Abstract
The water vapour permeability barrier of mammals and birds resides in the stratum corneum (SC), the outermost layer of the epidermis. The molar ratio and molecular arrangement of lipid classes in the SC determine the integrity of this barrier. Increased chain length and polarity of ceramides, the most abundant lipid class in mammalian SC, contribute to tighter packing and thus to reduced cutaneous evaporative water loss (CEWL). However, tighter lipid packing also causes low SC hydration, making it brittle, whereas high hydration softens the skin at the cost of increasing CEWL. Cerebrosides are not present in the mammalian SC; their pathological accumulation occurs in Gaucher's disease, which leads to a dramatic increase in CEWL. However, cerebrosides occur normally in the SC of birds. We tested the hypothesis that cerebrosides are also present in the SC of bats, because they are probably necessary to confer pliability to the skin, a quality needed for flight. We examined the SC lipid composition of four sympatric bat species and found that, as in birds, their SC has substantial cerebroside contents, not associated with a pathological state, indicating convergent evolution between bats and birds.
Keywords: Aves, Chiroptera, skin
1. Introduction
The integument of vertebrates serves as a protective layer, but also as an exchange surface with the environment. Selective pressures imposed on these opposing functions have led to the evolution of the extraordinarily diverse integuments found in vertebrates [1]. One key aspect in the evolution of the vertebrate integument is its ability to control water vapour diffusion, a function necessitated by the vertebrate invasion of terrestrial environments [1]. The water vapour permeability barrier of mammals and birds resides in the outermost layer of the epidermis, the stratum corneum (SC) [2]. The lipids in the intercellular spaces of the SC form ordered layers called lamellae, which were shown to increase resistance to water flux across the epidermis [1]. The lamellae are formed by cholesterol, free fatty acids and ceramides, consisting of a sphingosine base attached to a fatty acid [2]. Birds also have significant amounts of cerebrosides, ceramides with a hexose molecule attached, in their SC [3,4]. Specific molar ratios of these major SC lipid classes are thought to be crucial for the formation of the lamellae, which, in turn, determine the efficiency of the water vapour permeability barrier [5].
In mammals, ceramides comprise up to 50% of the total SC lipids and they provide the structural foundation for the formation of the lamellae in the SC [1,6]. Therefore, the arrangement of ceramide classes in the SC affects the properties of the skin permeability barrier [1,5]. Lillywhite [1] suggested that polar ceramides increase the order of the lamellae and decrease cutaneous evaporative water loss (CEWL). However, an increase in the proportion of ceramides results in low SC hydration levels [7], which increase brittleness and fragility of the SC. High hydration levels, facilitated by the presence of lipid classes able to bind water molecules, soften the skin [8], but at the cost of enhancing the flux of hydrophilic and lipophilic substances [9]. Therefore, there may be a trade-off between tight lipid molecule packing to maintain low rates of CEWL and high levels of hydration to favour integument elasticity and flexibility.
Cerebrosides are absent in the mammalian SC because of the activity of the enzyme β-glucocerebrosidase, which cleaves the sugar moieties from cerebrosides to make ceramides in the basal layers of the epidermis [10]. Individuals deficient in β-glucocerebrosidase accumulate cerebrosides in the SC, a condition that results in Gaucher's disease, which substantially increases CEWL [3,10]. The hexose moiety of cerebrosides can bind to molecules of water, which is thought to disrupt the lamellar packing; both characteristics that apparently increase rates of water loss [3,10]. However, birds normally have cerebrosides in their SC; although they have higher rates of CEWL than mammals of comparable size, they suffer no apparent impairment of the epidermal water vapour barrier [11]. Desert birds have lower rates of evaporative water loss than mesic species [12] and have higher proportions of polar ceramides in their SC than their mesic counterparts [5,13], but surprisingly they also have more cerebrosides [11]. The adjusted lipid ratio in desert birds, i.e. a greater proportion of polar classes of ceramides over free fatty acids and sterols, is associated with lower CEWL [13]. Muñoz-Garcia & Williams [11] suggested that cerebrosides contribute to evaporative cooling at high ambient temperature (Ta), but promote tighter packing at lower Ta because of temperature-dependent changes in their properties. Because the SC has been characterized for only a few species of mammals [1,6,10,14] and because birds have an SC containing cerebrosides that is fully functional, we hypothesize that cerebrosides might play important roles in the suite of adaptations for flight, offering an interesting case of convergent evolution. We propose that cerebrosides in the SC serve a dual purpose by controlling the water permeability barrier in a temperature-dependent manner and conferring flexibility and elasticity to the skin, thereby reducing turbulence over the wings and tail during flight.
