Abstract
Communities of organisms, from mammals to microorganisms, have discontinuous distributions of body size. This pattern of size structuring is a conservative trait of community organization and is a product of processes that occur at multiple spatial and temporal scales. In this study, we assessed whether body size patterns serve as an indicator of a threshold between alternative regimes. Over the past 7000 years, the biological communities of Foy Lake (Montana, USA) have undergone a major regime shift owing to climate change. We used a palaeoecological record of diatom communities to estimate diatom sizes, and then analysed the discontinuous distribution of organism sizes over time. We used Bayesian classification and regression tree models to determine that all time intervals exhibited aggregations of sizes separated by gaps in the distribution and found a significant change in diatom body size distributions approximately 150 years before the identified ecosystem regime shift. We suggest that discontinuity analysis is a useful addition to the suite of tools for the detection of early warning signals of regime shifts.
Keywords: palaeoecology, regime shift, climate change, thresholds, body size, resilience
1. Introduction
Body size is one of the most easily determined characteristics of an organism and spans many orders of magnitude from microscopic single-celled organisms to whales. This range in body sizes is the direct product of the habitats, resources, phylogenetic history and interactions of a given organism with other organisms and its environment [1]. The distribution of body size is often not continuous within groups of taxa [2]; even microorganisms (e.g. phytoplankton and zooplankton [3]) have discontinuous body size distributions (aggregations of sizes separated by gaps in the distribution). Many non-exclusive hypotheses attempt to explain why these discontinuous patterns have emerged [4]. These hypotheses range from those that consider trophic and biotic interactions [5] that take place over relatively short timeframes, to energetic [6] and macroevolutionary mechanisms [7] that occur over much longer timescales. The textural discontinuity hypothesis [8] posits that body size aggregations are the product of organisms' interactions with the discontinuous distribution of resources and opportunities within their environment. Discontinuities in the spatio-temporal landscape are caused by processes that occur on different temporal and spatial scales, which in turn produce scale-specific patterns and structures that are reflected in the ecological structure of communities [9]. One objective way to evaluate those structures is through an assessment of body size distributions [2,9].
Havlicek & Carpenter [3] investigated discontinuities in the size of pelagic species (plankton and fishes) within aquatic ecosystems and found that size structure was similar across differing lake habitats and robust to perturbations. In other words, they found similar patterns in the distribution of body size across gradients in various lake characteristics, such as lake area, lake depth, pH, nutrients, water clarity and biotic diversity. This suggests that the clusters of body sizes (i.e. the size aggregations and gaps) are conservative and that size structuring is a recurrent characteristic of aquatic floras and faunas. Other analyses have confirmed the conservative nature of body size discontinuities [10,11].
For diatoms, the ecological controls on planktic and benthic species are often quite different. Plankton obtains nutrients from the water column, whereas benthos can derive some of their nutrients from the substrate that they are attached to, which can result in differing rates of productivity [12]. The shapes and sizes of planktic diatom species are also strongly limited (or selected for) by convective mixing strength, which determines the extent to which valves can stay suspended in the water column [13,14]. By contrast, benthic species do not have these same constraints on size and shape, because they live attached to a substrate [15,16]. These kinds of ecological controls on diatoms may result in body size discontinuities.
It has been theorized that discontinuities in body sizes may change as a system approaches a threshold from one domain of stability to another [2,17]; crossing this threshold is often referred to as a regime shift and can entail substantial changes in ecosystem structure and function, and related feedbacks as the system reorganizes and transitions to an alternative stable regime [18]. Regime shifts are often preceded by system dynamics that produce warning signals prior to reaching a critical threshold [19–21]. These dynamics have enabled the development of metrics that can be used in management to identify an impending threshold [22]. In some cases, regime change can take thousands of years and is characterized by a gradual change in community structure before an alternative regime stabilizes [23,24]. In an ecological resilience context, such regime shifts and transitions offer the opportunity to test whether body size aggregation patterns track these changes while maintaining an overall discontinuous distribution. Given the tight linkage between environmental structures, functions and body size, we hypothesize that it will take major reorganization of an ecosystem (i.e. a known regime shift) to impact size aggregations [17]. Until recently, the absence of long-term time series data for most ecosystems and organisms has limited an empirical test of this hypothesis. However, spatial studies comparing habitats exhibiting contrasting regimes indicate that changes to ecosystem structure and function result in differences in body size distributions [25,26].
