Abstract
Background
Survivors of childhood acute lymphoblastic leukemia (ALL) are at risk for low lean muscle mass and muscle weakness, which may contribute to inactivity and early development of chronic diseases typically seen in older adults. Although increasing protein intake, in combination with resistance training, improves lean muscle mass in other populations, it is not known whether muscular tissue among survivors of ALL, whose impairments are treatment-related, will respond similarly.
Objective
The aim of this study was to evaluate associations among dietary protein intake, resistance training, and lean muscle mass in survivors of ALL and age-, sex-, and race-matched controls.
Design
This was a cross-sectional study.
Methods
Lean muscle mass was determined with dual-energy x-ray absorptiometry, dietary information with 24-hour recalls, and participation in resistance training with a questionnaire. Participants were 365 survivors of ALL (52% male; 87% white; median age=28.5 years, range=23.6–31.7) and 365 controls with no previous cancer.
Results
Compared with controls, survivors of ALL had lower lean muscle mass (55.0 versus 57.2 kg, respectively) and lower percentage of lean muscle mass (68.6% versus 71.4%, respectively) than controls. Similar proportions of survivors (71.1%) and controls (69.7%) met recommended dietary protein intake (0.8 g/kg/d). Survivors (45.4%) were less likely to report resistance training than controls (53.8%). In adjusted models, 1-g higher protein intake per kilogram of body mass per day was associated with a 7.9% increase and resistance training ≥1×wk, with a 2.8% increase in lean muscle mass.
Limitations
The cross-sectional study design limits temporal evaluation of the association between protein intake and lean muscle mass.
Conclusions
The findings suggest that survivors of childhood ALL with low lean muscle mass may benefit from optimizing dietary protein intake in combination with resistance training. Research is needed to determine whether resistance training with protein supplementation improves lean muscle mass in survivors of childhood ALL.
Therapies for acute lymphoblastic leukemia (ALL) have acute and late effects on body composition. Loss of lean muscle mass1 and gains in relative overall body mass2 are reported during treatment and appear to persist long-term.3 Loss of lean muscle mass is important because it is associated with reduced muscle strength,4 bone density,5 physical performance limitations,4 markers of metabolic syndrome,6 and early mortality.7 Muscle weakness is common among survivors of childhood ALL,3 who are twice as likely than siblings to report physical performance limitations,8 1.4 times more likely than members of the general population to have metabolic syndrome,9 and 2.5 times more likely than siblings to experience early mortality.8
Research indicates that exposure to cranial radiation therapy (CRT) and damage to the growth hormone-producing ability of the pituitary gland10 are significant risk factors for low lean muscle mass.11,12 Among exposed survivors of ALL, this risk factor is unalterable without long-term growth hormone replacement therapy,13 a treatment that is not completely without side effects.14 Furthermore, sustainable and clinically significant long-term improvements in body composition and metabolic parameters following replacement with growth hormone have not been fully demonstrated in this population.15,16 Recent evidence indicates that CRT is not the only risk factor associated with suboptimal body composition,17,18 and that adopting a healthy lifestyle can positively influence body habits in this population.17–19
Although increasing protein intake, in combination with resistance training, improves lean muscle mass in other populations,20–23 it is not known whether muscular tissue among survivors of ALL, whose impairments are treatment-related, will respond similarly. The objective of this study was to evaluate the specific contributions of dietary protein intake and resistance training to lean muscle mass among survivors of childhood ALL and an age-, sex-, and race-matched comparison group.
Method
Participants
Participants were enrolled from the St. Jude Lifetime Cohort Study (SJLIFE), a study designed to evaluate health outcomes among survivors of childhood cancer as they age. Cohort details and procedures have been described previously.24,25 Individuals who were eligible for this cross-sectional analysis were diagnosed with ALL, treated at St. Jude Children's Research Hospital (SJCRH) between 1980 and 1999, at least 10 years from original diagnosis, and 18 years of age or older. People with congenital cognitive, musculoskeletal, or cardiopulmonary impairments; who were pregnant or lactating; or who were currently being treated for cancer were not eligible. Potentially eligible participants were stratified by sex, time since diagnosis (<25 years, ≥25 years), and whether they were exposed to cranial radiation during treatment. Survivors were randomized within stratum and simultaneously recruited across strata. To obtain a representative sample of the SJCRH ALL survivor population treated between 1980 and 1999, accrual was carefully monitored to ensure that the final sample matched the distribution of eligible survivors in each of the 4 strata within the radiation category.
To recruit a comparison group (controls), frequency matched to survivors of ALL by race, age, and sex, a random sample of family members of SJCRH patients (not including SJLIFE participants) was selected. Parents of patients were contacted prior to an upcoming clinic appointment to determine whether they, or other adult family members and friends, were interested in participating. A roster of interested family members and friends was created from which study staff selected individuals fulfilling matching criteria, screened them for eligibility, and invited them to participate. Inclusion and exclusion criteria for controls were the same as those for survivors, except that controls did not have a history of childhood cancer. Although adult onset cancer was not one of the exclusion criteria, none of the controls reported a cancer history. Participants provided written informed consent prior to any testing or questionnaire completion.
Measurements
Anthropometrics and lean muscle mass.
Anthropometric measurements were taken, including height (in centimeters) as measured with a wall-mounted stadiometer (SECA, Dundalk, Maryland), weight (in kilograms) as measured with an electronic scale (Scale-Tronix, White Plains, New York), and waist circumference (in centimeters) at the narrowest point between the anterior superior iliac crest and the lowest rib (Gulick tape measure, Patterson Medical, Warrenville, Illinois). Body mass index was calculated by dividing weight (in kilograms) by height (in meters squared), and waist-to-height ratio was calculated by dividing waist circumference by height.
Whole-body dual-energy x-ray absorptiometry (DXA) was performed to estimate body composition (Hologic Model QDR 4500 Fan-Array Scanner and APEX 2.3.1 software, Hologic Inc, Bedford, Massachusetts).26–30 The scanner was calibrated weekly with known phantoms to minimize machine drift. Body regions were isolated using regional computer-generated default lines with manual adjustment, as described by Kim et al.31 Total fat mass and total fat-free mass measurements were recorded. Percentage of body fat and percentage of lean body mass were calculated by dividing fat mass and fat-free mass by total body mass and multiplying the result by 100.32–35 Relative lean muscle mass was calculated by dividing lean muscle mass (in kilograms) by height (in meters) and converting the result to an age-, sex-, and race-specific z score using published data from the National Health and Nutrition Examination Survey (NHANES).36
Dietary intake.
