Abstract
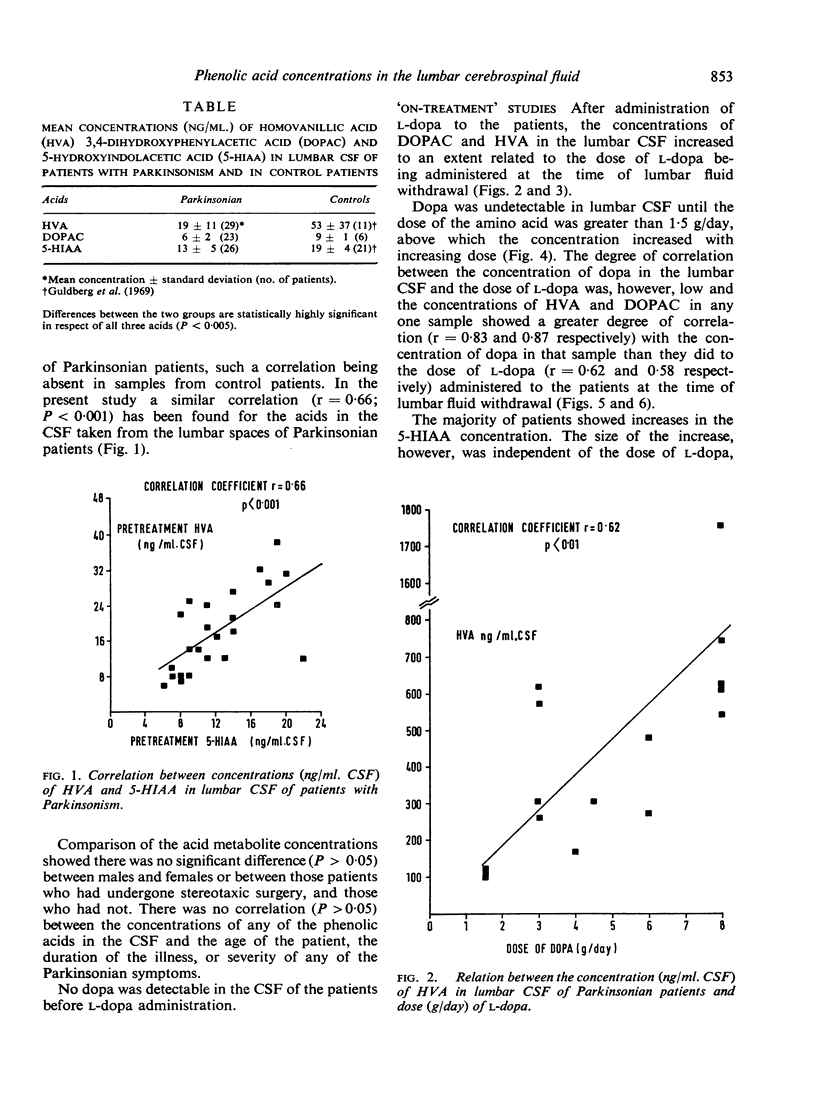
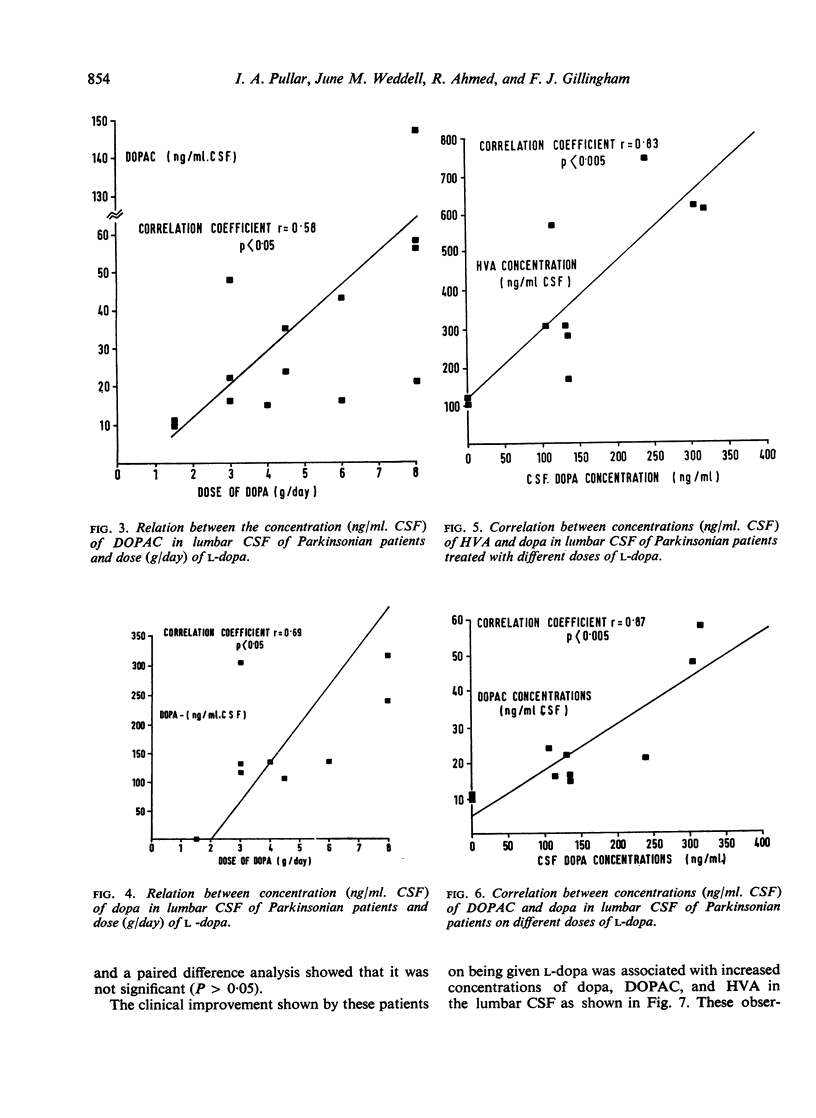
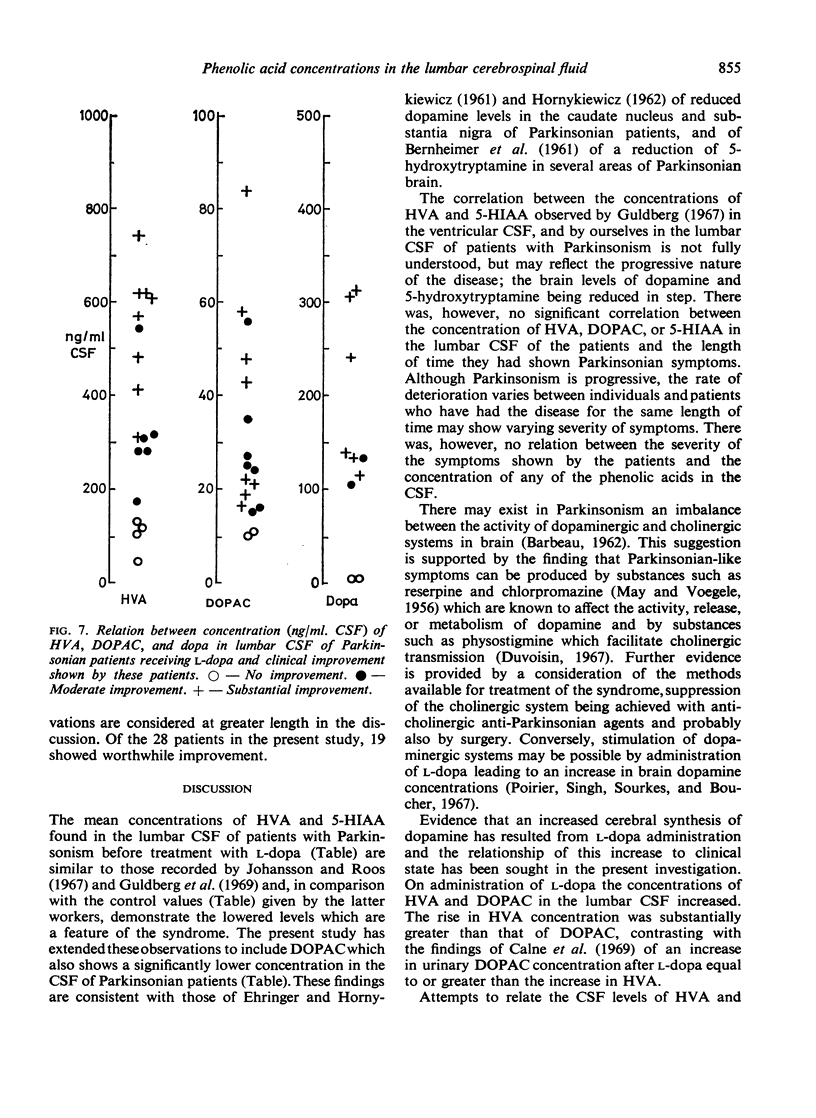
The acid metabolites of dopamine and 5-hydroxytryptamine were estimated in the lumbar cerebrospinal fluid (CSF) of Parkinsonian patients both before and during treatment with l-dopa, the amino acid precursor of dopamine. An attempt was made to relate clinical improvement to the biochemical results. The dopamine metabolites, 3,4-dihydroxyphenylacetic acid and homovanillic acid, showed increases related to the dose of l-dopa, the increase in homovanillic acid concentration being proportionately greater than that of 3,4-dihydroxyphenylacetic acid. The concentration of the 5-hydroxytryptamine metabolite, 5-hydroxyindol-3ylacetic acid, was unaltered by the drug. Clinical improvement, which in the early stages was evident particularly in the bradykinesia, was found to occur at doses of l-dopa greater than 1·5 g/day. Effective doses of l-dopa gave rise to concentrations of dopamine metabolites in the CSF which were greater than normal. Possible implications of these findings are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDEN N. E., ROOS B. E., WERDINIUS B. On the occurrence of homovanillic acid in brain and cerebrospinal fluid and its determination by a fluorometric method. Life Sci. 1963 Jul;(7):448–458. doi: 10.1016/0024-3205(63)90132-2. [DOI] [PubMed] [Google Scholar]
