Abstract
Across much of central Europe, the Linearbandkeramik (LBK) represents the first Neolithic communities. Arising in Transdanubia around 5500 cal. BC the LBK spread west to the Rhine within two to three hundred years, carrying elements of a mixed agricultural economy and a relatively homogeneous material culture. Colonisation of new regions during this progress would have required economic adaptations to varied ecological conditions within the landscape. This paper investigates whether such adaptation at a local scale affected health patterns and altered the dietary habits of populations that otherwise shared a common cultural and biological origin. Analysis of non-specific stress (linear enamel hypoplasia, porotic hyperostosis, cribra orbitalia) within five LBK populations from across central Europe in conjunction with published carbon and nitrogen stable isotope data from each site revealed a high prevalence of porotic hyperostosis and cribra orbitalia in western populations that was associated with a lower animal protein intake. Hypoplastic enamel was more frequently observed in eastern populations however, and may reflect geographic differences in childhood morbidity and mortality as a result of variation in social practices relating to weaning. Local socio-economic adaptations within the LBK were therefore an important factor in the exposure of populations to non-specific stress.
Settlements of the Linearbandkeramik (LBK) culture, identified by their characteristic longhouse architecture and linearly incised pottery, are found across much of central Europe during the late sixth and early fifth millennia BC. The culture first arose in Transdanubia around 5500 cal. BC1,2, practicing a small-scale intensive garden agriculture based on the cultivation of five staple crops (einkorn and emmer wheat, barley, peas, and lentils) and the husbandry of four animals (cattle, sheep, goats, and pigs3,4). Rapid spread of the LBK from Transdanubia to the Rhine by 5300 cal. BC suggests a rapid diffusion of the culture and recent studies of genomic data from 17 LBK specimens from Eastern Hungary5, Transdanubia, and Germany6,7 indicate a close genetic affinity between LBK populations across this distribution.
Carbon and nitrogen stable isotope analysis of bones and teeth from LBK populations suggests that they were consuming a mixed terrestrial diet and that this differed little depending on the age, sex, or social status of individuals8,9,10. Small differences in the proportions of proteins and carbohydrates consumed by males and females may, however, have existed at some sites11 and dietary differences across the geographic landscape may be identified in the archaeological record12.
Analysis of palaeobotanical data and faunal remains from a number of sites has given an insight into to some of the spectrum of foods consumed as part of the LBK diet, and how this diet apparently varied by region. Barley grains are frequently found within settlements from the Carpathian Basin and the Neckar region of Germany, but not over the landscape in between13. Greater proportions of einkorn than emmer wheat and the presence of the opium poppy further suggest slightly different subsistence at western German LBK sites3,4. Exploitation of wild animals and the inclusion of flint or bone arrowheads in graves are more frequent at the western limits of the LBK, possibly indicating a greater emphasis on hunting in this area14,15. Differences in the manufacture and decoration of fineware pottery, orientation and structuring of longhouses, and burial in cemeteries are observed along an east to west trajectory of the LBK archaeological record15,16,17. The archaeological data suggests that small variations in environmental and ecological conditions across central Europe may have forced local adaptations in diet and other behaviours as LBK populations expanded.
Little palaeopathological data are available for the Neolithic of central Europe (Supplementary Table S1) so it is not known how variations in diet and behaviour may have affected the health of LBK individuals. Non-specific stress, experienced during the lifetime of an individual, is an indicator of periods during which normal growth and repair processes are disrupted. Conditions affecting the skeletal system, such as porotic hyperostosis, cribra orbitalia, and linear enamel hypoplasia, may be suggestive of periods of nutritional or immunological stress suffered by individuals and may be observed at high frequency in prehistoric populations18,19,20. Porotic hyperostosis and cribra orbitalia affect the cranial vault and orbital roof, respectively, and may be identified as porosity of the outer table of bone resulting from expansion of the inner spongy bone20, while linear enamel hypoplasia are bands of decreased enamel thickness observed on tooth crowns resulting from the disruption of normal enamel formation during childhood18.
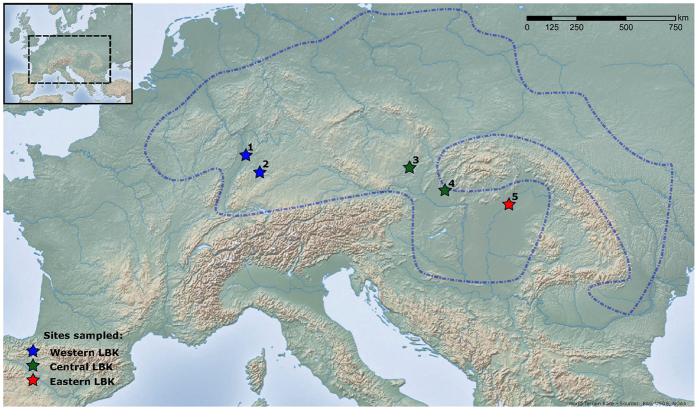
This study investigated differences in the experience of such biological stress between western, central and eastern regions of the LBK (Fig. 1) through comparison of the prevalence of porotic hyperostosis, cribra orbitalia, and linear enamel hypoplasia, and also of the age at formation of hypoplastic enamel defects. Analysis was conducted on a total of 511 skeletons from five Early Neolithic collections: Schwetzingen, Stuttgart-Mühlhausen, Vedrovice, Nitra-Horné Krškany, and Polgár-Ferenci-hát (Table 1, see also Supplementary Tables S2 and S3). Both inter-regional variations in the frequencies of these indicators in the overall populations, and intra-cemetery variations in stress within the adult male, adult female, and juvenile subsets of populations were examined. Comparison of results with dietary profiles constructed from published light stable isotope data for each population then facilitated discussion of stress as a response to variable access to dietary resources across the geographic distribution of the LBK.
Figure 1. Location map for populations used in this study, showing the regional grouping of sites.
See Supplementary Table S2 for more detail. 1 - Schwetzingen; 2 - Stuttgart-Mühlhausen; 3 - Vedrovice; 4 - Nitra-Horné Krškany; 5 - Polgár-Ferenci-hát. World Terrain Base map ( http://tiles.arcgis.com/tiles/jIL9msH9OI208GCb/arcgis/rest/services/World_Relief_Map/MapServer) from ArcGIS [version 10.1], ( http://www.esri.com/software/arcgis).