To estimate usual food and nutrient intake, trained interviewers conducted three 24-hour dietary recalls.37,38 The first recall was an in-person interview done at the time of anthropometric assessment. Subsequent interviews were conducted via telephone so that the recalls represented 2 nonconsecutive weekdays and 1 weekend day over a 1-month period. A standardized multiple-pass approach was used to capture types and amounts of foods and beverages consumed during a 24-hour period (midnight–midnight) for the day preceding the interview.39 The Nutrition Data System for Research (NDS-R) dietary data collection software (version 2008–2012, University of Minnesota, Minneapolis, Minnesota) includes a database of more than 18,000 foods, including ethnic foods and more than 7,000 brand-name foods, with values for 163 nutrients, nutrient ratios, and other food components. Final calculations for analysis were completed using the NDS-R 2012. Data are reported as daily averages of kilocalories, sodium, fiber intake, and kilocalories of fat, protein, and carbohydrates. Calories from protein were converted into grams of protein and divided by total body mass in order to compare participants' protein intake with the Dietary Reference Intake recommended value of 0.8 g of protein per kilogram of body weight.40
ALL treatment.
Trained abstractors examined participants' medical records for detailed information on cancer treatment. Variables included to describe the study population were cumulative doses of anthracyclines (in doxorubicin equivalents)41; antimetabolites, vincristine, epipodophyllotoxins, asparaginase, and glucocorticoids (in prednisone equivalents)42; and CRT. Cranial radiation was categorized as none, 1 to 19 Gy, or ≥20 Gy in analysis.
Lifestyle factors.
Demographic and lifestyle information, obtained by having participants complete detailed questionnaires, included age at examination, sex, race (white, black, other), smoking status (never, past, current), annual household income (≤$19,999, $20,000–$79,999, and ≥$80,000), education (less than high school, high school graduate, college), and employment status (employed, student, retired, unemployed, disabled).
Participation in regular resistance training was ascertained by a single question from the Rapid Assessment of Physical Activity;14 individuals who responded “yes” to a question that asked whether they participated in activities to increase muscle strength once a week or more frequently were categorized as participants. Physical activity patterns were calculated from accelerometer-obtained movement data. Participants wore the device (ActiGraph, model GT3X, and ActiLife version 6.1, ActiGraph LLC, Pensacola, Florida) on the right hip during nonbathing, nonswimming waking hours for 7 consecutive days, and 1-minute epochs were used to correspond to data collected in the NHANES study. Manufacturer-provided software were used to process data. Conversions based on the 2011 Compendium of Physical Activities were used to calculate metabolic equivalent minutes per week (MET minutes per week) (sedentary=1, light activity=2.25, moderate activity=4.5, and vigorous activity=9).43
Data Analysis
Descriptive statistics were used to summarize characteristics of the study population and were compared between survivor participants and eligible nonparticipant survivors and controls, using Wilcoxon sign rank tests or chi-square statistics as appropriate. Means and standard deviations were calculated for body composition and dietary variables and compared between survivors and controls with 2-sample t tests.44 Multivariable analyses were used to evaluate the contributions of daily intake of dietary protein (in grams per kilogram of body mass) and participation in regular resistance training (independent variables) to lean muscle mass (in kilograms) and waist-to-height ratio (dependent variables: 2 separate models) among survivors. In addition to protein intake and participation in regular resistance training, models included the following covariates: CRT,45 age, sex, race, and physical activity level. To evaluate the potential differential contribution of daily dietary protein intake between survivors and controls, an additional multivariable model was constructed that included both main effects and an interaction term for survivor status and protein intake. Variables were entered simultaneously into all models. These analyses were a secondary aim of our overall study. The sample size for the larger study was determined a priori and designed to detect a 10% difference between cases and the comparison group on measures of physical fitness and performance.46 Analysis was performed using SAS 9.3 software (SAS Institute Inc, Cary, North Carolina).
Role of the Funding Source
This work was supported by National Cancer Institute grants CA132901 (Dr Ness), CA023944 (Gronemeyer), and CA21765 (Gilbertson) and the American Lebanese Syrian Associated Charities.
Results
Among 416 potentially eligible survivors of ALL, 51 (12.5%) declined participation, and 365 (87.5%) completed a study visit. Among 451 potentially eligible controls, 86 (19.1%) declined participation, and 365 (80.9%) completed a study visit. Characteristics of survivor participants and eligible nonparticipants are shown in Table 1. Participants did not differ from nonparticipants by age, sex, race or ethnicity, age at diagnosis, time since diagnosis, or current age. Types and doses of chemotherapy were similar between survivor participants and nonparticipants, except for prednisone equivalent dose. Study participants were 47.7% male, had a median age of 28.5 years (range=18.4–44.6) at evaluation, and had a median survival time since diagnosis of 21.9 years (range=11.0–30.7). Because they were matched by sex, race, and 5-year age groups, the control group had the same demographic distribution as ALL survivor participants.
Table 1.