Here, we used a palaeoecological approach and examined a continuous sedimentological record of fossil diatoms from a temperate lake in the northern Rocky Mountains that spans thousands of years. Foy Lake underwent a regime shift in ecosystem structure, specifically rapid changes in diatom species [24], brought about by long-term climate change [27]. The diatom community changes were the product of climate-driven lake-level change that altered the abundance of deep open-water habitat for planktic diatoms relative to shallow-water substrates for benthic diatoms. Using a suite of early warning signals (increasing variance, skewed responses, kurtosis, autocorrelation, Fisher Information (FI) and multivariate time series modelling), Spanbauer et al. [24] identified a regime shift in Foy Lake, MT, USA. FI, a method that assesses the dynamic order of a system, indicated that the history of the lake can be divided into three different periods; two stable regimes bridged by a 2000-year period of instability that preceded the regime shift [24]. Periods of stability are characterized by stasis in the FI metric, whereas periods of instability show decreasing values of FI. Here, we compare the periods of stability and instability from FI to the size aggregations of diatoms.
Using the mean diatom valve face area as a proxy for size, we evaluate the patterns in size discontinuities through time. We relate the results of our temporal discontinuity analysis (the patterns of size aggregations and gaps) to the timing of previously identified stable regimes, the transition period, and the regime shift, to determine if changes in the discontinuous distribution of diatom body sizes characterizes the dynamics of the ecosystem and/or if body size discontinuities foretell regime shifts (i.e. size aggregations change prior to the regime shift). If size aggregations are successful in describing the dynamics and resilience of the system, quantifying the body size discontinuities of microscopic organisms may be useful for tracking ecosystem-level changes, thereby providing useful information for management, especially in the context of regime shift indication [28].
2. Material and methods
(a). Building the dataset
We used a high-resolution dataset of the per cent abundances of diatoms in a 7000 year sediment record from Foy Lake, Montana [29,30] that was used previously to identify and predict long-term climate-driven regime shifts [24]. To ease computation, the 7000 year record of 109 diatom taxa [24] was divided into 80 time intervals, which resulted in an average time period of 85 years. We recorded the species present for each of the resultant time intervals (80) to assess the species size relationships over time (i.e. the discontinuous body size distributions of diatoms). Prior studies have established that species mean sizes are a robust measure of size aggregations [31]. Taxonomic catalogues [32–35] and/or online identification guides [36] were used to estimate diatom size for the Foy Lake diatom species. Although volume would be a preferable body size measurement, the pervalvar height of diatom valves is difficult to measure and is rarely recorded in the literature. Thus, we used mean length and width measurements, computed from published size-range data, along with valve shape, to compute mean valve face area for each of the diatom species of Foy Lake.
(b). Analysis of discontinuities
We analysed each of the 80 datasets (time intervals) to characterize the size distribution of the diatom valve face areas using Bayesian classification and regression tree models (BCART). Classification and regression trees define homogeneous groups of data by defining successive splits based on within-group homogeneity. The result can be depicted as a branching tree, where the terminal nodes define groups of maximum homogeneity. A Bayesian implementation of the classification and regression tree algorithm performs a stochastic search over the space of all possible trees, based on prior probabilities of a split occurring at any given node [37]. Selecting the best tree is based on the log-integrated likelihood. The Bayesian algorithm has been found to be particularly effective at detecting discontinuities in datasets [38]. This method identifies the best structure of all possible combinations by selecting for the combination of data with the largest log-integrated likelihood. BCART has been used previously to determine discontinuities and aggregations of body size/mass [39]. Although several different analyses can be used to detect size aggregations (Gap Rarity Index, classification and regression trees, and hierarchical cluster analysis), a comparison found that the differing methodologies produced similar aggregations of sizes [39]. In this analysis, we plotted the resulting size aggregations generated from BCART for each time interval against time using R v. 3.0.2 (2013-09-25).