Table 1. Summary demographic information for each of the five skeletal collections.
| Site |
Adults |
Juveniles |
Males |
Females |
Total |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ind. | Dent. | Ind. | Dent. | Ind. | Dent. | Ind. | Dent. | Ind. | Dent. | |
| Schwetzingen | 66 | 498 | 34 | 122 | 35 | 318 | 24 | 180 | 100 | 620 |
| Stuttgart-Mühlhausen | 87 | 421 | 29 | 82 | 49 | 268 | 26 | 129 | 116 | 503 |
| Vedrovice | 81 | 543 | 30 | 48 | 33 | 272 | 33 | 243 | 111 | 591 |
| Nitra-Horné Krškany | 53 | 170 | 25 | 36 | 25 | 107 | 20 | 63 | 78 | 206 |
| Polgár-Ferenci-hát | 75 | 330 | 31 | 72 | 44 | 191 | 21 | 132 | 106 | 402 |
| 362 | 1962 | 149 | 360 | 186 | 1156 | 124 | 747 | 511 | 2322 | |
Ind. - number of individual skeletons included in analysis; Dent. - number of teeth.
Results
Prevalence of non-specific stress
True prevalence of all non-specific indicators of stress and results of chi-square comparisons between demographic groupings within each population are shown in Table 2. Table 3 further highlights variation in the prevalence of indicators between populations. Little inter-population variability was witnessed among dental defects. Prevalence of linear enamel hypoplasia did not differ significantly across the populations studied, ranging from 11.58% at Vedrovice to 16.81% at Nitra-Horné Krškany. In contrast, significant inter-population differences existed in the prevalence of porotic hyperostosis and cribra orbitalia (PH: χ2 = 73.91, df = 4, p < 0.001; CO: χ2 = 43.42, df = 2, p < 0.001, Fig. 2, Table 3). The highest prevalence of porotic hyperostosis was found at Stuttgart-Mühlhausen (80.23%), while Schwetzingen had the highest prevalence of cribra orbitalia (53.97%). Both of these populations were located within the western LBK region. When grouped into regions, differences in the prevalence of linear enamel hypoplasia remained non-significant but the prevalence of both porotic hyperostosis and cribra orbitalia was highest in the west (65.06% PH, 50.81% CO) followed by eastern (47.30% PH, 25.00% CO) and then central populations (38.16% PH, 23.73% CO). These inter-regional differences in porotic hyperostosis and cribra orbitalia were also statistically significant (PH: χ2 = 14.97, df = 2, p = 0.001; CO: χ2 = 21.06, df = 2, p < 0.001).
Table 2. Results of chi-square tests comparing true prevalence of linear enamel hypoplasia, porotic hyperostosis and cribra orbitalia between adults and juveniles within each population, and also between adult males and females within each population.
| Indicator | Site |
True Prevalence |
|
True Prevalence |
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Total |
Males |
Females |
Within population comparison |
Adults |
Juveniles |
Within population comparison |
||||||||||||||||
| n | na | % | n | na | % | n | na | % | χ2 | df | p | n | na | % | n | na | % | χ2 | df | p | ||
| Linear enamel hypoplasia | SW | 608 | 72 | 11.84 | 318 | 28 | 8.81 | 180 | 22 | 12.22 | 0.31 | 1 | 0.577 | 498 | 50 | 10.04 | 122 | 22 | 18.03 | 2.03 | 1 | 0.155 |
| SM | 499 | 63 | 12.63 | 268 | 35 | 13.06 | 129 | 11 | 8.53 | 0.65 | 1 | 0.421 | 421 | 53 | 12.59 | 82 | 10 | 12.20 | 0.00 | 1 | 1.000 | |
| V | 587 | 68 | 11.58 | 272 | 32 | 11.76 | 243 | 27 | 11.11 | 0.00 | 1 | 1.000 | 543 | 65 | 11.97 | 48 | 3 | 6.25 | 1.35 | 1 | 0.246 | |
| HK | 238 | 40 | 16.81 | 107 | 12 | 11.21 | 63 | 10 | 15.87 | 0.57 | 1 | 0.450 | 170 | 22 | 12.94 | 36 | 18 | 50.00 | 30.14 | 1 | 0.000 | |
| PF | 390 | 52 | 13.33 | 191 | 10 | 5.24 | 132 | 17 | 12.88 | 2.68 | 1 | 0.102 | 330 | 27 | 8.18 | 72 | 25 | 34.72 | 19.36 | 1 | 0.000 | |
| Porotic hyperostosis | SW | 80 | 39 | 48.75 | 32 | 21 | 65.63 | 20 | 11 | 55.00 | 1.94 | 1 | 0.164 | 53 | 32 | 60.38 | 27 | 7 | 25.93 | 22.81 | 1 | 0.000 |
| SM | 86 | 69 | 80.23 | 40 | 35 | 87.50 | 16 | 14 | 87.50 | 0.00 | 1 | 1.000 | 62 | 55 | 88.71 | 24 | 14 | 58.33 | 22.16 | 1 | 0.000 | |
| V | 82 | 17 | 20.73 | 26 | 7 | 26.92 | 29 | 4 | 13.79 | 4.54 | 1 | 0.033 | 60 | 13 | 21.67 | 22 | 4 | 18.18 | 0.19 | 1 | 0.660 | |
| HK | 70 | 41 | 58.57 | 23 | 17 | 73.91 | 17 | 11 | 64.71 | 1.58 | 1 | 0.208 | 45 | 32 | 71.11 | 25 | 9 | 36.00 | 23.39 | 1 | 0.000 | |
| PF | 74 | 35 | 47.30 | 26 | 19 | 73.08 | 18 | 8 | 44.44 | 15.75 | 1 | 0.000 | 47 | 29 | 61.70 | 27 | 6 | 22.22 | 30.40 | 1 | 0.000 | |
| Cribra orbitalia | SW | 63 | 34 | 53.97 | 30 | 16 | 53.33 | 20 | 7 | 35.00 | 6.09 | 1 | 0.014 | 50 | 23 | 46.00 | 13 | 11 | 84.62 | 31.23 | 1 | 0.000 |
| SM | 61 | 29 | 47.54 | 30 | 15 | 50.00 | 13 | 3 | 23.08 | 14.49 | 1 | 0.000 | 46 | 19 | 41.30 | 15 | 10 | 66.67 | 11.95 | 1 | 0.001 | |
| V | 63 | 10 | 15.87 | 23 | 4 | 17.39 | 25 | 2 | 8.00 | 3.18 | 1 | 0.075 | 49 | 6 | 12.24 | 14 | 4 | 28.57 | 7.23 | 1 | 0.007 | |
| HK | 55 | 18 | 32.73 | 22 | 4 | 18.18 | 13 | 6 | 46.15 | 16.67 | 1 | 0.000 | 36 | 11 | 30.56 | 19 | 7 | 36.84 | 0.63 | 1 | 0.429 | |
| PF | 52 | 13 | 25.00 | 18 | 1 | 5.56 | 17 | 6 | 35.29 | 25.41 | 1 | 0.000 | 35 | 7 | 20.00 | 17 | 6 | 35.29 | 5.11 | 1 | 0.024 | |
Prevalence represented the observed value for each demographic grouping and the mid-point of these was taken as the expected value for each separate test. Statistically significant results are highlighted in bold font. Due to the large number of tests performed some statistical significance is expected to occur by chance. To combat this, only tests returning a p value less than 0.02 were considered significant. SW - Schwetzingen; SM - Stuttgart-Mühlhausen; V - Vedrovice; HK - Nitra-Horné Krškany; PF - Polgár-Ferenci-hát; n - sample size; na - number of sample affected by indicator; % - prevalence of indicator in sample.
