Abstract
Diabetes increases the reabsorption of Na+ (TNa) and glucose via the sodium-glucose cotransporter SGLT2 in the early proximal tubule (S1-S2 segments) of the renal cortex. SGLT2 inhibitors enhance glucose excretion and lower hyperglycemia in diabetes. We aimed to investigate how diabetes and SGLT2 inhibition affect TNa and sodium transport-dependent oxygen consumption along the whole nephron. To do so, we developed a mathematical model of water and solute transport from the Bowman space to the papillary tip of a superficial nephron of the rat kidney. Model simulations indicate that, in the nondiabetic kidney, acute and chronic SGLT2 inhibition enhances active TNa in all nephron segments, thereby raising by 5–12% in the cortex and medulla. Diabetes increases overall TNa and by ∼50 and 100%, mainly because it enhances glomerular filtration rate (GFR) and transport load. In diabetes, acute and chronic SGLT2 inhibition lowers in the cortex by ∼30%, due to GFR reduction that lowers proximal tubule active TNa, but raises in the medulla by ∼7%. In the medulla specifically, chronic SGLT2 inhibition is predicted to increase by 26% in late proximal tubules (S3 segments), by 2% in medullary thick ascending limbs (mTAL), and by 9 and 21% in outer and inner medullary collecting ducts (OMCD and IMCD), respectively. Additional blockade of SGLT1 in S3 segments enhances glucose excretion, reduces by 33% in S3 segments, and raises by <1% in mTAL, OMCD, and IMCD. In summary, the model predicts that SGLT2 blockade in diabetes lowers cortical and raises medullary , particularly in S3 segments.
Keywords: sodium transport, glucose, metabolism, diabetes
in the united states, diabetes is one of the most common causes of chronic kidney disease (CKD) (16). Despite intense research, the pathways that link diabetes to CKD remain incompletely understood. Renal hypoxia, stemming from a mismatch between changes in renal oxygen (O2) delivery and demand, is thought to be an important mechanism in the development of CKD, as first proposed by Fine and Norman and subsequently expanded upon (14, 15, 18, 45).
Due to the role the renal medulla plays in the production of concentrated urine, blood flow is substantially lower in that region than in the renal cortex. The resulting low O2 delivery to the outer medulla (OM) yields a Po2 of 20 mmHg, compared with 50 mmHg in the cortex (6, 29). The reduced Po2 in the OM is attributable to low blood flow, to oxygen consumption associated with active Na+ reabsorption in the late proximal tubules (S3) and medullary thick ascending limbs (mTAL), and likely to the countercurrent exchange of O2 between the descending and ascending vasa recta. Thus low O2 levels in the renal OM are an obligatory component of the process of urinary concentration. When O2 supply is further impaired, OM hypoxic injury can develop. This is illustrated by the renal response to ischemia-reperfusion, which reveals widespread vascular congestion and tubular epithelial injury in the OM (6, 32).
A new therapeutic approach for lowering blood glucose levels in diabetes is to inhibit glucose reabsorption in the kidney, specifically by targeting the Na+-glucose cotransporter SGLT2 in the early proximal tubule. In addition to lowering blood glucose levels, SGLT2 inhibitors lead to weight loss and lower blood pressure and reduce cardiovascular mortality in type 2 diabetic patients (33, 47, 61). Despite their apparent promise, many nuances of SGLT2 inhibition remain poorly understood. Inhibiting SGLT2 lowers the proximal tubule uptake of not only glucose but Na+ as well, thereby shifting glucose and Na+ reabsorption to downstream nephron segments, including the S3 segment of the proximal tubule and the thick ascending limb (TAL) in the vulnerable OM. Furthermore, the efficiency of Na+ transport (namely, the number of Na+ moles reabsorbed per O2 moles consumed) changes along the nephron, with the early proximal tubule being more efficient than the distal segments (23). Finally, SGLT2 inhibitors have also been approved in diabetic patients with impairment of kidney function [estimated glomerular filtration rate (eGFR) >45 ml/min]. The remaining functional nephrons in these patients may also hyperfilter and be particularly sensitive to changes in transport work and developing hypoxia. Thus, to better understand the nuances and long-term effects of SGLT2 inhibitors, we sought to assess the extent to which diabetes and SGLT2 inhibition can affect transport activity and Po2 in the OM. To do so we developed a mathematical model of a rat nephron from the Bowman space to the papillary tip and built in established diabetes-induced changes in nephron structure, GFR, and protein expression of tubular transporters. Since dual SGLT2/SGLT1 inhibitors are currently in clinical development and SGLT1 is expressed in the vulnerable S3 segment of the proximal tubule in the OM, we also explored their effects.
MATHEMATICAL MODEL
We have developed an epithelial cell-based model of a superficial nephron of a rat kidney, extending from Bowman's capsule to the papillary tip. The model is based on previously applied models of the proximal tubule (26), TAL (12), distal convoluted tubule (DCT) (11), connecting tubule (CNT) (11), and collecting duct (CD) (53), together with a new, simple model of the descending limb (see below). Each nephron segment is represented as a rigid tubule lined by a layer of epithelial cells, with apical and basolateral transporters that vary according to cell type (Fig. 1).
Fig. 1.
Schematic diagram of the nephron. The model accounts for the transport of water and 15 solutes (listed in Table 3). The diagram displays only the main Na+, K+, and Cl− transporters. PCT, proximal convoluted tubule; mTAL, medullary thick ascending limb; cTAL, cortical thick ascending limb; DCT, distal convoluted tubule; CNT, connecting tubule; CCD, cortical collecting duct; OMCD and IMCD, outer and inner medullary collecting duct.
The model assumes that the CNTs coalesce successively within the cortex, resulting in a ratio of loop-to-cortical CD of 5:1. In the inner medulla (IM), the CDs again coalesce successively. The luminal diameter increases with coalescence: it is set to 15 μm in the DCT, 18 μm in the CNT, 25 μm in the cortical (CCD) and outer medullary collecting ducts (OMCD), and 28 μm in the inner medullary collecting duct (IMCD). The impact of tubular coalescence on transmembrane fluxes and fluid dynamics is described below. The model accounts for 15 solutes: Na+, K+, Cl−, HCO3−, H2HCO3−, CO2, NH3, NH4+, HPO42−, H2PO4−, H+, HCO2−, H2CO2, urea, and glucose.
The model is formulated for steady state and predicts luminal fluid flow, hydrostatic pressure, luminal fluid solute concentrations, and, with the exception of the descending limb segment, cytosolic solute concentrations, membrane potential, and transcellular and paracellular fluxes.
Sodium-Glucose Cotransport in the Proximal Tubule
Under physiological conditions, the filtered load of glucose is fully reabsorbed in the proximal tubule, via sodium-glucose cotransporters (SGLT) on the apical side and glucose transport facilitators (GLUT) on the basolateral side. The proximal convoluted tubule (PCT, also referred to as the S1–S2 segments) expresses the high-capacity, low-affinity transporters SGLT2 and GLUT2, whereas the proximal straight tubule (PST, also referred to as the S3 segment) expresses the low-capacity, high-affinity transporters SGLT1 and GLUT1. As in our previous study (26), the fluxes of glucose and Na+ across SGLT2 cotransporters are calculated based on the sodium-alanine cotransporter model (20), which posits a simultaneous mechanism for the transport of Na+ and glucose, such that the fluxes are given by·
| (1) |
where
| (2) |
xSGLT2 characterizes the density of SGLT2 transporters; is the forward translocation rate of the unloaded carrier; and denote the luminal concentration of glucose and Na+, respectively; and denote the cytosolic concentration of glucose and Na+; and denote the binding affinity of SGLT2 to glucose and Na+, respectively. ζ, which is given by F(Ψlum − Ψcyt)/(RT), denotes the nondimensional apical membrane potential difference; Ψlum and Ψcyt represent the electric potential in the lumen and cytosol, respectively, R is the ideal gas constant, F is the Faraday constant, and T is the temperature.
As in our previous study (26), the fluxes of glucose and Na+ across SGLT1 are computed using a six-state kinetic model (13, 37), which assumes a Na+:glucose stoichiometry of 2:1. Glucose fluxes across GLUT are determined based on the Maki and Keiser model (27)
| (3) |
where is the maximum glucose flux, is the glucose dissociation equilibrium constant, and denotes the external (peritubular) concentration of glucose. The values of and differ between GLUT1 and GLUT2.
Parameter values for glucose transport are listed in Table 1. The expression levels of SGLT1 and SGLT2 and the SGLT2 binding constants are chosen so that 1) the fraction of the glucose load that is reabsorbed by the S3 segment during SGLT2 inhibition is consistent with experimental measurements in mice (41), and 2) glucosuria begins when plasma glucose reaches 21 mM (see below).
Table 1.
Glucose transport parameters in the proximal tubule
| Parameter | Value |
|---|---|
| SGLT2 transport rate, xSGLT2kuf | 1.30 × 10−3 cm4·mmol−1·s−1 |
| SGLT2 binding affinity to glucose, | 4.9 mM |
| SGLT2 binding affinity to sodium, | 25 mM |
| GLUT2 maximum rate, VmGLUT2 | 1.625 × 10−6 mmol·s−1·cm−2 |
| GLUT2 affinity to glucose, KmGLUT2 | 17 mM |
| SGLT1 density | 83.3 × 10−9 mol/cm |
| GLUT1 maximum rate, VmGLUT1 | 3.0 × 10−6 mmol·s−1·cm−2 |
| GLUT1 affinity to glucose, KmGLUT1 | 2.0 mM |
| Tight junction permeability to glucose | 3.1 × 10−6 cm3·s−1·cm−2 epithelium |
Glucose transport parameters are taken from our previous study [26].
Descending Limb
Connecting the S3 segment and the TAL is the descending limb. Because its transport characteristics are not well characterized, the model descending limb is assumed to be impermeable to all solutes. The initial 0.8-mm descending limb segment is assumed to be moderately water permeable, whereas the terminal 0.6-mm segment is water impermeable (52). Because no solute transport is assumed, the composition and membrane potential of the descending limb epithelial cells are not computed (unlike in all other segments); only changes in luminal volume flow and pressure are tracked. Our model simulations suggest that significant transmembrane solute concentration gradients develop only along the water- impermeable descending limb segment. Because that segment is only 0.6 mm, even if passive solute transport occurs, the consequences on overall transport and oxygen consumption will likely be minor.
The luminal membrane area of descending limb cells is set to 2.0 cm2 per cm2 epithelial area, as in TAL cells. The surface areas of the tight junction and the interspace-basal interface are set to 0.001 and 0.02 cm2 per cm2 epithelial area, as in all other segments. The transcellular and paracellular water permeabilities of the initial segment are taken to be the same as those of the proximal tubule.
Tubule Coalescence and Fluid Pressure
Tubular fluid flow is described by the pressure-driven Poiseuille flow. Along a noncoalescing tubule i, the hydrostatic pressure in the lumen, Pi, is related to volume flow Qi (per tubule) and luminal radius ri by
| (4) |
where μ is the luminal fluid viscosity (taken as 6.4 × 10−6 mmHg/s).