(c). Changes in discontinuities through time
To characterize whether discontinuity structure was impacted by the regime shift and/or the period of instability, we used non-metric multidimensional scaling (NMDS) and analysis of similarity (ANOSIM) to evaluate differences in the two stable regimes and the intervening period of instability as defined by Spanbauer et al. [24]. As a nonlinear technique, NMDS ranks points in ordination space, such that the distance between sampling points reflects community similarity. For this ordination analysis, we used the size aggregations and the gaps from each time interval of the discontinuity analysis as our multivariate data to visualize if any periods cluster together (i.e. the matrix was populated with the sizes of aggregations and the sizes of gaps from each time interval). From these multivariate data, a Bray–Curtis dissimilarity matrix was constructed, and NMDS was carried out (999 re-runs) to characterize diatom size discontinuity structure for the periods of the palaeorecord described in Spanbauer et al. [24]. Periods are defined as regime 1 (approx. 7000 to approx. 4000 ybp; 31 time intervals), transition (approx. 4000 to approx. 2000 ybp; 13 time intervals) and regime 2 (approx. 2000 ybp to present; 36 time intervals). The ANOSIM analysis (999 re-runs) tested whether the similarity of the size discontinuity structure differed significantly between the stable regimes and the transition period. We also applied constrained cluster analysis (CONISS) to the dataset to determine clustering of the size aggregation data without any temporal assumptions. We ran all multivariate data analysis in the packages Vegan and Rioja in R v. 3.0.2 (2013-09-25), except ANOSIM, which we ran in Primer v6 (Primer-E Ltd, Plymouth, UK).
3. Results and discussion
Size structuring of the diatom community is evident throughout the Foy Lake record. This finding is similar to patterns in size distributions detected in communities across trophic levels in terrestrial and aquatic environments [3,10,17]. Aggregations of body size are generally thought to be conservative, despite different environmental conditions among ecosystems. This conservatism has been attributed to regional-scale ecological processes [3], ranging from biological interactions to biogeographical factors that operate over discrete scales of space and time [4]. Our study demonstrates that body size aggregations of diatoms changed over palaeoecological timescales in response to nonlinear lake-level changes driven by climate change, which shows that there are strong temporal controls on the size structuring of diatom communities that are sensitive to regime shifts in the ecosystem.
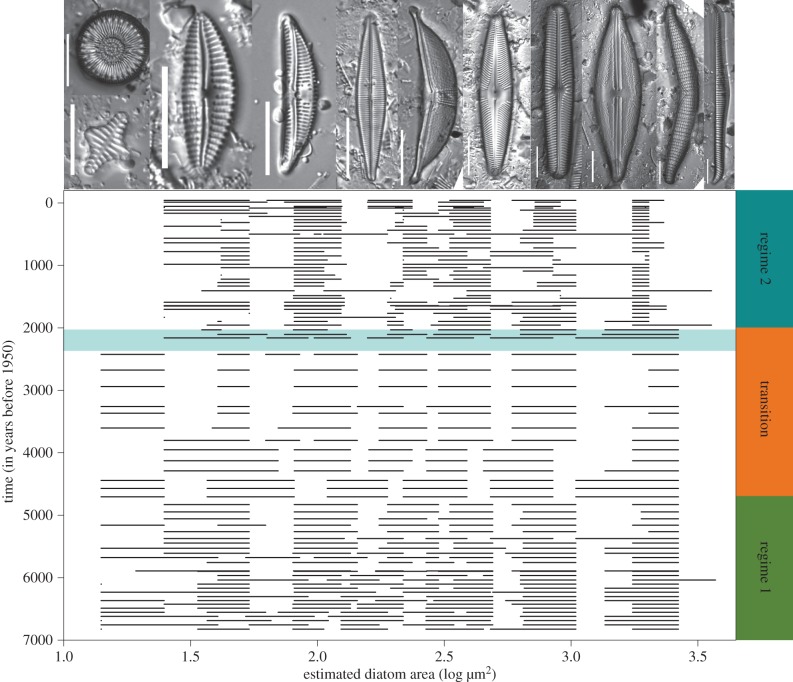
The number of size aggregations (clumps of similar sized organisms, separated by gaps in sizes) ranged from four to eight across the 80 time intervals (figure 1). From 1 to 14 species comprised an aggregation. There is a distinct change in the structure of size aggregations and gaps at approximately 2000 ybp (figure 1), which coincides with the climate-driven regime shift in Foy Lake. Using the periods from Spanbauer et al. [24], we found that during regime 1 and the transition period the diatom community had more size aggregations spread out over a larger size range than during regime 2 (figure 1). Foy Lake had a diverse diatom flora of planktic and benthic species during regime 1, indicating moderately deep waters [24]. The discontinuity analysis shows that the transition period was characterized by changes in gap sizes, but diatoms still spanned a large size range (figure 1). Major drought was prevalent in the Foy Lake region during the transition period [27], and benthic taxa dominated the flora. We found that during regime 2, the diatom community had fewer size aggregations and had lost size classes on the high and low ends of the size spectrum. This difference reflects the major regime shift in the Foy Lake record, when the lake transitioned from a shallow to a very deep lake, and the diatom flora switched from a moderately diverse benthic flora to a flora dominated by one planktic species [29]. The shifts in size aggregation appear to reflect the dynamics of the functional groups of diatom species (i.e. moving from a flora with both planktic and benthic species, to a benthic dominated flora and finally, after the regime shift, to a flora dominated by planktic species).
