Abstract
The collecting duct (CD) has been recognized as an important source of prorenin/renin, and it also expresses (pro)renin receptor (PRR). The goal of this study was to examine the hypothesis that prorenin or renin via PRR regulates epithelial Na+ channel (ENaC) activity in mpkCCD cells. Transepithelial Na+ transport was measured by using a conventional epithelial volt-ohmmeter and was expressed as the calculated equivalent current (Ieq). Amiloride-inhibitable Ieq was used as a reflection of ENaC activity. Administration of prorenin in the nanomolar range induced a significant increase in Ieq that was detectable as early as 1 min, peaked at 5 min, and gradually returned to baseline within 15 min. These changes in Ieq were completely prevented by a newly developed PRR decoy inhibitor, PRO20. Prorenin-induced Ieq was inhibitable by amiloride. Compared with prorenin, renin was less effective in stimulating Ieq. Prorenin-induced Ieq was attenuated by apocynin but enhanced by tempol, the latter effect being prevented by catalase. In response to prorenin treatment, the levels of total reactive oxygen species and H2O2 were both increased, as detected by spin-trap analysis and reactive oxygen species (ROS)-Glo H2O2 assay, respectively. Both siRNA-mediated Nox4 knockdown and the dual Nox1/4 inhibitor GKT137892 attenuated prorenin-induced Ieq. Overall, our results demonstrate that activation of PRR by prorenin stimulates ENaC activity in CD cells via Nox4-derived H2O2.
Keywords: (pro)renin receptor, renin activity, epithelial sodium channel, inner medullary collecting duct, hydrogen peroxide
the distal nephron of the kidney is an important site for fine regulation of Na+ reabsorption via the epithelial Na+ channel (ENaC), a rate-limiting process that is central to the maintenance of extracellular fluid volume and blood pressure (20, 28). ENaC consists of three partially homologous subunits (α, β, and γ) and is subject to complex regulation at the levels of channel expression, distribution, and cleavage, which ultimately affect channel density and open probability (Po). ENaC is regulated by multiple components of the renin-angiotensin-aldosterone system (RAAS) (35). In response to increased angiotensin II (ANG II) or high K+, aldosterone (Aldo) is secreted from the zona glomerulosa cells of adrenal glands and acts on the Aldo-sensitive distal nephron [the last third of the distal convoluted tubule, the connecting segment, and the cortical and medullary collecting ducts (CDs)]. Aldo chronically stimulates ENaC (within hours) at both transcriptional and posttranscriptional levels, leading to increased channel density (16, 37). In recent years, ANG II has been acknowledged as a direct regulator of ENaC (15, 33). Unlike Aldo, however, ANG II rapidly increases ENaC Po (within minutes) via NADPH oxidase-derived reactive oxygen species (ROS) (15, 33).
Renin, a rate-limiting enzyme in the RAAS responsible for generation of ANG I from angiotensinogen, is produced from the juxtaglomerular cells of the kidney in response to Na+ depletion or β-adrenergic activation and is secreted to the circulation (11). Prorenin, the precursor of renin, contains a 43-amino acid NH2-terminal prosegment that usually covers the enzymatic cleft and obstructs the access of angiotensinogen. Prorenin is activated proteolytically through the cleavage of the inhibitory prosegment or nonproteolytically through conformational changes. The concentration of prorenin in the serum is 5–10 times higher than that of renin. In recent years, accumulating evidence documents the existence of (pro)renin (a term denoting both prorenin and renin) in the CD as a key component of the intrarenal renin-angiotensin system (RAS) (22, 23, 27). Functional studies demonstrate an important role of CD renin in the regulation of blood pressure (24, 25).
In 2002, Nguyen et al. (19) cloned a specific receptor for prorenin and renin, termed (pro)renin receptor (PRR), a 350-amino-acid protein containing a large unglycosylated and highly hydrophobic NH2-terminal domain, a single transmembrane protein, and a short cytoplasmic tail of ∼20 amino acids (4). The COOH-terminal tail was previously purified from chromaffin granules as an 8- to 9-kDa accessory protein (M8-9) of the vacuolar-type H+-ATPase, designated ATP6AP2 (14). In vitro evidence demonstrates that prorenin or renin bound to PRR displays increases in catalytic activity (18, 19). Within the kidney, PRR is abundantly expressed in the CD (1), where its expression is elevated by ANG II (6, 38, 39). The goal of the present study was to test the potential role of prorenin/renin and PRR in the regulation of ENaC activity in cultured CD cells and to explore the underlying mechanism.
METHODS
Materials.
Dulbecco's modified Eagle's medium/nutrient mixture F1-2 (DMEM/F-12) and fetal bovine serum were purchased from Life Technologies (Grand Island, NY). Mouse prorenin (IMPREN-HIS) and recombinant mouse renin (cat. no. 4277-AS) were purchased from Innovative Research (Novi, MI) and R&D Systems, (Minneapolis, MN), respectively. Losartan was purchased from Cayman Chemical (Ann Arbor, MI). The Nox1/4 inhibitor GKT137892 was a gift from Genkyotex SA (Geneva, Switzerland). Apocynin (cat. no. A10809), tempol (cat. no. 176141), catalase (cat. no. C1345), and other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Electrophysiological transepithelial measurements.
