Abstract
Background
The epidemiology of avian hematozoa at high latitudes is still not well understood, particularly in sub-Arctic and Arctic habitats, where information is limited regarding seasonality and range of transmission, co-infection dynamics with parasitic and viral agents, and possible fitness consequences of infection. Such information is important as climate warming may lead to northward expansion of hematozoa with unknown consequences to northern-breeding avian taxa, particularly populations that may be previously unexposed to blood parasites.
Methods
We used molecular methods to screen blood samples and cloacal/oropharyngeal swabs collected from 1347 ducks of five species during May-August 2010, in interior Alaska, for the presence of hematozoa, Influenza A Virus (IAV), and IAV antibodies. Using models to account for imperfect detection of parasites, we estimated seasonal variation in prevalence of three parasite genera (Haemoproteus, Plasmodium, Leucocytozoon) and investigated how co-infection with parasites and viruses were related to the probability of infection.
Results
We detected parasites from each hematozoan genus in adult and juvenile ducks of all species sampled. Seasonal patterns in detection and prevalence varied by parasite genus and species, age, and sex of duck hosts. The probabilities of infection for Haemoproteus and Leucocytozoon parasites were strongly positively correlated, but hematozoa infection was not correlated with IAV infection or serostatus. The probability of Haemoproteus infection was negatively related to body condition in juvenile ducks; relationships between Leucocytozoon infection and body condition varied among host species.
Conclusions
We present prevalence estimates for Haemoproteus, Leucocytozoon, and Plasmodium infections in waterfowl at the interface of the sub-Arctic and Arctic and provide evidence for local transmission of all three parasite genera. Variation in prevalence and molecular detection of hematozoa parasites in wild ducks is influenced by seasonal timing and a number of host traits. A positive correlation in co-infection of Leucocytozoon and Haemoproteus suggests that infection probability by parasites in one or both genera is enhanced by infection with the other, or that encounter rates of hosts and genus-specific vectors are correlated. Using size-adjusted mass as an index of host condition, we did not find evidence for strong deleterious consequences of hematozoa infection in wild ducks.
Electronic supplementary material
The online version of this article (doi:10.1186/s13071-016-1666-3) contains supplementary material, which is available to authorized users.
Keywords: Hematozoa, Blood parasites, Co-infection, Occupancy models, Detection probability, Haemoproteus, Leucocytozoon, Plasmodium, Influenza A Virus, Waterfowl
Background
Protozoan blood parasites of the order Haemosporidia, or hematozoa, are transmitted by arthropod vectors and infect a broad diversity of avian taxa. The three most widely studied genera of hematozoa, Leucocytozoon, Haemoproteus, and Plasmodium, are putatively transmitted by the genus-specific dipteran vectors: black flies (Simuliidae), biting midges (Ceratopogonidae), and mosquitoes (Culicidae), respectively [1]. Endemic lineages of blood parasites circulating in wild bird populations may be associated with low virulence as evidence of direct mortality is rare, presumably due to long-term, host-pathogen evolutionary associations [2]. However, exposure of naïve hosts to hematozoa parasites may cause mortality and morbidity. For instance, the introduction of Plasmodium parasites to endemic Hawaiian avian taxa is thought to be responsible for reductions in abundance of native bird populations [3–5], and hematozoa infection can cause high rates of mortality in domestic birds (reviewed by [2]). Furthermore, species-specific variation in pathogenic outcomes resulting from experimental inoculation, ranging from 100 % mortality to resistance, suggests that susceptibility and virulence are influenced by host resistance developed through co-adaptation [6–9].
Environmental conditions influence the distribution and transmission of avian blood parasites [10, 11], and in North America, the abundance and diversity of avian hematozoa appears to vary across ecoregions [12]. Although Leucocytozoon, Haemoproteus, and Plasmodium parasites are known to infect birds in northern regions of North America, prevalence of these parasites vary. Leucocytozoon parasites appear to be the most common and widely distributed avian hematozoa at high latitudes, having been identified in juvenile or non-migratory resident birds as far north as the Arctic Coastal Plain [13–17], which is indicative of local transmission and completion of the parasite life-cycle. Although generally identified at lower prevalence, Haemoproteus and Plasmodium parasites are also transmitted among birds in temperate and sub-arctic regions [14, 17–20], but only sporadic infections of these genera have been detected north of the Arctic Circle, and to date, there is no direct evidence of Plasmodium transmission among birds in the North American Arctic [13, 21, 22]. Therefore, the geographic leading edge of Plasmodium and Haemoproteus transmission in North America appears to be at the interface of the Arctic and sub-Arctic.
Changing climatic conditions in arctic regions, such as accelerated warming [23], may increasingly favor northward range expansion of hematozoa [24, 25]. In North America, hematozoa transmission occurs primarily during summer months on the breeding grounds [1, 26], presenting the potential for parasite range expansion into previously unexposed bird populations that breed in arctic regions [17, 21]. Climatic changes may also promote increases in parasite transmission and prevalence in areas where parasites are endemic. These concerns highlight the need for information on prevalence, transmission, and distribution of blood parasites [27], especially at the presumed leading edge of their geographic range where infections may pose fitness consequences on naïve hosts.
Additionally, to understand dynamics of infection, it is important to consider sources of variation from heterogeneity in the distribution, abundance, and behavior of vectors and hosts [19, 28, 29]; as a result of genetically based disparities in host immunocompetence [6, 30, 31]; and on account of co-infections. Previous research [9, 32–34] suggests that infection by a given hematozoa lineage may increase or decrease susceptibility to infection by another, and that concomitant interactions among hematozoa lineages influence both parasite persistence and pathogenic outcome. Hematozoa infections have also been shown to interact in synergistic and antagonistic relationships with viruses and other pathogens in birds and mammals [3, 20, 35–37]. As such, thorough understanding of the epidemiology of hematozoa in wild birds requires not only knowledge of within- and among-host variation in transmission and prevalence of individual infections, but must also take into consideration the occurrence of multiple infections and the interactions between and among infectious agents within hosts. One viral agent that may potentially influence hematozoa infection in high-latitude waterfowl populations is Influenza A Virus (IAV).
IAV commonly infects waterfowl and is characterized by seasonal peaks of infection, especially among immunologically naïve juveniles, during late summer and autumn [38]. IAV strains common to wild waterfowl are generally not associated with mortality or morbidity; however, some evidence suggests that low pathogenic IAV infection may be associated with reductions in body weight, delayed migration, and pathological lesions in waterfowl hosts (reviewed by [39]). Similar to IAV, hematozoa infection has been linked to body condition in wild ducks [40], and fitness consequences associated with infection have been reported in a variety of avian taxa (e.g. [41–44]), although natural infections may pose minimal deleterious effects to wild waterfowl [2, 40, 45, 46]. As both IAV and hematozoa can infect waterfowl at relatively high rates and elicit immune responses, and such infections have been associated with sub-lethal effects in other avian taxa, investigation of co-infection and consequences of infection in waterfowl are warranted. Such information would be useful for further understanding of interactions among co-infecting parasitic and viral agents, and for predicting potential outcomes should range expansion or changes in population-level prevalence of hematozoa occur.
In this study, we investigated seasonal patterns and sources of variation in prevalence of Leucocytozoon, Haemoproteus, and Plasmodium parasites among five species of ducks breeding at the Arctic/sub-Arctic interface in the boreal forest region of interior Alaska. Our goal was to better understand within- and among-species variation in prevalence, identify specific factors related to the probability of infection and co-infection, assess evidence for local transmission at the northern periphery of the sub-Arctic, and to better understand potential fitness consequences of infection and co-infection. Specifically, we used molecular detection of hematozoa and an occupancy modeling approach, which accounts for imperfect detection rates, to: (i) estimate genus-specific hematozoa prevalence relative to month, species, age, and sex, (ii) evaluate co-infection dynamics among and between hematozoa genera and IAV, and (iii) assess relationships between size-adjusted body mass and single and multiple infections.
