Abstract
Objective:
To examine the factors influencing the pattern and extent of anti-craving medication adherence and drinking outcomes in alcohol-dependent patients.
Materials and Methods:
Demographic data from 102 inpatients were collected at discharge from hospital. The pattern of anti-craving medication, extent of adherence, and drinking outcome was collected at 1st, 3rd, 8th, and 12th week follow-up. Patients’ self-reported adherence, medication diary, and simplified medication adherence questionnaire were used and data were analyzed using SPSS.
Results:
Majority (99%) were male patients with a mean age of 41.17 ± 9.86 years and 70% belonged to middle socioeconomic status. There was a decrease in the number of patients coming for follow-up over time from 99.01% to 77.45% on day 90. Acamprosate was used in 74% and naltrexone and disulfiram in 7% of patients each. A significant reduction in adherence to acamprosate and naltrexone (P < 0.001) was associated with simultaneous decrease in days to alcohol abstinence and increase in relapse rate compared to adherent group (P < 0.001). Main barriers to adherence included younger age (odds ratio = 1.05 95% [1.01-1.09]; P < 0.01), self-decision, emotional factors, and adverse effects.
Conclusions:
The study demonstrated the need for safer therapeutic options along with suitable intervention at grass root level for sustenance of adherence to anti-craving medication among young adults to prevent relapse and achieve near-complete abstinence from alcohol dependence.
Key words: Acamprosate, adherence, alcohol dependence, anti-craving medications, drinking outcomes
INTRODUCTION
Alcohol consumption is one of the most common risk factors contributing to physical and psychological ill health. There has been a progressive increase in the harmful use of alcohol with approximately 2.5 million deaths each year and has been identified as the leading risk factor for death in men between the age of 15 and 59 years.[1] In the National Household Survey of Alcohol and Drug Abuse (2003), 21.4% were reported to be current users of alcohol (used in the last 30 days).[2] Alcoholism accounts for 4% of all global deaths and 4.6% of global disability-adjusted life years in addition to significant increase in psychosocial burden in the community.[3,4]
According to the criteria for alcohol dependence described by the Diagnostic and Statistical Manual of Mental Disorders,[5] dependence is defined as “a maladaptive pattern of alcohol use, leading to clinically significant impairment or distress, as manifested by three (or more) of the seven criteria, occurring at any time in the same 12-month period.”
The United States-Food and Drug Administration (FDA) approved medications are available for use in routine practice for the treatment of alcohol dependence. It is reported that ~50% of alcohol-dependent patients relapse within 3 months, despite following a treatment protocol using these approved medications.[3,6] While, the pathophysiology of alcohol dependence is complex and incompletely understood at the present time, the medications for its treatment have varied targets and mechanisms of action. There is an accumulating evidence demonstrating combined pharmacotherapy with psychotherapy as more efficacious approach than either of the treatments alone.[7] The treatment outcome is known to depend on several patient-related factors such as motivation, emotion, willingness to abstain, efficacy of medications, and adherence to medications. Low adherence to prescribed medications is said to be one of the limitations contributing to failure in achieving abstinence and preventing relapse.[8]
The present study was designed to evaluate the pattern of use of anti-craving medications, the extent of adherence to them, factors that influence adherence and the influence of medication adherence on time to the first drink, and on relapse in drinking among alcohol-dependent patients.
MATERIALS AND METHODS
This was a prospective study conducted for 18 months at a tertiary care hospital following the Institutional Ethical Review Board's approval. Data were collected using a specifically designed case record form. Subjects included were clinically diagnosed as alcohol-dependent according to the International Classification of Diseases 10 criteria, aged ≥20 years, and received treatment for alcohol dependence as hospital inpatients. The study exclusion criteria were patients with concurrent major psychiatric and other serious organ system disorders, psychotic disorders induced by harmful use of other substances, and complicated withdrawal symptoms.
The data on the sociodemography of the patients were collected during the hospital stay, while data on alcohol consumption and anti-craving medication use were recorded at discharge as well as during the time of follow-up at each outpatient clinic visit, i.e., 1st, 3rd, 8th, and 12th week. The treatment with anti-craving medications and necessary psychological interventions was discussed with each patient and their care takers by a psychiatric expert based on the reports of relevant investigations, along with their benefits and adverse effects before initiating treatment regimen to induce abstinence and/or prevent relapse.