Immortalized mouse cortical collecting duct mpkCCD cells were previously characterized by Bens et al. (3). Electrophysiology experiments were performed on mpkCCD cells grown in a Transwell after the cell monolayers reached confluence. The transepithelial voltage (Vte) and resistance (Rte) across cell monolayers were measured using an epithelial volt-ohmmeter (World Precision Instruments). The transepithelial current was calculated according to Ohm's law, Ite = Vte/Rte, and normalized by the surface area of the permeable insert. For monitoring the changes in total current, confluent mpkCCD cells in a six-well Transwell were exposed prorenin or renin on the apical side and the Ieq was monitored over 15 min. For measurement of ENaC activity, the cells were grown in a 24-well Transwell and were exposed for 1 min to prorenin or renin to the apical side, immediately followed by addition of amiloride to the final concentration of 10 μM. The use of the smaller Transwell facilitated mixing amiloride in the medium. With this protocol, amiloride reduced the Ieq by ∼90%. The involvement of Nox4 in prorenin-induced signaling was tested by using a dual Nox1/4 inhibitor, GKT137892 (a gift from Genkyotex), and Nox4 siRNA.
siRNA transient transfection.
Nox4 siRNA oligonucleotides were purchased from Invitrogen (cat. no. 4390771). At 50–70% confluence, the mpkCCD cells were transfected with Nox4 siRNA or nontargeting control siRNA (e.g., scrambled siRNA; cat. no. AM4636; Invitrogen) by using Lipofectamine RNAiMAX Transfection Reagent (cat. no. 13778-030; Invitrogen).
Measurement of ROS.
Electron paramagnetic resonance (EPR) spectroscopy was performed with N-tert-butyl-α-phenylnitrone (PBN), a commonly used free-radical spin trap, as described previously (29). After incubation with prorenin for 1 min at 37°C, PBN was added to mpkCCD cells in a final concentration of 50 mM, and the samples were extracted with toluene. After 10 min of centrifugation at 4,000 rpm, the toluene layer was collected as the measurement sample. Before the measurement, the samples were deoxygenated by bubbling nitrogen gas through them for at least 15 min.
Measurement of H2O2.
H2O2 was measured by using the ROS-Glo H2O2 Assay kit (cat. no. G8820; Promega) according to the manufacturer's instructions. The assay is based on a luminescent signal generated by a chemical reaction of H2O2 and its substrate. The luminescence was measured using a FLUOstar OPTIMA microplate reader (BMG Labtech, Ortenberg, Germany). The value was normalized by protein content.
Western blot analysis.
Equal amounts (40 μg) of protein from the lysate were separated by 12% SDS-PAGE and transferred to a PVDF membrane (Millipore). The membrane was blocked by 5% nonfat dry milk and then incubated with rabbit anti-Nox4 antibody (cat. no. ab133303; Abcam) or mouse monoclonal anti-β-actin antibody (cat. no. A1978; Sigma) at 4°C overnight. After incubation with the primary antibody, the membrane was washed three times for 15 min each and incubated with secondary antibody at room temperature for 1 h. After the membrane was washed three times, immunoreactivity was detected by chemiluminescence and visualized by using enhanced chemiluminescence Western blot reagents and hyperfilm ECL.
Quantitative PCR.
RNA from mpkCCD cells after Nox4 siRNA transfection was extracted, reverse transcribed, and cDNA levels were determined for Nox4 and GAPDH using real-time PCR. Sequences of primers for Nox4 were 5′-tgttgggcctaggattgtgtt-3′ and 5′-agggaccttctgtgatcctcg-3′, and primers for GAPDH were 5′-gtcttcactaccatggagaagg-3′ and 5′-tcatggatgaccttggccag-3′.
Statistical analysis.
Data are summarized as means ± SE. All data points were included in the statistical analyses. Sample sizes were determined on the basis of similar previous studies or pilot experiments. Statistical analysis was performed by using analysis of variance with the Bonferroni test for multiple comparisons or by paired or unpaired Student's t-test for two comparisons. A value of P < 0.05 was considered statistically significant.
RESULTS
ENaC regulation by (pro)renin/PRR in cultured CD cells.
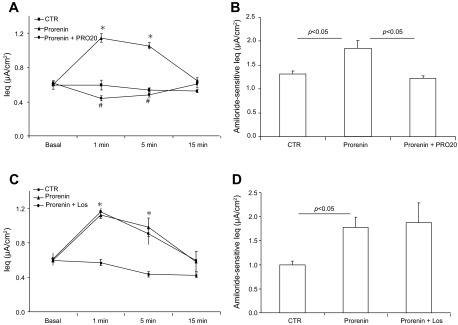
The recent discovery of PRR suggests that (pro)renin may exert a biological effect via its membrane receptor independently of RAS activity. We tested the hypothesis that prorenin or renin via PRR stimulated epithelial Na+ transport in cultured mpkCCD cells. Following 10 nM prorenin treatment, the Ieq, calculated as the ratio of Vte to Rte, was transiently increased, peaking at 1 min (1.18 ± 0.04 vs. 0.51 ± 0.02 μA/cm2; P < 0.05) and returning to baseline at 15 min. The increase was completely prevented by PRO20, a specific competitive inhibitor of PRR (13) (Fig. 1A). The same results were obtained by measurement of amiloride-sensitive current at 1 min of treatment (Fig. 1B).
Fig. 1.
Effect of prorenin/(pro)renin receptor (PRR) on epithelial Na+ channel (ENaC) activity in cultured collecting duct (CD) cells. Confluent mpkCCD cells grown on Transwells were pretreated for 30 min with PRO20 (1.5 μM) or losartan (1.0 μM) and then treated with prorenin (10 nM). The equivalent current (Ieq) was monitored for a 15-min period by using an epithelial volt-ohmmeter (EVOM). In a separate experiment, the time point of peak stimulation of Na+ transport at 1 min was chosen for measuring amiloride-sensitive Ieq, an index of ENaC activity. A: time course of Ieq changes in control (CTR), prorenin, and prorenin + PRO20 groups. B: corresponding amiloride-sensitive Ieq for experiments in A. C: time course of Ieq changes in control, prorenin, and prorenin + losartan (Los) groups. D: corresponding amiloride-sensitive Ieq for experiments in C. Data are means ± SE; n = 6–12 per group. *P < 0.05 vs. basal in the same group; #P < 0.05 vs. prorenin.