The CNTs and IMCDs coalesce successively, reducing the total luminal cross-sectional area. Let ωCNT(x) and ωIMCD(x) denote the number of CNTs and IMCDs, respectively, per nephron; we set
| (5) |
where LCNT and LIMCD denote the lengths of the CNTs and IMCDs, respectively, and xCNT and xIMCD denote the distances from the beginning of the respective segments. Note that at the end of the connecting tubule (xCNT = LCNT), ωCNT = 0.2, which corresponds to a CCD-to-loop ratio of 1:5. As the CNTs and IMCDs coalesce and their number diminishes, total fluid flow decreases (because water is reabsorbed), but fluid flow in a given tubule increases. The model describes the pressure gradient along the connecting tubules and IMCDs by
| (6) |
where Ω takes into account the increase in drag resistance due to tubular coalescence and is set to 2.
Oxygen Consumption Along the Nephron
The rate of oxygen (QO2) consumption can be divided into two parts: the active component provides the energy needed to actively reabsorb Na+, and the basal component supplies the energy for other transport processes and intracellular biochemical reactions. is calculated based on the ATP consumption of basolateral Na-K-ATPase pumps. Since 1 mol of ATP is required to pump out 3 mol of Na+ via the pump, and oxidative metabolism yields ∼5 moles of ATP per moles of O2 consumed (40), is determined as
| (7) |
where is the rate of Na+ transport across Na-K-ATPase pumps.
In rats, the whole kidney basal-to-total O2 consumption ratio has been estimated as 25–30% (57). To the best of our knowledge, that ratio has not been determined in individual nephron segments. We assume that, in a given segment, is fixed and equal to 25% of (total) QO2 under baseline conditions, such that
| (8) |
where the asterisk denotes base-case conditions.
The efficiency of oxygen utilization can be evaluated by computing the number of moles of Na+ reabsorbed per moles of O2 consumed. We distinguish between two ratios:
| (9) |
| (10) |
where denotes the rate of passive Na+ transport.
Assumptions for Diabetic Rats
Diabetes induces renal hypertrophy, hyperfiltration, and alterations in transporter expression. As in our previous model (26), we simulate diabetic conditions by simultaneously raising plasma glucose (from 5 to 25 mM), SNGFR (from 30 to 45 nl/min), proximal tubule diameter (from 25 to 30 μm), and SGLT2 expression (by 38%) and by decreasing SGLT1 expression (by 33%) (48). Diabetes also induces a 20% decrease in the ratio in the proximal tubule, as a result of increased fatty acid metabolism (2); we assume here that, in diabetic rats, equals 12 (vs. 15 in control animals) along the entire nephron.
Basal (ouabain-insensitive) O2 consumption in cortical and medullary cells was found to be, respectively, ∼40 and 160% higher in diabetic rats (25, 35, 36). In mTAL suspensions, however, ouabain-insensitive O2 consumption did not differ significantly between rats with streptozotocin (STZ)-induced diabetes and sham rats (60). Thus, in the present study, in diabetic rat nephrons, is taken to increase (relative to nondiabetic nephrons) by 40% in all cortical segments, to increase by 160% each in the PST, OMCD, and IMCD, and to remain unchanged in the mTAL.
In contrast, active O2 consumption in mTAL suspensions was approximately twice as high in diabetic rats than in sham rats, partly owing to the activation of NADPH oxidase (60). The increase in mTAL may also reflect a corresponding increase in mTAL . Based on several studies, we assume a small increase in the protein expression of NKCC2 in mTALs in diabetic rats but not in and cortical thick ascending limbs (cTALs; Table 2). Indeed, there appears to be differential regulation in the OM vs. the cortex. Administration of STZ to Wistar rats enhances Na-K-ATPase activity in the mTAL but not in the cTAL (21).
Table 2.
Diabetes-induced changes in sodium transporter expression
| Duration | Blood Glucose Level | Variations | Method | Reference |
|---|---|---|---|---|
| 10 days | 18.4 | NKCC2: OM × 2.8 | Protein | [22] |
| 14 days | 18.6 | NKCC2: CTX N.S. OM no change | Protein | [43] |
| 15 days | 27.1 | NKCC2: WK no change | Protein | [28] |
| 3 wk | 21.5 | NKCC2: OM × 1.45 | Protein | [5] |
| 12 wk | 30 | NKCC2: CTX and OM, N.S. | Protein | [39] |
| NKCC2: CTX no change, OM × 1.1 | model | |||
| 6 days | N.A. | NHE3; mTAL × 1.5 | Protein | [7] |
| 14 days | 18.6 | NHE3: CTX and OM N.S. | Protein | [43] |
| 15 days | 27.1 | NHE3: WK × 0.67 | Protein | [28] |
| 60 days | 24 | NHE3: CTX and OM, no change | Protein | [51] |
| NHE3: varies along PT*, mTAL no change | model | |||
| 14 days | 18.6 | NCC: N.S. | Protein | [43] |
| 15 days | 27.1 | NCC: no change | Protein | [28] |
| 3 wk | 21.5 | NCC: N.S. | Protein | [24] |
| 60 days | 24 | NCC: no change | Protein | [51] |
| 12 wk | 30 | NCC: no change | Protein | [39] |
| NCC: no change | model | |||
| 10 days | 18.4 | AQP2: IM tip × 2.7 | Protein | [22] |
| 14 days | 18.6 | AQP2: CTX × 1.4, OM no increase, IM N.S. | Protein | [43] |
| AQP3: CTX × 1.7, OM no increase, IM N.S. | ||||
| 15 days | 27.1 | AQP2: IM × 2, AQP3: IM × 1.7 | Protein | [28] |
| 3 wk | 21.5 | AQP2: IM × 1.2 | Protein | [5] |
| AQP2: CTX × 1.4, OM no increase, IM × 1.4 | model | |||
| AQP3: CTX × 1.7, OM no increase, IM × 1.4 | model | |||
| 14 days | 18.6 | α-ENaC: CTX × 2, OM no change | Protein | [43] |
| 3 wk | 21.5 | α-ENaC: CTX no change, OM × 1.7, IMB × 2.8 | Protein | [24] |
| 12 wk | 30 | α-ENaC: CTX and OM and IM, no change | Protein | [39] |
| 14 days | 18.6 | β-ENaC: CTX N.S., OM no change | Protein | [43] |
| 3 wk | 21.5 | β-ENaC: CTX no changes | Protein | [24] |
| OM × 3.9, IMB × 6 | ||||
| 12 wk | 30 | β-ENaC: CTX no change, OM N.S., IM × 1.6 | Protein | [39] |
| 14 days | 18.6 | γ-ENaC (85 kDa): CTX and OM, N.S. | Protein | [43] |
| γ-ENaC (70 kDa): CTX × 1.7, OM N.S. | ||||
| 3 wk | 21.5 | γ-ENaC (85 kDa): CTX no change, OM × 3.9, IMB × 6.2 | Protein | [24] |
| 12 wk | 30 | γ-ENaC (70 kDa): CTX and OM and IM N.S. | Protein | [39] |
| ENaC: CTX and OM and IM no change | model | |||
| 4–5 days | N.A. | Na-K-ATPase: CCD × 2, MCD × 1.6, mTAL × 1.4, PCT × 1.2, PST, cTAL, and DCT no changes | Activity | [21] |
| 8 days | 28.7 | Na-K-ATPase: mTAL × 1.5, CCD × 2.1 | mRna | [42] |
| 14 days | 18.6 | α-Na-K-ATPase: CTX and OM and IM, no increase | Protein | [43] |
| 15 days | 27.1 | α-Na-K-ATPase: WK no change | Protein | [28] |
| 2 wk | 28.7 | α1-Na-K-ATPase × 0.6 | mRNA | [31] |
| 3 wk | 21.5 | α1-Na-K-ATPase: CTX no change, OM × 3.2, IMB × 5.6 | Protein | [24] |
| 12 wk | 30 | α1-Na-K-ATPase: CTX × 1.4, OM no increase, IM N.S. | Protein | [39] |
| Na-K-ATPase: CTX × 1.1, OM × 1.7, IM × 2.5 | model |
In all these rat studies, diabetes was induced with streptozotocin; WK, whole kidney; CTX, cortex; OM, outer medulla; IM, inner medulla; IMB: inner medullary base; mTAL and cTAL, medullary and cortical thick ascending limbs; ENaC, epithelial sodium channel; PT, proximal tubule
As described in the text, NHE3 expression in the PT is modulated by flow-induced changes in the microvillous torque. Note that data from Ref. [39] are from ovarectomized female rats.
As summarized in Table 2, diabetes has been found to induce changes in the expression of other sodium transporters along the rat nephron (i.e., the sodium-hydrogen exchanger NHE3, the epithelial sodium channel ENaC, and the Na-K-ATPase pump), as well as that of aquaporin water channels. The quantitative changes differ significantly between studies, and very early changes (i.e., a few days after the administration of STZ) appear stronger, possibly as a response to osmotic diuresis and some volume depletion. We chose in the present study to focus on the “settled and stabilized” condition and considered data only from 14 days or more after diabetes induction/STZ administration. The fractional variation in the expression of a given transporter was determined as the average of all corresponding experimental values and is indicated in Table 2.
Other Model Assumptions
The length of the cortical (S1–S2) and OM (S3) segments of the proximal tubule is taken as 0.97 and 0.13 cm, respectively. The short descending limb extends from the junction between the outer and inner stripes of the OM (0.06 cm below the cortico-medullary junction) to the boundary between the OM and IM (0.2 cm below the cortico-medullary junction). The mTAL and cTAL are each taken to be 0.2 cm long; the DCT is 0.1 cm long; the CNT, the CCD, and the OMCD are each 0.2 cm long; and the IMCD is 0.5 cm long.
The composition of the interstitial fluid in the cortex, at the boundary between the OM and IM, and at the papillary tip is specified in Table 3. We assume that interstitial concentrations vary linearly between the cortico-medullary junction and the OM-IM boundary and between the OM-IM boundary and the papillary tip. The interstitial fluid in the cortex is taken to be homogeneous, with one exception: following the approach of Weinstein (54, 55), we assume that there is a NH3/NH4+ concentration gradient in the cortex. The total interstitial concentration of ammonia (NH3 + NH4+) is taken to increase from 0.2 mM in the upper cortex to 1.0 mM at the cortico-medullary boundary. Thus total interstitial ammonia concentration is assumed to increase from 0.2 to 1.0 mM along (the flow direction of) the PCT, to decrease from 1.0 to 0.2 mM along the cTAL, to remain constant at 0.2 mM along the DCT and CNT, and to increase from 0.2 to 1.0 mM along the CCD; interstitial Cl− concentrations are adjusted accordingly, so as to preserve electroneutrality. Tubular fluid concentrations at the proximal tubule inlet are equal to those in the cortical interstitium, except for the absence of protein.
