Abstract
Background
Induction therapy in deceased donor kidney transplantation (DDKT) is costly, with wide discrepancy in utilization and a limited evidence base, particularly regarding cost-effectiveness.
Methods
We linked the United States Renal Data System dataset to Medicare claims to estimate cumulative costs, graft survival, and incremental cost-effectiveness ratio (ICER –cost per additional year of graft survival) within 3 years of transplantation in 19,450 DDKT recipients with Medicare as primary payer from 2000 to 2008. We divided the study cohort into high-risk (age>60 years, panel reactive antibody>20%, African American race, Kidney Donor Profile Index>50%, cold ischemia time>24 hours) and low risk (not having any risk factors, comprising approximately 15% of the cohort). Following the elimination of dominated options, we estimated expected ICER among induction categories: no-induction, alemtuzumab, rabbit anti-thymocyte globulin (r-ATG), and interleukin-2 receptor-antagonist.
Results
No-induction was the least effective and most costly option in both risk groups. Depletional antibodies (r-ATG and alemtuzumab) were more cost-effective across all willingness-to-pay thresholds in the low-risk group. For the high-risk group and its subcategories, the ICER was very sensitive to the graft survival; overall both depletional antibodies were more cost-effective, mainly for higher willingness to pay threshold ($100,000 and $150,000). r-ATG appears to achieve excellent cost-effectiveness acceptability curves (%80 of the recipients) in both risk groups at $50,000 threshold (except age>60 years). In addition, only r-ATG was associated with graft survival benefit over no-induction category (hazard ratio 0.91, 95% confidence interval 0.84 to 0.99) in a multivariable Cox regression analysis.
Conclusions
Antibody-based induction appears to offer substantial advantages in both cost and outcome compared to no-induction. Overall, depletional induction (preferably r-ATG) appears to offer the greatest benefits.
INTRODUCTION
For most patients in the United States with end stage renal disease (ESRD), transplantation is the preferred modality of treatment, as it not only improves survival and quality of life, but is also more cost-effective than dialysis (1-3). In 2010, kidney transplant care, delivered to 30% of the overall ESRD population, accounted for only 10% (approximately $2.8 billion) of total Medicare ESRD expenditures (4, 5). Long-term successful engraftment necessitates use of immunosuppressant drug therapy to prevent immunologic rejection and maintain allograft function. How best to initiate effective immunosuppression at the time of transplantation remains controversial, with some preferring perioperative administration of potent biologic agents to enhance immediate efficacy, and others targeting early attainment of therapeutic levels of maintenance agents (no-induction). Beyond these broader approaches, many choose antibody-based induction only in selected patients, perhaps when delayed allograft function is anticipated or in high immunologic risk recipients (6). Contemporary options include both lymphocyte-depleting antibodies (polyclonal rabbit anti-thymocyte globulin [r-ATG] and monoclonal humanized anti-CD52 antibody [alemtuzumab]) and non-depleting monoclonal antibodies (interleukin 2 receptor antagonists [IL2-RA], such as basiliximab) (7, 8). Based on perceptions of efficacy, lymphocyte-depletion is now the favored approach in the U.S. (57% of recipients in 2011), though the Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend IL2-RA as first line induction in all types of donor-recipient profiles (6, 9).
Beyond issues surrounding efficacy of individual agents across a wide range of risk factors, antibody-based induction therapy adds cost to the care of kidney transplant recipients, a consideration only rarely included in decision-making regarding its use. Since renal transplantation is largely financed through public funds in the U.S. (Medicare), we sought to define, from the payer's perspective, the incremental cost effectiveness among different agents/approaches to early immunosuppressive treatment in risk-stratified DDRT recipients: no-induction, IL2-RA, r-ATG, and alemtuzumab.
MATERIAL & METHODS
Design and study cohort
The United States Renal Data System (USRDS) collaborates with the ESRD networks and the United Network for Organ Sharing (UNOS), and incorporates Centers for Medicare & Medicaid Services (CMS)'s billing (including ICD-9-CM diagnosis and procedure coding, CMS revenue center codes, HCPCS procedure codes, all eligible claims and payments) records into the USRDS database. This combined database allows researchers to analyze characteristics and outcomes of ESRD and renal transplant recipients and related cost for the medical services provided to them. Medicare is the primary payer for more than 70% of the recipients and secondary payer for all others (5, 10). Medicare coverage lasts only 3-years except for those patients older than 65 years or non-ESRD related disabilities (5).
This study is a retrospective cohort analysis of the USRDS database that initially included all adults who listed and underwent DDKT between January 1, 2000 and September 30, 2008 (N=66,204). Exclusion criteria consisted of patients: (1) undergoing multiorgan transplants; (2) undergoing repeat kidney transplantations; (3) receiving multiple induction agents and other research induction drugs; (4) for whom Medicare was not primary payer (or the Medicare payment for the initial transplant hospitalization less than $15 000). A total of 19 450 patients were included in the final analysis. The study population was initially divided into 2 risk groups (low vs. high) based on donor and recipient risk factors for overall graft failure including death with function. The high-risk group is defined as having any of the following: panel reactive antibody (PRA) > 20%, African American [AA] race, cold ischemia time [CIT] > 24 hours (higher risk for delayed allograft function), recipient's age > 60 (higher risk for death with functioning graft), kidney donor risk profile [KDPI] 50-100% (mainly representing the range for expanded criteria donor kidneys in old allocation system prior to December 4, 2014) (7, 9, 11-14). Each risk group was further stratified based on induction categories including no-induction, alemtuzumab, r-ATG, and IL2-RA.
