Abstract
Dimethyl fumarate (DMF) is one of the newer additions to the armamentarium of potent immunomodulators for the treatment of relapsing-remitting multiple sclerosis (RRMS). After more than 2 years of real-world experience and more than 190,000 patients currently treated with DMF worldwide, it is a good timepoint to review the experience gathered so far and to re-evaluate the potential of this first-line oral multiple sclerosis (MS) drug. Post-hoc analyses of clinical and magnetic resonance imaging (MRI) data, some comprising more than 6 years of drug exposure including patients from the clinical trials, and the overall notion in clinical practice widely confirm the good efficacy of DMF in RRMS. Despite an overall good safety profile, it became also clear that the necessary clinical vigilance while using DMF may not be neglected. So far, four reported cases of progressive multifocal leukoencephalopathy (PML), a towering shadow over many MS therapies, warrant proper attention in newly-updated risk management plans. This review recapitulates efficacy and safety aspects of DMF therapy in relation to reported data from the pivotal clinical trials. In addition, we summarize recent insights into DMF mechanisms of action drawn from the field of basic research which may have important implications for clinical practice.
Keywords: clinical practice, dimethyl fumarate, multiple sclerosis
History of fumarates in medicine
Dimethyl fumarate (DMF) is derived from the simple organic acid fumaric acid which is named after the earth smoke plant (Fumaria officinalis). In the late 1950s, fumaric acid derivates were first used for the treatment of psoriasis based on the erroneous assumption that the disease may be caused by a metabolic deficiency in the citric acid cycle and that exogenous repletion may restore the balance in the Krebs cycle, thus leading to beneficial effects on the disease [Schweckendiek, 1959].
While free fumaric acid is poorly absorbed by the gastrointestinal (GI) tract, its ester derivatives, namely monomethyl fumarate (MMF) and DMF proved to be beneficial in treating psoriasis, first administered as a topical ointment and, later, also as an oral formulation [Altmeyer et al. 1996]. Since the mid-1990s, a combination of ethylhydrogen fumarates and DMF has been licensed in Germany under the brand name Fumaderm® with DMF constituting approximately 60% of the fumaric acid mixture. This medication was proven to be clinically effective in the treatment of moderate to severe forms of psoriasis in large clinical trials and nowadays is one of the most widely used oral compounds for psoriasis therapy in Germany. Ultimately, DMF was found to be a major effective principle in the preparation. As the immunopathology of psoriasis was unveiled, first dermatologic in vitro and ex vivo studies rapidly pointed at the immunomodulatory properties of DMF. Fostered by the well-described safety profile of fumaric acid esters in psoriasis [Reich et al. 2009], the immunomodulatory potential of Fumaderm® and DMF was also explored in other immune-mediated diseases [Meissner et al. 2012] which ultimately led to rigorous testing of DMF in large multi-center phase II and III studies of relapsing-remitting multiple sclerosis (RRMS) [Fox et al. 2012; Gold et al. 2012; Kappos et al. 2008].
Dimethyl fumarate: pharmacokinetic data
Experimental studies revealed that abundant esterases in the GI tract rapidly metabolize orally-ingested DMF into its primary, active metabolite MMF [Werdenberg et al. 2003]. Systemic MMF concentrations in the circulation peak between 2–2.5 hours after ingestion, with the area under the curve being proportional to the applied dosages. In clinical practice, systemic MMF peaks may be delayed for several hours if DMF is ingested with high calorific, fat-rich meals without affecting the area under the curve. Importantly, such dietary procedures may decrease the side effects related to the metabolism of the medication. In contrast, orally ingested DMF is not readily found in the systemic circulation and less than 0.1% of its initial dosage can be detected in the urine [Litjens et al. 2004a]. After ascending doses up to 240 mg delayed release DMF, the mean Cmax of MMF in healthy human subjects was 1.43 μg/ml with a corresponding MMF area under the curve of 2.41 μg h/ml. There was no evidence of accumulation after multiple doses (e.g. 240 mg delayed-release DMF three times daily for 2 days) with MMF concentrations below detectable limits at the end of day1 and day 2 (for an overview of the pharmacokinetics see Figure 1 [Sheikh et al. 2013]).
Figure 1.
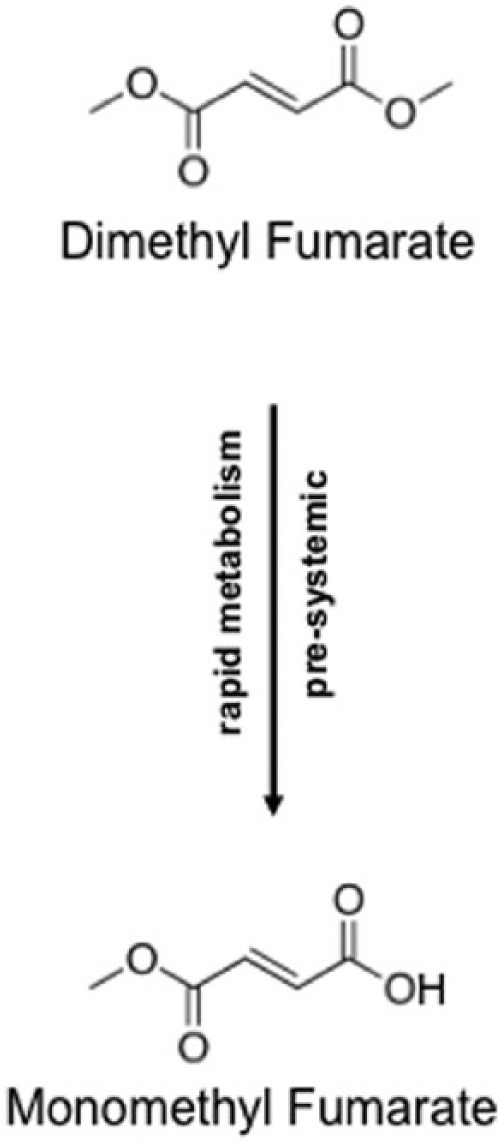
Pharmacokinetics of DMF. Pre-systemically, DMF is rapidly metabolized to MMF.
