Abstract
Background
Uveal melanoma patients with a poor prognosis can be detected through genetic analysis of the tumor, which has a very high sensitivity. A large number of patients with uveal melanoma decide to receive information about their individual risk and therefore routine prognostic genetic testing is being carried out on a growing number of patients. It is obvious that a positive prediction for recidivism in the future will emotionally burden the respective patients, but research on the psychosocial impact of this innovative method is lacking. The aim of the current study is therefore to investigate the psychosocial impact (psychological distress and quality of life) of prognostic genetic testing in patients with uveal melanoma.
Design and methods
This study is a non-randomized controlled prospective clinical observational trial. Subjects are patients with uveal melanoma, in whom genetic testing is possible. Patients who consent to genetic testing are allocated to the intervention group and patients who refuse genetic testing form the observational group. Both groups receive cancer therapy and psycho-oncological intervention when needed. The psychosocial impact of prognostic testing is investigated with the following variables: resilience, social support, fear of tumor progression, depression, general distress, cancer-specific and general health-related quality of life, attitude towards genetic testing, estimation of the perceived risk of metastasis, utilization and satisfaction with psycho-oncological crisis intervention, and sociodemographic data. Data are assessed preoperatively (at initial admission in the clinic) and postoperatively (at discharge from hospital after surgery, 6–12 weeks, 6 and 12 months after initial admission). Genetic test results are communicated 6–12 weeks after initial admission to the clinic.
Discussion
We created optimal conditions for investigation of the psychosocial impact of prognostic genetic testing. This study will provide information on the course of disease and psychosocial outcomes after prognostic genetic testing. We expect that empirical data from our study will give a scientific basis for medico-ethical considerations.
Electronic supplementary material
The online version of this article (doi:10.1186/s12885-016-2479-7) contains supplementary material, which is available to authorized users.
Keywords: Uveal melanoma, Genetic testing, Psychological distress, Quality of life, Psycho-oncology, Resilience, Social support, Shared decision-making
Background
There are two tumor-biological classes of uveal melanoma, which differ markedly from each other concerning the risk of metastasis [1, 2]. Both tumor classes can be determined either through detection of monosomy 3 in tumor DNA [1], or by a multi-gene expression profile of tumor RNA [2]. Due to the tumor class, the risk of developing metastases varies pronouncedly: In a current study [3], the mortality rates due to metastasis were 13.2 % for tumors with disomy 3 and 75.1 % for tumors with monosomy 3 (median follow-up time of 5.2 years).
Generally, it was found that global quality of life was significantly reduced in patients with malignant uveal melanoma (compared to the healthy norm and other ophthalmological patients, both pre and post treatment), with one half of patients displaying clinically relevant distress pre treatment and one third post treatment [4]. More recent studies concerning quality of life have revealed different improvements and deteriorations after treatment of uveal melanoma: significant decrease of physical functioning and physical role, but on the other hand significant improvement of mental health [5]; furthermore decreases in social functioning, but also in anxiety levels [6].
Prognostic testing of monosomy 3 in uveal melanoma patients aims at determinating the risk of metastasis in patients who have already been diagnosed with cancer. However, (until now) genetic testing does not directly affect treatment decisions [7]. This is in contrast to most of the studies targeting the psychosocial impact of prognostic genetic testing, which are performed with persons at risk for familial cancer (hereditary risk), but not yet diseased. Investigating uveal melanoma patients broadens this field by examining patients who have to decide whether they want to receive very reliable information about their future (prognosis and life perspective), while already being affected with cancer. Due to the dichotomous outcome (disomy 3 = good prognosis, monosomy 3 = poor prognosis), we assume patients to have high levels of involvement and psychological distress. Comparably, a positive test for a predisposition to breast cancer causes psychological reactions similar to those after a manifest diagnosis [8].
In relation to this, additional questions arise concerning patient autonomy and decision making. Concerning the psychological impact of prognostic genetic testing in patients with uveal melanoma, three studies could be identified: In a retrospective study, patients wanted prognostic information even though they were informed that medical care would not be influenced by the result. Furthermore, depressive symptoms and quality of life were found to be independent of a positive test result [9]. Another research group did not find any patients having regrets about prognostic diagnostics (even in the case of poor prognosis) and therefore concluded that there were no harmful effects of the diagnostic testing [10]. In another prospective study [11], patients often did not seem to realize they had to make a decision, for prognostic genetic testing was part of “normal” treatment for them – patients utilized the test because they trusted in what their physicians offered them. The authors concluded that active decision-making and consent procedures are not realistic in acute clinical situations. They even further stated that protecting patients’ interests is the physicians’ responsibility and not delegable to patients.
Moreover, two important issues have to be noted: First, only few respondents strive for an autonomous role in the decision-making process, as often a passive role (particularly in older and less educated patients) is preferred [12]. Second, cancer risks and heredity likelihood were often perceived inaccurately by the counselees. Outcomes like quality of life or current psychological wellbeing could therefore not be predicted by the actually communicated cancer risk, but were mainly mediated or predicted by perceived risk [13]. This might also counteract informed decision-making.