Characteristics of Acute Lymphoblastic Leukemia Survivor Participants and Nonparticipantsa
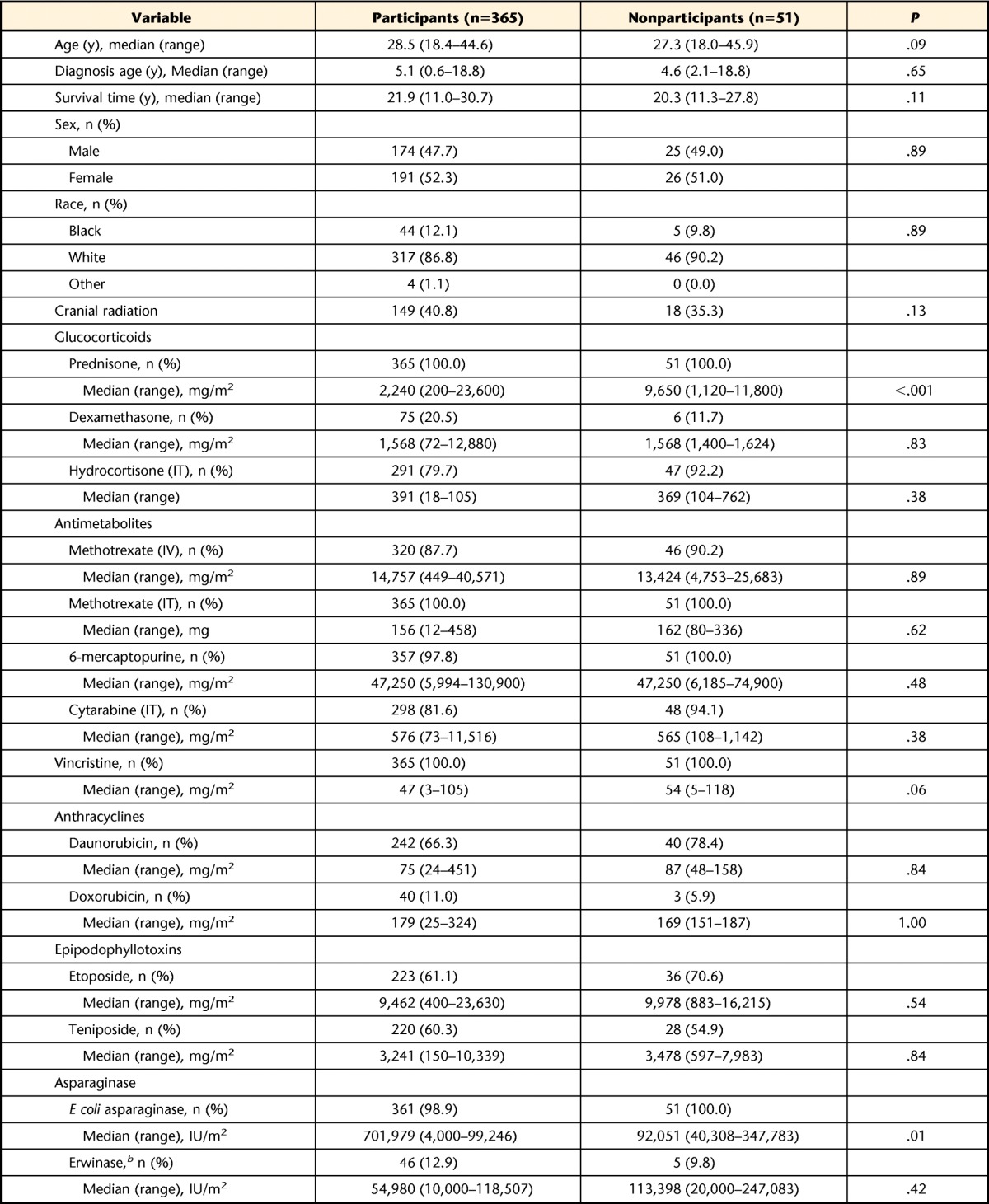
IV=intravenous, IT=intrathecal, IU=international unit.
b Porton Biopharma Ltd, Salisbury, Wiltshire, United Kingdom.
Lifestyle factors are shown in Table 2. There were no differences between survivors of ALL and comparison group members for physical activity levels, smoking status, educational attainment, or employment status. Survivors were less likely than controls to report participation in regular resistance training (45.4% versus 53.8%, χ2 [1 df]=4.97, P<.03), and to have an annual household income of ≥$80,000 per year (21.5% versus 29.7%, χ2 [1 df]=5.56, P=.02).
Table 2.
Lifestyle Variables Among Survivors of Acute Lymphoblastic Leukemia and Comparison Group
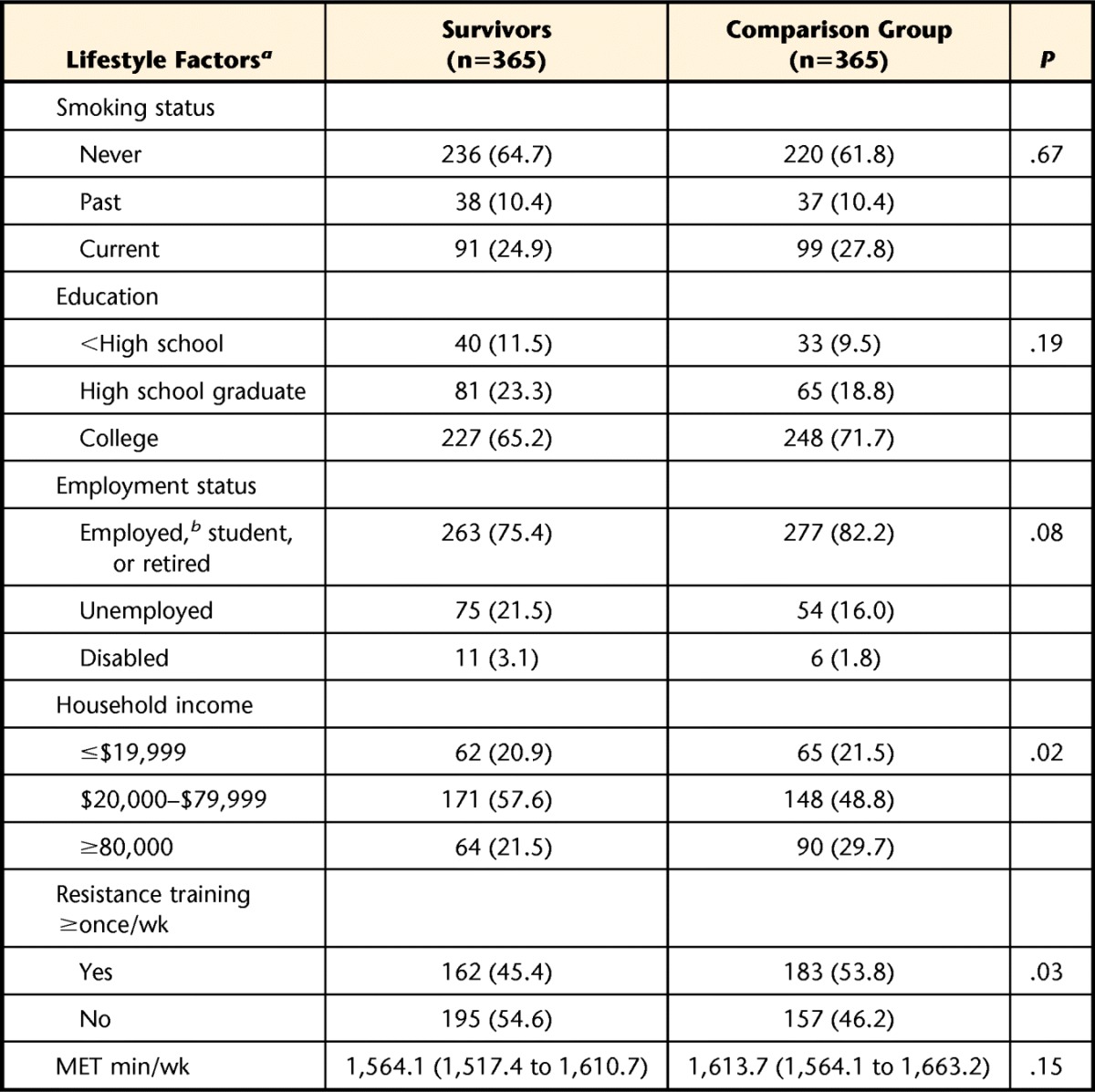
a Lifestyle factors are reported as number (%), except for metabolic equivalent (MET) minutes/week, which is reported as mean (95% confidence interval).
b Employed includes individuals who identified themselves as a homemaker or caregiver.
