Abstract
The green wave hypothesis (GWH) states that migrating animals should track or ‘surf’ high-quality forage at the leading edge of spring green-up. To index such high-quality forage, recent work proposed the instantaneous rate of green-up (IRG), i.e. rate of change in the normalized difference vegetation index over time. Despite this important advancement, no study has tested the assumption that herbivores select habitat patches at peak IRG. We evaluated this assumption using step selection functions parametrized with movement data during the green-up period from two populations each of bighorn sheep, mule deer, elk, moose and bison, totalling 463 individuals monitored 1–3 years from 2004 to 2014. Accounting for variables that typically influence habitat selection for each species, we found seven of 10 populations selected patches exhibiting high IRG—supporting the GWH. Nonetheless, large herbivores selected for the leading edge, trailing edge and crest of the IRG wave, indicating that other mechanisms (e.g. ruminant physiology) or measurement error inherent with satellite data affect selection for IRG. Our evaluation indicates that IRG is a useful tool for linking herbivore movement with plant phenology, paving the way for significant advancements in understanding how animals track resource quality that varies both spatially and temporally.
Keywords: forage maturation hypothesis, green wave hypothesis, habitat selection, large herbivores, migration, normalized difference vegetation index
1. Introduction
The green wave hypothesis (GWH) states that migrating herbivores should track or ‘surf’ the leading edge of spring green-up, where forage quality is the highest [1]. Although originally proposed more than 35 years ago to explain the northerly migration of waterfowl in temperate latitudes [2], the GWH is re-emerging as a lens to understand the foraging benefit of migration in ungulate taxa [3,4]. Additionally, Fryxell [5] conceptualized the forage maturation hypothesis, which posits that herbivores should consume vegetation at an intermediate state of phenological growth, because as biomass increases, rate of energy intake by herbivores becomes limited by increasing fibre content [6]. The forage maturation hypothesis has helped frame studies of foraging by large herbivores for over a quarter of a century. As high-resolution spatial data and analyses are allowing more rigorous tests of the GWH [3,7], it is becoming increasingly important to more fully integrate these two hypotheses. In a sense, the GWH, when applied to large herbivores, is the spatial manifestation of the forage maturation hypothesis. As green-up is propagated across large landscapes during spring, animals that forage in habitats according to the forage maturation hypothesis are predicted to surf green waves of high-quality forage in accordance with the GWH. Indeed, the distribution of intermediate plant biomass has predicted the habitat use and distribution of herbivores such as barnacle geese (Branta leucopsis), wildebeest (Connochaetus taurinus), Mongolian gazelles (Procapra gutturosa), Thomson's gazelles (Eudorcas thomsonii) and Rocky Mountain elk (Cervus elaphus canadensis) [6,8–11].
Field efforts to identify when forage quality peaks annually across large landscapes are time consuming and sometimes impractical [1,12]. In response, researchers have increasingly used remotely sensed metrics—particularly the Normalized Difference Vegetation Index (NDVI; or greenness)—as a proxy for vegetation phenology and net primary production [13,14]. NDVI correlates with vegetation biomass in arid and semi-arid landscapes [15–17]. More importantly, a slow rate of spring green-up, calculated as the slope between successive data points of NDVI, can enhance juvenile survival in large herbivores [18,19]. Recent work by Bischof et al. [3] has proposed the instantaneous rate of green-up (IRG) to index high-quality forage at intermediate biomass from time-series NDVI data. The IRG is calculated as the first derivative of a fitted curve to annual NDVI time series, and represents an objective method to quantify how rate of spring green-up varies across time and space during the growing season [3]. Given a time series of NDVI values in habitats where vegetation growth occurs primarily during a single growing season, the IRG will peak at approximately half of the maximum NDVI value, where biomass levels are intermediate (see the electronic supplementary material, appendix A for a conceptualization). Therefore, according to the forage maturation hypothesis, herbivores should forage in areas at peak IRG to maximize intake of high-quality forage. Estimation of IRG allows testing the GWH within an explicit, spatial context across multiple taxa and ecosystems. Accordingly, migrating animals exposed to gradients of plant phenology (e.g. altitudinal or latitudinal) should surf waves of peak IRG across the landscape [3]. Even within one seasonal range, herbivores should exploit gradients of plant phenology—caused by vegetation community, topography or snow melt—by selecting habitat patches at peak IRG [20].
Using IRG to evaluate the GWH across large landscapes and diverse phenological gradients appears promising [3]. Coupling IRG with animal movement creates avenues for identifying environmental and anthropogenic factors that alter plant phenology and consequently facilitate or constrain optimal tracking of high-quality forage. The value of coupling remotely sensed data with animal movement is increasing as climate change and human development alter plant phenology across the globe [21,22], modifying the ability of individuals to track changes in forage quality. The global decline of migratory animals [23,24] further amplifies the need for new, integrative approaches to understanding the behavioural ecology of migration [25] as these taxa are likely dependent on the foraging benefits of green wave surfing.
Nevertheless, the development of IRG as a tool for understanding how a variety of herbivore species exploit high-quality forage in time and space remains incomplete. It is still unclear whether the IRG and GWH are applicable across multiple species of large herbivores residing in a variety of landscapes. Further, the critical assumption of the green wave and forage maturation hypotheses that highly mobile herbivores select habitat patches at peak IRG has yet to be verified. It is unclear whether highly mobile herbivores across diverse taxa have the behavioural perception to select habitat patches at peak IRG. According to theory [3,18,26,27], animals should use the most profitable habitat patches available, which assumes an ability to assess spatial and temporal variability in habitat quality and then select superior habitat patches. Understanding how multiple species of large herbivorous perceive and select habitat patches that change in quality over time provides insight into the behavioural capacity or flexibility of animals to minimize foraging losses caused by, for example, alterations in plant phenology driven by climatic change [21].