Another important issue is the question of which individuals take the opportunity of genetic testing and/or counseling and which individuals do not (different motivating factors). In a sample of colorectal cancer patients, nonmetastatic cancer, lower perceived risk for cancer recurrence, and greater self-efficacy were associated with greater perceived benefits – whereas perceived barriers were related to cancer-specific psychological distress. Generally, individuals considering the test have positive attitudes towards it and perceive few barriers [14]. Furthermore, the following factors were found to be related to the decision to utilize genetic testing for BRCA 1/2: personal history of cancer, perceiving more benefits than barriers concerning genetic testing, having greater family hardiness, and perceiving a breast cancer diagnosis as associated with fewer negative consequences [15]. Other common motivations for the utilization of prognostic genetic testing are use of more screening offers, reassurance, and taking care of oneself ([16]; breast cancer screening).
Study aims
The objective of this study is to investigate the psychosocial impact (psychological distress and quality of life) of prognostic genetic testing in patients with uveal melanoma.
This comprises three main issues:
Decision-making: Which patients utilize genetic testing? What attitudes predominate in these patients? For this purpose we ask patients about their attitudes towards genetic testing, perceived risk, social support, and sociodemographic data.
Psychological distress: How distressing is genetic testing? Should psychological interventions be implemented to reduce pronounced psychological distress? To measure this, we investigate distress and record which patients utilize psycho-oncological interventions.
Risk: Which patients are at risk for pronounced psychological distress? To answer this question, we assess resilience, social support, and fear of progression as moderating factors of mental health and stability.
A prospective study is being conducted to investigate (possibly different) trajectories of disease between patients undergoing and not undergoing prognostic genetic testing. In particular, it is possible to examine whether and how patients benefit from knowing the prognosis, even though no medical decisions depend on the test results. These findings should be included in future informed consent. Furthermore, we will be able to overcome shortcomings of previous studies in this field, namely retrospectivity [9], a qualitative approach and small sample sizes [10, 11].
Hypotheses
Sociodemographic and psychological features will moderate the utilization of prognostic genetic testing: Patients with high education, high social support, high resilience, and a previous history of cancer will utilize genetic testing more often than patients with low levels of education, or low social support, or a previous history of cancer.
Attitudes towards prognostic testing (attitude scale) will remain stable between the measurement points. Patients with a positive attribution towards genetic testing will give their consent to it more often.
Perceived risk of metastasis will change after disclosure of the test result. In approximately 50 % of the patients, individually perceived risk and the objective result of the test will be congruent (congruency means that patients understood the communicated information).
- Psychological variables will change after prognostic testing and in the further trajectory of the disease:
- At T1 (initial admission to the clinic, diagnosis), psychological distress levels of patients with uveal melanoma will be significantly increased compared to a norm sample – irrespective of whether they gave their consent to genetic testing (intervention group; IG) or not (observational group; OG).
- At T2 (day of discharge after surgery), IG-patients and OG-patients will not differ concerning psychological distress, but distress levels will be increased compared to T1 and compared to a norm sample.
- At T3 (communication of the result, 6–12 weeks after surgery), IG-patients with poor prognosis (IGpp) will have the highest levels of psychological distress and the lowest mental quality of life compared to IG-patients with good prognosis (IGgp) and OG-patients. IPgp-Patients will have the lowest psychological distress and the highest mental quality of life and will be comparable to a norm sample.
- At T4 (6 months after initial admission), psychological distress in IGpp-patients will be significantly decreased compared to T3, but there will still be significant group differences.
- At T5 (12 months after initial admission), psychological distress levels in IGpp-patients will be further decreased, but still higher than in IGgp-patients and in a norm sample.
- Psycho-oncological crisis intervention:
- Patients with a higher load of psychological symptoms will utilize psycho-oncological interventions more often.
- Approximately 10 % of the patients will utilize psycho-oncological crisis interventions.
- Patient satisfaction is expected to be high.
Methods
Organizational structure of the study
This study is conducted within a network of three collaborative investigations (see Fig. 1) funded by the German Cancer Aid. A corporate time table was developed, in which the three projects were embedded (see Table 1).
Fig. 1.
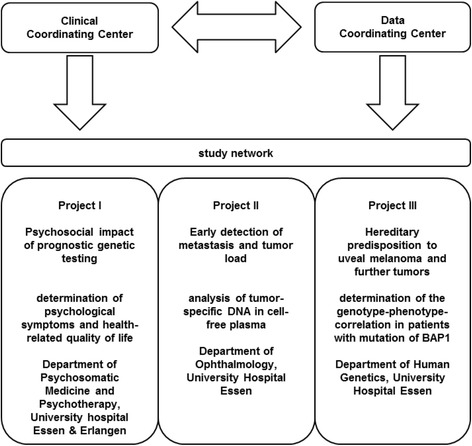
Organizational structure of the study network
Table 1.
Corporate time table of the study network
| Assessment | Interval | Project I (psycho-oncology) | Project II (metastasis) | Project III (hereditary predisposition) |
|---|---|---|---|---|
| T1 | Initial admission in the clinic | • Information about the 3 projects and about monosomy 3 diagnostics • medical history (patient and familiy) • psychometric questionnaires |
||
| Surgery (4–6 weeks after initial admission) | ||||
| T2 | Discharge from hospital after surgery | psychometric questionnaires | plasma sample | |
| T3 | 6-12 weeks after surgery (IG); 8 weeks after surgery (OG) | • offer of psycho-oncological support • psychometric questionnaires |
Disclosure of test results: • monosomy 3 • tumor disposition |
|
| T4 | 6 months after initial admission | psychometric questionnaires | plasma sample | Follow-up examinations |
| T5 | 12 months after initial admission | psychometric questionnaires | plasma sample | Follow-up examinations |
Study population
Patients are included in the study if they are reliably diagnosed with uveal melanoma and if removal of a tumor sample is possible (enucleation or biopsy). Furthermore, they have to be aged between 18 and 90 years, have sufficient knowledge of the German language and give informed consent to take part in the study. Patients are excluded from the study if they have a pre-existing diagnosis of mental disability, psychosis or dementia.