Figure 1.
(Top) A selection of diatom species from Foy Lake (Montana, USA) depicting size variation between species, from left to right: Lindavia cf. intermedia, Staurosirella leptostauron, Cymbella neoleptoceros, Amphora copulata, Craticula halophila, Halamphora elongata, Pinnularia brebissonii, Navicula oblonga, Anomoeoneis costata, Epithemia turgida and Rhopolodia gibba. All scale bars equal 20 µm. (Bottom) Diatom size aggregations plotted for each of the 80 time intervals, from the present to approximately 7000 ybp. Highlighted area indicates a major shift in the size structure of diatoms. Periods (regimes and transition) from Spanbauer et al. [24] are illustrated on the right side of the diagram.
When the record was partitioned into three periods, the NMDS analysis shows that regime 1 and the transition cluster together, but the time intervals from the transition are more constricted in NMDS space than the time intervals from regime 1. However, time intervals from both the transition and regime 1 are clearly separated from regime 2 (figure 2). The results of the ANOSIM support the NMDS, indicating that diatom size discontinuity structure differed among all periods (global R = 0.26, p = 0.01). However, pairwise comparisons show that the similarity in diatom size discontinuity structure does not significantly differ between regime 1 and the transitional period (R = 0.034, p = 0.25), whereas regime 1 and the transitional period significantly differ from regime 2 (regime 1 × regime 2: R = 0.31, p = 0.01; transition × regime 2: R = 0.35, p = 0.01). Using CONISS, we found that the major division in the dataset occurred at approximately 2150 ybp, slightly prior to the regime shift detected in Spanbauer et al. [24] (figure 3). This similarity indicates that the discontinuity analysis is a good indicator of regime changes when sufficient data are available and is probably an ecosystem manifestation of a bifurcation point, similar to other early warning signals [19–21].
Figure 2.
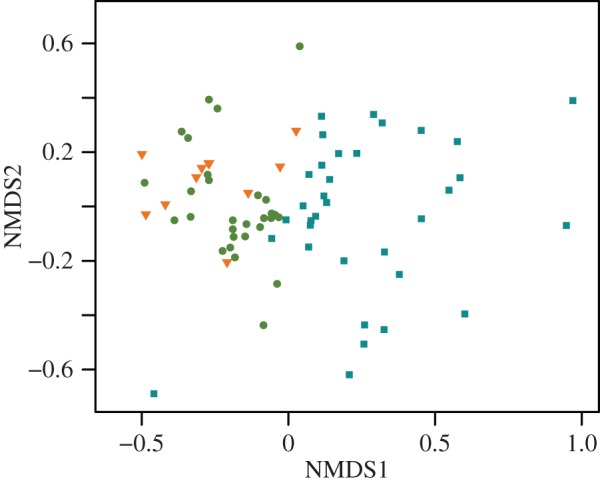
NMDS results of diatom size aggregations. Bray distances were used to calculate resemblance matrices (dimensions = 2, stress = 0.15). The size aggregations of each period are plotted; regime 1, green circles; transition, orange triangles; and regime 2, blue squares.
Figure 3.