In this study, we provide a cross-taxa evaluation of the assumption that large herbivores surf waves of spring green-up by selecting habitat patches at peak IRG. Using a movement modelling framework and relocation data collected during the green-up period from two populations each of bighorn sheep (Ovis canadensis), mule deer (Odocoileus hemionus), elk, moose (Alces alces) and bison (Bison bison) in western Wyoming and eastern Utah, USA, we tested whether individuals select habitat patches when they are at peak IRG. Our mechanistic approach to test this assumption involved three steps. First, for each species we parametrized a base movement model, which included habitat attributes known to influence habitat selection for each species (e.g. cover type, elevation, slope, aspect, distance to escape terrain, integrated NDVI). Second, we evaluated empirical support for the notion that individuals select habitat patches with relatively high IRG values compared with the base model. Third, we quantified whether animals select habitat patches at the leading edge, trailing edge or crest of the IRG wave (see the electronic supplementary material, appendix A for a conceptualization) by examining selection for a quadratic form of NDVI, which represents a flexible form of intermediate NDVI values. Evaluating selection for varying degrees of intermediate biomass is important for assessing whether there are other constraints to optimal surfing not captured by remotely sensed IRG. Our multi-species analysis provides novel insight into the appropriateness of IRG as a phenological cue for large herbivores that forage across a variety of habitats.
2. Material and methods
(a). Study area
Western Wyoming and eastern Utah are semi-arid mountainous regions with elevations ranging from approximately 1300 to 4100 m. Major plant communities include shrub (dominated by sagebrush (Artemisia spp.)), coniferous forest (dominated by lodgepole pine (Pinus contorta)), pinyon-juniper woodland (dominated by singleleaf pinyon (Pinus monophylla) and Juniper ( Juniperus spp.)), and herbaceous meadows. Cover types based on the 2011 National Land Cover Database in the study area included shrub (47%), coniferous forest (30%), herbaceous grassland (13%), woody and herbaceous wetlands (5%), hay fields (2%), deciduous forest (2%) and all others constituted less than 1% of the landscape. Mean annual precipitation ranged from 10–20 cm in the lowest elevations to 150–250 cm in the highest elevations (PRISM Climate Group, Oregon State University, http://prism.oregonstate.edu). The regional climate is characterized by long cold winters and relatively short warm summers. Mean daily temperatures ranged from 13 to 21°C in July and −9 to −3°C in December, based on weather stations northeast of Pinedale, WY (Gunsight Pass SNOTEL site; elevation 3000 m; National Water and Climate Center, US Department of Agriculture) and in Cody, WY (elevation 1500 m; NOAA Online Weather Data, National Oceanic and Atmospheric Administration).
(b). GPS collar data
We used spatial relocation data from previous studies of GPS-collared female ungulates. We collated GPS data on individuals collared over 1–3 years from 10 populations representing five species: bighorn sheep in the Teton Range (n = 20; years 2008–2010) and Whiskey Basin (n = 8; years 2002, 2004, 2012) areas, mule deer in the Upper Green River Basin (UGRB; n = 29; year 2012) and southern and eastern Wyoming Range (n = 46; years 2013–2014) areas, elk in the Southern Greater Yellowstone Ecosystem (n = 119; years 2006–2014) and Absaroka Mountain (n = 88; years 2007–2010) areas, moose in the Buffalo Valley (n = 39; years 2005–2010) and the eastern slope of the northern Wyoming Range (n = 64; years 2011–2014), and bison in the Henry Mountains (n = 46; years 2011–2014) and Book Cliffs (n = 4; year 2014) areas (see study area figure in the electronic supplementary material, appendix B). Elk used in the analysis from the Southern Greater Yellowstone Ecosystem never visited feedgrounds that also occur in this region. Elk in the Southern Greater Yellowstone Ecosystem occupied the western slope of the Wind River Range, eastern slope of the Wyoming Range and Buffalo Valley areas. The GPS fix rate varied for each population and included a fix every 0.5, 1, 2, 2.75, 3, 5 or 6 h. Collared individuals across all taxa represented a mix of migratory and resident foraging strategies (see the electronic supplementary material, appendix D for proportion of each population that was migratory). Our intent was to test the ability of animals to track IRG regardless of migratory status. Nonetheless, we subset the data to only migratory individuals and reran the analyses explained below; results were not different.
(c). Movement modelling approach
We used step selection functions [28] to test the assumption that herbivores select habitat patches with vegetation at intermediate biomass as indexed by peak IRG during the spring green-up period. We first identified consecutive steps in the movement data that were between 8 and 12 h apart, keeping the step interval the same within a population. We chose this range of temporal sampling for two reasons: (i) to ensure a similar sampling regime given variable fix rates, and (ii) to match as closely as possible the spatial sampling regime of the NDVI data (250 m resolution) so that all species had a mean probability of more than 0.5 of moving outside of a 250 m cell at each step. For each step, we identified the source and target points, and drew 25 potential target points originating from the known source point by sampling from the individuals step and turning angle distribution simultaneously [28]. These 25 potential target points were identified as available and compared to the used target step.
(d). Explanatory variables
Using surface reflectance bands 1 and 2 (250 m spatial and 8-day temporal resolution) from the MOD09Q1 data product from the MODIS terra satellite, we calculated NDVI [13,14] for the entire study area from 2001 to 2014. Following the protocol of Bischof et al. [3], we constructed a smoothed and scaled NDVI time series for each pixel. Briefly, this entailed a processing sequence of: (i) setting values less than 0 and clouded pixels to no data; (ii) flooring the times series to a winter value (November through February) calculated as the 0.025 quantile of each pixel's time series; (iii) applying a moving three-window median filter; and (iv) scaling the time series between 0 and 1 based on the upper 0.975 quantile of each pixel's time series. The higher elevations of our study area accumulate significant snow that lasts as snow pack sometimes into May and June. We found that setting all NDVI values after the end of February to ‘no data,’ as in Bischof et al.'s [3] procedure, led to fitted curves that portrayed green-up starting when sometimes more than a metre of snow was still on the ground. Thus, we used the snow cover band from the MOD09A1 data product (500 m spatial and 8-day temporal resolution) to determine how long after February (if any) to keep the NDVI time series floored to the winter value. NDVI for a time series of a given pixel was floored until the snow cover flag was absent for two consecutive 8-day data points. We followed Bischof et al.'s [3] fitting procedure using the double logistic curve. Once the double logistic curves were fitted for each pixel's time series for each year, we calculated the first derivative of this curve during the green-up portion and scaled it between 0 and 1, resulting in an IRG curve for each pixel [3]. Finally, we identified NDVI and IRG values associated in time and space with each potential and used target point.