Recruitment
The sample of this study is a consecutive sample of patients with uveal melanoma undergoing cancer therapy in the Department of Ophthalmology of the University Hospital in Essen, Germany, which is a national center for treatment of patients with ophthalmic tumors. Therefore, more than 170 patients with a first diagnosis of uveal melanoma undergo prognostic genetic testing for monosomy 3 every year.
Allocation to the study groups
This study is a non-randomized controlled prospective clinical observational trial. Patients are allocated to the groups by their choice (non-randomized) of whether to utilize prognostic genetic testing (intervention group, IG) or not (observationsal group, OG). Blinding is not possible.
Measurement points
Data are assessed preoperatively (at initial admission in the clinic) and postoperatively (at discharge from hospital after surgery, 6–12 weeks, 6 and 12 months after initial admission); the detailed time flow of the study can be seen in Fig. 2.
Fig. 2.
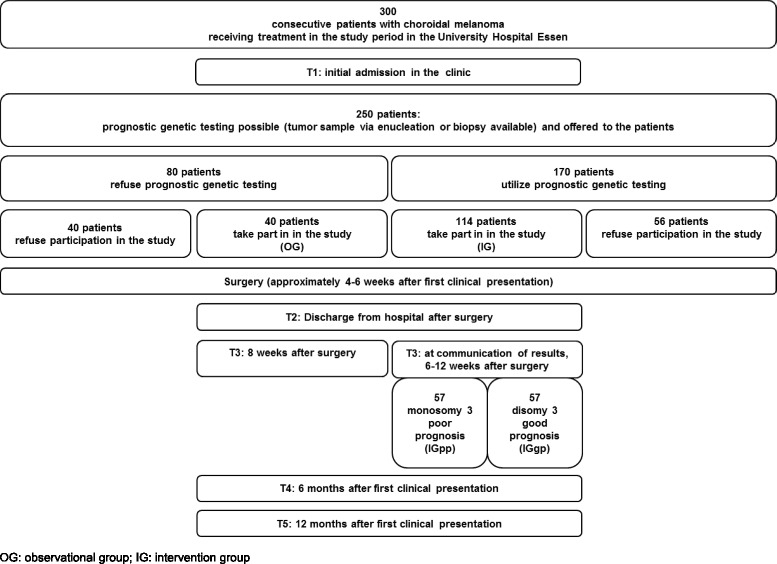
Time flow, expected patient numbers per year
Measures assessed
Exclusively at baseline assessment (initial admission in the clinic): sociodemographic data, medical history and initial protective factors are recorded. For assessment of initial protective factors (resilience and perceived social support), participants complete the short form of the sense of coherence scale [17], the short form of the social support questionnaire [18] and the subscale “social strain” from the long form of the social support questionnaire [19, 20].
The following measures are assessed both at baseline and at all follow-ups: psychological distress, (general and specific) health-related quality of life, attitude towards genetic testing, estimation of the perceived risk of metastasis and utilization of psycho-oncological interventions.
Psychological distress is measured by the Fear of Progression Questionnaire [21, 22], the Hospital Anxiety and Depression Scale (subscale depression, HADS-D, [23]), and the distress thermometer [24]. Specific health-related quality of life is assessed by the EORTC Quality of Life Questionnaire (EORTC QLQ-C30, [25]) and the ophthalmic module of the EORTC QLQ-C30 (EORTC QLQ-OPT 30, [26]). General health-related quality of life is measured by the short version of the Short Form Health Survey SF-36 (SF-12; [27]). Attitudes towards genetic testing are assessed with a modified version of the modified Attitudes Scale [28]. The estimation of perceived risk of metastasis is evaluated with a visual analogue scale (VAS), from 0 to 10. The utilization of psycho-oncological (crisis) interventions (control variable) and patient satisfaction with these interventions is recorded with a documentation form.
Detailed information about several of these measures is provided in the Additional file 1: Appendix. Which measures are assessed at which measurement point can be seen in Table 2.
Table 2.
Use of different measures at different assessment points
| T1 | T2 | T3 | T4 | T5 | |
|---|---|---|---|---|---|
| Resilience | ✓ | - | - | - | - |
| Social support | ✓ | - | - | - | - |
| Fear of progression | ✓ | ✓ | ✓ | ✓ | ✓ |
| Distress | ✓ | ✓ | ✓ | ✓ | ✓ |
| Depression | ✓ | ✓ | ✓ | ✓ | ✓ |
| Health-related Quality of life | ✓ | ✓ | ✓ | ✓ | ✓ |
| Perceived risk | ✓ | ✓ | ✓ | ✓ | ✓ |
| Attitudes towards prognostic testing | ✓ | ✓ | ✓ | ✓ | ✓ |
| Utilization of psycho-oncological intervention | - | ✓ | ✓ | ✓ | ✓ |
Psycho-oncological crisis interventions
Patients are informed at T1 that they may utilize psycho-oncological interventions. These crisis interventions follow a resource-oriented approach and are conducted by a clinical psychologist specialized in psycho-oncology. Frequency and kind of crisis intervention, as well as patient satisfaction, are assessed as covariates.