DMF, dimethyl fumarate; MMF, monomethyl fumarate.
For optimized pharmacokinetics with predominant release in the small intestine, DMF was formulated as an oral delayed release preparation which was initially named BG12 as the study compound. Given its pharmacokinetic profile, the compounds needs application every 12 hours (i.e. twice daily) with a dosage of 2 × 240 mg used in clinical practice. Lower dosages and application frequencies were initially tested, but did not yield sufficient effects [Kappos et al. 2008].
Immunomodulatory mechanisms of action: relevance for clinical practice
A plethora of scientific studies are still underway aimed at clarifying the ultimate mechanism of DMF action in multiple sclerosis (MS). Similar to other first-line immunomodulators, the bottom line message so far is that DMF may not only exert a single mechanism of action but is rather characterized by pleiotropic biological effects. The first in vitro studies with nonphysiological, high concentrations of fumaric acid esters pointed at proapoptotic effects on T-cells [Treumer et al. 2003]; a mechanism which is unlikely to be the main mechanism operative in vivo. Further experiments with cultured peripheral mononuclear blood cells disclosed anti-inflammatory properties of DMF with a dose-dependent ability to promote so called T-helper cell type 2 (Th2) immune responses characterized by the production of interleukin-(IL)4, or IL-5 [de Jong et al. 1996]. This Th2 shift was later linked to direct effects of fumaric acid esters on dendritic cells [Litjens et al. 2004b]. These data rank DMF as a clear immunomodulator with the ability to promote a Th1 to Th2 shift as one likely mechanism for its clinical effects, such as on contrast-enhancing lesions in magnetic resonance imaging (MRI). In contrast, a modulation of the anatomical integrity of the blood brain barrier does not seem to be a major mechanism of action of fumarates in neuroinflammation [Benardais et al. 2013].
More recent in vivo and in vitro studies further elaborated on the effect of DMF on dendritic cells. Showing a prevailing effect on Th2-cell-promoting, so called type II, dendritic cells, these studies provide further insights on the cascades of cellular events that may follow exposure to DMF [Ghoreschi et al. 2011]. Beyond its effects on T-cells and dendritic cells, DMF may also target several other immunologically active cell types, namely neutrophils, macrophages, monocytes, endothelial cells or keratinocytes [Asadullah et al. 1997; Loewe et al. 2002; Nibbering et al. 1993; Ockenfels et al. 1998; Schilling et al. 2006; Sebok et al. 1998; Stoof et al. 2001].
In human ex vivo studies with lower numbers of MS patients, recent analyses focused on immunophenotyping of peripheral blood mononuclear cells under DMF therapy and proved prevailing effects on CD8-positive T-cells and also regulatory T-cells independent from lymphopenia [Berkovich and Weiner, 2015; Gross et al. 2016; Longbrake et al. 2015b].
Further effects of dimethyl fumarate: cytoprotection and glial cells
On a molecular level, fumaric acid esters were shown to target the naturally-occurring cellular antioxidative stress response. In particular, MMF directly binds to and modifies the Kelch-like erythroid cell-derived (ECH) associated protein-1 (KEAP-1) which acts an inhibitor of the transcription factor nuclear factor (erythroid derived 2)-like2 (Nrf2). Nrf2 is a major transcription factor responsible for governing the expression of genes involved in antioxidative cellular responses and in cyto- or neuroprotection (Johnson et al. 2008). DMF-mediated modification of KEAP-1 enables the nuclear translocation of Nrf2 which ultimately leads to transcription of antioxidative target genes such as hemoxygenase-1 (HO1), nicotinamide adenine dinucleotide phosphate (NADPH), quinoline oxidoreductase-1 (NQO1) and others [Linker et al. 2011; Scannevin et al. 2012]. In concert, these factors may lead to cytoprotective effects in neurons and glial cells, but also exert immunomodulatory actions.
While this paradigm was first proven in cell culture models and animal studies, a neuropathological case report further supports this concept showing that glial cells indeed express Nrf2 after fumarate treatment in patient suffering from MS and psoriasis with progressive multifocal leukoencephalopathy (PML) [Metz et al. 2015]. This is of particular interest since further neuropathological analyses clearly revealed that especially damaged oligodendrocytes may show Nrf2 expression near demyelinated MS lesions [Licht-Mayer et al. 2015].
Beyond targeting the Nrf2 pathway, DMF and, even more so, MMF were described as agonists of the hydroxycarboxylic acid receptor 2 (HCAR2), a G protein-coupled membrane receptor. Recently, it was shown in a mouse model that this pathway may also play a role in the immunomodulatory properties of DMF in vivo (for an overview of the putative mechanisms of action see Figure 2 [Chen et al. 2014]). Targeting HCAR2 signaling pathways by fumarates may indeed modulate microglial activation and subsequently target synaptic dysregulation in the inflamed central nervous system [Parodi et al. 2015].
Figure 2.
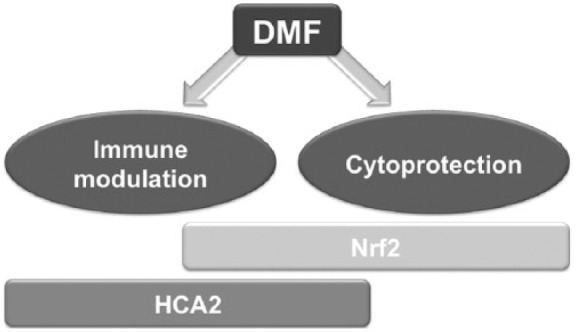
Presumed mechanisms of DMF action in MS.
DMF may exert immunomodulatory as well as cytoprotective effects via activation of Nrf2 mediated or HCAR2-mediated signalling pathways.