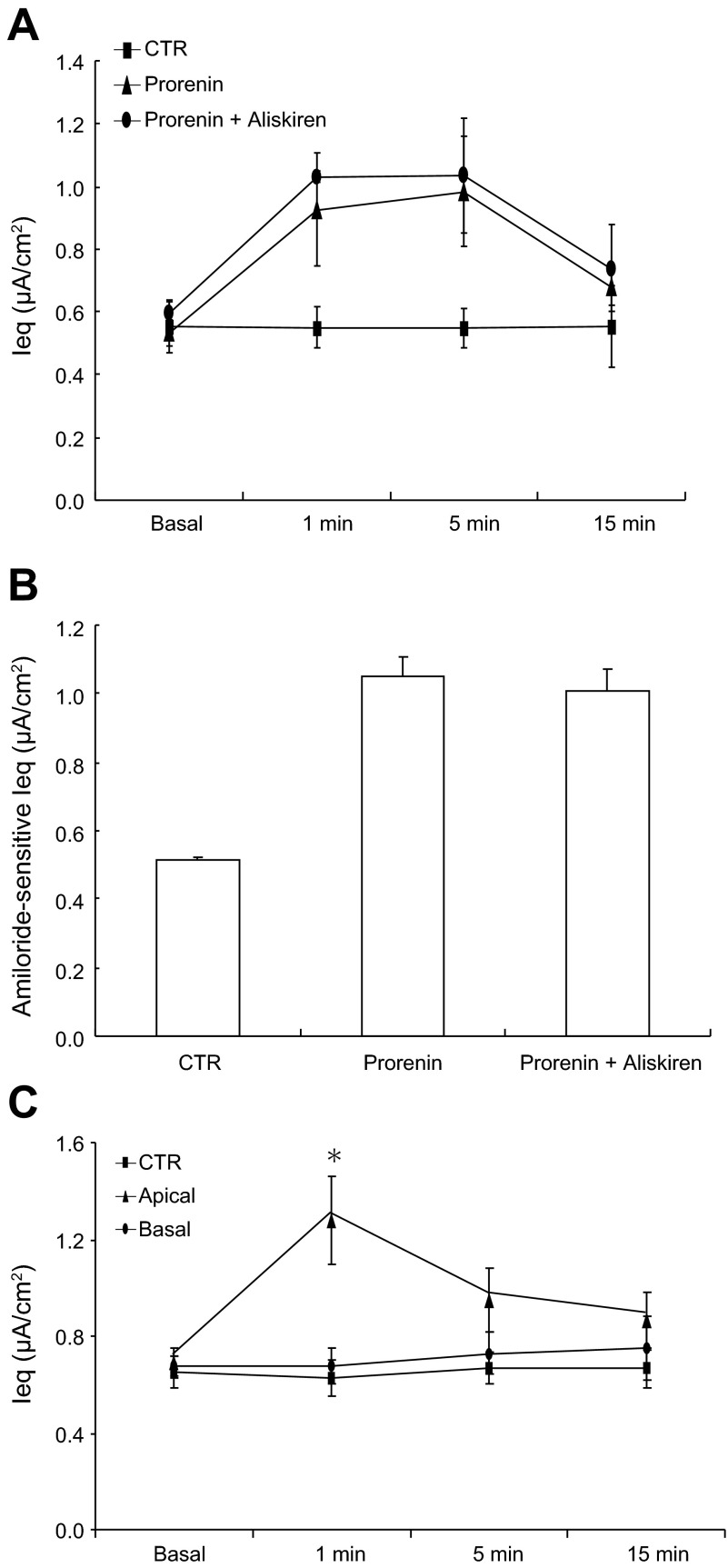
The addition of prorenin presumably increases renin activity, leading to activation of the canonical RAS. To rule out the involvement of RAS activity in prorenin-induced Na+ transport, we used an AT1 receptor blocker, losartan, and a direct renin inhibitor, aliskiren. Unlike PRO20, losartan failed to affect prorenin-induced increases in Ieq (Fig. 1C) or ENaC activity (Fig. 1D). Aliskiren was similarly ineffective (Fig. 2, A and B). It is evident that the stimulatory effect of prorenin on ENaC is independent of the canonical RAS activity. In these experiments, prorenin was added to apical side. Ieq was unaffected when prorenin was added to the basal side (Fig. 2C). This result agrees with the apical membrane localization of PRR (8).
Fig. 2.
Effect of aliskiren on prorenin-induced Na+ transport in cultured CD cells. Confluent mpkCCD cells grown on Transwells were pretreated for 30 min with aliskiren (1.0 μM) and then treated with prorenin (10 nM). Ieq was and amiloride-sensitive Ieq were determined as described in Fig. 1. A: time course of Ieq changes in control, prorenin, and prorenin + aliskiren groups. Prorenin was added to the apical side. B: corresponding amiloride-sensitive Ieq for experiments in A. C: comparison of the effect of apical and basal administration of prorenin on Ieq. Data are means ± SE; n = 6–12 per group. *P < 0.05 vs. basal in the same group.