Statistical analysis plan
Data will be analyzed using the software SPSS for Microsoft Windows®. For descriptive analysis, data will be expressed as mean values, standard deviations, and frequencies; distributional characteristics of all variables will be given. To analyze simple linear relations between variables, Pearson correlations will be calculated. For verification/falsification of our hypotheses (group differences), ANOVAs (post hoc: Scheffé tests), repeated measurement ANOVAs, ANCOVAs, parametric tests (for independent and dependent samples) and non-parametric tests (Chi2 tests, Mann–Whitney U-tests, Wilcoxon tests, Kruskal-Wallis tests) will be calculated (according to the respective levels of measurement and sample sizes). For prediction of the outcome variables, multiple regression analyses will be performed. For all tests, a significance level of p < 0.05 is predetermined.
Power considerations and calculation of patient attendance
Calculation of the sample size (GPower) for two-tailed t-tests with dependent samples, effect size = 0.6, probability of error α = 0.05, and power = 0.8 resulted in a sample size of n = 24 per group. For two-tailed t-tests with independent samples, the same calculation resulted in n = 45 per group. Due to anticipation of dropouts in the study progress, we enlarged this sample size by one third, so the final calculation of the sample size is n = 60 per group (N = 120). For the calculation of patient attendance see Fig. 2.
Progress
Patient recruitment started in October 2014. At the moment, 62 patients are already taking part in the study. For detailed information about the number of patients in each group and at each assessment point, please see Fig. 3. Baseline sociodemographic and disease-related data of all IG- and OG-patients included are presented in Table 3.
Fig. 3.
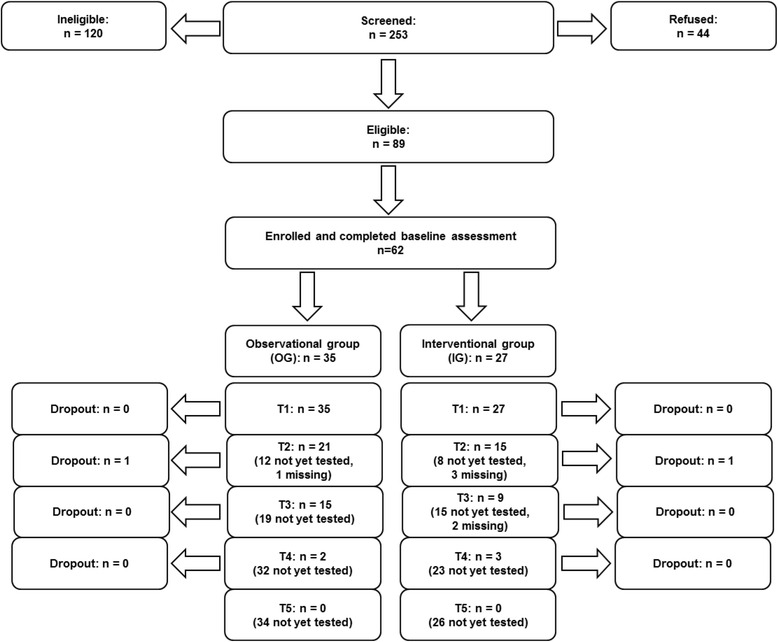
Study flowchart
Table 3.
Baseline sociodemographic and disease-related characteristics of patients to date
| Oberservational group (OG) | Intervention group (IG) | ||
|---|---|---|---|
| n | 35 | 27 | |
| Sociodemographic characteristics | Age in years | mean ± sd: 60.03 ± 12.41 | mean ± sd: 62.96 ± 12.30 |
| range: 37–78 | range: 40–82 | ||
| Sex | female: 16 | female: 11 | |
| male: 19 | male: 16 | ||
| Nationality | German: 34 | German: 26 | |
| Polish: 1 | Dutch: 1 | ||
| Disease-related characteristics | Treatment | brachytherapy: 32 | brachytherapy: 14 |
| endoresection: 0 | endoresection: 4 | ||
| endoresection and Gamma-Knife: 1 | endoresection and Gamma-Knife: 1 | ||
| cyber-Knife: 0 | cyber-Knife: 1 | ||
| enucleation: 2 | enucleation: 7 | ||
| Method of tumor sampling | - | pars plana vitrectomy: 15 | |
| endoresection: 5 | |||
| enucleation: 7 | |||
| Utilization of psycho-oncological support | support offered by study program: 2 | support offered by study program: 2 | |
| other support: 2 | other support: 1 |
Discussion
In the following sections, the psychosocial impact of genetic counseling, decision-making processes and ethical considerations concerning autonomy and communication shall be discussed within the framework of current research.