Table 3. Results of chi-square tests comparing true prevalence of linear enamel hypoplasia, porotic hyperostosis and cribra orbitalia between the five populations.
| Category |
Linear enamel hypoplasia |
Porotic hyperostosis |
Cribra orbitalia |
||||||
|---|---|---|---|---|---|---|---|---|---|
| χ2 | df | p | χ2 | df | p | χ2 | df | p | |
| Total | 1.55 | 4 | 0.818 | 73.91 | 4 | 0.000 | 43.42 | 4 | 0.000 |
| Males | 4.22 | 4 | 0.376 | 92.83 | 4 | 0.000 | 89.29 | 4 | 0.000 |
| Females | 2.68 | 4 | 0.612 | 118.12 | 4 | 0.000 | 40.61 | 4 | 0.000 |
| Adults | 1.62 | 4 | 0.806 | 101.36 | 4 | 0.000 | 38.05 | 4 | 0.000 |
| Juveniles | 69.74 | 4 | 0.000 | 47.36 | 4 | 0.000 | 92.96 | 4 | 0.000 |
Prevalence at each site represented the observed value while expected values for each separate test were estimated as the mean prevalence of all five sites. Significant variability in the prevalence of porotic hyperostosis and cribra orbitalia is evident between sites. Statistically significant results are highlighted in bold font.
Figure 2. True prevalence of non-specific indicators of stress within the five LBK populations.
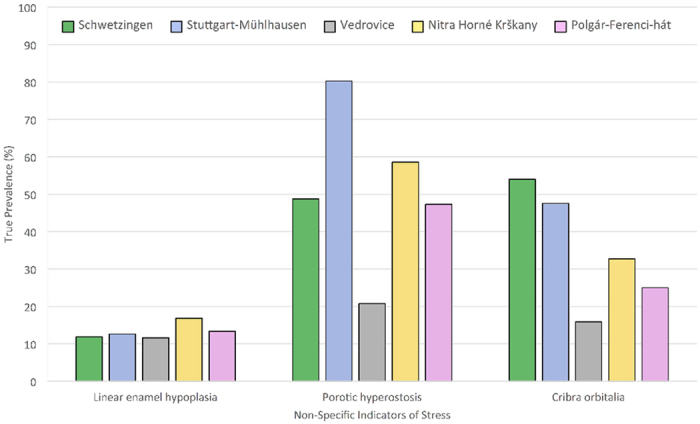
Populations are arranged in geographic order progressing from west to east. Cribra orbitalia may be seen to be at significantly higher prevalence within both of the populations from the western LBK, while porotic hyperostosis was also found at high prevalence at the western site of Stuttgart-Mühlhausen.
The true prevalence of porotic hyperostosis was greater in males than females in all populations except Stuttgart-Mühlhausen, where male and female prevalence was equal (87.50% for both), but differences in prevalence were only statistically significant for Vedrovice (p = 0.033) and Polgár-Ferenci-hát (p < 0.001). Prevalence of cribra orbitalia differed significantly between males and females at every site but Vedrovice. Males more frequently displayed orbital lesions at Stuttgart-Mühlhausen and Schwetzingen, while female prevalence was higher at Nitra-Horné Krškany and Polgár-Ferenci-hát (Table 2). No significant difference was found at any site between the male and female prevalence of linear enamel hypoplasia, nor were significant differences seen in the prevalence of males and females affected between sites (p = 0.376 and p = 0.612, respectively). Prevalence of males and females affected by porotic hyperostosis and cribra orbitalia did differ significantly across the LBK (Table 3). Both males and females showed a high level of porotic hyperostosis at Stuttgart-Mühlhausen (87.50% for both), while cribra orbitalia was highest amongst males at Schwetzingen (53.33%) and females from Nitra-Horné Krškany (46.15%). This was further evident when populations were grouped by region. Prevalence of porotic hyperostosis and cribra orbitalia amongst males varied significantly between geographic regions of the LBK (PH: χ2 = 21.46, df = 2, p < 0.001; CO: χ2 = 60.87, df = 2, p < 0.001), but amongst females only the level of porotic hyperostosis differed significantly (PH: χ2 = 28.31, df = 2, p < 0.001; CO: χ2 = 5.08, df = 2, p = 0.079) and neither males nor females were particularly variable in the expression of linear enamel hypoplasia.
Porotic hyperostosis was always found at higher prevalence amongst adults than juveniles and this result was statistically significant at all sites apart from Vedrovice where the prevalence amongst both adults and juveniles was low. Conversely juveniles displayed a higher prevalence of cribra orbitalia at every site except Nitra-Horné Krškany (Table 3). Adult and juvenile prevalence of linear enamel hypoplasia differed significantly only within Nitra-Horné Krškany and Polgár-Ferenci-hát, where juvenile prevalence was particularly high (50.00% at Nitra-Horné Krškany and 34.72% at Polgár-Ferenci-hát). As a result of this, juveniles were the only demographic grouping to show significant inter-site and inter-region variability in the prevalence of linear enamel hypoplasia (inter-site: χ2 = 69.74, df = 4, p < 0.001; inter-region: χ2 = 9.63, df = 4, p = 0.008). Both adult and juvenile prevalence of porotic hyperostosis and cribra orbitalia also varied significantly between populations and between regions (Table 3).