The base model for all populations included the Euclidian distance (in kilometres) and elevation change (in kilometres) between the source and target points, as a measure of energetic cost to moving to the potential target point [29,30]. Elevation was calculated for each point using the US Geological Survey National Elevation Dataset (30 m resolution). For mule deer, moose and elk, we included the difference in slope (in degrees) between the source and target points and aspect (ranging from −1 as southerly to 1 as northerly aspects) of the target point. For bighorn sheep, we included distance to escape terrain (in kilometres) of each target point. We defined escape terrain as areas with a slope more than 30° and a terrain ruggedness index more than 75 (sensu [31]). Slope, aspect and terrain ruggedness were calculated based on the 30 m elevation dataset. We also identified land cover types that have been shown to be important in the habitat selection of each species [32–34]. Using the 2011 National Land Cover Database (30 m resolution), we calculated a dummy variable (except in the case of per cent canopy cover) for the following cover types for each species: meadow (including grassland herbaceous and herbaceous wetlands) and shrub for bighorn sheep; per cent canopy cover for mule deer, elk and bison; and deciduous (including deciduous and mixed forests) and wetlands (including woody and herbaceous wetlands) for moose. Finally, to account for overall productivity or biomass at a given habitat patch, aside from its current relative phenological state, we calculated the integrated NDVI value for each target point [14].
(e). Data analysis and model selection
To ensure that green-up was available to individuals for the analysis, we reduced the step database to only steps that occurred when more than 50% of the available and used target points were undergoing spring green-up (i.e. the green-up period). We defined this period for each target point's pixel by identifying the Julian day of the start and end of spring in a given year, calculated by identifying the first and second derivatives of the IRG curve, respectively. We then further minimized any potential biases caused by functional responses in habitat selection (i.e. selection that varies by availability) by removing complete strata of target points where the range of IRG values in available pixels was less than 0.75 (i.e. 75% of the range of available IRG values).
We used a three-step approach to test the assumption that herbivores select habitat patches at peak IRG during the spring green-up period. First, we parametrized a base model for each population, which included distance, elevation, slope, aspect, escape terrain (for bighorn sheep), land cover and integrated NDVI. We then parametrized a second model that included the variables of the base model plus IRG, and assessed the empirical support for adding IRG. Finally, we compared the empirical support of the base and IRG models with a model containing variables from the base model and NDVI, and the base model and NDVI plus NDVI2. By calculating the local maximum of the quadratic relationship between relative probability of selection and NDVI, we could determine whether animals select the leading or trailing edge of the IRG curve.
Models were parametrized using conditional logistic regression, with each stratum identified as a used point and its paired 25 available target points. We calculated robust SE and 95% CI of parameters using generalized estimating equations, because of temporal autocorrelation and a lack of independence within an individual's movements [35]. All strata for a given individual and year were assigned a unique cluster. We assessed the level of relative empirical support received for each model by calculating the quasi-likelihood under independence criterion (QIC), which accounts for non-independence among observations within a cluster [35]. The level of collinearity among variables within fitted models was assessed by calculating variance inflation factors (VIFs) for all variables (except for NDVI and NDVI2 when they were in the same model). VIFs were always less than 2. We validated models for each species with the most empirical support using five-folds cross-validation repeated 100 times, following the framework developed by Fortin et al. [36] for step selection functions.
3. Results
For all populations, we established base models that quantified the effect of static habitat attributes on discrete movement steps, while taking into account the distance and elevation change between source and target points. Bighorn sheep generally selected habitat patches that were closer to escape terrain, had higher integrated NDVI values, and were covered by meadow and shrub. Mule deer, elk and bison generally selected less steep habitat patches with lower tree cover, higher integrated NDVI values and a southerly aspect. Moose generally selected flatter habitat patches that were on southerly aspects, and they used patches classified as deciduous or wetland areas in accordance with their availability (see coefficient tables for models with most empirical support in the electronic supplementary material, appendix C).
The annual green-up period that all populations were exposed to lasted for a mean of 90 days, starting on a mean date of 5 April (electronic supplementary material, appendix D). In all populations, except for both elk populations and Book Cliffs bison, adding IRG to the base model improved model fit by more than 1 QIC unit (mean decrease in QIC = 29.4; table 1). Thus, populations of bighorn sheep, mule deer, moose and Henry Mountains bison selected habitat patches with higher IRG values than available patches (βIRG ranged from 0.02 to 1.40; all lower 95% CI for βIRG did not overlap zero, except for Buffalo Valley moose; figure 1).
Table 1.
Relative empirical support for selection of habitat patches when at peak IRG during the green-up season. Step selection functions were parametrized by data from two populations each of bighorn sheep, mule deer, elk, moose and bison in western Wyoming and eastern Utah (totalling 463 individuals) between 2004 and 2014. The base model included variables representing habitat attributes known to influence habitat selection of each species (e.g. cover type, elevation, slope, aspect, distance to escape terrain). The model including IRG also contained all variables in the base models. Support was assessed using the QIC, n refers to the number of individuals in a population, n event refers to the number of movement steps used to fit the model and K is the number of model parameters.
| pop model | QIC | ΔQIC | n event | K | pop model | QIC | ΔQIC | n event | K |
|---|---|---|---|---|---|---|---|---|---|
| Teton Range bighorn sheep (n = 20) | Whiskey Basin bighorn sheep (n = 8) | ||||||||
| IRG | 9762.6 | 0.0 | 1584 | 7 | IRG | 1336.1 | 0.0 | 211 | 7 |
| base | 9793.4 | 30.8 | 1584 | 6 | base | 1339.5 | 3.4 | 211 | 6 |
| Green River Basin mule deer (n = 29) | SE Wyoming Range mule deer (n = 46) | ||||||||
| IRG | 8580.6 | 0.0 | 1366 | 7 | IRG | 12709.5 | 0.0 | 2046 | 7 |
| base | 8646.2 | 65.6 | 1366 | 6 | base | 12716.9 | 7.4 | 2046 | 6 |
| S Greater Yellowstone elk (n = 119) | Absaroka mountains elk (n = 88) | ||||||||
| base | 65747.1 | 0.0 | 10 345 | 6 | base | 14727.5 | 0.0 | 2371 | 6 |
| IRG | 65750.2 | 3.1 | 10 345 | 7 | IRG | 14727.5 | 0.0 | 2371 | 7 |
| Buffalo Valley moose (n = 39) | NE Wyoming Range moose (n = 64) | ||||||||
| IRG | 7998.6 | 0.0 | 1243 | 8 | IRG | 8034.3 | 0.0 | 1259 | 8 |
| base | 7999.6 | 1.0 | 1243 | 7 | base | 8120.4 | 86.1 | 1259 | 7 |
| Henry Mountains bison (n = 46) | Book Cliffs bison (n = 4) | ||||||||
| IRG | 15302.9 | 0.0 | 2669 | 7 | base | 854.7 | 0.0 | 156 | 6 |
| base | 15314.3 | 11.4 | 2669 | 6 | IRG | 855.5 | 0.8 | 156 | 7 |
Figure 1.