Psychosocial impact of genetic counseling
In two meta-analyses, a significant impact of genetic counseling for familial cancer on anxiety (reduction), and on accuracy of perceived risk and knowledge (both improved) was found, suggesting genetic counseling had no adverse psychological effects [29, 30]. Comparably, beneficial effects of multidisciplinary genetic risk counseling for familial colorectal cancer on psychosocial outcome were found: General anxiety, familial cancer-specific distress and general cancer worries were significantly reduced after genetic counseling [31]. Moreover, distress and depression levels were reduced within the first 6 months after counseling and testing [32], and intrusion and avoidance significantly reduced over time [33]. Furthermore, the authors found passive and palliative coping styles, excessive breast self-examination, and overestimation of breast cancer risk to be predictive of increased long-term distress. In contrast, decreased long-term distress could be predicted by promoting reassuring thoughts.
When comparing carriers and non-carriers, carriers were found to be significantly more distressed from testing and to report increased risk perception levels and surveillance (up to four years) [34]. Furthermore, Meiser (2005) [35] differentiated in their review between individuals having never been affected by cancer and individuals affected by cancer. In the former condition, non-carriers psychologically benefitted significantly, whereas carriers experienced no adverse effects. In individuals affected with cancer, the individual former cancer experience seemed to influence the effects of genetic testing.
While waiting for disclosure of the test result, acute anxiety may emerge [36]. Phelps et al. (2013) [37] investigated the effectiveness of a self-help coping intervention in patients awaiting their genetic result. Intrusive thoughts during the waiting period could be reduced significantly in patients with moderate baseline levels of intrusion. Furthermore, distress levels were decreased in patients with low or moderate intrusive worries at baseline. Yet, no intervention effect on the sample as a whole could be demonstrated, suggesting that patients with clinically high levels of psychological distress might need more intensive psychological treatments. The authors conclude that their intervention could provide help while waiting for test results and feeling uncertain, in various oncological patient groups. Moreover, there seem to be no harmful effects on those patients that are likely to not benefit from the intervention.
Our study was designed to investigate if these findings could also account for uveal melanoma patients (who have already been diagnosed with cancer).
Decision-making
Affected persons have to weigh the pros and cons of genetic testing when making their decision. Pros are, for example, relief from uncertainty, adjustment of important life decisions and life planning, as well as making decisions about intensified prophylaxis and screening programs. Cons are, for example, risks of persistent psychological distress, such as depressive rumination about one’s individual risk of disease.
It is an important ethical issue, as to how decision-making concerning genetic testing can be improved, such as through educative interventions both for patients and for medical staff. Wakefield et al. (2008) [38] developed a decision aid intervention (specifically for informed decision making concerning genetic testing for hereditary non-polyposis colorectal cancer risk) and tested its efficacy in a randomized controlled trial. Participants of the intervention group experienced less decisional conflict and were better informed and therefore had a better chance of making an informed decision with regard to genetic testing. Furthermore, Ilic et al. (2015) [39] (review, prostate cancer) found improvements in knowledge, reduction in decisional conflict and an increase in decisional satisfaction after utilization of a decision aid intervention. Moreover, Dieng et al. (2014) [40] (review, mainly breast cancer studies) found changes in risk perception level and accuracy after educational interventions in prospective observational studies (n = 28), but not in randomized controlled trials (n = 12). We expect our study to shed further light on questions concerning the influence of decision for or against genetic testing.
Ethical considerations: Autonomy and communication
Another important research question is the appropriate and correct ethical conduct with information. The right of self-determination and respect for the personal autonomy of the patient form the ethical and legal basis for the demand for information and participation in medical decisions. It is important to note, that the right of self-determination includes also the right of not being informed, because negatively appraised health-related information can cause severe psychological distress. So, apart from the ethical principle of autonomy, the principles of non-maleficence and beneficence have to be taken into account for medico-ethical analyses [41]. To minimize psychosocial distress due to prognostic genetic testing, genetic counseling before and directly after communication of the results is recommended by the guidelines of the German Medical Association [42]. This recommendation was taken into account in the elaboration of the German Genetic Diagnostics Act. In a systematic review (93 studies), it was found that most studies about communication of prognosis to cancer patients were conducted in early stages of disease [43]. It remained uncertain which approach of communication would be the best. We expect our study to provide further information about patient autonomy and disclosure of test results.
Limitations
There are two limitations of our study. First, randomization and blinding is not possible. Second, patients receive different cancer treatments (see Table 3), which might cause differences concerning the psychosocial impact of genetic testing. On the other hand, no differences concerning quality of life, psychological distress, depression and anxiety were found between enucleation and radiotherapy patients or between patients receiving different methods of radiotherapy [44, 45].
Conclusion
We created optimal conditions for the investigation of the psychosocial impact of prognostic genetic testing and the course of disease, in uveal melanoma patients. The naturalistic design we chose is very suitable for research in the clinical setting, but takes time and effort. We therefore thank the German Cancer Aid for promoting this network of three parallelized collaborative studies from different scientific disciplines, giving the opportunity to investigate uveal melanoma patients from different points of view.
We expect that our findings will be transferable to other oncological entities and clinical syndromes. Furthermore, we expect that empirical data from our study will contribute to the continuing medico-ethical debate on prognostic genetic testing, in the light of patient autonomy and decision-making processes.
Acknowledgements
We acknowledge the German Cancer Aid for financial support and wish to thank the patients who will take part or already took part in our study.
Funding
This study is funded by the German Cancer Aid.
Availability of data and material
The data supporting your findings can be requested from Prof. Yesim Erim (yesim.erim@uk-erlangen.de).
