Abstract
Background
The unprecedented rise in obesity among young adults, who have limited interaction with health services, has not been successfully abated.
Objective
The objective of this study was to assess the maintenance outcomes of a 12-week mHealth intervention on prevention of weight gain in young adults and lifestyle behaviors at 9 months from baseline.
Methods
A two-arm, parallel, randomized controlled trial (RCT) with subjects allocated to intervention or control 1:1 was conducted in a community setting in Greater Sydney, Australia. From November 2012 to July 2014, 18- to 35-year-old overweight individuals with a body mass index (BMI) of 25-31.99 kg/m2 and those with a BMI ≥ 23 kg/m2 and a self-reported weight gain of ≥ 2 kg in the past 12 months were recruited. A 12-week mHealth program “TXT2BFiT” was administered to the intervention arm. This included 5 coaching calls, 96 text messages, 12 emails, apps, and downloadable resources from the study website. Lifestyle behaviors addressed were intake of fruits, vegetables, sugar-sweetened beverages (SSBs), take-out meals, and physical activity. The control group received 1 phone call to introduce them to study procedures and 4 text messages over 12 weeks. After 12 weeks, the intervention arm received 2 further coaching calls, 6 text messages, and 6 emails with continued access to the study website during 6-month follow-up. Control arm received no further contact. The primary outcome was weight change (kg) with weight measured at baseline and at 12 weeks and self-report at baseline, 12 weeks, and 9 months. Secondary outcomes were change in physical activity (metabolic equivalent of task, MET-mins) and categories of intake for fruits, vegetables, SSBs, and take-out meals. These were assessed via Web-based surveys.
Results
Two hundred and fifty young adults enrolled in the RCT. Intervention participants weighed less at 12 weeks compared with controls (model β=−3.7, 95% CI −6.1 to −1.3) and after 9 months (model β=− 4.3, 95% CI − 6.9 to − 1.8). No differences in physical activity were found but all diet behaviors showed that the intervention group, compared with controls at 9 months, had greater odds of meeting recommendations for fruits (OR 3.83, 95% CI 2.10-6.99); for vegetables (OR 2.42, 95% CI 1.32-4.44); for SSB (OR 3.11, 95% CI 1.47-6.59); and for take-out meals (OR 1.88, 95% CI 1.07-3.30).
Conclusions
Delivery of an mHealth intervention for prevention of weight gain resulted in modest weight loss at 12 weeks with further loss at 9 months in 18- to 35-year-olds. Although there was no evidence of change in physical activity, improvements in dietary behaviors occurred, and were maintained at 9 months. Owing to its scalable potential for widespread adoption, replication trials should be conducted in diverse populations of overweight young adults.
Trial Registration
Australian and New Zealand Clinical Trials Registry (ANZCTR): ACTRN12612000924853; (Archived by WebCite at http://www.webcitation.org/6i6iRag55)
Keywords: young adult, weight gain prevention, mHealth, telehealth, fruit, vegetables, take-out foods, sugar-sweetened beverages, physical activity
Introduction
The World Health Organization (WHO) declared a global obesity epidemic in 1998, but to date, progress in reversing or even halting increases in prevalence has failed [1]. There is some evidence that the rise in childhood obesity has plateaued in countries such as the United States and Australia [2]. However, young adulthood is an important population group that has been largely neglected and their steep trajectory of weight gain is mostly unrecognized [3,4]. This is of concern as incident obesity at a younger age carries increased risk of mortality from and morbidities of cardiovascular disease, type 2 diabetes, some cancers, and osteoarthritis among others [5-8]. Failure to address the weight gain of young adults may limit the success of childhood prevention programs.
Systematic literature reviews in recent years have drawn attention to the limited evidence base for successful interventions in 18- to 35-year-olds [9]. Many studies have had small numbers of subjects but have shown no potential for translation and scale-up to the community [10]. Rather, the use of young adults as subjects has been coincidental as college-based researchers find it easy to recruit on campus [11].
The life cycle phase termed “emerging adulthood” (18-24 years) signifies the transition from adolescence to leaving school, going to college or finding a job, and increasing independence [12]. It has been identified that this might be a window of opportunity to improve health behaviors as they become more receptive as the rebellion of teenage years is left behind [13]. Young adults need healthy lifestyles to both avoid obesity-related diseases in middle age and to protect their future progeny [14-16].
In 2010, the United States acknowledged young adults as a group requiring intervention for the prevention of weight gain with the award of research funding for the seven EARLY studies targeting 18- to 35-year-olds. [17]. Decreases in physical activity and continued high consumption of sugar-sweetened beverages (SSBs) and food prepared outside home are common lifestyle behaviors in young adults across many western nations [18]. With almost universal ownership of mobiles phones, (91% of young adults in the United States and 95% in Australia), this communication channel could be exploited for intervention delivery, referred to as mHealth [19]. Mobile phones have many features that can be used to provide education and counseling, such as text messaging, apps, and Internet access, in addition to the traditional voice call function.
Here we describe the effectiveness of a 9-month randomized controlled trial (RCT) of an mHealth program for 18- to 35-year-olds conducted in Australia, which aimed to improve lifestyle behaviors [20]. We hypothesized that those overweight young adults who received our 12-week “TXT2BFiT” program followed by a 6-month low-dose maintenance phase would gain less weight compared with those who received minimal intervention.
Methods
Study Population
Participants were aged 18-35 years and lived in Greater Sydney, Australia. All participants provided written informed consent and the study was approved by the institutional human ethics review board [20]. A detailed description of the recruitment process has been published previously [21]. In brief, subjects were recruited using mailings from primary care physicians, print media including posters, mass delivery of brochures and newspaper advertisements, and electronic media [21]. No racial or gender bias existed in the recruitment process.
Study Design
The study was registered with the Australian and New Zealand Clinical Trials Registry (ACTRN12612000924853) and the study protocol published beforehand [20]. This study is a parallel two-arm RCT with subjects randomized 1:1. The deviation from the original protocol was that recruitment was extended from letters of invitation sent from primary care practices to include print and electronic media advertisements [21]. Regardless of recruitment method, all participants were required to visit a primary care physician to enter the study. Only 250 participants were recruited (due to slower-than-expected recruitment) rather than the 354 participants in the protocol [21].
