Abstract
Background:
Successful recruitment of patients is known to be one of the most challenging aspects in conduct of randomized controlled trials. Inadequate patient retention during conduct of trial affects conclusive results.
Objective:
To assess the level of challenges faced by Indian investigators in recruitment and retention of trial subjects.
Methods:
We developed a survey questionnaire on challenges encountered by investigators in subject recruitment and retention which was hosted on a web portal.
Results:
Seventy-three investigators from India participated in the survey. The frequently encountered challenges in subject recruitment were complexity of study protocol (38%), lack of awareness about clinical trials in patients (37%), and sociocultural issues related to trial participation (37%). About 63% of participants strongly agreed that creating a positive awareness about clinical trials among people through press and media, having a dedicated clinical research coordinator for trial (50.7%), and designing a recruitment strategy prior to study initiation (46.6%) would enhance recruitment. Almost 50.7% of participants agreed that interacting with medical community in vicinity of the study site and educating patients about clinical trials during routine outpatient department visits (46.6%) would enhance recruitment. Experiencing a serious adverse event, subject's fear for study procedures (47%) and side effects (44%) were thought to have a moderate effect on subject retention.
Conclusion:
Our survey has put forth factors related to negative publicity by media, lack of patient education about clinical trials; complex study designs are barriers to clinical trial recruitment in India. It is essential to devise innovative and effective strategies focusing on education of public and mass media about clinical research in India.
Key words: Indian, investigators, recruitment, retention, subjects
INTRODUCTION
Randomized controlled trials (RCTs) are widely accepted as the gold standard for evaluating the effectiveness and safety of healthcare interventions. Successful recruitment and retention of patients in clinical trials are known to be one of the most challenging aspects in completion of RCTs.[1]
Inadequate recruitment is known to have a significant impact on the scientific and financial viability of an RCT. The possibility of incurring a type 2 error (erroneously concluding, there is no significant difference between treatment groups) increases if the estimated sample size target is not met. Adequate enrolment of subjects in a clinical trial provides a base for projected retention of trial subjects and helpful in evaluation of patient data and may also result in extension of trial period, thus increasing the study cost. It may also cause a level of uncertainty about the treatment efficacy and may lead to delay in time for a potentially effective therapy. Slow acquisition of trial evidence may impact the financial investment of the funding agency causing them to prefer less reliable but more rapid approach to evaluation. Despite these facts, importance of subject recruitment often remains underestimated, and institutional resources are rarely deployed to facilitate subject recruitment.[2,3,4]
Since poor recruitment is a crucial factor in conduct of RCTs, it is essential to investigate the barriers to recruitment and devise potential strategies to improve the same in clinical trials. Several international studies investigating the issues in recruitment have put forth strategies such as piloting the recruitment process, financial and educational incentives for clinicians, newsletter and reminders for patients, open- versus placebo-controlled trials, assistance with patient travel, and networking with various healthcare professionals. However, these strategies have been put forth after detailed studies in western population with a different sociocultural scenario and their relevance to recruitment efforts in a developing country like India needs to be investigated.[5,6]
Objective of the study
The objective is to assess the level of challenges faced by Indian clinical trial investigators in recruitment and retention of subjects in clinical trials.
METHODS
Study population
The survey was a pilot study conducted during a 4-month period starting from February 2014 to May 2014. The survey investigated various issues faced by Indian Investigators such as challenges in recruitment-retention practices and new regulatory guidelines issued by DCGI in the year 2013. This paper is restricted to our findings in recruitment and retention challenges. We selected E-mail contacts of principal investigators from four zones (east, west, north, and south) from the following database sources (Clinical Trial Registry India, Indian Society Clinical Research, and Chest Research Foundation-Respiratory Research Network). We shortlisted the participant investigator list for investigators with whom we had some communication in the past to facilitate survey communication. All the shortlisted E-mail contacts were pooled together in an excel datasheet, and a random list was generated in Excel. We contacted the first 128 investigators in the list due to time constraint and it being a pilot study. We understand this was a limitation of the study as the sample may not be a true representative of Indian investigators. We also agree that cluster randomization technique would have given us a well-distributed data.
