ABSTRACT
We recently described 13-deoxytetrodecamycin, a new member of the tetrodecamycin family of antibiotics. A defining feature of these molecules is the presence of a five-membered lactone called a tetronate ring. By sequencing the genome of a producer strain, Streptomyces sp. strain WAC04657, and searching for a gene previously implicated in tetronate ring formation, we identified the biosynthetic genes responsible for producing 13-deoxytetrodecamycin (the ted genes). Using the ted cluster in WAC04657 as a reference, we found related clusters in three other organisms: Streptomyces atroolivaceus ATCC 19725, Streptomyces globisporus NRRL B-2293, and Streptomyces sp. strain LaPpAH-202. Comparing the four clusters allowed us to identify the cluster boundaries. Genetic manipulation of the cluster confirmed the involvement of the ted genes in 13-deoxytetrodecamycin biosynthesis and revealed several additional molecules produced through the ted biosynthetic pathway, including tetrodecamycin, dihydrotetrodecamycin, and another, W5.9, a novel molecule. Comparison of the bioactivities of these four molecules suggests that they may act through the covalent modification of their target(s).
IMPORTANCE The tetrodecamycins are a distinct subgroup of the tetronate family of secondary metabolites. Little is known about their biosynthesis or mechanisms of action, making them an attractive subject for investigation. In this paper we present the biosynthetic gene cluster for 13-deoxytetrodecamycin in Streptomyces sp. strain WAC04657. We identify related clusters in several other organisms and show that they produce related molecules.
INTRODUCTION
Antibiotics are essential for the successful management of bacterial infections. Most of these drugs are derived from secondary metabolites produced by fungi or bacteria, with a particularly rich source being the diverse bacterial genus Streptomyces. At present, more than 50% of antibiotics, including compounds that target the cell wall, the ribosome, RNA polymerase, DNA gyrase, and fatty acid biosynthesis, are derived from Streptomyces natural products. In addition to being clinically relevant, these compounds have served as invaluable probes of molecular pathways such as translation (e.g., streptomycin [1]) and cell wall biosynthesis (e.g., vancomycin [2]).
The steady advance of clinical resistance to antibiotics, coupled with a shortage of new antimicrobials in the developmental pipeline, has led to a serious need for new drugs. Mortality and morbidity from antibiotic-resistant bacterial infections are increasing steadily such that, for example, in the United States methicillin-resistant Staphylococcus aureus (MRSA) was responsible for the deaths of 11,285 people per year as of 2013 (3). The need for new antibiotics is great, in particular, for drugs that target new molecular pathways or known pathways in new ways. This is a key factor because compounds having novel mechanisms of action presumably have a better chance of evading the existing resistance mechanisms.
In previous work, we described a new congener of the tetrodecamycin (TDM) antibiotics called 13-deoxytetrodecamycin (13-dTDM) (Fig. 1). This compound is the third discovered representative of this family, with the other molecules, TDM and dihydrotetrodecamycin (dhTDM), having been reported in 1994 and 1995 (4–6). We showed that 13-dTDM is primarily specific for Gram-positive bacteria and that its bioactivity against common nosocomial pathogens such as S. aureus is competitive with that of antibiotics in clinical use. Most importantly, 13-dTDM exhibited potent activity in the same range of MICs against MRSA as vancomycin (7).
FIG 1.

All known TDM family molecules. 13-dTDM and TDM are known to have antibiotic activity, while W5.9 and dhTDM are inactive. Molecule W5.9 is a novel molecule related to the TDM molecules by biosynthesis although it lacks the canonical tetronate ring.
There has been very little investigation of the tetrodecamycins; their targets, mechanisms of action, and biosynthetic pathways have not been reported to date. They are structurally distinguished by a set of four rings, one of which is a five-membered lactone ring called a tetronate ring. Tetronate rings are found in several classes of secondary metabolites including the spirotetronates and the linear tetronates (8). The biosynthesis of tetronate rings has been investigated in the context of the otherwise unrelated antibiotic tetronomycin (target and mode of action also unknown) (9). To form the tetronate ring, 1,3-bisphosphoglycerate is taken from primary metabolism, dephosphorylated, and loaded onto a dedicated acyl carrier protein (ACP). This reaction is mediated by an FkbH family protein. In the case of tetronomycin, the protein that mediates this reaction is called Tmn16 (10). A condensation reaction mediated by a ketoacyl-ACP synthase III results in cyclization of the ACP-linked glyceryl moiety with a polyketide, resulting in the formation of the tetronate ring (11). A feature of the protein Tmn16 not found in other FkbH-like proteins is an ∼250-amino-acid (aa) amino-terminal extension. While the exact function of the amino-terminal extension remains unknown, it has been shown to be necessary for the transfer of the glyceryl moiety to the dedicated ACP (10). FkbH itself is believed to catalyze the same reaction as Tmn16 but for ultimate use as an unusual polyketide synthase (PKS) extender unit within the antibiotic FK520 (12, 13).
To date, there has been no investigation on the biosynthesis of TDM family molecules. Here, we report the discovery of the biosynthetic gene cluster, the ted genes, responsible for producing 13-dTDM in Streptomyces sp. strain WAC04657. We additionally use publicly available genome sequences to find closely related clusters in other organisms and, using a comparative genomics approach, thereby characterize the edges of the clusters and identify additional TDM-related molecules. Comparing the bioactivities of these different compounds suggests a possible mechanism of action for these antibiotics.
MATERIALS AND METHODS
General experimental procedures.
High-performance liquid chromatography (HPLC) was performed on a Waters Alliance e2695 equipped with an inline Waters 2998 photodiode array and a Waters Fraction Collector III. High-resolution mass spectra were acquired using a Waters Acquity UPLC-Xevo G2-S QToF instrument. 1H nuclear magnetic resonance (NMR) and two-dimensional (2D) NMR spectra were acquired on an Agilent DD2-700 MHz NMR spectrometer with a 1H-19F{13C/15N} 5-mm triple resonance cold probe. 13C NMR spectra were acquired on an Agilent DD2-500 MHz NMR spectrometer with an XSens cold probe.
Bacterial strains and culture methods.
Streptomyces strain WAC04657 was a gift from Gerry Wright (McMaster University, Canada); Streptomyces atroolivaceus ATCC 19725 was purchased from the ATCC through Cedarlane Labs (Burlington, Ontario, Canada). Streptomyces globisporus subsp. globisporus NRRL B-2293 was acquired from the USDA Agricultural Research Service (Peoria, IL). Streptomyces sp. strain LaPpAH-202 was a gift from Cameron Currie (University of Wisconsin). Escherichia coli XL1-Blue (Stratagene) and E. coli ET12567/pUZ8002 (14) are general laboratory strains. All Streptomyces strains were grown on maltose-yeast-malt extract (MYM) agar (7) in a stationary incubator at 30°C unless otherwise stated. E. coli strains were grown in LB broth at 37°C with shaking at 200 rpm. Where needed, medium was supplemented with apramycin (50 μg ml−1), chloramphenicol (25 μg ml−1), kanamycin (50 μg ml−1), or nalidixic acid (25 μg ml−1). Spore stocks were made on MYM agar according to common methods, with the modification that S. atroolivaceus was grown on R2 agar without sucrose (R2-S agar) (15). Intergenic conjugations were performed from E. coli ET12567/pUZ8002 host strains to Streptomyces on mannitol-soya flour (MS) agar as previously described (15). Strains used for MIC assays were Bacillus subtilis 168, E. coli ATCC 25922, Micrococcus luteus, S. aureus ATCC 29213, Pseudomonas aeruginosa PAO1, Staphylococcus epidermidis ATCC 12228, S. aureus ATCC BAA-41 (MRSA), S. aureus ATCC BAA-44 (MRSA), Enterococcus faecalis ATCC 29212, Burkholderia cepacia ATCC 25416, Acinetobacter baumannii ATCC 19606, and Klebsiella pneumoniae ATCC 13883.
