Abstract
Enhancement of natural killer (NK) cell function could be beneficial in treatment of a variety of tumors and infections. However, efforts to improve NK cell function by disrupting negative regulators that target proximal signaling pathways paradoxically results in hyporesponsive rather than hyperresponsive NK cells. In this study, we demonstrate that genetic deletion of diacylglycerol (DAG) kinase zeta (DGKζ), a negative regulator of DAG-mediated signaling, has the desired effect of enhancing NK cell function due to its distal position in the activating receptor-mediated signaling cascade. Upon stimulation through multiple activating receptors, NK cells from mice lacking DGKζ display increased cytokine production and degranulation in an ERK-dependent manner. In addition, they have improved cytotoxic functions against tumor cell lines. The enhancement of NK cell function by DGKζ deficiency is NK cell-intrinsic and developmentally independent. Importantly, DGKζ deficiency does not affect inhibitory NK cell receptor expression or function. Thus, DGKζ KO mice display improved missing self recognition, as evidenced by enhanced rejection of a TAP-deficient tumor in vivo. We propose that enzymes that negatively regulate distal activating receptor signaling pathways such as DGKζ represent novel targets for augmenting the therapeutic potential of NK cells.
Keywords: NK cells, natural cytotoxicity, signal transduction
Introduction
Natural killer (NK) cells are important for protection against certain viruses and tumors. They carry out their effector function through expression of a diverse set of germline-encoded activating and inhibitory receptors (1–3). Through these receptors, NK cells are able to sense their environment, allowing them to detect target cells that have upregulated activating ligands or reduced their expression of the inhibitory ligand MHC class I – events that can occur during viral infection or neoplastic transformation (1, 3).
The ability of NK cells to target virally-infected and neoplastic cells has led to major interest in using them for therapy. Early trials have shown safety and preliminary efficacy in NK cell-based adoptive immunotherapy to treat hematological malignancies. More recently, researchers have focused on methods to improve NK function in vivo (4). These approaches can broadly be divided into three categories: 1) expansion and activation of autologous NK cells to improve their responsiveness (2, 5); 2) matching ligand expression on patient cells to killer immunoglobulin-like receptor (KIR) expression on donor NK cells (1–3, 6); and 3) genetic or molecular augmentation of NK cell signaling machinery in donor cells (1, 3, 7).
Manipulation of NK cell activating receptor-mediated signaling is complicated by the variety of signaling modules utilized by NK cell activating receptors. For example, NKG2D signals through DAP10 (co-stimulatory like), 2B4 signaling requires SAP, while NKRP1-C (NK1.1) functions through FcεRγ (ITAMs) (4, 8). Interestingly, all of these activating receptors converge upon SLP-76 and PLCγ and hence, NK cells that lack either of these signaling molecules are hyporesponsive to all three families of activating receptor stimulation (2, 5, 9, 10).
Unlike the activating receptors that function through association with adaptor molecules with activating domains, the inhibitory NK cell receptors contain ITIMs in their cytoplasmic tails. Upon interaction with their ligands, inhibitory NK cell receptors use their ITIMs to recruit phosphatases such as Src homology region 2 domain-containing phosphatase 1 (SHP-1) and SHIP, which attenuate proximal signaling pathways downstream of activating receptors. Thus, one might imagine that the inhibition of these phosphatases would enhance NK cell function and could be useful therapeutically. Paradoxically, however, SHP-1 and SHIP-deficient NK cells are hyporesponsive to activating receptor stimulation (11, 12). One potential explanation for these observations is that NK cells can continuously tune their responsiveness according to their signaling capacity (13). In fact, NK cells that are continually exposed to activating ligands or MHC class I deficient tumors become hyporesponsive to proximal signaling events (14, 15). Importantly, it has been shown that the hyporesponsive NK cell phenotype can be bypassed through PMA and ionomycin stimulation (9, 10, 12).
Given that PMA and ionomycin mimic PLCγ activation by acting as a diacylglycerol (DAG) analog and inducing Ca2+ flux, respectively, these data suggest that NK signal tuning occurs proximal to PLCγ activation. Thus, we hypothesized that targeting an enzyme that negatively regulates activating receptor-mediated signaling distal to PLCγ would not allow NK cells to tune to their increased signaling capacity and hence, make NK cells hyperresponsive to activating receptor stimulation. Indeed, we provide data that genetic ablation of DGKζ, a negative regulator of DAG-mediated signaling, leads to hyperresponsive NK cells in a cell-intrinsic and developmentally independent manner. Thus, enzymes that negatively regulate distal activating receptor signaling pathways such as DGKζ may represent novel targets for augmenting the therapeutic potential of NK cells.
Materials and methods
Mice
DGKζ KO, DGKζF/F and DGKα KO mice were described previously (16–18). C57BL/6 (B6), B6.SJL were purchased from The Jackson Laboratory or Charles River Laboratories. CD45.1/45.2 heterozygous mice were created by breeding B6 mice to B6.SJL mice. Mice were housed in pathogen-free conditions and treated in strict compliance with Institutional Animal Care and Use Committee regulations of the University of Pennsylvania.
Flow cytometry, cell sorting, and data analysis
Antibodies for flow cytometry and functional studies were purchased from BD Pharmingen (San Diego, CA): CD3e PerCP-Cy5.5 (145-2C11), NK1.1 APC (PK136), CD45.1 AF700 (A20), CD45.2 PE-Cy7 (104), Ly49G2 APC (4D11), Ly49A PE (A1), CD244.2 Biotin (2B4), Ly49D purified or FITC (4E5), CD4 FITC (RM4-5), CD8 FITC (53-6.7), and CD27 PE (LG.3A10); Biolegend (San Diego, CA): CD49b biotin (DX5), IFNγ BV421 (XMG1.2), CD107a PE (1D4B), Ly49A Pacific Blue (YE1/48.10.6), and Streptavidin BV421; eBioscience (San Diego, CA): CD3e FITC (17A2), CD3e APC-efluor780 (145-2C11), NKp46 PerCP-efluor710 (29A1.4), CD49b FITC (DX5), IFNγ PE-Cy7 (XMG1.2), Ly49G2 PerCP-efluor710 (4D11), Ly49I FITC or PE (YLI-90), NKG2D APC (CX5), Ly49H FITC, PE, APC or purified (3D10), Ly49D APC (4E5), and CD11b PE-Cy7 (M1/70); BioXcell (West Lebanon, NH): NKG2D purified (HMG2D) and NK1.1 purified (PK136); Jackson Immunoresearch (West Grove, PA): anti-mouse-IgG3 APC, UCSF Cell Culture Facility Ly49C purified (4LO-3311) or Molecular Probes, Invitrogen (Carlsbad, CA): Live/Dead Aqua or NearIR. Flow cytometry and FACS were performed with an LSR II, FACS Canto, or a FACS Aria cell sorter (BD Biosciences). Data were analyzed with Flowjo software (Tree Star) and Prism (Graphpad), and all scatter plots have mean and SEM depicted. All flow data are pregated on live lymphocyte singlets, NK cells are CD3−DX5+NKp46+ and LAK cells are CD4−CD8−NK1.1+DX5+, unless otherwise stated (Fig 1A, 1B).