Enrolment took place from November 2012 until July 2014. Participants completed an online screener to assess eligibility and those who met inclusion criteria attended a paid consultation with a primary care physician to verify medical fitness to participate. Participants were then allocated to one of two study arms, control or “TXT2BFiT” intervention, using a stratified block design according to sex and the primary care practice responsible for confirming eligibility. The randomized block contained block sizes 2, 4, and 6. A randomization list was generated using RAND.exe [22] and held centrally by the statistician. When an individual was recruited, the statistician assigned the treatment arm but was blind to intervention status. Other researchers involved in measurement and analysis were also blinded. Participants had their treatment arm concealed but were aware of the intensity of intervention received [20].
Eligibility criteria included age between 18 and 35 years, a body mass index (BMI) of 25 to 31.9 kg/m2, or 23 to 24.9 kg/m2 and > 2 kg self-reported weight gain in preceding 12 months [20]. Ownership of a mobile phone and access to the internet at least once a week was required for intervention delivery. Subjects had to be failing to meet one or more of the key behaviors for modification which were less than 2 serves fruit daily; less than 5 serves vegetables daily; more than 1 high-energy, high-fat take-out meal weekly; more than or equal to 1 liter SSBs weekly; less than 60 minutes of moderate physical activity daily. Exclusion criteria were pregnancy or planning to fall pregnant within 9 months, participation in an alternate weight loss program, weight loss of > 10 kg in preceding 3 months, medications that caused > 2 kg weight gain, disordered eating or medical contraindication, and non-English speaking [20].
The mHealth program ran for 12 weeks after which participants entered a maintenance intervention phase for an additional 6 months.
Twelve-Week “TXT2BFiT” Program
The program promotes the consumption of core food groups and limitation of energy-dense nutrient-poor discretionary foods. Key behaviors for change are fruit and vegetable intake to meet recommended amounts (behavior 1); high-fat, high-energy take-out meals (behavior 2) and SSBs (behavior 3) are discouraged. In addition, participants are encouraged to achieve 60 minutes of physical activity daily (behavior 4), the upper level of Australian recommendations [23]. Participants allocated to the treatment arm received a multicomponent mHealth program. This included 5 coaching calls by a dietitian skilled in motivational interviewing. Goal setting and review were included in the coaching and modeled on control theory with their intake (input function) compared with recommended (comparator) and, therefore, provided feedback to improve their behavior (week 0, 2, 5, 8, 11) [24]. For each of the 4 key behaviors addressed, a staging algorithm based on the transtheoretical model was completed as part of the baseline survey by all participants [20,25]. This was used to generate a personalized set of messages (8 messages a week) from our bank of text messages to be sent over the 12-weeks. Messages were stratified by sex and whether the participant was in pre-contemplation, contemplation, preparation, action, or maintenance stages for each of the 4 behaviors. More cognitive messages were included if a behavior was in the early stage for change and messages were more behavioral if participant was in the action or maintenance stages for any given behavior. Twelve emails (once a week) were sent by the dietitian who offered coaching and repeated the information in the text messages with links to remind participants to use the other resources provided. After a coaching call, the goals set were reiterated in the emails sent by the dietitian.
Other components of the program were a comprehensive 18-page diet and nutrition booklet with physical activity guidelines and a website. This website gave access to 4 designer mobile phone apps for education and self-monitoring for each of the 4 key lifestyle behaviors addressed. Other resources were online weight tracker, printable charts such as “eating on a budget,” “emergency meal tool kit,” “meal planner,” “seasonal guide to fruit and vegetables,” “tips for take-outs,” physical activity planner and “staying healthy over holidays;” and a blog facility for communication [26].
Control Program
A minimal intervention was delivered to controls, which included 4 text messages, 1 on each key behavior, over 12 weeks (fruit and vegetables, take-out meals, SSBs, and physical activity). Control participants also received a 2-page handout based on the Australian dietary guidelines and physical activity guidelines. They had access to a website (separate from the password-protected website of the intervention participants) that contained only the participant information sheet and the 2-page handout. An introductory phone call was made to each participant but no coaching was provided.
Six-Month Maintenance Phase
After the 12-week “TXT2BFiT” program, intervention participants received a low dose maintenance intervention. This consisted of monthly text messages and emails, and participants had continued access to the website. Two booster coaching phone calls at 5 and 8 months from baseline were included. Control participants had no further treatment contact during this period.
Measurements
The primary outcome was change in weight. All participants were weighed to the nearest 0.1 kg and had their height measured to the nearest 0.1 cm by their primary care physician at baseline according to a standard protocol. Participants were invited to be weighed by study personnel at the end of the 12-week trial [20]. Self-reported measures of body weight were collected at baseline, end of 12-week trial, and again at the end of 6-month maintenance (9 months) via the Web-based survey instrument. BMI was calculated. Following a standardized procedure, participants were provided with instructions on self-weighing by the dietitian.
Secondary outcomes were assessed using online surveys at baseline, the end of the 12-week trial, and the end of the 6-month maintenance. These included changes in fruit and vegetable intake (daily servings), SSBs (weekly intake), and weekly frequency of take-out meals assessed using short categorical questions. Change in frequency and minutes of physical activity were assessed using the short-form International Physical Activity Questionnaire (IPAQ). Details of the questions have been published previously [20]. A $AU10.00 gift voucher was given for completion of each survey and clinic attendance for weight measurement.
Demographic details were collected via the online questionnaire that included age, sex, postcode used to determine socioeconomic status (SES), language spoken at home, and the WHO-5 well-being questionnaire [20,27]. The delivery of the program was monitored by number of coaching calls completed, number of emails and text messages delivered, and number of downloads of the mobile phone apps. Participants in the intervention were asked to reply to 22 text messages over the 9 months (16 in the first 12 weeks and 6 in the next 6 months). Both the 12-week and 9-month online surveys included questions on use of the program elements.
Sample Size Calculation
The sample size was calculated based on a difference of 2 kg between intervention and control groups, allowing for a standard deviation of 10 kg and a correlation of 0.8 of baseline weight and final weight. With 142 subjects in each arm, this difference could be detected with 80% power at P<.05 (two sided). To allow for a 20% drop out rate, the sample size was increased to 354 in total. As stated above, recruitment was ceased at 250 participants [21].