Plasmid construction and genetic manipulation of Streptomyces.
Chromosomal DNA was isolated from WAC04657 as previously described (7). To build the plasmid for disrupting the tedF1 gene (pOJ260-tedF1 frag.), we first amplified the full-length tedF1 gene to use as a template for subsequent rounds of PCR. The full-length tedF1 gene was amplified from WAC04657 (tedF1W) chromosomal DNA with the primers CGCCGAGGTTCTTCACTGGTGCAG (primer 61) and AAGGGCGGCTTTAGAACGCTCGCCAA (primer 62). NEB's Vent polymerase was used as described by the manufacturer with 5% (vol/vol) dimethyl sulfoxide (DMSO) in the reaction mixture. The thermocycler was programmed as follows: 10 min at 95°C and 30 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 2 min 3 s, with a final incubation at 72°C for 2 min. This generated an amplicon of 2.0 kb. The amplicon was purified using a QIAquick PCR purification system as described by the manufacturer. Using the generated amplicon as a template, the tedF1 frag. was amplified with the primers AACACGGTGTGGGGCGGCGT and GCTGAGCAGGAAGTTGTCGATGAC using NEB's Vent polymerase as described by the manufacturer (no added DMSO). The thermocycler was programmed as described above but with an extension time of 53 s. This generated an amplicon of 0.8 kb, which was purified using a QIAquick PCR purification system and then blunt-end cloned into the EcoRV site of pOJ260 (16). Insertion of the tedF1 fragment into pOJ260 was confirmed by restriction digest and Sanger sequencing with the primers M13 forward and M13 reverse.
Once confirmed, the plasmid was introduced into WAC04657 by conjugation (15). Recombinant strains were selected with apramycin and nalidixic acid. Three putative tedF1 disruptants were purified by two rounds of clonal selection on MYM agar with apramycin and nalidixic acid. pOJ260 lacks a Streptomyces origin of replication; thus, the putative disruptants were expected to have integrated the plasmid via a single homologous recombination event, disrupting the tedF1 gene. This was confirmed by PCR, using primer 61 and primer 62, showing the absence of a 2-kb full-length tedF1 gene. In parallel, we used PCR with primer 61 (specific to the genome) and the M13 reverse primer (specific to the vector) to generate a 1.2-kb amplicon and confirm that the insertion vector was located in the tedF1 gene. All mutant strains showed the expected presence or absence of amplicons.
To overexpress tedR, we amplified the gene by PCR with WAC04657 chromosomal DNA and the primers AGGAGGTCACACAAGACATGCGATTCGAAATC and ACCGCTCGTTTCACGCCGCT. The amplicon was blunt-end cloned into the EcoRV site of pSET152-ermE*p-null (version LK) (16, 17). Candidates were confirmed by restriction digestion, followed by DNA sequencing using the primers GCTGGCGAAAGGGGGATGT and TAGCTCACTCATTAGGCACC. The plasmid was then introduced into WAC04657, S. atroolivaceus, S. globisporus, or Streptomyces sp. LaPpAH-202 using conjugation as described above.
Genome sequencing and annotation.
For 454 sequencing (data not shown), genomic DNA was extracted as previously described (7). The genome was sequenced on a 454 GS FLX+ instrument at McMaster University using 37.5% of a sequencing plate. The genome was assembled using MIRA, version 3.4.1.1 (18), into 513 contigs. Open reading frames were assigned using Prodigal (19). For the SMRT (single-molecule real-time) sequencing, genomic DNA was extracted as above but, instead, a Qiagen Genomic-tip 20/G kit was used, as described by the manufacturer, with the exception that the lysis protocol for the 100/G tip was used in place of the 20/G tip protocol. The entire mixture was then loaded onto the 20/G tip, and the protocol was resumed as described in the manual (20). Genome sequencing was performed at Genome Quebec (Montreal, Canada) using the SMRT bell library prep and four SMRT sequencing cells on a PacBio RSII sequencer. The genome was assembled by Genome Quebec using the hierarchical genome-assembly process (HGAP) (21), which resulted in eight contigs (numbered 0 to 7; also called unitigs).
These contigs were screened for vector contamination using the NCBI's VecScreen tool (http://www.ncbi.nlm.nih.gov/tools/vecscreen/). This identified contigs 6 and 7 as clear vector contamination from pBR322. These contigs were removed from the genome record. Contig 5 also showed possible vector contamination, but a strong match to a vector could not be found on the NCBI sequence databases (http://blast.ncbi.nlm.nih.gov/). An analysis of the putative open reading frames (ORFs) of contig 5 showed that the closest homologues were all found within Gammaproteobacteria. This suggested that this DNA sequence was likely foreign to WAC04657, and, as a result, this contig was also removed from the sequencing record. The only other contig that showed vector contamination was contig 0. This potential contamination, identified as originating from pGGC011 and pHSG664, fell within the center of the contig (at 1.9-Mb and 2.1-Mb positions) within, respectively, the open reading frames of a putative σ70 protein and ribosomal protein. The full-length plasmid sequences were used as query in a BLAST search against the WAC04657 genome sequence and were not found to align to contig 0. Taken together, these data suggested that the vector contamination in contig 0 was not true contamination. Thus, no action was taken to remove these sequences. Open reading frames were assigned using Prodigal. Protein annotations were made by manually searching the NCBI Conserved Domain Database (CDD; http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml). Gene clusters were annotated using antiSMASH, version 2.0 (22). The genomic data were searched using the stand-alone version of the BLAST suite (23).
Identification of the ted genes in other organisms.
To find the ted cluster in other organisms, the tedF1 gene was used as a query to search the NCBI's RefSeq databases (http://blast.ncbi.nlm.nih.gov/). The genome sequences and protein sequences of these organisms were downloaded and searched for all the genes in the antiSMASH-designated ted cluster. Genes which showed good homology (>50% percent identity as identified by blastp search) and synteny were marked as common between the clusters.
Small-scale extractions of Streptomyces and HPLC analysis.