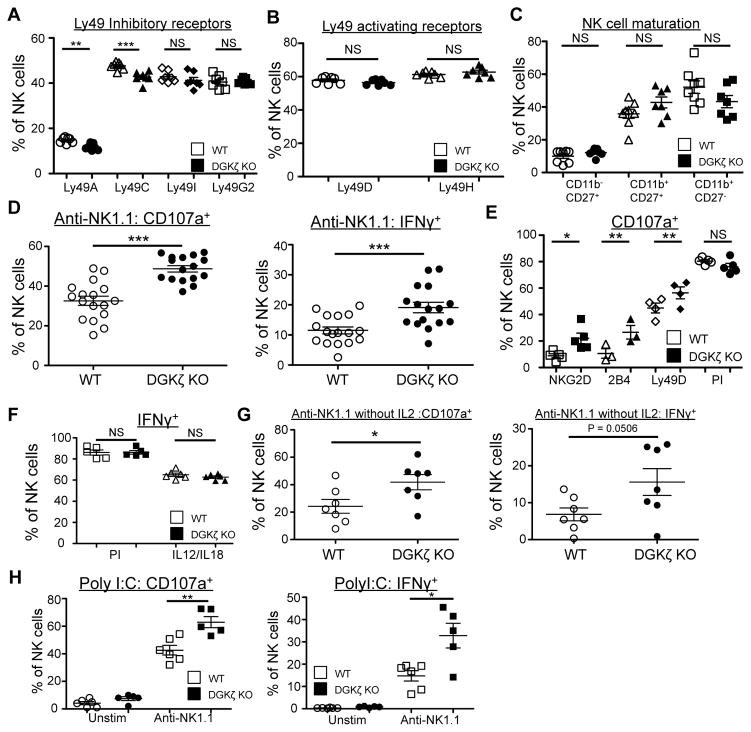
Figure 1. DGKζ-deficient NK cells exhibit enhanced function downstream of activating receptors.
The proportion of NK (CD3− NK1.1+) cells expressing A) inhibitory and B) activating Ly49 receptors, and C) CD27/CD11b in WT and DGKζ KO cells is shown. N=7. D) Splenocytes from WT and DGKζ KO mice were stimulated with plate-bound anti-NK1.1 antibody. The proportion of NK cells (CD3−DX5+NKp46+) labeled with anti-CD107a (left) and intracellular anti-IFNγ antibody (right) is shown. N=17. E) Splenocytes from WT and DGKζ KO mice were stimulated with plate-bound antibodies against the indicated activating receptors or with PMA/Ionomycin (PI). The proportion of NK cells (CD3−DX5+NKp46+) labeled with anti-CD107a antibody is shown. N=4–5 per condition. F) Splenocytes from WT and DGKζ KO mice were stimulated with PMA/ionomycin or with IL-12 and IL-18. The proportion of NK cells (CD3−DX5+NKp46+) expressing intracellular anti-IFNγ is shown. N=5–6 per condition G) Splenocytes from WT and DGKζ KO mice were stimulated with plate-bound anti-NK1.1 antibody without IL-2. The proportion of NK cells (CD3−DX5+NKp46+) labeled with anti-CD107a (left) and intracellular anti-IFNγ antibody (right) is shown. N=7. H) Splenocytes from polyI:C-treated WT and DGKζ KO mice were stimulated with plate-bound anti-NK1.1 antibodies. The proportion of NK cells (CD3−DX5+NKp46+) labeled with anti-CD107a (left) and intracellular anti-IFNγ antibody (right) is shown. N=5. *, ** and *** represent statistical significance of p<0.05, p<0.01, and p<0.001 by Student’s t-test, respectively. NS = not significant. Data shown are compiled from 2 separate experiments are shown in A–C and E, and from at least 3 separate experiments for figures D, F and G.
NK cell functional assays
Splenocytes from B6 or DGKζ KO mice were stained with anti-DX5-biotin antibody, enriched using anti-biotin MACs beads (Miltenyi Biotec), and expanded in hIL-2 (1000 U/ml) in tissue culture media for at least 5 days to create lymphokine activated killer (LAK) cells. After 5 days of expansion, ~80–90% of the LAK cells in culture were NK cells (CD4−CD8−NK1.1+DX5+). LAK cells were used for assays after resting in fresh cytokine-free media for at least 3 hours to reduce background activation of the expanded NK cells.
For NK cell activation assays, freshly isolated splenocytes or LAK cells were cultured together with anti-CD107a antibody and Monensin for 6 hours in tissue culture plates that were pre-coated with antibodies against NK cell activating receptors (20 μg/ml, overnight at 4°C unless otherwise specified). In experiments that examined inhibitory receptor function, anti-Ly49G2 antibody was also added (20 μg/ml, overnight at 4°C) to the wells together with anti-NK1.1 (20 μg/ml). For experiments that used freshly isolated splenocytes, 1000U of hIL-2 was added (except for Fig. 1G) to make the responsiveness of NK cell more robust and consistent. The addition of exogenous hIL-2 alone did not stimulate IFNγ or CD107a upregulation in NK cells of WT or DGKζ origin (Supplementary Fig. 1). Unstimulated controls shown in the figures did not contain exogenous hIL-2. After 6h in culture, cells were analyzed for anti-CD107a antibody staining and intracellular IFNγ by flow cytometry. In experiments involving the MEK inhibitor U0126, splenocytes were pre-incubated with the specified concentration of the inhibitor for 30 min before stimulation, and the inhibitor was maintained throughout the assay.