Statistical Analysis
Attrition bias was examined using t tests for continuous variables and chi-square tests for categorical variables. The baseline characteristics of completers and non-completers at 9 months within both the intervention and control groups were compared. The IPAQ was scored using standard methods to yield a continuous measure of reported physical activity minutes weekly (metabolic equivalent of task, MET-min) [28]. Differences between the experimental and control group over time in the continuous variables, such as body weight, BMI, physical activity, and WHO-5 outcomes, were estimated using linear mixed models, with an unstructured correlation matrix, adjusted for sex and primary care practice (fitted as fixed effects) and implemented with PROC MIXED. We examined plots of panel-studentized residuals which demonstrated normality and constant variance. Interaction between time and group was included in the model. Diet outcomes (fruit, vegetables, SSBs, and take-out meals) were analyzed using cumulative logistic regression models with general estimating equations (GEE) to account for correlation between time points and multiple imputations to account for missing values. Ten imputed data sets were created using chained equations utilizing baseline values and available data at 3 and 9 months, as well as participant baseline characteristics including sex, ethnic background (language spoken at home), recruitment practice, and allocation. This included odds of improvement in diet-related behaviors and odds of meeting suggested intakes of 2 serves fruit, 3 or more serves of vegetables, less than 500 mL of SSBs per week, and less than one take-out meal weekly. The effect of missing data was investigated as part of a sensitivity analysis using multiple imputation under the missing not at random (MNAR) assumption by searching for a tipping point that reverses the primary outcome conclusion [29]. Clinically plausible weight gains (fixed values) were added to randomly generated imputed values to investigate the impact at 3 month time-point and 9 month end point. Ten imputed data sets were created as described above. All analyses were performed with SAS (version 9.2 SAS Institute Inc. Cary NC, USA).
Results
Figure 1 displays the flow of participants through the trial. In all, 1181 attempts of the screener survey were recorded with 547 of these failing to complete screening. An additional 244 failed to meet the inclusion criteria and 138 eligible participants failed to complete the visit to the primary care physician and were not randomized. Two participants were randomized after census date.
Figure 1.
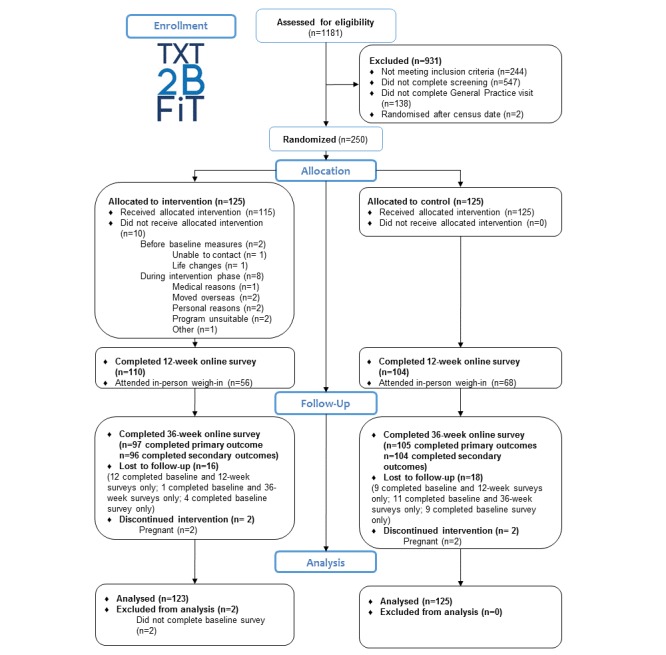
Flow of participants through the 9-month trial.
One hundred and twenty-five participants were allocated to each arm. After the 12-week program, 110 intervention participants and 104 control participants completed the Web-based survey for assessment of outcomes. At completion of the maintenance phase, 97 intervention participants and 105 control participants completed the final online survey.
Table 1 summarizes the demographic characteristics and lifestyle behaviors of the population at baseline. Participants were mostly in the overweight BMI range, of higher SES, from English-speaking backgrounds, and tertiary educated. Approximately 3 in 5 participants were females and mean WHO-5 was just below middle of the scale of 25, indicating a tendency of poor well-being. Most participants failed to meet the criteria for fruits (2 serves), vegetables (3 serves), and take-out meals (more than 1 per week). Most participants consumed less than 500 mL of SSBs weekly and the mean total daily physical activity was approximately 55 minutes of moderate physical activity on each of the past 7 days; 51.6% met the national recommendation for 30 minutes of physical activity per day (48.8% intervention, 54.4% control). A comparison of those participants retained in the study versus those lost to follow-up after 9 months showed no significant differences by demographic characteristics neither for intervention nor for the control group.
Table 1.
Characteristics and baseline behaviors of participants in the TXT2BFiT trial.
| Characteristic | Intervention group (n=123)a
mean (SD) or n (%) |
Control group (n=125) mean (SD) or n (%) |
||
| Age in years, mean (SD) | 28.1 (4.9) | 27.2 (4.9) | ||
| Gender, n (%) |
|
|
||
|
|
Female | 73 (59.3) | 79 (63.2) | |
| Weight status |
|
|
|
|
|
|
Normal BMI 23.0-24.9 | 24 (19.5) | 33 (26.4) | |
|
|
Overweight BMI 25.0-29.9 | 83 (67.5) | 70 (56.0) | |
|
|
Obese BMI 30.0-31.99 | 16 (13.0) | 22 (17.6) | |
| BMI (kg m-2), median (IQR) |
|
27.1 (3.7) | 26.8 (4.2) | |
| WHO-5 score |
|
11.8 (4.7) | 12.9 (4.5) | |
| SESb, n (%) |
|
|
||
|
|
0-60 | 8 (6.5) | 7 (5.6) | |
|
|
61-80 | 28 (22.8) | 17 (13.6) | |
|
|
81-100 (highest) | 87 (70.7) | 101 (80.8) | |
| Ethnic background, n (%) |
|
|
||
|
|
English speaking | 82 (66.7) | 90 (72.0) | |
|
|
Otherc | 41 (33.3) | 35 (28.0) | |
| Education, n (%) |
|
|
||
|
|
High school or below | 27 (22.0) | 21 (16.8) | |
|
|
Some tertiary education | 22 (17.8) | 25 (20.0) | |
|
|
University degree | 74 (60.2) | 79 (63.2) | |
| Fruit | < 2 serves per day | 82 (66.7) | 77 (61.6) | |
| Vegetable | ≤ 3 serves per dayd | 104 (84.6) | 107 (85.6) | |
| SSB e | ≥ 500 mL per week | 37 (30.1) | 44 (35.2) |
|
| Take-out meals | > once per week | 75 (60.9) | 79 (63.2) | |
| Physical activity | Total METf-mins weekly | 1620 (1581) | 1647 (1475) | |
aTwo participants had measured variables but did not complete baseline self-report survey.
bSES: socioeconomic status by quintile with the bottom three quintiles collapsed into one.
cEuropean, Asian, Pacific Islander, and Arabic ethnicities collapsed.
dAustralian recommendations are 5 serves per day but the World Health Organization recommendation of 3 is used here.
eSSB: sugar-sweetened beverages.
fMET: metabolic equivalent of task.