Authors’ contributions
YE conceived of the study and it’s design, participated in the study coordination and drafted the funding application. JS drafted the manuscript. AB and CHDM carried out patient recruitment and data acquisition. All authors (YE, JS, AB, CHDM, DL, H-CF, ST) revised the manuscript critically for important intellectual content, have given final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Authors’ information
YE (MD) is the head of the Department of Psychosomatic Medicine and Psychotherapy, Friedrich-Alexander University Erlangen-Nürnberg (FAU). JS works as research assistant (M.Sc. Psychology, PhD-student) in the Department of Psychosomatic Medicine and Psychotherapy, Friedrich-Alexander University Erlangen-Nürnberg (FAU). AB works as research assistant (M.Sc. Psychology) in the Clinic for Psychosomatic Medicine and Psychotherapy, LVR Hospital Essen, University of Duisburg-Essen. CHDM works as MD in the Department of Ophthalmology, University Hospital Essen. DL (MD) is the head of the Department of Human Genetics, University Hospital Essen. H-CF (MD) is the head of the Clinic for Psychosomatic Medicine and Psychotherapy, LVR Hospital Essen, University of Duisburg-Essen. ST is the research director (M.Sc. Psychology, PhD) of the Clinic for Psychosomatic Medicine and Psychotherapy, LVR Hospital Essen, University of Duisburg-Essen.
Competing interests
All authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study protocol was approved by the ethics committee of the medical faculty of the University of Duisburg-Essen. All patients gave written informed consent to participate. Our manuscript contains no individual person’s data in any form, consent to publish therefore does not have to be obtained.
Additional file
Appendix (DOCX 38 kb)
Contributor Information
Yesim Erim, Email: yesim.erim@uk-erlangen.de.
Jennifer Scheel, Email: jennifer.scheel@uk-erlangen.de.
Anja Breidenstein, Email: anja.breidenstein@lvr.de.
Claudia HD Metz, Email: claudia.metz@uk-essen.de.
Dietmar Lohmann, Email: dietmar.lohmann@uni-due.de.
Hans-Christoph Friederich, Email: hans-christoph.friederich@uni-due.de.
Sefik Tagay, Email: sefik.tagay@uni-due.de.
References
- 1.Prescher G, Bornfeld N, Hirche H, Horsthemke B, Jockel KH, Becher R. Prognostic implications of monosomy 3 in uveal melanoma. Lancet. 1996;347(9010):1222–5. doi: 10.1016/S0140-6736(96)90736-9. [DOI] [PubMed] [Google Scholar]
- 2.Petrausch U, Martus P, Tonnies H, Bechrakis NE, Lenze D, Wansel S, et al. Significance of gene expression analysis in uveal melanoma in comparison to standard risk factors for risk assessment of subsequent metastases. Eye. 2008;22(8):997–1007. doi: 10.1038/sj.eye.6702779. [DOI] [PubMed] [Google Scholar]
- 3.Thomas S, Putter C, Weber S, Bornfeld N, Lohmann DR, Zeschnigk M. Prognostic significance of chromosome 3 alterations determined by microsatellite analysis in uveal melanoma: a long-term follow-up study. Br J Cancer. 2012;106(6):1171–6. doi: 10.1038/bjc.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reimer J, Voigtlaender-Fleiss A, Karow A, Bornfeld N, Esser J, Helga Franke G. The impact of diagnosis and plaque radiotherapy treatment of malignant choroidal melanoma on patients’ quality of life. Psycho-Oncology. 2006;15(12):1077–85. doi: 10.1002/pon.1046. [DOI] [PubMed] [Google Scholar]
- 5.Klingenstein A, Furweger C, Nentwich MM, Schaller UC, Foerster PI, Wowra B, et al. Quality of life in the follow-up of uveal melanoma patients after CyberKnife treatment. Melanoma Res. 2013;23(6):481–8. doi: 10.1097/CMR.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 6.Suchocka-Capuano A, Bredart A, Dolbeault S, Rouic LL, Levy-Gabriel C, Desjardins L, et al. Quality of life and psychological state in patients with choroidal melanoma: longitudinal study. Bull Cancer. 2011;98(2):97–107. doi: 10.1684/bdc.2011.1300. [DOI] [PubMed] [Google Scholar]
- 7.Metz CH, Lohmann D, Zeschnigk M, Bornfeld N. Uveal melanoma: current insights into clinical relevance of genetic testing. Klin Monbl Augenheilkd. 2013;230(7):686–91. doi: 10.1055/s-0033-1350628. [DOI] [PubMed] [Google Scholar]
- 8.Pasacreta JV. Psychosocial issues associated with genetic testing for breast and ovarian cancer risk: an integrative review. Cancer Investig. 2003;21(4):588–623. doi: 10.1081/CNV-120022380. [DOI] [PubMed] [Google Scholar]
- 9.Beran TM, McCannel TA, Stanton AL, Straatsma BR, Burgess BL. Reactions to and desire for prognostic testing in choroidal melanoma patients. J Genet Couns. 2009;18(3):265–74. doi: 10.1007/s10897-009-9223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook SA, Damato B, Marshall E, Salmon P. Psychological aspects of cytogenetic testing of uveal melanoma: preliminary findings and directions for future research. Eye. 2009;23(3):581–5. doi: 10.1038/eye.2008.54. [DOI] [PubMed] [Google Scholar]