DMF, dimethyl fumarate; HCAR2, hydroxycarboxylic acid receptor 2; MS, multiple sclerosis; Nrf2, nuclear factor (erythroid derived 2)-like2 transcription factor.
To date, the relation of HCAR2 to Nrf2-mediated DMF effects is still unclear and await further analysis. Interestingly, HCAR2- mediated pathways were also described as involved in niacin-mediated flushing, the latter also being a typical side effect of fumarates.
In summary, the exact interaction between DMF, its putative membrane, or intracellular receptor(s), and the respective downstream targets still remain to be fully elucidated, especially in MS patients [Kieseier and Wiendl, 2015]. Yet, many data point at the relevant biological effects of DMF on the Nrf2 pathway including immunoregulatory effects, but also the potential as a cytoprotective compound. This potential has clearly been shown in cell culture and also several animal models of different forms of encephalomyelitis (including Theiler virus and experimental autoimmune encephalomyelitis), neuritis, colitis, and neurodegeneration (e.g. in models of Huntington’s disease), but also cardioprotection, brain edema in stroke, and intracerebral, as well as subarachnoid hemorrhage, by different research groups [Ashrafian et al. 2012; Ellrichmann et al. 2011; Iniaghe et al. 2015; Kobayashi et al. 2015; Kunze et al. 2015; Pitarokoili et al. 2015].
Dimethyl fumarate for the treatment of multiple sclerosis: efficacy data
In recent years, due to major research efforts in the field of clinical and experimental neuroimmunology, there was rapid progress in the treatment of RRMS, leading to over 10 approved drugs and more expected to be available soon.
When entering the field of neurology, fumarates were first tested in a small group of RRMS patients [Schimrigk et al. 2006], and then delayed-release DMF swiftly moved to clinical trials [Kappos et al. 2008].
The results of two large phase III trials testing DMF in RRMS including >2600 RRMS patients over more than 2 years (DEFINE and CONFIRM studies) warranted its rapid regulatory approval, first by the US Food and Drug Administration (FDA) in 2013 and then by the European Medicines Agency (EMA) in spring 2014 [Fox et al. 2012; Gold et al. 2012]. Due to diverse study populations, the two studies slightly differed on the exact doses and responses, however, annual relapse rates were significantly reduced by up to 53% overall and mean numbers of gadolinium (Gd)-enhancing MRI lesions by up to 90% (for details see Table 1). In the CONFIRM trial, DMF prevented at least 20% more relapses than glatiramer acetate although the study was not powered for a head-to-head comparison. Moreover, DMF met all its secondary MRI endpoints including a reduction in T2-weighted lesions and also the tertiary endpoint hypointense T1-weighted lesions (in the DEFINE trial) as indirect indicators of irreversible neuronal tissue damage [Arnold et al. 2014a].
Table 1.
Table summarizing clinical trial data on delayed-release dimethyl fumarate.
| Patient numbers/randomization | Outcome measures |
|||
|---|---|---|---|---|
| MRI (T2-, T1- and Gd-enhancing lesions) | Clinical (ARR, disease progression, freedom of disease) | Adverse events | ||
|
DMF 480 mg, 720 mg
(BG-12®, BiogenIdec, Cambridge, MS, USA) |
1237 RRMS BG-12 480 mg versus 720 mg daily versus placebo 1 : 1 : 1 (DEFINE study) |
90% (480 mg) and 73% (720 mg) fewer Gd-lesions under BG-12 versus placebo (p < 0.0001); 85% (480 mg) and 74% (720 mg) fewer new or enlarged T2-lesions under BG-12 versus placebo (p < 0.0001) | 53% ARR reduction under BG-12 (480 mg) and 48% (720 mg) versus placebo (p < 0.0001); ~40% less cumulative risk of disability progression under BG-12 doses versus placebo (p < 0.0001) | GI side effects, such as mild and moderate diarrhea, flushing, lymphopenia |
| 1430 RRMS BG-12 480 mg versus 720 mg daily versus GLAT s.c. 20 mg daily versus placebo 1 : 1 : 1 : 1 (CONFIRM study) |
71% (480 mg) and 73% (720 mg) fewer new or enlarged T2-lesions under BG-12 (p < 0.0001) and 54% by GLAT versus placebo (p < 0.0001); 57% (480 mg) and 65% (720 mg) fewer T1-hypointense lesions under BG-12 (p < 0.0001) and 41% by GLAT versus placebo (p < 0.003) | 44% ARR reduction under BG-12 (480 mg) and 51% (720 mg) (p < 0.0001) and 29% GLAT (p = 0.02) versus placebo; reduced risk of disability progression by 21% (480 mg) and 24% (720 mg) under BG-12 (not significant) and 7% by GLAT (not significant) versus placebo |
As for DEFINE study | |
ARR, annualized relapse rate; DMF, dimethylfumarate; Gd, gadolinium; GI, gastrointestinal; GLAT, galtiramer acetate; MRI, magnetic resonance imaging; RRMS, relapsing-remitting multiple sclerosis; s.c., subcutaneously.
Several post-hoc analyses of the pivotal studies as well as studies on real-world data further confirmed existing insights on the efficacy and safety and added new outcome clinical and MRI measures. These reports included subgroup analyses of the pivotal trials, kinetic studies on the rapid onset of efficacy, and data on hospitalizations, quality of life, as well as pharmacoeconomic outcomes [Bar-Or et al. 2013; Giovannoni et al. 2015; Hutchinson et al. 2013; Kappos et al. 2015; Kita et al. 2014; Mauskopf et al. 2016]. Subgroup analyses further revealed that early use of DMF in newly-diagnosed cases of MS, which comprised about 500 patients in the verum-treated groups, was highly effective, leading up to ~50% reduction of the risk for 12-week confirmed disability progression in the group receiving the approved dosage of 2 × 240 mg DMF daily [Gold et al. 2015a].