Methods
Study area
We conducted our study at Minto Flats State Game Refuge (Minto Flats; 64°53′N, 148°46′W) in the boreal forest region of interior Alaska, approximately 50 km northwest of Fairbanks and 180 km south of the Arctic Circle (Fig. 1). Our efforts were focused within approximately 100 km2 consisting of a series of interconnected lakes, seasonal wetlands, and streams surrounded by a mixture of grass, shrub, and forested uplands. Minto Flats supports high densities of breeding, molting, and migrating waterfowl and other species of waterbirds and landbirds [47]. Petrula [47] and Walker et al. [48] provide detailed descriptions of the Minto Flats study area.
Fig. 1.

Study area and capture locations within Minto Flats State Game Refuge, Alaska, USA. Circles depict capture locations relative to monthly sampling intervals (yellow = 17–27 May, orange = 10–19 June, red = 28 July – 20 August) and number of birds sampled (small = 1–25, medium = 26–100, large = 100–200)
Sample collection
We captured and sampled 1347 ducks of 5 species between 17 May and 20 August 2010: American green-winged teal (hereafter: green-winged teal; Anas crecca, n = 228), American wigeon (Anas americana, n = 88), mallard (Anas platyrhynchos, n = 481), northern pintail (Anas acuta, n = 490), and lesser scaup (Athya affinis, n = 60) (Table 1). Sampling was conducted over three distinct periods: 17–27 May (May), 10–19 June (June), and 28 July – 20 August (August) and ducks were sampled temporally throughout each interval. Capture techniques varied depending on the timing of the season and included decoy traps (May) [49], rocket nets (June, August) [50], and baited swim-in traps (August) [51]. Upon capture, up to 2 cc of blood was obtained from the jugular vein of each bird; approximately 1 cc of whole blood for screening of hematozoa parasites was transferred immediately to cryovials; the remaining blood was transferred to serum separator tubes, allowed to clot for ≥ 2 h, centrifuged at 3000 rpm for up to 10 min, and then serum for screening of IAV antibodies was transferred to cryovials. Cloacal and oropharyngeal swabs to test for IAV infection were obtained from each bird and stored individually in cryovials containing viral transport media (M4RT, Remel, KS). All samples were kept cool for up to 3 h following collection, then frozen in liquid nitrogen vapor shippers at -150 °C, and once transported from the field site, maintained at -80 °C until analysis. We categorized bird age as hatch year (juvenile) or after hatch year (adult) using cloacal and plumage characteristics [52, 53]. Birds were weighed to the nearest 1.0 g using a digital scale and the lengths of total tarsus and head were measured to the nearest 0.1 mm using dial calipers [54].
Table 1.
Sample sizes, number of positives, and apparent prevalence (in parentheses) of hematozoa, Influenza A Virus (IAV), and IAV serostatus (Ser)
| Species | Age | Sex | Sampling month(s) | n | Leuc | Haem | Plas | AIV | Ser | Leuc & Haem | Leuc & Plas | Leuc & AIV | Haem & AIV | Plas & AIV | Leuc & Ser | Haem & Ser | Plas & Ser |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MALL | Ad | M | May, Jun, Aug | 137 | 91 (66) | 71 (52) | 4 (3) | 27 (20) | 56 (41) | 51 (37) | 4 (3) | 19 (14) | 11 (8) | 2 (1) | 40 (29) | 30 (22) | 2 (1) |
| F | May, Jun, Aug | 115 | 57 (50) | 30 (26) | 9 (8) | 15 (13) | 41 (36) | 20 (17) | 4 (3) | 8 (7) | 4 (3) | 0 (0) | 18 (16) | 12 (10) | 3 (3) | ||
| Juv | M | Aug | 118 | 13 (11) | 6 (5) | 18 (15) | 21 (18) | 10 (8) | 1 (1) | 2 (2) | 3 (3) | 4 (3) | 5 (4) | 1 (1) | 1 (1) | 3 (3) | |
| F | Aug | 111 | 13 (12) | 5 (5) | 9 (8) | 16 (14) | 14 (13) | 1 (1) | 1 (1) | 2 (2) | 0 (0) | 0 (0) | 1 (1) | 3 (3) | 0 (0) | ||
| NOPI | Ad | M | May, Jun, Aug | 105 | 47 (45) | 20 (19) | 5 (5) | 3 (3) | 35 (33) | 13 (12) | 3 (3) | 1 (1) | 0 (0) | 0 (0) | 19 (18) | 9 (9) | 2 (2) |
| F | May, Jun, Aug | 142 | 82 (58) | 47 (33) | 32 (23) | 9 (6) | 46 (32) | 30 (21) | 16 (11) | 4 (3) | 3 (2) | 2 (1) | 31 (22) | 17 (12) | 10 (7) | ||
| Juv | M | Aug | 119 | 47 (39) | 8 (7) | 35 (29) | 8 (7) | 11 (9) | 5 (4) | 16 (13) | 2 (2) | 0 (0) | 2 (2) | 7 (6) | 1 (1) | 5 (4) | |
| F | Aug | 124 | 47 (38) | 8 (6) | 42 (34) | 10 (8) | 11 (9) | 4 (3) | 20 (16) | 4 (3) | 2 (2) | 4 (3) | 2 (2) | 0 (0) | 3 (2) | ||
| AGWT | Ad | M | Aug | 54 | 29 (54) | 13 (24) | 1 (2) | 3 (6) | 22 (41) | 9 (17) | 1 (2) | 2 (4) | 0 (0) | 0 (0) | 12 (22) | 5 (9) | 0 (0) |
| F | Aug | 29 | 19 (66) | 10 (34) | 3 (10) | 2 (7) | 8 (28) | 9 (31) | 2 (7) | 1 (3) | 1 (3) | 0 (0) | 4 (14) | 1 (3) | 1 (3) | ||
| Juv | M | Aug | 101 | 29 (29) | 6 (6) | 9 (9) | 10 (10) | 10 (10) | 4 (4) | 2 (2) | 4 (4) | 1 (1) | 0 (0) | 2 (2) | 0 (0) | 1 (1) | |
| F | Aug | 44 | 7 (16) | 1 (2) | 3 (7) | 5 (11) | 7 (16) | 0 (0) | 0 (0) | 2 (5) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 2 (5) | ||
| AMWI | Ad | M | May, Jun | 76 | 66 (87) | 39 (51) | 6 (8) | 2 (3) | 5 (7) | 36 (47) | 6 (8) | 1 (1) | 1 (1) | 0 (0) | 5 (7) | 2 (3) | 2 (3) |
| F | May, Jun | 12 | 8 (67) | 2 (17) | 0 (0) | 0 (0) | 1 (8) | 2 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (8) | 0 (0) | 0 (0) | ||
| LESC | Ad | M | May | 48 | 31 (65) | 13 (27) | 3 (6) | 0 (0) | 12 (25) | 10 (21) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 9 (19) | 5 (10) | 0 (0) |
| F | May | 12 | 2 (17) | 3 (25) | 0 (0) | 0 (0) | 3 (25) | 1 (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (8) | 1 (8) | 0 (0) | ||
| Total | 1347 | 588 (44) | 282 (21) | 179 (13) | 131 (10) | 292 (22) | 196 (15) | 78 (6) | 53 (4) | 27 (2) | 16 (1) | 153 (11) | 87 (6) | 34 (3) |
Hematozoa genera were abbreviated (Leuc Leucocytozoon, Haem Haemoproteus, Plas Plasmodium) and the “&” indicates co-infections with multiple hematozoa genera and/or IAV and Serostatus. Sampling month refers to the intervals when birds were sampled (May = 17 May – 27 May; Jun = 10 June – 19 June; Aug = 28 July – 20 August). Age was classified as adult (Ad) or juvenile (Juv). Species abbreviations: MALL mallard, NOPI northern pintail, AGWT American green-winged teal, AMWI American wigeon, LESC lesser scaup
Laboratory analysis
Blood parasites were detected using molecular methods as reported by Ramey et al. [55]. DNA was extracted from samples using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA) and screened for hematozoa using the nested PCR protocol as described by Hellgren et al. [56]. A minimum of one negative control was incorporated into PCR reactions for every 24 wells. Reactions were conducted in eight-well strip tubes with individual caps that remained closed, except while loading template and reagents, to prevent cross contamination. Amplicons were visualized on 0.8 % agarose gels stained with Gel Red Nucleic Acid Gel Stain 10,000× in DMSO (Biotium, Hayward, CA). All samples were screened twice for hematozoa by nested PCR.