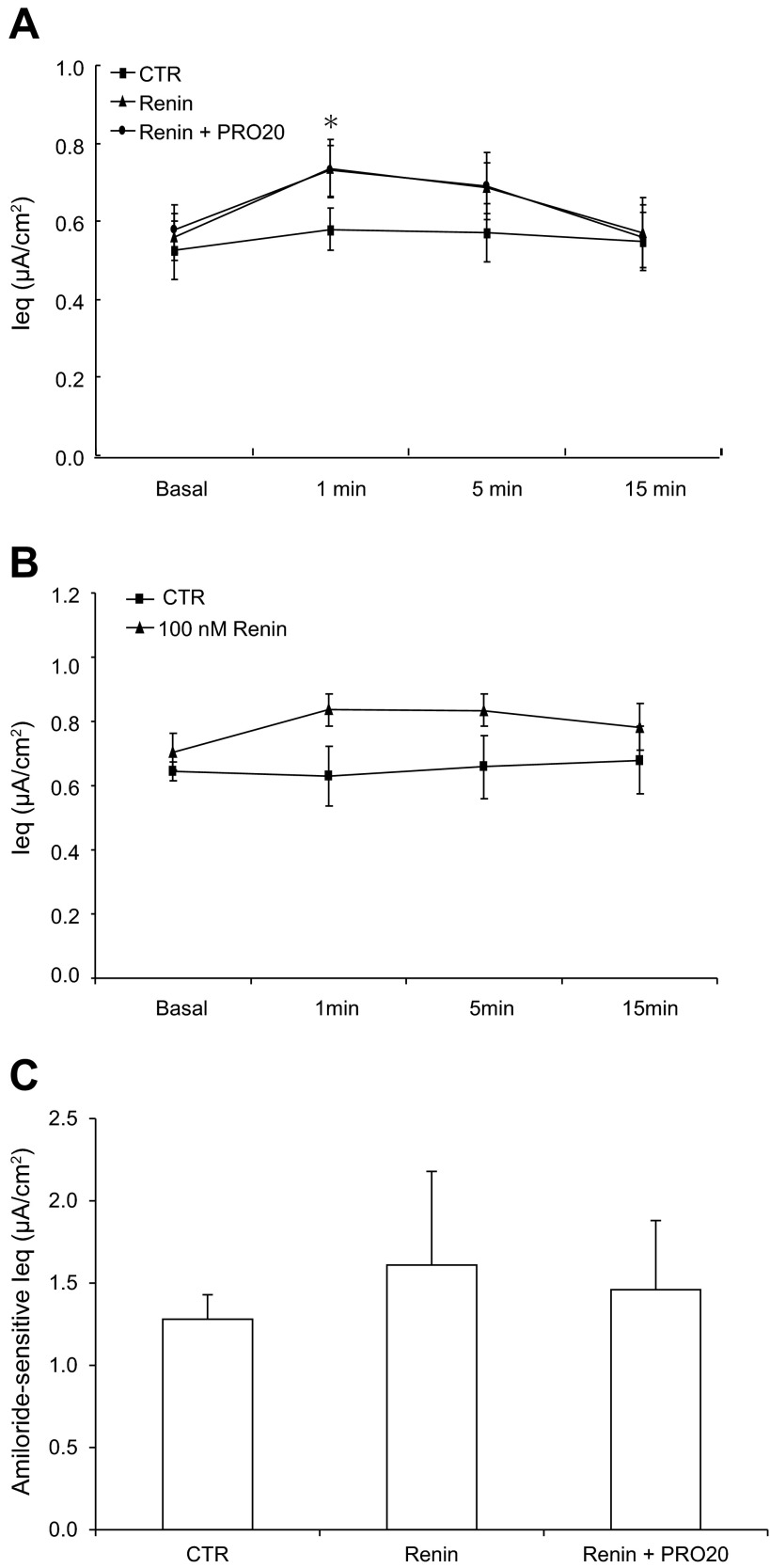
In parallel, we examined the effect of renin on ENaC activity in cultured mpkCCD cells. Exposure to renin at the same concentration of 10 nM induced a small but significant increase in the Ieq at 1 min (0.84 ± 0.08 vs. 0.61 ± 0.08 μA/cm2; P < 0.05); this increase was insensitive to PRO20 (Fig. 3A). No further increase in the Ieq was observed when a 10-time higher concentration of renin was used (Fig. 3B). Amiloride-sensitive Ieq showed no statistical significance in any group (Fig. 3C). Obviously, renin is a less potent stimulator of Na+ transport compared with prorenin.
Fig. 3.
Effect of renin on ENaC activity in cultured CD cells. Confluent mpkCCD cells grown on a Transwell membrane were pretreated for 30 min with PRO20 (1.5 μM) and then treated with renin (10 nM). A: time course of Ieq changes in Control, renin, and renin + PRO20 groups. Ieq was monitored for 15 min. Renin was given at 10 nM. B: time course of Ieq changes in control and renin groups was monitored for 15 min. Renin was given at 100 nM. C: amiloride-sensitive Ieq in control, renin, and renin + PRO20 groups. Data are means ± SE; n = 6–12 per group. *P < 0.05 vs. basal in the same group.