A total of 105 CFU of spores suspended in 50 μl of 0.85% saline was plated onto 10 ml of MYM agar plates and allowed to incubate at 30°C. After the desired growth time (growth times varied; see individual figures for incubation times), the agar and cell mat were cut into 1-cm2 chunks and extracted with 10 ml of ethyl acetate overnight. The extract was separated from the agar chunks by filtration through Whatman paper, collected in test tubes, and dried in a centrifugal evaporator set to a maximum temperature of 30°C. The dried extract was suspended in 200 μl of 50% aqueous acetonitrile–0.1% formic acid by sonication, washed with hexane, and centrifuged at 21,000 × g for 30 min at 4°C to remove particulates. The samples were then loaded into Robovials and separated by HPLC with H2O–0.1% formic acid (solvent A) and acetonitrile–0.1% formic acid (solvent B) mobile phases on an XSelect charged-surface hybrid (CSH) C18 column (5-μm particle size, 4.6 by 150 mm [Waters]) as follows: monitor at 271 nm, flow rate of 1 ml min−1, and temperature of 35°C; 20% B at 0.0 min, 40% B at 3.0 min, 40% B at 8.0 min, 95% B at 8.5 min, 95% B at 10.5 min, 20% B at 11.0 min, and 20% B at 15.0 min.
Purification and MICs of W5.9.
Solid MYM agar culture (6.4 liters) of WAC04657/pSET152-ermE*p-tedR was incubated for 48 h, macerated, and extracted with an equal volume of ethyl acetate overnight. The resulting extract was filtered through cotton and then a coffee filter followed by Whatman 1 filter paper and dried under vacuum. The resulting oily, brown extract (481.7 mg) was suspended in 2 ml of 50% aqueous acetonitrile and loaded onto a Sep-Pak C18 column (Waters). The column was washed with 10 ml each of 100% H2O and 30% aqueous acetonitrile. Molecule W5.9 (the WAC04657-produced molecule with an HPLC retention time of 5.9 min) was eluted with 70% aqueous acetonitrile, concentrated in a centrifugal evaporator (maximum temperature of 30°C), and brought to dryness by lyophilization (60.3 mg). The sample was dissolved in 500 μl of 50% aqueous acetonitrile–0.1% formic acid and, after a hexane wash and filtration through a 0.45-μm-pore-size filter, the extract was purified by reverse-phase chromatography on a C18 column using the same HPLC protocol described for the small-scale extracts. The peak eluting at 5.9 min was collected and lyophilized to yield a white powder (5.7 mg). This was suspended in 50% aqueous acetonitrile–0.1% formic acid and further purified by reverse-phase chromatography (Phenomenex Luna 5u PFP(2); 100 Å, 250 by 4.60 mm) using an isocratic method of 25% aqueous acetonitrile–0.1% formic acid at 1 ml min−1 and 35°C and collecting the peak which eluted at 12.4 min. After lyophilization, this yielded pure W5.9 (2.6 mg). MICs were determined by the agar dilution method as previously reported for 13-dTDM (7). Briefly, 2-fold dilutions of W5.9 in methanol were added to molten agar to yield a final concentration ranging from 64 μg ml−1 to 1 μg ml−1. Once plates had solidified, 5-μl droplets of indicator organism diluted to an optical density at 600 nm (OD600) of 0.001 were pipetted onto the plates and allowed to soak into the agar. Plates were incubated for 16 h at 37°C. The MIC is the lowest concentration of antibiotic at which no growth is visible on the plate.
Purification and structural elucidation of TDM and dhTDM.
Either S. atroolivaceus/pSET152-ermE*p-tedR or S. globisporus/pSET152-ermE*p-tedR was grown on MYM agar for 4 days. The agar and cell mat were macerated, extracted with an equal volume of ethyl acetate, and then concentrated to dryness. The extract was then suspended in pure acetonitrile–0.1% formic acid, washed with hexane, filtered through a 0.45-μm-pore-size filter, and separated by HPLC on a C18 column as described for the small-scale extracts.
To purify TDM, the peak eluting at 6.4 min was collected and lyophilized. The resulting molecule was suspended in 100% acetonitrile–0.1% formic acid and separated by HPLC with H2O–0.1% formic acid (solvent A) and acetonitrile–0.1% formic acid (solvent B) mobile phases on an PFP column (Phenomenex Luna 5u PFP(2); 100 Å, 250 by 4.60 mm) as follows: monitor at 271 nm, flow rate of 1 ml min−1, and temperature of 35°C; 28% B at 0.0 min, 28% B at 10.0 min, 32% B at 15.0 min, 80% B at 15.5 min, 80% B at 17.5 min, 28% B at 18.0 min, and 28% B at 22.0 min. The peak eluting at 14.4 min was collected and lyophilized to yield pure TDM. A sample of TDM was suspended in chloroform and loaded into a 3-mm NMR tube. The following spectra were collected: 1H, CRAPT (24), heteronuclear single-quantum coherence (HSQC), correlation spectroscopy (COSY), and heteronuclear multiple-bond correlation (HMBC). The identity of TDM was confirmed by comparing the observed NMR spectra (see Table S2 and Fig. S10 to S14 in the supplemental material) against the published record (6).
To purify dhTDM, the peak eluting from the C18 column at 5.4 min was collected and lyophilized. The resulting molecule was suspended in 100% acetonitrile–0.1% formic acid and separated by HPLC with H2O–0.1% formic acid (solvent A) and acetonitrile–0.1% formic acid (solvent B) mobile phases on a PFP column (Phenomenex Luna 5u PFP [2]; 100 Å, 250 by 4.60 mm) as follows: monitor at 250 nm, flow rate of 1 ml min−1, temperature of 35°C, and isocratic 25% B. The peak eluting at 11.2 min was collected and lyophilized to yield dhTDM. A sample of dhTDM was suspended in methanol and loaded into a 3-mm NMR tube. The following spectra were collected: 1H, CRAPT, HSQC, COSY, and HMBC. The identity of dhTDM was confirmed by comparing the observed spectra (see Table S2 and Fig. S11 to S19 in the supplemental material) against the published record (6).
Nucleotide sequence accession numbers.
The genome sequence for Streptomyces strain WAC04657 was deposited in DDBJ/EMBL/GenBank under accession number LQYF00000000. The accession numbers of all proteins can be found in Table S1 in the supplemental material.
RESULTS
The WAC04657 genome sequence.
To identify the biosynthetic genes for 13-dTDM (the ted genes), we sequenced the genome of WAC04657 using 454 pyrosequencing and single-molecule real-time (SMRT or PacBio) sequencing (25, 26). We initially searched the assembled 454 sequences (513 contigs [data not shown]) using a BLAST search with Tmn16 as a query and identified a single open reading frame encoding an FkbH-like protein. We gave this open reading frame the provisional name tedF1. Importantly, TedF1 is predicted to possess the amino-terminal extension that is characteristic of FkbH-like proteins that participate in tetronate ring formation (see Fig. S1 in the supplemental material). On the same strand, 1.5 kb downstream of tedF1, we found an incomplete open reading frame, provisionally named tedS1, encoding a putative type I PKS. The 454 sequencing-derived contig encoding these genes ended in the middle of tedS1, thus providing us with an incomplete biosynthetic gene cluster.