Development of study questionnaire
We developed a survey questionnaire which was hosted on a web portal (www.monkeysurvey.com) widely used to conduct online surveys. This particular survey portal was selected after reviewing its online accessibility and data security features. The idea behind using an online survey questionnaire was to reach maximum investigators in India and obtain their response in a timely manner. The questionnaire was designed keeping in perspective the challenges faced by clinical trial investigators in recruitment and retention of subjects as assessed from literature review and inputs from clinical trial investigators working at our organization. Validation of the questionnaire was done by administering it to 10 personnel, who comprised investigators and clinical research coordinators (CRCs) working at our center. Their suggestions were incorporated, and the final questionnaire was then hosted online on the survey portal.
The study questionnaire design
Participants were requested to give their opinion on the barriers and potential strategies related to recruitment and retention of trial subjects encountered by them.
Barriers in subject recruitment
Lack of awareness about clinical trials in patients
Complexity of study protocol
Social and cultural issues related to trial participation.
The frequency of encountering the above challenges in subject recruitment was captured on a 5-point scale (very frequently, frequently, occasionally, rarely, and never). Participants were also asked to mention in 150 words any additional factors which they felt hampered recruitment.
Potential strategies to enhance subject recruitment
Having a dedicated CRC for trial
Arranging for patient transport to trial site for study visits
Designing a recruitment strategy prior to study initiation
Interacting with medical community in your area regarding clinical trial recruitment
Educating subjects on clinical trial during routine outpatient department (OPD) visits
Creating positive awareness about clinical trials among people through press and mass media.
Degree of agreement to the above strategies was to be indicated on a 5-point scale (strongly agree, agree, neutral, disagree, and strongly disagree). Participants were also requested to mention strategies which could enhance subject recruitment in 150 words.
Barriers to subject retention
Participants were asked to indicate the effect of following factors on retention of subjects in clinical trials on a 5-point scale: Major effect, moderate effect, neutral, minor effect, and no effect. Participants were also requested to mention additional factors which they felt could affect subject retention in 150 words.
Fear of side effects in clinical trials
Change of residence of trial subject
Experiencing side effects
Subject's fear for study procedures
Poor compliance to study protocol on retention of subjects in clinical trials.
Survey recruitment methodology
The Institutional Ethics Committee was informed about the proposed survey and an exemption from review was obtained as it was an observational survey and involved no more than minimal risk to research participants. An invitation E-mail was sent to all investigators in the first month which contained information about the purpose of the survey and web link to the online survey portal. The invitation E-mail mentioned that participants who wished to be a part of the survey could proceed by accessing the online survey web link provided. It was also stated that the survey report will be published in a peer-reviewed journal. An acknowledgment receipt was requested on delivery of the E-mail. A reminder E-mail was sent to all investigators who acknowledged receipt of the mail but did not respond requesting them to participate in the survey in the second month. The investigator responses were stored in a central database which was accessed only by the study team. Individual responses were compiled in a format where names and other identification marks were removed prior to analysis.
Statistical analysis
The data obtained in the form of degree of agreement based on the selected option on a 5-point scale were analyzed quantitatively. The questions enquired about the challenges faced by Indian investigators in recruitment and retention of patients and potential strategies to enhance these areas of clinical trials. Findings are presented in the form of percentage of participants reporting each response for a particular factor affecting recruitment and retention in clinical trials.
The qualitative data obtained in the form of subjective responses were analyzed quantitatively. The justifications provided were grouped based on the degree of agreement. A category system was constructed based on major themes identified in the data. Each justification in the grouped data was coded as per the category. The frequency of justifications in each category was analyzed quantitatively.
RESULTS
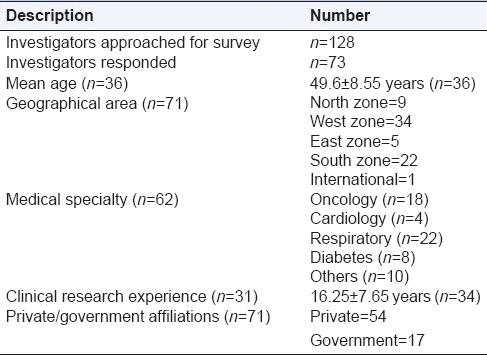
We sent the survey to 128 investigators in India through E-mail which consisted of a web link to the study questionnaire hosted on the survey website. A total of 73 investigators responded to the survey. We did not receive response from 55 investigators. This was attributed to nonfunctional E-mail addresses, (n = 15) unwillingness to participate in the survey (n = 14) and investigator's busy schedule (n = 26) as conveyed to us during the telephonic follow-up. The survey received a response rate of 57%. The demographics of survey participants are represented in Table 1 (mean age, geographical area, medical specialty, years of experience, and years of clinical trial experience).