Table 2 presents body weight, BMI, and MET-minutes of physical activity per week for intervention and control groups. After the 12-week program the intervention participants weighed 3.7 kg (95% CI −6.1 to −1.3, P=.003) less than controls and after maintenance, 9 months from baseline, the intervention group weighed 4.3 kg (95% CI −6.9 to −1.8) less than controls (P=.001). The changes in BMI equated to a difference of 0.56 kg/m2 (95% CI −1.22 to 0.09, P=.093) after the 12-week program and 0.78 kg/m2 (95% CI −1.53 to −0.02, P=.044) at end of maintenance stage. Sensitivity analyses outlined above generated consistent findings for the primary outcome, with the addition of up to 2.4 kg at 3 months and 7.2 kg at 9 months to the imputed values. Although the intervention improved their moderate physical activity by 12 minutes per day more than the controls at 12 weeks, the differences were not significant and disappeared by 9 months. The WHO-5 score showed improvement in both groups with no significant differences between them. The mean increase in both groups was clinically meaningful, with the mean score above the cut point of 13 indicating improved well-being.
Table 2.
Comparison of self-reported weight, BMIa, and physical activity in intervention (n=123) versus control (n=125) at baseline, end of program (3 months), and end of maintenance periods (9 months).
|
|
Baseline=0 months, mean (SD)b,c |
End of program=3 months, mean difference (SD)c |
End of maintenance=9 months, mean difference (SD)c |
||||||
|
|
TXT2BFiT | Control | TXT2BFiT | Control | Model β (95% CI) | TXT2BFiT | Control | Model β (95% CI) | |
| Weight, kg | |||||||||
|
|
78.4 (11.2) | 79.3 (12.6) | −2.2 (3.1) | −0.23 (2.3) | −3.7 (−6.1 to −1.3) P=.003 |
−3.8 (4.9) | −0.80 (3.7) | −4.3 (−6.9 to −1.8) P=.001 |
|
| BMI, kg/m2 | |||||||||
|
|
27.3 (2.3) | 27.0 (2.7) | −0.76 (1.0) | −0.08 (0.78) | −0.56 (−1.22 to 0.09) P=.093 |
−1.30 (1.7) | −0.26 (1.28) | −0.78 (−1.53 to −0.02) P=.044 |
|
| Physical activity, MET-min | |||||||||
|
|
1620 (1581) | 1647 (1475) | 625 (1932) | 302 (1411) | 333 (−206 to 871) P=.225 |
872 (1918) | 797 (2115) | 70 (−474 to 614) P=.801 |
|
| Physical activity, days | |||||||||
|
|
6.6 (3.3) | 7.4 (3.8) | 2.1 (3.8) | 0.5 (3.7) | 1.0 (0.0 to 2.0) P=.050 |
2.1 (4.3) | 1.3 (4.4) | 0.2 (−0.8 to 1.3) P=.679 |
|
| WHO-5 score | |||||||||
|
|
11.8 (4.7) | 12.9 (4.5) | 3.2 (4.7) | 1.2 (4.7) | 0.9 (−0.4 to 2.1) P=.176 |
3.4 (4.5) | 1.4 (5.2) | 0.8 (−0.5 to 2.1) P=.202 |
|
aBMI: body mass index.
bSD: standard deviation.
cMean difference between groups (95% confidence intervals) adjusted for practice and sex
Odds ratios comparing the odds for improving intake of fruit, vegetables, SSBs, and take-out meals for intervention and control groups are reported in Table 3. After the 12-week program, the odds that the intervention group improved intake compared to the control group were significantly greater for vegetables (P=.006), SSBs (P=.024), and take-out foods (P=.013). At the conclusion of maintenance stage, the intervention group had greater odds of maintaining improvements in all 4 diet variables. At the end of the program and the end of maintenance, the intervention group had greater odds of meeting suggested intakes for all dietary variables (Table 4).
Table 3.
Odds ratios (95% confidence intervals) of improved intakesa for TXT2BFiT intervention versus control post-intervention (3 months) and post-maintenance (9 months) adjusted for practice and sex.
| Phase |
|
Fruita | Vegetablesa | Sugar-sweetened beveragesa | Take-out mealsa |
| Post intervention, time=3 months | |||||
|
|
Control | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
|
|
TXT2BFiT | 1.31 (0.79-2.15) P=.292 | 2.03 (1.23-3.35) P=.006 | 1.67 (1.07-2.61) P=.024 | 2.16 (1.18-3.95) P=.013 |
| Post maintenance, time=9 months | |||||
|
|
Control | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
|
|
TXT2BFiT | 2.38 (1.41-4.01) P=.001 | 1.94 (1.19-3.16) P=.008 | 1.74 (1.10-2.77) P=.018 | 1.98 (1.17-3.34) P=.010 |
aOdds ratios were estimated using proportional odds models. Lower odds ratios, but greater than 1, were observed for the most improved categories.
Table 4.
Odds ratios (95% confidence intervals) of meeting recommendations for TXT2BFiT intervention versus control post-intervention (3 months) and post-maintenance (9 months) adjusted for practice and sex.
| Phase |
|
Fruit ≥ 2 serves | Vegetables ≥ 3 serves | Sugar-sweetened beverages < 500 mL per week | Take-out meals < one per week |
| Post intervention time=3 months | |||||
|
|
Control | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| TXT2BFiT | 1.84 (1.01-3.34) P=.046 | 2.05 (1.16-3.62) P=.014 | 4.77 (1.96-11.62) P=.001 | 2.37 (1.21-4.63) P=.012 | |
| Post 6 maintenance time=9 months | |||||
|
|
Control | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| TXT2BFiT | 3.83 (2.10-6.99) P=.001 | 2.42 (1.32-4.44) P=.005 | 3.11 (1.47-6.59) P=.003 | 1.88 (1.07-3.30) P=.028 | |
Monitoring of intervention delivery showed that 92% of coaching calls were completed during the 12-week program and 81% during 6-month maintenance period. All emails were delivered and for the text messages, 98% were delivered during the first 12 weeks and 96% during the 6-month maintenance. During the 12-week program, 53% of participants replied to more than half the text messages but only 40% replied during the maintenance phase. All participants reported using the text messages at 12 weeks and 60% at 9 months. Fifty-two percent of participants downloaded the physical activity app, 43% downloaded the fruit and vegetable app, and 31% the beverages app [30]. The data on downloads of the take-out app could not be determined. The company hosting the website made changes that lead to a temporary loss of data from this app but it did not affect the other three.