A luciferase expressing YAC-1 cell line was co-cultured with LAK cells at varying E:T ratios for 4 hours for bioluminescent cytotoxic assays as previously described (19). Luciferase activity was detected via an IVIS Lumina II imaging system and % specific lysis was calculated by the following formula: (minimum - test well) / (minimum – maximum) × 100%. For the cytotoxicity assay with the RMA/RMA-S cell lines, LAK cells were co-cultured with the cell line at varying E:T ratios for 6 hours, and the % of dead RMA or RMA-S cells was determined by live/dead staining and flow cytometric analysis. % specific lysis was measured by the following formula: (% dead cells in test well – % baseline death of cell line) / (100 – % baseline death of cell line). To measure cytokine production after co-culture with YAC-1 cells, LAK cells were co-cultured at a 1:1 ratio with either no targets or YAC-1 target cells for 24h in LAK media. The IFNγ content in cell-free supernatants was determined by ELISA (Biolegend).
Poly I:C injections in vivo
Poly I:C activation of NK cells in vivo was performed as previously described (20). In brief, WT B6 or DGKζ KO mice were injected with 250μg of poly I:C intraperitoneally. 18h after injection, splenocytes were harvested from the mice for functional analysis.
Mixed BM chimeras
BM (5 × 106 cells) from control B6 or DGKζ KO mice were mixed with CD45.1/45.2 heterozygous competitor BM (5 × 106 cells) and injected i.v. into lethally irradiated B6.SJL congenic host mice (9.5 Gy). Splenocytes were taken from the BM chimeras between 9–12 wk later for functional analysis.
Acute deletion of DGKζ floxed alleles using ERCreT2
DGKζF/F Rosa26-Stop-Flox-YFP ERCreT2 or control Rosa26-Stop-Flox-YFP ERCreT2 mice were treated with Tamoxifen for 5 days as previously described (21). 1 week after the end of treatment, splenocytes were removed for functional analysis.
Western blot analysis
MACS-enriched splenic DX5+ NK cells (pERK, total ERK) or LAK cells were rested for 2–4 hours, and then stimulated with PK136 Ab (30 μg/ml) for the indicated times. The cells were then lysed in 1% Ipegal in Tris-buffered saline with protease/phosphatase inhibitors (protease inhibitor cocktail solution [Roche, Sigma]), and the proteins were resolved by SDS-PAGE (Bio-Rad Laboratories, Hercules, CA). The levels of phosphorylated ERK1/2 (Thr202/Tyr204), total ERK, phosphorylated AKT (Ser473), and total IkBα were analyzed by Western blotting. Total PLCγ2 or beta-actin was used as a loading control. All blots were quantified using Fiji (ImageJ). All antibodies were from Cell Signaling (Danvers, MA), except for anti-beta-actin-HRP antibody (Sigma)
In vivo tumor challenges
In experiments involving long-term tumor burden, RMA-S cells were injected subcutaneously (1 × 106 cells) into WT or DGKζ KO mice. 12–15 days after injection, the mice were euthanized, and tumors were harvested and weighed.
For analysis of short-term tumor rejection, RMA and RMA-S cells were labeled with CFSE and CellTrace violet, respectively and injected i.v. at a 1:3 ratio (20 × 106 cells total) into WT or DGKζ KO mice. 18 hours after injection, spleens were harvested from these mice and the presence of tumor cells was analyzed by flow cytometry. In some experiments, NK cell depletion was performed by injecting anti-NK1.1 antibody (PK136 200 μg i.p.) 24 hours before tumor challenge.
Results
DGKζ KO but not DGKα KO NK cells are hyperresponsive to activating receptor stimulation
NK cells from WT and DGKζ KO mice were stimulated through multiple cell surface activating receptors. Although slight decreases in the proportion of NK cells expressing Ly49A, Ly49C, and 2B4 was seen in DGKζ KO compared to WT NK cells, the development of NK cells was largely similar between WT and DGKζ KO mice with regards to inhibitory receptor expression, activating receptor expression, and maturity (Fig. 1 A–C, Supplementary Table I). Upon activation through three distinct activating receptor families (ITAM-dependent: NK1.1, Ly49D; costimulatory-like: NKG2D; SAP-dependent: 2B4), an increased fraction of DGKζ KO NK cells degranulated and produced IFNγ compared to WT NK cells (Fig. 1D, 1E). Importantly, IFNγ production downstream of cytokine activation (IL-12 + IL-18) or by PMA/ionomycin was similar between DGKζ KO and WT NK cells (Fig. 1F). Since exogenous IL-2 was added to the NK cell stimulation assays to make the stimulations more robust and consistent, we additionally tested whether DGKζ deficiency augmented the activity of NK cells in the absence of exogenously added IL-2. Although the response of NK cells was more variable, an increased fraction of DGKζ KO NK cells degranulated and produced IFNγ compared to WT NK cells stimulated with anti-NK1.1 antibody in the absence of IL-2 (Fig. 1G), suggesting that DGKζ deficiency augmented activating receptor-mediated stimulation. Increased NK cell function was also observed in NK cells isolated from DGKζ KO mice treated with Poly I:C, which mimics a viral infection and primes NK cell responses through type I IFN (Fig. 1H). Thus, DGKζ deficiency enhances NK cell function even when isolated from an inflammatory environment.
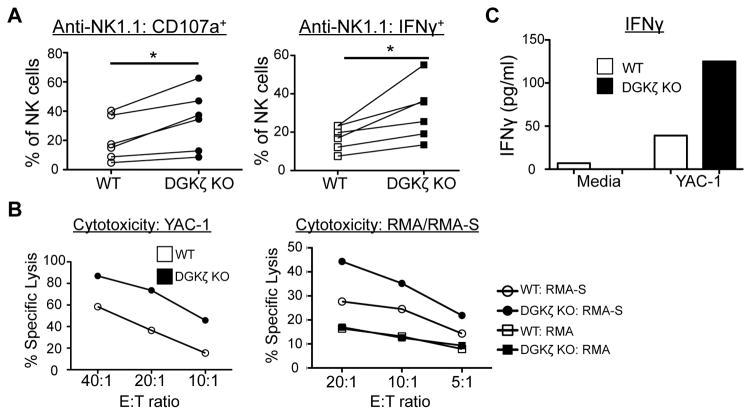
Next, to directly examine cytotoxicity by NK cells, WT and DGKζ KO NK cells were expanded in IL-2 to create lymphokine-activated killer (LAK) cells. Similar to freshly isolated NK cells, an increased proportion of DGKζ KO LAK cells degranulated and produced IFNγ upon activating receptor stimulation (Fig. 2A). Moreover, this correlated with an increased ability of DGKζ KO LAK cells to kill the NK cell-sensitive tumor cell line, YAC-1, and against the MHC class I-deficient tumor cell line, RMA-S (Fig. 2B). Importantly, no differences in cytotoxicity were seen between the ability of WT and DGKζ KO LAK cells to kill MHC class I-sufficient RMA cells (Fig. 2B). Increased IFNγ release was also detected by ELISA when DGKζ KO LAK cells were cocultured with the YAC-1 cell line (Fig 2C). Although we consistently observed an increase in cytoxicity and IFNγ production by DGKζ KO LAK cells in all experiments, the results were not statistically significant (Supplementary Fig. 2).