Using extended MRI protocols/techniques for a subgroup of patients during the phase II/III clinical trials, including atrophy measures and magnetic transfer ratio (MTR) studies, an overall reduction of brain atrophy of >30% after 2 years of DMF compared with placebo was observed, further fuelling the notion that DMF may exert neuroprotective, and possibly also myelin-restoring effects beyond its immunomodulatory mechanisms [Arnold et al. 2014b; MacManus et al. 2011].
Recently, efficacy data from the pivotal trials with DMF were also analyzed in a Cochrane report. This overview concluded that there is moderate-quality evidence to support that DMF reduces both, the number of patients with a relapse, and the annualized relapse rate over two years of treatment in comparison with placebo [Xu et al. 2015].
Safety and tolerability issues with dimethyl fumarate in clinical practice
All phase II/III clinical studies with DMF affirmed the prevailing transient character of the common adverse events under DMF (i.e. GI irritations and flushing which commonly occur in about 30% of treated patients for GI symptoms and up to 40% for flushing, respectively). However, in the vast majority of cases, these symptoms subside within the first 2–3 months of DMF use. Thereafter, less than 5% of the patients treated with DMF still expressed such complaints: in the pivotal trials, 3% of the patients discontinued DMF treatment due to GI side effects, and 2% due to continuous flushing [Phillips et al. 2015b].
In further studies on flushing in healthy volunteers, acetylic salicylic acid pretreatment (ASS) at 325 mg for 4 days reduced the incidence and intensity of flushing without affecting GI events or the Pharmacokinetic (PK) profile of the compound [O’Gorman et al. 2015]. Elevated levels of prostaglandin D2 in some DMF-treated individuals suggested that flushing may be, at least in part, prostaglandin mediated [Sheikh et al. 2013]. In countries where the FDA-approved dosage of 325 mg ASS is not available, lower dosages such as 200 mg ASS may be used. In these cases, patient education on well-known side effects of regular ASS intake is advised (e.g. peptic ulcers).
In a Delphi study among experts, consensus was reached on several strategies to manage GI adverse events [Phillips et al. 2015a], the most important interventions being, administering DMF with food, slow titration, transient dose reduction if needed, and use of symptomatic therapies including promethazine and ondansetron for nausea and vomiting, proton pump inhibitors, antacids and H2 blockers for abdominal pain and loperamide or probiotics for diarrhea [Phillips et al. 2014].
Apart from more frequent flushing, GI side effects and lymphocytopenia (see below) in the DMF-treated groups, no other significant adverse events were reported in the pivotal trials, rendering it a relatively well-tolerated drug. Nasopharyngitis, headache and fatigue were equally present in all treated or placebo groups. Notably, there were no reports of opportunistic infections in the pivotal studies.
Regarding outcome of pregnancies under DMF, data are still limited. Yet, animal studies did not show any evidence of impaired fertility or teratogenicity under treatment with DMF. All known DMF exposures in humans have occurred in the first trimester. So far, no increased risk of fetal abnormalities or adverse pregnancy outcomes associated with gestational exposure to DMF has been observed [Gold et al. 2015b].
Safety experience with dimethyl fumarate: special focus on lymphocytopenia and single cases of progressive multifocal leukoencephalopathy
Summarizing the results from the approval clinical trials and the clinical experience thereafter, the data reveal that DMF is an oral compound that can be generally prescribed without major safety concerns. This was also supported by the recent Cochrane review on DMF, reporting common adverse effects such as flushing and GI events as generally mild-to-moderate for most patients. Lymphopenia and leukopenia were reviewed in this report as uncommon adverse events but significantly associated with DMF [Xu et al. 2015]. While other transient hematologic abnormalities may occur after initiation of DMF treatment (e.g. eosinophilia), they are usually of subordinate clinical relevance.
This generally positive notion of the safety of DMF was, in a few cases, disturbed by the occurrence of PML in MS patients under DMF treatment, a feared side effect, which in recent years has been haunting several MS drugs in MS treatment (i.e. natalizumab and, to a lesser degree, fingolimod). As soon as the first PML cases were reported in lymphopenic psoriasis patients under Fumaderm®, neurologists were alerted. Yet, for some time, it seemed that PML may be confined to this compound only, which included a mixture of fumarate esters, among them ethyl hydrogen fumarate, which may harbour different pharmacokinetic and pharmacodynamic properties than the modern delayed-release DMF preparation (e.g. regarding accumulation in spleen, CNS or kidney, glutathione depletion, NF-κB inhibition or KEAP-1 modulation) [Brennan et al. 2015; Gillard et al. 2015].
It had been known among dermatologists that Fumaderm® may lead to lymphocytopenia in some cases [Hoxtermann et al. 1998], which, as a consequence, led to regular blood tests in psoriasis patients. Hence, after the first PML cases were reported with Fumaderm®, it was suggested to monitor lymphocyte counts on a regular basis and also in MS patients under DMF (Tecfidera®) [Longbrake and Cross, 2015]. This procedure led to consensus decisions in several countries. In Germany for instance, the competence network for MS (KKNMS) recommended blood tests every 6–8 weeks for the first year of therapy, despite the less stringent FDA and EMA guidelines. These measures were taken, since apart from the lymphocytopenia observed under DMF, which itself is a risk factor for opportunistic infections such as PML, no mechanistic link has been described so far for DMF-associated PML in MS patients. While a recent report described that DMF may inhibit expression of integrin α4 in circulating lymphocytes in in vitro and in vivo models independently from effects on Nrf2 activation (thus possibly providing a causal explanation for opportunistic infections), the functional relevance of these data is still under discussion and awaits further proof in MS patients [Haarmann et al. 2015; Kihara et al. 2015; Sebok et al. 1998].