At the time of discharge, the patients were instructed to take their medications as advised by the psychiatric expert and each patient was provided with a drug diary to record the information on medication details they consumed, the number of missed tablets, if any, reasons for the same, and adverse effects when experienced. The adherence to medication was assessed at every follow-up visit through patients’ self-report, medication dairy, and simplified medication adherence questionnaire (SMAQ), and the data on the levels of adherence (proportion of prescribed medications taken by the patient) and persistence (whether the patient continued with prescribed medications) were recorded. The medication diary was replaced with a new diary at each clinic visit. Patients who were unable to fill the medication diary were provided assistance by the investigator on the day of the follow-up visit to update the information from the previous visit to the current follow-up. The patient follow-up was either on their scheduled clinic visit or through telephonic interview if they were unable to visit the clinic. Three attempts per patient were made to contact before they were interviewed telephonically. The patients who neither visited the hospital nor were reachable for telephonic interview were considered as lost to follow-up.
The assessment of anti-craving treatment effect on the drinking outcomes and factors affecting adherence to them was collected over a period of 12 weeks. Anti-craving medication adherence was assessed using patient's self-report by recording whether he/she was regular with consumption. Furthermore, through the medication diary at each visit to see the entry for number of days and tablets that the patient had taken. The SMAQ which is a prevalidated, structured six-point questionnaire was also used to quantify medication adherence and to evaluate the extent of nonadherence, which is in use for patients with acquired immune deficiency syndrome.[9] The six questions used in the study are given below and scoring consisted of Yes = 1 and No = 0 responses from patients: 1. Are you careless at times about taking medication? 2. Did you ever forget to take your medication? 3. Not taken medication in the last week? 4. More than 2 days medication was not taken in the last month 5. Are you not taking your medication in time? 6. Do you stop medication if you feel worse after taking medication?