A 479-base pair (bp) cytochrome b (cyt b) mitochondrial DNA (mtDNA) target fragment was sequenced for all samples that had amplicons present on agarose gels. PCR products were treated with ExoSap-IT (USB Inc., Cleveland, OH) per the manufacturer’s instructions and not otherwise purified prior to sequencing. Sequencing was performed with identical primers used for PCR and with BigDye Terminator version 3.1 mix (Applied Biosystems, Foster City, CA) on an Applied Biosystems 3730xl automated DNA sequencer (Applied Biosystems, Foster City, CA). Sequence data were cleaned and edited using Sequencher version 5.1 (Gene Codes Corp., Ann Arbor, MI). Infections were assigned to genera (Leucocytozoon, Haemoproteus, or Plasmodium) using the nucleotide BLAST function available through the National Center for Biotechnology Information (NCBI). Assignment was based on the top NCBI BLAST result with a minimum identity score of at least 90 %. A sample was considered as positive for blood parasite infection in any given PCR run if it resulted in double-stranded, target mtDNA that was verified through genetic sequencing and successfully assigned to one of three parasite genera using BLAST. Genetic characterization of resultant sequences were previously summarized [57]. Single-stranded sequences or products that could not be assigned via BLAST (n = 20) were considered as negative to reduce the possible occurrence of false positives. To confirm the competency of extracted DNA, a 695-bp fragment of the mtDNA cytochrome c oxidase I (COI) gene of waterfowl hosts was amplified using PCR protocols described by Kerr et al. [58].
Avian influenza virus was detected in swab samples using molecular methods as described by Runstadler et al. [59]. We extracted viral RNA using the MagMax-96 Viral Isolation Kit (Ambion Inc., Austin, TX) and screened RNA for IAV using a two-step real-time Reverse Transcriptase - Polymerase Chain Reaction (rRT-PCR) targeting the matrix gene [59]. The rRT-PCR assays were run on an ABI 7500 real-time PCR System (Applied Biosystems, Foster City, CA). Swab samples were considered positive for IAV RNA if Ct values were ≤ 45. We screened serum samples for the presence of IAV antibodies using a commercial blocking enzyme-linked immunosorbent assay (bELISA, FlockChek AI MultiS-Screen Antibody Test Kit, IDEXX Laboratories, Westbrook, ME) according to the manufacturer’s instructions [60].
Statistical analyses
Previous studies using molecular identification of hematozoa mtDNA from blood samples have demonstrated high, but variable test sensitivity, usually within the range of 0.75 to 0.95 for single PCR runs [13, 14, 55, 61]. When unaccounted for, the occurrence of false negatives leads to underestimates of infection prevalence [62], so we used occupancy modeling to simultaneously estimate the probability of infection by each genera of hematozoa (Ψg; prevalence), and the probability of molecular detection of each genera of hematozoa, given infection (pg). In this framework, identification of parasite mtDNA for each of the two nested PCR reactions from each bird was used to construct encounter occasions where the presence of mtDNA in a sub-sample of blood was indicated by a “1” whereas absence was indicated by a “0” and these data were used to estimate pg and Ψg using maximum likelihood approaches [63]. If parasites are always detected in both samples (i.e. encounter history is always 1,1), then the data suggest an absence of false negatives, with observed prevalence equal to true prevalence. However, if there are instances of partial detections (encounter histories of 0,1 or 1,0), then these data provide information on detection probability (p), which can be used to estimate the proportion of samples where parasite mtDNA was detected in neither PCR run (i.e. some encounter histories of 0,0 represent infected birds that went undetected in both samples). We compiled encounter histories relative to each of the three parasite genera independently to allow for genus-specific estimation of hematozoa prevalence and detection. Whereas replicate PCR runs were used to estimate p, each individual bird was sampled on a single occasion, and therefore our dataset did not contain pseudo-replicates for analysis of Ψ. Occupancy modeling was conducted using Program MARK [64].
We developed an a priori suite of models to assess temporal variation in hematozoa prevalence and detection on the breeding grounds. Because species were sampled unequally during each of the three capture periods (Table 1), we modeled prevalence by monthly sampling period (May, June, August) and by species, sex, and age class within each month. To limit the total number of models considered, we conducted model selection for each parasite genus using a 3-staged approach; the top-supported model from each stage was carried on to the succeeding stage where it was used as base structure for consideration of additional variables. Relative support among models in each stage was assessed using Akaike’s Information Criterion corrected for sample size (AICc). Models were not considered further if their AICc values were greater than (i) a nested model with fewer parameters, or (ii) a more parameterized model with similar structure, but one additional parameter [65, 66]. In Stage 1, we assessed general ecological variation in detection probability and hematozoa prevalence by considering variation relative to duck species, age, sex, and month; in Stage 2, we considered models assessing relationships between genus-specific prevalence and co-infection with other parasite genera and IAV; and finally in Stage 3, we examined the effects of size-adjusted body condition on parasite infection and co-infection.
Peaks in abundance of parasitemia (i.e. parasite load) in avian hosts occur following initial infection, and to a lesser extent upon recrudescence associated with the spring breeding period in years subsequent to initial infection [1]. During the latent period in between these peaks, parasitemia in blood are reduced to low levels, perhaps below the threshold of detection via microscopy or molecular methods [7, 67, 68]. As such, we suspected that peaks in parasitemia for individuals may be correlated with seasonal peaks in population-level prevalence as a function of a positive relationship between molecular detection of hematozoa parasites and host parasite load [69]. Therefore, although our primary interest was in obtaining unbiased estimates of Ψ, in practice, p and Ψ may have been correlated such that variation in both variables were likely to explain variation in true prevalence [70]. To account for potential variation in detection probability, we first considered a range of models to identify the top approximating structure for p. Specifically, we constrained Ψ to a highly parameterized structure while considering each of the models from Stage 1 on p (Table 2). We then fixed p to the top supported structure for modeling of Ψ in each subsequent stage. For cases in which all PCR runs for a given class of birds were positive for parasite mtDNA (i.e. all encounter histories 1,1), p was fixed to 1.0. Due to the low frequency of Plasmodium parasites in samples collected during May and June, we limited sources of variation considered on p for Plasmodium to month and species during these 2 sampling periods (Additional file 1: Table S9). Reported estimates of p correspond to a single PCR run, thus detection probability for duplicate runs is calculated as: 1 – (1 – p)2.
Table 2.