Bats are reported to have relatively high rates of total evaporative water loss (TEWL) [15], the sum of CEWL and respiratory water loss (RWL). Bats also have relatively high field metabolic rates associated with flying, which increase their RWL [16] and they have vascularized membranous wings that increase surface area significantly, thus potentially increasing CEWL [15]. In support of this idea, CEWL was found to contribute over 50% of the TEWL in Kuhl's pipistrelle, Pipistrellus kuhlii, and Wahlberg's epauletted fruit bat, Epomophorus wahlbergi at moderate ambient temperatures (Ta), reaching 80% at Tas below 20°C, when bats were torpid [17,18]. If CEWL is indeed the main avenue of water loss in bats in general, it is reasonable to assume that desert bats are subject to selection pressures to modify the resistance of the skin to diffusion of water vapour. Indeed, CEWL was found to be associated with the lipid composition of the epidermis in two species of bats, the Mexican free-tailed bat, Tadarida brasiliensis, and the cave myotis, Myotis velifer [19]. This leads to the question of how bats, and particularly desert species, balance the need to resist water vapour loss from their thin membranous wings and tail, while maintaining their flexibility and elasticity that are crucial for flying.
In this study, we collected biopsy samples from the wing and tail membranes of four sympatric species of bats from Israel: Kuhl's pipistrelle, P. kuhlii, found throughout the country in both mesic and arid habitats in association with human settlements; the grey longed-eared bat, Plecotus christii, and Botta's serotine, Eptesicus bottae, both found in arid as well as semi-arid habitats and Hemprich's long-eared bat, Otonycteris hemprichii, a species that inhabits dryer and hotter deserts than the other three species [20]. We hypothesized that the SC of bats has a significant cerebroside content, as found in birds, and that CEWL of bats is associated with the composition of ceramide classes in the SC. We therefore tested the prediction that the low CEWL previously found in bat species from semi-arid and arid habitats [21] is positively correlated with polar and long-chained ceramides in the SC.
2. Material and methods
(a). Animals
We used mist nets to capture adult, non-reproductive E. bottae (n = 4), O. hemprichii (n = 6), P. kuhlii (n = 8) and P. christii (n = 5) at their foraging and drinking sites found by Korine & Pinshow [22] in the Central Negev Highlands, located within the hot desert belt of the northern hemisphere [23]. Bats were kept in separate cages in an outdoor enclosure (see the electronic supplementary material) on the Sede Boqer Campus of Ben-Gurion University at Midreshet Ben-Gurion (30°52′17″ N, 34°46′57″ E). They were provided mealworms and water, ad libitum. At the end of the experiments, we took two 4 mm skin biopsy samples: one from between the radius and fifth metacarpal of each bat, close to the wrist where there are few blood vessels and another from the uropatagium. Thereafter, we released the bats where we captured them.
(b). Measurement of cutaneous evaporative water loss and metabolic rate
We measured CEWL and metabolic rates in bats using an open-flow respirometry system following Muñoz-Garcia et al. [21]. We designed masks that fit over the snout of each species to separate RWL from CEWL. Post-absorptive bats were measured at 10°C, 15°C, 30°C or 35°C. CEWL was calculated as: CEWL = (ρch − ρin)(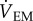 +
+ 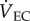 ), where ρin is the vapour density of the air entering the chamber and ρch is the vapour density of the air in the chamber (g m−3, STP),
), where ρin is the vapour density of the air entering the chamber and ρch is the vapour density of the air in the chamber (g m−3, STP), 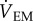 is the flow rate of the air leaving the mask and
is the flow rate of the air leaving the mask and 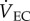 is the flow rate of the air leaving the chamber [24].
is the flow rate of the air leaving the chamber [24].
(c). Separation and identification of lipids of the stratum corneum
Each skin biopsy sample was immersed in double-distilled water (DDW), placed in a water bath at 65°C for 1 min and incubated in a solution of 0.5% trypsin in phosphate-buffered saline at 4°C overnight. The following day, samples were rinsed with DDW and immersed again in a fresh 0.5% trypsin solution for 3 h at 38°C to separate the SC from the rest of the skin. We then rinsed the samples with DDW, dried them under a stream of N2, freeze-dried them for 12 h and stored them in test tubes filled with N2 at −80°C. We extracted lipids with mixtures of chloroform : methanol 2 : 1, 1 : 1 and 1 : 2 v/v for 2 h, and combined extracts and dried them under N2 with an evaporimeter (N-EVAP, model 11155-O, Organomation Associates, Inc., Berlin, MA).