The SMRT sequencing generated 200,115 reads and a total of 1.317 Gbp of sequence. From this we assembled a nearly complete WAC04657 genomic sequence consisting of five contigs with a total length of 7.761 Mb at 169.7× coverage. We used the annotation program antiSMASH (22) to identify 27 putative biosynthetic gene clusters for secondary metabolites in this genome sequence (Fig. 2). These include three PKS clusters (two type I and one type II), eight nonribosomal peptide synthase (NRPS) clusters, seven terpenoid clusters, two siderophores, and several others. The two predicted type I PKS gene clusters (Fig. 3, red and blue bars) overlapped, raising the possibility that they in fact constitute a single cluster. The PKS genes themselves were separated by 22.5 kb. One cluster (red in Fig. 2 and 3) included tedF1, tedS1, and another polyketide synthase gene that we named tedS2.
FIG 2.
Location of the biosynthetic gene clusters in the genome of WAC04657. The genome of WAC04657 has been assembled into five contigs (or unitigs) with a total length of 7.761 Mb and possessing a predicted 27 biosynthetic gene clusters. Using antiSMASH, we identified the location of the biosynthetic gene clusters found in the organism. Hollow circles represent the location of putative biosynthetic gene clusters on the genome, while the connected, filled circles indicate the type of biosynthetic gene cluster at that location. The red and blue circles mark two closely located type I PKS clusters. The order of the contigs is unconfirmed. rev, contigs whose nucleotide sequence was reverse complemented compared to those of the deposited sequence.
FIG 3.
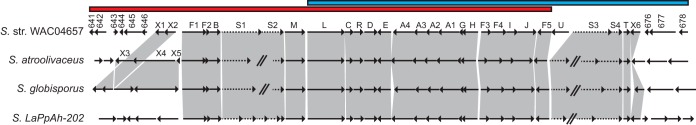
Position and overlap of PKS clusters and comparison of the ted cluster as found in WAC04657, S. atroolivaceus, S. globisporus, and Streptomyces sp. LaPpAH-202. The blue and red bars denote the location and position of overlap of the two type I PKS clusters that antiSMASH identified as distinct in WAC04657. For the purpose of visualization, the red cluster is truncated at its 5′ end, and the blue cluster is truncated at its 3′ end. The arrows represent genes found in each of the organisms. Shaded regions linking the arrows denote that the genes are syntenic. Hatch marks represent breaks in contigs. Solid arrows represent genes drawn to scale, while dashed arrows represent genes that are not to scale.
13-dTDM has 18 carbons of which 3 are predicted to come from 1,3-bisphosphoglycerate (10) and the other 15 are predicted to be PKS derived. Assembly of 13-dTDM should, therefore, require seven PKS modules. tedS1 and tedS2, however, encode three modules and one module, respectively. To ensure that this assignment was not due to a sequence assembly artifact, we searched the raw SMRT data and identified a single 19.5-kb read that spanned the entire length of both of the PKS genes. This confirmed that the predicted open reading frames were likely to be accurate in length. We noted, however, that the PKS genes in the adjacent cluster (Fig. 3, blue bar) encoded three modules, suggesting that they might serve to add the remaining carbons of the 13-dTDM backbone. Supporting this, the PKS genes of the second gene cluster (now named tedS3 and tedS4) lack the canonical loading/first module expected for PKS enzymes and, therefore, could not likely initiate polyketide biosynthesis on their own (27). Furthermore, antiSMASH predicted that the product of tedS2 ends with a putative docking domain and that the PKS genes in the second cluster (tedS3) start with a putative docking domain (28, 29), suggesting that they form a multiprotein complex. We thus hypothesized that the two type I PKS clusters identified as separate clusters by antiSMASH were, in fact, a single cluster which constitutes the 13-dTDM biosynthetic gene cluster.
Conservation of tedF1-containing gene clusters.
It is challenging to identify the boundaries of secondary metabolic gene clusters using gene annotation analysis alone. To simplify this problem, we looked for similar biosynthetic gene clusters in different bacterial species. We searched the NCBI RefSeq database (http://blast.ncbi.nlm.nih.gov/) using TedF1 as a query. Most of the homologs of TedF1 were identified in species of Streptomyces although we also found representatives from Actinoplanes, Saccharothrix, Frankia, Herbidospora, Dactylosporangium, and Verrucosispora (data not shown). In particular, we identified three proteins that shared greater than 84% identity with TedF1. These proteins were found in Streptomyces atroolivaceus ATCC 19725, Streptomyces globisporus NRRL B-2293, and Streptomyces sp. LaPpAH-202 (see Table S1 and Fig. S1 in the supplemental material). Using the putative 13-dTDM biosynthetic gene cluster as a guide, we searched the genomes of these organisms and identified the comparable ted-like gene cluster in each of them. We aligned the putative clusters, including several neighboring genes of ambiguous relevance to the ted cluster from WAC04657, taking note of the percent protein identity and synteny of each gene (Fig. 3; see also Table S1). In this way we confirmed that the four clusters were highly similar and bounded by otherwise unrelated genes. The ted cluster of WAC04657 is bordered by genes of unknown function and a transposase on the left side (Fig. 3, genes 641 to 646) as well as by a permease, a protein of unknown function, and a putative fasciclin on the right side (Fig. 3, genes 676 to 678). These genes are not found in the corresponding boundary regions of the other three clusters. The bordering genes in the other three clusters are similarly unrelated to each other. We concluded that the strongest prediction for a 13-dTDM biosynthetic gene cluster is as shown in Fig. 4.
FIG 4.
Proposed biosynthetic gene cluster for 13-dTDM as found in WAC04657. The genes tedX3, tedX4, and tedX5 are found only in S. atroolivaceus and S. globisporus, but their positions relative to the WAC04657 ted cluster are marked with a curly brace. Genes are drawn to scale.
A biosynthetic gene cluster for tetrodecamycin antibiotics.
The core ted gene cluster is bounded by tedF1 and tedT (Table 1). The genes within this sequence are syntenic between all four organisms and share at least 63% identity (see Table S1 in the supplemental material). The cluster encodes four PKS enzymes, tedS1 to tedS4, that together possess the seven modules predicted to produce the backbone of 13-dTDM. We predict that the tetronate ring is formed by five enzymes that first form a five-membered lactone ring and then generate the exocyclic methylene (Fig. 1, position C-5 in both 13-dTDM and TDM). Supporting this pathway, putative enzymes necessary for these reactions are encoded by genes in the ted cluster (tedF1 to tedF5) (10, 11, 30). Other putative proteins in the core of the cluster include a major facilitator superfamily (MFS)-type pump (tedM) and an ABC transporter (tedA1 to tedA4) and three regulatory genes consisting of an LAL family regulator (tedL), a Streptomyces antibiotic regulatory protein (SARP) family regulator (tedR), and a TetR family regulator (tedT), as well as candidate tailoring genes (tedC, -D, -E, -G, -H, -I, and -J). The only gene in the putative ted cluster that is not conserved between WAC04657 and the other three organisms is tedU, which encodes a protein of unknown function.