Table 3.
Interstitial concentrations
| Solute | Cortex | OM-IM Boundary | Papillary Tip |
|---|---|---|---|
| Na+ | 144.0 | 284.0 | 299.0 |
| K+ | 4.9 | 10.0 | 20.0 |
| Cl− | 117.0 | 264.9 | 294.9 |
| HCO3− | 25.0 | 25.0 | 25.0 |
| H2CO3− | 4.41 × 10−3 | 4.41 × 10−3 | 4.41 × 10−3 |
| CO2 | 1.50 | 1.50 | 1.50 |
| H2PO4− + H2PO4− | 3.9 | 3.9 | 3.9 |
| Urea | 5.0 | 20.0 | 500 |
| NH3 + NH4+* | 1.0 | 3.9 | 8.95 |
| HCO2− + H2CO2 | 1.0 | 1.0 | 1.0 |
| Glucose | 5.0 | 8.5 | 7.5 |
| Protein | 2.0 | 2.0 | 2.0 |
| pH | 7.323 | 7.323 | 7.323 |
Concentrations are in mM. OM-IM boundary, at the junction between the outer and inner medulla. In diabetic rats, interstitial glucose concentrations are multiplied by a factor of 5.
See text for details.
We assume that there is negligible transepithelial transport of formate (HCO2−), formic acid (H2CO2), and glucose downstream from the proximal tubule. In the absence of data, the permeability of the apical and basolateral membranes to these three solutes is respectively set to 0 and 1 × 10−1 cm/s, and the permeability of the tight junction and the basement membrane is, respectively, set to 1 × 10−4 cm/s (or 1 × 10−7 cm3/s per cm2 epithelial area) and 5 × 10−4 cm/s (or 1 × 10−5 cm3/s per cm2 epithelial area).
The values of other parameters, such as transporter expression and water and solute permeability, can be found in Ref. 26 for the proximal tubule, Ref. 12 for the TAL, and Ref. 11 for the distal segments. Owing to differences in segment length, the density of NKCC2 and Na-K-ATPase in the mTAL and cTAL is taken to be 50% higher in the current model than in our previous TAL model (12), so as to match measured values of luminal solute concentrations in the early distal tubule. We assume there are 36,000 nephrons per rat kidney when scaling the model from one nephron to one kidney (4, 38).
MODEL RESULTS
Base-Case Results in Nondiabetic Kidney
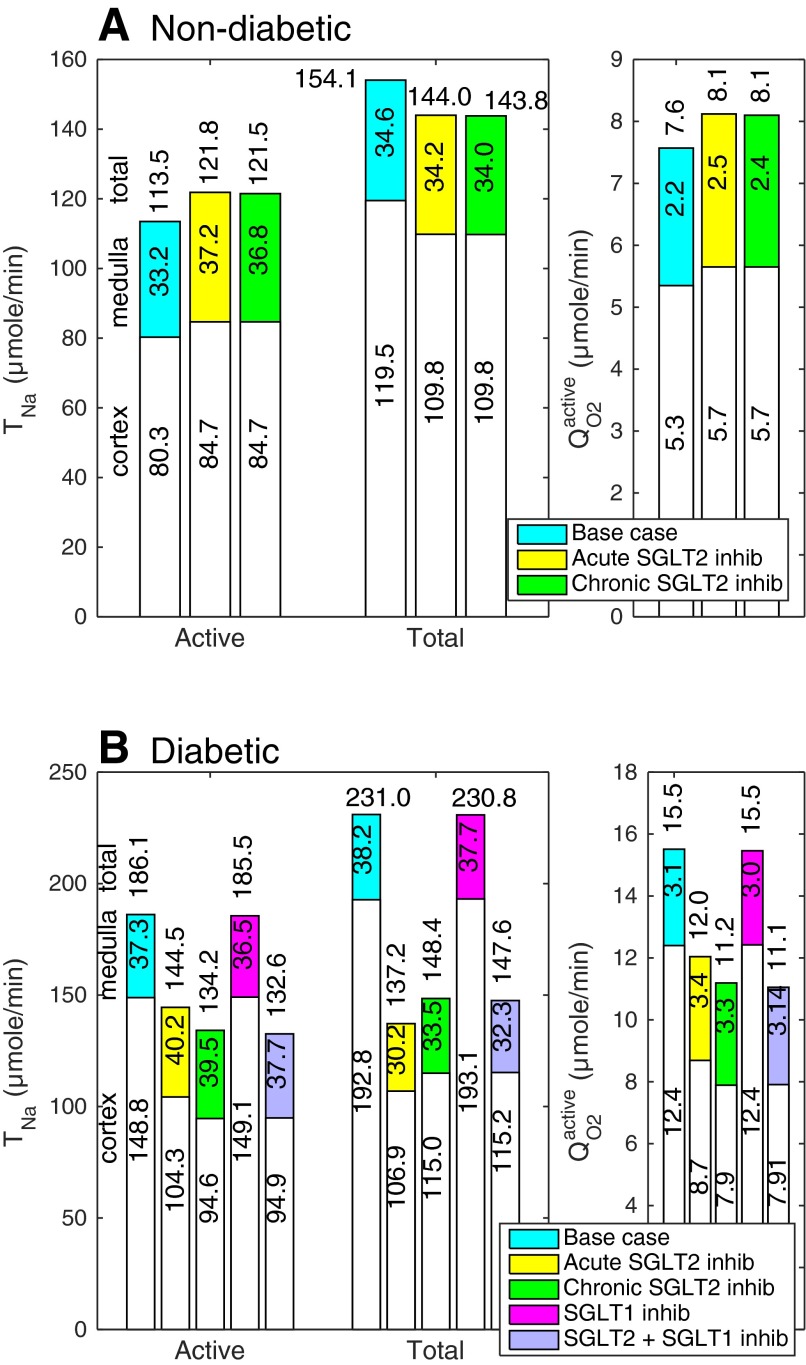
The present model extends our previous model of water and solute transport along the proximal tubule (26) to the entire nephron. Base-case nephron model predictions related to sodium transport and oxygen consumption are summarized in Fig. 2 (cortex, medulla, and whole kidney values) and Fig. 3 (tubular segment values, A and B). In the nondiabetic rat kidney, active sodium reabsorption () is computed as 113.5 μmol·min−1·kidney−1, and active O2 consumption equals 113.5/15 = 7.6 μmol·min−1·kidney−1; assuming a 3:1 ratio of active-to-basal O2 consumption (see above), total O2 consumption (QO2) is determined as 10.1 μmol·min−1·kidney−1. Total sodium reabsorption (TNa) is predicted as 154.1 μmol·min−1·kidney−1, and the base-case TNa/QO2 ratio equals 15.3 (see Table 5). The filtered load of glucose is (5 mM)·(30 nl/min) per nephron, or 5.4 μmol·min−1·kidney−1, all of which is reabsorbed along the proximal tubule.
Fig. 2.
Nondiabetic (A) and diabetic (B) rat nephron function under differing conditions. TNa, Na+ reabsorption; , O2 consumption for active Na+ reabsorption. White and colored segments, cortical and medullary values, respectively. Whole kidney values are indicated above the bars.
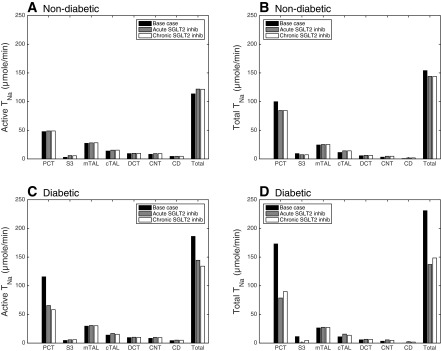
Fig. 3.
Active (A and C) and total (B and D) Na+ reabsorption (in μmol/min) along nephron segments in nondiabetic rats (A and B) and diabetic rats (C and D) under the following conditions: base case (black bars), acute SGLT2 inhibition (grey bars), and chronic SGLT2 inhibition (white bars). PST, proximal straight tubule.
Table 5.
Effects of SGLT inhibition on normal and diabetic rat nephron function
| TNa | QO2 | TNa/QO2 | |
|---|---|---|---|
| Nondiabetic rat | |||
| Base case | 154.1 | 10.1 | 15.3 |
| 100% SGLT2 inhibition–acute | 144.0 | 10.7 | 13.5 |
| 100% SGLT2 inhibition–chronic | 143.8 | 10.6 | 13.5 |
| Diabetic rat | |||
| Base case | 231.0 | 18.7 | 12.2 |
| 100% SGLT2 inhibition–acute | 137.2 | 15.2 | 8.9 |
| 100% SGLT2 inhibition–chronic | 148.4 | 14.3 | 10.2 |
| 100% SGLT1 inhibition | 230.8 | 18.6 | 12.2 |
| 100% chronic SGLT2 and SGLT1 inhibition | 147.5 | 14.2 | 10.2 |
TNa and QO2 denote total Na+ reabsorption and O2 consumption (in μmol·min−1·kidney−1). In nondiabetic rats, acute SGLT2 inhibition elicits a small decrease in the single nephron glomerular filtration rate (SNGFR); chronic SGLT2 inhibition elicits a similar decrease in SNGFR as well as variations in SGLT expression. In diabetic rats, acute SGLT2 inhibition significantly reduces SNGFR; chronic SGLT2 inhibition reduces both SNGFR and plasma glucose concentration. See text for details.
Acute SGLT2 Inhibition in Nondiabetic Kidney
In vivo, SGLT2 blockade has been found to reduce GFR in the nondiabetic setting: by 18% in wild-type mice treated with empagliozin for 15 wk (48), and by 3% in nondiabetic humans receiving canagliozin or dapagliozin for 4 days (personal communication by Paul Rothenberg). In the present study, we assumed that both acute and chronic SGLT2 inhibition reduces GFR in the nondiabetic setting by 3%; in the chronic case, we also accounted for changes in glucose transporter expression, as described below.
When SGLT2 is fully blocked (and GFR is 3% lower than in the base case), urinary glucose excretion is computed as 3.0 μmol·min−1·kidney−1, or 0.54 mg·min−1·kidney−1, yielding a fractional excretion of 56%. The model predicts that a small fraction (4.4%) of the filtered glucose load is reabsorbed in the S1–S2 segments via the paracellular route, and 38.9% in the S3 segment −38.5% (or 2.0 μmol·min−1·kidney−1) across SGLT1, and 0.4% (or 0.02 μmol·min−1·kidney−1) across tight junctions.
Our results also suggest that acutely inhibiting SGLT2 in euglycemic conditions increases by 7% but lowers total TNa by a similar fraction, relative to the base case (see Table 5 and Fig. 2). As we previously described (26), SGLT2 inhibition elicits osmotic diuresis in the proximal tubule, thereby reducing passive transport via the paracellular route in that segment. The high luminal flow conversely stimulates active transport (via torque-induced increases in transcellular transporter expression; Refs. 10, 56), but the reduction in passive transport is greater, so that net Na+ reabsorption decreases in the proximal tubule, by 16%. The results of the present study suggest that the TNa reduction in the proximal tubule is then partly compensated for downstream, particularly beyond the mTAL (Fig. 3, A and B). Since active Na+ reabsorption increases whereas passive Na+ reabsorption decreases, the TNa/QO2 ratio is lower during SGLT2 inhibition than in the absence of treatment (see Table 5).