Body Composition
Measurements of body composition are shown in Table 3. Survivors of ALL and controls had similar mean body weights. However, survivors were shorter than controls and thus had a higher body mass index and a higher waist-to-height ratio. These increases were accounted for by higher fat mass. Absolute lean muscle mass and percentage of lean muscle mass were lower among survivors of ALL than among controls. However, lean muscle mass relative to height did not differ between survivors and controls. Seven percent of survivors of ALL and 5% of the control group had relative lean muscle mass z scores of −1.3 or greater using published population values.36
Table 3.
Body Composition and Dietary Intake Among Survivors of Acute Lymphoblastic Leukemia and Comparison Groupa
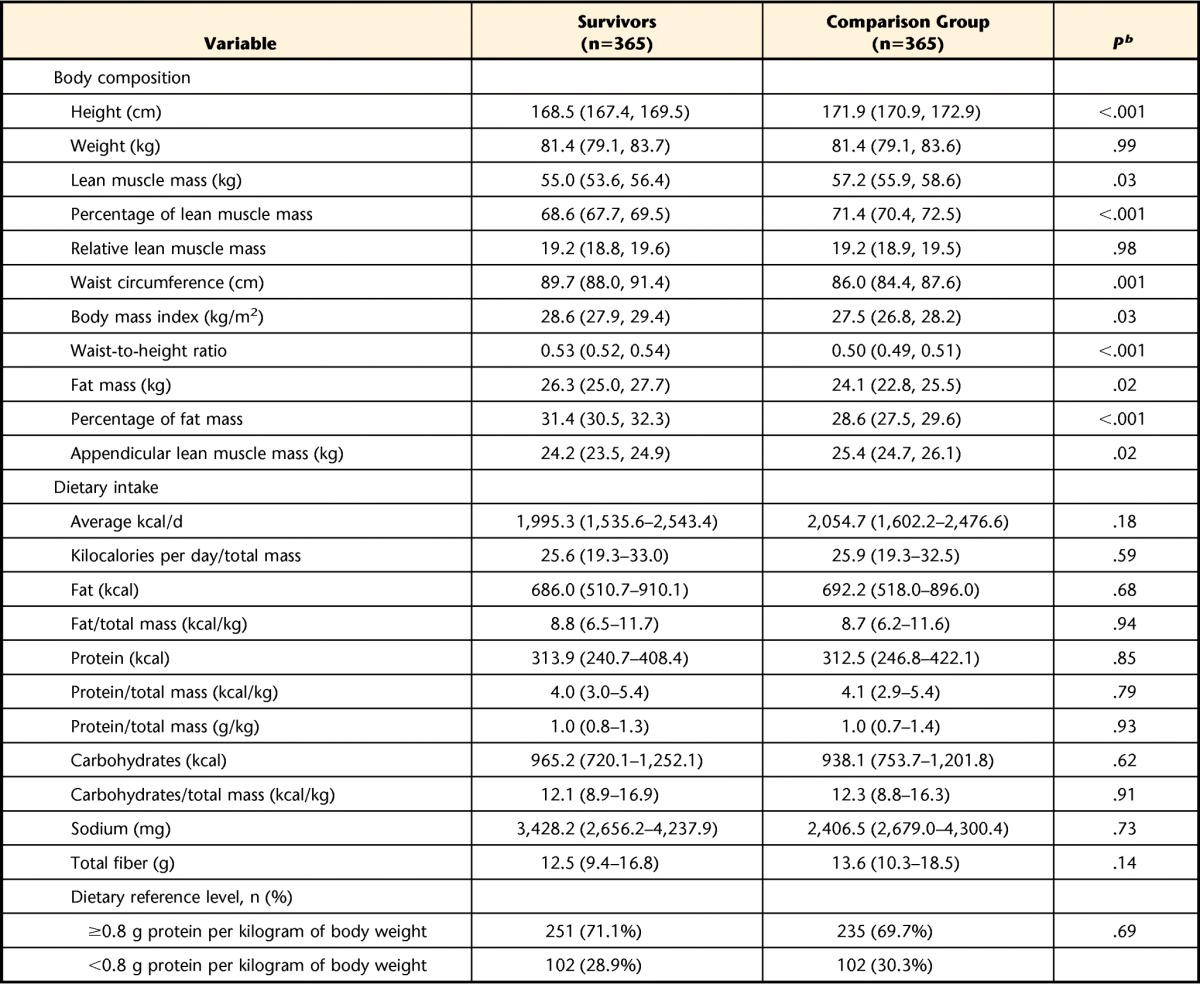
Body composition variables are reported as mean (95% confidence interval). Dietary variables are reported as median (interquartile range [first and third quartiles]) because the data were not normally distributed.
b P values are from median 2-sample t tests.
Dietary Intake
Dietary intake data are shown in Table 3. There were no differences between survivors of ALL and the control group for any dietary parameters. Notably, calories from protein and calories from protein standardized by total body mass did not differ between groups. Furthermore, the proportion of individuals who met the daily Dietary Reference Intake40 recommended amount of protein did not differ between survivors of ALL and controls.
Protein Intake and Lean Muscle Mass in Survivors of ALL
Table 4 shows the results of multivariable models evaluating associations between daily dietary protein intake (in grams) and waist-to-height ratio and percentage of lean body mass among survivors. Both protein intake and strength training were significantly associated with either waist-to-height ratio or percentage of lean muscle mass. Protein intake, age, sex, MET minutes per week, CRT, and resistance training explained 31% of the variability in waist-to-height ratio and 56% of the variability in percentage of lean muscle mass. There was no interaction between CRT status and protein intake, indicating that effects of protein intake on lean muscle mass did not differ among survivors who were and those who were not exposed to CRT.
Table 4.