Suite of models considered in Stage 1 to estimate probability of hematozoa infection (Ψ) and probability of detecting hematozoa mtDNA (p)
| Model | K | Model description |
|---|---|---|
| (.) | 1 | pr (infection) does not vary |
| month | 3 | pr (infection) varies by month (May, June, August) |
| May models (17 May – 27 May) | ||
| sex | 2 | pr (infection) varies during May by sex |
| species | 4 | pr (infection) varies during May by species (MALL, NOPI, AMWI, LESC) |
| species + sex | 5 | pr (infection) varies during May by species and by sex to the same degree for all species |
| June models (10 July – 19 June) | ||
| sex | 2 | pr (infection) varies during June by sex |
| species | 3 | pr (infection) varies during June by species (MALL, NOPI, AMWI) |
| species + sex | 4 | pr (infection) varies during June by species and by sex to the same degree for all species |
| August models (28 July – 20 August)a | ||
| age | 2 | pr (infection) varies during August by age (adult, juvenile) |
| sex | 2 | pr (infection) varies during August by sex |
| age + sex | 3 | pr (infection) varies during August by age and by sex to the same degree for both age classes |
| age * sex | 4 | pr (infection) varies during August by each age and sex class |
| (ad * sex) + (juv) | 3 | pr (infection) varies during August by age and by sex for adult birds |
| species | 3 | pr (infection) varies during August by species (MALL, NOPI, AGWT) |
| species * age | 6 | pr (infection) varies during August by species and age |
| species + age | 4 | pr (infection) varies during August by species and by age to the same degree for all species |
| species * sex | 6 | pr (infection) varies during August by species and sex |
| species + sex | 4 | pr (infection) varies during August by species and by sex to the same degree for all species |
| species * age + sex | 7 | pr (infection) varies during August by species and age, and by sex to the same degree for all species |
| species * sex + age | 7 | pr (infection) varies during August by species and sex, and by age to the same degree for all species |
| species * age * sex | 12 | pr (infection) varies during August by species, age, and sex |
| (ad * species * sex) + (juv * species) | 9 | pr (infection) varies during August by species and by sex for adult birds |
| August temporal trend models | ||
| + day | 1 | pr (infection) during August varies in a linear trend with a single slope for all sources of variation |
| + day * ageb | 2 | pr (infection) during August varies in a linear trend with a unique slope for each age class |
| * day | 2+ | pr (infection) during August varies in a linear trend with a unique slope for each source of variation |
This suite of models was considered independently for each of three hematozoa genera (Leucocytozoon, Haemoproteus, Plasmodium). K = number of model parameters applicable only to modeling of Ψ or p in a given month and is not representative of the total number of model parameters. Age of birds was either after-hatch-year (adult) or hatch-year (juvenile). Species abbreviations: AGWT American green-winged teal, AMWI American wigeon, LESC lesser scaup, MALL mallard, NOPI northern pintail
aAugust models were considered alone, with the additive temporal trend (+ day), with the temporal trend varying by age (+ day * age), and with the multiplicative temporal trend (* day)
bModel structure was applied only to August models containing variation in age
To assess temporal variation in prevalence, we modeled Ψ separately for each month (May, June, August) and assessed month-specific variation relative to species, sex, and age. We expected seasonal prevalence in adult birds to be highest towards the beginning of the breeding season as a result of recrudescence and new infections, and then subsequently decline with seasonal progression as infections were cleared by host immune response, but we suspected that prevalence in juvenile birds would exhibit a different pattern, peaking later in the season than adults due to delayed exposure to vectors [71, 72]. Our sample during the May and June intervals was limited to adult birds, and was weighted towards males, so we considered models in which Ψ was constant or varied relative to species, sex, or by species with an additive effect of sex. During August our sample contained both adult and juvenile birds and more equal representation of sexes, so we considered variation relative to species, age, sex, and the multiplicative and additive effects of each variable. Whereas hematozoa prevalence may vary between sexes of adult birds due to differences in behavior influencing exposure to vectors during the breeding season, or to sex-specific factors affecting immunocompetence [73], we predicted that prevalence for juveniles would not differ by sex given that young of both sexes remain in close proximity throughout the brood-rearing period and have similar behavior during autumn staging. To test this hypothesis, we considered an additional form of variation by age and sex in which prevalence varied by sex for adults, but was constrained to be equal between sexes for juvenile birds ((ad * sex) + (juv)). Finally, we assessed support for temporal variation within the August sampling interval by considering a linear trend in prevalence by sampling day (28 July = 1, 20 Aug = 24) to assess directionality in temporal trends of prevalence. We considered a suite of 14 models assessing variation in prevalence relative to species, age, and sex, and to each of these we considered an additive temporal trend, a temporal trend varying by age, and a temporal trend varying relative to each term in a given model. Our model set in Stage 1 contained 60 models on each of p and Ψ (Table 2).
In Stage 2 of model selection, we assessed the relationship between probability of infection with a given parasite genus and other parasite genera, active IAV infection, and previous IAV infection (serostatus) to ascertain potential interactions among infectious agents. Specifically, co-infection with each hematozoa genus, IAV, and serostatus were coded as binomial individual covariates. We considered models where each co-infection variable was added to the top-supported model from Stage 1 as a constant effect, and varying relative to month, species, age, and sex. The nested-PCR protocol that we used employed a single primer set for Haemoproteus and Plasmodium parasites, making distinguishing between the two genera in a single host difficult [74]. Thus, we were less likely to detect co-infections with Haemoproteus and Plasmodium, and therefore did not consider these relationships in our models. When modeling Leucocytozoon prevalence, we considered an additional suite of 20 models in Stage 2, whereas for Haemoproteus and Plasmodium, we considered 15 additional models (Table 3). We hypothesized that hematozoa and IAV prevalence may be positively correlated if immunosuppression resulting from one infection increased susceptibility of ducks to another infection. Additionally, we assessed the hypothesis that previous exposure to IAV, measured based on the presence of IAV antibodies, may reduce susceptibility to hematozoa infection as was recently demonstrated with West Nile Virus and Plasmodium in songbirds [36].
Table 3.
Suite of models considered in Stage 2 and Stage 3 to estimate probability of hematozoa infection (Ψ)
| Model | K | Model description |
|---|---|---|
| Stage 2 – Co-infection modelsa | ||
| Stage 1 | 0 | pr (infection) varies by top supported model structure from Stage 1 |
| Haem | 1 | pr (infection) varies by Stage 1 and by co-infection with Haemoproteus parasites |
| Leuc | 1 | pr (infection) varies by Stage 1 and by co-infection with Leucocytozoon parasites |
| Plas | 1 | pr (infection) varies by Stage 1 and by co-infection with Plasmodium parasites |
| IAV | 1 | pr (infection) varies by Stage 1 and by co-infection with Influenza A Virus |
| Serostatus | 1 | pr (infection) varies by Stage 1 and by Influenza A Virus serostatus |
| Co-infection variation | ||
| * month | 3 | pr (infection) varies by Stage 1 and by co-infection differently for each month |
| * species | 5 | pr (infection) varies by Stage 1 and by co-infection differently for each duck species |
| * age | 2 | pr (infection) varies by Stage 1 and by co-infection differently for each age class |
| * sex | 2 | pr (infection) varies by Stage 1 and by co-infection differently for each sex class |
| Stage 3 – Body condition models | ||
| Stage 2 | 0 | pr (infection) varies by top supported model structure from Stage 2 |
| BCI | 1 | pr (infection) varies by Stage 2 and by body condition |
| BCI * month | 3 | pr (infection) varies by Stage 2 and by body condition effect that is different for each month |
| BCI * species | 5 | pr (infection) varies by Stage 2 and by body condition effect that is different for each species |
| BCI * age | 2 | pr (infection) varies by Stage 2 and by body condition effect that is different for each age class |
| BCI * sex | 2 | pr (infection) varies by Stage 2 and by body condition effect that is different for each sex class |
Models were considered independently for each of three hematozoa genera (Leucocytozoon, Haemoproteus, Plasmodium) and were applied only to estimation of Ψ. K = number of model parameters in addition to those supported in the previous stage of model selection and BCI is the body condition index
aEach co-infecting agent was considered alone, and varying multiplicatively with co-infection variation structures
Finally, in Stage 3, we assessed support for variation in hematozoa prevalence relative to body condition to make inference on possible sub-lethal effects of infection. Our measure of body condition index (BCI) was size-adjusted body mass. We controlled for known sources of variation in body mass of ducks (i.e. species, age, sex, and seasonal timing) by calculating the BCI variable separately for each species, age, and sex class by month; this approach allowed for direct assessment of relationships between body condition and hematozoa prevalence in a single parameter. For each class, we performed a principal components analysis on the correlation matrix for lengths of head and tarsus, regressed body weight (g) on PC1 scores, and then divided residuals from the regression by class-specific mean weight. Hence, inference regarding the relationship between BCI and Ψ apply to the class-specific relationships. Analyses to construct the BCI variable were conducted using SAS (SAS Institute 1996). Deleterious effects from hematozoa infection are likely strongest during temporal periods associated with primary parasitemia, and may be greater in young and newly infected hosts [1]. Therefore, we considered models in which BCI was either additive (i.e. a single parameter), or varied relative to month, species, age, and sex (Table 3). To assess potential patterns in body condition associated with co-infections, we also considered variation in BCI relative to any supported co-infection variables from Stage 2 (Table 3).
