Abstract
A fixed drug eruption (FDE) is a cutaneous adverse drug reaction due to Type IV or delayed cell-mediated hypersensitivity. Antihistamines, which antagonize the action of histamine during an allergic reaction by blocking the H1 histamine receptors, are used routinely for the treatment of various allergic disorders such as urticaria, eczemas, and also in itchy lesions of skin like scabies. Levocetirizine, an active (R)-enantiomer of cetirizine, is a newer or second generation antihistamine, with more specific actions and fewer side effects, including cutaneous reactions. FDE due to levocetirizine as well as with cetirizine are rare. We report a case of levocetirizine induced FDE in a 49-year-old male patient with scabies. The patient had a history of cetirizine induced FDE in the past.
Key words: Anti-histamines, cetirizine, cutaneous reactions
INTRODUCTION
Fixed drug eruption (FDE) is characterized by the development of one or more annular or oval erythematous plaques, more commonly on the lips or genitalia. The hip, lower back and proximal extremities are also common sites for the initial lesion of an FDE, which may be solitary. These lesions typically resolve spontaneously after discontinuation of the causative drug but leave a residual hyperpigmentation at the reaction site(s).[1] Repeated or continued exposure to the suspected drug might cause the development of new lesions along with enhancement of the older hyperpigmented lesions. The etiology of FDEs is still not completely understood. CD8 T cells retained in the lesions serving as an immunological memory may get activated on re-challenge. Persistent expression of intercellular adhesion molecule 1by an abnormal subpopulation of keratinocytes facilitates the adhesion of lymphocytes that express lymphocyte-associated antigen1, which in turn liberates lymphokines, causing damage to epidermal cell layer. Oxidative stress parameters such as reduced glutathione, malondialdehyde, and inhibition of leukocyte migration have also been significantly noted in FDE.[2] Drugs such as ibuprofen, sulfonamides, naproxen, and tetracyclines are the most common among more than 100 drugs that have been implicated to cause FDEs.[1] Older, as well as newer generation antihistamines, have also been reported to cause FDEs.[3,4] Although rare, a few cases of FDE with cetirizine,[4] levocetirizine.[5,6] have also been reported. There are also reports of FDE in the same patient due to Levocetirizine as well as cetirizine or other piperazine derivatives,[7,8,9] but such reactions appeared after intentional re-challenge or patchtesting in patients who presented with an FDE with levocetirizine/cetirizine. We present a case of FDE in a patient with a history of FDE with cetirizine, who was inadvertently given levocetirizine.
CASE REPORT
A 49-year-old male patient, a known case of scabies with prior treatment with topical permethrin and oral cetirizine, presented at the Department of Community Family Medicine, AIIMS, Bhopal. He was prescribed tablet vermact (ivermectin) 12 mg stat, tablet Rantac (ranitidine) 150 mg BD (twice a day), and tablet levorid (levocetirizine) 5 mg OD (once a day) for 3 days. He started the first two drugs on the same day without any complications. Levocetirizine was started 1 day later. About 1h after taking levocetirizine, the patient experienced several bullae formations with generalized itching and multiple erythematous macules on the left lower leg [Figure 1a], right sole [Figure 1b], chest, penis, and middle finger right hand. The patient has a history of similar lesions at same sites with cetirizine 1month before current presentation. Cetirizine also had been prescribed for scabies in a government hospital before presentation at AIIMS, Bhopal. Levocetirizine was stopped and tablet Dazit (desloratidine) 5 mg OD, along with Clob (clobetasol propionate) cream, for topical application twice a day on erupted areas of the skin, was prescribed to treat the reaction. The lesions resolved with a faint lingering hyper-pigmentation after 7 days of withdrawal of levocetirizine along with the above treatment. De-challenge was thus satisfactory.
Figure 1.
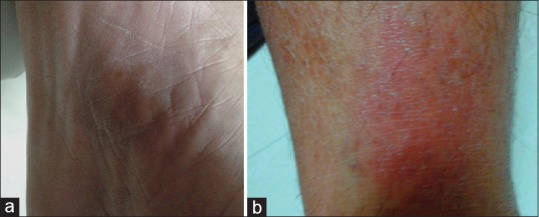
Fixed drug eruption with levocetirizine: Reddish erythematous macular patches on right sole (a) and left lower leg (b) (column width)
The adverse drug reaction (ADR) has been reported to the Pharmacovigilance Programme of India through in-house ADR Monitoring Centre. (Worldwide Unique Number at Uppsala Monitoring Center: 201538822). Using the World Health Organization causality assessment criteria, causality was determined as certain with levocetirizine by the causality assessment committee of the concerned ADR Monitoring Centre.