To identify and quantify lipids, we used reversed-phase high-performance liquid chromatography (HPLC) coupled with atmospheric pressure photoionization mass spectrometry (APPI-MS) following Muñoz-Garcia et al. [25]. For a full description of our methods, please see the electronic supplementary material. We followed the nomenclature of Motta et al. [26], who defined the types of fatty acids bound to sphingosines as non-hydroxy ester-linked fatty acid (N), α-hydroxy ester-linked fatty acid (A) and ω-hydroxy ester-linked fatty acid (EO); and the common sphingosine bases as sphingosine (S), phytosphingosine (P), 6-hydroxysphigosine (H) and dihydrosphingosine (DS).
(d). Calculations and statistics
At low ambient temperatures, bats often enter a state of torpor by downregulating body temperature and metabolic rate. To capture variation in the depth of torpor, due to differences in mass, physiological state, etc. we calculated a torpor index (TRi) for each bat, following Muñoz-Garcia et al. [27]. TRi is a measure of the time it takes a bat with a given body mass to generate or dissipate the amount of heat predicted it would gain or dissipate based on the body temperature, metabolic rate and the ambient temperature it is experiencing. For example, a thermoconforming bat that has a body temperature equal to ambient temperature will have a TRi of 0 because it generates no heat, whereas higher values of TRi are associated with increasing thermoregulatory effort by the animal. We estimated the surface area of bats following Muñoz-Garcia et al. [21] and examined the relationship between surface area-specific CEWL (ssCEWL) and log(TRi) by linear regression; we used the residuals of this regression to correct for the thermal state of the bats.
Because fractions of different sphingolipid classes in the SC were highly correlated with one another, we used principal component analysis (PCA) to identify patterns in our data. We used ANOVA to test whether the principal components separated among species. Further, we used backward stepwise multiple regression, with residuals of the regression between ssCEWL and log (TRi) as the dependent variable, the principal components as independent predictors and species as a categorical predictor, to examine the relationship between ssCEWL of the different species corrected for depth of torpor and their respective sphingolipid profile of the wing and tail SC. We report the standardized regression coefficients (β) in order to compare the relative contribution of each of the principal components. We tested for normal distribution of the residuals with the Shapiro–Wilk test and used logarithmic transformations whenever necessary. All statistical analyses were done using ‘R’ v. 3.1.2.
3. Results
(a). Sphingolipid composition of membranes
We identified 13 classes of ceramides in the SC of the membranes of all four bat species and, for the first time in a mammal, we found two classes of cerebrosides (table 1). Of the total SC sphingolipids, cerebrosides comprised 19.9 ± 3.0% in O. hemprichii and 17.7 ± 3.2% in E. bottae, percentages significantly higher than in either P. kuhlii (11.8 ± 1.1%) or P. christii (12.1 ± 1.1%). The first three components of the PCA accounted for 80.1% of the variance in the data (figure 1). The first, PC1, seemed to separate the different classes of sphingolipids based on decreasing polarity (figure 1a and table 1), with negative values corresponding to more polar ceramides, such as AP, NH, and EOH and cerebrosides. The second, PC2, appeared to separate the different classes of sphingolipids based on increasing chain length (figure 1a and table 1), with positive values corresponding to long EO ceramides with additional linoleic fatty acid moieties and cerebrosides.
Table 1.
Mean percentage ± 1 s.e. of the wing stratum corneum sphingolipid classes as determined by reversed-phase high-performance liquid chromatography coupled with atmospheric pressure photoionization mass spectrometry. Sphingolipids in the table are sorted by ascending polarity of ceramides and then cerebrosides. Non-hydroxy ester-linked fatty acid (N), α-hydroxy ester-linked fatty acid (A), ω-hydroxy ester-linked fatty acid (EO), sphingosine (S), phytosphingosine (P), 6-hydroxysphigosine (H), dehydrosphingosine (DS) and unknown (UK).