To examine the impact of SGLT2 inhibition on regional oxygenation, we computed separately and in the cortex (by summing the contributions of the PCT, cTAL, DCT, CNT, and CCD) and in the medulla (by summing the contributions of the PST, mTAL, OMCD, and IMCD). Acute SGLT2 inhibition is predicted to raise and by 5% in the cortex and by 12% in the medulla (Fig. 2). More specifically, the computed values of increase by 109% in the PST (Fig. 4), 3% in the mTAL, 2% in the OMCD, and 5% in the IMCD.
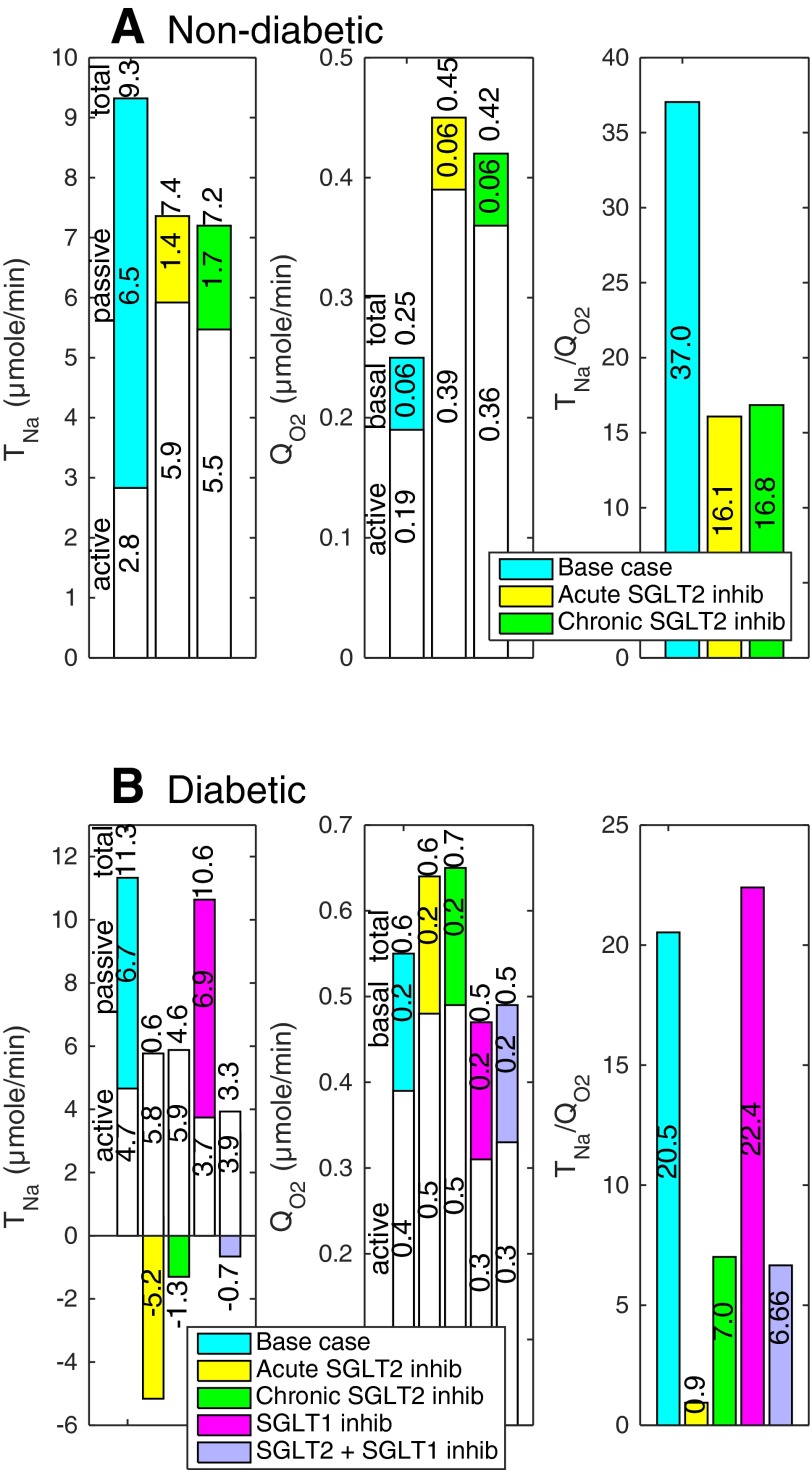
Fig. 4.
Nondiabetic (A) and diabetic (B) S3 segment function under differing conditions. TNa, Na+ reabsorption, given per kidney; white and colored segments, active and passive Na+ reabsorption. QO2, O2 consumption, given per kidney; white and colored segments, and . Total TNa and QO2 are indicated above the bars.
Chronic SGLT2 Inhibition in Nondiabetic Kidney
In wild-type mice, chronic inhibition of SGLT2 by empagliozin was found to upregulate SGLT2 protein expression by 47%, while reducing that of SGLT1 by 35% (48). To simulate the chronic blockade of SGLT2, we accounted for these changes in transporter density (which were seen in nondiabetic animals), in addition to lowering GFR by 3% and abolishing transport across SGLT2.
Under this scenario, fractional glucose excretion is predicted to be 66%, which is close to measured values in mice treated with a selective SGLT2 inhibitor (41). As with acute SGLT2 inhibition, ∼4% of the filtered load is reabsorbed via the paracellular route in the S1–S2 segments. However, the S3 segment reabsorbs less glucose (30.1%) in comparison, due to the downregulation of SGLT1.
Per se, the downregulation of SGLT1 has a negligible impact on sodium transport. Thus, as in the acute scenario, chronic SGLT2 inhibition is predicted to elicit a 7% decrease in total TNa and a 7% increase in and , relative to the base case (see Table 5). Likewise, active oxygen consumption rises more significantly in the medulla (by 11%) than in the cortex (5%). In medullary segments specifically, the computed rises by 93% in the PST (Fig. 4), 3% in the mTAL, 2% in the OMCD, and 4% in the IMCD.
Diabetic Kidney
We mimicked the renal effects of diabetes on the kidney as described in mathematical model. Corresponding results are illustrated in Fig. 2 (cortex, medulla, and whole kidney values) and Fig. 3 (tubular segment values, C and D). Relative to the normal rat kidney, the model predicts a 50% increase in total TNa. That increase, which is comparable to in vivo data (34), is attributable, in large part, to the GFR increase. As can be seen in Table 4 and by comparing the diabetic and nondiabetic results in Fig. 3, active Na+ reabsorption rises primarily in the PCT (by 143%), PST (by 65%), and mTAL (by 8%), i.e., segments where diabetes induces significant increases in the expression of Na+ transporters. In the proximal tubule, hyperfiltration-induced changes in the torque augment the density of all transcellular transporters; diabetes also enhances the density of NKCC2 in the mTAL (see mathematical model). Whole kidney active Na+ reabsorption is predicted to increase by 64%, yielding a 2.1-fold elevation in active O2 consumption: note that / is taken as 12 in diabetic rats, vs. 15 in control animals. Assuming that basal O2 consumption increases as described in mathematical model, it is computed as 2.50 μmol·min−1·kidney−1 in the cortex and 0.95 μmol·min−1·kidney−1 in the medulla. Total QO2 is then 19.0 μmol·min−1·kidney−1, that is, 88% higher than in control animals, and the whole kidney TNa/QO2 ratio is calculated as 12.2 (Table 5).
Table 4.
Normal and diabetic rat nephron function
| TNa | QO2 | TNa/QO2 | |||
|---|---|---|---|---|---|
| PCT | |||||
| Nondiabetic | 47.7 | 99.9 | 3.2 | 4.2 | 23.6 |
| Diabetic | 115.7 | 173.2 | 9.6 | 11.1 | 15.6 |
| PST | |||||
| Nondiabetic | 2.8 | 9.3 | 0.19 | 0.25 | 37.0 |
| Diabetic | 4.7 | 11.3 | 0.39 | 0.55 | 20.5 |
| mTAL | |||||
| Nondiabetic | 27.3 | 24.3 | 1.8 | 2.4 | 10.0 |
| Diabetic | 29.6 | 26.5 | 2.5 | 3.1 | 8.6 |
| cTAL | |||||
| Nondiabetic | 13.8 | 11.2 | 0.92 | 1.2 | 9.2 |
| Diabetic | 14.0 | 11.0 | 1.2 | 1.6 | 6.9 |
| DCT | |||||
| Nondiabetic | 9.3 | 5.6 | 0.62 | 0.83 | 6.7 |
| Diabetic | 9.6 | 5.7 | 0.80 | 1.1 | 5.3 |
| CNT | |||||
| Nondiabetic | 8.1 | 3.2 | 0.54 | 0.72 | 4.4 |
| Diabetic | 8.4 | 3.3 | 0.70 | 0.95 | 3.5 |
| CCD | |||||
| Nondiabetic | 1.3 | −0.31 | 0.09 | 0.12 | N.A. |
| Diabetic | 1.3 | −0.36 | 0.11 | 0.15 | N.A. |
| OMCD | |||||
| Nondiabetic | 1.6 | −0.27 | 0.11 | 0.14 | N.A. |
| Diabetic | 1.6 | −0.42 | 0.14 | 0.23 | N.A. |
| IMCD | |||||
| Nondiabetic | 1.5 | 1.2 | 0.10 | 0.13 | 8.8 |
| Diabetic | 1.4 | 0.83 | 0.12 | 0.20 | 4.1 |
| Whole kidney | |||||
| Nondiabetic | 113.5 | 154.1 | 7.6 | 10.1 | 15.3 |
| Diabetic | 186.1 | 231.0 | 15.5 | 19.0 | 12.2 |
and TNa denote active and total Na+ reabsorption (in μmol·min−1·kidney−1); and QO2, active and total O2 consumption (in μmol·min−1·kidney−1);
PCT and PST, proximal convoluted and straight tubule; DCT, distal convoluted tubule; CNT, connecting tubule; CCD, cortical collecting duct; OMCD and IMCD, outer and innder medullary collecting ducts.
These results were obtained assuming a plasma glucose concentration ([G]pl) of 25 mM, i.e., a filtered load of 40.5 μmol glucose·min−1·kidney−1. In this case, fractional reabsorption of glucose by the S1–S2 (cortical) and S3 (medullary) segments of the proximal tubule is computed as 80.6 and 1.4%, respectively, and absolute and fractional glucose excretion (FEG) as 7.3 μmol·min−1·kidney−1 and 17.9%, respectively. As in untreated, nondiabetic rats, there is a small amount of glucose backflux across the tight junction (∼0.3 μmol/min), since the lumen-to-interstitium glucose concentration gradient favors glucose secretion across the paracellular pathway.