Table 1.
Investigator demographics
Participant's opinion on challenges encountered in areas related to recruitment and retention of clinical trial subjects in India are as follows.
Barriers in subject recruitment
The frequently encountered challenge in subject recruitment as reported by participants was complexity of study protocol (38%), followed by lack of awareness about clinical trials in patients (37%) and sociocultural issues related to trial participation (37%) [Figure 1]. Additional factors hampering subject recruitment in clinical trials as put forth by participants (18 responses) were patient's fear of side effects (33%), negative publicity by media (22%), and large geographical distance with study site (16.7%). Other factors reported to hamper recruitment efforts were illiteracy, legal approach in clinical trials, language barrier, audio-video recording of consent process, and discouragement by treating physician (5.6%).
Figure 1.
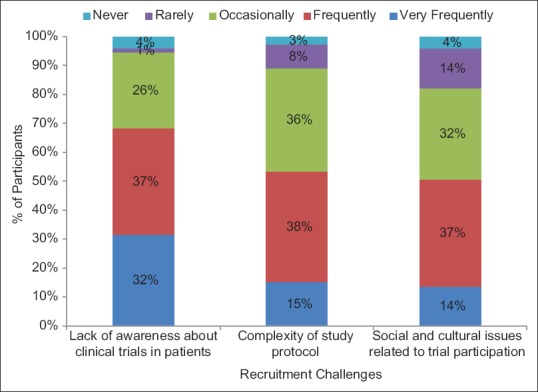
Challenges faced by Indian investigators in subject recruitment process (n = 73)
Potential strategies to enhance subject recruitment
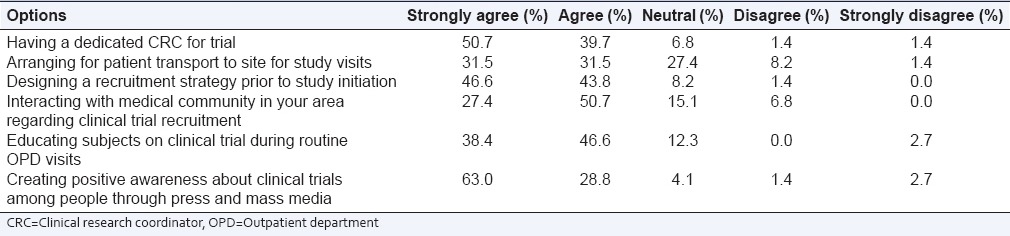
About 63% of participants strongly agreed that creating a positive awareness about clinical trials among people through press and media would enhance recruitment followed by having a dedicated CRC for trial (50.7%) and designing a recruitment strategy prior to study initiation (46.6%). Almost 50.7% of participants agreed that interacting with medical community in vicinity of study site and educating patients about clinical trials during routine OPD visits (46.6%) would also further enhance recruitment [Table 2]. Participants (10 responses) put forth the following strategies which could potentially improve recruitment having a large database of patients (30%), creating media awareness (50%) and networking with medical community in the region of the study site, and having a dedicated research team (10%).
Table 2.
Potential strategies that enhance recruitment in clinical trials (n=73)
Barriers to subject retention
Our survey also assessed the issue of subject retention encountered by clinical trial investigators. Fear of side effects in clinical trials was thought to have a moderate effect on subject retention by 44% of participants and major effect by 34% of participants. Experiencing a serious adverse event, subject's fear for study procedures (47%) were thought to have a moderate effect on subject retention. Almost 36% of participants felt that change of residence and poor compliance with study protocol also had moderate effect on subject retention [Figure 2]. Additional factors put forth by participants (10 responses) affecting subject retention in clinical trials were lack of support from family physician and family members (27%), lack of dedicated approach toward patient by investigator's team (55%), negative media publicity (9%), and lack of drug efficacy (9%).
Figure 2.
