Abstract
Acute lymphoblastic leukemia (ALL) is a hematologic malignancy that predominantly occurs in children between 2 and 10 years of age. L-asparaginase is an integral component of treatment for patients with ALL and since its introduction into pediatric treatment protocols in the 1960s, survival rates in children have progressively risen to nearly 90%. Outcomes for adolescent and young adult (AYA) patients, aged 15-39 years and diagnosed with ALL, have historically been less favorable. However, recent reports suggest substantially increased survival in AYA patients treated on pediatric-inspired protocols that include a greater cumulative dose of asparaginase. All currently available asparaginases share the same mechanism of action - the deamination and depletion of serum asparagine levels - yet each displays a markedly different pharmacokinetic profile. Pegylated asparaginase derived from the bacterium Escherichia coli is used as first-line therapy; however, up to 30% of patients develop a treatment-limiting hypersensitivity reaction. Patients who experience a hypersensitivity reaction to an E. coli-derived asparaginase can continue treatment with Erwinia chrysanthemi asparaginase. Erwinia asparaginase is immunologically distinct from E. coli-derived asparaginases and exhibits no cross-reactivity. Studies have shown that with adequate dosing, therapeutic levels of Erwinia asparaginase activity can be achieved, and patients switched to Erwinia asparaginase due to hypersensitivity can obtain outcomes similar to patients who do not experience a hypersensitivity reaction. Therapeutic drug monitoring may be required to ensure that therapeutic levels of asparaginase activity are maintained.
Key words: Adolescent and young adult, asparaginase, Erwinia chrysanthemi, Escherichia coli, hypersensitivity, pegylated, therapeutic drug monitoring
INTRODUCTION
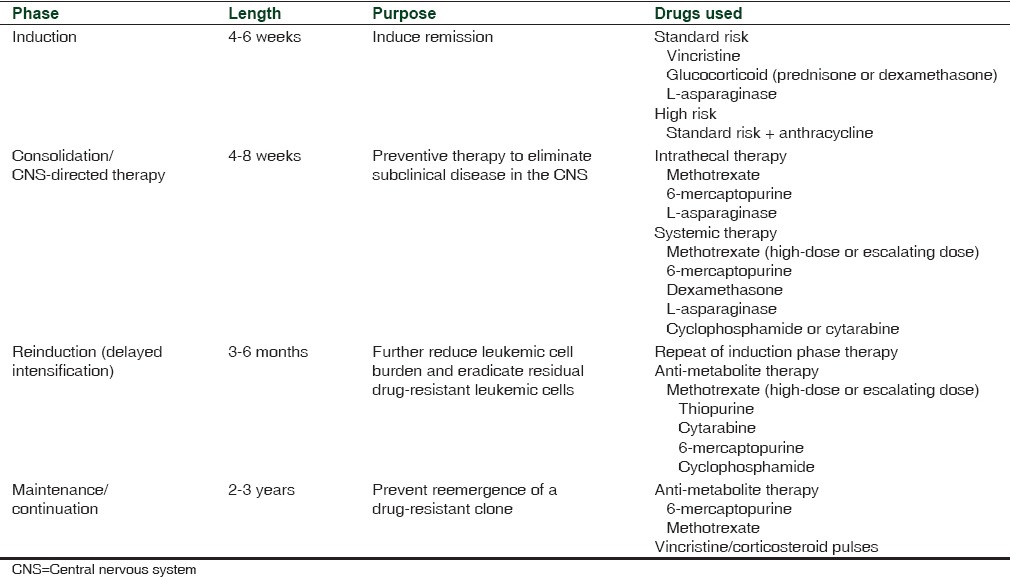
Acute lymphoblastic leukemia (ALL) is a type of cancer of lymphoid progenitor cells. The two main subtypes of ALL, as categorized by immunophenotype, are B-cell ALL and T-cell ALL.[1] The most common type of cancer in children, ALL represents approximately 25% of cancer diagnoses among children aged younger than 15 years.[2] Incidence is highest among children aged 2-3 years and declines with age and is higher in males versus females and in Whites versus Blacks/African.[2] Advances in therapy have led to a substantial improvement in the 5-year survival rate for patients aged <20 years, from 54% between 1975 and 1977 to 90% between 2004 and 2010.[2] Contemporary therapy for ALL consists of four phases of treatment; specifically remission induction, consolidation/central nervous system-directed therapy, reinduction (delayed intensification), and maintenance/continuation [Table 1].[1,3,4] Total treatment duration is about 2-3.5 years, with intensive therapy occurring over the first 6-9 months.[1,3,4]
Table 1.
HISTORY OF ASPARAGINASE TO TREAT PATIENTS WITH ACUTE LYMPHOBLASTIC LEUKEMIA
The use of asparaginase to treat patients with ALL can be traced to the discovery by Kidd[5] in 1953 that guinea pig serum regressed Gardner 6C3HED lymphosarcoma xenografts implanted subcutaneously in mice. In a series of studies, Broome[6,7,8] subsequently demonstrated that asparaginase is responsible for the anti-lymphoma effect of guinea pig serum. The anti-leukemic effect of asparaginase is due to the fact that lymphoblastic leukemia cells are unable to synthesize adequate amounts of L-asparagine (Asn) and, therefore, depend on extracellular sources. Asparaginase catalyzes the conversion of Asn to aspartic acid and ammonia, thereby depleting serum Asn and starving leukemic cells of the Asn necessary for DNA, RNA, and protein synthesis, leading ultimately to cell death.[9,10,11]
ASPARAGINASE AS PART OF FIRST-LINE THERAPY
Based on studies in the 1960s using bacteria to identify alternate sources of asparaginase,[12,13,14] clinically available asparaginase is derived from two sources, namely Escherichia coli and Erwinia chrysanthemi. Native enzyme and an enzyme derivatized by the addition of monomethoxypolyethylene glycol (pegylated) are derived from E. coli. Until December 2012, native E. coli asparaginase was available in the USA as Elspar® (Lundbeck, Deerfield, IL, USA) when it was withdrawn by the manufacturer;[15] pegylated E. coli asparaginase is available as Oncaspar® (Baxalta Incorporated, Deerfield, IL; formerly Sigma-Tau Pharmaceuticals, Inc., Gaithersburg, MD); and Erwinia asparaginase is available as Erwinaze® (Jazz Pharmaceuticals, Palo Alto, CA). Both pegylated E. coli asparaginase and Erwinia asparaginase are approved for intramuscular (IM) and intravenous (IV) administration.[16,17]
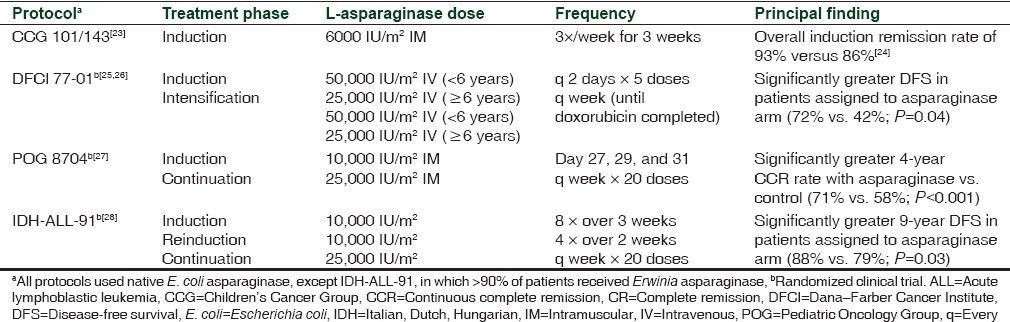
Until the discovery of asparaginase, standard chemotherapy involved the use of vincristine and prednisone to induce first remission in patients with ALL. The earliest case reports with asparaginase in humans were reported in the mid-1960s by Dolowy et al.[18] and Hill et al.[19] using asparaginase derived from guinea pig serum and highly purified asparaginase, respectively. Trials with larger populations were commenced in the early 1970s following the development of large-scale production methods.[20,21,22] Comparison of treatment protocols with/without asparaginase revealed that clinical outcome was improved with those incorporating asparaginase [Table 2].[23,24,25,26,27,28] In the Children's Cancer Group (CCG) 101/143 study,[23] the addition of native E. coli asparaginase to the treatment schedule during induction resulted in an increased percentage of patients (93%) who achieved complete remission (CR) at the end of induction compared with 86% of patients who achieved CR in the CCG 903 study[24] where only vincristine and prednisone were used. In the Dana-Farber Cancer Institute (DFCI) 77-01 trial, patients randomized to receive E. coli asparaginase during intensification had a significantly greater probability of disease-free survival (DFS) compared with those receiving treatment that did not include asparaginase (P = 0.04).[25] At a median follow-up of 9.4 years, event-free survival (EFS) was 71% versus 31% for patients treated with/without asparaginase, respectively (P = 0.03).[26] Similarly, the 4-year continuous CR rate of patients with T-cell ALL in the Pediatric Oncology Group (POG) 8704 was greater for those who also received native E. coli asparaginase during the maintenance phase.[27] In a study conducted by the Italian, Dutch, and Hungarian Pediatric Oncology Cooperative Groups, patients randomized to receive an additional 20 weeks of asparaginase (mostly Erwinia asparaginase) during continuation had a significantly greater probability of DFS at 10 years versus those who did not receive asparaginase (88% vs. 79%; P = 0.03).[28]
Table 2.