These research activities are consistent with the Principles of the Declaration of Istanbul on Organ Trafficking, and were approved by the Institutional Review Board of the Columbia University College of Physicians and Surgeons.
Cost estimations
Programming experts in the Pharmaceutical Research Computing at School of Pharmacy, University of Maryland, constructed the cost files. A study index date (date of transplant) was identified for each individual. All Medicare payments on a per patient basis were summarized as monthly (person 30-day period files) reimbursements (the amount paid for physician/supplier and institutional claims) during the first 36-months following transplantation, with the index date as reference. The aggregate of average monthly reimbursements were then summed to obtain total cumulative cost for each of the induction categories (including reimbursement for transplant and subsequent hospitalizations, infection, rejection and return to dialysis). Reimbursement for induction treatment is bundled in the initial transplant hospitalization payment by Medicare. A 3% inflation factor was used to adjust Medicare payments to 2013 U.S. dollar value. Additionally, Medicare reimburses organ acquisition cost to transplant centers (including the kidney recovery surgery and other related costs, such as tissue typing, candidacy evaluation services, registration fees, and preservation - perfusion costs). Since Medicare data does not include kidney acquisition cost, we added estimated $30,000 per kidneys with KDPI<50% and $35,000 per kidney with KDPI>50% (relatively marginal organs) as a cost of organ recovery (charges of the Organ Procurement Organization to the transplant center) (10).
Effect estimations
Effect was defined as number of the months of functioning allograft within 36 months posttransplantation period. Censoring occurred on return to dialysis, retransplant, death, or end of the study period.
Main Outcomes
The primary outcomes were cumulative cost (C), effect (E), and incremental cost-effectiveness ratio (ICER) within 3-years of transplantation among induction categories under 2-risk groups. Based on our choice of health outcome, ICER value represents the incremental cost per additional year of graft survival over 3 years for the alternative induction agent as compared to the base induction category or no-induction at all.
Statistical and Cost-Effectiveness analysis
Donor and recipient characteristics were described using frequencies or means ± standard deviation. Comparison between groups was made using the t test, Kruskal-Wallis test, or chi-squared test. Graft survival rates were estimated using the Kaplan-Meier product limit method. The log-rank test was used for comparison of the unadjusted survival curves. P values <0.05 were considered statistically significant. Statistical analyses were performed with Stata 14 MP4 (StataCorp LP, College Station, TX).
We used nonparametric bootstrapping method to estimate the expected values of cost and effect parameters for both low and high-risk recipient groups. Nonparametric bootstrapping is the primary choice for conducting cost-effectiveness when the theoretical distribution to be used for statistical inference is unknown. It yields estimate of error and confidence intervals by random sampling with replacement from the original cohort (15). We used absolute and extended dominance for an initial assessment of cost-effectiveness of induction choices. The absolute dominance occurs when a strategy is less costly and more effective than at least 1 alternative. The extended dominance is the case when the dominated strategy is less effective and less costly than any point located on the line of linear combination of 2 other strategies. When a treatment is dominated, it is eliminated from risk group. We then use incremental cost-effectiveness ratio (ICER) to compare cost-effectiveness of among final 2 induction choices within each risk group. To assess the comparative cost-effectiveness of 2 induction groups, say A and B, we determine the ICER values using the following equation:
where total cost or effectiveness of an induction group refers to the mean total cost or mean effectiveness of the bootstrap sample, respectively. ICER value indicates the amount of cost we would like to spend for additional year of graft survival to achieve a more effective treatment. We then performed 1,000 replications to obtain randomly distributed ICER values. We converted the effects from months to years and assumed the baseline of willingness-to-pay to be $50 000. Using the independent bootstrap samples, we plotted the cost-effectiveness acceptability curves (CEAC) for both low and high-risk groups. CEAC shows the probability that a decided option was cost-effective for a given willingness-to pay threshold. The shape of the CEAC provides the joint uncertainty in costs and effects (16). We used recommended cost-effectiveness thresholds of $50,000, $100,000, and $150,000 as the reference values in this research (17, 18). Different willingness-to-pay thresholds ($100,000 and $150,000) were used to explore whether any selected induction category remains cost effective at the respective threshold.
RESULTS
Patient characteristics and outcomes
Frequencies of Induction categories among DDRT recipients between 2000 and 2008 are shown in Figure 1. Use of lymphocyte-depleting induction agents (r-ATG and alemtuzumab) increased during this interval, while IL2-RA and no-induction approaches declined. Characteristics of the final cohort are summarized in the Supplemental Table 1. Approximately 80% of the recipients across all induction categories had at least 1 high-risk factor. Recipients undergoing lymphocyte depletion were also more frequent recipients of kidneys from ECD or DCD donors with correspondingly higher KDPI percentiles, as well as longer CIT and more DGF. Despite this increased risk, lymphocyte depletion was associated with lower rates of acute rejection in the first posttransplant year. At 3 years, overall allograft survival was better in antibody induction groups compared to no-induction category (78.7% in no-induction, 80.2% in alemtuzumab, 81.8% in r-ATG, and 81.5% in IL-2 RA, p=0.02). A multivariable Cox regression analysis was performed to evaluate risk factors for overall graft failure, shown in Supplemental Table S2. R-ATG was associated with overall graft survival compared to no induction, and there was steady improvement in graft survival over the study period.