Discussion
This is one of the first trials of a multicomponent mHealth program for delivery to young adults with demonstrated maintenance of weight management and nutrition-related behavior change after the 12-week program. As hypothesized, the TXT2BFiT program prevented weight gain [25] leading to weight loss that can be maintained after 6 months follow-up with minimal support. The use of mHealth to deliver effective lifestyle health promotion shows promise to halt the rising incidence of obesity during young adulthood—a group recognized as difficult to reach. The ubiquitous use of mobile phones by this age group allows for a number of communication channels to be used to deliver multicomponent programs.
The 12-week TXT2BFiT program led to additional positive health benefits through maintenance of improved diet behaviors at 9 months from baseline. This is the likely explanation for the 6-month maintenance of weight loss. In addition, increasing fruit and vegetable intake has benefits beyond weight management, in protection against cardiovascular disease and stroke and certain cancers [31-33]. In the past decade, consumption of SSBs has been associated with not only weight gain but also cardiovascular disease and type 2 diabetes [34]. The age group targeted here is the largest consumer of fast food meals among adults [35]. Such food is inevitably high in deleterious nutrients such as saturated fat and sodium, and higher frequency of intake is associated with type 2 diabetes in Australian young adults [36]. The intervention failed to produce increases in physical activity compared with control. Both groups appeared to have higher amounts of physical activity at 3 and 9 months but with wide variation. In previous research in a similar group of younger adults, 75% reported at least 150 minutes of moderate physical activity per week but most wanted to increase their physical activity [37]. This is higher than that in the current trial, with 51.6% of participants meeting national physical activity recommendations. The controls were sufficiently motivated to enroll in the study and likely had the capability and opportunity to increase their physical activity without intervention input. It is also of note that the controls did not gain weight during the 12-week program or at 9 months.
A number of other trials using new technologies have been conducted in young adults in the United States [17]. The CITY study compared treatment using 6 face-to-face group sessions followed by monthly phone calls for 24 months (PC), with both an intervention delivered via a mobile phone app and a control group [38]. Weight measurements in the 18- to 35-year-olds indicated a significant loss by the PC group compared with control and app group at 6 months but no difference between app and control groups. By 12 months, all differences disappeared [38].
This study was different from other studies in that the current intervention included 5 short coaching calls in the 3-month intensive phase as a component of the mHealth intervention. In our former pilot RCT to assess the feasibility of delivering the intervention in 50 young adults, we found no difference in weight loss between groups at 12 weeks because both reduced their weight [39]. The extra communication component in this study was the addition of short coaching calls. This allowed more personalized feedback for goal setting and review whereby their performance against a recommended behavior such as 2 daily serves of fruit was used to set goals to work toward the achievement of the target intake. It adds an additional cost to the program that amounted to approximately AU $45 per participant and cost-effectiveness comparisons with totally electronically delivered and traditional face-to-face intervention warrant further research. However, as discussed below, few electronic or entirely app-based interventions demonstrate effectiveness.
Other studies in young adults have used Web-based or email programs. Kattelmann et al delivered a 10-week Web-based intervention to 1639 US college students addressing healthy eating, physical activity, stress, and weight management in an RCT, and assessed post-intervention effects and 12-month maintenance. While both diet and physical activity behaviors improved, no changes in weight occurred and the effects were not maintained 12 months later [40]. Schweitzer et al delivered an adaptation of A Lifestyle Intervention via Email, the ALIVE program, for 24 weeks in a pilot RCT to 148 college students aged 18-20 years. While no differences in body weight were found, the intervention participants increased their intake of fruits as a snack and marginally decreased the percentage energy from saturated fat [41]. Park et al conducted a Web-based RCT in 160 US students aged 18-24 years comparing tailored advice based on the transtheoretical model of behavior change with non-tailored advice and found no differences in fruit and vegetable consumption [42]. This could suggest that using multiple components in mHealth such as text messaging and coaching calls provide a more personalized approach than Web-based techniques that young adults find helpful for changing their behaviors. The college-based RCT by Gow et al with four treatments including no intervention, 6 weeks of a Web-based intervention, 6 weeks of feedback on weight and calorie intake, and a combination of Web-based intervention with weight and calorie feedback found that the combined group attained the lowest BMI [43]. This further illustrates the advantages of feedback communication in addition to Web resources. Bertz et al studied 167 first-year US college students providing Wi-Fi scales and emailed graphs of weight to show changes compared with no feedback control group. It was found that regular weighing with feedback prevented weight gain [44]. Thus, the importance of building education and counseling with feedback along with ongoing monitoring and self-monitoring into a mHealth program is apparent. The inclusion of a range of demonstrated theory-based behavior change techniques, as in this study, also should be central to any mHealth program [45].
The most successful Web-based intervention to date was conducted in Scotland. Nikolaou et al randomized 20,975 university students to a 40-week three-arm RCT: control and two treatments [46]. While the control group gained weight (mean 2.0 kg, 95% CI 1.5-2.3), both treatment groups lost weight: treatment one −1.0 kg (95% CI −1.3 to −0.5) and treatment two −1.35 kg (95% CI −1.4 to −0.7). Both interventions were novel in their approach. The first treatment was based on the “rational” model that individuals when presented with information will make the best use of it. The messages overtly addressed the problem of weight gain and obesity. The second intervention was by “stealth” and raised discussion around social and political movements associated with food and health and obesity. For 19 weeks, participants would log into the Web-based modules to be completed weekly. The advantage of this study is that it was embedded within the university learning environment and was advertised as a new course being tested. Many undergraduate students participated after invitation, unlike the other studies that either recruited volunteers from within the college environment or the population at large. While this intervention could likely be adapted for other college students, whether it would be successful when participants must be recruited from the general community of young adults is uncertain.
One of the strengths of this study is that the age range of recruited participants was well distributed between 18 and 35 years. The program reached a greater proportion of males (40%) than usual in weight management studies [10] and included 30% participants who were not born to an English speaking family. However, our results may not be generalizable to all young adults because the study group tended to be of a higher SES and messaging might be country specific.