For each “yes” response, 1 point was given and 0 point for “no” response. The increasing order of scores from 0 to 6 was considered as decreasing levels of adherence and was then correlated with the percentage adherence scores. SMAQ score of more than four was considered as nonadherent and reason for this was recorded. Anti-craving medication efficacy outcome was assessed as time to the first drink and time to relapse. Pattern and extent of adverse effects to anti-craving medications were classified based on organ system affected.
Baseline and continuous data such as age were subjected to descriptive statistical analysis and expressed as number as well as percentages. We used Kuppuswamy and Prasad classification for presenting socioeconomic status (SES).[10,11] Categorical variables were compared using Chi-square test. Comparison of continuous variables between groups was done by independent t-test with a level of significance <0.05. SPSS version 17.0 (IBM Corporation, Armonk, New York, United States) was used to analyze the data.
RESULT TABLES AND/OR FIGURES
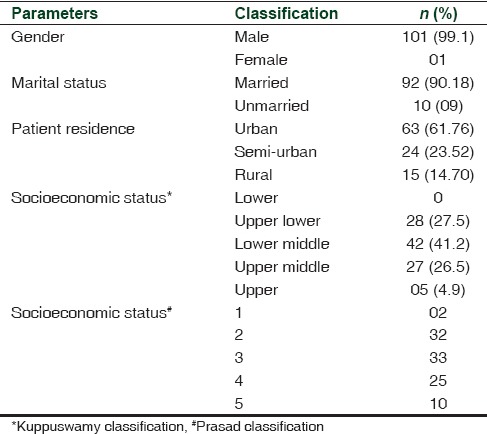
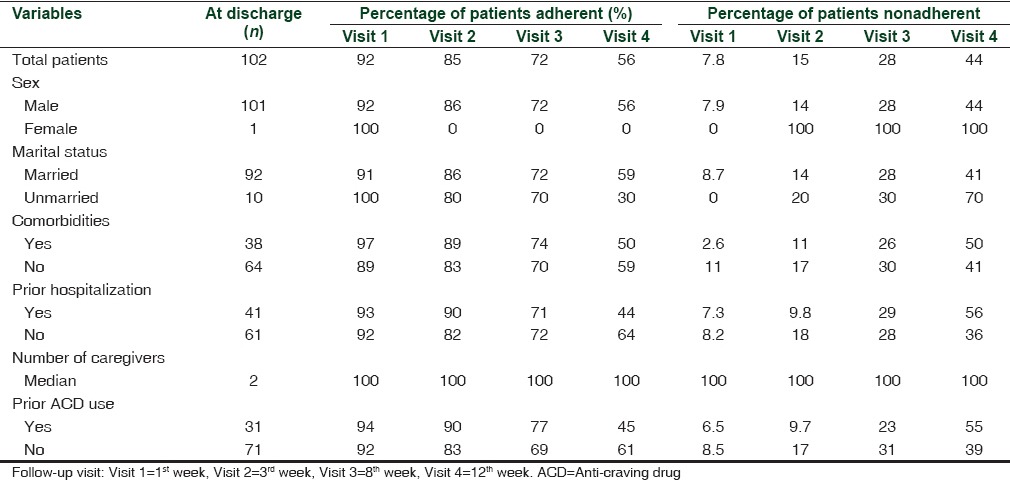
A total of 102 patients with a mean (standard deviation) age of 41.17 (9.8) years constituted the study group. The clinical characteristics of patients, their percentage wise distribution for the level of SES, and area of residence are shown in Table 1.[10,11] The gender distribution showed all male patients with one female.
Table 1.
The demographic profile of patients with alcohol dependence
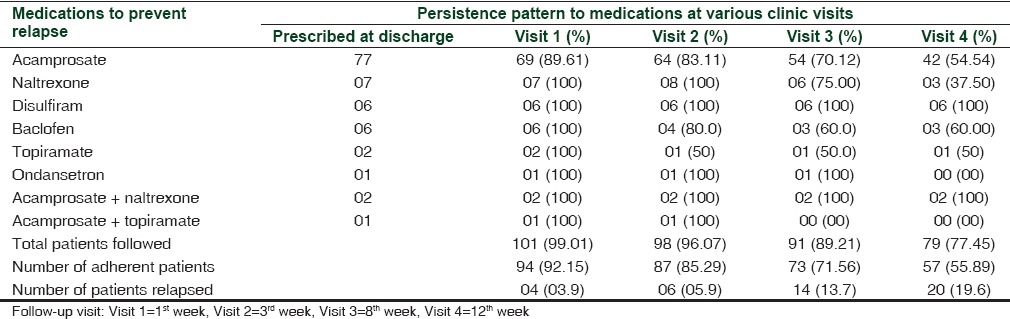
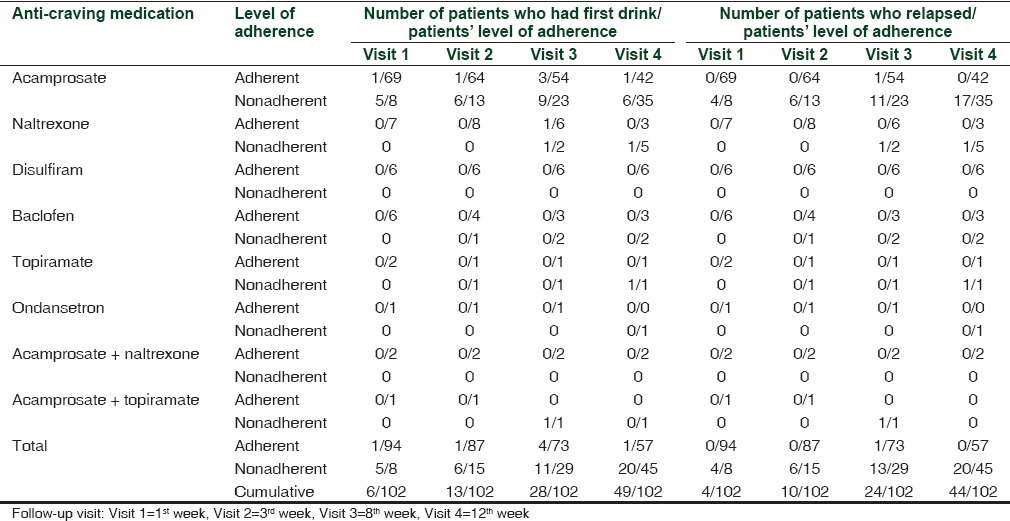
Of the total number of 102 patients - 101 (99.01%), 98 (96.07%), 91 (89.21%), and 79 (77.45%) patients completed the follow-up during 1st, 3rd, 8th, and 12th week, respectively. Acamprosate was prescribed as an anti-craving medication in 77 patients (75.49%) both during hospital stay and at discharge with median interquartile dose range of 1332 and 1998 mg/day. The number of patients who were adherent to acamprosate was 69 (89.61%), 64 (83.11%), 54 (70.12%), and 42 (54.54%) at 1st, 3rd, 8th, and 12th week follow-up, respectively. This reduction in the proportion of patients’ adherent across time was significant (P < 0.001) at the end of 12 weeks. Naltrexone was prescribed in 7 (6.8%) patients at a dose of 25 mg/day with 3 (37.50%) patients’ adherent after 12 weeks. Disulfiram was prescribed in 6 (5.88%) patients at a dose of 125-500 mg/day and had 100% adherence over 12 weeks. During a 12-week follow-up period, 57 (55.89%) patients reported to have taken 100% of the prescribed doses of anti-craving medications at any point of time. Other medications prescribed were baclofen, topiramate, and ondansetron [Table 2].