We report coefficient estimates using 85 % confidence intervals because they are more compatible with an AIC-based modeling approach than traditional 95 % confidence intervals [65]. Estimates of prevalence and detection probability were back-transformed from the logit link and associated variances were calculated using the delta method. We report estimates ± SE unless otherwise stated.
Results
Detection of hematozoa, IAV, IAV antibodies, and co-infections
We detected infection by hematozoa parasites in 775 of the 1347 ducks sampled (57.5 %); apparent prevalence was 43.7 % for Leucocytozoon, 20.9 % for Haemoproteus, 13.3 % for Plasmodium, and 9.7 % for IAV. Antibodies to IAV were detected in 21.7 % of samples. Co-infection of Leucocytozoon and Haemoproteus parasites occurred in 196 birds, whereas 78 birds were co-infected with Leucocytozoon and Plasmodium parasites. We detected only two co-infections containing Haemoproteus and Plasmodium parasites, which is likely, at least in part, a function of molecular methods employed (see Methods). Seventy-two birds were identified as being infected with hematozoa and actively shedding IAV, and 196 birds that were infected with blood parasites also tested positive for IAV antibodies.
Sources of variation in detection probability differed among parasite genera (Table 4). Detection of Plasmodium was constant across all months (0.86 ± 0.02), but detection varied by month, species, and age for Haemoproteus and by month, age, and sex for Leucocytozoon (Table 5). Detection of Haemoproteus ranged from 0.85 (±0.03) to 0.98 (±0.01) for adult birds, but was lower for juveniles among mallards (0.41 ± 0.13), northern pintails (0.69 ± 0.09), and green-winged teal (0.81 ± 0.11). Detection of Leucocytozoon in May was 0.90 (±0.02) and in June was 0.97 (±0.02). During August, detection of Leucocytozoon increased linearly through time for adult female (range: 0.80 ± 0.05–0.90 ± 0.05), adult male (range: 0.67 ± 0.07–0.99 ± 0.01), and juvenile (range: 0.74 ± 0.07–0.93 ± 0.02) ducks.
Table 4.
Top AICc approximating model explaining variation in detection probability (p) and probability of infection (Ψ) for three hematozoa genera following three stages of model selection
| Top approximating model | |||
|---|---|---|---|
| Parasite genus | Model stage | p | Ψ |
| Leuc | Stage 1 | p May(.) + p Jun(.) + p Aug((ad * sex) + juv) * day) | ΨMay(spp + sex) + ΨJun(spp) + ΨAug(spp * age) + (age * day)) |
| Stage 2 | -- | Stage 1 + Haem | |
| Stage 3 | -- | Stage 2 + (BCI * spp) | |
| Haem | Stage 1 | p May(.) + p Jun(.) + p Aug(spp + age) | ΨMay(spp + sex) + ΨJun(spp) + ΨAug((ad * spp * sex) + (juv * spp)) |
| Stage 2 | -- | Stage 1 + Leuc | |
| Stage 3 | -- | Stage 2 + (BCI * age) | |
| Plas | Stage 1 | p(.) | ΨMay(sex) + ΨJun(spp + sex) + ΨAug((spp + age) * day) |
| Stage 2 | -- | Stage 1 | |
| Stage 3 | -- | Stage 2 | |
Model structure explaining variation in p was fixed following Stage 1 for additional investigation of variation on Ψ in Stages 2 and 3. Abbreviations: Leuc Leucocytozoon, Haem Haemoproteus, Plas Plasmodium, spp duck species, ad age class adult, juv age class juvenile
Table 5.
Estimates of monthly detection probability for Leucocytozoon, Haemoproteus, and Plasmodium parasites for a single PCR run
| May | June | August | |||||
|---|---|---|---|---|---|---|---|
| Parasite | Class | SE | SE | SE | |||
| Leucocytozoon | All | 0.90 | 0.02 | 0.97 | 0.02 | ||
| ad - M | 0.67, 0.93, 0.99 | 0.07, 0.02, 0.01 | |||||
| ad - F | 0.80, 0.85, 0.90 | 0.05, 0.03, 0.05 | |||||
| juv | 0.74, 0.86, 0.93 | 0.07, 0.02, 0.02 | |||||
| Haemoproteus | All | 0.85 | 0.03 | 0.85 | 0.04 | ||
| AGWT - ad | 0.98 | 0.01 | |||||
| MALL - ad | 0.91 | 0.03 | |||||
| NOPI - ad | 0.97 | 0.02 | |||||
| AGWT - juv | 0.81 | 0.11 | |||||
| MALL - juv | 0.41 | 0.13 | |||||
| NOPI - juv | 0.69 | 0.09 | |||||
| Plasmodium | All | 0.86 | 0.02 | (applies to all months) | |||
Ducks were sampled in three distinct capture intervals: May (17 May – 27 May), June (10 June – 19 June), and August (28 July – 20 August). Estimates of p for Leucocytozoon increased linearly during the August sampling period; numbers represent the lowest, median, highest estimates and associated SE. Abbreviations: ad age class adult, juv age class juvenile, MALL mallard, NOPI northern pintail, AGWT American green-winged teal
Hematozoa prevalence
Leucocytozoon prevalence varied by host species and sex during May, by species during June, and by species, age, and time during August (Table 4). Estimates of Leucocytozoon prevalence were as low as 0.05 (±0.01) for juvenile mallards during the beginning of August and as high as 0.96 (±0.04) for adult American wigeon during June (Fig. 2). Leucocytozoon prevalence increased from May to June and was lower during August than June for adult birds. Estimated prevalence during the August sampling period increased strongly for juvenile mallards (28 Jul: 0.05 ± 0.01, 20 Aug: 0.23 ± 0.05), green-winged teal (28 Jul: 0.16 ± 0.03, 20 Aug: 0.48 ± 0.07), and northern pintails (28 Jul: 0.23 ± 0.04, 20 Aug: 0.60 ± 0.05), and increased slightly for adults of these three species (Fig. 2). Estimated prevalence of Leucocytozoon parasites was consistently higher for mallards than northern pintails in all three months, and Leucocytozoon prevalence for American wigeon exceeded those of other species during May and June, the two months for which data were obtained for this species (Fig. 2). Prevalence of Leucocytozoon for adult mallards, northern pintails, and green-winged teal during August was higher than for juvenile ducks of respective species, although these differences were largest early in the sampling period (Fig. 2).
Fig. 2.