Figure 1.
Induction types between 2000-2008 in our cohort of DDKT recipients.
Cumulative cost, effect and ICER
The cumulative (nonparametric bootstrap) means for C and E within 3-years of transplantation based on the risk groups and induction categories in DDRT recipients with Medicare primary coverage is shown in Table 1. In both low and high-risk groups, treatment with no-induction was the least effective and the most expensive compared with other induction categories. Alemtuzumab in the low-risk group and IL2-RA in the high-risk group had the lowest mean C. In both risk groups, r-ATG was the most effective induction treatment category. Among the high-risk subcategories, in general, IL2-RA was the least expensive (except in AA patients), while r-ATG appeared to be the most effective therapy (except in AA race and CIT > 24-hour subcategory). After applying absolute and extended dominance, we calculated the median ICERs in the low-risk group and among high-risk sub-categories as shown in Table 2 (nondiscounted analyses). Note that our choice of reporting means for expected costs and expected effects in Table 1 is consistent with the nonparametric bootstrap methods. For the ICER values in Table 2, however, we reported the medians because simultaneously changing costs (numerator of ICERs) and effects (denominator of ICERs) in the bootstrapped samples creates doubly skewed distribution of ICER values. The bootstrapped ICER for r-ATG compared to alemtuzumab was $32,511 per additional year of graft survival in the low-risk group. For the high-risk group and its subcategories the bootstrapped ICER was very sensitive to the graft survival; overall r-ATG was still cost-effective, but for higher willingness to pay threshold except AA race and CIT>24 hours subcategories where alemtuzumab was more cost-effective induction of choice.
Table 1.
Cumulative mean cost and effect within 3-years of transplantation based on the induction category in DDKT recipients with Medicare primary coverage.
| Cumulative Cost | Cumulative Effect | |||
|---|---|---|---|---|
| Induction Type | Observed Cost (USD*) | Cost (USD*), 95% Confidence Interval | Observed Effect (months) | Effect (months), 95% Confidence Interval |
| LOW RISK RECIPIENT | ||||
| Alemtuzumab | $131,885 | 121,723-142,193 | 33.14 | 31.03-34.19 |
| No-induction | $139,900 | 133,678-146,941 | 31.95 | 31.31-32.57 |
| IL2-RA | $141,762 | 134,260-151,206 | 32.75 | 32.22-33.24 |
| r-ATG | $142,771 | 136,480-149,297 | 33.19 | 32.68-33.66 |
| HIGH-RISK RECIPIENT | ||||
| IL2-RA | $165,217 | 161,738-168,630 | 30.79 | 30.13-31.49 |
| r-ATG | $166,435 | 166,002-172,338 | 31.10 | 30.85-31.34 |
| Alemtuzumab | $171,022 | 164,339-177,856 | 30.88 | 30.27-31.47 |
| No-induction | $189,333 | 185,142-193,398 | 29.56 | 29.21-29.92 |
| High-risk subcategories | ||||
| PRA>20% | ||||
| IL2-RA | $165,366 | 157,126-174,053 | 30.97 | 30.26-31.70 |
| r-ATG | $169,651 | 163,792-176,014 | 31.58 | 31.12-32.05 |
| Alemtuzumab | $173,531 | 158,459-189,505 | 31.02 | 29.68-32.27 |
| No-induction | $185,551 | 177,135-194,969 | 29.25 | 28.42.25-30.1 |
| African American | ||||
| Alemtuzumab | $161,850 | 152,408-171,999 | 31.42 | 30.52-32.29 |
| IL2-RA | $168,609 | 161,458-175,827 | 30.46 | 29.91-31.00 |
| r-ATG | $169,356 | 165,347-173,123 | 31.22 | 30.82-31.60 |
| No-induction | $198,683 | 192,062-205,993 | 29.37 | 28.81-29.90 |
| KDPI>50% | ||||
| IL2-RA | $177,122 | 172,105-182,262 | 29.87 | 29.40-30.33 |
| r-ATG | $181,119 | 176,286-185,313 | 30.18 | 29.82-30.53 |
| Alemtuzumab | $183,90 | 174,605-192,251 | 29.92 | 29.10-30.72 |
| No-induction | $206,932 | 200,836-213,083 | 28.69 | 28.21-29.18 |
| CIT> 24 hours | ||||
| IL2-RA | $164,512 | 158,283-171,369 | 30.91 | 30.32-31.42 |
| Alemtuzumab | $169,949 | 159,332-181,889 | 31.37 | 30.44-32.28 |
| r-ATG | $173,794 | 168,812-179,883 | 31.06 | 30.57-31.57 |
| No-induction | $211,958 | 203,043-221,638 | 28.93 | 28.26-29.53 |
| Age> 60 years-old | ||||
| IL2-RA | $172,433 | 167,803-177,096 | 30.32 | 29.89-30.77 |
| r-ATG | $179,481 | 174,732-184,241 | 30.43 | 30.02-30.84 |
| Alemtuzumab | $185,350 | 174,202-196,315 | 29.55 | 28.47 -30.50 |
| No-induction | $204,865 | 198,057-211,631 | 28.74 | 28,18-29.34 |
The cost adjusted to 2013 U.S. dollar value.