Table 2.
The pattern of anti-craving medication use and their persistence pattern in patients with alcohol dependence
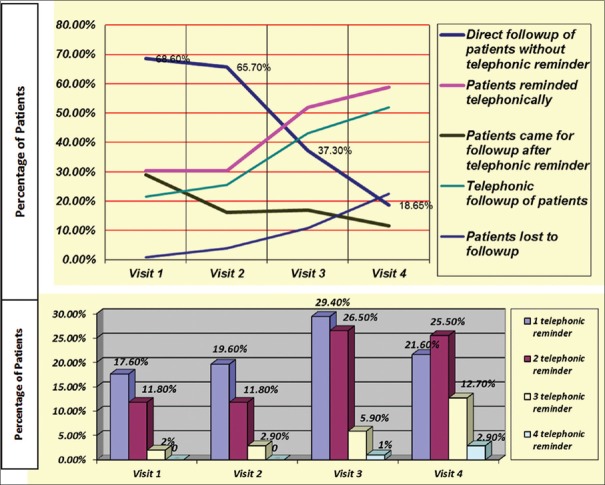
The number of patients who reported for follow-up reduced significantly (P < 0.001) from 70 (68.6%) at the first visit to 19 (18.65%) at the fourth visit. The number of patients who were reminded over telephone and also the number of telephonic reminders to each patient regarding the follow-up visit increased over time and this was statistically significant (P < 0.001). The odds ratio of patients coming for follow-up after phone call reduced from 29.0% at the first visit to 11.6% at the fourth visit and was significant [Table 3 and Figure 1].
Table 3.
Characteristics and rates of follow-up over 12 weeks
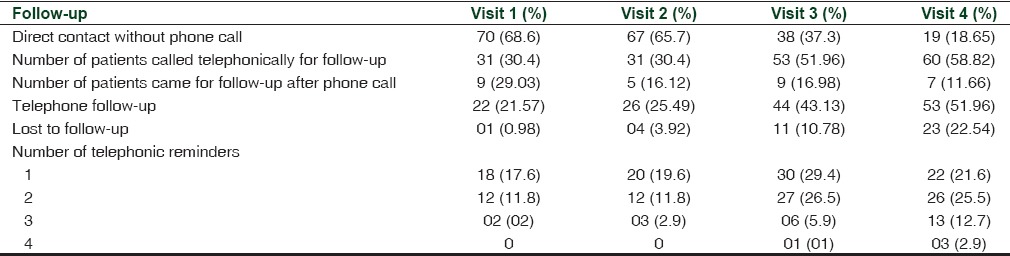
Figure 1.