| sphingolipid class | Eptesicus bottae | Otonycteris hemprichii | Pipistrellus kuhlii | Plecotus christii | ANOVA p-value |
|---|---|---|---|---|---|
| NDS | 4.56 ± 1.29 | 6.19 ± 0.76 | 12.47 ± 2.39 | 14.82 ± 1.1 | 0.001 |
| EODS | 2.95 ± 0.50 | 4.18 ± 0.18 | 4.71 ± 0.69 | 5.98 ± 1.19 | n.s. |
| NS | 11.67 ± 2.24 | 9.47 ± 0.63 | 8.22 ± 0.86 | 5.97 ± 0.66 | 0.006 |
| EOS | 5.05 ± 0.61 | 5.19 ± 0.17 | 4.47 ± 0.37 | 3.85 ± 0.45 | 0.009 |
| NP | 8.84 ± 1.08 | 5.47 ± 0.47 | 9.05 ± 0.57 | 11.03 ± 0.44 | <0.001 |
| ADS | 12.81 ± 0.59 | 9.93 ± 0.44 | 11.17 ± 0.56 | 9.88 ± 0.46 | n.s. |
| AS | 7.78 ± 0.87 | 7.32 ± 0.51 | 8.91 ± 0.40 | 6.98 ± 0.30 | 0.01 |
| EOP | 9.10 ± 1.32 | 10.17 ± 0.57 | 8.51 ± 0.41 | 11.74 ± 1.20 | n.s. |
| NH | 8.05 ± 0.51 | 7.31 ± 0.78 | 8.10 ± 0.38 | 6.25 ± 0.42 | n.s. |
| EOH | 0.58 ± 0.08 | 1.82 ± 0.08 | 0.94 ± 0.08 | 0.95 ± 0.04 | 0.009 |
| AP | 3.33 ± 0.63 | 3.41 ± 0.39 | 3.90 ± 0.14 | 2.85 ± 0.15 | n.s. |
| AH | 2.67 ± 0.29 | 2.38 ± 0.24 | 3.68 ± 0.27 | 3.48 ± 0.35 | 0.005 |
| cerebroside NS | 1.48 ± 0.19 | 2.14 ± 0.22 | 1.1 ± 0.21 | 0.94 ± 0.17 | 0.002 |
| cerebroside EOS | 16.27 ± 3.04 | 17.81 ± 2.74 | 10.69 ± 0.88 | 11.17 ± 0.96 | 0.008 |
| UK | 4.88 ± 0.52 | 7.23 ± 1.74 | 4.08 ± 0.33 | 4.12 ± 0.42 | 0.01 |
Figure 1.
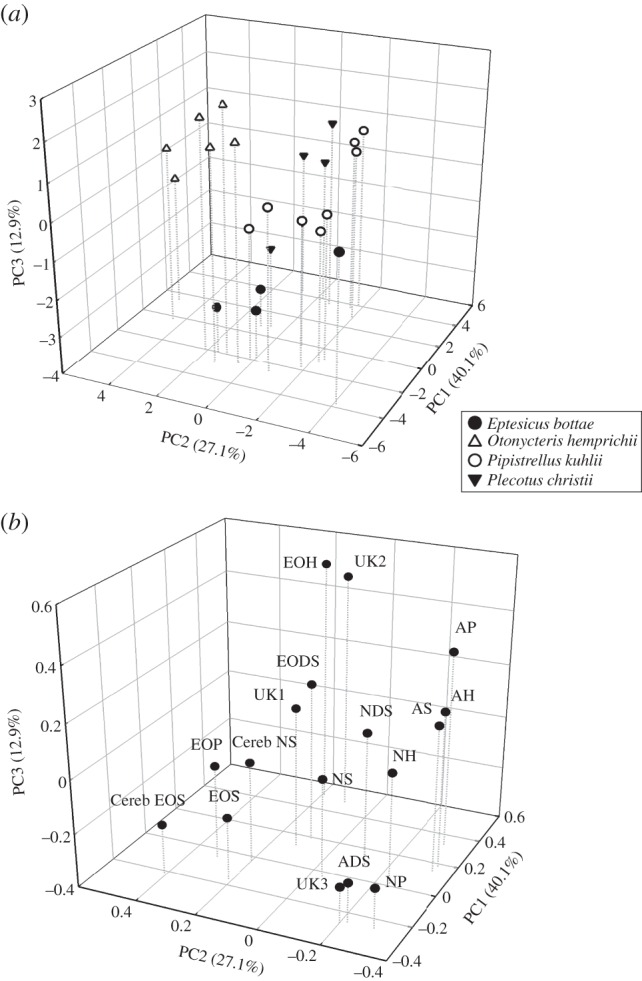
Principal component analysis based on the weighted fractions of sphingolipids in the wing and tail stratum corneum of bats. (a) Scores for Plecotus christii (filled triangles) and Eptesicus bottae (filled circles), the species found in arid and semi-arid habitats; scores for Pipistrellus kuhlii (open circles), the species found in both mesic and arid habitats in association with human settlements; and scores for Otonycteris hemprichii (open triangles), the species that inhabits hot, dry desert areas. (b) Loading, i.e. the weight relative to the principal components, for each sphingolipid. Non-hydroxy ester-linked fatty acid (N), α-hydroxy ester-linked fatty acid (A), ω-hydroxy ester-linked fatty acid (EO), sphingosine (S), phytosphingosine (P), 6-hydroxysphigosine (H), dehydrosphingosine (DS) and unknown (UK). The scores on the first principal component (PC1) denote decreasing polarity and the scores on the second principal component (PC2) denote increasing chain length.