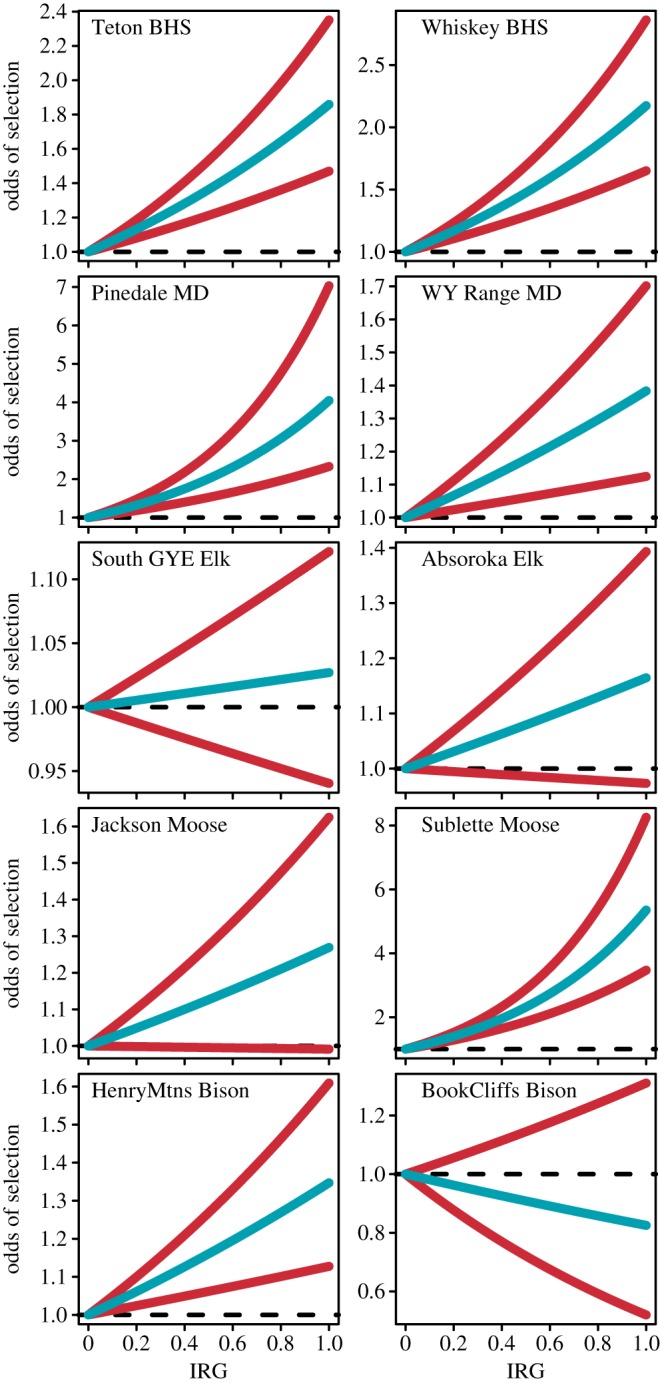
Relationship between odds of selection (with 95% CI in red, reported as odds ratio or exponent of the beta coefficient for IRG]) of a habitat patch and its IRG value for two populations each of elk, mule deer (MD), bighorn sheep (BHS), moose and bison in western Wyoming and eastern Utah between 2004 and 2014. Probability of selection based on predicted values of a Step Selection Function parametrized with GPS collar data in each population. (Online version in colour.)
Our flexible analysis to evaluate the position on the IRG curve that animals select for revealed that Teton Range bighorn sheep and SE Wyoming Range mule deer selected habitat patches at the trailing edge of the IRG curve (figure 2; electronic supplementary material, appendix E). Except for Book Cliffs bison, which selected habitat patches at the leading edge of the IRG curve, all other populations (Whiskey Basin bighorn sheep, UGRB mule deer, both populations of moose and Henry Mountains bison) selected for patches at the crest of the IRG curve (figure 2; electronic supplementary material, appendix E). The IRG, however, was not always the best variable to explain habitat selection (electronic supplementary material, appendix F). For Teton Range bighorn sheep, SE Wyoming Range mule deer and bison, a model containing NDVI and NDVI2 received more empirical support than a comparable model containing IRG (mean of 54.9 QIC units better than a model containing IRG). For both elk populations, a model including NDVI alone best predicted selection of habitat patches (electronic supplementary material, appendix F). Top models for each population had satisfactory to excellent robustness based on k-fold cross-validation: mean observed Spearman rank correlation across all populations was 0.60 (s.d. = 0.24), and mean expected under random selection patterns was −0.18 (s.d. = 0.21; see the electronic supplementary material, appendix G for details).
Figure 2.
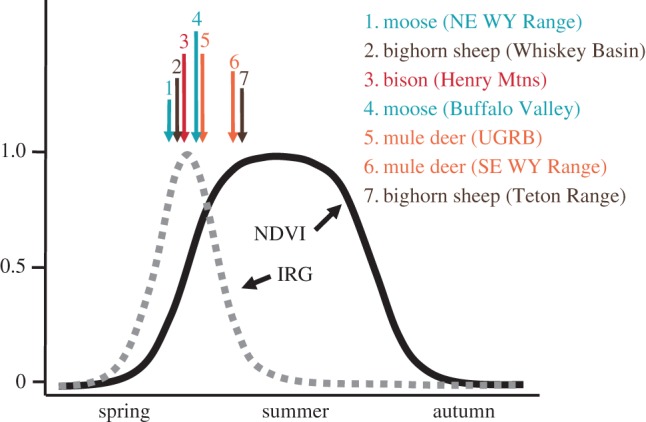
Selection for the leading edge, trailing edge or crest of the IRG wave by mule deer, bighorn sheep and moose in western Wyoming, and bison in eastern Utah between 2004 and 2014. The location on the wave was determined by calculating the local maximum of the quadratic relationship between relative probability of selection and NDVI. Probability of selection was based on a step selection function parametrized with GPS collar data in each population. (Online version in colour.)