Table 2.
Nondiscounted cost-effectiveness analysis based on induction regimens for individual low and high-risk DDKT recipients.
| Strategy | Cost (C), USD* | Incremental Cost, USD* | Effect (E), years | Incremental Effect, years | C/E | ICER |
|---|---|---|---|---|---|---|
| LOW-RISK | ||||||
| Alemtuzumab | 138,414 | 2.69 | 51,455 | |||
| r-ATG | 141,340 | 2,926 | 2.78 | 0.09 | 50,841 | 32,511 |
| HIGH-RISK | ||||||
| IL2-RA | 164,750 | 2.55 | 64,607 | |||
| r-ATG | 167,357 | 2,607 | 2.57 | 0.02 | 65,119 | 130,350 |
| High-risk subcategories | ||||||
| PRA>20% | ||||||
| IL2-RA | 167,270 | 2.56 | 65,339 | |||
| r-ATG | 171,485 | 4215 | 2.63 | 0.07 | 65,203 | 60,214 |
| KDPI> 50% | ||||||
| IL2-RA | 176,284 | 2.47 | 71,370 | |||
| r-ATG | 179,843 | 3,559 | 2.50 | 0.03 | 71,937 | 118,633 |
| CIT>24 hours | ||||||
| IL2-RA | 165,875 | 2.50 | 66,350 | |||
| Alemtuzumab | 170,845 | 4,970 | 2.66 | 0.16 | 64,227 | 31,062 |
| Age> 60 years-old | ||||||
| IL2-RA | 171,418 | 2.50 | 68,567 | |||
| r-ATG | 179,452 | 8,034 | 2.56 | 0.06 | 70,098 | 133,900 |
| African American** | ||||||
| Alemtuzumab | 161,850 | 2.62 | 61,775 | |||
| r-ATG | 169,356 | 7,506 | 2.60 | 0.02 | 65,137 | |
The cost adjusted to 2013 U.S. dollar value.
The ICER is not included because alemtuzumab is marginally dominant strategy for African American category in the high-risk group.
We also calculated the ICERs using 3% discount rate applied to both the health and cost outcomes, shown in Table 3. These analyses produced no important changes in the results for the high-risk group. In the low risk group, we could not provide an ICER value because the incremental effect (difference in years of graft survival between r-ATG and alemtuzumab) became zero. Overall alemtuzumab appears to be cost-effective strategy (lower cost and same effect).
Table 3.
Inflation adjusted and outcomes discounted cost-effectiveness analysis based on induction regimens for low and high-risk recipients.
| Strategy | Cost (C), 2013 USD value | Incremental Cost, 2013 USD value | Effect (E), years | Incremental Effect, years | C/E | ICER |
|---|---|---|---|---|---|---|
| LOW-RISK* | ||||||
| Alemtuzumab | 125,316 | 2.64 | 47.468 | |||
| r-ATG | 136,185 | 10,869 | 2.64 | 0.00 | 51,585 | |
| HIGH-RISK | ||||||
| IL2-RA | 154,088 | 2.43 | 63,410 | |||
| r-ATG | 159,894 | 5,806 | 2.48 | 0.05 | 64,473 | 116,120 |
| High-risk subcategories | ||||||
| PRA>20% | ||||||
| IL2-RA | 154,268 | 2.43 | 63,484 | |||
| r-ATG | 157,567 | 3,299 | 2.49 | 0.06 | 63,279 | 54,983 |
| KDPI> 50% | ||||||
| IL2-RA | 162,431 | 2.38 | 68,243 | |||
| r-ATG | 167,160 | 4,729 | 2.42 | 0.04 | 69,074 | 118,225 |
| CIT>24 hours | ||||||
| IL2-RA | 153,999 | 2.46 | 62,601 | |||
| Alemtuzumab | 158,686 | 4,687 | 2.56 | 0.10 | 61,986 | 46,870 |
| Age> 60 years-old | ||||||
| IL2-RA | 156,990 | 2.41 | 65,141 | |||
| r-ATG | 163,100 | 6,110 | 2.47 | 0.06 | 66,032 | 101,833 |
| African American* | ||||||
| Alemtuzumab | 152,918 | 2.51 | 60,924 | |||
| r-ATG | 161,772 | 8,854 | 2.49 | 0.02 | 64,968 | |
The ICER is not included. The ICER is not defined for the low risk group because the incremental health effects among alemtuzumab and r-ATG is zero (division by zero). In the African American category, alemtuzumab is marginally dominant strategy.