Plecotus christii had higher scores for the first principal component than E. bottae and O. hemprichii (post hoc: p < 0.03), but not significantly different from the scores of P. kuhlii (figure 1b and table 2), suggesting that the dominant sphingolipids in the membranes of E. bottae and O. hemprichii are relatively polar. Otonycteris hemprichii had the highest scores for PC2 among all the species (table 2; p < 0.01), indicating that its SC had sphingolipids with long chains. Finally, the average PC3 scores of all four bat species differed significantly from one another (table 2).
Table 2.
Mean scores ±1 s.e. of the principal component analysis of the four bat species based on the composition of sphingolipids isolated from their wing membranes as determined by reversed-phase high performance liquid chromatography coupled with atmospheric pressure photoionization mass spectrometry. The last column lists the p-values derived from comparing the scores of the principal components of the four bats species using one-way ANOVA. Different letters denote statistically significant differences in scores.
| Eptesicus bottae | Otonycteris hemprichii | Pipistrellus kuhlii | Plecotus christii | ANOVA p-value | |
|---|---|---|---|---|---|
| PC1 | −1.72 ± 1.11a | −1.36 ± 0.91a | 0.29 ± 0.79b | 2.54 ± 1.00b | p = 0.03 |
| PC2 | −0.38 ± 0.78a | 2.42 ± 0.63b | −1.56 ± 0.55a | −0.10 ± 0.69a | p < 0.01 |
| PC3 | −2.11 ± 0.49a | 1.39 ± 0.40b | 0.19 ± 0.34c | −0.29 ± 0.44d | p < 0.001 |
(b). Cutaneous water loss and stratum corneum lipid composition
Surface area-specific CEWL was negatively correlated with TRi in all four bat species (electronic supplementary material, figure S1; F1,66 = 48.45, p < 0.001); ssCEWL in O. hemprichii was significantly lower than in the other three species (electronic supplementary material, figure S1; F3,19 = 29.33, p < 0.001).
The residuals calculated from the regression of ssCEWL against TRi were significantly correlated with the scores of the three principal components (figure 2; F5,84 = 30.95, p < 0.001, R2 = 0.63). There was a positive correlation with PC1 (figure 2a; β = 0.14 ± 0.05; p = 0.006), and a negative correlation with PC2 (figure 2b; β = −0.35 ± 0.04, p < 0.001) and PC3 (figure 2c; β = −0.21 ± 0.06, p < 0.001).
Figure 2.
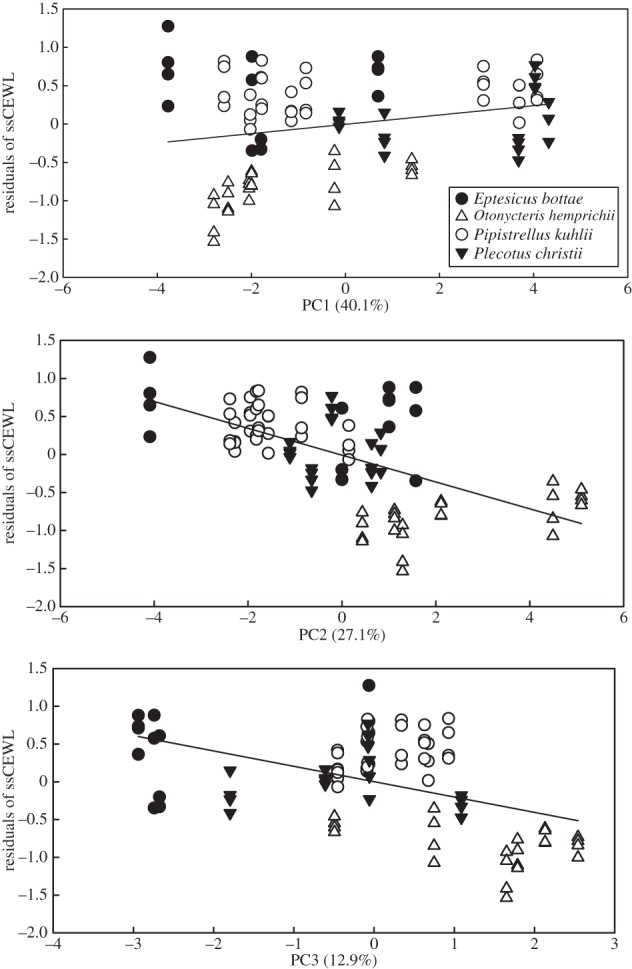
The relationship between residuals calculated from regressing surface area-specific cutaneous water loss against the torpor index of the bats and (a) the scores on the first principal component (PC1) denoting decreasing stratum corneum (SC) sphingolipid polarity, (b) the scores on the second principal component (PC2) denoting increasing (SC) sphingolipid chain length and (c) the scores on the third principal component (PC3). Plecotus christii (filled triangles) and Eptesicus bottae (filled circles) are the species found in arid and semi-arid habitats; Pipistrellus kuhlii (open circles) is the species found in both mesic and arid habitats in association with human settlements and Otonycteris hemprichii (open traingles) is the species that inhabits only hot, dry desert areas.