Characteristics and rates of follow-up of alcohol-dependent patients over 12 weeks. Telephonic reminders to patients at each follow-up visit. Follow-up visit: Visit 1 = 1st week, Visit 2 = 3rd week, Visit 3 = 8th week, Visit 4 = 12th week
The analysis of adherence to anti-craving medications by patients’ self-report, through medication diary and SMAQ scores, did not show statistically significant difference in the levels of adherence [Table 4]. The responses to SMAQ exhibited an all or none phenomenon on adherence to medications, with 57 (55.89%) patients completely adherent and 45 (44.11%) nonadherent at the end of the fourth follow-up visit. These groups were comparable in terms of baseline characteristics such as age, marital status, prior admission for the management of alcohol dependence, anti-craving medication use in the recent past, and number of caregivers.
Table 4.
Comparison of baseline variables between patients who were adherent and nonadherent to anti-craving medications
The adherent group showed significantly more number of abstinent days with lower relapse rate (P < 0.001). Table 5 provides data on the influence of anti-craving medication adherence on drinking outcomes in alcohol-dependent patients. With increase in the number of days of follow-up, there was an increase in the number of patients who relapsed with a parallel decrease in the number of days for the first drink, namely, on 1st week, 6 (5.9%) patients had the first drink since discharge and 4 (3.94%) had relapsed; by 3rd week, 13 (12.74%) patients had the first drink and 10 (9.80%) had relapsed; by 8th week, 28 (27.45%) patients had the first drink and 24 (23.53%) relapsed; and by 12th week follow-up, 49 (48.03%) patients had the first drink and 44 (43.17%) patients relapsed [Table 5].
Table 5.
Influence of anti-craving medication adherence on drinking outcomes in alcohol-dependent patients
The univariate analysis for variables such as age, sex, history of prior hospitalization, marital status, and social support in terms of number of caregivers, place of origin, and SES showed no significant difference. Multivariate analysis showed patients of younger age group (odds ratio = 1.05 95% [1.01-1.09]; P < 0.01) to be significantly less adherent to anti-craving medications.
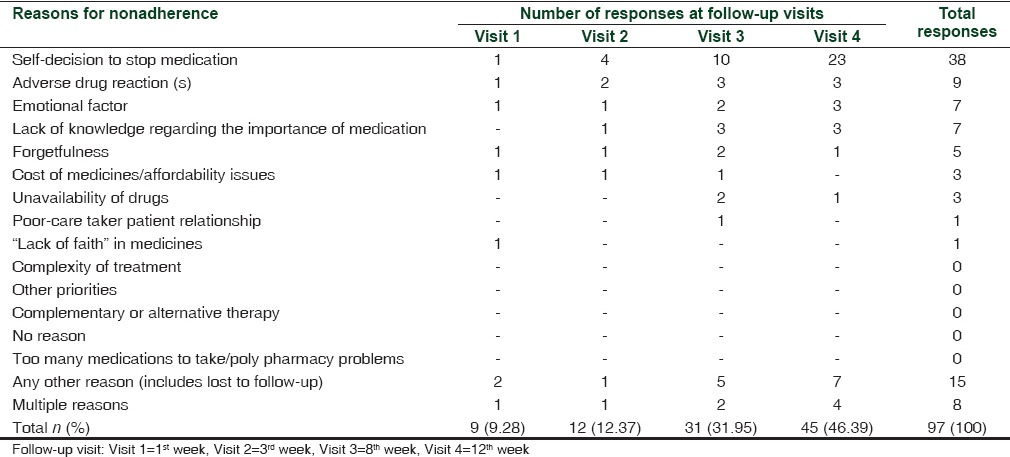
There were 97 responses with reasons for nonadherence, of which 38 (39.17%) reported to have stopped anti-craving medication on their own. The response to reason for nonadherence given was that they had either improvement in condition and/or were confident of quitting alcohol or they had started to drink alcohol again and felt unnecessary to take medication. By 12 weeks, 20 (19.6%) patients had experienced adverse drug reactions (ADRs), of which 9 (45%) were on acamprosate who stopped medication due to subjectively perceived side effects [Table 6]. Other reasons included forgetfulness and lack of knowledge.
Table 6.
Reasons for nonadherence to anti-craving medications as reported by alcohol-dependent patients
DISCUSSION
The results of the present study showed that the extent of adherence to anti-craving medications reduced significantly over time and that there was an early relapse in drinking behavior among alcohol-dependent patients.
