Abstract
The prognosis of the primary refractory anaplastic lymphoma kinase (ALK+) anaplastic T large cell lymphoma is ominous. The identification of molecular targets with potential to drive oncogenesis remains a cornerstone for the designing of new selective cancer therapies. Crizotinib is a selective ATP-competitive inhibitor for ALK, approved for its use in lung cancer with rearrangements on ALK gene. The reported cases describe the use of crizotinib as a bridging strategy prior to allotransplantation; there are no reported prolonged survivals under monotherapy with Crizotinib. We report a case of a primary refractory ALK+ anaplastic large-cell lymphoma that sustains complete response after 3 years of crizotinib monotherapy.
Key words: Lymphoma, large-cell, anaplastic, anaplastic lymphoma kinase, crizotinib
Introduction
Anaplastic lymphoma kinase (ALK) was initially discovered in anaplastic large-cell lymphoma (ALCL) as component of fusion protein NPM-ALK formed as a result of the t(2;5)(p23;q35) chromosomal translocation.1,2 The continuous identification of molecular targets with potential to drive oncogenesis remains a cornerstone for the designing of new selective cancer therapies. Crizotinib is a selective ATP-competitive inhibitor for ALK, approved for its use in lung cancer harboring rearrangements on ALK gene.3
Overall, the prognosis of ALK+ ALCL is remarkably better than that of the other T-cell lymphomas. However, the primary refractory presentation has an ominous prognosis.4
There are some case reports and phase I/II studies reporting remissions with crizotinib among patients with relapsed/refractory ALK+ ALCL,2,5,6 but the results of the trial NCT00939770 have not been published yet.
Most of the case reports published so far describe the use of crizotinib as a bridging strategy prior to allogeneic transplantation7 and there are no reported prolonged survivals under monotherapy with Crizotinib.8 We report a case of a primary refractory ALK+ ALCL that sustains complete response (CR) after 3 years of crizotinib monotherapy.
Case Report
A 16-year-old woman, previously healthy, was diagnosed with an ALK+ ALCL, Ann Arbor IVaE (skin and bone marrow involvement), IPI 2. Fluorescence in situ hybridization (FISH) assessment of the bone marrow was positive for t(2;5). She received first line treatment with CHOEP 21 for 6 courses, showing a CR on computed tomography (CT) scan after the third course. The skin was clear after the fourth chemotherapy cycle. After 4 weeks of the last CHOEP she developed a laterocervical dermic-subdermic lesion and only hypermetabolic lesion on PET-CT. Its biopsy confirmed ALK+ ALCL. She received salvage combination chemotherapy according to etoposide, methylprednisolone, cytarabine and cisplatin (ESHAP) protocol for 2 cycles, she was collected and autotransplanted in CR, 7 months after diagnosis. On day 75 after autologous transplantation she showed signs of disease progression [poliadenopathies, B Symphtoms, increasing lactate dehydrogenase (LDH) levels; Ann Arbor IVbE, IPI3; PS=0] (Figure 1). As compassionate treatment, she started Crizotinib 250 mg every 12 h without steroids or other antineoplastic drug. The clinical response was impressive: on day 7 there were no palpable adenopathies; on day 14 there were no skin lesions and the patient was in clinical CR. The only identified adverse event was diarrhea (3-4 depositions/day, controlled with medical treatment without limiting her daily activities). On day 30 a positron emission tomography-CT (PET-CT) was performed (Figure 1). The diffuse hypermetabolic compromise of ascendant and transverse colon was described as inflammatory, in correlation with the AE described. The diarrhea disappeared progressively and on the PET-CT performed on month 5 of treatment the hypermetabolic colonic compromise was not evident anymore. Nowadays, she is still in CR after 36 months of crizotinib, without registered AE. All laboratory results performed since month 2 of treatment are within normal range and she is leading a fully normal life.
Figure 1.
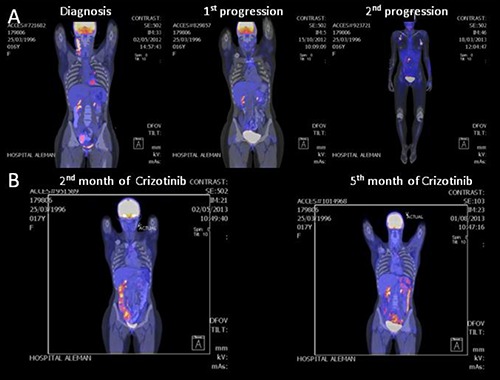
Images before and after crizotinib therapy in a 16-year-old woman with ALK-positive anaplastic large-cell lymphoma. Positron emission tomography-computed tomography (PET-CT) images obtained at diagnosis, 1st and 2nd progression (A) and PET-CT images obtained at 2nd and 5th month of crizotinib treatment (B), showing the tumor regression associated with crizotinib.