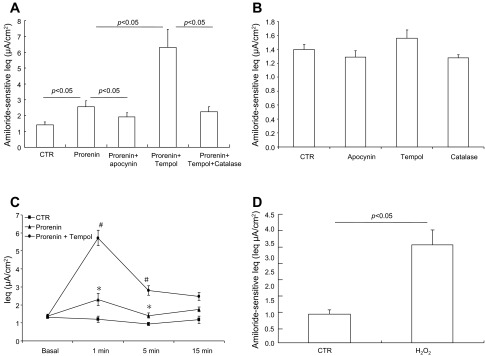
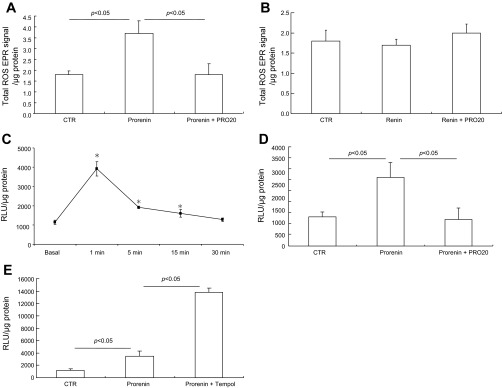
ROS have been suggested to be a potential regulator of ENaC activity (10, 41). We examined the contribution of ROS to prorenin-induced ENaC activity. The acute effect of prorenin on ENaC activity was completely abolished by apocynin, an inhibitor of NADPH oxidase (Fig. 4A). Interestingly, acute ENaC activation by prorenin was remarkably enhanced by tempol, a superoxide dismutase that converts O2− to H2O2. The effect of tempol was reversed by catalase, which decomposes H2O2 to H2O and O2−. Administration of apocynin, tempol, or catalase alone did not affect amiloride-sensitive Ieq under basal condition (Fig. 4B). In a separate experiment, we compared the time courses of the Ieq changes in control, prorenin, and prorenin + tempol groups. As shown earlier, prorenin induced a much greater increase in the Ieq in the presence of tempol but the time course of Ieq elevation was roughly similar in the presence or absence of tempol (Fig. 4C). Administration of exogenous H2O2 induced rapid stimulation of ENaC activity (Fig. 4D).
Fig. 4.
Effect of antioxidants on prorenin-induced ENaC activity. A: mpkCCD cells were pretreated for 30 min with vehicle, apocynin (10 μM), tempol (2 mM), or tempol (2 mM) + catalase (1 μg/ml) and then treated with 10 nM prorenin. Amiloride-sensitive Ieq was taken as ENaC activity. B: mpkCCD cells were treated with vehicle, apocynin, tempol, or catalase alone at the same concentrations. C: time course of Ieq changes in control, prorenin, and prorenin + tempol groups. D: amiloride-sensitive Ieq in mpkCCD cells exposed to 1.5 μM H202 for 1 min. Data are means ± SE; n = 6–12 per group. *P < 0.05 vs. basal in the same group; #P < 0.05 vs. prorenin.
EPR spin trapping showed that a 10-nM prorenin treatment in mpkCCD cells for 1 min elevated the EPR signal, an index of total ROS production, which was blocked by PRO20 treatment (Fig. 5A). In contrast, 10 nM renin was ineffective (Fig. 5B). Prorenin induced a rapid H2O2 generation with the peak at 1 min that declined thereafter (Fig. 5C), coinciding with the time course of the Ieq. Prorenin-induced H2O2 generation was completely blocked by PRO20 (Fig. 5D). As expected, tempol enhanced H2O2 generation induced by prorenin (Fig. 5E). These results suggest H2O2 as a mediator in prorenin-induced ENaC activity.
Fig. 5.
reactive oxygen species (ROS) generation in response to prorenin and renin. mpkCCD cells grown on a Transwell membrane were exposed to prorenin or renin each at 10 nM for 1 min in the presence or absence of 1.5 μM PRO20 or tempol. The samples were then subjected to spin trapping analysis of ROS (A and B) or the luciferase assay for H2O2 (C and D). A: effect of prorenin on ROS generation in the presence or absence of PRO20. B: effect of renin on ROS generation. EPR, electron paramagnetic resonance. C: time course of H2O2 generation after prorenin treatment. RLU, relative light units. D: effect of prorenin on H2O2 generation in the presence or absence of PRO20. E: effect of prorenin on H2O2 generation in the presence or absence of tempol. Data are means ± SE; n = 6–12 per group. *P < 0.05 vs. basal in the same group.
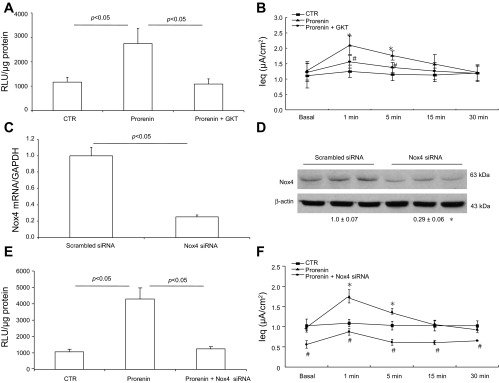
Nox4 is a unique NADPH oxidase subunit capable of generating H2O2 in a number of systems, such as lung mesenchymal cells, where Nox4-derived H2O2 mediates transforming growth factor-β-induced profibrotic response (9). We examined the involvement of Nox4 in prorenin-induced ENaC activity by using a dual Nox1/4 inhibitor, GKT137892, and Nox4 siRNA. GKT137892 effectively attenuated increases in both H2O2 generation and Ieq induced by prorenin (Fig. 6A) but had no effect on baseline Ieq (Fig. 6B). Nox4 silencing with siRNA effectively reduced Nox4 mRNA and protein as assessed by quantitative RT-PCR (Fig. 6C) and immunoblotting (Fig. 6D), respectively. These results documented efficacy of the gene silencing. Nox4 knockdown attenuated prorenin-induced H2O2 generation (Fig. 6E), similar to the effect of GKT137892. After Nox4 knockdown, baseline Ieq was reduced by 45% and its response to prorenin was significantly attenuated (Fig. 6F). The slope of the initial rise of Ieq (within 1 min) following prorenin treatment was determined to reflect differences in the Ieq responses (3.15 ± 0.60 in the prorenin/Nox4-siRNA group vs. 7.82 ± 1.32 in the prorenin group, P < 0.01).
Fig. 6.