Age at onset of non-specific stress
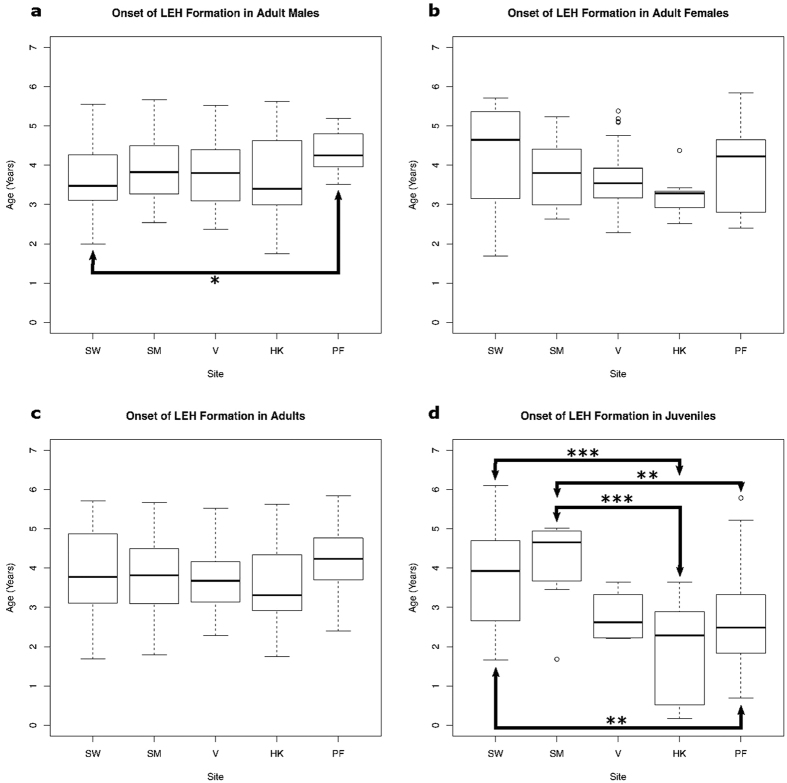
Table 4 shows the average age at onset of linear enamel hypoplasia formation within all five populations, this ranged from an early 0.7 years in individual HK23 from Nitra-Horné Krškany, to a late 6.2 years in individual SW109(96) from Schwetzingen (See Supplementary Tables S4 and S5 for results of the Kruskal-Wallis analysis of variance in age at onset). Both of these individuals were juveniles. Variation between sites was significant (H = 31.90, df = 4, p < 0.001) with a difference of almost one year between the population with the earliest average onset (Nitra-Horné Krškany: 2.9 years) and the population with the latest (Schwetzingen and Stuttgart-Mühlhausen: 3.9 years). Adult males and juveniles showed variation in onset (Fig. 3). Males differed significantly in onset between Schwetzingen and Polgár-Ferenci-hát (p = 0.012) while juveniles displayed a much greater degree of variation. Juvenile individuals at Schwetzingen and Stuttgart-Mühlhausen had a significantly later average age at onset of linear enamel hypoplasia formation than cohorts from Nitra-Horné Krškany and Polgár-Ferenci-hát.
Table 4. Average age at onset of linear enamel hypoplasia formation for each population (total) and for each demographic grouping within populations.
| Site | Adults | Juveniles | Males | Females | Total |
|---|---|---|---|---|---|
| Schwetzingen | 4.0 | 3.8 | 3.7 | 4.4 | 3.9 |
| Stuttgart-Mühlhausen | 3.8 | 4.2 | 3.9 | 3.7 | 3.9 |
| Vedrovice | 3.7 | 2.8 | 3.8 | 3.6 | 3.7 |
| Nitra-Horné Krškany | 3.6 | 1.9 | 3.7 | 3.2 | 2.9 |
| Polgár-Ferenci-hát | 4.2 | 2.7 | 4.4 | 4.0 | 3.2 |
All ages are in years. Only juveniles show significant variation in average age at onset between populations, being later in western than eastern populations. See Supplementary Tables S4 and S5 for statistical significance of differences.
Figure 3. Boxplots of variation in age at onset of hypoplasia formation within each population.
Populations are arranged geographically proceeding from west to east. Connecting arrows indicate statistically significant differences between paired populations (*p < 0.02, **p < 0.01, ***p < 0.001). Pairwise comparisons with a p-value of 0.02 or greater are not considered statistically significant due to the high probability of this value occurring by chance when all five pairs are compared. All significant differences involve one or both of the populations from the western LBK. In the juvenile subset, both populations from the western LBK show a later average onset than the more easterly populations. SW - Schwetzingen; SM - Stuttgart-Mühlhausen; V - Vedrovice; HK - Nitra-Horné Krškany; PF - Polgár-Ferenci-hát.
Average age at onset in the dentition of females was 0.75 years later than the average age at onset in the dentition of males at Schwetzingen (p = 0.006). No other population evidenced a significant difference between males and females. Onset in teeth from individuals dying during immaturity was significantly earlier on average than onset in the teeth of those individuals surviving to adulthood within the three populations from the central and eastern LBK (Supplementary Table S5), while no difference was observed in the dentition from the western LBK populations.
Stable isotopes
Variation in carbon and nitrogen isotopic ratios between the five LBK populations was statistically significant (Kruskal-Wallis: δ13C - H = 82.87, df = 3, p < 0.001; δ15N - H = 169.08, df = 3, p < 0.001). Vedrovice and Schwetzingen displayed lower δ15N values than Nitra-Horné Krškany and Polgár-Ferenci-hát, and individuals from Stuttgart-Mühlhausen, Schwetzingen and Nitra-Horné Krškany had more negative δ13C than Vedrovice and Polgár-Ferenci-hát (Fig. 4). Application of a post-hoc pairwise Wilcoxon rank sum test indicated that all population pairings differed significantly except Nitra-Horné Krškany/Schwetzingen and Vedrovice/Polgár-Ferenci-hát for δ13C, and Nitra-Horné Krškany/Polgár-Ferenci-hát for δ15N. At Vedrovice δ15N of adults was 0.33‰ higher than that of juveniles and δ15N of adult males was 0.44‰ higher than females. Both of these differences were small but significant (two-group Mann-Whitney U test, p = 0.029 and p < 0.001, respectively). Male-female and adult-juvenile isotope ratios did not differ significantly at any other site.
Figure 4. Distribution of carbon and nitrogen stable isotope ratios for LBK populations.