Protocols of trials showing improved efficacy with addition of L-asparaginase
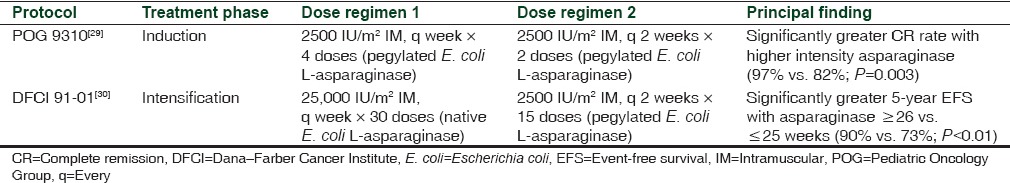
Study results also suggest the effect of asparaginase on outcome is frequency- or intensity-dependent [Table 3].[29,30] In the POG 9310 study, children with B-precursor ALL in first marrow relapse with/without concomitant extramedullary relapse had a significantly higher CR rate and an approximate eightfold lower risk of induction failure when investigators increased the frequency of pegylated asparaginase administration, 1-week versus 2-week intervals.[29] Nearly all patients (95%) in this study were previously exposed to native E. coli asparaginase during induction therapy and many patients likely developed antiasparaginase antibodies. Asparaginase clearance can increase in patients with antiasparaginase antibodies, and these patients would benefit from a more frequent dosing schedule. The DFCI ALL consortium protocol 91-01 prolonged E. coli asparaginase therapy from 20 to 30 weeks during intensification; 5-year EFS was significantly increased in children with newly diagnosed ALL compared retrospectively with previous DFCI protocols.[30] In this study, patients who tolerated ≤25 weeks of asparaginase therapy had worse EFS than those who received ≥26 weeks of the enzyme (73% vs. 90%, respectively; P < 0.01). Older children were less tolerant of more intensive therapy. The authors report that 5-year EFS was not significantly different in patients treated with pegylated asparaginase (78%; n = 106) compared with native E. coli asparaginase (84%; n = 92; P = 0.29); however, the study was not sufficiently powered to compare survival between asparaginase preparations.[30]
Table 3.
Protocols of trials showing improved efficacy with increased dose intensity of L-asparaginase
OPTIMAL DOSING OF ASPARAGINASE
The goal of asparaginase therapy is to deplete serum Asn. The relationship between the serum asparaginase activity and Asn concentration in humans has been the focus of many studies even though measurement of Asn in the presence of asparaginase has been controversial due to rapid ex vivo hydrolysis. The optimal degree and length of asparaginase depletion required for leukemic cell death are not known; however, results from several studies suggest an asparaginase activity level of 0.1 IU/mL as the target necessary to ensure adequate Asn depletion.[31,32,33,34,35] Thus, doses and schedules, for example, Berlin-Frankfurt-Münster (BFM) and Dutch Childhood Oncology Group protocols, that ensure a nadir serum asparaginase activity (NSAA) ≥0.1 IU/mL, have become standard.[36] Accordingly, a target NSAA ≥0.1 IU/mL was the primary endpoint in studies that led to the Food and Drug Administration approval of Erwinia asparaginase given IV or IM.[37,38] Several investigators have reported Asn depletion or positive outcomes in patients with asparaginase activity as low as 0.05 IU/mL, challenging the strict ≥0.1 IU/mL criterion.[36,39,40,41] Conversely, Avramis and Panosyan have proposed that asparaginase activity levels of >0.4-0.7 IU/mL are required for optimal Asn depletion.[42]
Asselin et al.[43] first recognized the clinical implications on the dosing schedule imparted by differences in the pharmacokinetics of the various preparations. Specifically, they demonstrated that the half-lives (t) of serum asparaginase activity following IM administration were 1.28, 5.73, and 0.65 days for native E. coli, pegylated E. coli, and Erwinia asparaginases, respectively; t of serum asparaginase activity correlated with the t of the protein level. The t for native E. coli (0.27-0.76 days) and Erwinia (0.31 days) asparaginases was lower with IV administration[16,17,44,45] but was independent of dose or dosing history, age, or disease-risk profile.
Results from studies comparing the effects of native E. coli and Erwinia asparaginases administered on the same schedule and dose suggested that Erwinia asparaginase is less effective. In the European Organisation for Research and Treatment of Cancer-Children's Leukemia Group (EORTC-CLG) 58881 trial, 700 patients aged <18 years were randomized to E. coli asparaginase or Erwinia asparaginase 10,000 IU/m2 IV twice weekly for 4 and 2 weeks during induction and reinduction, respectively.[46] Log-rank tests revealed that the 6-year rates of EFS and overall survival for Erwinia asparaginase versus E. coli asparaginase were 60% versus 73% (P = 0.0004) and 75% versus 84% (P = 0.002), respectively. Similarly, in the DFCI 95-01 trial, 5-year EFS was inferior with Erwinia asparaginase.[47] In this study, patients were given a single dose of E. coli asparaginase or Erwinia asparaginase 25,000 IU/m2 IM during induction and 25,000 IU/m2 IM at weekly intervals for 20 weeks during intensification. The apparent difference in efficacy between asparaginases noted in the EORTC-CLG 58881 and DFCI 95-01 trials is consistent with the differences in pharmacokinetics,[43] suggesting that the difference in efficacy is related to suboptimal dosing rather than a less effective compound.