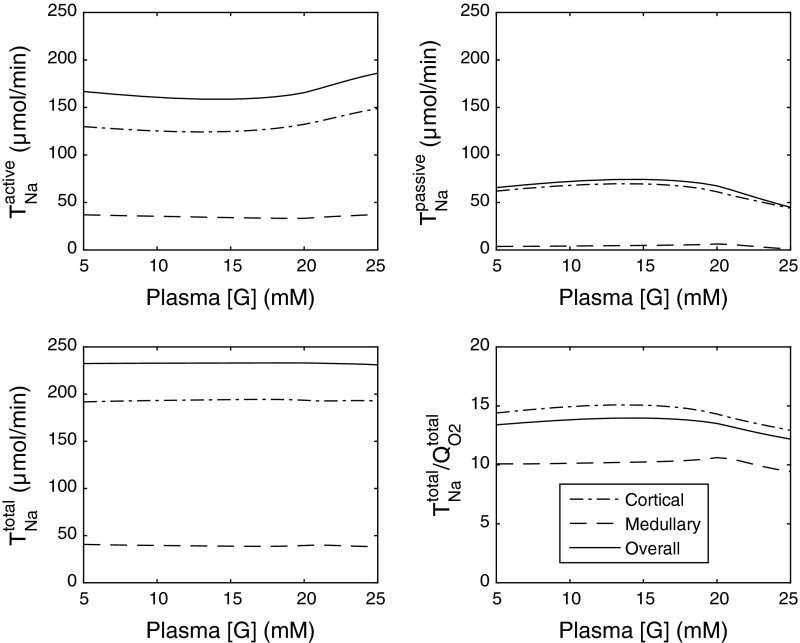
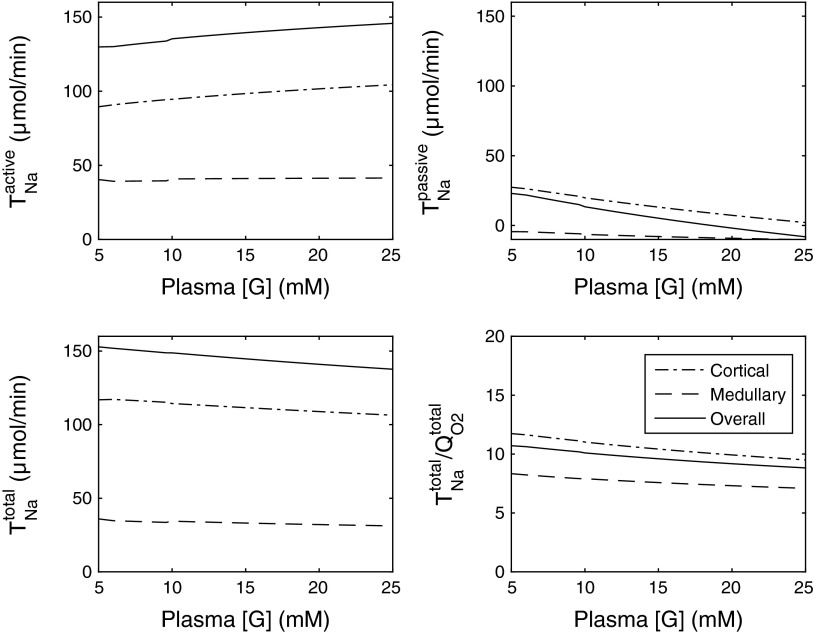
The effects of varying [G]pl on and TNa are illustrated on Fig. 5 (all other parameters being constant). As [G]pl is decreased from 25 to ∼20 mM, the effects of diuretic osmosis diminish, thereby increasing passive (paracellular) Na+ transport and decreasing active Na+ transport in the proximal tubule, since transcellular transporter expression is less stimulated by flow. Downstream from the PST, increases in partial compensation (not shown). The sum of paracellular and transcellular Na+ transport remains approximately constant, that is, net sodium reabsorption (TNa) does not vary much (Fig. 5). However, the change in the relative contribution of active vs. passive Na+ transport raises the whole kidney TNa/QO2 ratio. The latter is predicted as 13.5 when [G]pl is set to 20 mM (vs. 12.2 when [G]pl = 25 mM).
Fig. 5.
Active, passive, and total Na+ reabsorption (in μmol/min) as well as the TNa/QO2 ratio, in the cortex, medulla, and overall in diabetic rats, as a function of plasma glucose concentration [G]. The single nephron glomerular filtration rate (SNGFR) is taken as 45 nl/min in these simulations.
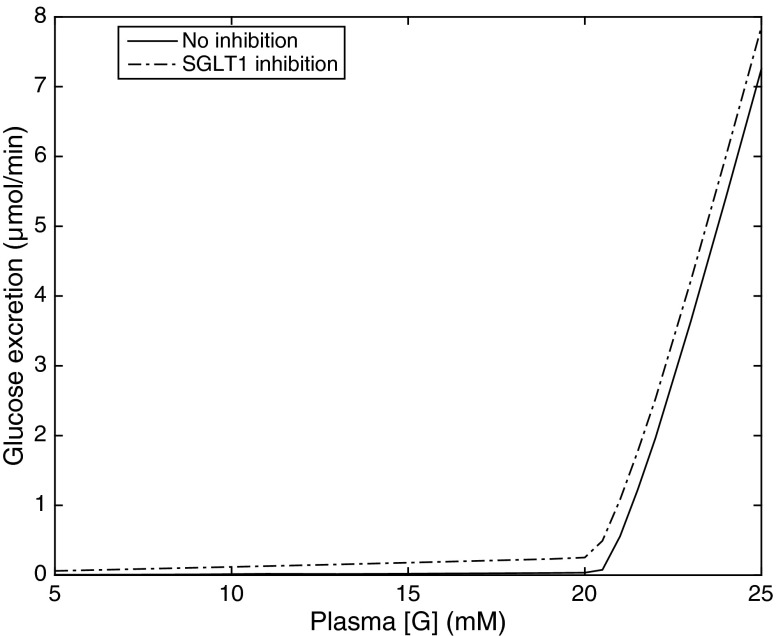
Glucosuria (i.e., FEG ≥1%) is predicted to occur in diabetic rats when the plasma glucose equals or exceeds 21 mM (Fig. 6). At lower [G]pl values, there is no osmotic diuresis since the entire glucose load is reabsorbed in the proximal tubule. As the reabsorption of glucose and Na+ via SGLT decreases with decreasing [G]pl, the transcellular and paracellular transport of sodium in the proximal tubule both diminish. In compensation, increases downstream, yielding a small increase in whole kidney (Fig. 5). Thus the relative contribution of active Na+ transport increases with decreasing [G]pl, and the whole kidney ratio TNa/QO2 conversely slightly decreases (from 14.0 when [G]pl = 15 mM to 13.4 when [G]pl = 5 mM).
Fig. 6.
Glucose excretion (in μmol/min) in diabetic rats, as a function of plasma glucose concentration [G]. SNGFR is set to 45 nl/min. Solid lines: without inhibition; dotted lines: with SGLT1 inhibition.
Acute SGLT2 Inhibition in Diabetic Kidney
Both acute and chronic SGLT2 inhibition reduce or prevent diabetic-induced hyperfiltration in rodents (34, 46, 50, 48) and humans (8). When simulating the effects of blocking SGLT2 in diabetic rats, we therefore lowered SNGFR to 30 nl/min (its value in control rats). In addition, chronic inhibition of SGLT2 has been shown to reduce circulating levels of glucose in diabetic mice, rats, and humans (47, 48). In our simulations, the plasma concentration of glucose was reduced during chronic SGLT2 blockade (see below) but kept constant (at 25 mM) during acute blockade.
With these assumptions, during acute SGLT2 inhibition the filtered load of glucose in diabetic rats is reduced from 40.0 to 27.0 μmol·min−1·kidney−1. Glucose excretion is predicted to be 25.3 μmol·min−1·kidney−1, that is, FEG = 93.7%. The S1–S2 (via the paracellular pathway) and S3 segments of the proximal tubule, respectively, reabsorb 0.7 and 0.9 μmol glucose·min−1·kidney−1. In comparison, in diabetic rats without treatment, S1–S2 and S3 respectively reabsorb 32.6 and 0.6 μmol glucose·min−1·kidney−1. Whereas there is glucose backflux across the tight junction in untreated animals, the paracellular pathway mediates glucose reabsorption when SGLT2 is inhibited (owing to higher luminal glucose concentrations). Altogether these results suggest that SGLT1 barely compensates for the acute blockade of SGLT2 in diabetic rats, because hyperglycemia and the increased tubular glucose load already use up the full transport capacity of SGLT1 in the absence of SGLT2 inhibition.
In diabetic rats, acute inhibition of SGLT2 is predicted to significantly lower , total TNa, QO2, and TNa/QO2, primarily because it normalizes GFR. Our simulations suggest that, as in control animals, blocking SGLT2 causes osmotic diuresis in the proximal tubule, thereby reducing paracellular transport and enhancing the relative contribution of transcellular, energy-consuming Na+ transport, particularly in the medullary, S3 segment (where increases by 24%; Fig. 4). In fact, the model predicts that the direction of paracellular Na+ transport is reversed in S3, that is, tight junctions mediate Na+ secretion; indeed, owing to osmotic diuresis, the luminal-to-interstitial Na+ concentration gradient favors Na+ back-diffusion into the lumen.
Since blocking SGLT2 increases the Na+ load delivered to the mTAL, active Na+ reabsorption, and therefore active O2 consumption, are also augmented downstream from S3 (Fig. 3). In medullary segments, the computed increases by 4% in the mTAL, 14% in the OMCD, and 24% in the IMCD. Overall, our model predicts that acute SGLT2 inhibition lowers by 30% in the cortex but increases it by 8% in the medulla.
Chronic SGLT2 Inhibition in Diabetic Kidney
Chronic SGLT2 inhibition was found to lower blood glucose levels by 60% in diabetic Akita mice, from ∼25 to 10 mM, without altering the renal membrane expression of SGLT1 nor that of SGLT2 (i.e., the diabetes-induced changes were maintained) (48). Thus, in simulating chronic SGLT2 blockade, we reduced the plasma concentration of glucose from 25 to 10 mM, in addition to decreasing SNGFR to 30 nl/min.
Under these conditions, the filtered load of glucose is taken as 10.8 μmol·min−1·kidney−1, 82.9% of which (or 9.0 μmol·min−1·kidney−1) is excreted according to our model. Relative to the acute inhibition case, less glucose is reabsorbed across the tight junction (0.4 vs. 0.7 μmol·min−1·kidney−1 in S1–S2) because the luminal concentration of glucose is lower. In the S3 segment, SGLT1 and the paracellular pathway are predicted to reabsorb ∼12 and 1% of filtered glucose, respectively, during chronic SGLT2 inhibition.