4. Discussion
We evaluated whether multiple large herbivore species residing in different study areas select habitat patches at peak IRG in spring and thus track forage at intermediate biomass—a critical assumption of the green wave and forage maturation hypotheses [3]. In support of the GWH, seven of the 10 populations we examined surfed an IRG-indexed green wave by selecting habitat patches with relatively high IRG values (table 1). Nevertheless, five of the seven populations surfed the crest of the IRG wave, whereas the other two surfed the trailing edge of the wave (figure 2). Three populations did not surf the IRG wave at all; both elk populations selected habitat patches at peak NDVI values (far later than peak IRG), and Book Cliffs bison neither selected nor avoided habitat patches when at peak IRG or NDVI. Although variability existed across taxa, our results indicated that habitat selection of temperate large herbivores in spring is indeed influenced by temporal dynamics in habitat quality and patterns of plant phenology that are mainly captured by IRG, thereby helping to enhance our understanding of, and power to predict, animal movement and migration [3].
The IRG metric provides a much-needed tool for researchers and managers seeking to understand how animals track forage resources that change as the growing season progresses across large landscapes. First, assessing change in selection strength for IRG over time may provide insights into how well animals are surfing waves of high-quality forage. Detecting changes in surfing ability can alert researchers and managers to when tracking abilities (i.e. movement) of individuals are threatened by, for example, environmental (e.g. climate change, drought) or land-use (e.g. land development or disturbance) change. Second, from a management and conservation perspective, understanding how large herbivores track waves of forage may help guide habitat improvement projects. For example, after observing a decrease in successful green wave surfing, managers may be able to alter habitat (e.g. remove fencing, or restrict human access) at specific times (i.e. during the green-up season) so that high-quality forage again becomes accessible. Third, assessing how individuals across species and ecosystems select IRG incorporates a key temporal dimension of habitat quality that, heretofore, has been more of a static view and will no doubt inform investigations of optimal foraging, movement and migration [3,20]. Finally, monitoring changes in IRG over the growing season can provide insight into how climate change is affecting the timing and rate of spring green-up, which is emerging as a key component of annual habitat quality, and ultimately, fitness and population performance (e.g. [18,19,37,38]). Incorporating IRG into such analyses will prove useful for quantifying the loss in forage availability—because of climate change or land development—that animals might experience in the future.
The forage maturation and green wave hypotheses have their roots in foraging theory, where individuals who seek to maximize their intake rate of forage will enjoy higher fitness than those who do not [27]. Our results support the notion that movement decisions and spatial distribution of animals are related to energy intake [11], where selecting for sites at peak IRG likely corresponds to a balance between forage biomass and quality. During spring, such a foraging strategy is essential as animals departing winter ranges are often experiencing a net energy deficit [39], and female ungulates that are pregnant will soon face lactation, their highest energy burden of the year. Tracking and selecting habitat patches with high-quality forage is imperative for replenishing fat reserves and acquiring the energy needed to provision young during lactation [39]. We would therefore expect that tracking ability would be correlated with herbivore fitness. To date, however, we are not aware of a study that has linked the ability of individuals to surf IRG with any fitness correlates. Nevertheless, Searle et al. [38] related body condition with the synchrony of NDVI-based rate of green-up within home ranges of mule deer in Colorado, and Hurley et al. [40] reviewed relationships between other NDVI metrics and life-history characteristics linked to performance and population abundance. Connecting phenology tracking to forage quality, diet, nutrition and demography is the next step in integrating the forage maturation and green wave hypotheses.
Three of the 10 populations we examined did not select habitat patches at peak IRG, and two others did not select the crest of the IRG wave but selected habitat patches that lagged behind the IRG wave. We propose the following four hypotheses that might explain variation in selection for IRG that we observed. First, species-specific differences may be a function of body size and ruminant physiology, resulting in adaptations in behaviour to exploit forage at phenological states to enhance energy intake. For instance, ruminant herbivores vary along a gradient in morphophysiological feeding types from concentrate selectors (e.g. deer and moose) to grass and roughage eaters (e.g. elk and bison), where concentrate selectors seek out plants with less cell wall and fibre than roughage eaters [41]. Relative to the forage maturation and green wave hypotheses, grass/roughage eaters should select habitat patches at higher biomass, i.e. after peak IRG, than smaller-bodied ungulates. Our results only partially support this hypothesis. Selection of habitat patches by elk, moose and bighorn sheep generally follows such ruminant physiological predictions. Yet, bison should select habitat patches at the highest biomass values, whereas mule deer should be selecting at the most intermediate biomass values—neither appears to do so.
Second, variation in selection for IRG could be explained by measurement error where there is a mismatch between the scale of NDVI data used to quantify forage quality and the scale of habitat selection. NDVI is satellite-derived and quantifies a mean of all photosynthetic activity occurring at a given pixel [14]. In our instance, NDVI is indexing an average greenness or biomass of millions of individual plants that grow within a 250 m patch. Each species (and potentially each population) of large herbivores we examined presumably forages on different plant species and functional groups. If a given herbivore targets a plant species with a phenology different from the mean of all plants in a community or NDVI pixel, then NDVI will not correctly index the phenology of the plant species sought by a given herbivore. Indeed, the relationship between NDVI and biomass can differ (including varying degrees of correlation) among plant communities [42]. Furthermore, the relationship between NDVI and biomass in some vegetation types can be nonlinear [43], complicating coarse indices of vegetation phenology. A comprehensive, species-specific test of whether IRG correctly indexes digestible energy and growth of forage plants—including evaluating the ability of herbivores to mediate patch-based NDVI through diet selection—would complement our study and help determine the ability of large herbivores to surf waves of high-quality food. Nonetheless, individuals of most populations we investigated selected for high IRG, suggesting that until further studies provide refinements, the IRG is a useful tool.