The role of Nox4 in prorenin-induced ENaC activity. mpkCCD cells were pretreated with a Nox1/4 inhibitor, GKT137892 (GKT), or transfected with Nox4 siRNA and then treated with 10 nM prorenin. H2O2 generation was determined by ROS-Glo H2O2 Assay. Transepithelial Na+ transport was measured by using an EVOM. Nox4 mRNA and protein were determined by quantitative RT-PCR and Western blotting, respectively. A: effect of GKT on prorenin-induced H2O2 level. B: effect of GKT on prorenin-induced Ieq. C: validation of Nox4 knockdown by quantitative RT-PCR. D: validation of Nox4 knockdown by Western blotting. Shown is a representative immunoblot of 3 independent experiments. The value underneath the blot shows the densitometry of Nox4 protein normalized by β-actin. E: effect of Nox4 siRNA knockdown on prorenin-induced H2O2 level. F: effect of Nox4 knockdown on prorenin-induced Ieq. Data are means ± SE: n = 3 per group for D and n = 6–8 per group for A–C, E, and F. *P < 0.05 vs. basal in the same group; #P < 0.05 vs. prorenin.
DISCUSSION
Despite the well-accepted concept of the juxtaglomerular cell origin of (pro)renin, recent work has established the CD principal cells as an important source of renin/prorenin (11, 23, 27). Interestingly, PRR is also localized to the CD, particularly the intercalated cells, suggesting a possible autocrine/paracrine mechanism for the regulation of distal tubular reabsorption. The present study examined the potential role of (pro)renin/PRR in regulation of ENaC activity in cultured CD cells and further explored the underlying mechanism. We demonstrated that prorenin in the nanomolar range markedly induces ENaC activity with a potency much greater than that of renin. The stimulatory effect of prorenin on ENaC activity depends on activation of PRR but not canonical RAS activity. The signaling downstream of PRR involves the release of H2O2 derived from Nox4.
To probe the sodium-regulatory function of PRR, we examined the effect of PRR activation by prorenin and renin on ENaC activity in cultured CD cells. The most striking observation was that prorenin in the nanomolar range remarkably induced ENaC activity whereas renin at this dose or even at a 10-time higher dose was largely ineffective. Prorenin may act via its enzyme activity or receptor-mediated signal transduction. We used a newly developed decoy peptide of PRR, PRO20, to evaluate the contribution of PRR to prorenin-induced ENaC activation. The specificity of PRO20 has been validated by using neuronal cells deficient in PRR (13). We found that prorenin-induced ENaC activation was completely abolished by PRO20 but not losartan or aliskiren, suggesting exclusive reliance on receptor-mediated signal transduction, but not RAS activity. These results also favor prorenin over renin as the true physiological ligand of PRR in CD cells. Although both prorenin and renin were initially found to bind PRR at similar affinities in cultured mesangial cells, a subsequent binding kinetics study reported differences in binding affinities in rat vascular smooth muscle cells that overexpressed human PRR (2). This study showed that human PRR exhibited a higher binding affinity for prorenin than renin (2). Moreover, the prorenin content was remarkably increased in the CD after ANG II treatment or in the milieu of diabetes (11). Unfortunately, in this study, the prorenin content was assessed based on the assay for renin activity following trypsinization and thus the local concentration of prorenin remains unclear. For this reason, we are uncertain whether 10 nM of prorenin used in the current cell culture model is a physiological concentration in the CD. A range of 5–100 nM of prorenin is required to induce proinflammatory and profibrotic responses in various in vitro systems (21, 31, 42). Overall, these results support a potential signaling function of prorenin/PRR in the CD under normal or pathological conditions.
ROS are shown to positively influence ENaC activity (31, 32, 33). Moreover, one study reported that in human embryonic kidney (HEK) cells, treatment with prorenin or renin stimulated ROS generation, although the involvement of PRR was not tested (5). In the present study, we examined the potential role of ROS in mediating prorenin/PRR-induced activation of ENaC. EPR spin trapping demonstrated that exposure of cultured mpkCCD cells to prorenin rapidly induced ROS generation, which was completely prevented by PRO20, indicating reliance on PRR activation. The stimulatory effect of prorenin on ENaC activity was completely abolished by apocynin, an inhibitor of NADPH oxidase. Interestingly, ENaC activation by prorenin was remarkably enhanced by tempol but reversed by catalase, suggesting involvement of H2O2. Indeed, the production of H2O2 was increased after prorenin treatment in a time-dependent manner, coinciding with the time course of increased Na+ transport. These results suggest that H2O2 may play a more important role than O2− in coupling with PRR to activate ENaC. Compared with O2−, H2O2 is a relatively stable and mild oxidant, favoring its role in signal transduction. In support of this notion, increasing evidence suggests an important role of renal medullary H2O2 in mediating the response to various hypertensive stimuli such as high salt loading (34) and ANG II infusion (32). More recently, H2O2 has been shown to induce epigenetic modification of the PRR gene (12), suggesting a mutually interactive relationship between PRR and H2O2.
Nox4, also known as renox, is the major NADPH oxidase isoform expressed in renal cells and appears to primarily produce H2O2 (17). Therefore, we suspected that Nox4 might be involved in ENaC activation induced by prorenin. Nox4 knockdown significantly reduced baseline Na+ transport in mpkCCD cells, suggesting that Nox4-derived ROS may tonically control ENaC activity. Following Nox4 knockdown, the response of Na+ transport to prorenin was partially attenuated. The same result was obtained with the Nox1/4 inhibitor GKT137892, except that the baseline current was unaffected. These findings indicate that the full ENaC response to prorenin may require factors in addition to Nox4. On the other hand, Nox4 has been shown to mediate the fibrogenic effect of prorenin and renin in HEK cells (5). It is likely that the stimulation of Nox4-derived ROS may represent a common mediator of diverse effects of prorenin/PRR.