However, after a case of PML in an MS patient under DMF (Tecfidera®) was reported [Rosenkranz et al. 2015], it became apparent that PML is not restricted to psoriasis patients and Fumaderm® or self-compounded DMF (which may significantly differ in pharmaceutical quality from Tecfidera®) and under which also, one case without detection of preceding severe lymphopenia was described [Nieuwkamp et al. 2015]. With four cases of PML reported so far under Tecfidera® in MS (www.EMA.europe.eu), PML is now considered as a very rare (incidence ~1:42.000), but serious side effect of DMF therapy in MS patients. In the meantime the EMA has also reviewed the risk–benefit situation of DMF and recently concluded that blood tests should be performed once every 3 months and therapy should be stopped if lymphocyte counts fall <500 per μl (WHO grade III lymphocytopenia) (www.EMA.europe.eu). From what we know so far from the post-hoc analyses of the clinical trials and incidental retrospective reports, about 30% of DMF-treated MS patients develop a WHO grade I (>800 lymphocytes per μl) or grade II (>500 lymphocytes per μl) lymphocytopenia, and only ~2% a grade III (<500 lymphocytes per μl) lymphocytopenia, usually occurring within the first 6 months of treatment, according to clinical trial data. Various case series from outpatient clinics report differing frequencies of grade III lymphocytopenia ranging approximately from 6–10% after 6 months of DMF therapy, suggesting that there is a positive correlation for the risk of developing lymphocytopenia with increasing age (>55 years), longer disease duration and low lymphocyte counts prior to DMF initiation [Longbrake et al. 2015a]. However, in order to gain more precise data on the dynamics of lymphocyte counts under DMF and the frequency of lymphocytopenia as the major risk factor for developing PML under DMF in a real-world setting, prospective monitoring studies with large cohorts are urgently needed. In the meantime, a higher degree of clinical vigilance and regular blood tests may minimize the risk of further PML cases under DMF treatment. Testing for anti-JCV antibody status has been proven worthwhile for risk management under therapy with natalizumab. Yet, it is currently not generally recommended under DMF therapy due to the lack of evidence for the mere presence of the anti-JCV antibody being associated with a higher risk of PML. Thus, persisting lymphopenia currently remains the only well-known risk factor for PML under DMF, considering the history of the four PML cases reported so far. Hence, the latest Committee for Medicinal Products for Human Use (CHMP) decision (www.EMA.europe.eu) includes testing for total number of lymphocytes every 3 months after starting DMF treatment. Considering stopping treatment is recommended if a grade 3 lymphopenia of <500/µl persists over 6 months (grade 0: upwards from 900/µl; grade 1: up to 800/µl; grade 2: less than 800/µl; grade 3: less than 500/µl; grade 4: less than 200/µl). Once the lymphocyte counts recover after DMF cessation, a re-initiation of DMF may be considered after evaluation of possible alternatives.
In case of switching to or from DMF, no study data or general consensus are available. Given the short half-life of DMF, switching from DMF seems safe if cytopenia has been ruled out. If switching to DMF, the effects of the respective preceding compound on the immune system should have subsided. According to the recommendation of the EMA, DMF therapy may be started immediately after cessation of interferon-beta or glatiramer acetate treatment (www.ema.europa.eu).
Clinical experience on neuroprotection and outlook for the future
More than 2 years into the real-world use of DMF, it seems that overall, the efficacy of DMF as a first-line treatment option for RRMS is, to state the least, comparable to the so-called ‘injectables’ that have been long employed in MS therapy [Hutchinson et al. 2014]. Moreover, as first suspected from clinical/MRI findings [MacManus et al. 2011], as well as supported by experimental setups [Linker et al. 2011], there is some available evidence from studies in the phase III trials implying that DMF may also have neuroprotective features in humans [Arnold et al. 2014b]. Yet, this concept still awaits further insights. While experimental data well agree with results from clinical trials, especially secondary MRI endpoints, a clinician will demand further validation in follow-up studies with newer MRI technologies and also in progressive forms of MS. In this context, a recent open-label retrospective case series, including 26 MS patients with a secondary progressive course, suggested an overall stabilizing effect on disease progression over a time course of up to 36 months [Strassburger-Krogias et al. 2014]. It may thus be desirable to further pursue the efficacy of DMF also in progressive MS courses and to encourage the resumption of currently-terminated clinical trials in this indication (INSPIRE study, see https://clinicaltrials.gov/ct2/show/NCT02430532).
This may be of particular interest in combination therapy studies which were already tested in proof-of concept studies in animal models (e.g. with beta interferons or glatiramer acetate as safe combination compounds) [Reick et al. 2014]. While such studies (e.g. with sphingosine-1 phosphate receptor modulators and DMF) are already discussed in MS patients, the possibility of an increased PML risk in cases of combination therapies with DMF may lead to some reservations regarding the implementation of such approaches.
Conclusion
DMF is a modern oral immunomodulator for the treatment of RRMS. Over a short time period, a plethora of data on its putative mechanism of action, the efficacy and also its side-effect profile emerged which fit well into the already extensive real-world experience. With a broad efficacy, good safety and satisfying tolerability, the compound is a cornerstone of modern immunotherapy in RRMS. At present, rare cases of PML under therapy warrant vigilance and monitoring for lymphocytopenia, particularly in the first year of treatment.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors received honoraria or research funding as well as travel support from Biogen, the company marketing dimethyl fumarate for RRMS.
Contributor Information
Ralf A. Linker, Department of Neurology, Friedrich-Alexander-University Erlangen, Schwabachanlage 6, 91054 Erlangen, Germany.
Aiden Haghikia, Department of Neurology, Ruhr-University Bochum, St. Josef-Hospital Bochum, Bochum, Germany.