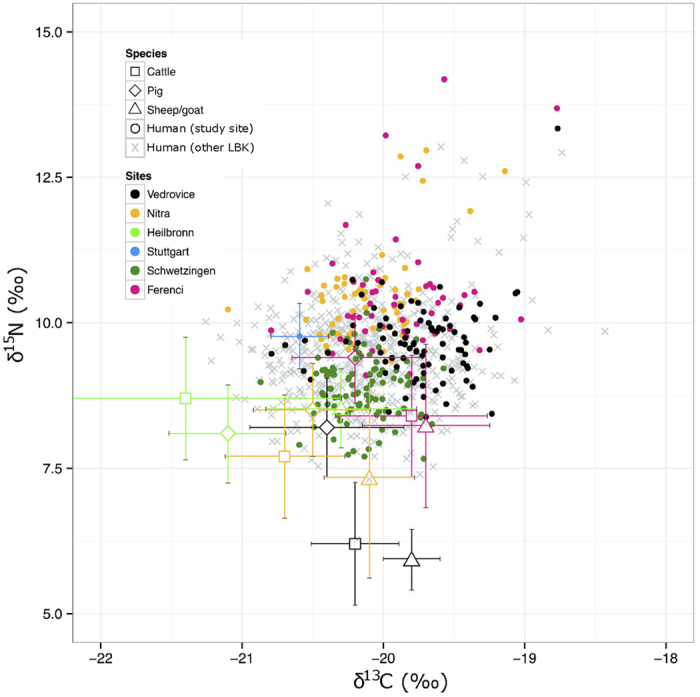
Individual isotope values are available in the Supplementary Information (Supplementary Table S6). Stuttgart-Mühlhausen is rep-resented by an average value and standard deviation as full data has not yet been published. Faunal isotope ratios for each site are represented by a mean value bracketed within one standard deviation (see Supplementary Table S7). No faunal data was available for Schwetzingen or Stuttgart-Mühlhausen: fauna from the contemporaneous site of Heilbronn-Neckargartach are plotted as a proxy. Sampled sites show clear groupings which are statistically different. Particularly noticeable are the lower δ15N values of human remains from Schwetzingen. Data collected from published sources8,9,10,11,21,22,27,54,55,56,57 (see Supplementary Tables S6 and S7 and Methods section for full detail).
Discussion
Experience of stress within the LBK appears to have differed significantly from east to west. Porotic hyperostosis and cribra orbitalia were both found at high prevalence in the western LBK skeletal collections of Schwetzingen and Stuttgart-Mühlhausen, suggesting greater nutritional or immunological stress within farming populations towards the western limits of this archaeological culture20. Prevalence of linear enamel hypoplasia was more uniform across the LBK, although prevalence amongst juveniles was particularly high in the more easterly populations of Nitra-Horné Krškany and Polgár-Ferenci-hát where average age at onset of linear enamel hypoplasia formation was also significantly younger for juveniles than adults. Findings from this study correspond with palaeopathological data previously published on LBK populations, which also hint at a geographic gradient in skeletal indicators of stress (Supplementary Table S1). High prevalence of cribra orbitalia at the sites of Rutzing (55.60%) and Schletz (55.22%)21 in Austria is similar to the prevalence here observed at both Schwetzingen (53.97%) and Stuttgart-Mühlhausen (47.54%). Further east, more moderate prevalence of this pathological condition reported from the site of Füzesabony-Gubakút (12.20%)22, Hungary, also corresponds with lower relative levels of the condition in eastern LBK populations. Although broad continental-scale patterns in the health of Early Neolithic populations are difficult to make on the basis of this analysis of just five sites, these results would nevertheless suggest that predictable differences may exist from east to west and that greater investigation of palaeopathology within the LBK is needed to test this hypothesis.
Lower δ15N values at Schwetzingen and Vedrovice than Nitra-Horné Krškany or Polgár-Ferenci-hát may suggest a lower intake of animal proteins or a lack of manure-fertilised produce in the diet of these populations23. Some variation in isotopic levels of dietary carbon may be expected as a result of environmental differences across the landscape of central Europe12, although significantly more negative δ13C values at Nitra-Horné Krškany, Schwetzingen and Stuttgart-Mühlhausen could further indicate consumption of a greater proportion of food from closed canopy environments24. Domesticates may have been browsing woodland vegetation in these locations, but it is also possible that western isotope values may reflect food obtained from wild resources, as indicated by the high frequency of wild fauna and arrowheads in the western LBK archaeological record14,15. Wild fauna accounts for just 5–10% of animal remains recovered from LBK settlements in eastern areas25 but up to 39% of assemblages from the Baden-Württemberg area of southern Germany14. The hunting of wild fauna remained a component of agricultural subsistence into the Iron Age and beyond26 but the increased emphasis on this activity in the western LBK perhaps indicates a greater economic need for hunted meat and may suggest that early agriculture was not as successful in western climates as it appears to have been in eastern Europe and the Balkans. Further reliance on barley, einkorn wheat and poppy seeds, which are more tolerant of fluctuating climatic and soil conditions than other Neolithic staples3,4, may indicate areas of poor agricultural productivity at the western limits of the LBK27.
If conditions were less optimal for Neolithic domesticates in the western LBK, the risk of crop failure and food deficit may have been greater and may have led to a greater reliance on crops that would grow more predictably and an increased utilisation of wild resources to supplement the diet. Low δ15N values from Schwetzingen arguably indicate that consumption of meat may have been significantly reduced compared to other populations, while enriched nitrogen values of fauna from Heilbronn may suggest intensive manuring of crops in an attempt to increase yield at other sites23. Evidence for the diversification of subsistence strategies and retention of productive land within family lineages in the western LBK28,29 could also represent responses to lower agricultural productivity.
High rates of nutritional stress and disease are associated with periods of deficit in modern populations from low-income countries and may also be witnessed in the skeletons of historic famine victims30,31,32,33. Moderate levels of nutritional stress across the LBK coupled with lower agricultural productivity, and hence increased nutritional stress, in western populations would be consistent with the geographic patterning of non-specific indicators of stress highlighted in the present study. Differences in the experience of stress between populations would not be entirely unexpected as each population is subject to unique ecological conditions, however the clear distinction in prevalence of porotic hyperostosis and cribra orbitalia between more easterly and more westerly located sites lends this pattern significance despite the small sample size. Populations from these sites share a high degree of genetic affinity5,6,7 and are broadly comparable in their material culture, although regional variation along an east-west axis15,16,17 coincides with patterns in biological stress observed here and may reflect larger differences in subsistence practices developing as a response to economic need.
East-west patterning in male-female and adult-juvenile prevalence of non-specific stress could further reflect behavioural differences that echo the variation in material culture observed across the LBK distribution. Cribra orbitalia was more prevalent in males within western LBK populations but higher in females from Nitra-Horné Krškany and Polgár-Ferenci-hát. Assessment of tibial cross-sectional geometry within a time-series of central European populations34 showed that biomechanical values were particularly high at Schwetzingen and Stuttgart-Mühlhausen when compared to populations from other regions of the LBK and later time periods. This was suggestive of increased mobility, particularly across rough terrain, and could relate to a diversification of subsistence strategies in the western LBK and the greater exploitation of upland resources29, which may have affected males more than females although why such a pattern would occur is uncertain. High levels of activity increase the metabolic demand for iron and may be associated with an increased risk of anemia in modern athletes35. Similar activity in western LBK males may have increased the risk of iron-deficiency and development of cribra orbitalia beyond the level experienced in females from iron loss during pregnancy and menstruation36.