Third, variation in selection for IRG may be caused by a mismatch between movement and optimal plant phenology driven by site fidelity. All of the animals we monitored portrayed varying levels of fidelity to annual or seasonal ranges, and migration routes. Site fidelity, by means of remembering past experience [44], plays an important role in movement and habitat selection of mobile animals [45]. In some instances, site fidelity can be such a strong adaptive force that animals will use familiar, but poorer-quality habitat patches over ones of higher quality [46]. The ability of animals to track IRG is likely constrained by past experience and knowledge repertoire. A mismatch between the true, current locations of high-quality food and locations of high-quality food in memory is likely exacerbated in areas where weather patterns are rapidly changing, because of climate and land-use change. For example, previous work on the Absaroka elk population in our analysis indicates that rate of spring green-up has increased, leading to shorter spring periods [37]. Shorter springs likely make it difficult for individuals with strong site fidelity to adapt to new environmental conditions. Further, accelerated spring green-up should be a strong factor limiting the ability to assess habitat quality at a given time and successfully surf the green wave. Indeed, the Absaroka elk population did not select habitat patches when at peak IRG, but selected habitat patches when at their peak greenness or highest NDVI values (figure 2).
Finally, variation in selection for IRG may be attributable to inter- and intraspecific interactions (including predation risk) that affect optimal habitat selection [47,48]. For example, female red deer (C. elaphus) on the Isle of Rum, Scotland trade-off selecting high-quality resources with diminished lifetime reproductive success because of relatively high density at high-quality sites [49]. Similar density-dependent trade-offs may limit the ability of individuals in some of the populations we examined to select habitat patches when at peak IRG. For animals foraging at the trailing edge of IRG or at peak NDVI, it may be beneficial to forage on vegetation with relatively high biomass to attain rumen fill quickly (i.e. a time-minimizing foraging objective), resulting in extra time to, for example, be more vigilant or maintain social status [50]. Further, anthropogenic land-use change or other disturbance could result in the cessation of the link between foraging cues and their foraging benefits for animals, causing a miscue in timing of movements (e.g. [51]). For example, mule deer within our study area increase movement rate, detour from established migration routes and reduce stopover use in areas of intense human development [52]—all observations that could lead to poor tracking or surfing ability.
In conclusion, remotely sensed measures of vegetation biomass and phenology, such as the NDVI and its derivatives, have proven useful in connecting animal movement to phenological changes in vegetation across large landscapes [3,4,13,20]. Earlier work by Bischof et al. [3] made a key step forward by identifying IRG as an index to quantify the spatial and temporal distribution of high-quality forage, and using it to mechanistically link the green wave and forage maturation hypotheses, plant phenology and animal movement. Our multi-species examination largely supports the critical assumption of the green wave and forage maturation hypotheses: herbivores track plants at intermediate biomass by selecting habitat patches at peak IRG. Consistent with the findings of Bischof et al. [3], IRG appears to capture broad-scale variation in forage quality that multiple large herbivores can perceive and respond to while foraging. Further, our work highlights species- and population-specific variation in the position on the IRG wave that individuals and populations select. We hypothesize that variation in phenology tracking is related to differences in foraging strategies among taxa, phenological mismatches and other factors that constrain individuals from optimally tracking plant phenology. Unravelling differences in selection for IRG among species, populations and individuals should prove fruitful in understanding how landscape- and climate-related changes affect gradients in plant phenology that animals rely on, and for identifying when habitat manipulation will benefit herbivores that move across large landscapes.
Supplementary Material
Acknowledgements
Numerous private landowners permitted access to their properties to capture animals and retrieve radio collars used for this study. Many field technicians, biologists and wildlife managers assisted with logistics and data collection. T. Morrison provided insight during the development of the study design. Any use of trade, product or firm names is for descriptive purposes only and does not imply endorsement by the US Government.
Data accessibility
Raw GPS collar data used in this analysis can be accessed via the Wyoming Migration Database and Viewer located at migrationinitiative.org.
Authors' contributions
J.A.M. and M.J.K. conceived the idea. J.A.M., M.J.K., K.L.M., E.O.A. and M.M.H. designed the study. J.A.M. conducted all analyses and wrote the manuscript. Datasets were collected and managed by all authors and cooperators. All authors contributed to revisions and gave final approval for publication.
Competing interests
The authors have no competing interests.
Funding
J.A.M. was supported by a US Department of Agriculture National Institute of Food and Agriculture postdoctoral fellowship (grant award no. 2014–01928). E.O.A. was supported by The National Science Foundation Graduate Research Fellowship (grant no. 1252375) and the University of Wyoming's Berry Fellowship. Support for data collection and management came from The Boone and Crockett Club, Bowhunters of Wyoming, Cimarex Energy, Finley Resources, Inc., Greater Yellowstone Interagency Brucellosis Committee, Knobloch Family Foundation, Muley Fanatic Foundation, Northwind, LLC, Plains Exploration and Production Company, Ricketts Conservation Foundation, Rocky Mountain Elk Foundation, Safari Club International Foundation, Sportsmen for Fish and Wildlife, Teton Conservation District, US Bureau of Land Management, US Forest Service, US Geological Survey, Utah Division of Wildlife Resources, Wyoming Animal Damage Management Board, Wyoming Game and Fish Department, Wyoming Governor's Big Game License Coalition, Wyoming Wildlife Foundation, Wyoming Wildlife and Natural Resource Trust and Wyoming and Sublette County Outfitters and Guides Association.