References
- Altmeyer P., Hartwig R., Matthes U. (1996) Efficacy and safety profile of fumaric acid esters in oral long-term therapy with severe treatment refractory psoriasis vulgaris. A study of 83 patients. Hautarzt 47: 190–196. [DOI] [PubMed] [Google Scholar]
- Arnold D., Gold R., Kappos L., Bar-Or A., Giovannoni G., Selmaj K., et al. (2014a) Effects of delayed-release dimethyl fumarate on MRI measures in the phase III DEFINE study. J Neurol 261: 1794–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold D., Gold R., Kappos L., Bar-Or A., Giovannoni G., Selmaj K., et al. (2014b) Magnetization transfer ratio in the delayed-release dimethyl fumarate DEFINE study. J Neurol 261: 2429–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadullah K., Schmid H., Friedrich M., Randow F., Volk H., Sterry W., et al. (1997) Influence of monomethylfumarate on monocytic cytokine formation – explanation for adverse and therapeutic effects in psoriasis? Arch Dermatol Res 289: 623–630. [DOI] [PubMed] [Google Scholar]
- Ashrafian H., Czibik G., Bellahcene M., Aksentijevic D., Smith A., Mitchell S., et al. (2012) Fumarate is cardioprotective via activation of the Nrf2 antioxidant pathway. Cell Metab 15: 361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or A., Gold R., Kappos L., Arnold D., Giovannoni G., Selmaj K., et al. (2013) Clinical efficacy of BG-12 (dimethyl fumarate) in patients with relapsing-remitting multiple sclerosis: subgroup analyses of the DEFINE study. J Neurol 260: 2297–2305. [DOI] [PubMed] [Google Scholar]
- Benardais K., Pul R., Singh V., Skripuletz T., Lee D., Linker R., et al. (2013) Effects of fumaric acid esters on blood-brain barrier tight junction proteins. Neurosci Lett 555: 165–170. [DOI] [PubMed] [Google Scholar]
- Berkovich R., Weiner L. (2015) Effects of dimethyl fumarate on lymphocyte subsets. Mult Scler Relat Disord 4: 339–341. [DOI] [PubMed] [Google Scholar]
- Brennan M., Matos M., Li B., Hronowski X., Gao B., Juhasz P., et al. (2015) Dimethyl fumarate and monoethyl fumarate exhibit differential effects on KEAP1, NRF2 activation, and glutathione depletion in vitro. PLoS One 10: e0120254 DOI: 10.1371/journal.pone.0120254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Assmann J., Krenz A., Rahman M., Grimm M., Karsten C., et al. (2014) Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate’s protective effect in EAE. J Clin Invest 124: 2188–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong R., Bezemer A., Zomerdijk T., van de Pouw-Kraan T., Ottenhoff T., Nibbering P. (1996) Selective stimulation of T helper 2 cytokine responses by the anti-psoriasis agent monomethylfumarate. Eur J Immunol 26: 2067–2074. [DOI] [PubMed] [Google Scholar]
- Ellrichmann G., Petrasch-Parwez E., Lee D., Reick C., Arning L., Saft C., et al. (2011) Efficacy of fumaric acid esters in the R6/2 and YAC128 models of Huntington’s disease. PLoS One 6: e16172 DOI: 10.1371/journal.pone.0016172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R., Miller D., Phillips J., Hutchinson M., Havrdova E., Kita M., et al. (2012) Placebo-controlled phase III study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 367: 1087–1097. [DOI] [PubMed] [Google Scholar]
- Ghoreschi K., Bruck J., Kellerer C., Deng C., Peng H., Rothfuss O., et al. (2011) Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J Exp Med 208: 2291–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard G., Collette B., Anderson J., Chao J., Scannevin R., Huss D., et al. (2015) DMF, but not other fumarates, inhibits NF-kappaB activity in vitro in an Nrf2-independent manner. J Neuroimmunol 283: 74–85. [DOI] [PubMed] [Google Scholar]
- Giovannoni G., Gold R., Fox R., Kappos L., Kita M., Yang M., et al. (2015) Relapses requiring intravenous steroid use and multiple-sclerosis-related hospitalizations: integrated analysis of the delayed-release dimethyl fumarate phase III studies. Clin Ther 37: 2543–2551. [DOI] [PubMed] [Google Scholar]
- Gold R., Giovannoni G., Phillips J., Fox R., Zhang A., Meltzer L., et al. (2015a) Efficacy and safety of delayed-release dimethyl fumarate in patients newly diagnosed with relapsing-remitting multiple sclerosis (RRMS). Mult Scler 21: 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold R., Kappos L., Arnold D., Bar-Or A., Giovannoni G., Selmaj K., et al. (2012) Placebo-controlled phase III study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 367: 1098–1107. [DOI] [PubMed] [Google Scholar]
- Gold R., Phillips J., Havrdova E., Bar-Or A., Kappos L., Kim N., et al. (2015b) Delayed-release dimethyl fumarate and pregnancy: preclinical studies and pregnancy outcomes from clinical trials and postmarketing experience. Neurol Ther 4: 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C., Schulte-Mecklenbeck A., Klinsing S., Posevitz-Fejfar A., Wiendl H., Klotz L. (2016) Dimethyl fumarate treatment alters circulating T helper cell subsets in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 3: e183 DOI: 10.1212/NXI.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarmann A., Nehen M., Deiss A., Buttmann M. (2015) Fumaric acid esters do not reduce inflammatory NF-kappaB/p65 nuclear translocation, ICAM-1 expression and T-Cell adhesiveness of human brain microvascular endothelial cells. Int J Mol Sci 16: 19086–19095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxtermann S., Nuchel C., Altmeyer P. (1998) Fumaric acid esters suppress peripheral CD4- and CD8-positive lymphocytes in psoriasis. Dermatology 196: 223–230. [DOI] [PubMed] [Google Scholar]