Given the antihyperglycemic effect (or reduction in [G]pl), our model also predicts that chronic SGLT2 inhibition exerts a smaller osmotic diuretic effect than acute inhibition does in diabetic rats; hence, it induces a smaller reduction in paracellular transport, but a larger decrease in transcellular transport, in the PCT. Thus, at the whole kidney level, chronic SGLT2 inhibition reduces TNa less, but lowers more, relative to acute SGLT2 inhibition, as shown in Fig. 3. As a result, the TNa/QO2 ratio is predicted to be higher during chronic SGLT2 inhibition than during acute inhibition (10.2 vs. 8.9).
Our results also suggest that chronic SGLT2 inhibition in diabetic rats lowers active O2 consumption by 36% in the cortex and increases it by 6% in the medulla (Fig. 2). Cortical is predicted to decrease more than during acute inhibition (Fig. 2), since transcellular transport is less stimulated in the PCT (owing to reduced blood glucose levels and thus reduced osmotic diuresis). Whereas active is predicted to increase slightly more in the PST (26%), medullary is predicted to increase less, overall, than during acute inhibition; it is augmented by 2% in the mTAL, 9% in the OMCD, and 21% in the IMCD.
Effects of the initial level of hyperglycemia on the impact of SGLT2 inhibition.
We first computed and total TNa as a function of [G]pl in diabetic animals treated chronically with an SGLT2 inhibitor. The plasma concentration of glucose was varied between 5 and 25 mM in these simulations. As shown in Fig. 7, decreasing [G]pl reduces active Na+ reabsorption while raising passive Na+ reabsorption. Since the latter effect dominates, net TNa is predicted to rise with decreasing [G]pl. We then compared the effects of chronic SGLT2 inhibition in diabetic rat nephrons with different initial values of [G]pl. If the latter is taken as 25 mM and is reduced to 10 mM by chronic SGLT2 blockade, (and therefore ) is predicted to decrease by 28%; if the initial [G]pl is 15 mM and is reduced also by 60% (i.e., to 6 mM) by chronic SGLT2 blockade, is predicted to decrease by 18%. In other words, the higher the initial level of hyperglycemia, the greater the SGLT2 blockade-induced reduction in active TNa and QO2.
Fig. 7.
Active, passive, and total Na+ reabsorption (in μmol/min) as well as the TNa/QO2 ratio, in the cortex, medulla, and overall in diabetic rats treated chronically with a SGLT2 inhibitor, as a function of plasma glucose concentration [G]. SNGFR is taken as 30 nl/min in these simulations.
The effects of chronic SGLT2 inhibition on net glucose excretion in diabetic rats, depending on the initial level of hyperglycemia (at a constant SNGFR of 30 nl/min) are summarized in Table 6. If SGLT2 blockade reduces [G]pl from 25 to 10 mM, glucose excretion is predicted to increase from 7.3 to 9.0 μmol·min−1·kidney−1; if it reduces [G]pl from 15 to 6 mM, glucose excretion rises from 0.02 to 4.4 μmol·min−1·kidney−1. Above the glucosuria threshold, the higher the initial level of hyperglycemia, the smaller the SGLT2 blockade-induced reduction in glucosuria, consistent with experimental data in rodents (50, 48).
Table 6.
Effects of chronic SGLT2 inhibition on glucose excretion in diabetic rats
| Before Inhibition |
After Inhibition |
|||||
|---|---|---|---|---|---|---|
| [G]pl, mM | Filtered load, μmol/min | Excreted load μmol/min | [G]pl, mM | Filtered load, μmol/min | Excreted load, μmol/min | Increase in glucosuria, μmol/min |
| 25.0 | 40.50 | 7.26 | 10.0 | 10.80 | 8.95 | 1.69 |
| 24.0 | 38.88 | 5.42 | 9.6 | 10.37 | 8.51 | 3.09 |
| 23.0 | 37.26 | 3.64 | 9.2 | 9.94 | 8.06 | 4.42 |
| 22.0 | 35.64 | 1.96 | 8.8 | 9.50 | 7.61 | 5.65 |
| 21.0 | 34.02 | 0.56 | 8.4 | 9.07 | 7.16 | 6.60 |
| 20.0 | 32.40 | 0.04 | 8.0 | 8.64 | 6.71 | 6.66 |
| 19.0 | 30.78 | 0.03 | 7.6 | 8.21 | 6.26 | 6.23 |
| 18.0 | 29.16 | 0.03 | 7.2 | 7.78 | 5.80 | 5.77 |
| 17.0 | 27.54 | 0.03 | 6.8 | 7.34 | 5.35 | 5.32 |
| 16.0 | 25.92 | 0.02 | 6.4 | 6.91 | 4.89 | 4.87 |
| 15.0 | 24.30 | 0.02 | 6.0 | 6.48 | 4.43 | 4.41 |
The plasma concentration of glucose, [G]pl, is taken to decrease by 60% during chronic SGLT2 inhibition, and the single nephron GFR is reduced from 45 to 30 nl/min. Filtered load, excreted load, and glucosuria are given per kidney.
SGLT1 Inhibition
Under nondiabetic conditions, the filtered load of glucose is almost entirely reabsorbed by SGLT2 and GLUT2 in the PCT. Thus blocking SGLT1, which is situated downstream in the PST, has no discernible impact on glucose excretion, sodium transport, and oxygen consumption (results not shown).
When simulating the effects of SGLT1 inhibition in diabetic rats, SNGFR was kept constant (at 45 nl/min), since it is not clear whether it is affected by SGLT1 blockade. The model predicts that fully inhibiting SGLT1 in the PST of diabetic rats significantly increases glucose excretion, as illustrated in Fig. 6. Assuming no change in blood glucose levels, and TNa decrease by 20 and 6%, respectively, in S3. Downstream, active Na+ transport changes very little: is augmented by ∼1% in each (cortical and medullary) segment below S3. Given the relatively modest contribution of S3 to overall Na+ transport, SGLT1 blockade has a negligible (<0.1%) impact on whole kidney TNa and QO2 (Fig. 2).
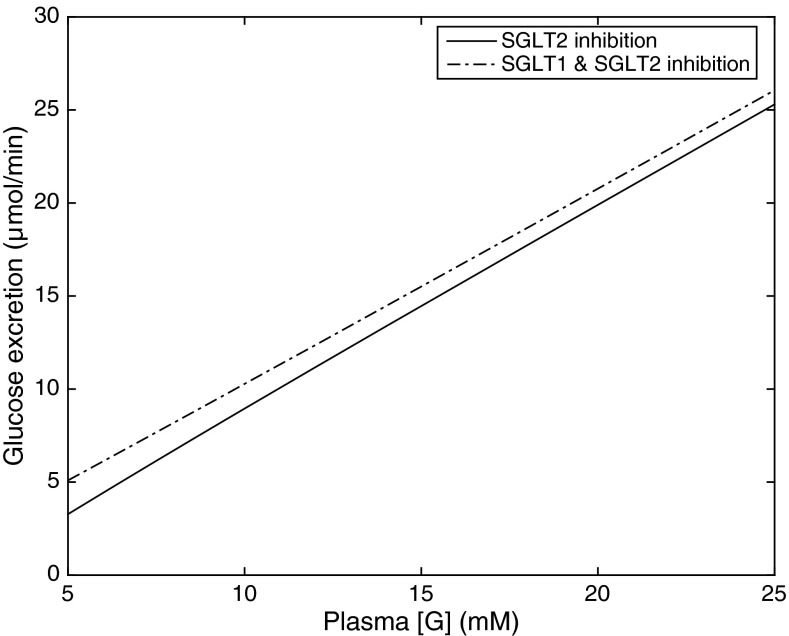
We also examined the combined effect of chronic SGLT2 and SGLT1 inhibition in diabetic rats. In those simulations, SNGFR was lowered to 30 nl/min and [G]pl was reduced by 60%. As shown in Fig. 2, model predictions of overall TNa and QO2 in this case are very similar to those obtained when only SGLT2 is inhibited, even if the blockade of SGLT1 induces significant changes in S3. In that segment, and total TNa are 33 and 29% lower than during SGLT2 inhibition only, and fractional glucose reabsorption drops from 13 to 1%, so that glucose excretion is significantly enhanced (Fig. 8). Downstream from S3, increases by 1% in the cTAL and <1% in all other (cortical and medullary) segments.
Fig. 8.
Glucose excretion (in μmol/min) in diabetic rats treated chronically with a SGLT2 inhibitor, as a function of plasma glucose concentration [G]. SNGFR is set to 30 nl/min. Solid lines: with chronic SGLT2 inhibition; dotted lines: with combined chronic SGLT2 and SGLT1 inhibition.
DISCUSSION
Effects of Diabetes and SGLT2 Inhibition on QO2
The main objective of this study was to assess the theoretical impact of diabetes and SGLT2 inhibitors on oxygen consumption in the renal cortex and medulla. Our previous model of tubular transport applied to the proximal tubule only (26) and could not be used to predict overall changes in cortical and medullary TNa and QO2. Moreover, in contrast with the present work, the former study did not take into account the effects of SGLT inhibitors on GFR, SGLT expression, and blood glucose levels. As summarized in Fig. 2, our current model predicts that diabetes increases overall TNa by 50% and by 64%, mainly because diabetes induces hyperfiltration, thereby enhancing water and solute transport loads. The computed values of active and total oxygen consumption are, respectively, 105 and 88% higher in diabetic rats (Table 4). In both nondiabetic and diabetic rats, acute and chronic SGLT2 blockade is predicted to raise in the medulla (Fig. 2). Furthermore, SGLT2 inhibition is predicted to increase cortical in nondiabetic rats, and to lower it in diabetic rats.
SGLT2 inhibition induces osmotic diuresis in the proximal tubule in nondiabetic conditions, which reduces paracellular (passive) Na+ transport but stimulates transcellular (active) Na+ reabsorption. In regards to the latter, Du and colleagues (10, 56) have shown that augmenting tubular flow rate by raising the filtration rate enhances the microvillous torque, which in turn induces a coordinated increase in luminal and peritubular transporter expression in the proximal tubule. Thus, as we previously described (26), in nondiabetic conditions, and increase in the PCT in response to SGLT2 inhibition. In diabetic conditions, however, SGLT2 inhibition also elicits a large decrease in GFR. The present study suggests that the lower filtration rate more than counterbalances the effects of osmotic diuresis and that the ensuing reduction in tubular flow rate lowers both paracellular and transcellular Na+ reabsorption in the PCT, thereby diminishing therein.
The decrease in Na+ reabsorption in the PCT that results from SGLT2 inhibition is partly compensated downstream, which is why is predicted to increase in the medulla. Our results suggest that in diabetic rats, fully inhibiting SGLT2 raises by 24–26% in the PST, 2–4% in the mTAL, 9–14% in the OMCD, and 21–24% in the IMCD (Fig. 3). As illustrated in Table 2, measured changes in the expression of distal sodium and water transporters in diabetic rat nephrons differ significantly between studies. The fractional changes assumed in this study are averages of reported values (see mathematical model). In a subset of simulations (not shown), we assumed that diabetes elicits no changes in transporter density downstream of the proximal tubule. That assumption did not affect much the model results: when SGLT2 was fully blocked in diabetic rats, was predicted to increase by 24–26% in the PST, 2–4% in the mTAL, 8–11% in the OMCD, and 16–17% in the IMCD.