TABLE 1.
Predicted functions of ted cluster genes
| Name | Description of gene and/or gene producta |
|---|---|
| tedX1 | 4′-PPT |
| tedX2 | 3′,5′-Cyclic AMP phosphodiesterase, CpdA-like protein |
| tedX3 | Lantibiotic cyclase, lanC-like gene |
| tedX4 | Lantibiotic dehydratase, thiopeptide-type bacteriocin biosynthesis domain |
| tedX5 | Small peptide |
| tedF1 | Tetronate ring tailoring enzyme, fkbH-like gene |
| tedF2 | Tetronate ring tailoring enzyme, fkbH-associated ACP |
| tedB | Thioesterase |
| tedS1 | Polyketide synthase (ATAc-ACP, KS-ATmal-DH-KR-ACP, KS-ATmal-DH-KR-ACP) |
| tedS2 | Polyketide synthase, docking domain (KS-ATmal-DH-ER-KR-ACP) |
| tedM | Major facilitator superfamily (MFS) efflux pump |
| tedL | Large ATP-binding luxR (LAL)-type regulator |
| tedC | Flavin reductase, potentially uses the coenzyme F420 |
| tedR | Streptomyces antibiotic regulatory protein (SARP)-type regulator |
| tedD | FMN-linked alkanal monooxygenase, potentially uses F420 |
| tedE | FMN-linked alkanal monooxygenase, potentially uses F420 |
| tedA4 | ABC transporter, ATP-binding protein |
| tedA3 | ABC transporter, permease |
| tedA2 | ABC transporter, permease |
| tedA1 | ABC transporter, substrate-binding protein |
| tedG | Ferredoxin |
| tedH | Cytochrome P450 |
| tedF3 | Tetronate ring tailoring enzyme, acyltransferase |
| tedF4 | Tetronate ring tailoring enzyme, hydrolase |
| tedI | F420-dependent oxidoreductase |
| tedJ | Short-chain dehydrogenase/reductase |
| tedF5 | Tetronate ring tailoring enzyme, fabH-like gene |
| tedU | Predicted protein, conserved domain DUF4157 |
| tedS3 | Polyketide synthase, docking domain (KS-ATmal-DH-ER-KR-ACP, KS-ATmmal-DH-KR-ACP) |
| tedS4 | Polyketide synthase, docking domain (KS-ATmal-ACP) |
| tedT | tetR family regulator |
| tedX6 | Thioesterase |
Function was annotated by searching the protein sequence for conserved domains in the NCBI Conserved Domain Database or, for the PKS genes, with the use of antiSMASH. PPT, phosphopantetheine transferase; FMN, flavin mononucleotide; ATAc, acetyl coenzyme A acyltransferase; DH, dehydratase; KR, ketoreductase; KS, ketosynthase; ATmal, malonyl coenzyme A acyltransferase; ER, enoyl reductase; ATmmal, methylmalonyl coenzyme A acyltransferase; ABC, ATP-binding cassette.
To confirm the involvement of this cluster in the biosynthesis of 13-dTDM, we disrupted the tedF1 gene in WAC04657 by inserting the pOJ260 plasmid into the middle of the open reading frame with a single crossover. Three candidate mutants were isolated, and insertion of the pOJ260 plasmid into tedF1 was confirmed by PCR. We were unable to detect 13-dTDM in extracts of any of these mutants (Fig. 5). As a second approach, we constructed a vector in which the tedR gene, which encodes a putative SARP (31) for the activation of the rest of the biosynthetic genes, was cloned into the pSET152 plasmid (16) behind the strong, constitutive promoter ermE* (17). The plasmid was introduced into WAC04657, and three candidates were purified and tested for yields of 13-dTDM. When tested after 24 h and 48 h of growth, the ermE*-tedR strain was found to produce substantially more 13-dTDM than the control organism containing the pSET152-ermE*p vector lacking tedR (Fig. 5). The facts that the tedF1 disruption mutant failed to produce 13-dTDM and that the tedR overexpression strain overproduces the compound demonstrate that the ted biosynthetic gene cluster encodes the biosynthetic pathway for 13-dTDM.
FIG 5.
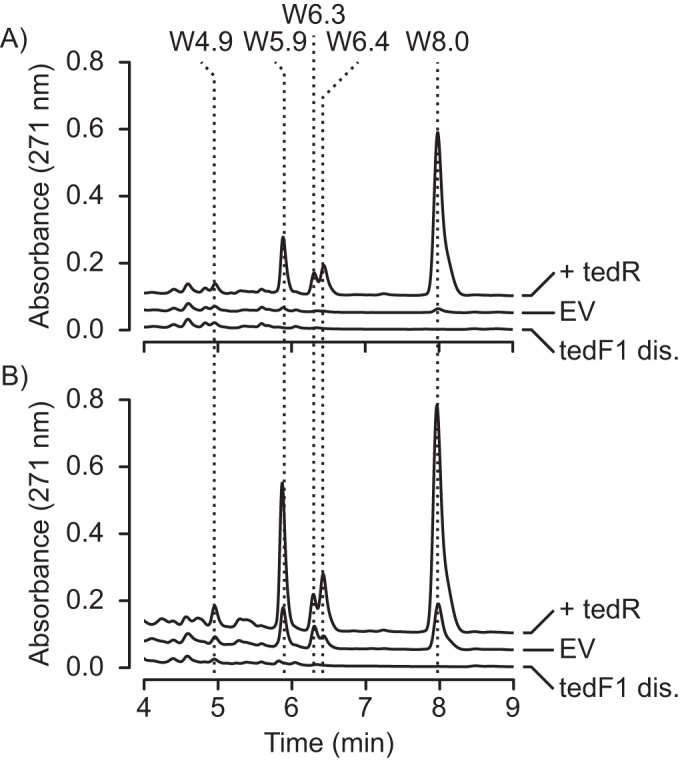
Genetic manipulation of the ted cluster in WAC04657 results in changes in 13-dTDM production as well as altered expression of several other molecules. Differences in production of the TDM family molecules can be seen after both 24 h of growth (A) and 48 h of growth (B). +tedR, ermE*p-tedR-expressing strain; EV, WAC04657 possessing the pSET152-ermE*p empty vector control plasmid; tedF1 dis., WAC04657 with a disruption mutation in the tedF1 gene. Dotted lines mark the positions of molecules which appear to be responsive to genetic manipulation of the ted gene cluster.
A series of related tetrodecamycins in WAC04657, S. atroolivaceus, and S. globisporus.
Interestingly, when we compared the HPLC chromatograms of wild-type WAC04657, the tedF1 disruption, and the tedR overexpression strains, we found that several other compounds were produced in tandem with 13-dTDM (Fig. 5 and Table 2). 13-dTDM corresponds to the m/z 319.1548 [M+H]+ compound that eluted at 8.0 min. Additional compounds at 4.9 min (m/z 353.1598 [M+H]+), 5.9 min (m/z 337.1664 [M+H]+), 6.3 min (m/z 341.1364 [M+Na]+), and 6.4 min (m/z 335.1496 [M+H]+) were all lost upon disruption of the tedF1 gene and enhanced in the tedR overexpression strain, suggesting that they might also be products of the ted cluster-encoded pathway. We refer to these compounds as W4.9, W5.9, W6.3, and W6.4 to indicate their retention time during HPLC and that they are produced by WAC04657.