Limitations
A perceived limitation is that the study maintenance phase was for 6 months only, that is 9 months from baseline, whereas up to 2 years is suggested for weight maintenance [47]. However, maintenance of nutrition behaviors with habit formation may be developed in shorter time frames [48]. Another limitation is the assessment of outcomes using self-report. While there was no difference in the comparison of weight change between both groups when measured and self-reported weight was used at 12 weeks [26], we cannot be certain if this remained the case at 9 months from baseline. The IPAQ may not be sufficiently sensitive to monitor changes in physical activity [28]. Although acknowledged as a limitation, the use of an objective measure like biomarkers is not practical for a remotely delivered intervention and costs of providing Wi-Fi enabled scales, from which data can be accessed, may prove too costly for such an intervention if it is to be scaled up in the population. Recruiting young adults to participate in lifestyle intervention proved challenging in this study. Both the primary care physicians and their patients had a lower than expected uptake of the program [21]. Costs, time, and methods of recruitment require consideration when planning replication trials or scale-up and roll out. Further consideration should be given to recruiting a larger sample size to determine the effects on well-being because, while no evidence was provided in this study, the 95% confidence intervals include beneficial values. Finally, the study had multiple components and we did not attribute the changes to any component in isolation. While the 12-week findings from our study were included in a recent meta-analysis of mobile phone apps for weight loss, it can be seen that less than half the sample downloaded the apps [49].
Conclusion
In conclusion, delivery of an mHealth theory-based intervention for healthy lifestyle and prevention of weight gain in 18- to 35-year-olds was effective in achieving and maintaining weight loss and improved diet behaviors. Countries such as the United States, United Kingdom, and Australia are recognizing that programs with wide reach but of low cost are required in young adulthood [18]. Although the messaging component of the mHealth program was developed in the local context, the behaviors targeted are globally problematic with SSB consumption, high-fat, high-energy take-out meals, and poor vegetable intakes prominent in young adults in the United States [18]. Given the potential for universal adoption and wide reach in this age group, we suggest replication trials of mHealth be conducted in a broader range of young adults throughout countries battling obesity.
Acknowledgments
The authors thank the partnering primary care practices and Medicare local offices for support in this research.
Funding to conduct this trial was obtained by a grant from the Hospitals Contribution Fund (HCF) Medical Research Foundation (reference number MAUsyd1008201111), listed on the Australian Competitive Research Grants Register Category 1. The HCF had no part in study design, analysis, or manuscript writing. This work was also supported by the Commonwealth Government of Australia via an Australian Postgraduate Award Scholarship to SRP, and LH was supported by a National Health and Medical Research Council (NHMRC) Scholarship between 2011 and 2013. MFH was partly funded by an NHMRC Senior Research Fellowship.
Footnotes
Authors' Contributions: MAF, KM, AB, LH, KB, EDW, MFH designed the trial. MAF, AW, KB, and SRP acquired the data. SRP performed the statistical analysis with input from KM, MAF, and AB. MAF drafted the manuscript with input from SRP. All authors assisted in interpretation of findings and approved the final manuscript content.
Conflicts of Interest: MAF was on the Research Advisory Board of the Australian Primary Health Care Institute. MFH is a director of Central and Eastern Sydney Primary Health Network.
References
- 1.World Health Organization . Obesity: preventing and managing the global epidemic. Geneva: World Health Organization; 2000. [PubMed] [Google Scholar]
- 2.Olds T, Maher C, Zumin S, Péneau S, Lioret S, Castetbon K, Bellisle. de Wilde J, Hohepa M, Maddison R, Lissner L, Sjöberg A, Zimmermann M, Aeberli I, Ogden C, Flegal K, Summerbell C. Evidence that the prevalence of childhood overweight is plateauing: data from nine countries. Int J Pediatr Obes. 2011 Oct;6(5-6):342–60. doi: 10.3109/17477166.2011.605895. [DOI] [PubMed] [Google Scholar]
- 3.Vadeboncoeur C, Townsend N, Foster C. A meta-analysis of weight gain in first year university students: is freshman 15 a myth? BMC Obes. 2015;2:22. doi: 10.1186/s40608-015-0051-7. http://bmcobes.biomedcentral.com/articles/10.1186/s40608-015-0051-7 .51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allman-Farinelli MA, Chey T, Bauman AE, Gill T, James W P T Age, period and birth cohort effects on prevalence of overweight and obesity in Australian adults from 1990 to 2000. Eur J Clin Nutr. 2008 Jul;62(7):898–907. doi: 10.1038/sj.ejcn.1602769.1602769 [DOI] [PubMed] [Google Scholar]
- 5.Adams KF, Leitzmann MF, Ballard-Barbash R, Albanes D, Harris TB, Hollenbeck A, Kipnis V. Body mass and weight change in adults in relation to mortality risk. Am J Epidemiol. 2014 Jan 15;179(2):135–44. doi: 10.1093/aje/kwt254. http://aje.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=24173550 .kwt254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allman-Farinelli M. Invited commentary: Body mass index and mortality. Am J Epidemiol. 2014 Jan 15;179(2):145–6; discussion 147. doi: 10.1093/aje/kwt252. http://aje.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=24173547 .kwt252 [DOI] [PubMed] [Google Scholar]