Figure 2. DGKζ KO LAK cells display increased cytotoxicity and cytokine production upon interaction with tumor cells.
A) WT or DGKζ KO LAK cells were stimulated with plate-bound anti-NK1.1 antibodies. The proportion of NK cells (CD4−CD8−NK1.1+DX5+) labeled with anti-CD107a (left) and intracellular anti-IFNγ antibody (right) is shown. Data from 6 independent experiments is shown (*P<0.05 by paired t-test). N=6 B) WT or DGKζ KO LAK cells were co-cultured with YAC-1, RMA, or RMA-S cells at the indicated E:T ratios and % specific lysis was determined 4–6 hours later. C) WT or DGKζ KO LAK cells were plated with or without YAC-1 cells at a 1:1 ratio for 24 hours. IFNγ content in the cell-free supernatants was determined by ELISA. One representative of N=3 independent experiments is shown for B and C.
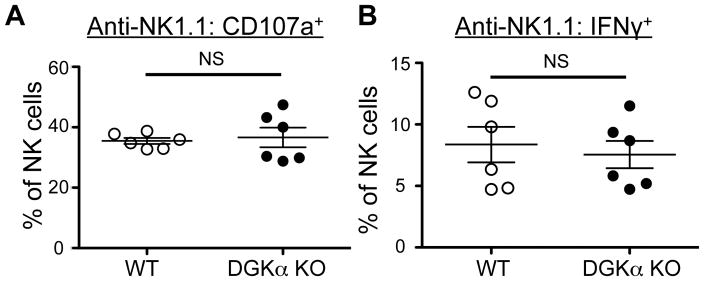
Previous studies have highlighted the importance of other isoforms of DGK such as DGKα in T cell activation (18, 22). Thus, to test if DGKα was also important in NK cell function, we stimulated NK cells from WT and DGKα KO mice using platebound anti-NK1.1 antibody. We found that there was no significant difference between DGKα KO mice and WT controls in both degranulation and cytokine production, suggesting that DGKα does not contribute to limiting NK cell function (Fig 3A, 3B).
Figure 3. DGKα NK cells are not hyperresponsive compared to WT NK cells.
Splenocytes from WT and DGKα KO mice were stimulated with plate-bound anti-NK1.1 antibody. The proportion of NK cells (CD3−DX5+NKp46+) labeled with A) anti-CD107a and B) intracellular anti-IFNγ antibody is shown. NS = not significant by Student’s t-test. Data shown are compiled from 2 separate experiments, N=6.
DGKζ KO NK cells are hyperresponsive in an NK cell-intrinsic and developmentally independent manner
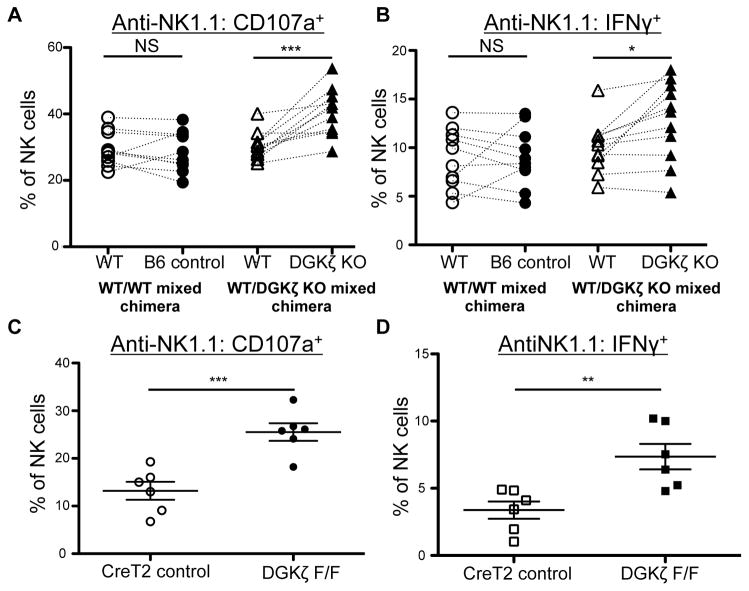
Since DGKζ KO mice display increased activation in multiple hematopoietic lineages, it was conceivable that the hyperresponsive phenotype of NK cells was not NK cell-intrinsic. To test this possibility, bone marrow (BM) chimeric mice were created by BM transplantation of WT competitor BM mixed with WT control or DGKζ KO BM. Upon stimulation of NK cells from these mice, NK cells in the WT competitor/WT control BM chimeric mice were similarly responsive, while NK cells of DGKζ KO origin in the WT competitor/DGKζ KO BM chimeras were significantly more responsive than the WT competitor controls (Fig. 4A, B). Next, to test whether the hyperresponsive phenotype of DGKζ KO NK cells was independent of altered NK cell development, we acutely deleted DGKζ from NK cells using a Tamoxifen-inducible Cre (Cre-ERT2) system. NK cells from Tamoxifen-treated mice bearing floxed alleles of DGKζ were more responsive to activating receptor stimulation than Cre+ WT controls (Fig. 4C, 4D). Together, these data suggest that DGKζ deficiency enhances NK cell responsiveness to activating receptor stimuli in an NK cell-intrinsic and developmentally independent manner.
Figure 4. DGKζ KO NK cells are hyperresponsive in a cell-intrinsic and developmentally independent manner.
A) Splenocytes from WT/WT control and WT/DGKζ KO mixed BM chimeras were stimulated with plate-bound anti-NK1.1 antibody. The proportion of NK cells (CD3−DX5+NKp46+) labeled with anti-CD107a and B) intracellular anti-IFNγ antibody is shown. Cells derived from either the B6 control BM or DGKζ KO bone marrow were paired with cells that were of WT competitor BM origin within the same mouse. N=11. C) Splenocytes from Tamoxifen-treated DGKζF/F Rosa26-STOP-Flox-YFP ERCreT2 or Rosa26-STOP-Flox-YFP ERCreT2 control mice were stimulated with plate-bound anti-NK1.1 antibody. The proportion of NK cells (CD3−DX5+NKp46+YFP+ lymphocytes) labeled with anti-CD107a and D) intracellular anti-IFNγ antibody is shown. N=6. *, ** and *** represent a statistical significance by paired t-test (A and B) or Student’s t-test (C and D) of p<0.05, p<0.01 and p<0.001 respectively. NS= not significant. All data shown in this figure is compiled from at least 3 independent experiments.