In light of the differences in the pharmacokinetic properties of the asparaginase preparations, results from several studies suggest that dose adjustments to yield therapeutic levels of asparaginase activity are necessary if conditions dictate the need to switch between E. coli-derived and Erwinia asparaginase preparations. In a trial following the ALL/non-Hodgkin lymphoma BFM 95 protocol,[48] Erwinia asparaginase 20,000 IU/m2 given for 9 doses during reinduction resulted in a trough enzyme activity level comparable with that measured in a previous study using native E. coli (Crasnitin) asparaginase given for 4 doses of 10,000 IU/m2.[39] Albertsen et al.[49] reported that trough asparaginase activity was 1.75 IU/mL versus 0.272 IU/mL following administration of Erwinia asparaginase 30,000 IU/m2 IM daily for 10 days during induction versus E. coli asparaginase (medac) 1000 IU/m2 IM, respectively. In the Children's Oncology Group (COG) AALL07P2 study,[38] substitution of Erwinia asparaginase at a dose of 25,000 IU/m2 IM given 3 times weekly for 2 weeks for each dose of pegylated E. coli asparaginase yielded an overall median NSAA of 0.645 IU/mL at 48 h after dosing for all treatment cycles, with NSAA ≥0.1 IU/mL in 96% of all 48 h samples; the overall median NSAA at 72 h after dosing was 0.248 IU/mL during all treatment courses, with NSAA ≥0.1 IU/mL in 85% of all 72 h samples. A key finding of this study was that 80% of the evaluable patients completed all remaining courses of planned asparaginase therapy.[38] A study to evaluate the pharmacokinetics of IV administration of Erwinia asparaginase at a dose of 25,000 IU/m2 found that 83% and 43% of patients had an NSAA ≥ 0.1 IU/mL at 48 h and 72 h, respectively.[37]
HYPERSENSITIVITY TO ESCHERICHIA COLI-DERIVED ASPARAGINASE AND MAINTENANCE OF EFFICACY AFTER SUBSTITUTION WITH ERWINIA ASPARAGINASE
Hypersensitivity caused by the introduction of a foreign protein such as asparaginase is a common toxicity.[50] Of note, up to 30% of individuals develop a treatment-limiting allergic reaction to E. coli-derived asparaginase, necessitating a switch to Erwinia asparaginase.[30,51,52] Erwinia asparaginase is immunologically distinct from E. coli-derived asparaginases and, therefore, lacks immunologic cross-reactivity.[53,54] There are two patterns of hypersensitivity responses including antibody production concomitant with an overt clinical reaction and antibody production in the absence of an overt clinical reaction, referred to as “silent inactivation” or “subclinical hypersensitivity.” The presence of antiasparaginase antibodies has been documented in numerous studies, ranging in incidence from 26% to 71% of patients.[51,53,55,56,57,58,59,60,61,62,63,64,65] Results from several studies suggest that pegylated asparaginase is less immunogenic than the native E. coli enzyme[33,66,67,68] and patients who develop antibodies are more likely to suffer an allergic reaction.[51,56,57,58,62,64,69]
The development of a hypersensitivity reaction and/or the production of anti-asparaginase antibodies can have a significant impact on t, the serum levels of asparaginase protein and activity, and consequently, clinical outcome.[29,33,43,53,57,59,60,61,62,64,70,71] Several studies have demonstrated that switching patients who develop hypersensitivity to E. coli-derived asparaginase to Erwinia asparaginase, at a dose level adequate to maintain Asn depletion, yields clinical outcomes equivalent to patients who never experienced a hypersensitivity reaction.[51,59,72] In the DFCI ALL consortium protocol 00-01, children with newly diagnosed ALL who developed hypersensitivity to native E. coli asparaginase were switched to treatment with twice-weekly IM Erwinia asparaginase at a dose of 25,000 IU/m2.[72] Measurements of asparaginase activity showed that 89% of Erwinia patients had at least one trough asparaginase activity level > 0.1. Importantly, the investigators showed that patients who switched to Erwinia asparaginase due to hypersensitivity to native E. coli asparaginase showed similar EFS at 5.4 years compared with patients who never developed hypersensitivity (86.5% vs. 81.3%, respectively; P = 0.55).[72] Similarly, recently reported data from CCG-1961 show that 5-year EFS was similar in patients who were able to tolerate pegylated E. coli asparaginase throughout postinduction compared with patients who displayed clinical hypersensitivity and were switched to Erwinia asparaginase (80.8% vs 81.6%, respectively; P = 0.66).[73] In the St. Jude Children's Research Hospital front-line protocol XIII-HR, Woo et al.[51] noted no difference in the 4-year EFS rate between patients who developed hypersensitivity and were switched to Erwinia asparaginase compared with patients who did not develop hypersensitivity and continued treatment with E. coli asparaginase (82% vs. 78%, respectively; P = 0.68).
In a prospective drug-monitoring study by Tong et al., patients who developed silent inactivation or allergy to pegylated E. coli asparaginase were given Erwinia asparaginase 20,000 IU/m2 3 times weekly for up to 30 weeks during intensification.[74] Approximately 96% of the patients had at least one NSAA level ≥0.1 IU/mL during the first 2 weeks, and all patients had at least one NSAA level ≥0.1 IU/mL; thereafter, 47% of the patients had all 48 h samples ≥0.1 IU/mL from week 6 to 30.
Erwinia asparaginase is indicated for those patients who have developed hypersensitivity to native or pegylated E. coli asparaginase.[17] A treatment algorithm created by Bleyer et al.[75] proposes that if a reaction is suspected to have occurred after infusion of pegylated E. coli asparaginase, serum should be collected after 4-7 days if the full dose was given, or earlier for an incomplete dose. Based on the finding of Rizzari et al.,[40] the algorithm recommends switching to Erwinia asparaginase if the serum NSAA is <0.05 IU/mL. Of note, NSAA put forth in this algorithm is below the generally accepted threshold of 0.1 IU/mL as an index of asparaginase efficacy in pediatric patients,[36] and others have suggested a target level of 0.05 IU/mL to be inadequate. Specifically, results from two studies of adolescent and young adult (AYA) and adult patients with newly diagnosed ALL suggest that minimal serum asparaginase activity levels of 0.2 IU/mL[76] and 0.4 IU/mL[77] are associated with significant Asn depletion. Moreover, in the study by Angiolillo et al.,[77] Asn began to rebound after asparaginase activity fell below 0.4 IU/mL.
THERAPEUTIC DRUG MONITORING
The wide interpatient variability with respect to trough asparaginase activity levels in serum, the development of subclinical hypersensitivity, and differences in the pharmacokinetic properties among the different asparaginase preparations have underscored the need for therapeutic drug monitoring (TDM). The importance of TDM has been highlighted in several studies. In a study following the ALL-BFM 2000 protocol, children were given eight and four doses of native E. coli asparaginase (medac or Crasnitin) 10,000 IU/m2 IV during induction and reinduction, respectively; patients were switched to Erwinia asparaginase if they developed an allergy or untoward reaction.[39] During induction, median trough asparaginase activity was higher and t was longer with medac versus Crasnitin. During reinduction, the rank order of median trough asparaginase activity was medac (0.528 IU/mL), Crasnitin (0.049 IU/mL), and Erwinia asparaginase (<0.02 IU/mL). Boos et al., therefore, concluded that monitoring is necessary to ensure efficacy targets are reached following substitution of therapy due to an allergic reaction.[39] In their study to assess the pharmacokinetics of IV administration of Erwinia asparaginase, Vrooman et al. concluded that every 48 h dosing should be evaluated, given that NSAA ≥0.1 IU/mL was achieved in 83% of patients after 48 h versus 43% of patients after 72 h.[37] By monitoring patients given native E. coli asparaginase 5000 or 10,000 IU/m2, pegylated E. coli asparaginase 1000 IU/m2, or Erwinia asparaginase 10,000 IU/m2 according to the ALL-BFM 2000 protocol, Schrey et al. found that the wide range of serum asparaginase activity regardless of the asparaginase preparation highlighted the need for TDM.[78]
Results from a recent study to evaluate the relative tolerability and efficacy of fixed versus individualized dosing suggest that individual dosing may be an effective strategy to improve outcome.[79] Patients treated according to the DFCI ALL consortium protocol 00-01 were given 30 weekly doses of E. coli asparaginase either at a fixed dose of 25,000 IU/m2 IM or an individualized dose, based on monitoring of the NSAA, starting at 12,500 IU/m2, and adjusted to maintain NSAA between 0.10 and 0.14 IU/mL.[79] Clinical outcomes were superior with individualized dosing compared with fixed dosing: Fewer relapses (9% vs. 15%, respectively) and significantly greater overall 5-year EFS (90% vs. 82%, respectively; P = 0.04). Moreover, 5-year EFS was 95% in patients placed on individualized dosing, but who were switched to another preparation because of silent inactivation compared with 76% for those in the FD arm with NSAA <0.1 IU/mL and never switched.[79] These results suggest that individualized dosing may improve clinical outcome by monitoring asparaginase activity and prospectively identifying subclinical hypersensitivity.