- 11.Cook SA, Damato B, Marshall E, Salmon P. Reconciling the principle of patient autonomy with the practice of informed consent: decision-making about prognostication in uveal melanoma. Health Expect. 2011;14(4):383–96. doi: 10.1111/j.1369-7625.2010.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deber RB, Kraetschmer N, Urowitz S, Sharpe N. Do people want to be autonomous patients? Preferred roles in treatment decision-making in several patient populations. Health Expect. 2007;10(3):248–58. doi: 10.1111/j.1369-7625.2007.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vos J, Gomez-Garcia E, Oosterwijk JC, Menko FH, Stoel RD, van Asperen CJ, et al. Opening the psychological black box in genetic counseling. The psychological impact of DNA testing is predicted by the counselees’ perception, the medical impact by the pathogenic or uninformative BRCA1/2-result. Psycho-Oncology. 2012;21(1):29–42. doi: 10.1002/pon.1864. [DOI] [PubMed] [Google Scholar]
- 14.Manne SL, Chung DC, Weinberg DS, Vig HS, Catts Z, Cabral MK, et al. Knowledge and attitudes about microsatellite instability testing among high-risk individuals diagnosed with colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(10):2110–7. doi: 10.1158/1055-9965.EPI-07-0412. [DOI] [PubMed] [Google Scholar]
- 15.Katapodi MC, Northouse LL, Milliron KJ, Liu G, Merajver SD. Individual and family characteristics associated with BRCA1/2 genetic testing in high-risk families. Psycho-Oncology. 2013;22(6):1336–43. doi: 10.1002/pon.3139. [DOI] [PubMed] [Google Scholar]
- 16.Lerman C, Daly M, Masny A, Balshem A. Attitudes about genetic testing for breast-ovarian cancer susceptibility. J Clin Oncol. 1994;12(4):843–50. doi: 10.1200/JCO.1994.12.4.843. [DOI] [PubMed] [Google Scholar]
- 17.Antonovsky A. The structure and properties of the sense of coherence scale. Soc Sci Med. 1993;36:725–33. doi: 10.1016/0277-9536(93)90033-Z. [DOI] [PubMed] [Google Scholar]
- 18.Fydrich T, Sommer G, Menzel U, Höll B. Fragebogen zur sozialen Unterstützung (Kurzform; SozU-K-22) Z Klin Psychol. 1987;16:434–6. [Google Scholar]
- 19.Sommer G, Fydrich T. Soziale Unterstützung, Diagnostik, Konzepte, Fragebogen F-SozU. Tübingen; 1989.
- 20.Sommer G, Fydrich T. Entwicklung und Überprüfung eines Fragebogens zur sozialen Unterstützung. Diagnostica. 1991;37:160–78. [Google Scholar]
- 21.Herschbach P, Berg P, Dankert A, Duran G, Engst-Hastreiter U, Waadt S, et al. Fear of progression in chronic diseases: psychometric properties of the Fear of Progression Questionnaire. J Psychosom Res. 2005;58:505–11. doi: 10.1016/j.jpsychores.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Herschbach P, Dankert A, Duran-Atzinger G, Waadt S, Engst-Hastreiter U, Keller M et al. Diagnostik von Progredienzangst – Entwicklung eines Fragebogens zur Erfassung von Progredienzangst bei Patienten mit Krebserkrankungen, Diabetes mellitus und entzündlich-rheumatischen Erkrankungen in der Rehabilitation [Diagnostic of fear of progression – evaluation of a questionnaire for measurement of fear of progression in patients with cancer, diabetes mellitus and inflammatory rheumatic diseases in rehabilitation] http://forschung.deutsche-rentenversicherung.de/ForschPortalWeb/rehaDoc.pdf?rehaid=82EF8B8F899B55CEC1256E6A003B729D. 2013.
- 23.Herrmann CH, Buss U, Snaith RP. HADS-D: Hospital Anxiety and Depression Scale – German Version. Bern, Germany: Huber; 1995. [Google Scholar]
- 24.Roth AJ, Kornblith AB, Batel-Copel L, Peabody E, Scher HI, Holland JC. Rapid screening for psychologic distress in men with prostate carcinoma: a pilot study. Cancer. 1998;82(10):1904–8. doi: 10.1002/(SICI)1097-0142(19980515)82:10<1904::AID-CNCR13>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 25.Aaronson NK, Ahmedazai S, Bergman B, Bullinger M, Cull A, Duez NJ. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality of life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 26.Brandberg Y, Damato BE, Kivela T, Kock E, Seregard S. The EORTC ophthalmic oncology quality of life questionnaire module (EORTC QLQ-OPT30). Development and pre-testing (Phase I-III) Eye. 2004;18:283–9. doi: 10.1038/sj.eye.6700639. [DOI] [PubMed] [Google Scholar]
- 27.Ware JE, Jr, Kosinski M, Keller S. A 12-item shortform health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Keller M, Jost R, Kadmon M, Wüllenweber H-P, Mastromarino Haunstetter C, Willeke F, et al. Acceptance of and Attitude Toward Genetic Testing for Hereditary Nonpolyposis Colorectal Cancer: A Comparison of Participants and Nonparticipants in Genetic Counseling. Dis Colon Rectum. 2004;47:153–62. doi: 10.1007/s10350-003-0034-5. [DOI] [PubMed] [Google Scholar]