The hyperresponsive phenotype of DGKζ KO NK cells is associated with a trend towards enhanced ERK activation
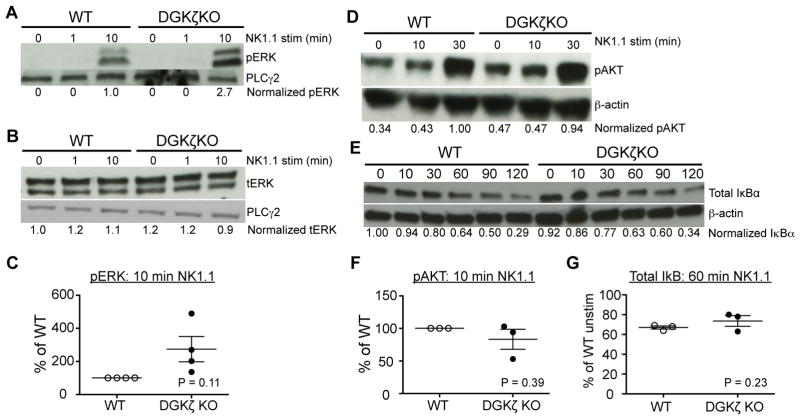
DGKζ deficiency has been shown to enhance at least 3 distinct downstream signaling pathways: ERK, AKT, and NFκB. To test which of these pathways are altered in DGKζ KO NK cells, we stimulated WT or DGKζ KO NK cells with anti-NK1.1 antibody, and analyzed the levels of pERK, pAKT and IκB degradation by Western blot analysis. Consistent with previous results, DGKζ KO NK cells consistently displayed increased levels of pERK upon stimulation (although not statistically significant due to variability in the fold-increase), while showing no difference in the expression of total ERK (Fig. 5A–C), suggesting that the fraction of pERK was increased in DGKζ KO NK cells. However, to our surprise, we found that stimulation of DGKζ KO NK cells resulted in no difference in pAKT or IκB degradation compared to WT controls (Fig. 5D–F).
Figure 5. DGKζ KO NK cells display enhanced ERK, but not AKT or NFκB, signaling downstream of activating receptor stimulation.
A) Fresh splenic NK cells were left unstimulated or stimulated with anti-NK1.1 antibody for the indicated duration. Cell lysates were analyzed for pERK and B) total ERK by Western blot analysis. C) Compiled data showing the relative levels of pERK in WT versus DGKζ KO NK cells (after quantification against loading controls) at 10 min post-stimulation are plotted. D) Lysates from anti-NK1.1-stimulated LAK cells were analyzed for pAKT or E) IκBα by Western blot analysis. F) Compiled data showing the relative levels of pAKT in WT versus DGKζ KO NK cells (after quantification against loading controls) at 10 min post-stimulation are plotted. G) The relative levels of total IκB in WT versus DGKζ KO NK cells after 60 min of stimulation with anti-NK1.1 antibodies, as compared to unstimulated cells (and after quantification against loading controls) are plotted. Quantification of protein against loading controls was performed using ImageJ software, and quantified protein levels were then normalized against either unstimulated (B and D) or stimulated (A and C) WT NK cells. Total PLCγ2 or β-actin was used as a loading control. Data shown is representative of at least 3 independent experiments. Statistical analysis was performed by (E, F) Student’s t-test or (G) paired t-test.
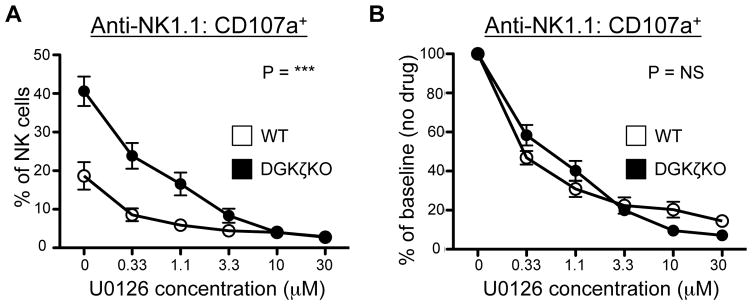
To test whether the enhancement in pERK was responsible for the increased function of DGKζ KO NK cells, we treated anti-NK1.1 antibody-activated NK cells with the MEK inhibitor U0126. The degranulation of WT NK cells was inhibited by U0126 in a dose-dependent manner, suggesting that pERK was important for NK cell degranulation. Although DGKζ KO NK cells were also inhibited by U0126 in a dose-dependent manner, they required a higher concentration of U0126 to completely attenuate their ability to degranulate (Fig. 6A). Moreover, the % of functional inhibition (% of baseline) observed at each concentration of MEK inhibitor was similar between WT and DGKζ KO NK cells (Fig. 6B). Given that U0126 is a non-competitive MEK inhibitor (23), these data suggest that the pERK level observed in anti-NK1.1-stimulated DGKζ KO NK cells is not at a functionally saturating level. Together with increased pERK activation, these data suggest that increased pERK contributes to the enhanced responsiveness of DGKζ KO NK cells.
Figure 6. Enhanced ERK signaling is associated with the hyperresponsiveness of DGKζ KO NK cells.
WT or DGKζ KO splenocytes were stimulated with plate-bound anti-NK1.1 antibody in the presence of various concentrations of the MEK inhibitor U0126. A) The proportion of NK cells (CD3−DX5+NKp46+) incorporating anti-CD107a antibody is shown. B) The percentage of responding cells as compared to no drug control (% of baseline) is plotted. N=3–5 per concentration. *** = P<0.001, NS= not significant by 2 way-ANOVA. Data shown is complied from at least 3 independent experiments.