Higher juvenile prevalence of cribra orbitalia and linear enamel hypoplasia, particularly in eastern populations, where significant differences were also observed in the average age at onset of enamel hypoplasia formation between adults and juveniles, may signal greater childhood morbidity and mortality. Incomplete growth and greater plasticity of juvenile bone means that porous lesions of the cranium may be more likely to develop in skeletally immature individuals, initially affecting the orbital roof and progressing onto the flat bones of the cranium with prolonged exposure to stress37. High juvenile mortality may therefore be reflected in the higher prevalence of cribra orbitalia amongst immature skeletal individuals, who died before lesions could manifest on the cranial vault, while high prevalence of porotic hyperostosis amongst adults may signal significant juvenile morbidity in those who managed to survive for a longer period.
Average onset of enamel hypoplasia formation for all populations fell between two and four years of age, corresponding with stable isotope evidence for weaning after two or three years in LBK populations9. Weaning may be a stressful period for infants, particularly if weaning food is low in nutrients and softened with water contaminated by pathogens38, and although a direct correlation between weaning and hypoplasia formation cannot be attested39,40, an association is generally postulated41.
Later average age at onset of enamel hypoplasia formation in western LBK juveniles could indicate a practice of later weaning or that those individuals weaned early did not survive to six or seven years of age, when the permanent dentition begins to erupt42. Weaning may be delayed in modern populations if food is scarce43, as may have been the case in the western LBK. Such a delay may correspond with an increase in the length of the inter-birth interval and hence overall a reduction in the rate of population growth within western LBK populations, which is contrary to findings from other cemetery assemblages of an increasing rate of population growth after the adoption of agriculture44. Prolonged breastfeeding does not adequately meet the nutritional demands of infants and can result in iron-deficiency and megaloblastic anemia, both of which may lead to the formation of porotic hyperostosis and cribra orbitalia20. Any individual weaned early in the western LBK may have been subject to even greater stress than those weaned later if the availability of weaning foods was restricted. As a result, these individuals may have been more likely to develop cribra orbitalia at a young age but then die prior to the eruption of the permanent dentition. Thus high prevalence of porotic hyperostosis and cribra orbitalia but lower juvenile prevalence and later average onset of linear enamel hypoplasia in the western LBK may all signal greater nutritional stress resulting from lower agricultural productivity and different practices of weaning than eastern counterparts.
Conclusions
Examination of three non-specific indicators of biological stress in five populations from across the east-west distribution of the LBK suggests that the experience of stress within this archaeological culture differed significantly, despite shared characteristics of material culture and population genetics. Porotic hyperostosis and cribra orbitalia were found at higher prevalence within populations from the western region of the LBK, an area associated with lower agricultural productivity, a greater reliance on wild resources, and a diet lower in animal protein. Exposure to stress therefore seems to have been greater towards the western reaches of the LBK and a practice of delayed weaning, possibly as a response to the reduced availability of dietary resources, may have contributed towards this increased morbidity. These results suggest that treating the LBK as a single homogeneous unit may, in fact, be masking a significant diversity of behaviour, which may have important consequences for our understanding of early farming communities.
Materials and Methods
Skeletal Material
Skeletal remains from five LBK cemeteries were included in this study: the eastern Alföld Pottery site of Polgár-Ferenci-hát; the two sites of Vedrovice and Nitra-Horné Krškany from the central region of the LBK distribution; and Stuttgart-Mühlhausen and Schwetzingen from the western limits of the initial LBK expansion (Fig. 1, Supplementary Table S2). Due to the antiquity of the skeletal material, ethical consent for study was not necessary. The age and sex of each specimen was assessed using standard osteological methods from observation of both the crania and ossa coxae where possible45,46,47,48,49.
A total of 511 skeletons and 2322 teeth, from both the maxilla and mandible, were examined (Table 1). Only the permanent incisors and canines were included in the analysis due to the higher frequency of hypoplasia formation50,51, and the greater precision with which the location of defects may be measured, on the enamel surface of these single-cusped teeth.
Macroscopic Analysis
Crania and teeth were examined macroscopically for the presence of each non-specific indicator of stress, and the true prevalence of porotic hyperostosis, cribra orbitalia and linear enamel hypoplasia was calculated as the proportion of the total crania or teeth examined that displayed pathological alterations. In addition to prevalence data, the distance of each hypoplastic defect from the cemento-enamel junction (CEJ) to the border of the defect closest to the tooth cusp was measured. Digital sliding calipers with an accuracy of 0.01mm were used and the location of each defect was measured three times to provide an average. CEJ to defect distances were then used to calculate an age at onset of hypoplasia formation using the Goodman and Rose18 equation and tooth crown growth standards from Smith42.
Statistical Testing
Inter-population comparisons of the true prevalence of each non-specific indicator of stress and the age at onset of hypoplasia formation were conducted using the chi-square test for independence and the Kruskal-Wallis analysis of variance, respectively. Tests were conducted between total populations and between the demographic components of each population (adults, juveniles, adult males, and adult females). These analyses tested whether variation in prevalence and onset was random across the dataset or related to the populations being examined. Application of a post-hoc pairwise Wilcoxon rank sum test with Bonferroni adjustment to the results of the Kruskal-Wallis analysis highlighted differences between the median age at onset of paired populations that exceeded the standard error. Comparison of adults and juveniles, and also of adult males and females, using unpaired Wilcoxon rank sum tests further revealed any differences in onset within populations52. All statistical testing was conducted using R software53.
Isotopic Analysis
Data on carbon and nitrogen stable isotope ratios for LBK sites were collected from a review of published literature8,9,10,11,21,22,27,54,55,56,57. All studies extracted collagen from ribs and long bones (see Supplementary Table S6) using the modified Longin58,59 method of sample preparation. Ribs are sampled preferentially for analysis of dietary isotopes as they should represent food consumed closer to the time of death of the individual compared to long bones, due to the generally higher rate of bone turnover in ribs9, however collagen from ribs and long bones may be comparable to each other while isotopic values from dental collagen are not comparable to either. For this reason published data on dietary isotopes based on dental collagen values were not included in the present analysis. Small differences may arise in carbon and nitrogen isotopic values between sites due to the processing of material at different laboratories, but the use of comparable standards should minimise this confounding factor. Only data for Schwetzingen, Vedrovice, Nitra-Horné Krškany, and Polgár-Ferenci-hát were included in statistical comparisons, all other data from additional sites formed background noise for Fig. 4 but were not tested statistically. Values were plotted using R software53 and compared between populations through application of the Kruskal-Wallis analysis of variance statistical test and post-hoc pairwise Wilcoxon rank sum test with Bonferroni adjustment. These tests were performed as the data were determined to be non-normal in distribution. Isotopic values for adult males and females, and also for adults and juveniles, were further compared using two-group Mann-Whitney U tests.