References
- 1.Van der Graaf A, Stahl J, Klimkowska A, Bakker JP, Drent RH. 2006. Surfing on a green wave–how plant growth drives spring migration in the Barnacle Goose Branta leucopsis. Ardea 94, 567–577. [Google Scholar]
- 2.Drent R, Ebbinge B, Weijand B. 1978. Balancing the energy budgets of arctic-breeding geese throughout the annual cycle: a progress report. Verh. Ornithol. Ges. Bayern. 23, 239–264. [Google Scholar]
- 3.Bischof R, Egil Loe L, Meisingset EL, Zimmermann B, Van Moorter B, Mysterud A. 2012. A migratory northern ungulate in the pursuit of spring: jumping or surfing the green wave? Am. Nat. 180, 407–424. ( 10.3410/f.717973224.793469905) [DOI] [PubMed] [Google Scholar]
- 4.Sawyer H, Kauffman MJ. 2011. Stopover ecology of a migratory ungulate. J. Anim. Ecol. 80, 1078–1087. ( 10.1111/j.1365-2656.2011.01845.x) [DOI] [PubMed] [Google Scholar]
- 5.Fryxell JM. 1991. Forage quality and aggregation by large herbivores. Am. Nat. 138, 478–498. ( 10.1086/285227) [DOI] [Google Scholar]
- 6.Hebblewhite M, Merrill E, McDermid G. 2008. A multi-scale test of the forage maturation hypothesis in a partially migratory ungulate population. Ecol. Monogr. 78, 141–166. ( 10.1890/06-1708.1) [DOI] [Google Scholar]
- 7.Si Y, Xin Q, de Boer WF, Gong P, Ydenberg RC, Prins HHT. 2015. Do Arctic breeding geese track or overtake a green wave during spring migration? Sci. Rep. 5, 8749 ( 10.1038/srep08749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilmshurst JF, Fryxell JM, Farm BP, Sinclair A, Henschel CP. 1999. Spatial distribution of Serengeti wildebeest in relation to resources. Can. J. Zool. 77, 1223–1232. ( 10.1139/z99-088) [DOI] [Google Scholar]
- 9.Si Y et al. 2011. Distribution of barnacle geese Branta leucopsis in relation to food resources, distance to roosts, and the location of refuges. Ardea 99, 217–226. ( 10.5253/078.099.0212) [DOI] [Google Scholar]
- 10.Mueller T, Olson KA, Fuller TK, Schaller GB, Murray MG, Leimgruber P. 2008. In search of forage: predicting dynamic habitats of Mongolian gazelles using satellite-based estimates of vegetation productivity. J. Appl. Ecol. 45, 649–658. ( 10.1111/j.1365-2664.2007.01371.x) [DOI] [Google Scholar]
- 11.Fryxell JM, Wilmshurst JF, Sinclair AR. 2004. Predictive models of movement by Serengeti grazers. Ecology 85, 2429–2435. ( 10.1890/04-0147) [DOI] [Google Scholar]
- 12.Fortin D, Fryxell JM, Pilote R. 2002. The temporal scale of foraging decisions in bison. Ecology 83, 970–982. ( 10.2307/3071906) [DOI] [Google Scholar]
- 13.Pettorelli N, Ryan SJ, Mueller T, Bunnefeld N, Jedrzejewsk B, Lima M, Kausrud K. 2011. The Normalized Difference Vegetation Index (NDVI): unforeseen successes in animal ecology. Clim. Res. 46, 15–27. ( 10.3354/cr00936) [DOI] [Google Scholar]
- 14.Pettorelli N, Vik JO, Mysterud A, Gaillard JM, Tucker CJ, Stenseth NChr. 2005. Using the satellite-derived NDVI to assess ecological responses to environmental change. Trends Ecol. Evol. 20, 503–510. ( 10.1016/j.tree.2005.05.011) [DOI] [PubMed] [Google Scholar]
- 15.Dancose K, Fortin D, Guo X. 2011. Mechanisms of functional connectivity: the case of free-ranging bison in a forest landscape. Ecol. Appl. 21, 1871–1885. ( 10.1890/10-0779.1) [DOI] [PubMed] [Google Scholar]
- 16.Boelman NT, Stieglitz M, Rueth HM, Sommerkorn M, Griffin KL, Shaver GR, Gamon JA. 2003. Response of NDVI, biomass, and ecosystem gas exchange to long-term warming and fertilization in wet sedge tundra. Oecologia 135, 414–421. ( 10.1007/s00442-003-1198-3) [DOI] [PubMed] [Google Scholar]
- 17.Hobbs TJ. 1995. The use of NOAA-AVHRR NDVI data to assess herbage production in the arid rangelands of Central Australia. Int. J. Remote Sensing 16, 1289–1302. ( 10.1080/01431169508954477) [DOI] [Google Scholar]
- 18.Pettorelli N, Pelletier F, Hardenberg AV, Festa-Bianchet M, Côté SD. 2007. Early onset of vegetation growth vs. rapid green-up: impacts on juvenile mountain ungulates. Ecology 88, 381–390. ( 10.1890/06-0875) [DOI] [PubMed] [Google Scholar]
- 19.Monteith KL, Klaver RW, Hersey KR, Holland AA, Thomas TP, Kauffman MJ. 2015. Effects of climate and plant phenology on recruitment of moose at the southern extent of their range. Oecologia 178, 1137–1148. ( 10.1007/s00442-015-3296-4) [DOI] [PubMed] [Google Scholar]
- 20.van Moorter B, Bunnefeld N, Panzacchi M, Rolandsen CM, Solberg EJ, Sæther B-E. 2013. Understanding scales of movement: animals ride waves and ripples of environmental change. J. Anim. Ecol. 82, 770–780. ( 10.1111/1365-2656.12045) [DOI] [PubMed] [Google Scholar]
- 21.Cleland EE, Chuine I, Menzel A, Mooney HA, Schwartz MD. 2007. Shifting plant phenology in response to global change. Trends Ecol. Evol. 22, 357–365. ( 10.1016/j.tree.2007.04.003) [DOI] [PubMed] [Google Scholar]
- 22.Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TJ, Fromentin J-M, Hoegh-Guldberg O, Bairlein F. 2002. Ecological responses to recent climate change. Nature 416, 389–395. ( 10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- 23.Berger J. 2004. The last mile: how to sustain long-distance migration in mammals. Conserv. Biol. 18, 320–331. ( 10.1111/j.1523-1739.2004.00548.x) [DOI] [Google Scholar]
- 24.Wilcove DS, Wikelski M. 2008. Going, going, gone: is animal migration disappearing. PLoS Biol. 6, e188 ( 10.1371/journal.pbio.0060188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolger DT, Newmark WD, Morrison TA, Doak DF. 2008. The need for integrative approaches to understand and conserve migratory ungulates. Ecol. Lett. 11, 63–77. ( 10.1111/j.1461-0248.2007.01109.x) [DOI] [PubMed] [Google Scholar]
- 26.Albon S, Langvatn R. 1992. Plant phenology and the benefits of migration in a temperate ungulate. Oikos 63, 502–513. ( 10.2307/3545568) [DOI] [Google Scholar]