The discovery of ENaC regulation by the prorenin/PRR pathway may have functional implications in a number of pathophysiological conditions. The evidence for increased expression of CD renin (7) and PRR (38, 39) in this model, as well as the attenuation of hypertension by CD-specific deletion of renin (24), suggests that overactivation of this pathway may partially contribute to ANG II-induced hypertension. Our findings may also be relevant to other fluid retention states associated with high prorenin levels seen in diabetes and pregnancy. The alteration of the RAS in diabetes is characterized by elevated serum prorenin levels that are often associated with microvascular complications, whereas plasma renin is normal or low (40). Excess prorenin over and above active renin in diabetes originates from the CD rather than from the juxtaglomerular apparatus (11). Normal pregnancy is well known to be associated with increased plasma prorenin concentration (26, 30). Serum soluble PRR is elevated in patients with preeclampsia (36). It seems possible that abnormal renal Na+ reabsorption due to aberrant activation of CD prorenin/PRR may contribute to fluid retention and hypertension in preeclampsia.
The present study has a number of limitations, which include the heavy reliance on the cell culture model and the lack of direct measurement of ENaC activity. As a limitation of most cell culture models, mpkCCD cells may not behave as the native CD cells. Our findings need to be validated by patch-clamp measurement of ENaC activity in freshly isolated CCD.
In summary, the present study examined the role of prorenin/renin and PRR in the regulation of ENaC activity in CD cells. Our data suggest that prorenin in the nanomolar range significantly induces ENaC activity via PRR-dependent Nox4-derived H2O2 but not RAS activity. These results set an important platform for future investigation of the role of prorenin/PRR in the regulation of blood pressure and Na+ balance under pathophysiological conditions.
GRANTS
This work was supported by National Natural Science Foundation of China Grants 91439205 and 31330037, National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-104072 and DK-094956, National Basic Research Program of China 973 Program 2012CB517600 (No. 2012CB517602), and Veterans Affairs Merit Review from the Department of Veterans Affairs. T. Yang is a Research Career Scientist in the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
X.L., F.W., and T.Y. conception and design of research; X.L., F.W., M.L., K.T.Y., A.N., V.R.R., and T.Y. performed experiments; X.L., F.W., M.L., K.T.Y., and T.Y. analyzed data; X.L., F.W., D.E.K., R.S.R., and T.Y. interpreted results of experiments; X.L., F.W., and T.Y. prepared figures; X.L., F.W., and T.Y. drafted manuscript; X.L., F.W., and T.Y. edited and revised manuscript; X.L., F.W., and T.Y. approved final version of manuscript.
REFERENCES
- 1.Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, Connelly KA, Yuen D, Trogadis J, Herzenberg AM, Kuliszewski MA, Leong-Poi H, Gilbert RE. The (Pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension 54: 261–269, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Batenburg WW, Krop M, Garrelds IM, de Vries R, de Bruin RJ, Burckle CA, Muller DN, Bader M, Nguyen G, Danser AH. Prorenin is the endogenous agonist of the (pro)renin receptor. Binding kinetics of renin and prorenin in rat vascular smooth muscle cells overexpressing the human (pro)renin receptor. J Hypertens 25: 2441–2453, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, Rossier BC, Vandewalle A. Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol 10: 923–934, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Burckle C, Bader M. Prorenin and its ancient receptor. Hypertension 48: 549–551, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Clavreul N, Sansilvestri-Morel P, Magard D, Verbeuren TJ, Rupin A. (Pro)renin promotes fibrosis gene expression in HEK cells through a Nox4-dependent mechanism. Am J Physiol Renal Physiol 300: F1310–F1318, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC. Soluble form of the (pro)renin receptor is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertension 57: 859–864, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez AA, Liu L, Lara LS, Seth DM, Navar LG, Prieto MC. Angiotensin II stimulates renin in inner medullary collecting duct cells via protein kinase C and independent of epithelial sodium channel and mineralocorticoid receptor activity. Hypertension 57: 594–599, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez AA, Luffman C, Bourgeois CR, Vio CP, Prieto MC. Angiotensin II-independent upregulation of cyclooxygenase-2 by activation of the (Pro)renin receptor in rat renal inner medullary cells. Hypertension 61: 443–449, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15: 1077–1081, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ilatovskaya DV, Pavlov TS, Levchenko V, Staruschenko A. ROS production as a common mechanism of ENaC regulation by EGF, insulin, and IGF-1. Am J Physiol Cell Physiol 304: C102–C111, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J. The collecting duct is the major source of prorenin in diabetes. Hypertension 51: 1597–1604, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DY, Kim HS, Won KJ, Lee KP, Jung SH, Park ES, Choi WS, Lee HM, Kim B. DJ-1 regulates the expression of renal (pro)renin receptor via reactive oxygen species-mediated epigenetic modification. Biochim Biophys Acta 1850: 426–434, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Sullivan MN, Zhang S, Worker CJ, Xiong Z, Speth RC, Feng Y. Intracerebroventricular infusion of the (Pro)renin receptor antagonist PRO20 attenuates deoxycorticosterone acetate-salt-induced hypertension. Hypertension 65: 352–361, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ludwig J, Kerscher S, Brandt U, Pfeiffer K, Getlawi F, Apps DK, Schagger H. Identification and characterization of a novel 9.2-kDa membrane sector-associated protein of vacuolar proton-ATPase from chromaffin granules. J Biol Chem 273: 10939–10947, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Mamenko M, Zaika O, Ilatovskaya DV, Staruschenko A, Pochynyuk O. Angiotensin II increases activity of the epithelial Na+ channel (ENaC) in distal nephron additively to aldosterone. J Biol Chem 287: 660–671, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCormick JA, Bhalla V, Pao AC, Pearce D. SGK1: a rapid aldosterone-induced regulator of renal sodium reabsorption. Physiology 20: 134–139, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Montezano AC, Burger D, Ceravolo GS, Yusuf H, Montero M, Touyz RM. Novel Nox homologues in the vasculature: focusing on Nox4 and Nox5. Clin Sci 120: 131–141, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Nabi AH, Kageshima A, Uddin MN, Nakagawa T, Park EY, Suzuki F. Binding properties of rat prorenin and renin to the recombinant rat renin/prorenin receptor prepared by a baculovirus expression system. Int J Mol Med 18: 483–488, 2006. [PubMed] [Google Scholar]