Sensitivity analysis
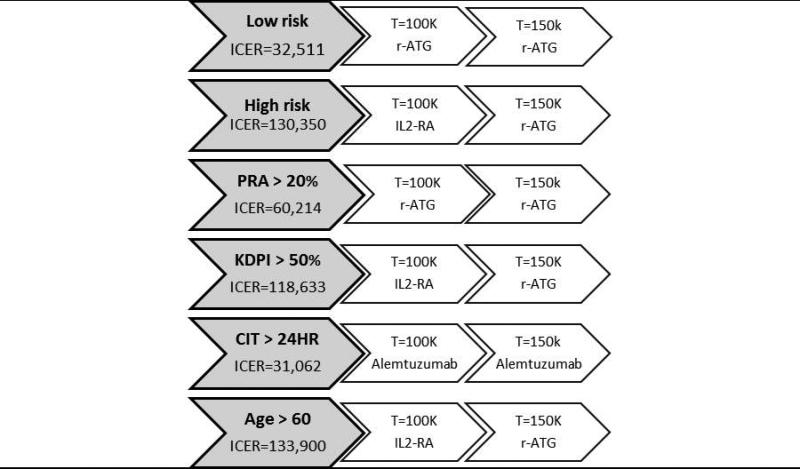
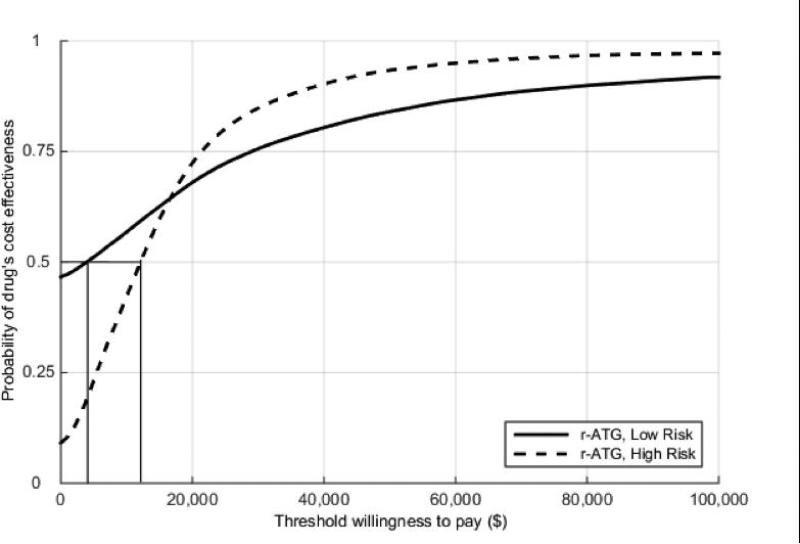
The ICERs for different willingness-to-pay threshold values ($100,000 and $150,000) were performed (Figure 2). In the low-risk group, r-ATG was the most cost-effective induction therapy for both thresholds. For the subcategories of high-risk group, depletional antibodies (r-ATG and alemtuzumab) remained the most cost-effective treatment for all risk profiles and both thresholds, except in recipients with KDPI > 50% and older patients (age > 60) where IL2-RA was more cost-effective for $100K threshold. The acceptability curves for r-ATG in both risk groups are shown in Figure 3a and 3b. r-ATG continued to be cost-effective in at least 80% of cases at $50,000 ($50K) willingness to pay threshold in both risk groups except in patients older than 60 years.
Figure 2.
Summary of the strategies of choice based on willingness-to-pay in low or high-risk recipients and individual high-risk subcategories (The ICER for African American category is not included in the figure because alemtuzumab had the least expected cost and highest effect, as indicated in Table 1, the most cost-effective induction of choice) based on the undiscounted analysis.
Figure 3a.
Acceptability curves for rATG induction in low and high-risk recipients.
Figure 3b.
Acceptability curves for r-ATG induction in high-risk subgcategories recipients.
DISCUSSION
Over eighty percent of DDRT recipients in the U.S. receive antibody-based induction therapy; our analysis indicates, on the whole, this is a cost-effective approach to immunosuppression. Specifically, based on our nondiscounted analysis, 1) no-induction was the least effective and most costly approach in both low and high-risk recipients; 2) r-ATG was the most cost-effective strategy for all willingness-to-pay thresholds (with an ICER of $32,511 per additional year of graft survival compared to alemtuzumab, cost-effective at the $50K threshold in approximately 80% of the recipients) in the low-risk group; 3) for the high-risk group and its subcategories, the bootstrapped ICER was very sensitive to the graft survival; overall, depletional antibodies were more cost-effective, but mainly for higher willingness to pay threshold. Though r-ATG induction increased costs significantly, it was the most cost-effective induction at higher thresholds, except in AA race and recipients with CIT>24 hours (alemtuzumab was the induction of choice at any thresholds for this subcategory). The discounted analyses largely confirmed these findings except in the low-risk group where alemtuzumab appeared to be more cost-effective (lower cost and same effect compared to r-ATG category), a less robust conclusion reflective perhaps of a much smaller sample size and wider variation in effect size in the alemtuzumab-treated low risk group.