- 7.Jeffreys M, McCarron P, Gunnell D, McEwen J, Smith GD. Body mass index in early and mid-adulthood, and subsequent mortality: a historical cohort study. Int J Obes Relat Metab Disord. 2003 Nov;27(11):1391–7. doi: 10.1038/sj.ijo.0802414.0802414 [DOI] [PubMed] [Google Scholar]
- 8.de Mutsert R, Sun Q, Willett WC, Hu FB, van Dam Rob M. Overweight in early adulthood, adult weight change, and risk of type 2 diabetes, cardiovascular diseases, and certain cancers in men: a cohort study. Am J Epidemiol. 2014 Jun 1;179(11):1353–65. doi: 10.1093/aje/kwu052. http://aje.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=24786797 .kwu052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hebden L, Chey T, Allman-Farinelli M. Lifestyle intervention for preventing weight gain in young adults: a systematic review and meta-analysis of RCTs. Obes Rev. 2012 Aug;13(8):692–710. doi: 10.1111/j.1467-789X.2012.00990.x. [DOI] [PubMed] [Google Scholar]
- 10.Partridge SR, Juan SJ, McGeechan K, Bauman A, Allman-Farinelli M. Poor quality of external validity reporting limits generalizability of overweight and/or obesity lifestyle prevention interventions in young adults: a systematic review. Obes Rev. 2015 Jan;16(1):13–31. doi: 10.1111/obr.12233. [DOI] [PubMed] [Google Scholar]
- 11.Lam E, Partridge SR, Allman-Farinelli M. Strategies for successful recruitment of young adults to healthy lifestyle programmes for the prevention of weight gain: a systematic review. Obes Rev. 2016 Feb;17(2):178–200. doi: 10.1111/obr.12350. [DOI] [PubMed] [Google Scholar]
- 12.Arnett JJ. Emerging adulthood. A theory of development from the late teens through the twenties. Am Psychol. 2000 May;55(5):469–80. [PubMed] [Google Scholar]
- 13.Nelson MC, Story MT, Larson NI, Neumark-Sztainer D, Lytle L. Emerging adulthood and college-aged youth: an overlooked age for weight-related behavior change. Obesity (Silver Spring) 2008 Oct;16(10):2205–11. doi: 10.1038/oby.2008.365. doi: 10.1038/oby.2008.365.oby2008365 [DOI] [PubMed] [Google Scholar]
- 14.Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA. 2014 Apr 16;311(15):1536–46. doi: 10.1001/jama.2014.2269.1860462 [DOI] [PubMed] [Google Scholar]
- 15.Lepe M, Bacardí GM, Castañeda-González LM, Pérez Morales Ma E. Jiménez CA. Effect of maternal obesity on lactation: systematic review. Nutr Hosp. 2011;26(6):1266–9. doi: 10.1590/S0212-16112011000600012. http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S0212-16112011000600012&lng=en&nrm=iso&tlng=en .S0212-16112011000600012 [DOI] [PubMed] [Google Scholar]
- 16.Nicholas LM, Morrison JL, Rattanatray L, Zhang S, Ozanne SE, McMillen IC. The early origins of obesity and insulin resistance: timing, programming and mechanisms. Int J Obes (Lond) 2016 Feb;40(2):229–38. doi: 10.1038/ijo.2015.178.ijo2015178 [DOI] [PubMed] [Google Scholar]
- 17.Lytle LA, Svetkey LP, Patrick K, Belle SH, Fernandez ID, Jakicic JM, Johnson KC, Olson CM, Tate DF, Wing R, Loria CM. The EARLY trials: a consortium of studies targeting weight control in young adults. Transl Behav Med. 2014 Sep;4(3):304–13. doi: 10.1007/s13142-014-0252-5. http://europepmc.org/abstract/MED/25264469 .252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allman-Farinelli M. Nutrition Promotion to prevent obesity in young adults. Healthcare. 2015 Sep 07;3:809–821. doi: 10.3390/healthcare3030809.s151025033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pew Research Center. [2016-03-15]. http://www.pewglobal.org/files/2016/02/pew_research_center_global_technology_report_final_february_22__2016.pdf .
- 20.Hebden L, Balestracci K, McGeechan K, Denney-Wilson E, Harris M, Bauman A, Allman-Farinelli M. 'TXT2BFiT' a mobile phone-based healthy lifestyle program for preventing unhealthy weight gain in young adults: study protocol for a randomized controlled trial. Trials. 2013;14:75. doi: 10.1186/1745-6215-14-75. http://www.trialsjournal.com/content/14//75 .1745-6215-14-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Partridge SR, Balestracci K, Wong AT, Hebden L, McGeechan K, Denney-Wilson E, Harris MF, Phongsavan P, Bauman A, Allman-Farinelli M. Effective Strategies to Recruit Young Adults Into the TXT2BFiT mHealth Randomized Controlled Trial for Weight Gain Prevention. JMIR Res Protoc. 2015;4(2):e66. doi: 10.2196/resprot.4268. http://www.researchprotocols.org/2015/2/e66/ v4i2e66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piantadosi S. Clinical trials: a methodologic perspective. 2nd edition. Hoboken, NJ: Wiley-Interscience; 2005. [Google Scholar]
- 23.Australian Government Department of Health . Australian Government. Canberra: Australian Government; 2014. [2016-06-08]. Make your move ? Sit less. Be active for life! http://www.health.gov.au/internet/main/publishing.nsf/Content/health-pubhlth-strateg-phys-act-guidelines/$File/Brochures_PAG_5-12yrs.pdf . [Google Scholar]
- 24.Carver CS, Scheier MF. Control theory: a useful conceptual framework for personality-social, clinical, and health psychology. Psychol Bull. 1982 Jul;92(1):111–35. [PubMed] [Google Scholar]
- 25.Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. Am Psychol. 1992 Sep;47(9):1102–14. doi: 10.1037//0003-066x.47.9.1102. [DOI] [PubMed] [Google Scholar]
- 26.Partridge SR, McGeechan K, Hebden L, Balestracci K, Wong AT, Denney-Wilson E, Harris MF, Phongsavan P, Bauman A, Allman-Farinelli M. Effectiveness of a mHealth Lifestyle Program With Telephone Support (TXT2BFiT) to Prevent Unhealthy Weight Gain in Young Adults: Randomized Controlled Trial. JMIR Mhealth Uhealth. 2015;3(2):e66. doi: 10.2196/mhealth.4530. http://mhealth.jmir.org/2015/2/e66/ v3i2e66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organisation. [2016-03-16]. https://www.psykiatri-regionh.dk/who-5/Pages/default.aspx .
