prior HM, Yates MS, Beech DJ. Functions of large conductance Ca2+-activated (BKCa), delayed rectifier (KV) and background K+ channels in the control of membrane potential in rabbit renal arcuate artery. J Physiol 511: 159–169, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puri N, Zhang F, Monu SR, Sodhi K, Bellner L, Lamon BD, Zhang Y, Abraham NG, Nasjletti A. Antioxidants condition pleiotropic vascular responses to exogenous H2O2: role of modulation of vascular TP receptors and the heme oxygenase system. Antioxid Redox Signal 18: 471–480, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quiroz Y, Ferrebuz A, Vaziri ND, Rodriguez-Iturbe B. Effect of chronic antioxidant therapy with superoxide dismutase-mimetic drug, tempol, on progression of renal disease in rats with renal mass reduction. Nephron Exp Nephrol 112: e31–e42, 2009. [DOI] [PubMed] [Google Scholar]
- 41.Racasan S, Turkstra E, Joles JA, Koomans HA, Braam B. Hypoxanthine plus xanthine oxidase causes profound natriuresis without affecting renal blood flow autoregulation. Kidney Int 64: 226–231, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Schnackenberg CG, Welch WJ, Wilcox CS. TP receptor-mediated vasoconstriction in microperfused afferent arterioles: roles of O2− and NO. Am J Physiol Renal Physiol 279: F302–F308, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Segal AW. The function of the NADPH oxidase of phagocytes and its relationship to other NOXs in plants, invertebrates, and mammals. Int J Biochem Cell Biol 40: 604–618, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma K, Cook A, Smith M, Valancius C, Inscho EW. TGF-β impairs renal autoregulation via generation of ROS. Am J Physiol Renal Physiol 288: F1069–F1077, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Shen M, Wang L, Wang B, Wang T, Yang G, Shen L, Wang T, Guo X, Liu Y, Xia Y, Jia L, Wang X. Activation of volume-sensitive outwardly rectifying chloride channel by ROS contributes to ER stress and cardiac contractile dysfunction: involvement of CHOP through Wnt. Cell Death Dis 5: e1528, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su EN, Kelly ME, Cringle SJ, Yu DY. Role of endothelium in abnormal cannabidiol-induced vasoactivity in retinal arterioles. Invest Ophthalmol Vis Sci 56: 4029–4037, 2015. [DOI] [PubMed] [Google Scholar]
- 47.Takenaka T, Kanno Y, Kitamura Y, Hayashi K, Suzuki H, Saruta T. Role of chloride channels in afferent arteriolar constriction. Kidney Int 50: 864–872, 1996. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka M, Kanatsuka H, Ong BH, Tanikawa T, Uruno A, Komaru T, Koshida R, Shirato K. Cytochrome P-450 metabolites but not NO, PGI2, and H2O2 contribute to ACh-induced hyperpolarization of pressurized canine coronary microvessels. Am J Physiol Heart Circ Physiol 285: H1939–H1948, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Troncoso Brindeiro CM, Lane PH, Carmines PK. Tempol prevents altered K+ channel regulation of afferent arteriolar tone in diabetic rat kidney. Hypertension 59: 657–664, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vogel PA, Yang X, Moss NG, Arendshorst WJ. Superoxide enhances Ca2+ entry through L-type channels in the renal afferent arteriole. Hypertension 66: 374–381, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang D, Chabrashvili T, Wilcox CS. Enhanced contractility of renal afferent arterioles from angiotensin-infused rabbits: roles of oxidative stress, thromboxane prostanoid receptors, and endothelium. Circ Res 94: 1436–1442, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Wang D, Jose P, Wilcox CS. β1 Receptors protect the renal afferent arteriole of angiotensin-infused rabbits from norepinephrine-induced oxidative stress. J Am Soc Nephrol 17: 3347–3354, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Yang J, Lane PH, Pollock JS, Carmines PK. PKC-dependent superoxide production by the renal medullary thick ascending limb from diabetic rats. Am J Physiol Renal Physiol 297: F1220–F1228, 2009. [DOI] [PubMed] [Google Scholar]
- 54.Yang KC, Kyle JW, Makielski JC, Dudley SC Jr. Mechanisms of sudden cardiac death: oxidants and metabolism. Circ Res 116: 1937–1955, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang DX, Borbouse L, Gebremedhin D, Mendoza SA, Zinkevich NS, Li R, Gutterman DD. H2O2-induced dilation in human coronary arterioles: role of protein kinase G dimerization and large-conductance Ca2+-activated K+ channel activation. Circ Res 110: 471–480, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zuo L, Pannell BK, Re AT, Best TM, Wagner PD. Po2 cycling protects diaphragm function during reoxygenation via ROS, Akt, ERK and mitochondrial channels. Am J Physiol Cell Physiol 309: C759–C766, 2015. [DOI] [PubMed] [Google Scholar]