SPECIAL POPULATION: OPTIMAL TREATMENT PARADIGM IN ADOLESCENT AND YOUNG ADULTS
Despite the significant advances made in the treatment of children with ALL, the outcome for AYAs, defined by the National Cancer Institute as patients aged 15-39 years,[2] has historically been considerably less favorable. A period analysis of trends in 5-year survival based on the US National Cancer Institute Surveillance, Epidemiology and End Results 9 registry has shown that relative survival in children aged younger than 15 years increased from 80% between 1990 and 1994 to 88% between 2000 and 2004.[80] The 5-year survival for adolescents aged 15-19 years also increased during that time, but from 41% to 61%, respectively; lesser but significant improvements were seen for older age groups as well.[81] Similarly, an analysis of 21,626 patients aged 0-22 years enrolled in the COG ALL clinical trials showed that the 5-year survival rate was 91% for children aged younger than 15 years and 75% for those aged 15-19 years in 2000-2005.[82]
The reasons for the disparity in outcome are multifactorial and not completely understood. Adult patients have a poorer tolerance to intensive chemotherapy involving asparaginase. Evidence of increased toxicity in adults was noted as early as 1970 by Oettgen et al.[55] As a result of using different treatment protocols, there is an abrupt drop in the 5-year survival/age relationship at the age at which pediatric versus adult therapy regimens are administered.[83]
Retrospective comparisons have consistently shown that clinical outcome is improved in AYAs treated with pediatric versus adult treatment protocols [Table 4].[84,85,86,87] Prospective studies have shown that clinical outcome is improved in AYAs treated with pediatric or pediatric-inspired protocols [Table 5][88,89,90,91,92,93] when compared with historical controls of patients treated on adult protocols.[94,95] A feature common to the pediatric protocols was the higher cumulative dose of asparaginase, as well as that of glucocorticoid and vincristine, compared with the adult protocols. The asparaginase-free regimen comprising hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD) has been widely used to treat AYA patients.[96,97,98,99,100] Although most retrospective studies have documented inferior survival rates in AYA patients treated with hyper-CVAD versus pediatric-inspired protocols,[101,102] Rytting et al.[103] found that the 3-year OS was 71% and 74% for AYA patients treated with hyper-CVAD and an augmented BFM protocol, respectively.
Table 4.
Trials involving adolescent and young adult patients with acute lymphoblastic leukemia treated with pediatric versus adult protocols
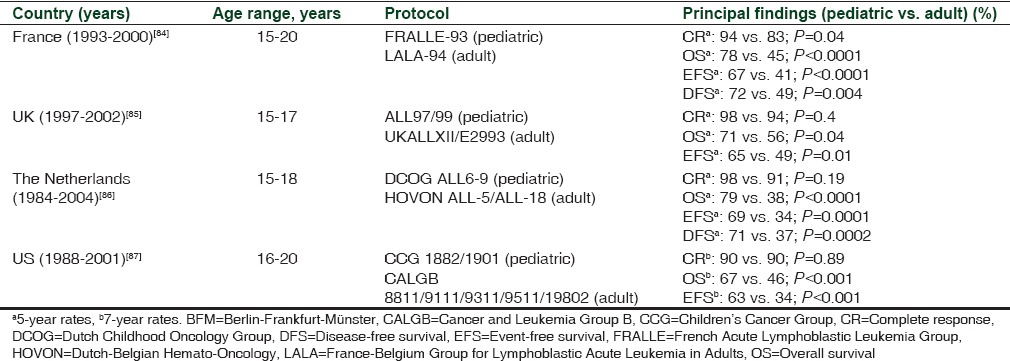
Table 5.
Trials involving adolescent and young adult patients with acute lymphoblastic leukemia treated with pediatric or pediatric-inspired protocols
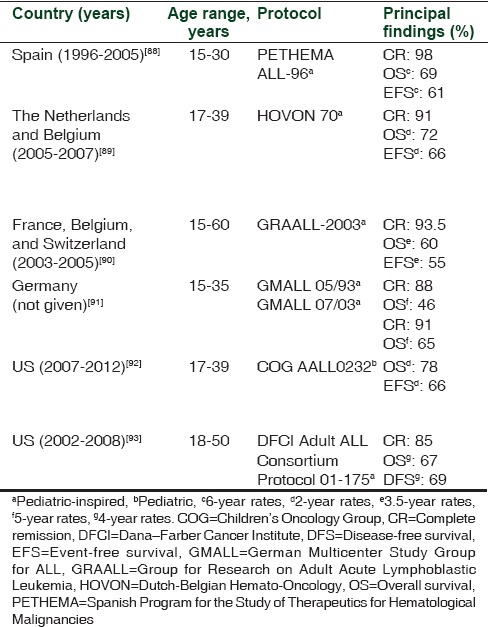
As described, pediatric protocols are distinguished by the use of intensive asparaginase, vincristine, and glucocorticoid therapies. A meta-analysis of total therapy studies XIIIA, XIIIB, XIV, and XV revealed that despite the more intensive treatment regimen, the incidence of asparaginase-related allergy was not higher in patients aged 15-18 years treated with any of these protocols versus those aged 1-14 years; conversely, the incidences of thromboembolic complications, pancreatitis, osteonecrosis, and hyperglycemia were greater in the older patients.[104] Central venous thrombosis (CVT) is a potentially life-threatening event that has been reported in a minority of patients receiving asparaginase and corticosteroids.[105,106] Early monitoring and detection of CVT are critical to ensure positive outcomes with anticoagulation therapy.[105,106]
In a compassionate-use trial with patients switched to Erwinia asparaginase after developing a hypersensitivity reaction to native E. coli or pegylated E. coli asparaginase, Plourde et al.[107] found that the safety profile in 147 patients aged ≥16-<40 years was consistent overall with that of the full trial population. In this trial, Erwinia asparaginase was given at a dose of 25,000 IU/m2 IM 3 times/week for 2 weeks for each dose of pegylated E. coli asparaginase remaining or 1:1 for each dose of native E. coli asparaginase remaining. In addition, in the USA intergroup study C10403, the largest prospective study to date to assess the feasibility of pediatric protocols in AYAs, toxicities were manageable in AYA patients treated using the COG AALL0232 regimen administered by adult hematologists/oncologists; 2-year OS and EFS rates were 78% and 66%, respectively.[108]
SUMMARY
Following the seminal discovery by Kidd in 1953,[5] asparaginase has been a mainstay of pediatric chemotherapy protocols to treat patients with ALL. Since the incorporation of asparaginase into treatment protocols, clinical outcomes have improved significantly, with an NSAA ≥0.1 IU/mL widely accepted as the therapeutic level necessary to achieve efficacy. Pegylated E. coli asparaginase remains first-line treatment, but the occurrence of an allergic reaction necessitates a switch to Erwinia asparaginase. Studies have shown that substitution of Erwinia asparaginase for E. coli-derived asparaginase following an allergic reaction and/or silent inactivation is an effective therapeutic option to complete the treatment protocol as planned. The use of pediatric-inspired protocols has been shown to improve outcome in the AYA population with an acceptable safety profile. TDM may also improve clinical outcome by prospectively identifying patients who develop subclinical hypersensitivity.
Financial support and sponsorship
This work was supported by Jazz Pharmaceuticals.
Conflicts of interest
Rachel A. Egler has nothing to disclose. Sanjay P. Ahuja has received honoraria from Bayer and Biogen Inc., served as a consultant for Bayer and Biogen Inc., and served on the speakers’ bureau for Biogen Inc., Novo Nordisk Inc., and Grifols. Yousif Matloub has served as a consultant for Novartis AG, has received honoraria from Novartis AG and Jazz Pharmaceuticals, and owns stock in Amgen Inc.
Acknowledgments
Yousif Matloub is the Angie Fowler Chair of Adolescent and Young Adult Cancer. The authors would like to acknowledge and thank Melissa Makii, PharmD, BCPS, Clinical Pharmacy Specialist, for her insightful contributions in the writing and reviewing of this manuscript. We would also like to thank Gerard D’Angelo, PhD, of The Curry Rockefeller Group, LLC, Tarrytown, NY, who provided editorial assistance that was supported by Jazz Pharmaceuticals.