- 28.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003 Aug;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 29.White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ. 2011;342:d40. doi: 10.1136/bmj.d40. http://europepmc.org/abstract/MED/21300711 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Partridge SR, Allman-Farinelli M, McGeechan K, Balestracci K, Wong Annette T Y. Hebden L, Harris MF, Bauman A, Phongsavan P. Process evaluation of TXT2BFiT: a multi-component mHealth randomised controlled trial to prevent weight gain in young adults. Int J Behav Nutr Phys Act. 2016;13(1):7. doi: 10.1186/s12966-016-0329-2. http://ijbnpa.biomedcentral.com/articles/10.1186/s12966-016-0329-2 .10.1186/s12966-016-0329-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartley L, Igbinedion E, Thorogood M, Clarke A, Stranges S, Hooper L, Rees K. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst Rev. 2012;2012(5):CD009874. doi: 10.1002/14651858.CD009874. http://europepmc.org/abstract/MED/25267919 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu D, Huang J, Wang Y, Zhang D, Qu Y. Fruits and vegetables consumption and risk of stroke: a meta-analysis of prospective cohort studies. Stroke. 2014 Jun;45(6):1613–9. doi: 10.1161/STROKEAHA.114.004836. http://stroke.ahajournals.org/cgi/pmidlookup?view=long&pmid=24811336 .STROKEAHA.114.004836 [DOI] [PubMed] [Google Scholar]
- 33.Bradbury KE, Appleby PN, Key TJ. Fruit, vegetable, and fiber intake in relation to cancer risk: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC) Am J Clin Nutr. 2014 Jul;100 Suppl 1:394S–8S. doi: 10.3945/ajcn.113.071357. http://www.ajcn.org/cgi/pmidlookup?view=long&pmid=24920034 .ajcn.113.071357 [DOI] [PubMed] [Google Scholar]
- 34.Imamura F, O'Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SN, Forouhi NG. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576. doi: 10.1136/bmj.h3576. http://www.bmj.com/cgi/pmidlookup?view=long&pmid=26199070 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith C, Gray AR, Fleming EA, Parnell WR. Characteristics of fast-food/takeaway-food and restaurant/café-food consumers among New Zealand adults. Public Health Nutr. 2014 Oct;17(10):2368–77. doi: 10.1017/S1368980013002681.S1368980013002681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith KJ, Blizzard L, McNaughton SA, Gall SL, Dwyer T, Venn AJ. Takeaway food consumption and cardio-metabolic risk factors in young adults. Eur J Clin Nutr. 2012 May;66(5):577–84. doi: 10.1038/ejcn.2011.202.ejcn2011202 [DOI] [PubMed] [Google Scholar]
- 37.Cook AS, O'Leary F, Chey T, Bauman A, Allman-Farinelli M. Prevalence of and intention to change dietary and physical activity health risk behaviours. Appetite. 2013 Dec;71:150–7. doi: 10.1016/j.appet.2013.07.016.S0195-6663(13)00364-4 [DOI] [PubMed] [Google Scholar]
- 38.Svetkey LP, Batch BC, Lin P, Intille SS, Corsino L, Tyson CC, Bosworth HB, Grambow SC, Voils C, Loria C, Gallis JA, Schwager J, Bennett GB. Cell phone intervention for you (CITY): A randomized, controlled trial of behavioral weight loss intervention for young adults using mobile technology. Obesity (Silver Spring) 2015 Nov;23(11):2133–41. doi: 10.1002/oby.21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hebden L, Cook A, van der Ploeg H P. King L, Bauman A, Allman-Farinelli M. A mobile health intervention for weight management among young adults: a pilot randomised controlled trial. J Hum Nutr Diet. 2014 Aug;27(4):322–32. doi: 10.1111/jhn.12155. [DOI] [PubMed] [Google Scholar]
- 40.Kattelmann KK, Bredbenner CB, White AA, Greene GW, Hoerr SL, Kidd T, Colby S, Horacek TM, Phillips BW, Koenings MM, Brown ON, Olfert MD, Shelnutt KP, Morrell JS. The effects of Young Adults Eating and Active for Health (YEAH): a theory-based Web-delivered intervention. J Nutr Educ Behav. 2014;46(6):S27–41. doi: 10.1016/j.jneb.2014.08.007.S1499-4046(14)00637-X [DOI] [PubMed] [Google Scholar]
- 41.Schweitzer AL, Ross JT, Klein CJ, Lei KY, Mackey ER. An Electronic Wellness Program to Improve Diet and Exercise in College Students: A Pilot Study. JMIR Res Protoc. 2016;5(1):e29. doi: 10.2196/resprot.4855. http://www.researchprotocols.org/2016/1/e29/ v5i1e29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park A, Nitzke S, Kritsch K, Kattelmann K, White A, Boeckner L, Lohse B, Hoerr S, Greene G, Zhang Z. Internet-based interventions have potential to affect short-term mediators and indicators of dietary behavior of young adults. J Nutr Educ Behav. 2008;40(5):288–97. doi: 10.1016/j.jneb.2008.02.001.S1499-4046(08)00025-0 [DOI] [PubMed] [Google Scholar]
- 43.Gow RW, Trace SE, Mazzeo SE. Preventing weight gain in first year college students: an online intervention to prevent the “freshman fifteen”. Eat Behav. 2010 Jan;11(1):33–9. doi: 10.1016/j.eatbeh.2009.08.005. http://europepmc.org/abstract/MED/19962118 .S1471-0153(09)00086-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bertz F, Pacanowski CR, Levitsky DA. Frequent Self-Weighing with Electronic Graphic Feedback to Prevent Age-Related Weight Gain in Young Adults. Obesity (Silver Spring) 2015 Oct;23(10):2009–14. doi: 10.1002/oby.21211. http://europepmc.org/abstract/MED/26414563 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health. 2011 Nov;26(11):1479–98. doi: 10.1080/08870446.2010.540664.938640058 [DOI] [PubMed] [Google Scholar]
- 46.Nikolaou CK, Hankey CR, Lean Michael Ernest John Elearning approaches to prevent weight gain in young adults: A randomized controlled study. Obesity (Silver Spring) 2015 Dec;23(12):2377–84. doi: 10.1002/oby.21237. [DOI] [PubMed] [Google Scholar]
- 47.MacLean PS, Wing RR, Davidson T, Epstein L, Goodpaster B, Hall KD, Levin BE, Perri MG, Rolls BJ, Rosenbaum M, Rothman AJ, Ryan D. NIH working group report: Innovative research to improve maintenance of weight loss. Obesity (Silver Spring) 2015 Jan;23(1):7–15. doi: 10.1002/oby.20967. doi: 10.1002/oby.20967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fjeldsoe B, Neuhaus M, Winkler E, Eakin E. Systematic review of maintenance of behavior change following physical activity and dietary interventions. Health Psychol. 2011 Jan;30(1):99–109. doi: 10.1037/a0021974.2011-02060-010 [DOI] [PubMed] [Google Scholar]
- 49.Flores MG, Granado-Font E, Ferré-Grau C, Montaña-Carreras X. Mobile Phone Apps to Promote Weight Loss and Increase Physical Activity: A Systematic Review and Meta-Analysis. J Med Internet Res. 2015;17(11):e253. doi: 10.2196/jmir.4836. http://www.jmir.org/2015/11/e253/ v17i11e253 [DOI] [PMC free article] [PubMed] [Google Scholar]