The S3 and mTAL segments are known to be particularly vulnerable to hypoxic injury, and the significant increase in S3 QO2 predicted by the model (recapitulated in Fig. 4) suggests that the SGLT2 inhibitors used as a novel treatment for diabetes may increase hypoxia in the S3 segment. Interestingly, if we assumed no downregulation of renal SGLT1 expression in diabetic rats, active O2 consumption in S3 would be 8% higher in untreated animals and 34% higher in animals treated chronically with a SGLT2 inhibitor (vs. 26% accounting for diabetes-induced SGLT1 downregulation). In contrast, the model predicts only minor increases in mTAL and in response to SGLT2 inhibition, because the luminal concentration of Na+ along the mTAL is sufficiently high under baseline conditions so that active TNa is essentially carrier limited. In other words, SGLT2 inhibition would have a stronger effect on mTAL if it upregulated NKCC2 expression.
Comparison with In Vivo Data
Our model predictions generally agree well with the in vivo data of O'Neill et al. (34). In nondiabetic rats, the filtered loads of sodium and glucose in their study (136.8 and 5.8 μmol/min, respectively) are comparable to model assumptions (155.5 and 5.4 μmol·min−1·kidney−1, respectively), and our estimates of TNa and QO2 are very similar to their measurements. Moreover, both our model and their study indicate that TNa is ∼50% higher in diabetic rats than in control rats.
Based on experimental data (36, 60), our model assumes that basal O2 consumption is higher in diabetic rats than in control rats in the proximal tubule and the CD. Total QO2 is predicted as 10.1 μmol/min in control rats and 19.0 μmol/min in diabetic rats; whereas the former number is similar to the control value obtained by O'Neill et al. (34), the latter number is about one-third lower than their experimental value in diabetic rats, even though the predicted and measured TNa are similar. This discrepancy could signify that diabetes elicits a larger increase in basal QO2 than what we have assumed. On the other hand, our prediction that total QO2 is twice as high in diabetic rats as in control rats agrees well with the experimental results of Körner et al. (25) and recent data from Palm and colleagues (17, 30).
Model simulations suggest that acute SGLT2/SGLT1 inhibition lowers TNa/QO2 in nondiabetic conditions and reduces TNa, QO2, and TNa/QO2 in diabetic conditions; these results match the observations of O'Neill et al. (34). Our predictions regarding the differential effects of acute SGLT2/SGLT1 inhibition on cortical and medullary oxygenation are also consistent with their experimental results (34). In their study, acute SGLT2/SGLT1 inhibition with phlorizin elicited a large increase in cortical Po2 in diabetic rats and a significant decrease in medullary Po2 in control and diabetic rats. O'Neill et al. (34) surmised that the reduction in medullary Po2 stemmed from the redistribution of active Na+ transport from the proximal tubule to less efficient nephron segments such as the mTAL. Our model fully supports the redistribution hypothesis. In response to dual SGLT2/SGLT1 inhibition, is predicted to increase in all segments downstream of the S3 segment. Our results also suggest that following the blockade of SGLT2 only, in nondiabetic conditions active O2 consumption is shifted from the PCT mostly to S3 and much less to distal segments; in diabetic conditions, however, SGLT2 blockade significantly enhances in all downstream segments.
We also compared model results with the micropuncture data of Vallon et al. (49). In agreement with the major results of the micropuncture study, our model predicts that 1) early distal tubular concentrations of Na+, K+, and Cl− are lower in diabetic rats than in nondiabetic rats; 2) fractional reabsorption of these ions up the early distal tubule (FRED) is higher in diabetic rats than in nondiabetic rats; and 3) dual inhibition of SGLT2 and SGLT1 elicits a greater reduction in FRED in diabetic rats than in nondiabetic rats.
Effects of SGLT2 Inhibition on Chloride Reabsorption
Thomson et al. (46) observed that acute and chronic SGLT2 inhibition raises the luminal concentration of chloride in the early distal tubule by 70 and 35%, respectively, in the early diabetic rat. To mimic their experimental results, in corresponding simulations we set [G]pl to 24, 18, and 24 mM, respectively, in the absence of treatment, during acute SGLT2 inhibition, and during chronic SGLT2 inhibition; moreover SNGFR was taken as 36.0, 29.3 and 26.7 nl/min, respectively, in these three conditions. Our model predicts that luminal (Cl−) at the DCT inlet increases by 106 and 108%, respectively, during acute and chronic SGLT2 inhibition in diabetic rats. Part of the discrepancy may stem from the fact that the micropuncture measurements were performed in early diabetic rats, whereas our model aims to simulate more stable and settled diabetic conditions. As shown in Table 2, diabetes-induced changes in water and sodium transporter expression are significantly greater in the early stage.
The luminal [Cl−] augmentation can be explained as follows. SGLT2 inhibition impedes Na+ absorption, and thus water and Cl− reabsorption, in the proximal tubule, thereby increasing the delivery of Cl− to the loop of Henle. Since Cl− reabsorption along the TAL is close to saturation (i.e., it cannot increase much) and is not accompanied by water reabsorption, the delivery of Cl− to the DCT, and its luminal concentration in the early distal tubule, are then greater than in the absence of SGLT2 inhibitors.
Model Limitations
Our model does not account for nonoxidative metabolism in renal epithelial cells, due to the paucity of data regarding the contribution of anaerobic pathways in nephron segments. There is evidence that anaerobic metabolism supplies some fraction of the energy needed to actively reabsorb Na+ in the proximal tubule, the TAL, and the CD (1, 9, 44), but quantitative data on glycolysis-induced ATP generation are lacking. Our present estimates of QO2 and TNa/QO2 in nondiabetic rats are similar to in vivo measurements (34), which suggests that neglecting nonoxidative metabolism is not a strong limitation of the model.
In simulating a diabetic rat nephron, we considered diabetes-induced changes in transporter expression (Table 2), but we did not assume that oxygen, and thus ATP, were rate limiting. While some studies indicate that cortical, but not medullary, Po2 is lower in diabetic rats than in control rats (17, 34), others have found that Po2 is reduced both in the cortex and medulla in diabetic rats (30). Whether medullary Po2 in diabetic rats (about 15–20 mmHg) is sufficiently low to slow down active transport processes is not clear. We should also acknowledge that whether hypoxia occurs depends on the balance between oxygen delivery and consumption and that our model only predicts the latter. If O2 delivery increases in parallel with O2 consumption, oxygen levels may not vary significantly. However, in the in vivo study of O'Neill et al. (34), phlorizin-induced changes in QO2 were associated with parallel changes in cortical and medullary Po2, which suggests a mismatch between oxygen supply and demand, i.e., no compensating increase in O2 delivery.
Perspectives
Our modeling data point to the importance of the GFR-lowering effect of SGLT2 inhibitors for their net effect on tubular Na+ transport and oxygen consumption along the nephron. Assuming that the remaining nephrons in advanced stages of CKD also hyperfilter, then the resulting increase in tubular glucose load on the single nephron level is expected to preserve the GFR-lowering effect of SGLT2 inhibition under these conditions, even though the effect on overall glucose homeostasis due to the reduction in total filtered glucose is diminished. This is consistent with clinical data showing that chronic SGLT2 inhibition induced a reduction in eGFR in type 2 diabetes mellitus patients with CKD stage 2–3 (3, 58). Blockers of the angiotensin II system can also induce a modest reduction in GFR in CKD, which may lower the tubular transport work and the associated oxygen consumption, thereby contributing to a reduced progression of CKD (19, 59). Lowering glomerular hyperfiltration on the single nephron level by SGLT2 inhibition may induce similar long-term beneficial effects in the diabetic kidney. Further studies are needed to determine the relevance of the proposed SGLT2 inhibition-induced increase in oxygen consumption in the OM in the diabetic kidney (including increases of 26, 9, and 2% in PST, OMCD, and mTAL, respectively). Blockers of the angiotensin II system also have the potential to directly inhibit proximal tubular Na+ reabsorption; however, the specific quantitative effects on transport in cortical vs. medullary segments remain unclear as do the combined effects of these compounds together with SGLT2 inhibition.
GRANTS
This research was supported by the Department of Veterans Affairs (to V. Vallon) and National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-089066 (to A. T. Layton) and R01-DK-56248 (to V. Vallon) and the University of Alabama at Birmingham/University of California, San Diego O'Brien Center for Acute Kidney Injury Research Grants P30-DK-079337 and R01-HL-94728 (to V. Vallon).
DISCLOSURES
V. Vallon has served as a consultant for Boehringer Ingelheim, Janssen Pharmaceutical, and Intarcia Therapeutics. V. Vallon's work was supported by investigator-initiated research grants from Bristol-Myers Squibb, Astra-Zeneca, and Boehringer Ingelheim, Biberach, Germany.
AUTHOR CONTRIBUTIONS
A.T.L., V.V., and A.E. conception and design of research; A.T.L. and A.E. performed experiments; A.T.L., V.V., and A.E. analyzed data; A.T.L., V.V., and A.E. interpreted results of experiments; A.T.L. and A.E. prepared figures; A.T.L. and A.E. drafted manuscript; A.T.L., V.V., and A.E. edited and revised manuscript; A.T.L., V.V., and A.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Carolyn Ecelbarger and Dr. Paul Rosenberg for providing data.