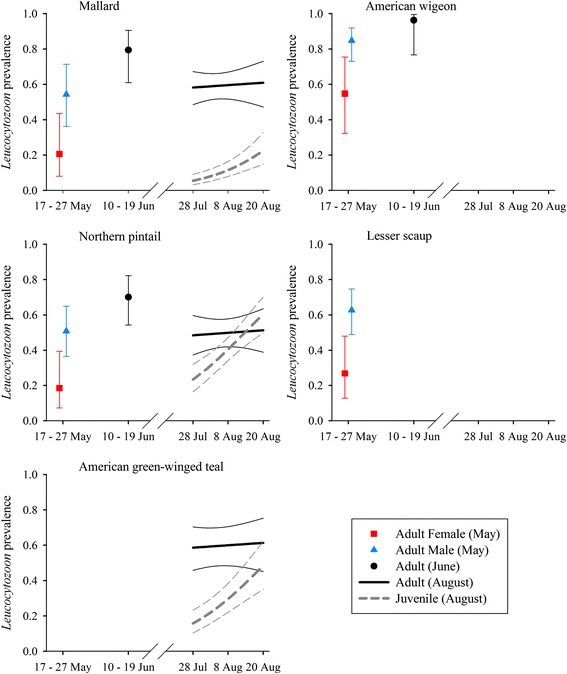
Estimated prevalence (±95 % CI) of Leucocytozoon parasites infecting five duck species sampled during May – August, 2010, interior Alaska
Prevalence of Haemoproteus parasites varied by host species and sex during May, by species during June, and by species, age, and adult sex during August (Table 4). Estimates of Haemoproteus prevalence were as low as 0.05 (±0.02) for juvenile green-winged teal and as high as 0.79 (±0.08) for adult American wigeon during June. Estimates of Haemoproteus prevalence were relatively constant from May to June for mallards (May females: 0.49 ± 0.14, May males: 0.65 ± 0.09, June: 0.67 ± .09) and northern pintails (May females: 0.18 ± 0.09, May males: 0.30 ± 0.07, June: 0.31 ± 0.07); however, for American wigeon, prevalence during June (0.79 ± 0.08) was considerably higher than May estimates for both females (0.23 ± 0.09) and males: (0.37 ± 0.07). We did not find strong support for temporal trends in prevalence of Haemoproteus during the August sampling period (ΔAICc = 0.95, Additional file 1: Table S6), and species and sex-specific estimates were generally lower during August than in previous months (Fig. 3). Haemoproteus prevalence for adult mallards, northern pintails, and green-winged teal exceeded that of juvenile birds during August, similar to results for Leucocytozoon (Fig. 2).
Fig. 3.
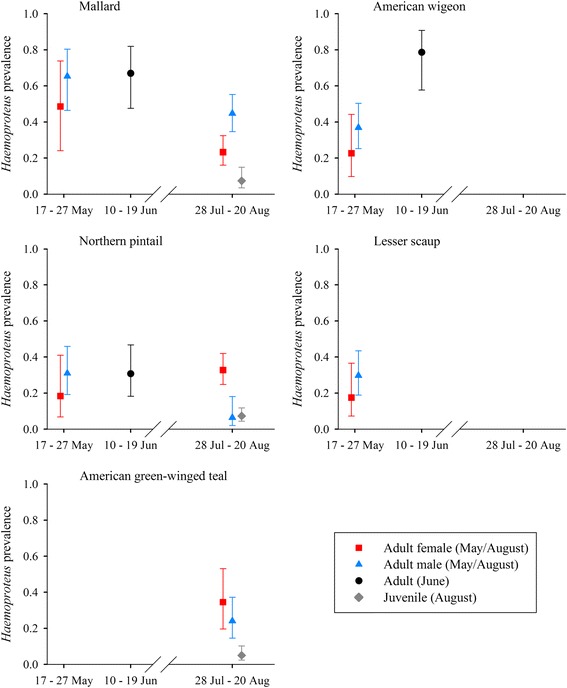
Estimated prevalence (±95 % CI) of Haemoproteus parasites infecting five duck species sampled during May – August, 2010, interior Alaska
Prevalence of Plasmodium parasites varied by sex during May, by species and sex during June, and by species, age, and day during August (Table 4). Plasmodium prevalence ranged from a lack of detection in any species of female duck during the May sampling period, or in mallards during June (i.e. 0.00), to as high as 0.36 (±0.06) for juvenile northern pintails at the end of the August sampling period (Fig. 4). We did not find strong support for variation in Plasmodium prevalence among species during May (ΔAICc = 2.67, Additional file 1: Table S10), but prevalence for northern pintails was higher during June (female: 0.36 ± 0.09; male: 0.11 ± 0.08) than for American wigeon (female: 0.23 ± 0.18, male: 0.07 ± 0.05) and mallards (0.00), and mean prevalence during August was higher for both adult (0.17 ± 0.03) and juvenile (0.31 ± 0.03) northern pintails than for mallards (adult: 0.06 ± 0.01; juvenile: 0.13 ± 0.02) and green-winged teal (adult: 0.04 ± 0.01; juvenile: 0.09 ± 0.02). During the August sampling period, Plasmodium prevalence increased for juvenile mallards, northern pintails, and green-winged teal, but decreased or remained stable during August for adult ducks of these three species (Fig. 2). In contrast to results for Leucocytozoon and Haemoproteus, prevalence of Plasmodium was higher for juvenile than adult ducks during August (Fig. 2).
Fig. 4.

Estimated prevalence (±95 % CI) of Plasmodium parasites infecting five duck species sampled during May – August, 2010, interior Alaska
Co-infections
We found strong support for a positive relationship between co-infections with Leucocytozoon and Haemoproteus parasites (Additional file 1: Tables S3 and S7). Estimated prevalence of Leucocytozoon was 1.04–2.12 times higher given co-infection with Haemoproteus (, 85 % CI = 0.64, 1.14, Additional file 2: Table S13), whereas estimated prevalence of Haemoproteus was 1.25–2.10 times higher given co-infection with Leucocytozoon (, 85 % CI = 0.55, 1.03, Additional file 2: Table S14), depending upon month, duck species, age, and sex (Fig. 5). We did not find support for any relationships between infection with Leucocytozoon and Plasmodium, and there was no support for relationships between infection with any parasite genus and IAV (Table 4, Additional file 1: Tables S3, S7, and S11).
Fig. 5.

Co-infection relationships between infection status with Haemoproteus and Leucocytozoon parasites in northern pintails. Panel a depicts estimated probability of infection with Haemoproteus parasites (±95 % CI) relative to Leucocytozoon (Leuc) infection status; panel b depicts estimated probability of infection with Leucocytozoon parasites (±95 % CI) relative to Haemoproteus (Haem) infection status. Estimates were produced from the top approximating genus-specific models in Stage 2 of model selection
Hematozoa infection and body condition
We found support for variation in prevalence relative to BCI for Leucocytozoon and Haemoproteus, but not for Plasmodium (Table 4). The BCI effect for Haemoproteus was negative for juvenile birds (, 85 % CI = -8.72, -1.87) and weakly negative for adults (, 85 % CI = -2.71, 0.56). When comparing the probability of Haemoproteus infection across the spectrum of BCI values for juvenile mallards, northern pintails, and green-winged teal, estimated probabilities of infection for ducks in the lowest 10th percentile of body condition were 2.36–3.24 times higher than for ducks in the highest 90th percentile (Additional file 2: Table S15). For Leucocytozoon, the BCI effect varied by species and was negative for northern pintails (, 85 % CI = -4.95, -1.54), positive for American wigeon (, 85 % CI = 6.10, 24.83), and equivocal for lesser scaup (, 85 % CI = -7.37, 6.60), green-winged teal (, 85 % CI = -3.22, 2.21), and mallards (, 85 % CI = -0.96, 3.42). The probabilities of Leucocytozoon infection for northern pintails in the lowest 10th percentile of body condition were 1.26–1.62 times higher than for those in the highest 90th percentile, whereas American wigeon in the highest 90th percentile of body condition were 1.04–3.48 times more likely to be infected than those in the lowest 10th percentile (Additional file 2: Table S16). We did not find support for variation in prevalence relative to interactions with co-infection status and BCI (Additional file 1: Tables S4 and S8).
Discussion
In this study we screened a large number of waterfowl samples collected in the northern sub-Arctic and found relatively high prevalence of three genera of hematozoa parasites in adults and juveniles of five species of ducks, which provides evidence for local transmission. Molecular detection of hematozoa varied by parasite genus and a suite of host traits, but was relatively high, especially given two replicate PCR runs of each sample, indicating that we were successfully able to detect the majority of infections and reduce potential bias in estimates of prevalence. Parasite genus, seasonal timing, and duck species and age were important sources of variation in hematozoa prevalence, providing evidence for complex epidemiological patterns of infection. Co-infections with multiple genera of blood parasites were common, as was evidence for infection with hematozoa and current or past infection with IAV, although only the positive relationship between Leucocytozoon and Haemoproteus infection was correlated with the probability of infection of a given parasite genus. Finally, an assessment of relationships between size-adjusted body mass and infection status revealed a negative relationship between body condition and infection with Haemoproteus, but this relationship varied by duck species for Leucocytozoon infection, revealing no clear patterns.