There is no question that induction therapy (using IL2-RA, r-ATG, or alemtuzumab) increases initial cost during renal transplant hospitalization; our data indicate this is more than offset by other benefits, such as decreasing short-term rejection rates and intermediate-term graft survival in both low and high-risk recipients. Specifically, r-ATG appears to achieve excellent CEAC (in higher than 80% of the recipients) in both risk groups (except patients older than 60 years) even at $50,000 willingness to pay threshold (considered acceptable as a value parameter in the U.S.). For patients older than 60 years, based on less steep r-ATG CEAC (Figure 3b) and the ICERs for $100,000 willingness to pay threshold (Table 2), IL2-RA might be more cost effective compared to r-ATG. Similarly, for AA race and patients with CIT > 24 hours, alemtuzumab should be a preferable induction of choice.
The literature regarding the impact of cost on choice of appropriate induction agent is limited and conflicting (11, 19-22). Morton et al (19), using a Markov model based on health outcomes from a published meta-analysis (mainly maintained on cyclosporine, mycophenolate mofetile, and prednisone immunosuppression) (23, 24) and actual resource costs from Australian Transplant Hospitals, reported that IL2-RA improved survival 1.4 quality adjusted life years (QALY) and saved AU$79,302 (Australian dollar) per patient over a twenty year period compared to no-induction. IL2-RA was also cost-effective compared to polyclonal antibodies (using rabbit anti-thymocyte globulin and horse anti-lymphocyte globulin) with the ICER of AU$14,803 per QALY saved. In a multicenter randomized trial (N=135, with 60% of subjects undergoing DDRT), Polsky et al compared basiliximab (IL2-RA) and anti-thymocyte globulin (ATG) in cost and quality-adjusted survival (11). Cost saving with Basiliximab was $8,872, while quality-adjusted survival was same for both groups at 1-year. As part of a broader meta-analysis of newer immunosuppressants, a British group found consistent reduction in acute rejection with improved 1-year graft survival when IL2-RA was compared to no-induction (22). The Scottish Medicines Consortium recommended against r-ATG as an induction therapy in renal transplantation in 2008 due to lack of graft survival benefit and increased adverse effects compared to IL2-RA (25). In a single center retrospective study reported from the UK (N=45), Popat et al studied cost and outcomes of IL2-RA vs. r-ATG induction in recipients of donation after cardiac death (DCD) renal transplantation (21). Rabbit ATG was associated with less delayed graft function, rejection, and rehospitalization; though graft and patient survival were similar, r-ATG was associated with significant overall savings in cost.
In the current study, the large sample size and robust financial and health outcomes data allow meaningful evaluation of even small differences. It addresses contemporary immunosuppression and reflects current practices in the U.S., including the impact of various induction approaches in high-risk subgroups. Because our analysis utilizes national data sources (combined Medicare claims and the UNOS registry) and incorporates the perspective of Medicare (actual payments), primary payer for at least first 3 years of renal transplantation, it should be generalizable in this country. Within Medicare, bundled payment (Diagnosis-Related Group 302) for the initial kidney transplant hospitalization is not adjusted for patient-specific comorbidities or resource utilization of a transplant center (such as selection of induction agent, diagnostic testing, intensive care observation, length of stay etc.). Consequently, differences in cost among induction categories most likely reflect subsequent hospitalizations and complication-related resource utilization. Furthermore, Medicare perspective does not include societal costs (indirect costs, such as time and opportunity costs, and community preferences) (26). Approximately 30% of recipients with a functioning graft lose Medicare coverage 3-years after renal transplantation, with no obvious source of subsequent payment for maintenance immunosuppression, a factor that may indirectly increase graft loss beyond 3 years (5). Though these issues may limit determination of the overall costs of transplantation to the Medicare program, economic analyses from a Medicare perspective have been widely accepted due to sample size, quality of data, predominance of payer role, and its effect on related governmental policy decisions (access to transplant centers, kidney allocation, and long term immunosuppressive coverage).
The study has several limitations. Though it is the first to include analysis of costs related to alemtuzumab, those data were accumulated at a time when, though off-label, the drug was approved for use only in chronic lymphocytic leukemia, at a significantly lower price than current FDA-approved marketing for multiple sclerosis, (see Supplement for the cost of induction agents) (27-29). The time frame of the study may reduce its ability to detect long-term impact of induction, both adverse effects (such as malignancy) and potential beneficial effects on long-term survival, which could either increase or reduce costs (7). Total exposure to r-ATG and alemtuzumab was not reported in the UNOS registry. Transplant centers have increasingly been utilizing lower doses of r-ATG for induction purposes that may change adverse event profile (7, 30, 31). It should be emphasized that Medicare aggregate data do not permit for fine cost analysis, such as readmission, complications, follow-up visits, malignancy to better define incidence and mechanisms of short and long-term complications related to use of induction agents. We also acknowledge that our choice of outcome variable as graft survival leads to an ICER description (additional cost per year of graft survival over 3 years) that may be difficult to interpret, as decision makers are usually dealing with final health outcomes (survival or quality adjusted survival) rather than intermediate health outcomes (graft survival). However, our choice is consistent with the primary outcome of immunosuppression after transplantation. Finally, our analysis primarily relies on estimates derived from 2000-2008 cohorts. Clinical use of induction agents in renal transplantation may be different in 2016, at least partly as a consequence of implementation of a new kidney allocation system in 2014, risk-averse behavior of transplant centers under new regulations (the CMS and the Scientific Registry of Transplant Recipients report card system), and economic disincentives for using marginal organs. However, as newer data mature, the techniques utilized in our analysis can be applied to characterize the impact of alterations in practice and related ICER trends.