- 27.Stephens DW, Krebs JR. 1986. Foraging theory. Princeton, NJ: Princeton University Press. [Google Scholar]
- 28.Fortin D, Beyer HL, Boyce MS, Smith DW, Duchesne T, Mao JS. 2005. Wolves influence elk movements: behavior shapes a trophic cascade in Yellowstone National Park. Ecology 86, 1320–1330. ( 10.1890/04-0953) [DOI] [Google Scholar]
- 29.Parker KL, Robbins CT, Hanley TA. 1984. Energy expenditures for locomotion by mule deer and elk. J. Wildl. Manag. 48, 474–488. ( 10.2307/3801180) [DOI] [Google Scholar]
- 30.Mitchell MS, Powell RA. 2004. A mechanistic home range model for optimal use of spatially distributed resources. Ecol. Model. 177, 209–232. ( 10.1016/j.ecolmodel.2004.01.015) [DOI] [Google Scholar]
- 31.McKinney T, Boe SR, deVos JC Jr. 2003. GIS-based evaluation of escape terrain and desert bighorn sheep populations in Arizona. Wildl. Soc. Bull. 31, 1229–1236. [Google Scholar]
- 32.DeCesare NJ, Pletscher DH. 2006. Movements, connectivity, and resource selection of Rocky Mountain bighorn sheep. J. Mamm. 87, 531–538. ( 10.1644/05-mamm-a-259r1.1) [DOI] [Google Scholar]
- 33.Thomas JW, Black H Jr, Scherzinger RJ, Pedersen RJ. 1979. Deer and elk. In Wildlife habitats in managed forests: the Blue Mountains of Oregon and Washington, vol. 553 (ed Thomas JW.), pp. 104–127. USDA Forest Service Agriculture Handbook; Washington, DC: US Government Printing Office. [Google Scholar]
- 34.Osko TJ, Hiltz MN, Hudson RJ, Wasel SM. 2004. Moose habitat preferences in response to changing availability. J. Wildl. Manag. 68, 576–584. ( 10.2193/0022-541x(2004)0680576:mhpirt%5D2.0.co;2) [DOI] [Google Scholar]
- 35.Craiu RV, Duchesne T, Fortin D. 2008. Inference methods for the conditional logistic regression model with longitudinal data. Biometric. J. 50, 97–109. ( 10.1002/bimj.200610379) [DOI] [PubMed] [Google Scholar]
- 36.Fortin D, Fortin ME, Beyer HL, Duchesne T, Courant S, Dancose K. 2009. Group-size-mediated habitat selection and group fusion–fission dynamics of bison under predation risk. Ecology 90, 2480–2490. ( 10.1890/08-0345.1) [DOI] [PubMed] [Google Scholar]
- 37.Middleton AD, Kauffman MJ, McWhirter DE, Cook JG, Cook RC, Nelson AA, Jimenez MD, Klaver RW. 2013. Animal migration amid shifting patterns of phenology and predation: lessons from a Yellowstone elk herd. Ecology 94, 1245–1256. ( 10.1890/11-2298.1) [DOI] [PubMed] [Google Scholar]
- 38.Searle KR, Rice MB, Anderson CR, Bishop C, Hobbs N. 2015. Asynchronous vegetation phenology enhances winter body condition of a large mobile herbivore. Oecologia 179, 377–391. ( 10.1007/s00442-015-3348-9) [DOI] [PubMed] [Google Scholar]
- 39.Parker KL, Barboza PS, Gillingham MP. 2009. Nutrition integrates environmental responses of ungulates. Funct. Ecol. 23, 57–69. ( 10.1111/j.1365-2435.2009.01528.x) [DOI] [Google Scholar]
- 40.Hurley MA, Hebblewhite M, Gaillard J-M, Dray S, Taylor KA, Smith W, Zager P, Bonenfant C. 2014. Functional analysis of Normalized Difference Vegetation Index curves reveals overwinter mule deer survival is driven by both spring and autumn phenology. Phil. Trans. R. Soc. B 369, 20130196 ( 10.1098/rstb.2013.0196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hofmann R. 1989. Evolutionary steps of ecophysiological adaptation and diversification of ruminants: a comparative view of their digestive system. Oecologia 78, 443–457. ( 10.1007/BF00378733) [DOI] [PubMed] [Google Scholar]
- 42.Asner GP. 1998. Biophysical and biochemical sources of variability in canopy reflectance. Remote Sensing Environ. 64, 234–253. ( 10.1016/S0034-4257(98)00014-5) [DOI] [Google Scholar]
- 43.Asrar G, Myneni RB, Kanemasu ET. 1989. Estimation of plant canopy attributes from spectral reflectance measurements. In Theory and applications of optical remote sensing (ed. Asrar G.), pp. 252–295. New York, NY: John Wiley and Sons. [Google Scholar]
- 44.Merkle JA, Fortin D, Morales JM. 2014. A memory-based foraging tactic reveals an adaptive mechanism for restricted space use. Ecol. Lett. 17, 924–931. ( 10.1111/ele.12294) [DOI] [PubMed] [Google Scholar]
- 45.Piper WH. 2011. Making habitat selection more ‘familiar’: a review. Behav. Ecol. Sociobiol. 65, 1329–1351. ( 10.1007/s00265-011-1195-1) [DOI] [Google Scholar]
- 46.Merkle JA, Cherry SG, Fortin D. 2015. Bison distribution under conflicting foraging strategies: site fidelity versus energy maximization. Ecology 96, 1793–1801. ( 10.1890/14-0805.1) [DOI] [PubMed] [Google Scholar]
- 47.Brown JS. 1999. Vigilance, patch use and habitat selection: foraging under predation risk. Evol. Ecol. Res 1, 49–71. [Google Scholar]
- 48.Morris DW. 2003. Toward an ecological synthesis: a case for habitat selection. Oecologia 136, 1–13. ( 10.1007/s00442-003-1241-4) [DOI] [PubMed] [Google Scholar]
- 49.McLoughlin PD, Boyce MS, Coulson T, Clutton-Brock T. 2006. Lifetime reproductive success and density-dependent, multi-variable resource selection. Proc. R. Soc. B 273, 1449–1454. ( 10.1098/rspb.2006.3486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bergman CM, Fryxell JM, Gates CC, Fortin D. 2001. Ungulate foraging strategies: energy maximizing or time minimizing? J. Anim. Ecol. 70, 289–300. ( 10.1111/j.1365-2656.2001.00496.x) [DOI] [Google Scholar]
- 51.Post E, Forchhammer MC. 2008. Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Phil. Trans. R. Soc B 363, 2367–2373. ( 10.1098/rstb.2007.2207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sawyer H, Kauffman MJ, Middleton AD, Morrison TA, Nielson RM, Wyckoff TB. 2013. A framework for understanding semi-permeable barrier effects on migratory ungulates. J. Appl. Ecol. 50, 68–78. ( 10.1111/1365-2664.12013) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw GPS collar data used in this analysis can be accessed via the Wyoming Migration Database and Viewer located at migrationinitiative.org.