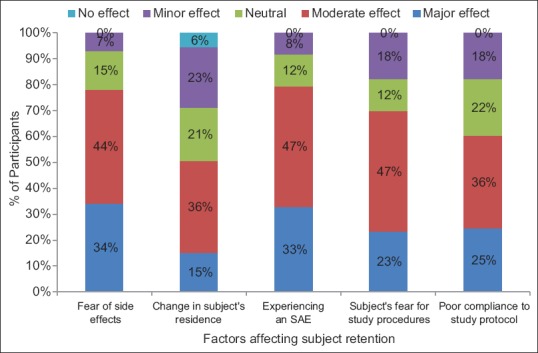
Factors affecting subject retention in clinical trials
DISCUSSION
The survey questionnaire was sent to 128 investigators from various therapeutic areas all over the country and 73 investigators responded to it (response rate 57%). Investigators from different parts of India and from varied faculties such as cardiology, oncology, respiratory, and diabetes participated in the survey.
Key findings
The frequently encountered challenge by participants in trial recruitment was complexity of study protocol along with lack of awareness about clinical trials in patients and sociocultural issues of subjects related to clinical trial participation. Participants also reported that patient's fear of side effects, negative publicity by media, and large geographical distance with study site were the factors that influenced recruitment at sites. Participants expressed that fear of side effects in clinical trials was thought to have a moderate effect on subject retention.
Interpretations
International literature investigating issues encountered in recruitment and retention have reported barriers such as language, cultural factors, trial designs, belief about medical research, time constraints, and discouragement of patients by family physicians for trial participation, inadequate training of trial team members, and limited human resources at study sites.[7] Clinical trial investigators in our survey felt that complex study protocol was a hindrance to recruitment procedures. This can be attributed to complex trial designs with extensive selection criteria and designed for safety of trial population but may also cause significant hurdles for recruitment. It also implies that study protocols need to be realistic and easy to implement. Survey participants have recommended designing a recruitment strategy prior to study initiation and dedicated research co-coordinator exclusively for a trial to support recruitment procedures.
Other barriers emerging from the survey were lack of patient awareness about clinical trials and sociocultural issues influencing trial participation. This could be exclusive to Indian settings and attributed to level of literacy and conservative cultural mindset prevailing in some parts of the country. Negative publicity of clinical research by mass media has also emerged as a hurdle to recruitment which is again unique to Indian scenario and could be due to past instances of few unethical trials reported in the country.[8] The findings of our survey were consistent with a study conducted in South India to assess the barriers to recruitment which reported negative impact from the media, trial conducted in rare disease, and large geographical distance between study site and subject residences as major barriers.[9] Another meta-analysis by Shah et al. has reported concerns about safety and efficacy of trials, psychological reasons, and confidentiality issues as recruitment barriers in Indian patients.[10] Our survey findings have confirmed the role of negative publicity by media along with complexities in the study protocol and patient concerns related to safety in trial participation as challenges for recruitment in India. Fear of side effects and study procedures by patients have emerged as factors influencing retention of subjects in trials which significantly point toward effectiveness and importance of informed consent procedures, simplifying trial information given to study patients, and motivation of subjects in clinical trials.
Strengths and limitations of the study
This survey attempted to gain insight into the challenges faced by Indian investigators in recruitment and retention of subjects in clinical trials. With very limited data available in Indian population on constraints related to recruitment and retention, the survey findings will be helpful in designing effective strategies to enhance subject recruitment in India. A limitation of this study was a relatively small sample size as it was a pilot study. The survey also received a fair response rate of 57%. It is essential to study the same in a large arena of clinical research community to have a better consensus on the issues related to recruitment and retention of trial subjects in our country. Moreover, the survey had large representation from private institutions as compared to government institutions which may have influenced some observations.