TABLE 2.
Observed properties of the TDM series molecules identified in this study
| Name (abbreviation) | Alternate name(s) | Producing strain(s) | Retention time (min) | UV-Vis abs max (nm)a | Mol formula | Mol wt (g/mol) | ESI-MS (m/z)b |
Mass accuracy (ppm) | |
|---|---|---|---|---|---|---|---|---|---|
| Observed | Calculated | ||||||||
| W4.9 | WAC04657 | 4.9 | 260 | C18H24O7 | 352 | 353.1598 | 353.1600 | −0.6 | |
| Dihydro-tetrodecamycin (dhTDM) | A5.4, G5.4 | S atroolivaceus, S. globisporus | 5.4 | 251 | C18H24O6 | 336 | 337.1651 | 337.1651 | 0 |
| W5.9 | WAC04657 | 5.9 | 271 | C18H24O6 | 336 | 337.1664 | 337.1651 | 3.9 | |
| W6.3 | WAC04657 | 6.3 | 243 | C18H22O5 | 318 | 341.1364 | 341.1365 | −0.3 | |
| Tetrodecamycin (TDM) | A6.4, G6.4, W6.4 | WAC04657, S. atroolivaceus, S. globisporus | 6.4 | 271 | C18H22O6 | 334 | 335.1496 | 335.1495 | 0.3 |
| 13-Deoxy-tetrodecamycin (13-dTDM) | W8.0 | WAC04657 | 8.0 | 271 | C18H22O5 | 318 | 319.1548 | 319.1545 | 0.9 |
UV-Vis absorbance maximum (abs max) was recorded on an HPLC column in a solution of 40% aqueous acetonitrile–0.1% formic acid.
ESI, electrospray ionization.
We purified the most abundant of these compounds, W5.9, from the tedR overexpression strain and solved its structure using NMR (see the supplemental material). This molecule corresponds to structure 2 in Fig. 1. We tested this compound for antibacterial activity against B. subtilis, S. aureus, M. luteus, MRSA, S. epidermidis, E. faecalis, E. coli, B. cepacia, A. baumannii, and K. pneumoniae. While 13-dTDM, as previously demonstrated (7), had MICs against all the tested Gram-positive bacteria in the range of 1 to 8 µg ml−1, W5.9 had no activity against any of these strains even at concentrations as high as 64 µg ml−1.
Given the presence of apparent ted biosynthetic gene clusters in S. atroolivaceus, S. globisporus, and Streptomyces sp. LaPpAH-202, we set out to determine whether these organisms produce 13-dTDM or any related compounds. We prepared extracts of all three strains and compared them to the extract of WAC04657. None of the organisms produced 13-dTDM, but S. globisporus extracts contained a compound, G6.4, which absorbed light at 271 nm and had a retention time of 6.4 min; this was identical to characteristics of compound W6.4 produced by WAC04657 (Fig. 6B). The ted gene clusters in S. atroolivaceus, S. globisporus, and Streptomyces sp. LaPpAH-202 also encode a SARP, having, respectively, 88%, 95%, and 86% identity to TedR of WAC04657 (TedRW). We therefore introduced the ermE*p-tedR plasmid and its control vector into each of these strains and again tested TDM yields. Consistent with the relatedness of these gene clusters, the presence of the ermE*p-tedR plasmid induced molecules eluting at 5.4 min and 6.4 min in S. atroolivaceus (A5.4 and A6.4, respectively) (Fig. 6A) and one eluting at 6.4 min in S. globisporus (G6.4) (Fig. 6B). Indeed, with prolonged growth, small amounts of a compound eluting at 5.4 min were also observed in S. globisporus (G5.4) (see Fig. S8 in the supplemental material). A5.4 and G5.4 shared a UV-visible light (Vis) absorbance maximum of 251 nm.
FIG 6.

Heterologous overexpression of tedRW in other strains of Streptomyces with the ted genes results in stimulation of TDM family molecules. (A) The stimulation of the molecules A5.4 and A6.4 is particularly clear in S. atroolivaceus, shown here after 5 days of growth. (B) In S. globisporus, there is a significant increase in the amount of G6.4 produced after 24 h of growth although stimulation of G5.4 is considerably less obvious. EV, pSET152-ermE*p, empty vector-expressing strain; +tedR, the ermE*p-tedR-expressing strain.
We used mass spectrometry (MS) to compare W6.4, A6.4, and G6.4 and found that all of them have m/z values of 335.1496 [M+H]+, corresponding to a molecular formula of C18H22O6, the same as that reported for TDM. Tandem MS (MS/MS) confirmed that all of the molecules fragment to the same daughter ions (and thus represent the same molecule) (see Fig. S9 in the supplemental material), and the identity of G6.4 (and thus W6.4 and A6.4 by association) was confirmed by NMR to be TDM (molecule 3 in Fig. 1; see also Table S2 and Fig. S10 to S14 in the supplemental material). We additionally purified G5.4 and, from the tedR overexpression strain of S. atroolivaceus, A5.4. Both molecules had m/z values of 337.1651 [M+H]+, corresponding to a molecular formula of C18H24O6, the same as that for dhTDM. MS/MS confirmed that both molecules fragmented to the same daughter ions (see Fig. S9), and their identity as dhTDM was confirmed by NMR (molecule 4 in Fig. 1; see also Table S2 and Fig. S15 to S19).
We were unable to introduce the ermE*p-tedR expression plasmid into Streptomyces sp. LaPpAH-202 despite being able to introduce the control vector and did not observe production of the TDM family of compounds from this strain.
DISCUSSION
In this work we used a structural feature of the TDM family antibiotics, the tetronate ring, to predict a class of enzyme that would be present in their biosynthetic gene cluster. By searching the genome of a 13-dTDM producer for such an enzyme, we identified a candidate biosynthetic gene cluster and confirmed that it is responsible for 13-dTDM biosynthesis. These experiments also revealed that the biochemical pathway encoded by this gene cluster and its relatives can produce several related molecules, including TDM, 13-dTDM, dhTDM, and W5.9. We found that comparing the ted cluster to related clusters in S. atroolivaceus, S. globisporus, and Streptomyces sp. LaPpAH-202 simplified the identification of the complete gene set as well as the probable edges of the cluster itself.