Association Between Dietary Protein Intake and Body Composition Among Survivors of Acute Lymphoblastic Leukemiaa
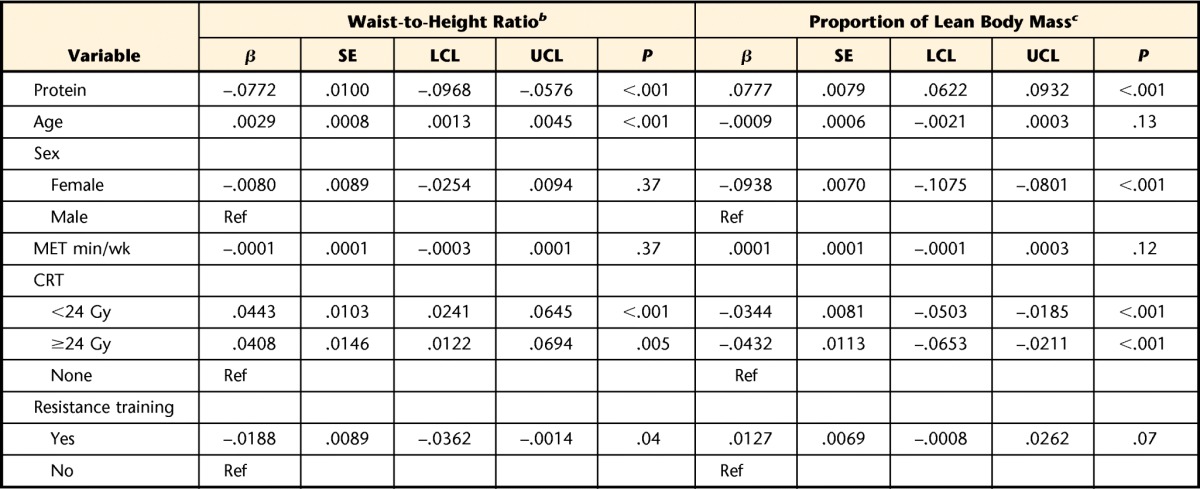
Multiple linear regression models were used to generate beta coefficients, standard errors, confidence intervals, and P values. β=beta coefficient, SE=standard error, LCL=lower 95% confidence limit, UCL=upper 95% confidence limit, Ref=reference group, MET=metabolic equivalent, CRT=cranial radiation therapy.
b R-squared value for the waist-to-height ratio model was .31.
c R-squared value for the percentage of lean body mass model was .56. Age at assessment was removed from the percentage of lean muscle mass model because it was found to be nonsignificant.
Protein Intake and Lean Muscle Mass Among Survivors of ALL and Controls
Table 5 shows the association between relative protein intake and percentage of lean muscle mass. This linear model explained 58% of the variability in percentage of lean muscle mass with age, sex, physical activity, resistance training, relative protein intake, and survivor status included in the model. For each gram of protein per kilogram of body mass, lean muscle mass increased by 7.9%; participation in resistance training increased lean muscle mass by 2.8%. There was no interaction (data not shown) between survivor status and relative protein intake, indicating that the effects of protein intake on lean muscle mass did not differ between survivors and controls.
Table 5.
Association Between Protein Intake and Body Composition for Survivors of Acute Lymphoblastic Leukemia and Control Groupa
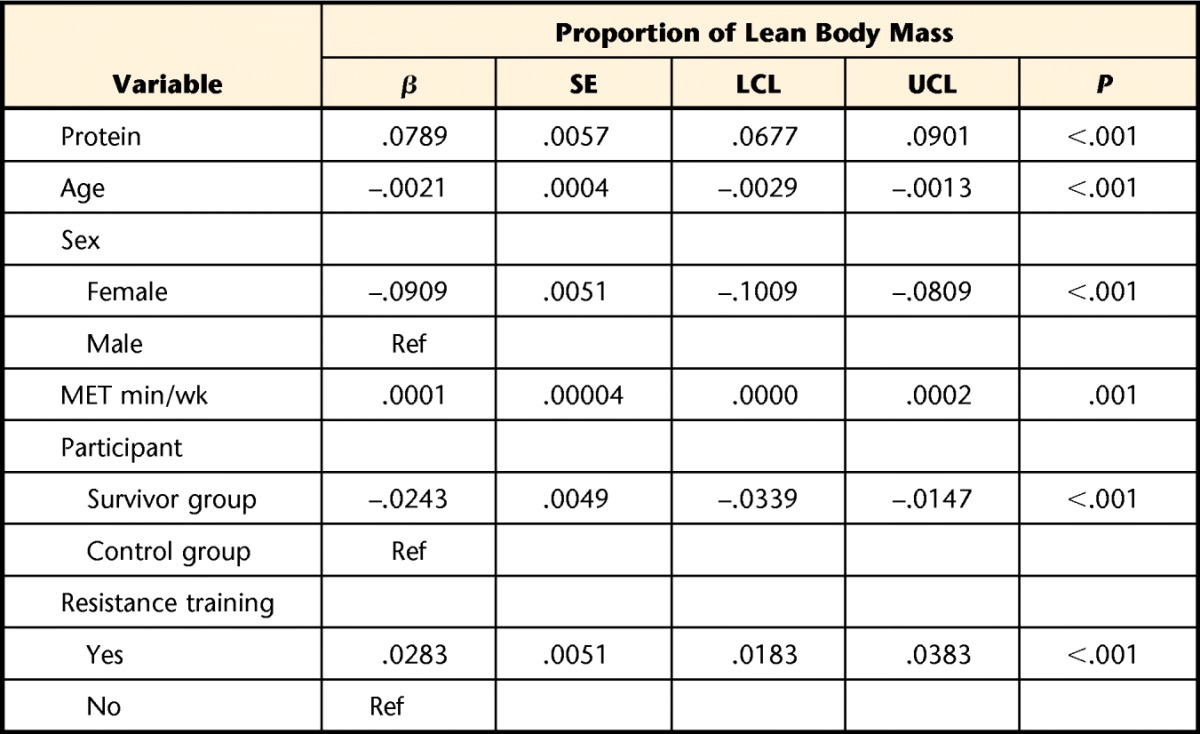
A multiple linear regression model was used to generate beta coefficients, standard errors, and P values. An interaction term between participant status and protein intake was removed from the final model because it was found to be nonsignificant. R-squared value for the model was=.58. β=beta coefficient, SE=standard error, LCL=lower 95% confidence limit, UCL=upper 95% confidence limit, Ref=reference group.