CONCLUSIONS
After extensive analysis of Medicare data, with the limitations noted above, antibody-based induction appears to offer substantial advantages in both cost and outcome within 3-years of transplantation compared to no induction. Overall, for most but not all recipients, depletional induction (preferably r-.ATG) appears to offer the most beneficial balance between cost and effect.
Supplementary Material
Acknowledgments
This study was performed as a deliverable under Contract HHSN267200715002C (National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Health, Bethesda, Maryland). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the U.S. government.
The authors thank Christine Franey and Pat Stewart for their assistance in programing and constructing the cost files at the Pharmaceutical Research Computing, University of Maryland.
Funding: This work was supported by the NIH grant KM1CA156709-01 (BT) and partially the Columbia. University Irving Institute for Clinical and Translational Research (grant no: UL1 TR000040).
Abbreviations
- AA
African American
- CEA
Cost-effectiveness analysis
- CEAC
Cost-effectiveness acceptability curves
- CI
Confidence interval
- CIT
Cold ischemia time
- CNI
Calcineurin inhibitor
- DDRT
Deceased donor renal transplantation
- ESRD
End stage kidney disease
- KDPI
Kidney donor risk profile
- IL2-RA
Interleukin-2 receptor antagonist
- PRA
Panel reactive antibody
- OAC
Organ acquisition cost
- r-ATG
Rabbit anti-thymocyte globulin
- USRSD
United States Renal Data System
Footnotes
Disclosure: BT has served for the advisory board meeting and the Speaker Bureau of Alexion Pharmaceuticals. RSG declared conflicts with Immucor (consultant), Novartis (consultant and honoraria), and BMS (grant). Other coauthors declare no conflict of interest. We also acknowledged use of rabbit-ATG and alemtuzumab as off label.
ZG, MUSA, and BT: participated in research design, writing of the paper, and data analysis. MH participated in research design. TG and RSG participated in writing of paper.
REFERENCES
- 1.Gaston RS. Appendix D, Part 2; Transplantation and immunosuppressive medications: Evolution of Medicare Policy involving transplantation and immunosuppresive medications—past developments and future directions. In: Field MJ, Lawrence RL, Zwanziger L, editors. Extending Medicare Coverage for Preventive and Other Services. National Academy Press; Washington, DC: 2000. pp. 347–362. [Google Scholar]
- 2.Eggers PW, Kucken LE. Cost issues in transplantation. Surg Clin North Am. 1994;74(5):1259–67. [PubMed] [Google Scholar]
- 3.Yen EF, Hardinger K, Brennan DC, Woodward RS, Desai NM, Crippin JS, et al. Cost-effectiveness of extending Medicare coverage of immunosuppressive medications to the life of a kidney transplant. Am J Transplant. 2004;4(10):1703–8. doi: 10.1111/j.1600-6143.2004.00565.x. [DOI] [PubMed] [Google Scholar]
- 4. [March 9th, 2015];United States Renal Data System website: 2014 Annual Data Report, Chapter 9: Costs of ESRD. 2014 Available at: http://www.usrds.org/2014/download/V2_Ch_9_Costs_14.pdf.
- 5.Tanriover B, Stone PW, Mohan S, Cohen DJ, Gaston RS. Future of Medicare immunosuppressive drug coverage for kidney transplant recipients in the United States. Clin J Am Soc Nephrol. 2013;8(7):1258–66. doi: 10.2215/CJN.09440912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kidney Disease: Improving Global Outcomes Transplant Work G KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 7.Hardinger KL, Brennan DC, Klein CL. Selection of induction therapy in kidney transplantation. Transpl Int. 2013;26(7):662–72. doi: 10.1111/tri.12043. [DOI] [PubMed] [Google Scholar]
- 8.Tanriover B, Zhang S, MacConmara M, Gao A, Sandikci B, Ayvaci MU, et al. Induction Therapies in Live Donor Kidney Transplantation on Tacrolimus and Mycophenolate With or Without Steroid Maintenance. Clin J Am Soc Nephrol. 2015;10(6):1041–9. doi: 10.2215/CJN.08710814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Schnitzler MA, et al. OPTN/SRTR 2012 Annual Data Report: kidney. Am J Transplant. 2014;14(Suppl 1):11–44. doi: 10.1111/ajt.12579. [DOI] [PubMed] [Google Scholar]
- 10.Whiting JF, Woodward RS, Zavala EY, Cohen DS, Martin JE, Singer GG, et al. Economic cost of expanded criteria donors in cadaveric renal transplantation: analysis of Medicare payments. Transplantation. 2000;70(5):755–60. doi: 10.1097/00007890-200009150-00007. [DOI] [PubMed] [Google Scholar]
- 11.Polsky D, Weinfurt KP, Kaplan B, Kim J, Fastenau J, Schulman KA. An economic and quality-of-life assessment of basiliximab vs antithymocyte globulin immunoprophylaxis in renal transplantation. Nephrol Dial Transplant. 2001;16(5):1028–33. doi: 10.1093/ndt/16.5.1028. [DOI] [PubMed] [Google Scholar]