REFERENCES
- 1.Gaynon P, Schrappe M. Childhood ALL. In: Cairo M, Perkins S, editors. Hematological Malignancies in Children, Adolescents and Young Adults. Hackensack, NJ: World Scientific Publishing Company; 2012. pp. 197–235. [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, et al., editors. SEER Cancer Statistics Review, 1975-2011. Bethesda, MD: National Cancer Institute; 2014. National Cancer Institute. Section 28: Childhood cancer by site, incidence, survival, and mortality. [Google Scholar]
- 3.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–78. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 4.Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–43. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 5.Kidd JG. Regression of transplanted lymphomas induced in vivo by means of normal guinea pig serum. I. Course of transplanted cancers of various kinds in mice and rats given guinea pig serum, horse serum, or rabbit serum. J Exp Med. 1953;98:565–82. doi: 10.1084/jem.98.6.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broome JD. Evidence that L-asparaginase activity of guinea pig serum is responsible for its antilymphoma effects. Nature. 1961;191:1114–5. doi: 10.1084/jem.118.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broome JD. Evidence that the L-asparaginase of guinea pig serum is responsible for its antilymphoma effects. I. Properties of the L-asparaginase of guinea pig serum in relation to those of the antilymphoma substance. J Exp Med. 1963;118:99–120. doi: 10.1084/jem.118.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broome JD. Evidence that the L-asparaginase of guinea pig serum is responsible for its antilymphoma effects. II. Lymphoma 6C3HED cells cultured in a medium devoid of L-asparagine lose their susceptibility to the effects of guinea pig serum in vivo. J Exp Med. 1963;118:121–48. doi: 10.1084/jem.118.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho DH, Whitecar JP, Jr, Luce JK, Frei E., 3rd L-asparagine requirement and the effect of L-asparaginase on the normal and leukemic human bone marrow. Cancer Res. 1970;30:466–72. [PubMed] [Google Scholar]
- 10.Onuma T, Waligunda J, Holland JF. Amino acid requirements in vitro of human leukemic cells. Cancer Res. 1971;31:1640–4. [PubMed] [Google Scholar]
- 11.Sobin LH, Kidd JG. A metabolic difference between two lines of lymphoma 6C3hed cells in relation to asparagine. Proc Soc Exp Biol Med. 1965;119:325–7. doi: 10.3181/00379727-119-30169. [DOI] [PubMed] [Google Scholar]
- 12.Wade HE, Elsworth R, Herbert D, Keppie J, Sargeant K. A new L-asparaginase with antitumour activity? Lancet. 1968;2:776–7. doi: 10.1016/s0140-6736(68)90977-x. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz JH, Reeves JY, Broome JD. Two L-asparaginases from E. coli and their action against tumors. Proc Natl Acad Sci U S A. 1966;56:1516–9. doi: 10.1073/pnas.56.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyse EA, Old LJ, Campbell HA, Mashburn LT. Suppression of murine leukemias by L-asparaginase. Incidence of sensitivity among leukemias of various types: Comparative inhibitory activities of guinea pig serum L-asparaginase and Escherichia coli L-asparaginase. J Exp Med. 1967;125:17–31. doi: 10.1084/jem.125.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keding R. Discontinuation of Elspar®, (asparaginase for injection) 10,000 IU. Deerfield, IL: Lundbeck LLC; 2012. [Last accessed on 2015 Jul 30]. Available from: http://www.fda.gov/downloads/Drugs/DrugSafety/DrugShortages/UCM321556.pdf . [Google Scholar]
- 16.Prescribing information. Deerfield, IL: Baxalta, Incorporated (formerly Gaithersburg, MD: Sigma Tau Pharmaceuticals, Inc.); 2015. Oncaspar® (pegaspargase) [Google Scholar]
- 17.Prescribing Information. Palo Alto, CA: Jazz Pharmaceuticals; 2014. Erwinaze® (asparaginase Erwinia chrysanthemi) [Google Scholar]
- 18.Dolowy WC, Henson D, Cornet J, Sellin H. Toxic and antineoplastic effects of L-asparaginase. Study of mice with lymphoma and normal monkeys and report on a child with leukemia. Cancer. 1966;19:1813–9. doi: 10.1002/1097-0142(196612)19:12<1813::aid-cncr2820191208>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 19.Hill JM, Roberts J, Loeb E, Khan A, MacLellan A, Hill RW. L-asparaginase therapy for leukemia and other malignant neoplasms. Remission in human leukemia. JAMA. 1967;202:882–8. [PubMed] [Google Scholar]
- 20.Tallal L, Tan C, Oettgen H, Wollner N, McCarthy M, Helson L, et al. E. coli L-asparaginase in the treatment of leukemia and solid tumors in 131 children. Cancer. 1970;25:306–20. doi: 10.1002/1097-0142(197002)25:2<306::aid-cncr2820250206>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 21.Clarkson B, Krakoff I, Burchenal J, Karnofsky D, Golbey R, Dowling M, et al. Clinical results of treatment with E. coli L-asparaginase in adults with leukemia, lymphoma, and solid tumors. Cancer. 1970;25:279–305. doi: 10.1002/1097-0142(197002)25:2<279::aid-cncr2820250205>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Sutow WW, Garcia F, Starling KA, Williams TE, Lane DM, Gehan EA. L-asparaginase therapy in children with advanced leukemia. The Southwest Cancer Chemotherapy Study Group. Cancer. 1971;28:819–24. doi: 10.1002/1097-0142(1971)28:4<819::aid-cncr2820280403>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Ortega JA, Nesbit ME, Jr, Donaldson MH, Hittle RE, Weiner J, Karon M, et al. L-Asparaginase, vincristine, and prednisone for induction of first remission in acute lymphocytic leukemia. Cancer Res. 1977;37:535–40. [PubMed] [Google Scholar]
- 24.Heyn RM, Joo P, Karon M, Nesbit M, Shore N, Breslow N, et al. BCG in the treatment of acute lymphocytic leukemia. Blood. 1975;46:431–42. [PubMed] [Google Scholar]
- 25.Sallan SE, Hitchcock-Bryan S, Gelber R, Cassady JR, Frei E, 3rd, Nathan DG. Influence of intensive asparaginase in the treatment of childhood non-T-cell acute lymphoblastic leukemia. Cancer Res. 1983;43:5601–7. [PubMed] [Google Scholar]
- 26.Sallan SE, Gelber RD, Kimball V, Donnelly M, Cohen HJ. More is better! Update of Dana-Farber Cancer Institute/Children's Hospital childhood acute lymphoblastic leukemia trials. Haematol Blood Transfus. 1990;33:459–66. doi: 10.1007/978-3-642-74643-7_83. [DOI] [PubMed] [Google Scholar]
- 27.Amylon MD, Shuster J, Pullen J, Berard C, Link MP, Wharam M, et al. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: A Pediatric Oncology Group study. Leukemia. 1999;13:335–42. doi: 10.1038/sj.leu.2401310. [DOI] [PubMed] [Google Scholar]
- 28.Pession A, Valsecchi MG, Masera G, Kamps WA, Magyarosy E, Rizzari C, et al. Long-term results of a randomized trial on extended use of high dose L-asparaginase for standard risk childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:7161–7. doi: 10.1200/JCO.2005.11.411. [DOI] [PubMed] [Google Scholar]
- 29.Abshire TC, Pollock BH, Billett AL, Bradley P, Buchanan GR. Weekly polyethylene glycol conjugated L-asparaginase compared with biweekly dosing produces superior induction remission rates in childhood relapsed acute lymphoblastic leukemia: A Pediatric Oncology Group Study. Blood. 2000;96:1709–15. [PubMed] [Google Scholar]
- 30.Silverman LB, Gelber RD, Dalton VK, Asselin BL, Barr RD, Clavell LA, et al. Improved outcome for children with acute lymphoblastic leukemia: Results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97:1211–8. doi: 10.1182/blood.v97.5.1211. [DOI] [PubMed] [Google Scholar]