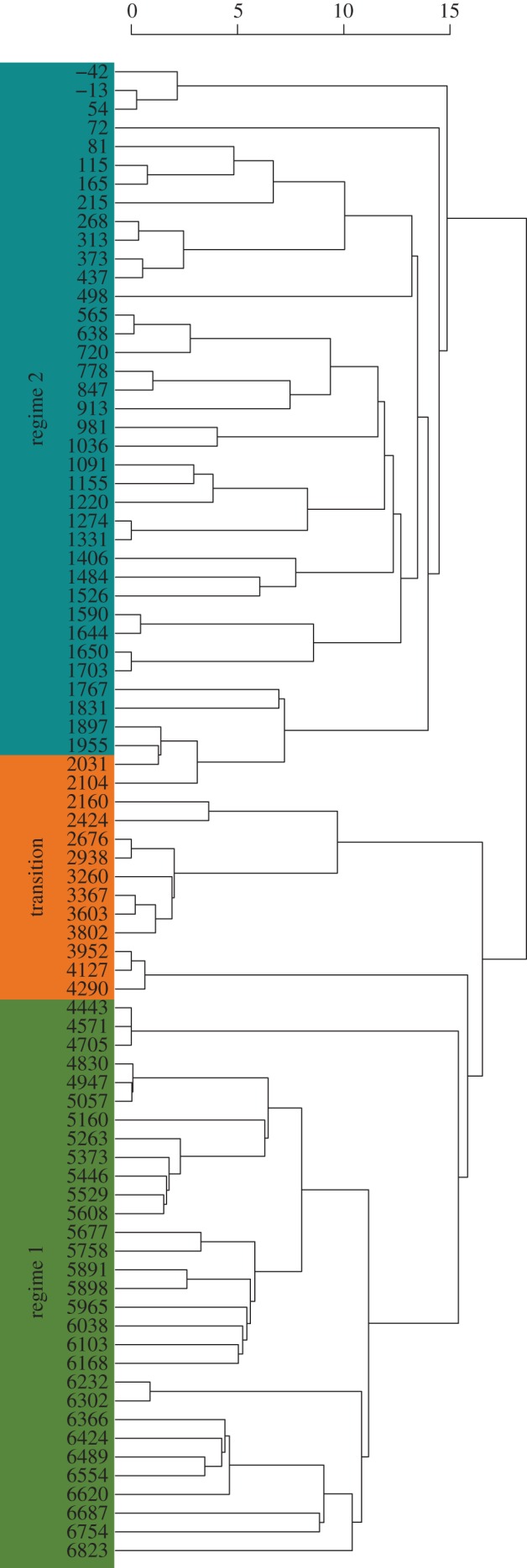
CONISS results (Euclidean distance) of the diatom size aggregations from 80 time intervals. Clustering separates the data into two major periods (broken stick model indicated two significant groupings) at approximately 2150 ybp. Periods from Spanbauer et al. [24] are outlined on the left side of the figure.
Spanbauer et al. [24] found that the major driver of temporal dynamics and the regime shift in the Foy Lake Basin was long-term climate change [29,30]. FI was successful in detecting a prolonged period of instability, and thus loss of resilience, prior to the climate induced regime shift. Results from the present study show that discontinuity analysis was also effective in predicting the climate induced regime shift, albeit much closer to the actual regime shift and without differentiating the long transition period. Therefore, the change in size aggregations is probably responding to the bifurcation point just prior to the regime shift instead of reflecting the slow degradation of resilience in the system. This lag in response of the discontinuity distribution could also indicate that it is a more conservative early warning signal than FI. Both types of analysis are useful in reflecting ecosystem dynamics prior to a regime shift, and we propose that they are complementary approaches to regime shift detection [40]. In addition, some early warning signals may fail to predict regime shifts (e.g. increasing variance [41]), limiting their use in all ecosystems. Therefore, it may be advantageous to use methods that are shaped by spatio-temporal scaling processes, such as body size discontinuity analysis.
Palaeoecological studies, which permit the analysis of temporal scales that are not often included in resilience assessments (but see Angeler et al. [42]), are essential to understanding the dynamics of communities prior to nonlinear responses in ecosystems and can be used to build understanding of the processes that are important in producing abrupt change. It may be difficult to extrapolate findings from the geological record to modern phenomena, largely because these dynamics can operate on decadal to millennial timeframes. However, this study illustrates that differing timescales may be responsible for distinct components of the community dynamics in an ecosystem. Discontinuity analysis is conservative and does not offer a warning signal until just prior to the regime shift (however, still several decades prior to the shift), whereas, FI indicates long-term loss of resilience prior to a regime shift in the Foy Lake record. These differences in the temporal scale of warning are potentially useful to managers working in systems that are controlled by a combination of slow and fast variables.
In conclusion, changes in size aggregations in Foy Lake occurred over temporal scales of millennia, indicating that processes that are important to community structuring occur within the predicted scales of the textural discontinuity hypothesis [8]. The speed of a regime shift probably reflects the scale of the system in question and the strength of the positive feedbacks that provide characteristic structures. Size aggregations changed with the regime shift, which suggests that changes in body size aggregations may accompany bifurcation points and are, therefore, valuable tools for forecasting regime shifts. If this pattern holds across different ecosystems and organisms, discontinuity analysis will prove to be a powerful tool for ecosystem managers in predicting abrupt ecosystem change in sufficient time to manage for resilience.