The co-existing medical conditions such as hypertension and diabetes mellitus justify the reasons for hospital admissions. Since adherence to pharmacotherapy and clinical outcome of patients is well known to be influenced by major and serious comorbidities, such patients were excluded when they were uncontrolled or recognized in the beginning of the study.
The pattern of anti-craving medication use was similar to that approved by the FDA.[12] The maximal use of acamprosate both as monotherapy and in combination with other medications in a proportion of patients may possibly be attributed to relatively reduced potential and/or absence of known serious interactions between acamprosate and alcohol or with other routinely prescribed medications.[4,13] However, we observed that the most frequent reason for nonadherence was among those patients who were prescribed acamprosate and maximum number of the patients reported self-termination of the medication.
The present study used three different tools to measure adherence to anti-craving medications. While, patient self-report as one of the methods is regarded as the most convenient method for measuring adherence, the same is known to have flaws since it is said to roughly give the dose count, but not the number of doses the patient missed and if the doses were taken at the right time.[14] To minimize this limitation, we assessed medication adherence additionally using medication diary and SMAQ. The extent of adherence varied from highest with disulfiram to moderate among naltrexone and acamprosate groups. This finding may be attributed to the smaller number of patients in the present study, a selection bias in prescribing medications, and naturalistic study setting.
A wide variation in treatment adherence rates (20-80%) has been reported in studies based on the types of intervention. In previous studies, the nonadherence rates of 20-60% for naltrexone, 53% for acamprosate, and highest for disulfiram are reported where treatment duration ranged from 3 to 24 months.[3] Such difference in the extent of nonadherence to various anti-craving medications is said to be due to patient-related factors similar to that seen in the present study such as self-decision to stop taking medications, either due to confidence to abstain from alcohol or the decision to restart consuming alcohol, along with other emotional factors. In addition, a frequently reported barrier that we found was ADRs to prescribed treatments, similar to those reported in the recent Cochrane reviews.[12,15]
The WHO criteria that may influence medication adherence include socioeconomic, marital/educational status, health-care system, medical condition, treatment of co-morbidities, and role of caregivers. However, these did not show a significant influence in maintaining adherence in our study. In addition, barriers such as forgetfulness to take medications, their cost, complexity of regimens and polypharmacy were not found to contribute to nonadherence. Another interesting observation was that adults and elderly >40 years were found to be more adherent compared to patients <30 years of age. In the Indian context, this behavior may be attributed to social responsibilities of adults and elderly people compared to the influence of peer group pressure among younger alcohol dependents. Given the unique set of barriers that are proposed to be involved in the intrinsic behavior of each patient, a complete understanding and assessment of factors involved in medication adherence is challenging in these types of study design. Several studies have shown that medication nonadherence is the most common cause of therapeutic failure in many acute and chronic disorders.[14,15] From the present study, it appears that the use of anti-craving medications induces abstinence and underscores the need to enhance treatment adherence through motivation. However, identifying and providing appropriate solutions to address individual patient's needs cannot be generalized. In addition, the adherence to medication in alcohol dependents is known to be influenced by efficacy outcome. Interestingly, adherence to anti-craving medications in the present study was similar to the previous studies with a lower rate of relapse (30%) compared to studies where 50% of the patients relapsed within 3 months of treatment.[1] Such lower rate of relapse in our study may be due to the combined effect of anti-craving medications that act by modifying neurotransmitters in the brain coupled with one or more nonpharmacological interventions such as counseling, support by family/caregivers, regular follow-up, and patient education. This adds to strengthen and support the overall aims of this study.
Interestingly, 21.05% of the patients who were nonadherent to anti-craving medications never had the first drink and 47.36% of the patients from the same group had no relapse at the end of 12 weeks, indicating that these nonadherent and nonrelapsed patients had decided to stop taking anti-craving medications with confidence. In addition, in the present study, it was observed that disulfiram was prescribed to those who were confident of quitting alcohol, were adequately counseled regarding likely ADRs, were found adherent to it at the end of 12 weeks, and also did not consume alcohol. These findings appear to support the finding that patient willingness may play a key role in achieving abstinence from alcohol dependence. The relapse rates were minimal among patients taking acamprosate raising concern over its efficacy in reducing craving effects. Similar results have been reported in COMBINE study,[7] where acamprosate was not better than placebo. However, other randomized studies have reported acamprosate to be effective. Since only very small number of patients received other anti-craving medications, their specific and synergistic beneficial effects alone or in combination with various other anti-craving medications need further evaluation. Furthermore, comparing the outcomes of various treatment options used in the present study with other therapeutic approaches needs to be examined in a larger sample size to suggest suitable and appropriate treatment options for alcohol dependence.