- 31.Riccardi R, Holcenberg JS, Glaubiger DL, Wood JH, Poplack DG. L-asparaginase pharmacokinetics and asparagine levels in cerebrospinal fluid of rhesus monkeys and humans. Cancer Res. 1981;41(11 Pt 1):4554–8. [PubMed] [Google Scholar]
- 32.Ahlke E, Nowak-Göttl U, Schulze-Westhoff P, Werber G, Börste H, Würthwein G, et al. Dose reduction of asparaginase under pharmacokinetic and pharmacodynamic control during induction therapy in children with acute lymphoblastic leukaemia. Br J Haematol. 1997;96:675–81. doi: 10.1046/j.1365-2141.1997.d01-2089.x. [DOI] [PubMed] [Google Scholar]
- 33.Avramis VI, Sencer S, Periclou AP, Sather H, Bostrom BC, Cohen LJ, et al. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: A Children's Cancer Group study. Blood. 2002;99:1986–94. doi: 10.1182/blood.v99.6.1986. [DOI] [PubMed] [Google Scholar]
- 34.Müller HJ, Löning L, Horn A, Schwabe D, Gunkel M, Schrappe M, et al. Pegylated asparaginase (Oncaspar) in children with ALL: Drug monitoring in reinduction according to the ALL/NHL-BFM 95 protocols. Br J Haematol. 2000;110:379–84. doi: 10.1046/j.1365-2141.2000.02187.x. [DOI] [PubMed] [Google Scholar]
- 35.Albertsen BK, Schrøder H, Jakobsen P, Müller HJ, Carlsen NT, Schmiegelow K. Monitoring of Erwinia asparaginase therapy in childhood ALL in the Nordic countries. Br J Clin Pharmacol. 2001;52:433–7. doi: 10.1046/j.0306-5251.2001.01464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pieters R, Hunger SP, Boos J, Rizzari C, Silverman L, Baruchel A, et al. L-asparaginase treatment in acute lymphoblastic leukemia: A focus on Erwinia asparaginase. Cancer. 2011;117:238–49. doi: 10.1002/cncr.25489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vrooman LM, Kirov II, Dreyer ZE, Kelly M, Hijiya N, Brown P, et al. Activity and toxicity of intravenous Erwinia asparaginase following allergy to E. coli-derived asparaginase in children and adolescents with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2016;63:228–33. doi: 10.1002/pbc.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salzer WL, Asselin B, Supko JG, Devidas M, Kaiser NA, Plourde P, et al. Erwinia asparaginase achieves therapeutic activity after pegaspargase allergy: A report from the Children's Oncology Group. Blood. 2013;122:507–14. doi: 10.1182/blood-2013-01-480822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boos J, Werber G, Ahlke E, Schulze-Westhoff P, Nowak-Göttl U, Würthwein G, et al. Monitoring of asparaginase activity and asparagine levels in children on different asparaginase preparations. Eur J Cancer. 1996;32A:1544–50. doi: 10.1016/0959-8049(96)00131-1. [DOI] [PubMed] [Google Scholar]
- 40.Rizzari C, Zucchetti M, Conter V, Diomede L, Bruno A, Gavazzi L, et al. L-asparagine depletion and L-asparaginase activity in children with acute lymphoblastic leukemia receiving i.m. or i.v. Erwinia C. or E. coli L-asparaginase as first exposure. Ann Oncol. 2000;11:189–93. doi: 10.1023/a:1008368916800. [DOI] [PubMed] [Google Scholar]
- 41.Appel IM, Kazemier KM, Boos J, Lanvers C, Huijmans J, Veerman AJ, et al. Pharmacokinetic, pharmacodynamic and intracellular effects of PEG-asparaginase in newly diagnosed childhood acute lymphoblastic leukemia: Results from a single agent window study. Leukemia. 2008;22:1665–79. doi: 10.1038/leu.2008.165. [DOI] [PubMed] [Google Scholar]
- 42.Avramis VI, Panosyan EH. Pharmacokinetic/pharmacodynamic relationships of asparaginase formulations: The past, the present and recommendations for the future. Clin Pharmacokinet. 2005;44:367–93. doi: 10.2165/00003088-200544040-00003. [DOI] [PubMed] [Google Scholar]
- 43.Asselin BL, Whitin JC, Coppola DJ, Rupp IP, Sallan SE, Cohen HJ. Comparative pharmacokinetic studies of three asparaginase preparations. J Clin Oncol. 1993;11:1780–6. doi: 10.1200/JCO.1993.11.9.1780. [DOI] [PubMed] [Google Scholar]
- 44.Ho DH, Yap HY, Brown N, Benjamin RS, Friereich EJ, Blumenschein GR, et al. Clinical pharmacology of intramuscularly administered L-asparaginase. J Clin Pharmacol. 1981;21:72–8. doi: 10.1002/j.1552-4604.1981.tb01752.x. [DOI] [PubMed] [Google Scholar]
- 45.Albertsen BK, Jakobsen P, Schrøder H, Schmiegelow K, Carlsen NT. Pharmacokinetics of Erwinia asparaginase after intravenous and intramuscular administration. Cancer Chemother Pharmacol. 2001;48:77–82. doi: 10.1007/s002800100286. [DOI] [PubMed] [Google Scholar]
- 46.Duval M, Suciu S, Ferster A, Rialland X, Nelken B, Lutz P, et al. Comparison of Escherichia coli-asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: Results of a randomized European Organisation for Research and Treatment of Cancer-Children's Leukemia Group phase 3 trial. Blood. 2002;99:2734–9. doi: 10.1182/blood.v99.8.2734. [DOI] [PubMed] [Google Scholar]
- 47.Moghrabi A, Levy DE, Asselin B, Barr R, Clavell L, Hurwitz C, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood. 2007;109:896–904. doi: 10.1182/blood-2006-06-027714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vieira Pinheiro JP, Ahlke E, Nowak-Göttl U, Hempel G, Müller HJ, Lümkemann K, et al. Pharmacokinetic dose adjustment of Erwinia asparaginase in protocol II of the paediatric ALL/NHL-BFM treatment protocols. Br J Haematol. 1999;104:313–20. doi: 10.1046/j.1365-2141.1999.01192.x. [DOI] [PubMed] [Google Scholar]
- 49.Albertsen BK, Schrøder H, Ingerslev J, Jakobsen P, Avramis VI, Müller HJ, et al. Comparison of intramuscular therapy with Erwinia asparaginase and asparaginase Medac: Pharmacokinetics, pharmacodynamics, formation of antibodies and influence on the coagulation system. Br J Haematol. 2001;115:983–90. doi: 10.1046/j.1365-2141.2001.03148.x. [DOI] [PubMed] [Google Scholar]
- 50.Raetz EA, Salzer WL. Tolerability and efficacy of L-asparaginase therapy in pediatric patients with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2010;32:554–63. doi: 10.1097/MPH.0b013e3181e6f003. [DOI] [PubMed] [Google Scholar]
- 51.Woo MH, Hak LJ, Storm MC, Sandlund JT, Ribeiro RC, Rivera GK, et al. Hypersensitivity or development of antibodies to asparaginase does not impact treatment outcome of childhood acute lymphoblastic leukemia. J Clin Oncol. 2000;18:1525–32. doi: 10.1200/JCO.2000.18.7.1525. [DOI] [PubMed] [Google Scholar]
- 52.Müller HJ, Beier R, Löning L, Blütters-Sawatzki R, Dörffel W, Maass E, et al. Pharmacokinetics of native Escherichia coli asparaginase (Asparaginase medac) and hypersensitivity reactions in ALL-BFM 95 reinduction treatment. Br J Haematol. 2001;114:794–9. doi: 10.1046/j.1365-2141.2001.03009.x. [DOI] [PubMed] [Google Scholar]
- 53.Willer A, Gerss J, König T, Franke D, Kühnel HJ, Henze G, et al. Anti-Escherichia coli asparaginase antibody levels determine the activity of second-line treatment with pegylated E. coli asparaginase: A retrospective analysis within the ALL-BFM trials. Blood. 2011;118:5774–82. doi: 10.1182/blood-2011-07-367904. [DOI] [PubMed] [Google Scholar]
- 54.Wang B, Relling MV, Storm MC, Woo MH, Ribeiro R, Pui CH, et al. Evaluation of immunologic crossreaction of antiasparaginase antibodies in acute lymphoblastic leukemia (ALL) and lymphoma patients. Leukemia. 2003;17:1583–8. doi: 10.1038/sj.leu.2403011. [DOI] [PubMed] [Google Scholar]
- 55.Oettgen HF, Stephenson PA, Schwartz MK, Leeper RD, Tallai L, Tan CC, et al. Toxicity of E. coli L-asparaginase in man. Cancer. 1970;25:253–78. doi: 10.1002/1097-0142(197002)25:2<253::aid-cncr2820250204>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 56.Peterson RG, Handschumacher RE, Mitchell MS. Immunological responses to L-asparaginase. J Clin Invest. 1971;50:1080–90. doi: 10.1172/JCI106579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheung NK, Chau IY, Coccia PF. Antibody response to Escherichia coli L-asparaginase. Prognostic significance and clinical utility of antibody measurement. Am J Pediatr Hematol Oncol. 1986;8:99–104. [PubMed] [Google Scholar]