Acknowledgements
Comments from Diana Pilson, Ross Secord and David Watkins greatly improved this manuscript. The Nebraska Cooperative Fish and Wildlife Research Unit is jointly supported by a cooperative agreement between the US Geological Survey, the Nebraska Game and Parks Commission, the University of Nebraska—Lincoln, the United States Fish and Wildlife Service and the Wildlife Management Institute. GLERL contribution no. 1820.
Data accessibility
Diatom species presences from the Foy Lake record were obtained from Spanbauer et al. [24], for which all data are archived with that publication (doi:10.1371/journal.pone.0108936). Species size data were found in taxonomic [32–35] and online [36] catalogues.
Authors' contributions
T.L.S., C.R.A., D.G.A., T.E., A.S.G., K.L.N., C.A.S. and S.M.S. conceived and designed the analyses at a Powell Center working group. T.L.S., D.G.A., J.R.S. and C.A.S. performed the analyses. T.L.S., C.R.A., D.G.A., T.E., T.L.S., S.C.F. and A.S.G. analysed the data. T.L.S. was responsible for drafting the manuscript with contributions from all the authors.
Competing interests
We have no competing interests.
Funding
This manuscript was conceived at the Managing for Resilience Working Group, funded by the United States Geological Survey's John Wesley Powell Center for Analysis and Synthesis. This work was supported, in part, by the August T. Larsson Foundation of the Swedish University of Agricultural Sciences, the NSF's Integrative Graduate Education and Research Traineeship (IGERT) programme (NSF no. 0903469), the Sedimentary Geology and Palaeobiology programme (NSF no. 1251678), the Swedish Research Councils VR (2014-5828) and Formas (2014-1193). A University of Nebraska Presidential Graduate Fellowship and a National Research Council Research Associateship also provided support for this project.
Disclaimer
Any use of trade, firm or product names is for descriptive purposes only and does not imply endorsement by the US Government. The views expressed in this paper are those of the authors and do not represent the views or policies of the US Environmental Protection Agency.
References
- 1.White EP, Ernest SKM, Kerkhoff AJ, Enquist BJ. 2007. Relationships between body size and abundance in ecology. Trends Ecol. Evol. 22, 323–330. ( 10.1016/j.tree.2007.03.007) [DOI] [PubMed] [Google Scholar]
- 2.Nash KL, et al. 2014. Discontinuities, cross-scale patterns, and the organization of ecosystems. Ecology 95, 654–667. ( 10.1890/13-1315.1) [DOI] [PubMed] [Google Scholar]
- 3.Havlicek TD, Carpenter SR. 2001. Pelagic species size distributions in lakes: are they discontinuous? Limnol. Oceanogr. 46, 1021–1033. ( 10.4319/lo.2001.46.5.1021) [DOI] [Google Scholar]
- 4.Allen CR, Garmestani AS, Havlicek TD, Marquet PA, Peterson GD, Restrepo C, Stow CA, Weeks BE. 2006. Patterns in body mass distributions: sifting among alternative hypotheses. Ecol. Lett. 9, 630–643. ( 10.1111/j.1461-0248.2006.00902.x) [DOI] [PubMed] [Google Scholar]
- 5.Hutchinson GE. 1959. Homage to Santa Rosalia or why are there so many kinds of animals? Am. Nat. 93, 145–159. [Google Scholar]
- 6.Kelt DA. 1997. Assembly of local communities: consequences of an optimal body size for the organization of competitively structured communities. Biol. J. Linn. Soc. 62, 15–37. ( 10.1111/j.1095-8312.1997.tb01615.x) [DOI] [Google Scholar]
- 7.Smith FA, et al. 2004. Similarity of mammalian body size across the taxonomic hierarchy and across space and time. Am. Nat. 163, 672–691. ( 10.1086/382898) [DOI] [PubMed] [Google Scholar]
- 8.Holling CS. 1996. Cross-scale morphology, geometry, and dynamics of ecosystems. In Ecosystem management (eds FB Samson, FL Knopf), pp. 351–423. New York, NY: Springer. [Google Scholar]
- 9.Allen CR, Angeler DG, Garmestani AS, Gunderson LH, Holling CS. 2014. Panarchy: theory and application. Ecosystems 17, 578–589. ( 10.1007/s10021-013-9744-2) [DOI] [Google Scholar]
- 10.Raffaelli D, Hall S, Emes C, Manly B. 2000. Constraints on body size distributions: an experimental approach using a small-scale system. Oecologia 122, 389–398. [DOI] [PubMed] [Google Scholar]
- 11.Forys EA, Allen RC. 2002. Functional group change within and across scales following invasions and extinctions in the everglades ecosystem. Ecosystems 5, 339–347. ( 10.1007/s10021-001-0078-0) [DOI] [Google Scholar]