- Ashcroft G. W., Crawford T. B., Dow R. C., Guldberg H. C. Homovanillic acid, 3,4-dihydroxyphenylacetic acid and 5-hydroxyindol-3-ylacetic acid in serial samples of cerebrospinal fluid from the lateral ventricle of the dog. Br J Pharmacol Chemother. 1968 Jul;33(3):441–456. doi: 10.1111/j.1476-5381.1968.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARBEAU A. The pathogenesis of Parkinson's disease: a new hypothesis. Can Med Assoc J. 1962 Oct 13;87:802–807. [PMC free article] [PubMed] [Google Scholar]
- BERNHEIMER H., BIRKMAYER W., HORNYKIEWICZ O. [Distribution of 5-hydroxytryptamine (serotonin) in the human brain and its behavior in patients with Parkinson's syndrome]. Klin Wochenschr. 1961 Oct 15;39:1056–1059. doi: 10.1007/BF01487648. [DOI] [PubMed] [Google Scholar]
- BERTLER A., FALCK B., ROSENGREN E. THE DIRECT DEMONSTRATION OF A BARRIER MECHANISM IN THE BRAIN CAPILLARIES. Acta Pharmacol Toxicol (Copenh) 1963;20:317–321. doi: 10.1111/j.1600-0773.1964.tb01752.x. [DOI] [PubMed] [Google Scholar]
- BIRKMAYER W., HORNYKIEWICZ O. [The L-3,4-dioxyphenylalanine (DOPA)-effect in Parkinson-akinesia]. Wien Klin Wochenschr. 1961 Nov 10;73:787–788. [PubMed] [Google Scholar]
- Calne D. B., Karoum F., Ruthven C. R., Sandler M. The metabolism of orally administered L-Dopa in Parkinsonism. Br J Pharmacol. 1969 Sep;37(1):57–68. doi: 10.1111/j.1476-5381.1969.tb09522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calne D. B., Stern G. M., Laurence D. R., Sharkey J., Armitage P. L-dopa in postencephalitic parkinsonism. Lancet. 1969 Apr 12;1(7598):744–746. doi: 10.1016/s0140-6736(69)91751-6. [DOI] [PubMed] [Google Scholar]