- 19.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimoto M, Fujita T. Renal mechanisms of salt-sensitive hypertension: contribution of two steroid receptor-associated pathways. Am J Physiol Renal Physiol 308: F377–F387, 2015. [DOI] [PubMed] [Google Scholar]
- 21.Peng H, Li W, Seth DM, Nair AR, Francis J, Feng Y. (Pro)renin receptor mediates both angiotensin II-dependent and -independent oxidative stress in neuronal cells. PLoS One 8: e58339, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prieto-Carrasquero MC, Botros FT, Pagan J, Kobori H, Seth DM, Casarini DE, Navar LG. Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip goldblatt hypertensive rats. Hypertension 51: 1590–1596, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension 44: 223–229, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramkumar N, Stuart D, Rees S, Hoek AV, Sigmund CD, Kohan DE. Collecting duct-specific knockout of renin attenuates angiotensin II-induced hypertension. Am J Physiol Renal Physiol 307: F931–F938, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramkumar N, Ying J, Stuart D, Kohan DE. Overexpression of Renin in the collecting duct causes elevated blood pressure. Am J Hypertens 26: 965–972, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Ringholm L, Pedersen-Bjergaard U, Thorsteinsson B, Boomsma F, Damm P, Mathiesen ER. A high concentration of prorenin in early pregnancy is associated with development of pre-eclampsia in women with type 1 diabetes. Diabetologia 54: 1615–1619, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension 34: 1265–1274, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Rossier BC, Baker ME, Studer RA. Epithelial sodium transport and its control by aldosterone: the story of our internal environment revisited. Physiol Rev 95: 297–340, 2015. [DOI] [PubMed] [Google Scholar]
- 29.Rossman MJ, Groot HJ, Reese V, Zhao J, Amann M, Richardson RS. Oxidative stress and COPD: the effect of oral antioxidants on skeletal muscle fatigue. Med Sci Sports Exerc 45: 1235–1243, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sealey JE, McCord D, Taufield PA, Ales KA, Druzin ML, Atlas SA, Laragh JH. Plasma prorenin in first-trimester pregnancy: relationship to changes in human chorionic gonadotropin. Am J Obstet Gynecol 153: 514–519, 1985. [DOI] [PubMed] [Google Scholar]
- 31.Shi P, Grobe JL, Desland FA, Zhou G, Shen XZ, Shan Z, Liu M, Raizada MK, Sumners C. Direct pro-inflammatory effects of prorenin on microglia. PLoS One 9: e92937, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sousa T, Pinho D, Morato M, Marques-Lopes J, Fernandes E, Afonso J, Oliveira S, Carvalho F, Albino-Teixeira A. Role of superoxide and hydrogen peroxide in hypertension induced by an antagonist of adenosine receptors. Eur J Pharmacol 588: 267–276, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Sun P, Yue P, Wang WH. Angiotensin II stimulates epithelial sodium channels in the cortical collecting duct of the rat kidney. Am J Physiol Renal Physiol 302: F679–F687, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor NE, Cowley AW Jr. Effect of renal medullary H2O2 on salt-induced hypertension and renal injury. Am J Physiol Regul Integr Comp Physiol 289: R1573–R1579, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Thomas CP, Itani OA. New insights into epithelial sodium channel function in the kidney: site of action, regulation by ubiquitin ligases, serum- and glucocorticoid-inducible kinase and proteolysis. Curr Opin Nephrol Hypertens 13: 541–548, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Thomason J, Reyes M, Allen SR, Jones RO, Beeram MR, Kuehl TJ, Suzuki F, Uddin MN. Elevation of (pro)renin and (pro)renin receptor in preeclampsia. Am J Hypertens 28: 1277–1284, 2015. [DOI] [PubMed] [Google Scholar]
- 37.Verrey F, Fakitsas P, Adam G, Staub O. Early transcriptional control of ENaC (de)ubiquitylation by aldosterone. Kidney Int 73: 691–696, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Wang F, Lu X, Peng K, Du Y, Zhou SF, Zhang A, Yang T. Prostaglandin E-prostanoid4 receptor mediates angiotensin II-induced (pro)renin receptor expression in the rat renal medulla. Hypertension 64: 369–377, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang F, Lu X, Peng K, Zhou L, Li C, Wang W, Yu X, Kohan DE, Zhou SF, Yang T. COX-2 mediates angiotensin II-induced (pro)renin receptor expression in the rat renal medulla. Am J Physiol Renal Physiol 307: F25–F32, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yokota H, Nagaoka T, Tani T, Takahashi A, Sato E, Kato Y, Yoshida A. Higher levels of prorenin predict development of diabetic retinopathy in patients with type 2 diabetes. J Renin Angiotensin Aldosterone Syst 12: 290–294, 2011. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Chen S, Liu H, Zhang B, Zhao Y, Ma K, Zhao D, Wang Q, Ma H, Zhang Z. Hydrogen sulfide prevents hydrogen peroxide-induced activation of epithelial sodium channel through a PTEN/PI(3, 4,5)P3 dependent pathway. PLoS One 8: e64304, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, Wu J, Gu C, Noble NA, Border WA, Huang Y. Receptor-mediated nonproteolytic activation of prorenin and induction of TGF-β1 and PAI-1 expression in renal mesangial cells. Am J Physiol Renal Physiol 303: F11–F20, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]