- 12.Gill J, Sampaio M, Gill JS, Dong J, Kuo HT, Danovitch GM, et al. Induction immunosuppressive therapy in the elderly kidney transplant recipient in the United States. Clin J Am Soc Nephrol. 2011;6(5):1168–78. doi: 10.2215/CJN.07540810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D, Thymoglobulin Induction Study G Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355(19):1967–77. doi: 10.1056/NEJMoa060068. [DOI] [PubMed] [Google Scholar]
- 14.Cardinal H, Hebert MJ, Rahme E, Houde I, Baran D, Masse M, et al. Modifiable factors predicting patient survival in elderly kidney transplant recipients. Kidney Int. 2005;68(1):345–51. doi: 10.1111/j.1523-1755.2005.00410.x. [DOI] [PubMed] [Google Scholar]
- 15.Severens JL, De Boo TM, Konst EM. Uncertainty of incremental cost-effectiveness ratios. A comparison of Fieller and bootstrap confidence intervals. Int J Technol Assess Health Care. 1999;15(3):608–14. [PubMed] [Google Scholar]
- 16.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20(3):332–42. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- 17.Tan-Torres E, Baltussen R, Adams T. Making choices in Health: WHO Guide to Cost-Effectiveness Analysis. World Health Organization; Geneva: 2003. [Google Scholar]
- 18.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn't it increase at the rate of inflation? Arch Intern Med. 2003;163(14):1637–41. doi: 10.1001/archinte.163.14.1637. [DOI] [PubMed] [Google Scholar]
- 19.Morton RL, Howard K, Webster AC, Wong G, Craig JC. The cost-effectiveness of induction immunosuppression in kidney transplantation. Nephrol Dial Transplant. 2009;24(7):2258–69. doi: 10.1093/ndt/gfp174. [DOI] [PubMed] [Google Scholar]
- 20.Oliaei F, Akbari R, Ghazi Mirsaeid AM. Adding thymoglobuline to the conventional immunosuppressant regimen in kidney transplantation: A cost-benefit analysis. Caspian J Intern Med. 2012;3(4):514–8. [PMC free article] [PubMed] [Google Scholar]
- 21.Popat R, Syed A, Puliatti C, Cacciola R. Outcome and cost analysis of induction immunosuppression with IL2Mab or ATG in DCD kidney transplants. Transplantation. 2014;97(11):1161–5. doi: 10.1097/01.tp.0000442505.10490.20. [DOI] [PubMed] [Google Scholar]
- 22.Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, et al. Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study. Health Technol Assess. 2005;9(21):1–179. iii–iv. doi: 10.3310/hta9210. [DOI] [PubMed] [Google Scholar]
- 23.Webster AC, Playford EG, Higgins G, Chapman JR, Craig JC. Interleukin 2 receptor antagonists for renal transplant recipients: a meta-analysis of randomized trials. Transplantation. 2004;77(2):166–76. doi: 10.1097/01.TP.0000109643.32659.C4. [DOI] [PubMed] [Google Scholar]
- 24.Webster AC, Playford EG, Higgins G, Chapman JR, Craig J. Interleukin 2 receptor antagonists for kidney transplant recipients. Cochrane Database Syst Rev. 2004;(1):CD003897. doi: 10.1002/14651858.CD003897.pub2. [DOI] [PubMed] [Google Scholar]
- 25.NHS Scotland Rabbit anti-human thymocyte immunoglobulin, 25 mg powder solution for infusion (thymoglobuline). Consortium SM. 2008:1–7. [Google Scholar]
- 26.Garrison LP, Jr., Mansley EC, Abbott TA, 3rd, Bresnahan BW, Hay JW, Smeeding J. Good research practices for measuring drug costs in cost-effectiveness analyses: a societal perspective: the ISPOR Drug Cost Task Force report--Part II. Value Health. 2010;13(1):8–13. doi: 10.1111/j.1524-4733.2009.00660.x. [DOI] [PubMed] [Google Scholar]
- 27.Hanaway MJ, Woodle ES, Mulgaonkar S, Peddi VR, Kaufman DB, First MR, et al. Alemtuzumab induction in renal transplantation. N Engl J Med. 2011;364(20):1909–19. doi: 10.1056/NEJMoa1009546. [DOI] [PubMed] [Google Scholar]
- 28. [December 29, 2015];MabCampath (alemtuzumab) withdrawal of the marketing authorisation in the European union. 2012 http://www.ema.europa.eu/docs/en_GB/document_library/Public_statement/2012/08/WC500130945.pdf.
- 29. [December 29, 2015];Prescribing information for Lemtrada (alemtuzumab) 2014 http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/103948s5139lbl.pdf.
- 30.Wong W, Agrawal N, Pascual M, Anderson DC, Hirsch HH, Fujimoto K, et al. Comparison of two dosages of thymoglobulin used as a short-course for induction in kidney transplantation. Transpl Int. 2006;19(8):629–35. doi: 10.1111/j.1432-2277.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 31.Agha IA, Rueda J, Alvarez A, Singer GG, Miller BW, Flavin K, et al. Short course induction immunosuppression with thymoglobulin for renal transplant recipients. Transplantation. 2002;73(3):473–5. doi: 10.1097/00007890-200202150-00025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.