DGKζ KO NK cells are licensed and exhibit intact inhibitory receptor function
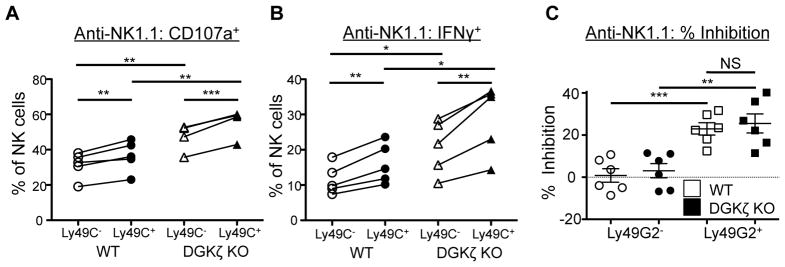
One unique function of NK cells is their ability to recognize reduced MHC class I expression (missing self) on target cells through their inhibitory receptors. To test whether DGKζ deficiency affected the function of inhibitory receptors, we examined whether DGKζ KO NK cells displayed intact licensing (24), a process by which an NK cell that expresses an inhibitory receptor for self-MHC I (e.g., Ly49C in B6 mice) is more likely to respond than one that does not. Both WT and DGKζ KO NK cells that expressed Ly49C+ were more likely to respond to activating receptor stimulation than their Ly49C− counterparts, suggesting that licensing and therefore inhibitory receptor signaling was intact in these mice. Importantly, both Ly49C+ and Ly49C− DGKζ KO NK cells were hyperresponsive compared to their WT subset counterparts, suggesting that licensing was not the major cause of NK cell hyperreponsiveness in DGKζ KO mice (Fig. 7A, 7B).
Figure 7. DGKζ deficiency does not affect NK cell inhibitory receptor function.
A) Splenocytes from WT and DGKζ KO mice were stimulated with plate-bound anti-NK1.1 antibody. The proportion of Ly49C+ versus Ly49C−NK cells (CD3−DX5+NKp46+) labeled with anti-CD107a and B) intracellular anti-IFNγ antibody (right) is shown. Data shown is compiled from 2 independent experiments, N=5. C) Splenocytes from WT and DGKζ KO mice were stimulated with plate-bound anti-NK1.1 antibody in the absence or presence of anti-Ly49G2 antibody. The percentage inhibition of degranulation (as measured by anti-CD107a labeling) in Ly49G2− or Ly49G2+ NK cells by the addition of the anti-Ly49G2 antibody is shown by scatter plot. Data shown is compiled from 2 independent experiments, N=6.
To further test if the loss of DGKζ in NK cells affected inhibitory receptor function, we tested whether co-ligation of an inhibitory Ly49 receptor would attenuate NK cell function in DGKζ KO NK cells. NK cells from WT or DGKζ KO mice were stimulated with anti-NK1.1 antibody with or without an antibody against the inhibitory receptor Ly49G2. Upon co-ligation of Ly49G2, the degranulation of Ly49G2+ NK cells from WT and DGKζ KO was similarly reduced by ~25% (Fig. 7C). Importantly, no inhibition of degranulation was seen in Ly49G2− NK cells from either WT or DGKζ KO mice. These data suggest that DGKζ deficiency did not alter the function of inhibitory receptors.
DGKζ KO NK cells exhibit increased clearance of TAP-deficient tumor cells in vivo
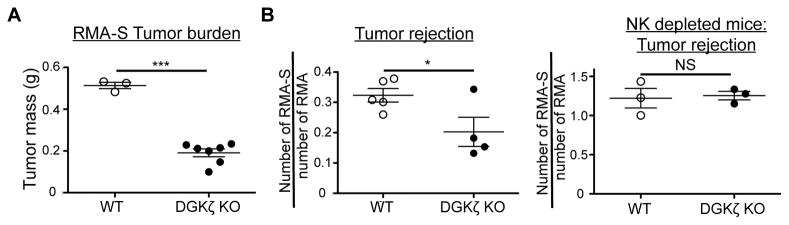
To test whether DGKζ deficiency leads to improved anti-tumor responses in vivo, we subcutaneously injected WT and DGKζ KO mice with RMA-S tumor cells. 12–15 days post injection, rapid tumor growth was observed in WT mice injected with RMA-S cells. Although tumors also grew in DGKζ KO mice, there was a significant reduction in tumor burden compared to WT controls. These data suggest that DGKζ deficiency enhances protection against tumors by delaying tumor growth in vivo (Fig. 8A).
Figure 8. DGKζ KO NK cells are more effective at effectively clearing TAP-deficient tumor cells.
A) RMA-S tumor cells were injected s.c. into WT or DGKζ KO mice. 12 days later, the tumors were harvested and weighed. One representative of 2 independent experiments is shown. B) NK cell-sufficient (left) or NK cell-depleted (right) WT or DGKζ KO mice were injected i.v. with a mixture of CFSE-labeled RMA and Celltrace Violet-labeled RMAS-S tumors. 18 hours later, splenocytes were analyzed for the presence of residual tumor cells via flow cytometry. The ratio of RMA-S versus RMA within each WT or DGKζ KO mouse was calculated and shown as a scatter plot. One representative of 2 independent experiments is shown. *, ** and *** represent a statistical significance by Student’s t-test of p<0.05, p<0.01 and p<0.001 respectively. NS = not significant.
To further test if this protection was NK cell specific, we performed an additional experiment where both RMA (TAP-sufficient) and RMA-S (TAP-deficient) tumor cells were differentially labeled with dyes and injected at a 1:3 ratio into WT or DGKζ KO hosts. 18 hours after injection into hosts, we found that the RMA-S:RMA ratio was significantly reduced in DGKζ KO compared to WT mice, suggesting that DGKζ KO NK cells were better at clearing MHC-Class I deficient tumor cells than WT NK cells. This difference was lost when mice were depleted of NK cells prior to the tumor challenge, suggesting that DGKζ KO NK cells were responsible for the significant increase in the rejection of the RMA-S cells (Fig 8B).
Discussion
In the present study, we tested the role of DGKζ in NK cell function. Using genetic ablation, we showed that the absence of DGKζ in NK cells lead to increased function of NK cells upon stimulation through multiple activating receptors in vitro. Moreover, this enhanced function correlated with an increased ability of DGKζ KO NK cells to exhibit increased rejection and limit growth of an MHC class I-deficient target cell in vivo. Thus, DGKζ serves to limit NK cell activation and could represent a novel target for the enhancement of NK cell function.
As DGKζ is a negative regulator of DAG signaling, one might predict that all immune cells that lack DGKζ would be hyperresponsive to activating stimuli. Indeed, DGKζ KO T cells show enhanced activation and resist anergy induction upon TCR stimulation (25, 26). However, the absence of DGKζ does not always lead to an enhancement in all cellular functions. For example, DGKζ-deficient mast cells produce more cytokines but display reduced degranulation upon IgE receptor engagement (27). Thus, depending on the cell type or cellular function, the responses that are regulated by DGKζ could be different. Thus, it was important to test the role of DGKζ in regulating NK cell function. Interestingly, we found that both degranulation (cytotoxicity) and cytokine production were increased in DGKζ KO NK cells compared to WT NK cells, suggesting that degranulation in mast cells and NK cells are potentially regulated by different signaling pathways.