- 12.Round FE, Crawford RM, Mann DG. 1990. The diatoms: biology & morphology of the genera. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 13.Saros JE, Stone JR, Pederson GT, Slemmons KEH, Spanbauer T, Schliep A, Cahl D, Williamson CE, Engstrom DR. 2012. Climate-induced changes in lake ecosystem structure inferred from coupled neo- and paleoecological approaches. Ecology 93, 2155–2164. ( 10.1890/11-2218.1) [DOI] [PubMed] [Google Scholar]
- 14.Huisman J, Sommeijer B. 2002. Maximal sustainable sinking velocity of phytoplankton. Mar. Ecol. Prog. Ser. 244, 39–48. ( 10.3354/meps244039) [DOI] [Google Scholar]
- 15.Passy SI. 2007. Differential cell size optimization strategies produce distinct diatom richness–body size relationships in stream benthos and plankton. J. Ecol. 95, 745–754. ( 10.1111/j.1365-2745.2007.01248.x) [DOI] [Google Scholar]
- 16.Cantonati M, Lowe RL. 2014. Lake benthic algae: toward an understanding of their ecology. Freshw. Sci. 33, 475–486. ( 10.1086/676140) [DOI] [Google Scholar]
- 17.Allen CR, Holling C. 2008. Discontinuities in ecosystems and other complex systems. New York, NY: Columbia University Press. [Google Scholar]
- 18.Holling CS. 1973. Resilience and stability of ecological systems. Annu. Rev. Ecol. Syst. 4, 1–23. ( 10.1146/annurev.es.04.110173.000245) [DOI] [Google Scholar]
- 19.Carpenter SR, Brock WA. 2006. Rising variance: a leading indicator of ecological transition. Ecol. Lett. 9, 311–318. ( 10.1111/j.1461-0248.2005.00877.x) [DOI] [PubMed] [Google Scholar]
- 20.Scheffer M, et al. 2009. Early-warning signals for critical transitions. Nature 461, 53–59. ( 10.1038/nature08227) [DOI] [PubMed] [Google Scholar]
- 21.Dakos V, Scheffer M, van Nes EH, Brovkin V, Petoukhov V, Held H. 2008. Slowing down as an early warning signal for abrupt climate change. Proc. Natl Acad. Sci. USA 105, 14 308–14 312. ( 10.1073/pnas.0802430105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindegren M, Dakos V, Gröger JP, Gårdmark A, Kornilovs G, Otto SA, Möllmann C. 2012. Early detection of ecosystem regime shifts: a multiple method evaluation for management application. PLoS ONE 7, e38410 ( 10.1371/journal.pone.0038410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes TP, Linares C, Dakos V, van de Leemput IA, van Nes EH. 2013. Living dangerously on borrowed time during slow, unrecognized regime shifts. Trends Ecol. Evol. 28, 149–155. ( 10.1016/j.tree.2012.08.022) [DOI] [PubMed] [Google Scholar]
- 24.Spanbauer TL, Allen CR, Angeler DG, Eason T, Fritz SC, Garmestani AS, Nash KL, Stone JR. 2014. Prolonged instability prior to a regime shift. PLoS ONE 9, e108936 ( 10.1371/journal.pone.0108936) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nash KL, Graham NAJ, Wilson SK, Bellwood DR. 2013. Cross-scale habitat structure drives fish body size distributions on coral reefs. Ecosystems 16, 478–490. ( 10.1007/s10021-012-9625-0) [DOI] [Google Scholar]
- 26.Nash KL, Allen CR, Barichievy C, Nyström M, Sundstrom S, Graham NAJ. 2014. Habitat structure and body size distributions: cross-ecosystem comparison for taxa with determinate and indeterminate growth. Oikos 123, 971–983. ( 10.1111/oik.01314) [DOI] [Google Scholar]
- 27.Booth RK, Jackson ST, Forman SL, Kutzbach JE, Bettis EA, Kreigs J, Wright DK. 2005. A severe centennial-scale drought in midcontinental North America 4200 years ago and apparent global linkages. Holocene 15, 321–328. ( 10.1191/0959683605hl825ft) [DOI] [Google Scholar]
- 28.Angeler DG, et al. 2016. Management applications of discontinuity theory. J. Appl. Ecol. 53, 688–698. ( 10.1111/1365-2664.12494) [DOI] [Google Scholar]
- 29.Stone JR, Fritz SC. 2006. Multidecadal drought and Holocene climate instability in the Rocky Mountains. Geology 34, 409–412. ( 10.1130/g22225.1) [DOI] [Google Scholar]
- 30.Stevens LR, Stone JR, Campbell J, Fritz SC. 2006. A 2200-yr record of hydrologic variability from Foy Lake, Montana, USA, inferred from diatom and geochemical data. Quat. Res. 65, 264–274. ( 10.1016/j.yqres.2005.08.024) [DOI] [Google Scholar]
- 31.Sendzimir JP. 1998. Patterns of animal size and landscape complexity: correspondence within and across scales. Dissertation, University of Florida, Gainesville, FL, USA.