Additional Information
How to cite this article: Ash, A. et al. Regional differences in health, diet and weaning patterns amongst the first Neolithic farmers of central Europe. Sci. Rep. 6, 29458; doi: 10.1038/srep29458 (2016).
Supplementary Material
Acknowledgments
This research was supported by R.P.’s European Research Council Starting Grant (ERC- 2010-StG 263441) and Irish Research Council Advanced Research Project Grant (RPG2013-2).
Footnotes
Author Contributions A.A. collected paleopathological data from the material, performed statistical analysis upon the data, wrote the paper and prepared the figures. M.F., I.P., Z.T. and J.W. provided skeletal material for analysis. R.P. provided advice and funding throughout the duration of the study. All authors reviewed and commented upon the preparation of the manuscript.
References
- Gronenborn D. A Variation on a Basic Theme: The Transition to Farming in Southern Central Europe. Journal of World Prehistory 13, 123–202 (1999). [Google Scholar]
- Oross K. & Bánffy E. Three successive waves of Neolithisation: LBK development in Transdanubia. Doc. praeh. 36, 175 (2009). [Google Scholar]
- Bogaard A. Neolithic farming in central Europe: an archaeobotanical study of crop husbandry practices . (Routledge, 2004). [Google Scholar]
- Kreuz A., Marinova E., Schäfer E. & Wiethold J. A comparison of early Neolithic crop and weed assemblages from the Linearbandkeramik and the Bulgarian Neolithic cultures: differences and similarities. Veg. Hist. Archaeobot. 14, 237–258 (2005). [Google Scholar]
- Gamba C. et al. Genome flux and stasis in a five millennium transect of European prehistory. Nat. Commun. 5, 5257 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaridis I. et al. Ancient human genomes suggest three ancestral populations for present-day Europeans. Nature 513, 409–413 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haak W. et al. Massive migration from the steppe was a source for Indo-European languages in Europe. Nature 522, 207–211 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürrwächter C., Craig O. E., Collins M. J., Burger J. & Alt K. W. Beyond the grave: variability in Neolithic diets in Southern Germany? J. Archaeol. Sci. 33, 39–48 (2006). [Google Scholar]
- Oelze V. M. et al. Early Neolithic diet and animal husbandry: stable isotope evidence from three Linearbandkeramik (LBK) sites in Central Germany. J. Archaeol. Sci. 38, 270–279 (2011). [Google Scholar]
- Bickle P. et al. Roots of diversity in a Linearbandkeramik community: isotope evidence at Aiterhofen (Bavaria, Germany). Antiquity 85, 1243–1258 (2011). [Google Scholar]
- Whittle A. et al. In The First Farmers of Central Europe: Diversity in LBK Lifeways (eds Bickle P. & Whittle A. ) 101–158 (Oxbow Books, 2013). [Google Scholar]
- Hedges R. et al. The supra-regional perspective. The first farmers of central Europe: diversity in LBK lifeways 345–386 (2013). [Google Scholar]
- Gyulai F. Seed and fruit remains associated with Neolithic origins in the Carpathian Basin. The origins and spread of domestic plants in southwest Asia and Europe. Left Coast Press, Walnut Creek 125–140 (2007). [Google Scholar]
- Lüning J. Steinzeitliche Bauern in Deutschland. Die Landwirtschaft im Neolithikum. (Habelt, 2000).
- Modderman P. The Linear Pottery Culture: diversity in uniformity. Berichten van de Rijksdienst voor het Oudheidkundig Bodemonderzoek 38, 63–139 (1988). [Google Scholar]
- Bogucki P. & Grygiel R. The First Farmers of Central Europe: A Survey Article. J. Field Archaeol. 20, 399–426 (1993). [Google Scholar]
- Bradley R. Orientations and origins: a symbolic dimension to the long house in Neolithic Europe. Antiquity 75, 50–56 (2001). [Google Scholar]
- Goodman A. H. & Rose J. C. In Advances in Dental Anthropology (eds Kelley M. A. & Larsen C. S. ) 279–294 (Wiley-Liss, 1991). [Google Scholar]
- Stuart-Macadam P. In Diet, demography and disease: changing perspectives on anemia (eds Stuart-Macadam P. & Kent S. K. ) 151–170 (Aldine De Gruyer, 1992). [Google Scholar]
- Walker P. L., Bathurst R. R., Richman R., Gjerdrum T. & Andrushko V. A. The causes of porotic hyperostosis and cribra orbitalia: a reappraisal of the iron-deficiency-anemia hypothesis. Am. J. Phys. Anthropol. 139, 109–125 (2009). [DOI] [PubMed] [Google Scholar]
- Bickle P. et al. In The First Farmers of Central Europe: Diversity in LBK Lifeways (eds Bickle P. & Whittle A. ) 159–204 (Oxbow Books, 2013). [Google Scholar]
- Whittle A. et al. In The First Farmers of Central Europe: Diversity in LBK Lifeways (eds Bickle P. & Whittle A. ) 49–100 (Oxbow Books, 2013). [Google Scholar]
- Bogaard A. et al. Crop manuring and intensive land management by Europe’s first farmers. Proceedings of the National Academy of Sciences 110, 12589–12594 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Merwe N. J. & Medina E. The canopy effect, carbon isotope ratios and foodwebs in amazonia. J. Archaeol. Sci. 18, 249–259 (1991). [Google Scholar]
- Döhle H.-J. Haustierhaltung und Jagd in der Linienbandkeramik - ein Überblick. Zeitschrift für Archäologie 27, 105–124 (1993). [Google Scholar]
- Cumberpatch C. G. Production and society in the later Iron Age of Bohemia and Moravia. BAR International Series 602, 67 (1995). [Google Scholar]
- Bentley R. A. et al. In The First Farmers of Central Europe: Diversity in LBK Lifeways (eds Bickle P. & Whittle A. ) 251–290 (Oxbow Books, 2013). [Google Scholar]
- Bogaard A., Krause R. & Strien H.-C. Towards a social geography of cultivation and plant use in an early farming community: Vaihingen an der Enz, south-west Germany. Antiquity 85, 395–416 (2011). [Google Scholar]