As biochemical and bioinformatic knowledge improves, using structural features of a molecule to find its cognate gene clusters is becoming increasingly applicable. There are many secondary metabolites that possess chemical modifications, functional groups, or structures that, coupled with the genome sequence, enable accurate prediction of the cognate biosynthetic gene cluster. These include glycosylated compounds (32), nonribosomal peptides (33), enediynes (34), phosphonates (35), and halogenated compounds (36). Identifying related clusters in multiple organisms is also becoming a widely accepted means of informatically characterizing a new gene cluster (for example, the cluster associated with tambromycin [37]). This has particular value in identifying the edges of the cluster, a process that classically involves disrupting genes at the predicted edges of the cluster. Of course, bioinformatically trimmed clusters should be considered with care as it is possible that neighboring, unconserved genes contribute additional decorations to the molecule. For this reason we included tedX1 to tedX6 and tedU as possible members of the ted cluster (Fig. 3 and 4, hatched genes). While tedX1, tedX2, and tedX6 are unlikely to be responsible for the production of W5.9 since all three can be found in S. globisporus (which was not observed to produce W5.9), tedU is found only in WAC04657. This makes it an interesting candidate for involvement in the biosynthesis of W5.9.
Last, it is interesting that 13-dTDM and TDM have equivalent antibacterial activities, yet W5.9 and dhTDM have none. This is consistent with previous work in which one of these molecules, TDM, was chemically modified (38). A feature of interest in the TDM tetronate ring is the possible Michael acceptor that extends through C-6/C-1, C-2, C-3, C-4, and C-5 (Fig. 1). Michael acceptors are constellations of atoms that generate a carbanion (predicted to be C-5 in 13-dTDM and TDM) that can participate in a nucleophilic addition with proteins or other molecules, resulting in the formation of a covalent bond. W5.9 and dhTDM may lack this capacity either because they have a distinct, conjugated double-bond system (as in W5.9) or because the position at which the carbanion is generated is likely to be sterically hindered (C-4 in dhTDM). While this mechanism of action is unproved, it is enticing to consider that the mechanism of action of the TDM antibiotics involves the formation of a covalent bond with their currently unknown molecular targets.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Gerry Wright and Cameron Currie for sharing strains, Sergiy Nokhrin and Darcy Burns for advice regarding NMR spectrometry, Geoff Tranmer for discussing ideas, Nick Waglechner for assistance with 454 sequence assembly, Fred Ulrich for help with Ubuntu, and Ken Dewar and Alex Ensminger for help with SMRT sequencing. We are especially grateful to Sheila Pimentel-Elardo and Glenna Kramer for extensive advice in writing about structural elucidation.
Author contributions were as follows: conceptualization, T.G. and J.R.N.; investigation, T.G.; writing of the original draft, T.G. and J.R.N.; and funding acquisition, J.R.N.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00140-16.
REFERENCES
- 1.Davies JE. 1964. Studies on the ribosomes of streptomycin-sensitive and resistant strains of Escherichia coli. Proc Natl Acad Sci U S A 51:659–664. doi: 10.1073/pnas.51.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tiyanont K, Doan T, Lazarus MB, Fang X, Rudner DZ, Walker S. 2006. Imaging peptidoglycan biosynthesis in Bacillus subtilis with fluorescent antibiotics. Proc Natl Acad Sci U S A 103:11033–11038. doi: 10.1073/pnas.0600829103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. CDC, Atlanta, GA: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. [Google Scholar]
- 4.Tsuchida T, Sawa R, Iinuma H, Nishida C, Kinoshita N, Takahashi Y, Naganawa H, Sawa T, Hamada M, Takeuchi T. 1994. Tetrodecamycin, a new antimicrobial antibiotic from Streptomyces. J Antibiot (Tokyo) 47:386–388. doi: 10.7164/antibiotics.47.386. [DOI] [PubMed] [Google Scholar]
- 5.Tsuchida T, Iinuma H, Nishida C, Kinoshita N, Sawa T, Hamada M, Takeuchi T. 1995. Tetrodecamycin and dihydrotetrodecamycin, new antimicrobial antibiotics against Pasteurella piscicida produced by Streptomyces nashvillensis MJ885-mF8. I. Taxonomy, fermentation, isolation, characterization and biological activities. J Antibiot (Tokyo) 48:1104–1109. [DOI] [PubMed] [Google Scholar]
- 6.Tsuchida T, Iinuma H, Sawa R, Takahashi Y, Nakamura H, Nakamura KT, Sawa T, Naganawa H, Takeuchi T. 1995. Tetrodecamycin and dihydrotetrodecamycin, new antimicrobial antibiotics against Pasteurella piscicida produced by Streptomyces nashvillensis MJ885-mF8. II. Structure determination. J Antibiot (Tokyo) 48:1110–1114. doi: 10.7164/antibiotics.48.1110. [DOI] [PubMed] [Google Scholar]
- 7.Gverzdys T, Hart MK, Pimentel-Elardo S, Tranmer G, Nodwell JR. 2015. 13-Deoxytetrodecamycin, a new tetronate ring-containing antibiotic that is active against multidrug-resistant Staphylococcus aureus. J Antibiot (Tokyo) 68:698–702. doi: 10.1038/ja.2015.60. [DOI] [PubMed] [Google Scholar]
- 8.Vieweg L, Reichau S, Schobert R, Leadlay PF, Süssmuth RD. 2014. Recent advances in the field of bioactive tetronates. Nat Prod Rep 31:1554–1584. doi: 10.1039/C4NP00015C. [DOI] [PubMed] [Google Scholar]
- 9.Keller-Juslén C, King HD, Kuhn M, Loosli HR, Pache W, Petcher TJ, Weber HP, von Wartburg A. 1982. Tetronomycin, a novel polyether of unusual structure. J Antibiot (Tokyo) 35:142–150. doi: 10.7164/antibiotics.35.142. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Hong H, Gillies F, Spencer JB, Leadlay PF. 2008. Glyceryl-S-acyl carrier protein as an intermediate in the biosynthesis of tetronate antibiotics. Chembiochem 9:150–156. doi: 10.1002/cbic.200700492. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y, Hahn F, Demydchuk Y, Chettle J, Tosin M, Osada H, Leadlay PF. 2010. In vitro reconstruction of tetronate RK-682 biosynthesis. Nat Chem Biol 6:99–101. doi: 10.1038/nchembio.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motamedi H, Shafiee A. 1998. The biosynthetic gene cluster for the macrolactone ring of the immunosuppressant FK506. Eur J Biochem 256:528–534. doi: 10.1046/j.1432-1327.1998.2560528.x. [DOI] [PubMed] [Google Scholar]
- 13.Wenzel SC, Williamson RM, Grünanger C, Xu J, Gerth K, Martinez RA, Moss SJ, Carroll BJ, Grond S, Unkefer CJ, Müller R, Floss HG. 2006. On the biosynthetic origin of methoxymalonyl-acyl carrier protein, the substrate for incorporation of “glycolate” units into ansamitocin and soraphen A. J Am Chem Soc 128:14325–14336. doi: 10.1021/ja064408t. [DOI] [PubMed] [Google Scholar]