Discussion
New agents like crizotinib or anti CD30 (brentuximab vedotin) are therapeutic options for relapsed/refractory ALCL that should be considered as bridging treatments before allogenic bone marrow transplantation.7,8 In this particular case, the patient had two potential donors for an haploidentical transplantation. The use of allogeneic stem cell transplantation in patients with relapsed/refractory ALK+ ALCL is mostly supported by a few small retrospective studies in patients with various peripheral T cell-lymphomas (PTCL) subtype categories, including rare cases of ALK+ ALCL. Conclusions are sometimes limited by the fact that authors describe results in ALCL patients whose ALK status is not reported. A French study retrospectively evaluated the role of allogeneic transplant typically in relapsed or refractory patients, which included 27 patients (median age 12-55 years) with ALCL.9 Information on ALK status was available in 13 patients and 8 were ALK+. The 5 year EFS and OS for ALCL patients was 58 and 55% respectively, which was comparable to the other PTCL subtypes. On chemotherapy-resistant patients the 5-year OS was 29% for the whole group. Chemoresistant disease at the time of transplant and the occurrence of severe grade 3-4 acute graft-versus-host disease were the strongest adverse prognostic factors for OS, while an HLA-mismatched donor increased treatment-related mortality.9 Considering the low-level evidence, the patient’s age and the spectacular, sustained response without severe adverse events, the decision was to continue with crizotinib. At that moment, the treatment purposed in case of a new progression was to intend a new salvage combination chemotherapy regimen plus brentuximab vetotin and proceed with the allotransplantation. Since 2009, two clinical trials have been developed to clarify the role of crizotinib for the treatment of ALCL. The first phase I/II trial was designed for patients with relapsed or refractory solid tumors or ALCL and the estimated primary completion date is May 2017 (NCT00939770). The second trial is a phase IV, multicenter prospective study of treatment ALK (+) systemic ALCL with Crizotinib (NCT02487316) with an estimated primary completion date in December 2017. In 2014 Gambacorti et al. reported 11 ALK+ lymphoma patients who were resistant/refractory to cytotoxic therapy and were treated with Crizotinib as monotherapy. The overall response rate was 90.9% and the overall and progression-free survival rates at 2 years were 72.7 and 63.7%, respectively. They concluded that Crizotinib exerted a potent antitumor activity with durable responses in advanced, heavily pretreated ALK+ lymphoma patients with a benign safety profile.10
The reported patient was heavily treated and only achieved a complete and sustained response with crizotinib. She now leads a fully normal life. With this particular case report we want to emphasize the challenging role of new targeted therapies. Hopefully, the on-going trials are going to answer relevant questions like what the potential utility of this agent as first line treatment is, what the optimal treatment duration is, and, consequently, what the optimal moment for allotransplantation would be, considering the morbidity and mortality associated to this procedure. However, precisely because of the lack of evidence regarding the optimal ALK- inhibition treatment duration and its long term efficacy in controlling the lymphoma and, moreover the poor reported survival after allogenic bone marrow transplantation in the relapsed/refractory scenario, a potential alternative to this procedure in patients with complete response to ALK inhibition might even be indefinite treatment with ALK inhibitors until relapse/progression. In fact, this is the approach we decided for this patient.
Conclusions
New targeted therapies might represent more effective strategies than the conventional polichemotherapy protocols, since the former might have a significantly less toxic profile. Even heavily treated patients respond and sustain prolonged remissions with this drugs used as monotherapy.
Acknowledgments
Authors would thank Dr. Juan Cicco for his help in proof reading the article. We thank Dr. Hernán Pringe for providing language help.
References
- 1.Zdzalik D, Dymek B, Grygielewicz P, et al. Activating mutations in ALK kinase domain confer resistance to structurally unrelated ALK inhibitors in NPM-ALK-positive anaplastic large-cell lymphoma. J Cancer Res Clin Oncol 2014;140:589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foyil KV, Bartlett NL. Brentuximab vedotin and crizotinib in anaplastic large-cell lymphoma. Cancer J 2012;18:450-6. [DOI] [PubMed] [Google Scholar]
- 3.Murga-Zamalloa C, Lim MS. ALK-driven tumors and targeted therapy: focus on crizotinib. Pharmgenomics Pers Med 2014;7:87-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreri AJ, Govi S, Pileri SA, Savage KJ. Anaplastic large cell lymphoma, ALK-positive. Crit Rev Oncol Hematol 2012;83:293-302. [DOI] [PubMed] [Google Scholar]
- 5.Mosse YP, Lim MS, Voss SD, et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a children’s oncology group phase 1 consortium study. Lancet Oncol 2013;14:472-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosse Y, Balis FM, Kim MS. Efficacy of crizotinib in children with relapsed/refractory ALD-driven tumors including anaplastic large cell lymphoma and neuroblastoma: a children’s oncology group phase I consortium study. Journal of Clinical Oncology 2012;30.23169502 [Google Scholar]
- 7.Ordemann R, Stohlmacher J, Beuthien-Baumann B, et al. Use of targeted therapy for refractory ALK-positive anaplastic large cell lymphoma as a bridging strategy prior to allogeneic transplantation. Ann Hematol 2013;92:125-7. [DOI] [PubMed] [Google Scholar]
- 8.Gambacorti-Passerini C, Messa C, Pogliani EM. Crizotinib in anaplastic large-cell lymphoma. N Engl J Med 2011; 364:775-6. [DOI] [PubMed] [Google Scholar]
- 9.Le Gouill SMN, Buzyn A. Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. J Clin Oncol 2004;26: 2264-71. [DOI] [PubMed] [Google Scholar]
- 10.Gambacorti Passerini C, Farina F, Stasia A, et al. Crizotinib in advanced, chemoresistant anaplastic lymphoma kinase-positive lymphoma patients. J Natl Cancer Inst 2014;106:djt378. [DOI] [PubMed] [Google Scholar]