- 29.Braithwaite D, Emery J, Walter F, Prevost AT, Sutton S. Psychological impact of genetic counseling for familial cancer: a systematic review and meta-analysis. Familial Cancer. 2006;5(1):61–75. doi: 10.1007/s10689-005-2577-1. [DOI] [PubMed] [Google Scholar]
- 30.Meiser B, Halliday JL. What is the impact of genetic counselling in women at increased risk of developing hereditary breast cancer? A meta-analytic review. Soc Sci Med. 2002;54((10):1463–70. doi: 10.1016/S0277-9536(01)00133-2. [DOI] [PubMed] [Google Scholar]
- 31.Keller M, Jost R, Haunstetter CM, Sattel H, Schroeter C, Bertsch U, et al. Psychosocial outcome following genetic risk counselling for familial colorectal cancer. A comparison of affected patients and family members. Clin Genet. 2008;74(5):414–24. doi: 10.1111/j.1399-0004.2008.01089.x. [DOI] [PubMed] [Google Scholar]
- 32.Shiloh S, Koehly L, Jenkins J, Martin J, Hadley D. Monitoring coping style moderates emotional reactions to genetic testing for hereditary nonpolyposis colorectal cancer: a longitudinal study. Psycho-Oncology. 2008;17(8):746–55. doi: 10.1002/pon.1338. [DOI] [PubMed] [Google Scholar]
- 33.den Heijer M, Seynaeve C, Vanheusden K, Timman R, Duivenvoorden HJ, Tilanus-Linthorst M, et al. Long-term psychological distress in women at risk for hereditary breast cancer adhering to regular surveillance: a risk profile. Psycho-Oncology. 2013;22(3):598–604. doi: 10.1002/pon.3039. [DOI] [PubMed] [Google Scholar]
- 34.Shiloh S, Dagan E, Friedman I, Blank N, Friedman E. A follow-up study on men tested for BRCA1/BRCA2 mutations: impacts and coping processes. Psycho-Oncology. 2013;22(2):417–25. doi: 10.1002/pon.2106. [DOI] [PubMed] [Google Scholar]
- 35.Meiser B. Psychological impact of genetic testing for cancer susceptibility: an update of the literature. Psycho-Oncology. 2005;14(12):1060–74. doi: 10.1002/pon.933. [DOI] [PubMed] [Google Scholar]
- 36.Bancroft EK, Castro E, Bancroft GA, Ardern-Jones A, Moynihan C, Page E, et al. The psychological impact of undergoing genetic-risk profiling in men with a family history of prostate cancer. Psycho-Oncology. 2015;24:1492–9. doi: 10.1002/pon.3814. [DOI] [PubMed] [Google Scholar]
- 37.Phelps C, Bennett P, Hood K, Brain K, Murray A. A self-help coping intervention can reduce anxiety and avoidant health behaviours whilst waiting for cancer genetic risk information: results of a phase III randomised trial. Psycho-Oncology. 2013;22(4):837–44. doi: 10.1002/pon.3072. [DOI] [PubMed] [Google Scholar]
- 38.Wakefield CE, Meiser B, Homewood J, Ward R, O’Donnell S, Kirk J, et al. Randomized trial of a decision aid for individuals considering genetic testing for hereditary nonpolyposis colorectal cancer risk. Cancer. 2008;113(5):956–65. doi: 10.1002/cncr.23681. [DOI] [PubMed] [Google Scholar]
- 39.Ilic D, Jammal W, Chiarelli P, Gardiner RA, Hughes S, Stefanovic D et al. Assessing the effectiveness of decision aids for decision making in prostate cancer testing: a systematic review. Psycho-oncology. 2015. doi:10.1002/pon.3815 [DOI] [PubMed]
- 40.Dieng M, Watts CG, Kasparian NA, Morton RL, Mann GJ, Cust AE. Improving subjective perception of personal cancer risk: systematic review and meta-analysis of educational interventions for people with cancer or at high risk of cancer. Psycho-Oncology. 2014;23(6):613–25. doi: 10.1002/pon.3476. [DOI] [PubMed] [Google Scholar]
- 41.Beauchamp TL, Childress JF. Principles of Biomedical Ethics. Oxford University Press; 2008
- 42.Bundesärztekammer. Richtlinien zur Diagnostik der genetischen Disposition für Krebserkrankungen. Deutsches Ärzteblatt. 1998;95:A1396-403.
- 43.Hagerty RG, Butow PN, Ellis PM, Dimitry S, Tattersall MH. Communicating prognosis in cancer care: a systematic review of the literature. Ann Oncol. 2005;16(7):1005–53. doi: 10.1093/annonc/mdi211. [DOI] [PubMed] [Google Scholar]
- 44.Brandberg Y, Kock E, Oskar K, af Trampe E, Seregard S. Psychological reactions and quality of life in patients with posterior uveal melanoma treated with ruthenium plaque therapy or enucleation: a one year follow-up study. Eye. 2000;14:839–46. doi: 10.1038/eye.2000.233. [DOI] [PubMed] [Google Scholar]
- 45.Chabert S, Velikay-Parel M, Zehetmayer M. Influence of uveal melanoma therapy on patients’ quality of life: a psychological study. Acta Ophthalmol Scand. 2004;82:25–31. doi: 10.1046/j.1600-0420.2003.0210.x. [DOI] [PubMed] [Google Scholar]