Discussion
In this large, well-characterized population of adult survivors of childhood ALL, we found an independent association between dietary protein intake and lean muscle mass. This association persisted after accounting for CRT exposure, sex, age, race, smoking, physical activity, and participation in regular resistance training. Additionally, the association between protein intake and lean muscle mass did not differ between ALL survivors and an age-, sex-, and race-matched comparison group, even though survivors had lower mean values for actual lean muscle mass and percentage of lean muscle mass. These findings are good news for survivors of childhood ALL with reduced lean muscle mass, suggesting that optimizing dietary protein intake may help remediate this problem. Our data also indicate that resistance training and exercise are associated with higher lean muscle mass.
Our findings regarding body composition are consistent with previous reports in the ALL survivor population.1–3,47 Tonorezos et al,47 in a study of 117 adult survivors of childhood ALL, reported that those exposed had a mean lean muscle mass of 47.8 kg (SD=12.4) compared with survivors not exposed to CRT who had a mean lean muscle mass of 52.7 kg (SD=11.0). Our slightly older survivor population, with a higher proportion of male participants, had a modestly higher mean lean muscle mass of 55.0 kg, which was still lower than the mean lean muscle mass (57.2 kg) in our age-, sex- and race-matched comparison group. Our finding that survivors of ALL had, on average, larger waist circumferences than comparison group members is of clinical significance, as risk for mortality increases by 9% for every 5-cm increase in waist circumference.48
We were unable to identify other published studies that examined the association between dietary protein intake and lean muscle mass in the childhood ALL survivor population. However, observational studies among older adults and intervention studies among women who were overweight or obese and among people with cystic fibrosis and rheumatoid arthritis showed similar associations.48–52 In a report from the Health, Aging, and Body Composition Study, a study of older adults at risk for low lean muscle mass, Houston et al48 reported that daily protein intake of 1.2 g per kilogram of body mass was associated with a 43% less loss of lean muscle mass compared with a daily protein intake of 0.8 g per kilogram of body mass. Another study among older adults showed that dietary protein intake explained 76.4% of the variation in lean muscle mass and that a 1-unit increment in protein intake was associated with 2.7% higher lean muscle mass.49 In a weight loss study (750 kcal/d deficient) among women who were overweight or obese, the group randomized to receive 1.4 g per kilogram of protein per day lost 1.3 kg less lean muscle mass than the group receiving 0.82 g per kilogram of protein per day.50 In a study of protein supplementation (20%–40% increase over 6 months) among children with cystic fibrosis who were more than 1 standard deviation below normal weight at baseline, Shepherd et al51 reported a mean increase of 1.5 kg of lean muscle mass. Lastly, in a 12-week study among adults with rheumatoid arthritis with cachexia, Marcora et al52 reported mean lean muscle mass increases of 0.61 to 0.84 kg with 2 different 7.2 g/d protein supplementation formulations.
We also found associations between resistance training and lean muscle mass among survivors of childhood ALL. This association is novel among survivors of ALL, but has been observed in studies among adults with other types of cancer or other chronic illnesses.53–57 Lonbro et al,57 in a study of head and neck cancer patients, demonstrated that 12 weeks of structured resistance training improved lean muscle mass by approximately 4% compared with self-selected physical activity. Similar studies have shown 4.0% to 6.5% increases in lean muscle mass after resistance training among people with chronic obstructive pulmonary disease,55 type 2 diabetes,56 and McArdle disease.54
There are limitations that should be taken into account when interpreting the results of this study. First, our participants were from a single institution and included those treated between 1980 and 1999. Even though the study population was carefully recruited to represent ALL survivors treated during this period, our results might not be generalizable to survivors of ALL treated at other institutions or those treated prior to 1980 or after 1999. Current treatment for ALL rarely includes CRT. Because CRT was associated with both of our outcomes, children treated without CRT are likely to be at less risk for higher waist-to-hip ratio and lower percentage of lean body mass. Second, our dietary assessment was based on self-report. Even though multiple 24-hour recall methods yield more reliable data than a food frequency questionnaire,38 all recall data are subject to bias, and variation in diet within individuals is difficult to quantify. Because our study participants underreported their intake by about 30% in relation to their known energy requirements,46 the reported mean intake of 1 g per kilogram of body weight was more likely 1.5 g per kilogram of body weight. Additionally, we did not have 100% participation in this study. It is possible that the health status of those individuals who opted to participate may have differed from those who chose not to participate, which has potential to bias our estimates. Finally, because this was a cross-sectional analysis, we do not know whether increasing protein intake and adding resistance exercise will improve lean muscle mass among survivors of ALL whose lean muscle mass is suboptimal. Because lean muscle mass is inherently tied to musculoskeletal fitness, which is key to overall well-being, injury prevention, functional independence, metabolic capacity, and maintenance of ideal body weight,58 research is needed to address this question.
In conclusion, our study demonstrates a clear association among protein intake, resistance training, regular physical activity, and lean muscle mass in adult survivors of childhood ALL. As a result, we recommend that survivors of ALL without chronic kidney disease or conditions where a high protein diet is contraindicated59 be counseled to eat a diet that includes at least 0.8 g of protein per kilogram of body mass per day. They also should be encouraged to participate in resistance training to support lean muscle mass. Thus, when treating survivors of childhood ALL, physical therapy professionals should include assessment of lean muscle mass as part of their comprehensive evaluation, prescribe resistance training for patients with low lean muscle mass, and refer individuals with inadequate protein in their diet for nutritional counseling.
Footnotes
Ms Boland, Ms Lu, Dr Kaste, Dr Chemaitilly, Dr Robison, Dr Hudson, and Dr Ness provided concept/idea/research design. Ms Boland, Dr Kaste, Dr DeLany, Mrs Partin, Dr Howell, Dr Nelson, Dr Chemaitilly, Dr Pui, Dr Mulrooney, Dr Hudson, and Dr Ness provided writing. Ms Boland, Dr Kaste, Dr DeLany, Mrs Partin, Dr Lanctot, Dr Howell, Dr Robison, Dr Mulrooney, Dr Hudson, and Dr Ness provided data collection. Ms Boland, Ms Lu, Dr Kaste, Dr DeLany, Dr Chemaitilly, Dr Robison, and Dr Ness provided data analysis. Ms Boland, Dr Kaste, Dr Lanctot, Dr Howell, Dr Robison, and Dr Ness provided project management. Dr Hudson and Dr Ness provided fund procurement. Dr Kaste, Dr Pui, Dr Hudson, and Dr Ness provided participants. Dr Kaste, Dr DeLany, and Dr Ness provided facilities/equipment. Dr Ness provided institutional liaisons and administrative support. Dr Gibson, Dr Lanctot, Dr Nelson, Dr Pui, Dr Hudson, and Dr Ness provided consultation (including review of manuscript before submission). The authors acknowledge Tracie Gatewood for her help formatting the manuscript.