4. Discussion
The most striking result of this study is the presence of substantial quantities of cerebrosides in the wing and tail SC of bats (table 1). In all species of mammals studied to date, cerebrosides mobilized to the epidermis are converted to ceramides in the SC. Therefore, the SC of these mammals contains negligible amounts of cerebrosides [1,6,10,14]. High concentrations of cerebrosides in the SC of humans are apparently an important factor in Gaucher's disease, where the permeability barrier of the skin is compromised [3,10]. However, we found that, as in birds, the SC of the wings of bats contains cerebrosides, with no apparent impairment to the epidermal water vapour barrier, making bat epidermis different from that of other mammals so far studied.
Because cerebrosides have bulky hexose moieties that disrupt lamellar packing, they contribute to higher rates of water loss. In fact, birds at thermoneutral ambient temperatures lose water through their integument at twice the rate of mammals of the same body size [12], as bats do [15]. This might be a trait associated with the presence of cerebrosides in the SC. However, house sparrows from arid environments were found to have higher proportions of cerebrosides in their SC than their mesic counterparts, which was associated with lower CEWL at moderate ambient temperatures [11]. Similarly, O. hemprichii, whose distribution is restricted to desert habitats, had relatively low CEWL (figure 1) and a high proportion of SC cerebrosides (table 1) compared with P. kuhlii and P. christii, but not E. bottae. These observations might be resolved by considering the effect of temperature on the biochemical characteristics of the lipid matrix. Muñoz-Garcia et al. [5] suggested that, at low and moderate temperatures, polar cerebrosides form ordered structures with the ceramides and they might sequester water molecules in their sugar moieties, decreasing the diffusion of water vapour through the skin. However, at higher temperatures, molecular motion causes cerebrosides to release water molecules bound to their sugar moieties and thus water vapour diffusion through the SC increases [28]. We propose that the presence of cerebrosides in the SC of bats may play a role in maintaining skin flexibility and elasticity by allowing increased hydration, a property that is important for animals using flight for locomotion [29]. Because bats are heterothermic, the properties of the mixture of lipids in the SC might change when body temperature changes, allowing bats to conserve water at low temperatures, but promoting evaporative cooling at high temperatures.
The proportion of cerebrosides in the SC of E. bottae was not different from that of O. hemprichii (table 1), despite differences in CEWL (electronic supplementary material, figure S1). We found, however, that O. hemprichii had significantly longer chains in SC lipids than the other three species of bats. Longer fatty acid chains permit stronger van der Waals forces, which bring about tighter packing of the ceramides and a more ordered structure, thereby minimizing diffusion of water vapour through the skin [1]. It is possible that O. hemprichii maintains lower CEWL than E. bottae due to the presence of longer chains of fatty acids. Indeed, we found a negative correlation between the scores on the second principal component and CEWL, suggesting that longer fatty acid chains are associated with reduced CEWL.
Our results indicate that alterations of the composition and the organization of the lipid matrix that forms the water vapour permeability barrier of the skin are one mechanism by which bats can modulate CEWL. Another mechanism by which CEWL may be reduced is torpor. We found that CEWL was lower as torpor depth increased, although we did not find significant differences among species. All of the species used in this study were caught in the Negev desert, and therefore it is possible that the absence of significant differences is a product of acclimatization (phenotypic flexibility), adaptation of the desert populations to their environment or both.
To conclude, this is the first study to report significant quantities of cerebrosides in the SC of mammals, not associated with a pathological condition. We propose that in bats cerebrosides promote water conservation when ambient temperatures are low, but facilitate evaporative cooling when Ta is high and heat must be dissipated by evaporative cooling. The presence of cerebrosides benefits bats for two reasons: they facilitate increased flexibility and elasticity of the wing and tail membranes, a property essential for flight and they allow temperature-dependent flexibility in the water vapour permeability properties of the epidermis, hindering water vapour diffusion at low Tas, when bats may be torpid, and aiding evaporative cooling at high Tas. The lipid composition of bat wing and tail membranes is similar to that of bird skin, perhaps the result of convergent evolution.
Supplementary Material
Acknowledgements
We thank Ana Gabriela Jimenez, Alex Champagne and Elisabeth Calhoon for their help with laboratory work. This is paper no. 907 of the Mitrani Department of Desert Ecology.