The heterogeneity in detection probability observed in this study highlights the importance of tailoring protocols to allow for estimation of false negatives (i.e. through multiple PCR runs per blood sample) to minimize potential sources of bias in estimates of prevalence. Detection probability of hematozoa mtDNA using molecular methods has previously been shown to vary relative to parasite genus, geographic location, and host age [13, 14], which may be the result of variation in parasite load among host groups [69]. We found support for variation in detection probability by season, age, sex, and species, although for adult ducks, estimates of detection of all genera of parasites from a single PCR run were ≥ 0.85, and therefore the probability of detecting infection with two replicate runs (1 – (1 – p)2) was at least 0.98. Variation in detection for juvenile ducks was more pronounced, especially for Haemoproteus parasites, where estimates of detection were considerably lower for juveniles than for adults in their respective species . Hence, while our results indicate that duplicate PCR runs sufficiently decreased the likelihood of false negatives in our data for adult birds, the probability of detecting Haemoproteus infections in juveniles using duplicate runs was still as low as 0.65, 0.90, and 0.96 for mallards, northern pintails, and green-winged teal, respectively. The use of triplicate runs would allow for more sophisticated models that recognize individual heterogeneity in detection probability, possibly related to intensity of infection, and may be warranted in studies where high levels of precision on estimates of detection probability are critical towards achieving project objectives, or when it is necessary to substantially reduce sources of bias in estimates of prevalence.
For adult birds of all species investigated in this study, Leucocytozoon prevalence was the highest, followed by Haemoproteus and Plasmodium, however, for juvenile ducks, prevalence of Leucocytozoon and Plasmodium exceeded that of Haemoproteus. Similar patterns have been reported in previous investigations of North American waterfowl [19, 75] and in recent high-latitude investigations of waterfowl [13, 14] and songbirds [21]. Our mean species-specific estimates of Plasmodium prevalence in juvenile ducks (range: 0.08 – 0.31), which are indicative of local transmission, are considerably higher than estimates of juvenile (0.01) and resident adult (0.05) songbirds at a nearby location of the same latitude [17]. These differences may be due to variation in susceptibility or exposure of waterfowl versus passerines, may reflect relatively high densities of mosquito vectors in the Minto Flats wetlands where we sampled, or could be a function of inter-annual variation in hematozoa prevalence [55, 72].
Our results indicate that, for adult birds, prevalence of all three hematozoa genera is highest during May and June, with peaks in prevalence occurring in association with pre-nesting and breeding, and diminishing during the autumn staging period in August. In contrast, our results indicate increasing trends in prevalence for Leucocytozoon and Plasmodium parasites during August for juvenile ducks. Contrasting patterns in seasonal prevalence by age class is likely due to differential timing of exposure to vectors and recrudescence from previous infections in adults [72]. That is, adult birds may experience re-lapse from previous infections prior to or shortly after arrival on the breeding grounds, and acquire new infections through exposure to vectors during the breeding period. However, juvenile birds in our study hatched during June and July and were therefore exposed to vectors of hematozoa later in the season. Duration of hematozoa infection in wild waterfowl is not well understood, but we suspect that many adult ducks may have been clearing infections at the time that juvenile ducks were first susceptible to infection. Higher prevalence in adults may be expected given that prevalence of juveniles represents only infections obtained in the given year, whereas prevalence in adult ducks represents new infections plus those retained from previous seasons. For the three duck species sampled when juveniles were available for capture (i.e. mallard, northern pintail, green-winged teal), Haemoproteus and Leucocytozoon prevalence was higher for adults, while prevalence of Plasmodium was up to several times higher for juvenile birds of these same three species. This suggests that juvenile ducks may be more susceptible to Plasmodium infections than adults, Plasmodium infections take longer for juveniles to clear, or encounter rates with mosquito vectors vary by age class. It is plausible that downy feathers of ducklings provide less defense against biting vectors than the fully developed feathers of adult waterfowl [13], although given such high rates of Plasmodium infection in juvenile birds, it is surprising that recrudescence of Plasmodium infections in adults was not apparent through higher estimates of prevalence. This may be a result of the peak of recrudescence occurring prior to our sampling in mid-May, or could, at least in part, be an artifact of the molecular approach used to detect infections. That is, we used the same primers to amplify parasites from both Haemoproteus and Plasmodium genera, hence, it is plausible that in some cases where birds were co-infected with parasites of both genera, only Haemoproteus infection was detected, thus resulting in negatively biased estimates of Plasmodium prevalence. Future investigations could provide further inference by incorporating samples collected earlier in the breeding season, developing molecular tests that use different primers for Haemoproteus versus Plasmodium parasites, and by incorporating microscopy that may be useful for identifying co-infections based upon morphological examination of thin blood smears.
We did not find evidence that hematozoa prevalence varied relative to sex for juvenile ducks; however, for adults, variation in prevalence by sex was dependent upon seasonal timing and parasite genus. Differences in prevalence by sex in adult birds may be due to sex-specific differences in host immunocompetency or to differences in behavior leading to differential exposure to vectors [73]. Male and female ducks exhibit considerably different behaviors during the breeding period; upon clutch completion, male ducks depart the nest site to undergo remigial molt whereas females incubate eggs and rear the young [76]. Whereas our estimates of hematozoa prevalence were higher for males than females for all species of ducks and parasite genera during May, differences in Haemoproteus prevalence among species by sex did not exhibit consistent patterns in June or August. Thus, the mechanisms driving sex-specific variation in parasite prevalence are unclear and represent an important topic for further investigation.
Variation in hematozoa prevalence among co-occurring avian host species has been frequently reported, and a number of hypotheses have been put forth to explain such findings. These hypotheses include differential exposure to vectors resulting from timing of nest initiation [77] or habitat selection [28], differential vector preference relative to host body size in which larger birds attract more vectors [29], or to plumage characteristics in which vectors are attracted to brighter colors [78]. In comparative studies, Ricklefs [30] and Tella et al. [79] demonstrated that prevalence was inversely related to the relative length of the incubation period, presumably because slower growth periods associated with prolonged incubation leads to more robust immunocompetency. We did not directly assess variation in prevalence relative to timing of nest initiation or species-specific incubation periods, and our sampling design did not allow for direct assessment of body size effects due to confounding of new infections and recrudescence among adults, and the correlation between juvenile body size and time, but there does not appear to be evidence in our data to support these hypotheses. Green-winged teal were the smallest species sampled in our study (mean mass = 314 g) and had the shortest incubation period (~22 days) [80], yet hematozoa prevalence of green-winged teal was generally similar to, or on occasion higher than, that of mallards, the species with the largest body size (mean mass = 1100 g) and the longest incubation period (~28 days) [80]. Furthermore, species-specific estimates of Leucocytozoon prevalence during May and June, and of Haemoproteus prevalence during June, were highest for American wigeon, a species of intermediate size (mean mass = 708 g) and incubation period (~25 days) [80]. Variation in behavior or habitat selection among species that influences exposure to vectors may account for species-specific differences in prevalence, but throughout the duration of sampling, we frequently captured multiple species simultaneously, indicating substantial overlap in habitat use. Thus, although we cannot rule out mechanisms associated with host or vector behavior on our study area, we suspect that variation in prevalence among species in our study was likely due either to temporal or geographical variation in migratory patterns leading to differential exposure to vectors off of the breeding grounds, or to species-specific disparities in host immunocompetence [6, 30, 31, 81].