- Duvoisin R. C. Cholinergic-anticholinergic antagonism in parkinsonism. Arch Neurol. 1967 Aug;17(2):124–136. doi: 10.1001/archneur.1967.00470260014002. [DOI] [PubMed] [Google Scholar]
- EHRINGER H., HORNYKIEWICZ O. [Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system]. Klin Wochenschr. 1960 Dec 15;38:1236–1239. doi: 10.1007/BF01485901. [DOI] [PubMed] [Google Scholar]
- FERRINI R., GLAESSER A. IN-VITRO DECARBOXYLATION OF NEW PHENYLALANINE DERIVATIVES. Biochem Pharmacol. 1964 May;13:798–801. doi: 10.1016/0006-2952(64)90018-8. [DOI] [PubMed] [Google Scholar]
- GILLINGHAM F. J., WATSON W. S., DONALDSON A. A., NAUGHTON J. A. The surgical treatment of parkinsonism. Br Med J. 1960 Nov 12;2(5210):1395–1402. doi: 10.1136/bmj.2.5210.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldberg H. C., Turner J. W., Hanieh A., Ashcroft G. W., Crawford T. B., Perry W. L., Gillingham F. J. On the occurrence of homovanillic acid and 5-hydroxyindol-3-ylacetic acid in the ventricular C.S.F. of patients suffering from parkinsonism. Confin Neurol. 1967;29(2):73–77. doi: 10.1159/000103680. [DOI] [PubMed] [Google Scholar]
- Guldberg H. C., Yates C. M. Some studies of the effects of chlorpromazine, reserpine and dihydroxyphenylalanine on the concentrations of homovanillic acid, 3,4-dihydroxyphenylacetic acid and 5-hydroxyindol-3-ylacetic acid in ventricular cerebrospinal fluid of the dog using the technique of serial sampling of the cerebrospinal fluid. Br J Pharmacol Chemother. 