DISCUSSION
Levocetirizine is generally considered as a safe and effective drug for different allergic diseases such as allergic rhinitis and chronic urticaria. Paradoxically, there have been several reports of hypersensitivity reactions, including FDEs, due to levocetirizine, cetirizine and other antihistamines of the piperazine group.[4,5,6,7,8,9] However, while there are reports of FDE with levocetirizine and cetirizine in the same patient,[7,8,9] these patients were deliberately re-challenged by oral route or by patch-testing with levocetirizine or cetirizine. On the other hand, in our patient levocetirizine was used therapeutically in a patient with a history of cetirizine-induced FDE and led to a similar reaction. Levocetirizine is the R-isomer of cetirizine dihydrochloride and has two times more affinity for the histamine H1 -receptor than cetirizine.[10]
FDEs usually appear as erythematous, pruritic macules, which may occur due to systemic exposure to a causative drug. These lesions typically resolve spontaneously after discontinuation of causative drug but recur on re-administration of the causative agent. FDEs are CD8+ T cells mediated classical delayed-type hypersensitivity reactions.[11] In the present case, the patient developed levocetirizine induced FDE. Oral re-challenge test is the most reliable method for diagnosis of an FDE. However, since the lesions after levocetirizine reappeared on the same sites as those with a chemically similar drug, that is cetirizine, a diagnosis of levocetirizine induced FDE can be made without re-challenge. Further, it is recommended that a patient with history of hypersensitivity to a certain antihistamine should be expected to cross-react with other antihistamines of the same chemical class. Antihistamines with least structural resemblance to the offending agent should be used in such a patient.
Financial support and sponsorship
Pharmacovigilance Program of India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We are grateful to Dr. Sanjeev Kumar; Department of Community and Family Medicine for his contribution by reporting this adverse drug reaction; and Mr. Imran Alam, Department of Pharmacology AIIMS Bhopal for his technical support.
REFERENCES
- 1.Kauppinen K, Stubb S. Fixed eruptions: Causative drugs and challenge tests. Br J Dermatol. 1985;112:575–8. doi: 10.1111/j.1365-2133.1985.tb15266.x. [DOI] [PubMed] [Google Scholar]
- 2.Sehgal VN, Verma P, Bhattacharya SN. Pathophysiology of adverse cutaneous drug reactions - Applied perceptions: Part II. Skinmed. 2012;10:373–83. [PubMed] [Google Scholar]
- 3.Pionetti CH, Kien MC, Alonso A. Fixed drug eruption due to loratadine. Allergol Immunopathol (Madr) 2003;31:291–3. doi: 10.1016/s0301-0546(03)79199-x. [DOI] [PubMed] [Google Scholar]
- 4.Assouère MN, Mazereeuw-Hautier J, Bonafé JL. Cutaneous drug eruption with two antihistaminic drugs of a same chemical family: Cetirizine and hydroxyzine. Ann Dermatol Venereol. 2002;129:1295–8. [PubMed] [Google Scholar]
- 5.Kataria G, Saxena A, Sharma S. Levocetirizine induced fixed drug eruption: A rare case report. Int J Sci Study. 2014;2:228–9. [Google Scholar]
- 6.Mahajan VK, Sharma NL, Sharma VC. Fixed drug eruption: A novel side-effect of levocetirizine. Int J Dermatol. 2005;44:796–8. doi: 10.1111/j.1365-4632.2004.02454.x. [DOI] [PubMed] [Google Scholar]
- 7.Gupta LK, Agarwal N, Khare AK, Mittal A. Fixed drug eruption to levocetirizine and cetirizine. Indian J Dermatol. 2014;59:411–3. doi: 10.4103/0019-5154.135507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim MY, Jo EJ, Chang YS, Cho SH, Min KU, Kim SH. A case of levocetirizine-induced fixed drug eruption and cross-reaction with piperazine derivatives. Asia Pac Allergy. 2013;3:281–4. doi: 10.5415/apallergy.2013.3.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cravo M, Gonçalo M, Figueiredo A. Fixed drug eruption to cetirizine with positive lesional patch tests to the three piperazine derivatives. Int J Dermatol. 2007;46:760–2. doi: 10.1111/j.1365-4632.2007.03131.x. [DOI] [PubMed] [Google Scholar]
- 10.Walsh GM. Levocetirizine: An update. Curr Med Chem. 2006;13:2711–5. doi: 10.2174/092986706778201594. [DOI] [PubMed] [Google Scholar]
- 11.Shiohara T. Fixed drug eruption: Pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol. 2009;9:316–21. doi: 10.1097/ACI.0b013e32832cda4c. [DOI] [PubMed] [Google Scholar]