- 14.Flett F, Mersinias V, Smith CP. 1997. High efficiency intergeneric conjugal transfer of plasmid DNA from Escherichia coli to methyl DNA-restricting streptomycetes. FEMS Microbiol Lett 155:223–229. doi: 10.1111/j.1574-6968.1997.tb13882.x. [DOI] [PubMed] [Google Scholar]
- 15.Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA. 2000. Practical Streptomyces genetics. The John Innes Foundation, Norwich, England. [Google Scholar]
- 16.Bierman M, Logan R, O'Brien K, Seno ET, Rao RN, Schoner BE. 1992. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43–49. doi: 10.1016/0378-1119(92)90627-2. [DOI] [PubMed] [Google Scholar]
- 17.Bibb MJ, Janssen GR, Ward JM. 1985. Cloning and analysis of the promoter region of the erythromycin resistance gene (ermE) of Streptomyces erythraeus. Gene 38:215–226. doi: 10.1016/0378-1119(85)90220-3. [DOI] [PubMed] [Google Scholar]
- 18.Chevreux B, Pfisterer T, Drescher B, Driesel AJ, Müller WEG, Wetter T, Suhai S. 2004. Using the miraEST assembler for reliable and automated mRNA transcript assembly and SNP detection in sequenced ESTs. Genome Res 14:1147–1159. doi: 10.1101/gr.1917404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyatt D, Chen G-L, LoCascio PF, Land ML, Larimer FW, Hauser LJ. 2010. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiagen. 2012. Qiagen genomic DNA handbook. Qiagen, Valencia, CA. [Google Scholar]
- 21.Chin C-S, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, Clum A, Copeland A, Huddleston J, Eichler EE, Turner SW, Korlach J. 2013. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods 10:563–569. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- 22.Blin K, Medema MH, Kazempour D, Fischbach MA, Breitling R, Takano E, Weber T. 2013. antiSMASH 2.0—a versatile platform for genome mining of secondary metabolite producers. Nucleic Acids Res 41:W204–W212. doi: 10.1093/nar/gkt449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. 2009. BLAST+: architecture and applications. BMC Bioinformatics 10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burrow TE, Burns DC, Krishnamurthy K, Reynolds WF. 2014. CRAPT: an improved version of APT with compensation for variations in JCH. Magn Reson Chem 52:195–201. doi: 10.1002/mrc.4050. [DOI] [PubMed] [Google Scholar]
- 25.Eid J, Fehr A, Gray J, Luong K, Lyle J, Otto G, Peluso P, Rank D, Baybayan P, Bettman B, Bibillo A, Bjornson K, Chaudhuri B, Christians F, Cicero R, Clark S, Dalal R, Dewinter A, Dixon J, Foquet M, Gaertner A, Hardenbol P, Heiner C, Hester K, Holden D, Kearns G, Kong X, Kuse R, Lacroix Y, Lin S, Lundquist P, Ma C, Marks P, Maxham M, Murphy D, Park I, Pham T, Phillips M, Roy J, Sebra R, Shen G, Sorenson J, Tomaney A, Travers K, Trulson M, Vieceli J, Wegener J, Wu D, Yang A, Zaccarin D, Zhao P, Zhong F, Korlach J, Turner S. 2009. Real-time DNA sequencing from single polymerase molecules. Science 323:133–138. doi: 10.1126/science.1162986. [DOI] [PubMed] [Google Scholar]
- 26.Ronaghi M, Uhlén M, Nyrén P. 1998. A sequencing method based on real-time pyrophosphate. Science 281:363, 365. doi: 10.1126/science.281.5375.363. [DOI] [PubMed] [Google Scholar]
- 27.Fischbach MA, Walsh CT. 2006. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: logic, machinery, and mechanisms. Chem Rev 106:3468–3496. doi: 10.1021/cr0503097. [DOI] [PubMed] [Google Scholar]
- 28.Dutta S, Whicher JR, Hansen DA, Hale WA, Chemler JA, Congdon GR, Narayan ARH, Håkansson K, Sherman DH, Smith JL, Skiniotis G. 2014. Structure of a modular polyketide synthase. Nature 510:512–517. doi: 10.1038/nature13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gokhale RS, Tsuji SY, Cane DE, Khosla C. 1999. Dissecting and exploiting intermodular communication in polyketide synthases. Science 284:482–485. doi: 10.1126/science.284.5413.482. [DOI] [PubMed] [Google Scholar]
- 30.Kanchanabanca C, Tao W, Hong H, Liu Y, Hahn F, Samborskyy M, Deng Z, Sun Y, Leadlay PF. 2013. Unusual acetylation-elimination in the formation of tetronate antibiotics. Angew Chem Int Ed Engl 52:5785–5788. doi: 10.1002/anie.201301680. [DOI] [PubMed] [Google Scholar]
- 31.Liu G, Chater KF, Chandra G, Niu G, Tan H. 2013. Molecular regulation of antibiotic biosynthesis in Streptomyces. Microbiol Mol Biol Rev 77:112–143. doi: 10.1128/MMBR.00054-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kersten RD, Ziemert N, Gonzalez DJ, Duggan BM, Nizet V, Dorrestein PC, Moore BS. 2013. Glycogenomics as a mass spectrometry-guided genome-mining method for microbial glycosylated molecules. Proc Natl Acad Sci U S A 110:E4407–E4416. doi: 10.1073/pnas.1315492110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohimani H, Liu W-T, Kersten RD, Moore BS, Dorrestein PC, Pevzner PA. 2014. NRPquest: coupling mass spectrometry and genome mining for nonribosomal peptide discovery. J Nat Prod 77:1902–1909. doi: 10.1021/np500370c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudolf JD, Yan X, Shen B. 2016. Genome neighborhood network reveals insights into enediyne biosynthesis and facilitates prediction and prioritization for discovery. J Ind Microbiol Biotechnol 43:261–276. doi: 10.1007/s10295-015-1671-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ju K-S, Gao J, Doroghazi JR, Wang K-KA, Thibodeaux CJ, Li S, Metzger E, Fudala J, Su J, Zhang JK, Lee J, Cioni JP, Evans BS, Hirota R, Labeda DP, van der Donk WA, Metcalf WW. 2015. Discovery of phosphonic acid natural products by mining the genomes of 10,000 actinomycetes. Proc Natl Acad Sci U S A 112:12175–12180. doi: 10.1073/pnas.1500873112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao L, Chen R, Jiang M, Tian X, Liu H, Yu Y, Fan C, Chen B. 2016. Bioprospecting potential of halogenases from Arctic marine actinomycetes. BMC Microbiol 16:34. doi: 10.1186/s12866-016-0662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goering AW, McClure RA, Doroghazi JR, Albright JC, Haverland NA, Zhang Y, Ju K-S, Thomson RJ, Metcalf WW, Kelleher NL. 2016. Metabologenomics: correlation of microbial gene clusters with metabolites drives discovery of a nonribosomal peptide with an unusual amino acid monomer. ACS Cent Sci 2:99–108. doi: 10.1021/acscentsci.5b00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuchida T, Iinuma H, Nakamura KT, Nakamura H, Sawa T, Hamada M, Takeuchi T. 1995. Derivatives of tetrodecamycin. J Antibiot (Tokyo) 48:1330–1335. doi: 10.7164/antibiotics.48.1330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.