Institutional review board approval at St. Jude Children's Research Hospital was obtained for all study procedures and documents.
This work was supported by National Cancer Institute grants CA132901 (Dr Ness), CA023944 (Suzanne Gronemeyer), and CA21765 (Richard Gilbertson) and the American Lebanese Syrian Associated Charities.
The views expressed in the article are those of the authors and not an official position of the institutions or funding organizations.
References
- 1. Rayar M, Webber CE, Nayiager T, et al. Sarcopenia in children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2013;35:98–102. [DOI] [PubMed] [Google Scholar]
- 2. Fuemmeler BF, Pendzich MK, Clark K, et al. Diet, physical activity, and body composition changes during the first year of treatment for childhood acute leukemia and lymphoma. J Pediatr Hematol Oncol. 2013;35:437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ness KK, Baker KS, Dengel DR, et al. Body composition, muscle strength deficits and mobility limitations in adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49:975–981. [DOI] [PubMed] [Google Scholar]
- 4. Brill PA, Macera CA, Davis DR, et al. Muscular strength and physical function. Med Sci Sports Exerc. 2000;32:412–416. [DOI] [PubMed] [Google Scholar]
- 5. Joyce ED, Nolan VG, Ness KK, et al. Association of muscle strength and bone mineral density in adult survivors of childhood acute lymphoblastic leukemia. Arch Phys Med Rehabil. 2011;92:873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gueugneau M, Coudy-Gandilhon C, Theron L, et al. Skeletal muscle lipid content and oxidative activity in relation to muscle fiber type in aging and metabolic syndrome. J Gerontol A Biol Sci Med Sci. 2015;70:566–576. [DOI] [PubMed] [Google Scholar]
- 7. Srikanthan P, Karlamangla AS. Muscle mass index as a predictor of longevity in older adults. Am J Med. 2014;127:547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Essig S, Li Q, Chen Y, et al. Risk of late effects of treatment in children newly diagnosed with standard-risk acute lymphoblastic leukaemia: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2014;15:841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nottage KA, Ness KK, Li C, et al. Metabolic syndrome and cardiovascular risk among long-term survivors of acute lymphoblastic leukaemia: from the St. Jude Lifetime Cohort. Br J Haematol. 2014;165:364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steffens M, Beauloye V, Brichard B, et al. Endocrine and metabolic disorders in young adult survivors of childhood acute lymphoblastic leukaemia (ALL) or non-Hodgkin lymphoma (NHL). Clin Endocrinol (Oxf). 2008;69:819–827. [DOI] [PubMed] [Google Scholar]
- 11. Siviero-Miachon AA, Spinola-Castro AM, Lee ML, et al. Cranial radiotherapy predisposes to abdominal adiposity in survivors of childhood acute lymphocytic leukemia. Radiat Oncol. 2013;8:8–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jarfelt M, Lannering B, Bosaeus I, et al. Body composition in young adult survivors of childhood acute lymphoblastic leukaemia. Eur J Endocrinol. 2005;153:81–89. [DOI] [PubMed] [Google Scholar]
- 13. Follin C, Thilen U, Ahren B, Erfurth EM. Improvement in cardiac systolic function and reduced prevalence of metabolic syndrome after two years of growth hormone (GH) treatment in GH-deficient adult survivors of childhood-onset acute lymphoblastic leukemia. J Clin Endocrinol Metab. 2006;91:1872–1875. [DOI] [PubMed] [Google Scholar]
- 14. Topolski TD, LoGerfo J, Patrick DL, et al. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3:A118. [PMC free article] [PubMed] [Google Scholar]
- 15. Claessen KM, Appelman-Dijkstra NM, Pereira AM, et al. Abnormal metabolic phenotype in middle-aged GH-deficient adults despite long-term recombinant human GH replacement. Eur J Endocrinol. 2014;170:263–272. [DOI] [PubMed] [Google Scholar]
- 16. Elbornsson M, Gotherstrom G, Bosaeus I, et al. Fifteen years of GH replacement improves body composition and cardiovascular risk factors. Eur J Endocrinol. 2013;168:745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tan SY, Poh BK, Nadrah MH, et al. Nutritional status and dietary intake of children with acute leukaemia during induction or consolidation chemotherapy. J Hum Nutr Diet. 2013;26(suppl 1):23–33. [DOI] [PubMed] [Google Scholar]
- 18. Smith WA, Li C, Nottage KA, et al. Lifestyle and metabolic syndrome in adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort Study. Cancer. 2014;120:2742–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Demark-Wahnefried W, Werner C, Clipp EC, et al. Survivors of childhood cancer and their guardians. Cancer. 2005;103:2171–2180. [DOI] [PubMed] [Google Scholar]
- 20. Mamerow MM, Mettler JA, English KL, et al. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J Nutr. 2014;144:876–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mettler S, Mitchell N, Tipton KD. Increased protein intake reduces lean body mass loss during weight loss in athletes. Med Sci Sports Exerc. 2010;42:326–337. [DOI] [PubMed] [Google Scholar]
- 22. Pasiakos SM, Vislocky LM, Carbone JW, et al. Acute energy deprivation affects skeletal muscle protein synthesis and associated intracellular signaling proteins in physically active adults. J Nutr. 2010;140:745–751. [DOI] [PubMed] [Google Scholar]
- 23. Tieland M, Dirks ML, van der Zwaluw N, et al. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2012;13:713–719. [DOI] [PubMed] [Google Scholar]
- 24. Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2011;56:825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ojha RP, Oancea SC, Ness KK, et al. Assessment of potential bias from non-participation in a dynamic clinical cohort of long-term childhood cancer survivors: results from the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2013;60:856–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Njeh CF, Fuerst T, Hans D, et al. Radiation exposure in bone mineral density assessment. Appl Radiat Isot. 1999;50:215–236. [DOI] [PubMed] [Google Scholar]
- 27. Jensen MD, Kanaley JA, Roust LR, et al. Assessment of body composition with use of dual-energy x-ray absorptiometry: evaluation and comparison with other methods. Mayo Clin Proc. 1993;68:867–873. [DOI] [PubMed] [Google Scholar]