To the best of our knowledge, this study is the first to demonstrate that a negative regulator of activating signals can be targeted in NK cells to improve their function. Previous attempts to enhance the function of NK cells have been hampered by the ability of NK cells to quickly tune their activation threshold to the environment. NK cells that lack enzymes that negatively regulate proximal signaling pathways (SHP-1 and SHIP) tune their responsiveness, which results in NK cells with diminished function (11, 12). Similarly, NK cells subjected to an MHC I-deficient environment also leads to hyporesponsiveness (15), as do NK cells that experience constant stimulation through their activation receptors (14). However, in each of these situations, NK cells are normally responsive to PMA and ionomycin, suggesting that the tuning process occurs upstream of PLCγ activation. Thus, in this study, we hypothesized that the removal of DGKζ, which is a negative downstream of PLCγ function, would enhance the function of NK cells downstream of the three major pathways of activating signals (8). Indeed, we found that DGKζ KO NK cells showed an increased propensity to degranulate and produce cytokines after activation through receptors that associate with either SAP or with adaptor molecules containing ITAM or costimulatory-like domains.
In addition to bypassing tuning, targeting a negative regulator of distal signaling pathways could be beneficial for the retention of inhibitory signaling pathways that are required for missing-self recognition by NK cells. The removal of negative regulators that are directly associated with MHC I-binding inhibitory receptors such as SHP-1 and SHIP abrogates the ability of NK cells to conduct missing self recognition (12, 28). In contrast, our data demonstrate that the loss of DGKζ does not affect the ability of inhibitory receptors to attenuate activation and license NK cells. Thus, missing-self recognition by DGKζ KO NK cells remains intact as evidenced by increased killing of the RMA-S cell line.
One potential caveat with our studies is that our NK cell preparation could be contaminated with T cells, which may confound our interpretations. In experiments using LAK cells, the NK cell purity was typically in the 80–90% range. Although we used T cell markers (CD4 and CD8) to avoid measuring T cell activation in our flow cytometry-based assays, T cells could potentially contribute to cytotoxicity in assays involving co-culture with tumor cell targets or in vivo tumor rejection. However, we believe that the contribution of T cells to these assays is minimal, given that RMA-S cells express very low levels of MHC class I and are prime targets for missing self recognition by NK cells. Furthermore, no enhancement in killing was observed against MHC class I-sufficient RMA cells by the LAK cells derived from DGKζ KO mice. In our in vivo RMA-S rejection assay, NK cell depletion abrogated the ability of NK cells to skew the RMA-S:RMA ratio in WT and DGKζ KO mice, suggesting that the enhancement in RMA-S killing in vivo was NK cell-dependent. Hence, we conclude that it is likely that the enhanced activity we see in our data is derived from NK cells, and not from T cell contaminants.
To test the contribution of signaling pathways downstream of DAG that were responsible for the enhanced NK cell function, we examined the activation of ERK, AKT, and NFκB signaling pathways. In contrast to what has been reported in T cells (17, 18), DGKζ KO NK cells exhibited increased pERK, but not pAKT or enhanced IκBα degradation, suggesting that increased pERK could be a major contributor to the enhanced NK cell function seen in DGKζ KO NK cells. Consistent with previous findings (29), we found that the addition of a MEK inhibitor suppressed NK cell degranulation in a dose-dependent manner. Importantly, the NK cell response in WT and DGKζ KO NK cells was reduced by the same magnitude even at the lowest doses of the MEK inhibitor. These data suggest that the elevation of pERK seen in DGKζ KO NK cells is functionally meaningful (not saturated) and supports the notion that elevated pERK contributes to heightened NK cell function in the absence of DGKζ.
Aside from DGKζ, DGKα is another negative regulator of DAG signaling in lymphocytes. DGKα seems to possess both positive and negative roles in T cell function. Although the phenotype is weaker than seen with DGKζ KO T cells, DGKα KO T cells display increased cytokine production and phosphorylation of ERK (18, 25), suggesting that DGKα negatively regulates TCR signaling. DGKα is involved in the stabilization of the immune synapse IS in T cells (22), suggesting that DGKα also plays a positive role in T cell function. However, in NK cells, we found that the loss of DGKα did not influence NK cell effector function. Thus, our data suggest DGKα does not negatively regulate signaling downstream of NK cell activating receptors. However, a more in-depth study into the role that DGKα plays in IS formation in NK cells could potentially demonstrate a role for DGKα in NK cell function.
Overall, our data support a model where NK cell tuning acts proximal to the generation of DAG, suggesting that therapeutic targeting of distal negative regulators of NK cell activation may represent a strategy for improving the clinical efficacy of NK cells. In particular, our data regarding the acute deletion of DGKζ in NK cells, as well as its key position in T cell function, highlights its potential as a specific target for clinical purposes in anti-tumor responses (30).
Supplementary Material
Acknowledgments
We would like to thank Matthew Riese for the DGKζ floxed mice.