Ethics
This research was done under permit no. 34615 from the Israel Nature and National Parks Protection Authority and permit no. IL-71-12-2010 from the Ben-Gurion University Committee for the Ethical Care and Use of Animals in Experiments.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by the US–Israel Binational National Science Foundation grant no. 2008469 to C.K. and J.B.W. A.M.G. was a Blaustein Postdoctoral Fellow during the study and M.B.H. was the recipient of a Negev Fellowship and the Prof. Rahamimoff travel grant for young scientists.
References
- 1.Lillywhite HB. 2006. Water relations of tetrapod integument. J. Exp. Biol. 209, 202–226. ( 10.1242/jeb.02007) [DOI] [PubMed] [Google Scholar]
- 2.Elias PM. 2004. The epidermal permeability barrier: from the early days at Harvard to emerging concepts. J. Invest. Dermatol. 122, 36–39. ( 10.1046/j.1523-1747.2003.22258.x) [DOI] [PubMed] [Google Scholar]
- 3.Holleran WM, Ginns E, Menon G, Grundmann J, Fartasch M, McKinney C, Elias P, Sidransky E. 1994. Consequences of beta-glucocerebrosidase deficiency in epidermis. Ultrastructure and permeability barrier alterations in Gaucher disease. J. Clin. Invest. 93, 1756 ( 10.1172/JCI117160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uchida Y, et al. 2000. Epidermal sphingomyelins are precursors for selected stratum corneum ceramides. J. Lipid Res. 41, 2071–2082. [PubMed] [Google Scholar]
- 5.Muñoz-Garcia A, Ro J, Brown JC, Williams JB. 2008. Cutaneous water loss and sphingolipids in the stratum corneum of house sparrows, Passer domesticus L., from desert and mesic environments as determined by reversed phase high-performance liquid chromatography coupled with atmospheric pressure photospray ionization mass spectrometry. J. Exp. Biol. 211, 447–458. ( 10.1242/jeb.013649) [DOI] [PubMed] [Google Scholar]
- 6.Holleran WM, Man M, Gao WN, Menon GK, Elias P, Feingold K. 1991. Sphingolipids are required for mammalian epidermal barrier function. Inhibition of sphingolipid synthesis delays barrier recovery after acute perturbation. J. Clin. Invest. 88, 1338 ( 10.1172/JCI115439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egawa M, Tagami H. 2008. Comparison of the depth profiles of water and water-binding substances in the stratum corneum determined in vivo by Raman spectroscopy between the cheek and volar forearm skin: effects of age, seasonal changes and artificial forced hydration. Br. J. Dermatol. 158, 251–260. ( 10.1111/j.1365-2133.2007.08311.x) [DOI] [PubMed] [Google Scholar]
- 8.Caspers PJ, Lucassen GW, Carter EA, Bruining HA, Puppels GJ. 2001. In vivo confocal Raman microspectroscopy of the skin: noninvasive determination of molecular concentration profiles. J. Invest. Dermatol. 116, 434–442. ( 10.1046/j.1523-1747.2001.01258.x) [DOI] [PubMed] [Google Scholar]
- 9.Bouwstra JA, Honeywell-Nguyen PL, Gooris GS, Ponec M. 2003. Structure of the skin barrier and its modulation by vesicular formulations. Prog. Lipid Res. 42, 1–36. ( 10.1016/S0163-7827(02)00028-0) [DOI] [PubMed] [Google Scholar]
- 10.Takagi Y, Kriehuber E, Imokawa G, Elias PM, Holleran WM. 1999. β-Glucocerebrosidase activity in mammalian stratum corneum. J. Lipid Res. 40, 861–869. [PubMed] [Google Scholar]
- 11.Muñoz-Garcia A, Williams JB. 2005. Cutaneous water loss and lipids of the stratum corneum in house sparrows Passer domesticus from arid and mesic environments. J. Exp. Biol. 208, 3689–3700. ( 10.1242/jeb.01811) [DOI] [PubMed] [Google Scholar]
- 12.Williams JB. 1996. A phylogenetic perspective of evaporative water loss in birds. Auk 113, 457–472. ( 10.2307/4088912) [DOI] [Google Scholar]
- 13.Haugen M, Williams JB, Wertz P, Tieleman BI. 2003. Lipids of the stratum corneum vary with cutaneous water loss among larks along a temperature–moisture gradient. Physiol. Biochem. Zool. 76, 907–917. ( 10.1086/380213) [DOI] [PubMed] [Google Scholar]