1968 Jul;33(3):457–471. doi: 10.1111/j.1476-5381.1968.tb00494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORNYKIEWICZ O. [Dopamine (3-hydroxytyramine) in the central nervous system and its relation to the Parkinson syndrome in man]. Dtsch Med Wochenschr. 1962 Sep 7;87:1807–1810. doi: 10.1055/s-0028-1114024. [DOI] [PubMed] [Google Scholar]
- Johansson B., Roos B. E. 5-hydroxyindoleacetic and homovanillic acid levels in the cerebrospinal fluid of healthy volunteers and patients with Parkinson's syndrome. Life Sci. 1967 Jul 1;6(13):1449–1454. doi: 10.1016/0024-3205(67)90193-2. [DOI] [PubMed] [Google Scholar]
- MAY R. H., VOEGELE G. E. Parkinsonian reactions following chlorpromazine and reserpine; similar reactions in the same patients. AMA Arch Neurol Psychiatry. 1956 May;75(5):522–524. doi: 10.1001/archneurpsyc.1956.02330230072008. [DOI] [PubMed] [Google Scholar]
- Pletscher A., Bartholini G., Tissot R. Metabolic fate of l-[14C] DOPA in cerebrospinal fluid and blood plasma of humans. Brain Res. 1967 Feb;4(1):106–109. doi: 10.1016/0006-8993(67)90154-0. [DOI] [PubMed] [Google Scholar]
- Poirier L. J., Singh P., Sourkes T. L., Boucher R. Effect of amine precursors on the concentration of striatal dopamine and serotonin in cats with and without unilateral brain stem lesions. Brain Res. 1967 Dec;6(4):654–666. doi: 10.1016/0006-8993(67)90123-0. [DOI] [PubMed] [Google Scholar]