- 58.Woo MH, Hak LJ, Storm MC, Evans WE, Sandlund JT, Rivera GK, et al. Anti-asparaginase antibodies following E. coli asparaginase therapy in pediatric acute lymphoblastic leukemia. Leukemia. 1998;12:1527–33. doi: 10.1038/sj.leu.2401162. [DOI] [PubMed] [Google Scholar]
- 59.Panosyan EH, Seibel NL, Martin-Aragon S, Gaynon PS, Avramis IA, Sather H, et al. Asparaginase antibody and asparaginase activity in children with higher-risk acute lymphoblastic leukemia: Children's Cancer Group Study CCG-1961. J Pediatr Hematol Oncol. 2004;26:217–26. doi: 10.1097/00043426-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 60.Armstrong JK, Hempel G, Koling S, Chan LS, Fisher T, Meiselman HJ, et al. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer. 2007;110:103–11. doi: 10.1002/cncr.22739. [DOI] [PubMed] [Google Scholar]
- 61.Panetta JC, Gajjar A, Hijiya N, Hak LJ, Cheng C, Liu W, et al. Comparison of native E. coli and PEG asparaginase pharmacokinetics and pharmacodynamics in pediatric acute lymphoblastic leukemia. Clin Pharmacol Ther. 2009;86:651–8. doi: 10.1038/clpt.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu C, Kawedia JD, Cheng C, Pei D, Fernandez CA, Cai X, et al. Clinical utility and implications of asparaginase antibodies in acute lymphoblastic leukemia. Leukemia. 2012;26:2303–9. doi: 10.1038/leu.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zalewska-Szewczyk B, Andrzejewski W, Bodalski J. Development of anti-asparaginase antibodies in childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2004;43:600–2. doi: 10.1002/pbc.20064. [DOI] [PubMed] [Google Scholar]
- 64.Zalewska-Szewczyk B, Andrzejewski W, Mlynarski W, Jedrychowska-Danska K, Witas H, Bodalski J. The anti-asparagines antibodies correlate with L-asparagines activity and may affect clinical outcome of childhood acute lymphoblastic leukemia. Leuk Lymphoma. 2007;48:931–6. doi: 10.1080/10428190701292049. [DOI] [PubMed] [Google Scholar]
- 65.Albertsen BK, Schrøder H, Jakobsen P, Avramis VI, Müller HJ, Schmiegelow K, et al. Antibody formation during intravenous and intramuscular therapy with Erwinia asparaginase. Med Pediatr Oncol. 2002;38:310–6. doi: 10.1002/mpo.10096. [DOI] [PubMed] [Google Scholar]
- 66.Ettinger LJ, Asselin B, Poplack DG, Kurtzberg J. Toxicity profile of PEG-L-asparaginase in native - L-asparaginase hypersensitive and non-hypersensitive patients with acute lymphoblastic leukemia (ALL) Med Pediatr Oncol. 1993;21:556. [Google Scholar]
- 67.Keating MJ, Holmes R, Lerner S, Ho DH. L-asparaginase and PEG asparaginase - Past, present, and future. Leuk Lymphoma. 1993;10(Suppl):153–7. doi: 10.3109/10428199309149129. [DOI] [PubMed] [Google Scholar]
- 68.Kamisaki Y, Wada H, Yagura T, Matsushima A, Inada Y. Reduction in immunogenicity and clearance rate of Escherichia coli L-asparaginase by modification with monomethoxypolyethylene glycol. J Pharmacol Exp Ther. 1981;216:410–4. [PubMed] [Google Scholar]
- 69.Killander D, Dohlwitz A, Engstedt L, Franzén S, Gahrton G, Gullbring B, et al. Hypersensitive reactions and antibody formation during L-asparaginase treatment of children and adults with acute leukemia. Cancer. 1976;37:220–8. doi: 10.1002/1097-0142(197601)37:1<220::aid-cncr2820370132>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 70.Capizzi RL, Bertino JR, Skeel RT, Creasey WA, Zanes R, Olayon C, et al. L-asparaginase: Clinical, biochemical, pharmacological, and immunological studies. Ann Intern Med. 1971;74:893–901. doi: 10.7326/0003-4819-74-6-893. [DOI] [PubMed] [Google Scholar]
- 71.Schwartz MK, Lash ED, Oettgen HF, Tomato FA. L-asparaginase activity in plasma and other biological fluids. Cancer. 1970;25:244–52. doi: 10.1002/1097-0142(197002)25:2<244::aid-cncr2820250203>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 72.Vrooman LM, Supko JG, Neuberg DS, Asselin BL, Athale UH, Clavell L, et al. Erwinia asparaginase after allergy to E. coli asparaginase in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2010;54:199–205. doi: 10.1002/pbc.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ko RH, Jones TL, Radvinsky D, Robison N, Gaynon PS, Panosyan EH, et al. Allergic reactions and antiasparaginase antibodies in children with high-risk acute lymphoblastic leukemia: A children's oncology group report. Cancer. 2015;121:4205–11. doi: 10.1002/cncr.29641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tong WH, Pieters R, Kaspers GJ, te Loo DM, Bierings MB, van den Bos C, et al. A prospective study on drug monitoring of PEGasparaginase and Erwinia asparaginase and asparaginase antibodies in pediatric acute lymphoblastic leukemia. Blood. 2014;123:2026–33. doi: 10.1182/blood-2013-10-534347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bleyer A, Asselin BL, Koontz SE, Hunger SP. Clinical application of asparaginase activity levels following treatment with pegaspargase. Pediatr Blood Cancer. 2015;62:1102–5. doi: 10.1002/pbc.25299. [DOI] [PubMed] [Google Scholar]
- 76.Douer D, Yampolsky H, Cohen LJ, Watkins K, Levine AM, Periclou AP, et al. Pharmacodynamics and safety of intravenous pegaspargase during remission induction in adults aged 55 years or younger with newly diagnosed acute lymphoblastic leukemia. Blood. 2007;109:2744–50. doi: 10.1182/blood-2006-07-035006. [DOI] [PubMed] [Google Scholar]
- 77.Angiolillo AL, Schore RJ, Devidas M, Borowitz MJ, Carroll AJ, Gastier-Foster JM, et al. Pharmacokinetic and pharmacodynamic properties of calaspargase pegol Escherichia coli L-asparaginase in the treatment of patients with acute lymphoblastic leukemia: Results from Children's Oncology Group Study AALL07P4. J Clin Oncol. 2014;32:3874–82. doi: 10.1200/JCO.2014.55.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schrey D, Borghorst S, Lanvers-Kaminsky C, Hempel G, Gerss J, Möricke A, et al. Therapeutic drug monitoring of asparaginase in the ALL-BFM 2000 protocol between 2000 and 2007. Pediatr Blood Cancer. 2010;54:952–8. doi: 10.1002/pbc.22417. [DOI] [PubMed] [Google Scholar]
- 79.Vrooman LM, Stevenson KE, Supko JG, O’Brien J, Dahlberg SE, Asselin BL, et al. Postinduction dexamethasone and individualized dosing of Escherichia coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: Results from a randomized study - Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. J Clin Oncol. 2013;31:1202–10. doi: 10.1200/JCO.2012.43.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pulte D, Gondos A, Brenner H. Trends in 5- and 10-year survival after diagnosis with childhood hematologic malignancies in the United States, 1990-2004. J Natl Cancer Inst. 2008;100:1301–9. doi: 10.1093/jnci/djn276. [DOI] [PubMed] [Google Scholar]
- 81.Pulte D, Gondos A, Brenner H. Improvement in survival in younger patients with acute lymphoblastic leukemia from the 1980s to the early 21 st century. Blood. 2009;113:1408–11. doi: 10.1182/blood-2008-06-164863. [DOI] [PubMed] [Google Scholar]
- 82.Hunger SP, Lu X, Devidas M, Camitta BM, Gaynon PS, Winick NJ, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the children's oncology group. J Clin Oncol. 2012;30:1663–9. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stock W, Douer D, DeAngelo DJ, Arellano M, Advani A, Damon L, et al. Prevention and management of asparaginase/pegasparaginase-associated toxicities in adults and older adolescents: Recommendations of an expert panel. Leuk Lymphoma. 2011;52:2237–53. doi: 10.3109/10428194.2011.596963. [DOI] [PubMed] [Google Scholar]