- Hutchinson M., Fox R., Havrdova E., Kurukulasuriya N., Sarda S., Agarwal S., et al. (2014) Efficacy and safety of BG-12 (dimethyl fumarate) and other disease-modifying therapies for the treatment of relapsing-remitting multiple sclerosis: a systematic review and mixed treatment comparison. Curr Med Res Opin 30: 613–627. [DOI] [PubMed] [Google Scholar]
- Hutchinson M., Fox R., Miller D., Phillips J., Kita M., Havrdova E., et al. (2013) Clinical efficacy of BG-12 (dimethyl fumarate) in patients with relapsing-remitting multiple sclerosis: subgroup analyses of the CONFIRM study. J Neurol 260: 2286–2296. [DOI] [PubMed] [Google Scholar]
- Iniaghe L., Krafft P., Klebe D., Omogbai E., Zhang J., Tang J. (2015) Dimethyl fumarate confers neuroprotection by casein kinase 2 phosphorylation of Nrf2 in murine intracerebral hemorrhage. Neurobiol Dis 82: 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J., Johnson D., Kraft A., Calkins M., Jakel R., Vargas M., et al. (2008) The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann NY Acad Sci 1147: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos L., Giovannoni G., Gold R., Phillips J., Arnold D., Hotermans C., et al. (2015) Time course of clinical and neuroradiological effects of delayed-release dimethyl fumarate in multiple sclerosis. Eur J Neurol 22: 664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos L., Gold R., Miller D., MacManus D., Havrdova E., Limmroth V., et al. (2008) Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 372: 1463–1472. [DOI] [PubMed] [Google Scholar]
- Kieseier B., Wiendl H. (2015) Nrf2 and beyond: deciphering the mode of action of fumarates in the inflamed central nervous system. Acta Neuropathol 130: 297–298. [DOI] [PubMed] [Google Scholar]
- Kihara Y., Groves A., Rivera R., Chun J. (2015) Dimethyl fumarate inhibits integrin alpha4 expression in multiple sclerosis models. Ann Clin Transl Neurol 2: 978–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita M., Fox R., Gold R., Giovannoni G., Phillips J., Sarda S., et al. (2014) Effects of delayed-release dimethyl fumarate (DMF) on health-related quality of life in patients with relapsing-remitting multiple sclerosis: an integrated analysis of the phase III DEFINE and CONFIRM studies. Clin Ther 36: 1958–1971. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Tomiki H., Inaba Y., Ichikawa M., Kim B., Koh C. (2015) Dimethyl fumarate suppresses Theiler’s murine encephalomyelitis virus-induced demyelinating disease by modifying the Nrf2-Keap1 pathway. Int Immunol 27: 333–344. [DOI] [PubMed] [Google Scholar]
- Kunze R., Urrutia A., Hoffmann A., Liu H., Helluy X., Pham M., et al. (2015) Dimethyl fumarate attenuates cerebral edema formation by protecting the blood-brain barrier integrity. Exp Neurol 266: 99–111. [DOI] [PubMed] [Google Scholar]
- Licht-Mayer S., Wimmer I., Traffehn S., Metz I., Bruck W., Bauer J., et al. (2015) Cell type-specific Nrf2 expression in multiple sclerosis lesions. Acta Neuropathol 130: 263–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker R., Lee D., Ryan S., van Dam A., Conrad R., Bista P., et al. (2011) Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 134: 678–692. [DOI] [PubMed] [Google Scholar]
- Litjens N., Burggraaf J., van Strijen E., van Gulpen C., Mattie H., Schoemaker R., et al. (2004a) Pharmacokinetics of oral fumarates in healthy subjects. Br J Clin Pharmacol 58: 429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litjens N., Rademaker M., Ravensbergen B., Rea D., van der Plas M., Thio B., et al. (2004b) Monomethylfumarate affects polarization of monocyte-derived dendritic cells resulting in down-regulated Th1 lymphocyte responses. Eur J Immunol 34: 565–575. [DOI] [PubMed] [Google Scholar]
- Loewe R., Holnthoner W., Groger M., Pillinger M., Gruber F., Mechtcheriakova D., et al. (2002) Dimethylfumarate inhibits TNF-induced nuclear entry of NF-kappa B/p65 in human endothelial cells. J Immunol 168: 4781–4787. [DOI] [PubMed] [Google Scholar]
- Longbrake E., Cross A. (2015) Dimethyl fumarate associated lymphopenia in clinical practice. Mult Scler 21: 796–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longbrake E., Naismith R., Parks B., Wu G., Cross A. (2015a) Dimethyl fumarate-associated lymphopenia: risk factors and clinical significance. Mult Scler J Exp Transl Clin 31 July 2015. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longbrake E., Ramsbottom M., Cantoni C., Ghezzi L., Cross A., Piccio L. (2015b) Dimethyl fumarate selectively reduces memory T-cells in multiple sclerosis patients. Mult Scler 12 October 2015. pii: 1352458515608961. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacManus D., Miller D., Kappos L., Gold R., Havrdova E., Limmroth V., et al. (2011) BG-12 reduces evolution of new enhancing lesions to T1-hypointense lesions in patients with multiple sclerosis. J Neurol 258: 449–456. [DOI] [PubMed] [Google Scholar]
- Mauskopf J., Fay M., Iyer R., Sarda S., Livingston T. (2016) Cost-effectiveness of delayed-release dimethyl fumarate for the treatment of relapsing forms of multiple sclerosis in the United States. J Med Econ 19: 432–442. [DOI] [PubMed] [Google Scholar]
- Meissner M., Valesky E., Kippenberger S., Kaufmann R. (2012) Dimethyl fumarate - only an anti-psoriatic medication? J Dtsch Dermatol Ges 10: 793–801. [DOI] [PubMed] [Google Scholar]
- Metz I., Traffehn S., Strassburger-Krogias K., Keyvani K., Bergmann M., Nolte K., et al. (2015) Glial cells express nuclear Nrf2 after fumarate treatment for multiple sclerosis and psoriasis. Neurol Neuroimmunol Neuroinflamm 2: e99 DOI: 10.1212/NXI.0000000000000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkamp D., Murk J., van Oosten B., Cremers C., Killestein J., Viveen M., et al. (2015). PML in a patient without severe lymphocytopenia receiving dimethyl fumarate. N Engl J Med 372: 1474–1476. [DOI] [PubMed] [Google Scholar]