A controlled study by Bucknam in soldiers with alcohol dependence examined the comparative efficacy of acamprosate, naltrexone, and topiramate over 1 year, and demonstrated a significant reduction in relapse rates among those who received topiramate. Authors attribute the findings to improved acceptability of topiramate in this population.[16] However, it is important to note that this difference may be due to maximum (76.3%) use of topiramate which was supplied by the hospital free of charge as against out-of-pocket payment for topiramate in the present study. In addition, further evaluation is needed to determine whether topiramate and acamprosate have comparable efficacy.
A preliminary, double-blind, randomized, controlled study using baclofen (15-30 mg/day) over 1 month as well as a separate efficacy study using high-dose baclofen (140 mg/day) has demonstrated their beneficial effects in reducing craving as well as sustenance of anti-craving effects over 10 months compared with other FDA-approved medications.[17,18] These reports suggest the need to conduct randomized controlled trials with high-dose baclofen for alcohol dependence.
A recent report by Steven has shown the influence of group therapy aimed at personality traits among alcohol dependents. Further, they propose that high-risk teenagers are more likely to progress to risky drinking behavior.[19] Thus, suggesting that it may be beneficial to conduct an intervention by therapists that targets specific personality traits to reduce drinking in teenagers who are at risk; in addition, they stress its potential for extension as a halo effect to low-risk students in schools. This modality is suggested as an example of personalized prevention in psychiatry which appears to be a useful and feasible tool in a resource poor country like India.
Strengths of the study
This study is one of the few that has provided a comprehensive overview of patterns of anti-craving medications prescribed in a tertiary care setup, adherence to them and their influence on drinking outcomes in India. Given that abuse and/or dependence often occur at a younger age, the study with 102 patients from a tertiary care center has a fair representation of younger age sample from urban and semi-urban backgrounds. The methods used were suitable to get relevant information aimed at addressing the objectives of the study where anti-craving medication adherence rates were assessed using three different assessment tools. The data analysis was done using statistical tools which provided important information that is likely to impact the future studies on adherence to anti-craving medications.
Limitations of the study
First, this was a tertiary care center study and hence, the results cannot be easily extrapolated to a larger population at different levels of health-care facilities. Further, the present study population is vulnerable to Berksonian bias, which is a type of selection bias for admission to hospital, thus indicating a need for community-based multicenter studies to appreciate disease burden. Second, the tools used to assess the extent of anti-craving medication adherence had some flaws such as overestimation through patient self-reporting and underestimating by the use of SMAQ. Third, the telephonic contact with patients, which is not used in routine clinical practice, may have contributed to the observed estimate of medication adherence. Finally, the association between nonadherence and its impact on clinical outcome, re-hospitalization, and quality of life was not assessed in this study. Nonetheless, the present study has quantified anti-craving medication adherence rates to assist in the identification of determinants of adherence among alcohol-dependent patients as an important first step in fulfilling the objectives.
However, more comprehensive, larger, and long-term studies are necessary to examine the adherence pattern to other anti-craving agents to evaluate the potential for their safety and improved efficacy. In addition, it is important to address the comparison between two anti-craving agents as monotherapy and/or their combinations, a careful assessment of factors that influence adherence/reasons for nonadherence, and a study among patients with physical and other mental comorbidities including the impact of nonpharmacological interventions. Such approaches may help in choosing safer and effective treatment options in the future.
CONCLUSION
The present study identified modifiable factors such as age, motivation, and willingness as determinants that can adversely influence adherence to anti-craving agents among patients with alcohol dependence. The findings indicate the need for appropriate pharmacological as well as suitable nonpharmacological intervention to achieve improved rate of abstinence with a decreased rate of relapse in patients with alcohol dependence.