- 84.Boissel N, Auclerc MF, Lhéritier V, Perel Y, Thomas X, Leblanc T, et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults. Comparison of the French FRALLE-93 and LALA-94 trials? J Clin Oncol. 2003;21:774–80. doi: 10.1200/JCO.2003.02.053. [DOI] [PubMed] [Google Scholar]
- 85.Ramanujachar R, Richards S, Hann I, Goldstone A, Mitchell C, Vora A, et al. Adolescents with acute lymphoblastic leukaemia: Outcome on UK national paediatric (ALL97) and adult (UKALLXII/E2993) trials. Pediatr Blood Cancer. 2007;48:254–61. doi: 10.1002/pbc.20749. [DOI] [PubMed] [Google Scholar]
- 86.de Bont JM, Holt Bv, Dekker AW, van der Does-van den Berg A, Sonneveld P, Pieters R. Significant difference in outcome for adolescents with acute lymphoblastic leukemia treated on pediatric vs adult protocols in the Netherlands. Leukemia. 2004;18:2032–5. doi: 10.1038/sj.leu.2403538. [DOI] [PubMed] [Google Scholar]
- 87.Stock W, La M, Sanford B, Bloomfield CD, Vardiman JW, Gaynon P, et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children's Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112:1646–54. doi: 10.1182/blood-2008-01-130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ribera JM, Oriol A, Sanz MA, Tormo M, Fernández-Abellán P, del Potro E, et al. Comparison of the results of the treatment of adolescents and young adults with standard-risk acute lymphoblastic leukemia with the Programa Español de Tratamiento en Hematología pediatric-based protocol ALL-96. J Clin Oncol. 2008;26:1843–9. doi: 10.1200/JCO.2007.13.7265. [DOI] [PubMed] [Google Scholar]
- 89.Rijneveld AW, van der Holt B, Daenen SM, Biemond BJ, de Weerdt O, Muus P, et al. Intensified chemotherapy inspired by a pediatric regimen combined with allogeneic transplantation in adult patients with acute lymphoblastic leukemia up to the age of 40. Leukemia. 2011;25:1697–703. doi: 10.1038/leu.2011.141. [DOI] [PubMed] [Google Scholar]
- 90.Huguet F, Leguay T, Raffoux E, Thomas X, Beldjord K, Delabesse E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: The GRAALL-2003 study. J Clin Oncol. 2009;27:911–8. doi: 10.1200/JCO.2008.18.6916. [DOI] [PubMed] [Google Scholar]
- 91.Gökbuget N, Beck J, Brandt K, Brüggeman M, Burmeister T, Diedeich H, et al. Significant improvement of outcome in adolescents and young adults (AYAs) aged 15-35 years with acute lymphoblastic leukemia (ALL) with a pediatric derived adult ALL protocol; results of 1529 AYAs in 2 consecutive trials of the German Multicenter Study Group for Adult ALL (GMALL) [abstract] Blood. 2013;122:839. [Google Scholar]
- 92.Stock W, Luger SM, Advani AS, Geyer S, Harvey RC, Mullighan C, et al. Favorable outcomes for older adolescents and young adults (AYA) with acute lymphoblastic leukemia (ALL): Early results of U.S. Intergroup Trial C10403 [abstract] Blood. 2014;124:796. [Google Scholar]
- 93.DeAngelo DJ, Stevenson KE, Dahlberg SE, Silverman LB, Couban S, Supko JG, et al. Long-term outcome of a pediatric-inspired regimen used for adults aged 18-50 years with newly diagnosed acute lymphoblastic leukemia. Leukemia. 2015;29:526–34. doi: 10.1038/leu.2014.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cornelissen JJ, van der Holt B, Verhoef GE, van’t Veer MB, van Oers MH, Schouten HC, et al. Myeloablative allogeneic versus autologous stem cell transplantation in adult patients with acute lymphoblastic leukemia in first remission: A prospective sibling donor versus no-donor comparison. Blood. 2009;113:1375–82. doi: 10.1182/blood-2008-07-168625. [DOI] [PubMed] [Google Scholar]
- 95.Thomas X, Boiron JM, Huguet F, Dombret H, Bradstock K, Vey N, et al. Outcome of treatment in adults with acute lymphoblastic leukemia: Analysis of the LALA-94 trial. J Clin Oncol. 2004;22:4075–86. doi: 10.1200/JCO.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 96.Garcia-Manero G, Kantarjian HM. The hyper-CVAD regimen in adult acute lymphocytic leukemia. Hematol Oncol Clin North Am. 2000;14:1381. doi: 10.1016/s0889-8588(05)70192-1. [DOI] [PubMed] [Google Scholar]
- 97.Kantarjian HM, O’Brien S, Smith TL, Cortes J, Giles FJ, Beran M, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18:547–61. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- 98.Kantarjian H, Thomas D, O’Brien S, Cortes J, Giles F, Jeha S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101:2788–801. doi: 10.1002/cncr.20668. [DOI] [PubMed] [Google Scholar]
- 99.Thomas DA, O’Brien S, Cortes J, Giles FJ, Faderl S, Verstovsek S, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood. 2004;104:1624–30. doi: 10.1182/blood-2003-12-4428. [DOI] [PubMed] [Google Scholar]
- 100.Morris K, Weston H, Mollee P, Marlton P, Gill D, Kennedy G. Outcome of treatment of adult acute lymphoblastic leukemia with hyperfractionated cyclophosphamide, doxorubicin, vincristine, dexamethasone/methotrexate, cytarabine: Results from an Australian population. Leuk Lymphoma. 2011;52:85–91. doi: 10.3109/10428194.2010.532889. [DOI] [PubMed] [Google Scholar]
- 101.Alacacioglu I, Medeni SS, Ozsan GH, Payzin B, Sevindik OG, Acar C, et al. Is the BFM regimen feasible for the treatment of adult acute lymphoblastic leukemia? A retrospective analysis of the outcomes of BFM and hyper-CVAD chemotherapy in two centers. Chemotherapy. 2014;60:219–23. doi: 10.1159/000375258. [DOI] [PubMed] [Google Scholar]
- 102.Buyukasik Y, Acar K, Kelkitli E, Uz B, Serefhanoglu S, Ozdemir E, et al. Hyper-CVAD regimen in routine management of adult acute lymphoblastic leukemia: A retrospective multicenter study. Acta Haematol. 2013;130:199–205. doi: 10.1159/000351172. [DOI] [PubMed] [Google Scholar]
- 103.Rytting ME, Thomas DA, O’Brien SM, Ravandi-Kashani F, Jabbour EJ, Franklin AR, et al. Augmented Berlin-Frankfurt-Münster therapy in adolescents and young adults (AYAs) with acute lymphoblastic leukemia (ALL) Cancer. 2014;120:3660–8. doi: 10.1002/cncr.28930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pui CH, Pei D, Campana D, Bowman WP, Sandlund JT, Kaste SC, et al. Improved prognosis for older adolescents with acute lymphoblastic leukemia. J Clin Oncol. 2011;29:386–91. doi: 10.1200/JCO.2010.32.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dubashi B, Jain A. L-Asparginase induced cortical venous thrombosis in a patient with acute leukemia. J Pharmacol Pharmacother. 2012;3:194–5. doi: 10.4103/0976-500X.95531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Caruso V, Iacoviello L, Di Castelnuovo A, Storti S, Mariani G, de Gaetano G, et al. Thrombotic complications in childhood acute lymphoblastic leukemia: A meta-analysis of 17 prospective studies comprising 1752 pediatric patients. Blood. 2006;108:2216–22. doi: 10.1182/blood-2006-04-015511. [DOI] [PubMed] [Google Scholar]
- 107.Plourde PV, Jeha S, Hijiya N, Keller FG, Silverman LB, Rheingold SR, et al. Safety profile of asparaginase Erwinia chrysanthemi in a large compassionate-use trial. Pediatr Blood Cancer. 2014;61:1232–8. doi: 10.1002/pbc.24938. [DOI] [PubMed] [Google Scholar]
- 108.Advani AS, Sanford B, Luger S, Devidas M, Larsen EC, Liedtke M, et al. Frontline-treatment of acute lymphoblastic leukemia (ALL) in older adolescents and young adults (AYA) using a pediatric regimen is feasible: Toxicity results of the prospective US intergroup trial C10403 (Alliance) [abstract] Blood. 2013;122:3903. [Google Scholar]