- Bentley R. A. et al. Community differentiation and kinship among Europe’s first farmers. Proc. Natl. Acad. Sci. USA 109, 9326–9330 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque A., Alam N., Wai L. & Foster A. Sustained Effects of the 1974–5 Famine on Infant and Child Mortality in a Rural Area of Bangladesh. Popul. Stud. 44, 145–154 (1990). [DOI] [PubMed] [Google Scholar]
- Goodman A. H., Martinez C. & Chavez A. Nutritional supplementation and the development of linear enamel hypoplasias in children from Tezonteopan, Mexico. Am. J. Clin. Nutr. 53, 773–781 (1991). [DOI] [PubMed] [Google Scholar]
- de Benoist B., McLean E., Egli I. & Cogswell M. Worldwide prevalence of anemia 1993–2005 (2008). [DOI] [PubMed] [Google Scholar]
- Geber J. Skeletal manifestations of stress in child victims of the Great Irish Famine (1845–1852): Prevalence of enamel hypoplasia, Harris lines, and growth retardation. Am. J. Phys. Anthropol. 155, 149–161 (2014). [DOI] [PubMed] [Google Scholar]
- Macintosh A. A., Pinhasi R. & Stock J. T. Lower limb skeletal biomechanics track long-term decline in mobility across ∼6150 years of agriculture in Central Europe. J. Archaeol. Sci. 52, 376–390 (2014). [Google Scholar]
- Beard J. & Tobin B. Iron status and exercise. Am. J. Clin. Nutr. 72, 594S–597SS (2000). [DOI] [PubMed] [Google Scholar]
- Holland T. D. & O’Brien M. J. Parasites, Porotic Hyperostosis, and the Implications of Changing Perspectives. Am. Antiq. 62, 183–193 (1997). [Google Scholar]
- Stuart-Macadam P. In Sex and gender in paleopathological perspective (eds Grauer A. & Stuart-Macadam P. ) 45–63 (Cambridge University Press, 1998). [Google Scholar]
- Eisenberg L. E. Interpreting measures of community health during the Late Prehistoric period in Middle Tennessee: A biocultural approach. Health in Past Societies: Biocultural Interpretations of Human Skeletal Remains in Archaeological Contexts, BAR International Series 567, 115–127 (1991). [Google Scholar]
- Suckling G. & Thurley D. C. In Tooth Enamel (eds Fearnhead R. & Suga S. ) 4, 347–362 (Elsevier, 1984). [Google Scholar]
- Blakey M. L., Leslie T. E. & Reidy J. P. Frequency and chronological distribution of dental enamel hypoplasia in enslaved African Americans: a test of the weaning hypothesis. Am. J. Phys. Anthropol. 95, 371–383 (1994). [DOI] [PubMed] [Google Scholar]
- Goodman A. H. & Martin D. L. Reconstructing health profiles from skeletal remains. The Backbone of History . Cambridge University Press, Cambridge, UK 11–60 (2002). [Google Scholar]
- Smith B. H. In Advances in Dental Anthropology (eds Kelley M. A. & Larsen C. S. ) 143–168 (Wiley-Liss, 1991). [Google Scholar]
- Lindstrom D. P. & Berhanu B. The effects of breastfeeding and birth spacing on infant and early childhood mortality in Ethiopia. Soc. Biol. 47, 1–17 (2000). [DOI] [PubMed] [Google Scholar]
- Bocquet-Appel J.-P. When the world’s population took off: the springboard of the Neolithic Demographic Transition. Science 333, 560–561 (2011). [DOI] [PubMed] [Google Scholar]
- Phenice T. W. A newly developed visual method of sexing the os pubis. Am. J. Phys. Anthropol. 30, 297–301 (1969). [DOI] [PubMed] [Google Scholar]
- Ferembach D., Schwindezky I. & Stoukal M. Recommendation for Age and Sex Diagnoses of Skeletons. J. Hum. Evol. 9, 517–549 (1980). [Google Scholar]
- Brooks S. & Suchey J. M. Skeletal age determination based on the os pubis: A comparison of the Acsádi-Nemeskéri and Suchey-Brooks methods. Hum. Evol . 5, 227–238 (1990). [Google Scholar]
- Buikstra J. E. & Ubelaker D. H. Standards for data collection from human skeletal remains . 44 (Arkansas Archaeological Survey, 1994). [Google Scholar]
- Buckberry J. L. & Chamberlain A. T. Age estimation from the auricular surface of the ilium: a revised method. Am. J. Phys. Anthropol. 119, 231–239 (2002). [DOI] [PubMed] [Google Scholar]
- Goodman A. H., Armelagos G. J. & Rose J. C. Enamel hypoplasia as indicators of stress in three prehistoric populations from Illinois. Human Biology 52, 515–528 (1980). [PubMed] [Google Scholar]
- Goodman A. H. & Armelagos G. J. Factors affecting the distribution of enamel hypoplasias within the human permanent dentition. Am. J. Phys. Anthropol. 68, 479–493 (1985). [DOI] [PubMed] [Google Scholar]
- Crawley M. J. Statistics: an introduction using R . (John Wiley & Sons, 2005). [Google Scholar]
- R Core Team. R: A language and environment for statistical computing . (R Foundation for Statistical Computing, 2014). [Google Scholar]
- Nehlich O. et al. Mobility or migration: a case study from the Neolithic settlement of Nieder-Mörlen (Hessen, Germany). J. Archaeol. Sci. 36, 1791–1799 (2009). [Google Scholar]
- Bickle P. et al. In The First Farmers of Central Europe: Diversity in LBK Lifeways (eds Bickle P. & Whittle A. ) 291–342 (Oxbow Books, 2013). [Google Scholar]
- Hofmann D. et al. In The First Farmers of Central Europe: Diversity in LBK Lifeways (eds Bickle P. & Whittle A. ) 205–250 (Oxbow Books, 2013). [Google Scholar]
- Mörseburg A., Alt K. W. & Knipper C. Same old in Middle Neolithic diets? A stable isotope study of bone collagen from the burial community of Jechtingen, southwest Germany. Journal of Anthropological Archaeology 39, 210–221 (2015). [Google Scholar]
- Longin R. New Method of collagen extraction for radiocarbon dating. Nature 230, 241–242 (1971). [DOI] [PubMed] [Google Scholar]
- Brown T. A., Nelson D. E., Vogel J. S. & Southon J. R. Improved collagen extraction by modified Longin method. Radiocarbon 30, 171–177 (1988). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.