Abbreviations used in this article
- NK
natural killer
- DGKζ
diacylglycerol kinase zeta
- NK1.1
NKRP1-C
- SHP-1
Src homology region 2 domain-containing phosphatase 1
- DAG
diacylglycerol
- DGKζ KO
DGKζ deficient
- DGKα KO
DGKα deficient
- DGKζ F/F
DGKζ floxed mice
Footnotes
This work was supported by grants from the Translational Center of Excellence in Hematological Malignancies of the Abramson Cancer Center and the National Institutes of Health (R01HL107589, R01HL111501)
References
- 1.Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8+ T cells. Nature Publishing Group. 2011;11:645–657. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanier LL. NK CELL RECOGNITION. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 4.Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cellular and Molecular Immunology. 2013;10:230–252. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes RL, Koslow M, Hiesiger EM, Hymes KB. Improved long term survival after intracavitary interleukin-2 and lymphokine-activated killer cells for adults with recurrent malignant glioma. Cancer. 1995 doi: 10.1002/1097-0142(19950901)76:5<840::aid-cncr2820760519>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Hsu KC, Gooley T, Malkki M, Pinto-Agnello C, Dupont B, Bignon JD, Bornhäuser M, Christiansen F, Gratwohl A, Morishima Y, Oudshoorn M, Ringden O, van Rood JJ, Petersdorf E. KIR Ligands and Prediction of Relapse after Unrelated Donor Hematopoietic Cell Transplantation for Hematologic Malignancy. Biology of Blood and Marrow Transplantation. 2006;12:828–836. doi: 10.1016/j.bbmt.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Uherek C, Tonn T, Uherek B, Becker S, Schnierle B, Klingemann HG, Wels W. Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood. 2002;100:1265–1273. [PubMed] [Google Scholar]
- 8.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.May RM, Okumura M, Hsu CJ, Bassiri H, Yang E, Rak G, Mace EM, Philip NH, Zhang W, Baumgart T, Orange JS, Nichols KE, Kambayashi T. Murine natural killer immunoreceptors use distinct proximal signaling complexes to direct cell function. Blood. 2013;121:3135–3146. doi: 10.1182/blood-2012-12-474361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tassi I, Presti R, Kim S, Yokoyama WM, Gilfillan S, Colonna M. Phospholipase C-gamma 2 is a critical signaling mediator for murine NK cell activating receptors. J Immunol. 2005;175:749–754. doi: 10.4049/jimmunol.175.2.749. [DOI] [PubMed] [Google Scholar]
- 11.Lowin-Kropf B, Kunz B, Beermann F, Held W. Impaired natural killing of MHC class I-deficient targets by NK cells expressing a catalytically inactive form of SHP-1. J Immunol. 2000;165:1314–1321. doi: 10.4049/jimmunol.165.3.1314. [DOI] [PubMed] [Google Scholar]
- 12.Gumbleton M, Vivier E, Kerr WG. SHIP1 Intrinsically Regulates NK Cell Signaling and Education, Resulting in Tolerance of an MHC Class I-Mismatched Bone Marrow Graft in Mice. The Journal of Immunology. 2015;194:2847–2854. doi: 10.4049/jimmunol.1402930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Höglund P, Brodin P. Current perspectives of natural killer cell education by MHC class I molecules. Nature Publishing Group. 2010;10:724–734. doi: 10.1038/nri2835. [DOI] [PubMed] [Google Scholar]
- 14.Tripathy SK, Keyel PA, Yang L, Pingel JT, Cheng TP, Schneeberger A, Yokoyama WM. Continuous engagement of a self-specific activation receptor induces NK cell tolerance. Journal of Experimental Medicine. 2008;205:1829–1841. doi: 10.1084/jem.20072446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ardolino M, Azimi CS, Iannello A, Trevino TN, Horan L, Zhang L, Deng W, Ring AM, Fischer S, Garcia KC, Raulet DH. Cytokine therapy reverses NK cell anergy in MHC-deficient tumors. J Clin Invest. 2014;124:4781–4794. doi: 10.1172/JCI74337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu CH, Machado FS, Guo R, Nichols KE, Burks AW, Aliberti JC, Zhong XP. Diacylglycerol kinase regulates microbial recognition and host resistance to Toxoplasma gondii. Journal of Experimental Medicine. 2007;204:781–792. doi: 10.1084/jem.20061856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt AM, Zou T, Joshi RP, Leichner TM, Pimentel MA, Sommers CL, Kambayashi T. Diacylglycerol Kinase Limits the Generation of Natural Regulatory T Cells. Science Signaling. 2013;6:ra101–ra101. doi: 10.1126/scisignal.2004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi RP, Schmidt AM, Das J, Pytel D, Riese MJ, Lester M, Diehl JA, Behrens EM, Kambayashi T, Koretzky GA. The Isoform of Diacylglycerol Kinase Plays a Predominant Role in Regulatory T Cell Development and TCR-Mediated Ras Signaling. Science Signaling. 2013;6:ra102–ra102. doi: 10.1126/scisignal.2004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karimi MA, Lee E, Bachmann MH, Salicioni AM, Behrens EM, Kambayashi T, Baldwin CL. Measuring Cytotoxicity by Bioluminescence Imaging Outperforms the Standard Chromium-51 Release Assay. PLoS ONE. 2014;9:e89357. doi: 10.1371/journal.pone.0089357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyake T, Kumagai Y, Kato H, Guo Z, Matsushita K, Satoh T, Kawagoe T, Kumar H, Jang MH, Kawai T, Tani T, Takeuchi O, Akira S. Poly I:C-Induced Activation of NK Cells by CD8 + Dendritic Cells via the IPS-1 and TRIF-Dependent Pathways. The Journal of Immunology. 2009;183:2522–2528. doi: 10.4049/jimmunol.0901500. [DOI] [PubMed] [Google Scholar]
- 21.Wu GF, Corbo E, Schmidt M, Smith-Garvin JE, Riese MJ, Jordan MS, Laufer TM, Brown EJ, Maltzman JS. Conditional deletion of SLP-76 in mature T cells abrogates peripheral immune responses. Eur J Immunol. 2011;41:2064–2073. doi: 10.1002/eji.201040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chauveau A, Le Floc’h A, Bantilan NS, Koretzky GA, Huse M. Diacylglycerol kinase α establishes T cell polarity by shaping diacylglycerol accumulation at the immunological synapse. Science Signaling. 2014;7:ra82. doi: 10.1126/scisignal.2005287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 24.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 25.Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, Zhong XP. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol. 2006;7:1174–1181. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]
- 26.Riese MJ, Grewal J, Das J, Zou T, Patil V, Chakraborty AK, Koretzky GA. Decreased Diacylglycerol Metabolism Enhances ERK Activation and Augments CD8+ T Cell Functional Responses. J Biol Chem. 2011;286:5254–5265. doi: 10.1074/jbc.M110.171884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olenchock BA. Impaired degranulation but enhanced cytokine production after Fc RI stimulation of diacylglycerol kinase -deficient mast cells. Journal of Experimental Medicine. 2006;203:1471–1480. doi: 10.1084/jem.20052424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viant C, Fenis A, Chicanne GET, Payrastre B, Ugolini S, Vivier E. SHP-1-mediated inhibitory signals promoteresponsiveness and anti-tumour functionsof natural killer cells. Nature Communications. 2014;5:1–11. doi: 10.1038/ncomms6108. [DOI] [PubMed] [Google Scholar]
- 29.Yu TK, Caudell EG, Smid C, Grimm EA. IL-2 activation of NK cells: involvement of MKK1/2/ERK but not p38 kinase pathway. J Immunol. 2000;164:6244–6251. doi: 10.4049/jimmunol.164.12.6244. [DOI] [PubMed] [Google Scholar]
- 30.Riese MJ, Wang LCS, Moon EK, Joshi RP, Ranganathan A, June CH, Koretzky GA, Albelda SM. Enhanced Effector Responses in Activated CD8+ T Cells Deficient in Diacylglycerol Kinases. Cancer Research. 2013;73:3566–3577. doi: 10.1158/0008-5472.CAN-12-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.