- 32.Krammer K, Lange-Bertalot H. 1986. Bacillariophyceae. 1. Teil: Naviculaceae. In Süsswasser flora von Mitteleuropa, Band 2/1 (eds Ettl H, Gerloff J, Heynig H, Mollenhauer D), 876 p. Stuttgart, Germany: Gustav Fischer Verlag. [Google Scholar]
- 33.Krammer K, Lange-Bertalot H. 1988. Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In Süsswasserflora von Mitteleuropa, Band 2/2 (eds Ettl H, Gerloff J, Heynig H, Mollenhauer D), 596 p. Jena, Germany: VEB Gustav Fischer Verlag. [Google Scholar]
- 34.Krammer K, Lange-Bertalot H. 1991. Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In Süsswasserflora von Mitteleuropa, Band 2/3 (eds Ettl H, Gerloff J, Heynig H, Mollenhauer D), 576 p. Stuttgart, Germany: Gustav Fischer Verlag. [Google Scholar]
- 35.Krammer K, Lange-Bertalot H. 1991. Bacillariophyceae. 4. Teil: Achnanthaceae, Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema, Gesamtliteraturverzeichnis Teil 1–4. In Süsswasserflora von Mitteleuropa, Band 2/4 (eds Ettl H, Gärtner G, Gerloff J, Heynig H, Mollenhauer D), 437 p. Stuttgart, Germany: Gustav Fischer Verlag. [Google Scholar]
- 36.Spaulding SA, Lubinski DJ, Potapova M. 2010. Diatoms of the United States. See http://westerndiatoms.colorado.edu (accessed on 9 December 2014).
- 37.Chipman HA, George EI, McCulloch RE. 1998. Bayesian CART Model search. J. Am. Stat. Assoc. 93, 935–960. ( 10.1080/01621459.1998.10473750) [DOI] [Google Scholar]
- 38.Bremner AP, Taplin RH. 2004. Performance of localized regression tree splitting criteria on data with discontinuities. Aust. N.Z. J. Stat. 46, 367–381. ( 10.1111/j.1467-842X.2004.00336.x) [DOI] [Google Scholar]
- 39.Stow C, Allen CR, Garmestani AS. 2007. Evaluating discontinuities in complex systems: toward quantitative measures of resilience. Ecol. Soc. 12, 26. [Google Scholar]
- 40.Eason T, Garmestani AS, Stow CA, Alvarez-Cobelas M, Rojo C, Cabezas H. 2016. Managing for resilience: an information theory-based approach to assessing ecosystems. J. Appl. Ecol. 53, 656–665. ( 10.1111/1365-2664.12597) [DOI] [Google Scholar]
- 41.Dakos V, Carpenter SR, van Nes EH, Scheffer M. 2015. Resilience indicators: prospects and limitations for early warnings of regime shifts. Phil. Trans. R. Soc. B 370, 20130263 ( 10.1098/rstb.2013.0263) [DOI] [Google Scholar]
- 42.Angeler DG, Allen CR, Birgé HE, Drakare S, McKie BG, Johnson RK. 2014. Assessing and managing freshwater ecosystems vulnerable to environmental change. Ambio 43, 113–125. ( 10.1007/s13280-014-0566-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Diatom species presences from the Foy Lake record were obtained from Spanbauer et al. [24], for which all data are archived with that publication (doi:10.1371/journal.pone.0108936). Species size data were found in taxonomic [32–35] and online [36] catalogues.