- 14.Stahl J, Niedorf F, Kietzmann M. 2009. Characterisation of epidermal lipid composition and skin morphology of animal skin ex vivo. Eur. J. Pharm. Biopharm. 72, 310–316. ( 10.1016/j.ejpb.2008.09.013) [DOI] [PubMed] [Google Scholar]
- 15.Studier EH. 1970. Evaporative water loss in bats. Comp. Biochem. Physiol. 35, 935–943. ( 10.1016/0010-406X(70)90087-3) [DOI] [Google Scholar]
- 16.Speakman JR, Thomas DW. 2003. Physiological ecology and energetics of bats. In Bat ecology (eds Kunz TH, Thomas DW), pp. 430–490. Chicago, IL: Chicago Press. [Google Scholar]
- 17.Muñoz-Garcia A, Ben-Hamo M, Pinshow B, Williams JB, Korine C. 2012. The relationship between cutaneous water loss and thermoregulatory state in the Kuhl's pipistrelle, Pipistrellus kuhlii, a widely distributed vespertillionid bat. Physiol. Biochem. Zool. 85, 516–525. [DOI] [PubMed] [Google Scholar]
- 18.Minnaar IA, Bennett NC, Chimimba CT, McKechnie AE. 2014. Partitioning of evaporative water loss into respiratory and cutaneous pathways in Wahlberg's epauletted fruit bats (Epomophorus wahlbergi). Physiol. Biochem. Zool. 87, 475–485. ( 10.1086/675342) [DOI] [PubMed] [Google Scholar]
- 19.Muñoz-Garcia A, Ro J, Reichard JD, Kunz TH, Williams JB. 2012. Cutaneous water loss and lipids of the stratum corneum in two syntopic species of bats. Comp. Biochem. Physiol. A 161, 208–215. [DOI] [PubMed] [Google Scholar]
- 20.Yom-Tov Y, Kadmon R. 1998. Analysis of the distribution of insectivorous bats in Israel. Divers. Distrib. 4, 63–70. ( 10.1046/j.1472-4642.1998.00012.x) [DOI] [Google Scholar]
- 21.Muñoz-Garcia A, Ben-Hamo M, Larraín P, Cruz-Neto AP, Williams JB, Pinshow B, Korine C. 2016. The relationship between metabolic rate, evaporative water loss and thermoregulatory state in four species of bats in the Negev desert, Israel. Comp. Biochem. Physiol. A 191, 156–165. ( 10.1016/j.cbpa.2015.10.010) [DOI] [PubMed] [Google Scholar]
- 22.Korine C, Pinshow B. 2004. Guild structure, foraging space use, and distribution in a community of insectivorous bats in the Negev Desert. J. Zool. 262, 187–196. ( 10.1017/S0952836903004539) [DOI] [Google Scholar]
- 23.Evenari M, Shanan L, Tadmor N. 1971. The Negev: the challenge of a desert. Cambridge, MA: Harvard University Press. [Google Scholar]
- 24.Tieleman BI, Williams JB. 2002. Cutaneous and respiratory water loss in larks from arid and mesic environments. Physiol. Biochem. Zool. 75, 590–599. ( 10.1086/344491) [DOI] [PubMed] [Google Scholar]
- 25.Muñoz-Garcia A, Ro J, Brown JC, Williams JB. 2006. Identification of complex mixtures of sphingolipids in the stratum corneum by reversed-phase high-performance liquid chromatography and atmospheric pressure photospray ionization mass spectrometry. J. Chromatogr. A 1133, 58–68. ( 10.1016/j.chroma.2006.06.067) [DOI] [PubMed] [Google Scholar]
- 26.Motta S, Monti M, Sesana S, Caputo R, Carelli S, Ghidoni R. 1993. Ceramide composition of the psoriatic scale. BBA–Mol. Basis Dis. 1182, 147–151. ( 10.1016/0925-4439(93)90135-N) [DOI] [PubMed] [Google Scholar]
- 27.Muñoz-Garcia A, Ben-Hamo M, Korine C, Pinshow B, Williams JB. 2014. A new thermoregulatory index for heterothermy. Method. Ecol. Evol. 5, 141–145. ( 10.1111/2041-210X.12131)25628871 [DOI] [Google Scholar]
- 28.Champagne AM, Allen HC, Williams JB. 2015. Lipid composition and molecular interactions change with depth in the avian stratum corneum to regulate cutaneous water loss. J. Exp. Biol. 218, 3032–3041. ( 10.1242/jeb.125310) [DOI] [PubMed] [Google Scholar]
- 29.Elias PM, Menon GK, Grayson S, Brown BE, Rehfeld SJ. 1987. Avian sebokeratocytes and marine mammal lipokeratinocytes: structural, lipid biochemical, and functional considerations. Am. J. Anat. 180, 161–177. ( 10.1002/aja.1001800206) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