- 28. Thomas SR, Kalkwarf HJ, Buckley DD, Heubi JE. Effective dose of dual-energy x-ray absorptiometry scans in children as a function of age. J Clin Densitom. 2005;8:415–422. [DOI] [PubMed] [Google Scholar]
- 29. Njeh CF, Samat SB, Nightingale A, et al. Radiation dose and in vitro precision in paediatric bone mineral density measurement using dual x-ray absorptiometry. Br J Radiol. 1997;70:719–727. [DOI] [PubMed] [Google Scholar]
- 30. Kalender WA. Effective dose values in bone mineral measurements by photon absorptiometry and computed tomography. Osteoporos Int. 1992;2:82–87. [DOI] [PubMed] [Google Scholar]
- 31. Kim J, Wang Z, Heymsfield SB, et al. Total-body skeletal muscle mass: estimation by a new dual-energy x-ray absorptiometry method. Am J Clin Nutr. 2002;76:378–383. [DOI] [PubMed] [Google Scholar]
- 32. McDowell MA, Fryar CD, Hirsch R, Ogden CL. Anthropometric reference data for children and adults: U.S. population, 1999–2002. Adv Data. 2005;361:1–5. [PubMed] [Google Scholar]
- 33. Gallagher D, Heymsfield SB, Heo M, et al. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72:694–701. [DOI] [PubMed] [Google Scholar]
- 34. Gallagher D, Visser M, De Meersman RE, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol (1985). 1997;83:229–239. [DOI] [PubMed] [Google Scholar]
- 35. Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol (1985). 2000;89:81–88. [DOI] [PubMed] [Google Scholar]
- 36. Kelly TL, Wilson KE, Heymsfield SB. Dual energy x-ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4:e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schatzkin A, Kipnis V, Carroll RJ, et al. A comparison of a food frequency questionnaire with a 24-hour recall for use in an epidemiological cohort study: results from the biomarker-based Observing Protein and Energy Nutrition (OPEN) study. Int J Epidemiol. 2003;32:1054–1062. [DOI] [PubMed] [Google Scholar]
- 38. Kristal AR, Peters U, Potter JD. Is it time to abandon the food frequency questionnaire? Cancer Epidemiol Biomarkers Prev. 2005;14:2826–2828. [DOI] [PubMed] [Google Scholar]
- 39. Feskanich D, Sielaff BH, Chong K, Buzzard IM. Computerized collection and analysis of dietary intake information. Comput Methods Programs Biomed. 1989;30:47–57. [DOI] [PubMed] [Google Scholar]
- 40. Trumbo P, Schlicker S, Yates AA, Poos M; Food and Nutrition Board of the Institute of Medicine, The National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002;102:1621–1630. [DOI] [PubMed] [Google Scholar]
- 41. Children's Oncology Group. Long-Term Follow-up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. 2008. Available at: http://www.survivorshipguidelines.org Accessed March 4, 2015.
- 42. Asare K. Diagnosis and treatment of adrenal insufficiency in the critically ill patient. Pharmacotherapy. 2007;27:1512–1528. [DOI] [PubMed] [Google Scholar]
- 43. Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. [DOI] [PubMed] [Google Scholar]
- 44. Moore DS, McCabe GP, Craig B. Introduction to the Practice of Statistics. 6th ed New York, NY: WH Freeman & Co; 2007. [Google Scholar]
- 45. Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. [DOI] [PubMed] [Google Scholar]
- 46. Ness KK, DeLany JP, Kaste SC, et al. Energy balance and fitness in adult survivors of childhood acute lymphoblastic leukemia. Blood. 2015;125:3411–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tonorezos ES, Robien K, Eshelman-Kent D, et al. Contribution of diet and physical activity to metabolic parameters among survivors of childhood leukemia. Cancer Causes Control. 2013;24:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Houston DK, Nicklas BJ, Ding J, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87:150–155. [DOI] [PubMed] [Google Scholar]
- 49. Geirsdottir OG, Arnarson A, Ramel A, et al. Dietary protein intake is associated with lean body mass in community-dwelling older adults. Nutr Res. 2013;33:608–612. [DOI] [PubMed] [Google Scholar]
- 50. Leidy HJ, Carnell NS, Mattes RD, Campbell WW. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity (Silver Spring). 2007;15:421–429. [DOI] [PubMed] [Google Scholar]
- 51. Shepherd RW, Thomas BJ, Bennett D, et al. Changes in body composition and muscle protein degradation during nutritional supplementation in nutritionally growth-retarded children with cystic fibrosis. J Pediatr Gastroenterol Nutr. 1983;2:439–446. [DOI] [PubMed] [Google Scholar]
- 52. Marcora S, Lemmey A, Maddison P. Dietary treatment of rheumatoid cachexia with beta-hydroxy-beta-methylbutyrate, glutamine and arginine: a randomised controlled trial. Clin Nutr. 2005;24:442–454. [DOI] [PubMed] [Google Scholar]
- 53. Hulmi JJ, Kovanen V, Selanne H, et al. Acute and long-term effects of resistance exercise with or without protein ingestion on muscle hypertrophy and gene expression. Amino Acids. 2009;37:297–308. [DOI] [PubMed] [Google Scholar]
- 54. Santalla A, Munguia-Izquierdo D, Brea-Alejo L, et al. Feasibility of resistance training in adult McArdle patients: clinical outcomes and muscle strength and mass benefits. Front Aging Neurosci. 2014;6:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Constantin D, Menon MK, Houchen-Wolloff L, et al. Skeletal muscle molecular responses to resistance training and dietary supplementation in COPD. Thorax. 2013;68:625–633. [DOI] [PubMed] [Google Scholar]
- 56. Cauza E, Strehblow C, Metz-Schimmerl S, et al. Effects of progressive strength training on muscle mass in type 2 diabetes mellitus patients determined by computed tomography. Wien Med Wochenschr. 2009;159:141–147. [DOI] [PubMed] [Google Scholar]
- 57. Lonbro S, Dalgas U, Primdahl H, et al. Progressive resistance training rebuilds lean body mass in head and neck cancer patients after radiotherapy: results from the randomized DAHANCA 25B trial. Radiother Oncol. 2013;108:314–319. [DOI] [PubMed] [Google Scholar]
- 58. Ness KK, Gurney JG. Adverse late effects of childhood cancer and its treatment on health and performance. Annu Rev Public Health. 2007;28:279–302. [DOI] [PubMed] [Google Scholar]
- 59. Centers for Disease Control & Prevention. Nutrition for Everyone: Basics: Protein. 2012. Available at: http://www.cdc.gov/nutrition/everyone/basics/protein.html Accessed March 4, 2015.