CONCLUSION AND RECOMMENDATIONS
Recruitment is a dialog process initiated between the investigator and potential participant prior to consent process. It involves identification of potential participants and providing information to them, thus generating their interest in the proposed study. It involves collaborative efforts from all members of research team as well as trial sponsors. Successful recruitment of participants is critically dependent on factors such as administrative support, attitude of clinical staff, volume/turnover of patients, realistic study protocols, and stability of the patient population. Investigators themselves need to be conscientious, have professional integrity, plan meticulously, and develop good interpersonal skills. Respect for participants can help to establish trust and rapport with study patients, thus translating into better subject retention. Providing adequate, clear, and concise explanation about trial procedures to study patients during informed consent process also aids in subject retention.[11] Protocol-related barriers can be avoided if details such as number of subjects required, eligibility criteria, and study procedures are adequately discussed by both the investigator and sponsor during feasibility stage. Sponsors need to validate the realistic implementation of study protocols in the intended population. It is essential that sponsors validate recruitment targets before finalization of investigator site.[12] Further, the research question addressed in RCT should be both interesting and relevant to clinical practice. This could be of vital importance for dedicated involvement and interest of clinicians in a clinical study. The trial methods should be easy to understand which in turn will help in effective communication of the same with patients. Appropriate training of the study staff on research and recruitment methods may also enhance recruitment. Training should focus on addressing common misconceptions about RCTs, particularly equipoise and informed consent. Efforts should also be directed toward community awareness of RCTs, which may increase the number of patients willing to participate in clinical trials. It is also suggested that endorsement of research by patient's own family physician may enhance trial participation.[13]
Another recommendation which can aid recruitment is considering advanced statistical methods in trial planning and analysis such as adaptive designs. Such designs provide opportunities to determine the progress of trials, thus allowing opportunity for reassessment of the assumptions made in trial design as well as assessment of efficacy and futility. This approach may save both time and trial cost. Other recommended strategies which help investigators in recruitment efforts are open- versus placebo-controlled trials, forming research networks, and not blinding and using incentives for participants.[14] It is been reported that time constraints and problems of enrolling eligible patients are major barriers to recruitment in multicenter RCTs. Hence, it is essential that investigators adequately allocate resources in terms of staff as well as infrastructure to overcome the barriers related to recruitment. Investigators need to develop a foresight to gauge the barriers in recruitment and evolve a system to overcome recruitment hurdles.[15] Both sponsors as well as investigators need to invest coordinated efforts which focus on education and motivation of trial team members in issues related to recruitment. The issue of negative perception of clinical trials in India needs to be addressed by imparting extensive education in the domain of clinical trials, ethics, and regulations to mass media as well as general public. This can be achieved by initiatives such as organizing patient education programs, publishing informative articles about clinical research in print media, developing informational videos, posters, information leaflets which can be displayed in hospital wards, and giving information about clinical research to general population during health camps.
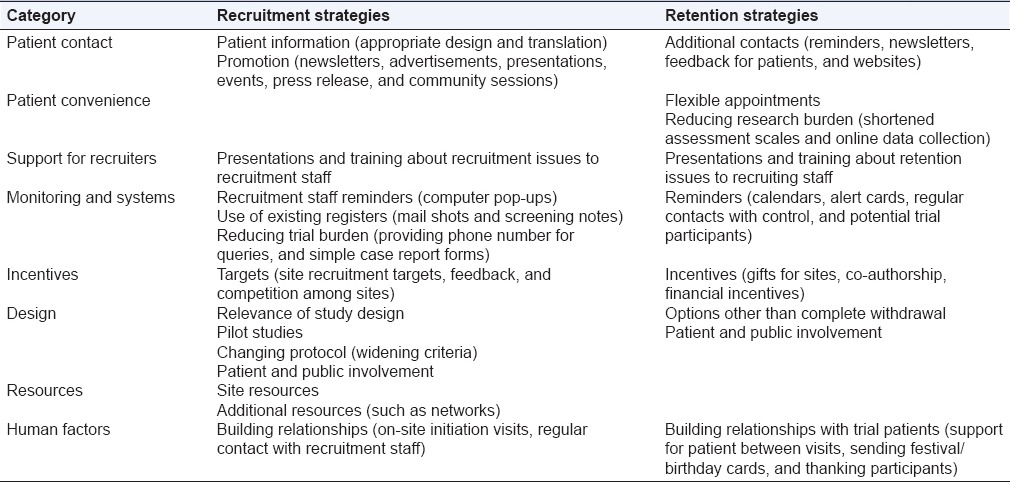
Successful patient retention spells for good quality and valuable trial results. It is recommended that early retention techniques be incorporated into recruitment strategies during planning phase of the trial. Techniques such as effective, persistent, and clear communication with trial participants, emphasis on effective informed consent conduct, along with strong interpersonal relations with trial subjects may encourage retention. International literature has reported innovative strategies [Table 3] to enhance recruitment and retention in clinical trials.[16] It would be interesting to study and know the relevance of implementing these strategies to enhance retention in Indian population.