REFERENCES
- 1.Bagnasco S, Good D, Balaban R, Burg M. Lactate production in isolated segments of the rat nephron. Am J Physiol Renal Fluid Electrolyte Physiol 248: F522–F526, 1985. [DOI] [PubMed] [Google Scholar]
- 2.Baines A, Ho P. Glucose stimulates O2 consumption, NOS, and Na/H exchange in diabetic rat proximal tubules. Am J Physiol Renal Physiol 283: F286–F293, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, Broedl UC, EMPA-REG RENAL Trial Investigators. Efficacy and safety of empagliozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2: 369–384, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Bennett KM, Bertram JF, Beeman SC, Gretz N. The emerging role of MRI in quantitative renal glomerular morphology. Am J Physiol Renal Physiol 304: F1252–F1257, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blount MA, Sands JM, Kent KJ, Smith TK, Price R, Klein JD. Candesartan augments compensatory changes in medullary transport proteins in the diabetic rat kidney. Am J Physiol Renal Physiol 294: F1448–F1452, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brezis M, Rosen S. Hypoxia of the renal medulla–its implications for disease. N Engl J Med 332: 647–655, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Capasso G, Evangelista C, Zacchia M, Trepiccione F, Acone D, Cantone A, Pollastro RM, Rizzo M. Acid-base transport in Henle's loop: the effects of reduced renal mass and diabetes. J Nephrol 19, Suppl 9: S11–S17, 2006. [PubMed] [Google Scholar]
- 8.Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC, von Eynatten M. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129: 587–597, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Dickman KG, Mandel LJ. Differential effects of respiratory inhibitors on glycolysis in proximal tubules. Am J Physiol Renal Fluid Electrolyte Physiol 258: F1608–F1615, 1990. [DOI] [PubMed] [Google Scholar]
- 10.Du Z, Yan Q, Duan Y, Weinbaum S, Weinstein AM, Wang T. Axial flow modulates proximal tubule NHE3 and H-ATPase activities by changing microvillous bending moments. Am J Physiol Renal Physiol 290: F289–F296, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Edwards A. Regulation of calcium reabsorption along the rat nephron: a modeling study. Am J Physiol Renal Physiol 308: F553–F566, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Edwards A, Castrop H, Laghmani K, Vallon V, Layton AT. Effects of NKCC2 isoform regulation on NaCl transport in thick ascending limb and macula densa: a modeling study. Am J Physiol Renal Physiol 307: F137–F146, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eskandari S, Wright EM, Loo DD. Kinetics of the reverse mode of the Na+/glucose cotransporter. J Membr Biol 204: 23–32, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fine LG, Bandyopadhay D, Norman JT. Is there a common mechanism for the progression of different types of renal diseases other than proteinuria? Towards the unifying theme of chronic hypoxia. Kidney Int Suppl 75: S22–S26, 2000. [PubMed] [Google Scholar]
- 15.Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int 74: 867–872, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Rrenal Data System. J Am Soc Nephrol 18: 2644–2648, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Franzen S, Palm F. Endothelin type A receptor inhibition normalises intrarenal hypoxia in rats used as a model of type 1 diabetes by improving oxygen delivery. Diabetologia 58: 2435–2442, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Friederich-Persson M, Thorn E, Hansell P, Nangaku M, Levin M, Palm F. Kidney hypoxia, attributable to increased oxygen consumption, induces nephropathy independently of hyperglycemia and oxidative stress. Hypertension 62: 914–919, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holtkamp FA, Zeeuw de D, Thomas MC, Cooper ME, Graeff de PA, Hillege HJL, Parving HH, Brenner BM, Shahinfar S, Heerspink HJ. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int 80: 282–287, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Jauch P, Lauger P. Electrogenic properties of the sodium-alanine cotransporter in pancreatic acinar cells: II. Comparison with transport models. J Memb Biol 94: 117–127, 1986. [DOI] [PubMed] [Google Scholar]
- 21.Khadouri C, Barlet-Bas C, Doucet A. Mechanism of increased tubular Na-K-ATPase during streptozotocin-induced diabetes. Pflügers Arch 409: 296–301, 1987. [DOI] [PubMed] [Google Scholar]
- 22.Kim D, Sands JM, Klein JD. Role of vasopressin in diabetes mellitus-induced changes in medullary transport proteins involved in urine concentration in Brattleboro rats. Am J Physiol Renal Physiol 286: F760–F766, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Klahr S, Hamm LL, Hammerman MR, Mandel LJ. Renal metabolism: integrated responses. The Gastrointestinal System In: Handbook of Physiology, edited by Orloff J, Berliner RW. Bethesda, MD: Am. Physiol. Soc, sect. 8, 1989. [Google Scholar]
- 24.Klein JD, Rash A, Sands JM, Ecelbarger CM, Tiwari S. Candesartan differentially regulates epithelial sodium channel in cortex versus medulla of streptozotocin-induced diabetic rats. J Epithel Biol Pharmacol 2: 23, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Körner A, Eklöf AC, Celsi G, Aperia A. Increased renal metabolism in diabetes: mechanism and functional implications. Diabetes 43: 629–633, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Layton AT, Vallon V, Edwards A. Modeling oxygen consumption in the proximal tubule: effects of NHE and SGLT2 inhibition. Am J Physiol Renal Physiol 308: F1343–F1357, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maki LW, Keizer J. Mathematical analysis of a proposed mechanism for oscillatory insulin secretion in perfused HIT-15 cells. Bull Math Biol 57: 569–591, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Nejsum LN, Kwon TW, Marples D, Flyvbjerg A, Knepper MA, Froklaer J, Nielsen S. Compensatory increase in AQP2, p-AQP2, and AQP3 expression in rats with diabetes mellitus. Am J Physiol Renal Physiol 280: F715–F726, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Neuhofer W, Beck FX. Cell survival in the hostile environment of the renal medulla. Annu Rev Physiol 67: 531–555, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Nordquist L, Friederich-Persson M, Fasching A, Liss P, Shoji K, Nangaku M, Hansell P, Palm F. Activation of hypoxia-inducible factors prevents diabetic nephropathy. J Am Soc Nephrol 26: 328–338, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nordquist L, Shimada K, Ishii T, Furuya DT, Kamikawa A, Kimura K. Proinsulin c-peptide prevents type-1 diabetes-induced decrease of renal Na+-K+-ATPase α1-subunit in rats. Diabetes Metab Res Rev 26: 193–199, 2010. [DOI] [PubMed] [Google Scholar]
- 32.Okusa MD, Linden J, MacDonald T, Huang L. Selective A2A adenosine receptor activation reduces ischemia-reperfusion injury in rat kidney. Am J Physiol Renal Physiol 277: F404–F412, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Oliva RV, Bakris GL. Blood pressure effects of sodium-glucose co-transport 2 (SGLT2) inhibitors. J Am Soc Hypertens 8: 330–339, 2014. [DOI] [PubMed] [Google Scholar]
- 34.O'Neill J, Fasching A, Pihl L, Patinha D, Franzén S, Palm F. Acute SGLT inhibition normalizes oxygen tension in the renal cortex but causes hypoxia in the renal medulla in anaesthetized control and diabetic rats. Am J Physiol Renal Physiol 309: F227–F234, 2015. [DOI] [PubMed] [Google Scholar]
- 35.Palm F, Cederberg J, Hansell P, Liss P, Carlsson PO. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia 46: 1153–1160, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Palm F, Hansell P, Ronquist G, Waldenstrom A, Liss P, Carlsson PO. Polyol-pathway-dependent disturbances in renal medullary metabolism in experimental insulin- deficient diabetes mellitus in rats. Diabetologia 47: 1223–1231, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Parent L, Supplisson S, Loo DD, Wright EM. Electrogenic properties of the cloned Na+/glucose cotransporter: II. A transport model under nonrapid equilibrium conditions. J Membr Biol 125: 63–79, 1992. [DOI] [PubMed] [Google Scholar]
- 38.Pennell JP, Lacy FB, Jamison RL. An in vivo study of the concentrating process in the descending limb of Henle's loop. Kidney Int 5: 337–347, 1974. [DOI] [PubMed] [Google Scholar]
- 39.Riazi S, Maric C, Ecelbarger CA. 17-βEstradiol attenuates streptozotocin-induced diabetes and regulates the expression of renal sodium transporters. Kidney Int 69: 471–480, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Rich PR. The molecular machinery of Keilin's respiratory chain. Biochem Soc Trans 6: 1095–1105, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Rieg T, Masuda T, Gerasimova M, Mayoux E, Platt K, Powell DR, Thomson SC, Koepsell H, Vallon V. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol 306: F188–F193, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scherzer P, Popovtzer MM. Segmental localization of mRNAs encoding Na-K-ATPase α1- and β1-subunits in diabetic rat kidneys using RT-PCR. Am J Physiol Renal Physiol 282: F492–F500, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Song J, Knepper MA, Verbalis JG, Ecelbarger CA. Increased renal ENaC subunit and sodium transporter abundances in streptozotocin-induced type 1 diabetes. Am J Physiol Renal Physiol 285: F1125–F1137, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Stokes JB, Grupp SC, Kinne RK. Purification of rat papillary collecting duct cells: functional and metabolic assessment. Am J Physiol Renal Fluid Electrolyte Physiol 253: F251–F262, 1987. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka S, Tanaka T, Nnagaku M. Hypoxia as a key player in the AKI-to-CKD transition. Am J Physiol Renal Physiol 307: F1187–F1195, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, Singh P. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol 302: R75–R83, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vallon V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu Rev Med 66: 255–270, 2015. [DOI] [PubMed] [Google Scholar]
- 48.Vallon V, Gerasimova MM, Rose MA, Masuda T, Satriano J, Mayoux E, Koepsell H, Thomson SC, Rieg T. SGLT2 inhibitor empagliozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol 306: F194–F204, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus potential role of tubular reabsorption. J Am Soc Nephrol 10: 2569–2576, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Vallon V, Rose M, Gerasimova M, Satriano J, Platt KA, Koepsell H, Cunard R, Sharma K, Thomson SC, Rieg T. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol 304: F156–F167, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vidotti DB, Arnoni CP, Maquigussa E, Boim MA. Effect of long-term type 1 diabetes on renal sodium and water transporters in rats. Am J Nephrol 28: 107–114, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Wade JB, Lee AJ, Ecelbarger CA, Mitchell C, Bradford AD, Terris J, Kim GH, Knepper MA. UT-A2: a 55-kDa urea transporter in thin descending limb whose abundance is regulated by vasopressin. Am J Physiol Renal Physiol 278: F52–F62, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Weinstein AM. A mathematical model of rat collecting duct. I. Flow effects on transport and urinary acidification. Am J Physiol Renal Physiol 283: F1237–F1251, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Weinstein AM. Potassium excretion during antinatriuresis: perspective from a distal nephron model. Am J Physiol Renal Physiol 302: F658–F673, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weinstein AM. A mathematical model of the rat nephron: glucose transport. Am J Physiol Renal Physiol 308: F1098–F1118, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weinstein AM, Weinbaum S, Duan Y, Du ZP, Yan QS, andWang TG. Flow-dependent transport in a mathematical model of rat proximal tubule. Am J Physiol Renal Physiol 292: F1164–F1181, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Welch WJ, Lubber an Baumgartld DH, Wilcox CS. Nephron Po2 and renal oxygen usage in the hypertensive rate kidney. Kidney Int 59: 230–237, 2001. [DOI] [PubMed] [Google Scholar]
- 58.Yal e JF, Bakris G, Cariou B, Yue D, David-Neto E, Xi L, Figueroa K, Wajs E, Usiskin K, Meininger G. Efficacy and safety of canagliozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab 15: 463–473, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamout H, Lazich I, Bakris GL. Blood pressure, hypertension, RAAS blockade, and drug therapy in diabetic kidney disease. Adv Chronic Kidney Dis 21: 281–4286, 2014. [DOI] [PubMed] [Google Scholar]
- 60.Yang J, Pollock JS, Carmines PK. NADPH oxidase and PKC contribute to increased Na transport by the thick ascending limb during type 1 diabetes. Hypertension 59: 431–436, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheusa M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373: 2117–2128, 2015. [DOI] [PubMed] [Google Scholar]