- Nibbering P., Thio B., Zomerdijk T., Bezemer A., Beijersbergen R., van Furth R. (1993) Effects of monomethylfumarate on human granulocytes. J Invest Dermatol 101: 37–42. [DOI] [PubMed] [Google Scholar]
- O’Gorman J., Russell H., Li J., Phillips G., Kurukulasuriya N., Viglietta V. (2015) Effect of aspirin pretreatment or slow dose titration on flushing and gastrointestinal events in healthy volunteers receiving delayed-release dimethyl fumarate. Clin Ther 37: 1402–1419. [DOI] [PubMed] [Google Scholar]
- Ockenfels H., Schultewolter T., Ockenfels G., Funk R., Goos M. (1998) The antipsoriatic agent dimethylfumarate immunomodulates T-cell cytokine secretion and inhibits cytokines of the psoriatic cytokine network. Br J Dermatol 139: 390–395. [DOI] [PubMed] [Google Scholar]
- Parodi B., Rossi S., Morando S., Cordano C., Bragoni A., Motta C., et al. (2015) Fumarates modulate microglia activation through a novel HCAR2 signaling pathway and rescue synaptic dysregulation in inflamed CNS. Acta Neuropathol 130: 279–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J., Erwin A., Agrella S., Kremenchutzky M., Kramer J., Darkes M., et al. (2015a) Consensus management of gastrointestinal events associated with delayed-release dimethyl fumarate: a delphi study. Neurol Ther 4: 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J., Hutchinson M., Fox R., Gold R., Havrdova E. (2014) Managing flushing and gastrointestinal events associated with delayed-release dimethyl fumarate: experiences of an international panel. Mult Scler Relat Disord 3: 513–519. [DOI] [PubMed] [Google Scholar]
- Phillips J., Selmaj K., Gold R., Fox R., Havrdova E., Giovannoni G., et al. (2015b) Clinical significance of gastrointestinal and flushing events in patients with multiple sclerosis treated with delayed-release dimethyl fumarate. Int J MS Care 17: 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitarokoili K., Ambrosius B., Meyer D., Schrewe L., Gold R. (2015) Dimethyl fumarate ameliorates lewis rat experimental autoimmune neuritis and mediates axonal protection. PLoS One 10: e0143416 DOI: 10.1371/journal.pone.0143416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich K., Thaci D., Mrowietz U., Kamps A., Neureither M., Luger T. (2009) Efficacy and safety of fumaric acid esters in the long-term treatment of psoriasis–a retrospective study (FUTURE). J Dtsch Dermatol Ges 7: 603–611. [DOI] [PubMed] [Google Scholar]
- Reick C., Ellrichmann G., Thone J., Scannevin R., Saft C., Linker R., et al. (2014) Neuroprotective dimethyl fumarate synergizes with immunomodulatory interferon beta to provide enhanced axon protection in autoimmune neuroinflammation. Exp Neurol 257: 50–56. [DOI] [PubMed] [Google Scholar]
- Rosenkranz T., Novas M., Terborg C. (2015) PML in a patient with lymphocytopenia treated with dimethyl fumarate. N Engl J Med 372: 1476–1478. [DOI] [PubMed] [Google Scholar]
- Scannevin R., Chollate S., Jung M., Shackett M., Patel H., Bista P., et al. (2012) Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J Pharmacol Exp Ther 341: 274–284. [DOI] [PubMed] [Google Scholar]
- Schilling S., Goelz S., Linker R., Luehder F., Gold R. (2006) Fumaric acid esters are effective in chronic experimental autoimmune encephalomyelitis and suppress macrophage infiltration. Clin Exp Immunol 145: 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimrigk S., Brune N., Hellwig K., Lukas C., Bellenberg B., Rieks M., et al. (2006) Oral fumaric acid esters for the treatment of active multiple sclerosis: an open-label, baseline-controlled pilot study. Eur J Neurol 13: 604–610. [DOI] [PubMed] [Google Scholar]
- Schweckendiek W. (1959) Treatment of psoriasis vulgaris. Med Monatsschr 13: 103–104. [PubMed] [Google Scholar]
- Sebok B., Bonnekoh B., Vetter R., Schneider I., Gollnick H., Mahrle G. (1998) The antipsoriatic dimethyl-fumarate suppresses interferon-gamma -induced ICAM-1 and HLA-DR expression on hyperproliferative keratinocytes. Quantification by a culture plate-directed APAAP-ELISA technique. Eur J Dermatol 8: 29–32. [PubMed] [Google Scholar]
- Sheikh S., Nestorov I., Russell H., O’Gorman J., Huang R., Milne G., et al. (2013) Tolerability and pharmacokinetics of delayed-release dimethyl fumarate administered with and without aspirin in healthy volunteers. Clin Ther 35: 1582–1594. [DOI] [PubMed] [Google Scholar]
- Stoof T., Flier J., Sampat S., Nieboer C., Tensen C., Boorsma D. (2001) The antipsoriatic drug dimethylfumarate strongly suppresses chemokine production in human keratinocytes and peripheral blood mononuclear cells. Br J Dermatol 144: 1114–1120. [DOI] [PubMed] [Google Scholar]
- Strassburger-Krogias K., Ellrichmann G., Krogias C., Altmeyer P., Chan A., Gold R. (2014) Fumarate treatment in progressive forms of multiple sclerosis: first results of a single-center observational study. Ther Adv Neurol Disord 7: 232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treumer F., Zhu K., Glaser R., Mrowietz U. (2003) Dimethylfumarate is a potent inducer of apoptosis in human T-cells. J Invest Dermatol 121: 1383–1388. [DOI] [PubMed] [Google Scholar]
- Werdenberg D., Joshi R., Wolffram S., Merkle H., Langguth P. (2003) Presystemic metabolism and intestinal absorption of antipsoriatic fumaric acid esters. Biopharm Drug Dispos 24: 259–273. [DOI] [PubMed] [Google Scholar]
- Xu Z., Zhang F., Sun F., Gu K., Dong S., He D. (2015) Dimethyl fumarate for multiple sclerosis. Cochrane Database Syst Rev 4: CD011076 DOI: 10.1002/14651858.CD011076. [DOI] [PMC free article] [PubMed] [Google Scholar]