Table 3.
Potential strategies to enhance recruitment and retention recommended internationally
Recent years have seen India emerge as a hub for clinical research with Indian investigators participating in various global clinical trials. This has significantly contributed to the development of medical research in our country by making newer and effective drugs accessible to India patients. Indian clinical trial investigators have also benefitted by having access to international medical research and latest state of the art medical technologies. It is therefore essential to employ significant efforts in devising innovative and effective strategies in clinical trials relevant to Indian population, thus encouraging quality clinical research in our country.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;7:9. doi: 10.1186/1745-6215-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watson JM, Torgerson DJ. Increasing recruitment to randomised trials: A review of randomised controlled trials. BMC Med Res Methodol. 2006;6:34. doi: 10.1186/1471-2288-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris PA, Lane L, Biaggioni I. Clinical research subject recruitment: The Volunteer for Vanderbilt Research Program www.volunteer.mc.vanderbilt.edu. J Am Med Inform Assoc. 2005;12:608–13. doi: 10.1197/jamia.M1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frank G. Current challenges in clinical trial patient recruitment and enrollment. SOCRA Source. 2004:30–8. [Google Scholar]
- 5.Lovato LC, Hill K, Hertert S, Hunninghake DB, Probstfield JL. Recruitment for controlled clinical trials: Literature summary and annotated bibliography. Control Clin Trials. 1997;18:328–52. doi: 10.1016/s0197-2456(96)00236-x. [DOI] [PubMed] [Google Scholar]
- 6.Treweek S, Lockhart P, Pitkethly M, Cook JA, Kjeldstrøm M, Johansen M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open. 2013;3:pii: E002360. doi: 10.1136/bmjopen-2012-002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spaar A, Frey M, Turk A, Karrer W, Puhan MA. Recruitment barriers in a randomized controlled trial from the physicians’ perspective: A postal survey. BMC Med Res Methodol. 2009;9:14. doi: 10.1186/1471-2288-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sully BG, Julious SA, Nicholl J. A reinvestigation of recruitment to randomised, controlled, multicenter trials: A review of trials funded by two UK funding agencies. Trials. 2013;14:166. doi: 10.1186/1745-6215-14-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: A systematic review. J Clin Epidemiol. 1999;52:1143–56. doi: 10.1016/s0895-4356(99)00141-9. [DOI] [PubMed] [Google Scholar]
- 10.Gulhati CM. Needed: Closer scrutiny of clinical trials. Indian J Med Ethics. 2004;1:4–5. doi: 10.20529/IJME.2004.002. [DOI] [PubMed] [Google Scholar]
- 11.Ruckmani A, Vishaly S, Arunkumar R, Prabhu L, Priya A. Assessment of barriers in subject recruitment for clinical trials. J Clin Res Bioeth. 2012;3:125. [doi4172/2155-9627.1000125] [Google Scholar]
- 12.Shah JY, Phadtare A, Rajgor D, Vaghasia M, Pradhan S, Zelko H, et al. What leads Indians to participate in clinical trials? A meta-analysis of qualitative studies. PLoS One. 2010;5:e10730. doi: 10.1371/journal.pone.0010730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel M, Doku V, Tennakoon L. Challenges in recruitment of research participants. Adv Psychiatr Treat. 2003;9:229–38. [Google Scholar]
- 14.Fletcher B, Gheorghe A, Moore D, Wilson S, Damery S. Improving the recruitment activity of clinicians in randomised controlled trials: A systematic review. BMJ Open. 2012;2:e000496. doi: 10.1136/bmjopen-2011-000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan J. Subject recruitment and retention: Barriers to success. (PLCO) cancer screening trial. Appl Clin Trials. 2004. [Last cited on 2015 Mar 03]. Available from: http://www.appliedclinicaltrialsonline.com/subject-recruitment-and-retention-barriers-success .
- 16.Bower P, Brueton V, Gamble C, Treweek S, Smith CT, Young B, et al. Interventions to improve recruitment and retention in clinical trials: A survey and workshop to assess current practice and future priorities. Trials. 2014;15:399. doi: 10.1186/1745-6215-15-399. [DOI] [PMC free article] [PubMed] [Google Scholar]