Financial support and sponsorship
Indian Council of Medical Research, New Delhi.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors gratefully acknowledge the financial assistance from the Indian Council of Medical Research, New Delhi, India, in conducting the study. We wish to acknowledge Dr. Padmini Devi, Associate Professor, St. John's Medical College, for her assistance during the initial part of the study. In addition, they would like to thank Dr. Annalakshmi J, Quintiles, Bengaluru, and Prof. Shanaz Tejani-Butt, USP, Philadelphia, USA, for critical evaluation during the final stages of preparation of this manuscript.
REFERENCES
- 1.World Health Organization. (NLM Classification: WM 274) Geneva, Switzerland: WHO Press, World Health Organization; 2011. Global Status Report on Alcohol and Health. [Google Scholar]
- 2.Current Information on Use and Harm from Alcohol in the South-East Asian Region. Alcohol Series No. 6. New Delhi: WHO-SEARO; 2007. WHO Regional Office for South-East Asia; p. 12. [Google Scholar]
- 3.Jacob KS. Alcohol and public health policies in India. Natl Med J India. 2010;23:224–5. [PubMed] [Google Scholar]
- 4.Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–33. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- 5.Diagnostic and Statistical Manual of Mental Disorders. 4th Edition, Text Revision. Washington D.C.: American Psychiatric Press; 2000. American Psychiatric Association. [Google Scholar]
- 6.Moos RH, Moos BS. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction. 2006;101:212–22. doi: 10.1111/j.1360-0443.2006.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: The COMBINE study: A randomized controlled trial. JAMA. 2006;295:2003–17. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 8.O’Malley SS, O’Connor PG. Medications for unhealthy alcohol use: Across the spectrum. Alcohol Res Health. 2011;33:300–12. [PMC free article] [PubMed] [Google Scholar]
- 9.Knobel H, Alonso J, Casado JL, Collazos J, González J, Ruiz I, et al. Validation of a simplified medication adherence questionnaire in a large cohort of HIV-infected patients: The GEEMA Study. AIDS. 2002;16:605–13. doi: 10.1097/00002030-200203080-00012. [DOI] [PubMed] [Google Scholar]
- 10.Sharma R. Kuppuswamy's socioeconomic status scale - revision for 2011 and formula for real-time updating. Indian J Pediatr. 2012;79:961–2. doi: 10.1007/s12098-011-0679-3. [DOI] [PubMed] [Google Scholar]
- 11.Sharma R. Revision of Prasad's social classification and provision of an online tool for real-time updating. South Asian J Cancer. 2013;2:157. doi: 10.4103/2278-330X.114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arias AJ, Kranzler HR. Treatment of co-occurring alcohol and other drug use disorders. Alcohol Res Health. 2008;31:155–67. [PMC free article] [PubMed] [Google Scholar]
- 13.Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: Results of a meta-analysis. Alcohol Clin Exp Res. 2004;28:51–63. doi: 10.1097/01.ALC.0000108656.81563.05. [DOI] [PubMed] [Google Scholar]
- 14.Cline CM, Björck-Linné AK, Israelsson BY, Willenheimer RB, Erhardt LR. Non-compliance and knowledge of prescribed medication in elderly patients with heart failure. Eur J Heart Fail. 1999;1:145–9. doi: 10.1016/s1388-9842(99)00014-8. [DOI] [PubMed] [Google Scholar]
- 15.Rosner S, Hackl-Herrwerth A, Leucht S, Lehert P, Vecchi S, Soyka M. Acamprosate for alcohol dependence. Cochrane Database Syst Rev. 2010;9:CD004332. doi: 10.1002/14651858.CD004332.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, et al. Oral topiramate for treatment of alcohol dependence: A randomised controlled trial. Lancet. 2003;361:1677–85. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- 17.Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, et al. Baclofen efficacy in reducing alcohol craving and intake: A preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37:504–8. doi: 10.1093/alcalc/37.5.504. [DOI] [PubMed] [Google Scholar]
- 18.Bucknam W. Suppression of symptoms of alcohol dependence and craving using high-dose baclofen. Alcohol Alcohol. 2007;42:158–60. doi: 10.1093/alcalc/agl091. [DOI] [PubMed] [Google Scholar]
- 19.Steven D. A personalized approach to prevention of risky drinking. JAMA Psychiatry. 2013;70:334. [Google Scholar]