Existing parasitic infections in a host may increase the likelihood of a secondary infection resulting from immunosuppression or down-regulation of the host immune system, or, existing infections may limit secondary infections due to competitive exclusion or elicitation of cross-protective immune response [9, 82]. In our study, co-infections with Leucocytozoon and Haemoproteus parasites occurred together three times more frequently than expected given the relative prevalence of each genus, and this positive relationship between infection with Leucocytozoon and Haemoproteus is consistent with results of Arriero and Moller [32] and Van Rooyen et al. [34], who speculated that Leucocytozoon parasites primarily infect hosts with existing infections. Whereas prevalence of both Leucocytozoon and Haemoproteus varied considerably by month and among species and ages in our study, we did not find support for variation in the co-infection relationships relative to any of these factors, indicating a high degree of constancy in the mechanisms associated with co-occurrence of these parasite genera. This relationship may also be due to host traits in that an individual behavior leading to exposure to vectors of one genus may, for the same reason, lead to exposure to vectors for the other genus. Whereas black flies (Diptera: Simuliidae) and biting midges (Diptera: Ceratopogonidae) are generally considered to be the genus-specific vectors of Leucocytozoon and Haemoproteus, respectively, knowledge of vectors in Alaska is largely lacking. Therefore, it may be plausible that in our study area, both Leucocytozoon and Haemoproteus parasites are transmitted by the same vector. Alternatively, genus-specific vectors may utilize similar habitats such that ducks exposed to one are more likely to be exposed to the other. In a study of hematozoa co-infection relationships among songbirds in the Boreal forest region of Alaska, Oakgrove et al. [21] observed a negative relationship between Leucocytozoon and Haemoproteus infections and suggested that this result may be due to within-host competition among parasite lineages. This discrepancy in findings is unexpected given the close proximity of our study site with theirs, but may be due to species differences in parasites infecting waterfowl versus those infecting songbirds [1, 57].
We found no relationships between active or previous IAV infection and the probability of hematozoa infection, suggesting that the mechanisms influencing infection of IAV in waterfowl on the breeding grounds are independent of those influencing susceptibility to hematozoa. Aquatic birds, and waterfowl in particular, are the primary reservoir for IAV, and it is generally accepted that the viruses circulating in wild duck populations cause minimal deleterious effects on waterfowl hosts [83]. Thus, the avirulent nature of endemic IAVs may not illicit a strong enough effect on the host immune system to influence susceptibility to hematozoa. Likewise, host immune response to infection by endemic hematozoa lineages may be insufficient to influence susceptibility to IAV, or the contrasting epidemiological characteristics of IAV and hematozoa (e.g. differential route of infection, lack of within-host competition) may prohibit cross-protective immunological properties. It is plausible that other infectious agents (e.g. viruses, bacteria, intestinal parasites, ectoparasites) influence the susceptibility and transmission of hematozoa in waterfowl and future research may be warranted.
Similar to an investigation of nesting wild ducks in Saskatchewan, Canada [40], we observed a negative relationship between body condition and infection status with Haemoproteus parasites, although the effect in our study was strongest for juvenile ducks. This may be indicative of a positive relationship between body condition and host immunocompetence, or conversely, costs of Haemoproteus infection may result in reductions to host body condition. We suspect that the relationship between body condition and Haemoproteus infection was strongest in juvenile ducks due to the relative parasite load and associated immune response in primary versus secondary parasitemia. All infections identified in juvenile ducks represented newly acquired infections whereas some proportion of infections in adults were likely chronic. Chronic infections are generally associated with lower parasite loads and adult birds presumably have more developed immune response to parasite infection [1]. We also found support for relationships between body condition and Leucocytozoon infection, but the direction of this relationship varied by duck species; northern pintails in poorer condition were more likely to be infected, but we found an opposite effect for American wigeon. In previous experimental studies using wild-strain mallards in captivity, Leucocytozoon infection imposed no severe morbidity on ducklings or measureable effects on duckling growth rates [46, 84]. Susceptibility and host immune response to hematozoa is known to vary among avian taxa [9, 33], and experimental studies have shown variation in species-specific tolerance to infections with the same hematozoa lineages [6, 8]. Thus, our results may represent species-specific variation in immune response to Leucocytozoon infection. The use of quantitative molecular approaches that determine relative parasite load associated with infections (e.g. [85]) would allow for assessment of variation in body condition relative to parasitemia and may provide improved insight into the epidemiology of hematozoa infections.
Conclusions
Infections of Leucocytozoon, Haemoproteus, and Plasmodium parasites were common in all species of adult and juvenile ducks sampled, demonstrating an established host-parasite system occurring at the interface of the sub-Arctic and Arctic. Our results corroborate recent evidence in songbirds that Plasmodium transmission is established as far north as 65° in Alaska, and extends these findings to multiple waterfowl species. Variation in the prevalence and molecular detection of hematozoa parasites in wild ducks is influenced by parasite genus, seasonal timing, and a number of host traits including species, age, and sex. These factors should be controlled for in future studies to allow for direct comparisons of prevalence and assessment of potential climate-mediated changes in parasite abundance and distribution. Our study provides data on within-host relationships between hematozoa and IAV, showing that infection with these parasitic and viral agents is independent. We observed a strong positive relationship in the occurrence of Leucocytozoon and Haemoproteus parasites, suggesting that infection by parasites in one or both genera is enhanced by existing infection with the other, or that encounter rates of hosts and genus-specific vectors are correlated. Studies designed to identify arthropod vectors associated with hematozoa transmission in sub-Arctic and Arctic habitats are needed to provide detailed insight into co-infection dynamics.
Acknowledgements
We thank the numerous technicians who assisted with waterfowl captures and analysis of influenza swab and serum samples. This study was funded by the U.S. Geological Survey through the Wildlife Program of the Ecosystem Mission Area, the U.S. Fish and Wildlife Service (K. Trust, Alaska Region, Migratory Bird Management), Delta Waterfowl Foundation, and the Institute for Wetland and Waterfowl Research. The work was also supported by CRIP (Center for Research on Influenza Pathogenesis), an NIAID funded Center of Excellence for Influenza Research and Surveillance (CEIRS; contracts HHSN272201400008C and HHSN266200700010C). David Andersen, Joseph Fleskes, and Julie Yee provided helpful comments that improved the manuscript. P. Flint provided valuable insight and analytical support. The use of trade or product names is for descriptive purposes only and does not constitute endorsement by the U.S. Government.
Availability of data and materials
Data presented in this paper are publicly available via the USGS Alaska Science Center (DOI:10.5066/F7QJ7FD2).
Authors’ contributions
BWM and AMR conceived the study. BWM, JAR, and AMR procured resources to support field data collection and sample analysis. BWM collected and analyzed the data; MSL and TWA assisted in study design and data analysis. MMS conducted molecular analyses to detect hematozoa infections. JAR screened influenza swab and serum samples. BWM wrote the manuscript with input from all authors. All authors read and approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethics approval
Waterfowl capture and sampling was authorized by U. S. Federal Bird Banding and Marking Permit (Permit No. 23191) and by the Institutional Animal Care and Use Committees at the University of Minnesota (Protocol No. 0902A60221) and the University of Alaska Fairbanks (Protocol No. 147429–9).
Additional files
This supporting file contains AICc model selection results for estimating probability of hematozoa infection (Ψ) and probability of detecting hematozoa mtDNA (p) for three hematozoa genera (Leucocytozoon, Haemoproteus, Plasmodium) in each of three stages of model selection (Tables S1-S12). Models are ranked by ΔAICc values. (XLSX 49 kb)
This supporting file contains estimates of Leucocytozoon prevalence relative to co-infection with Haemoproteus (Table S13), estimated Haemoproteus prevalence relative to co-infection with Leucocytozoon (Table S14), estimated Haemoproteus prevalence relative to body condition (Table S15), and estimated Leucocytozoon prevalence relative to body condition (Table S16). (PDF 66 kb)
Contributor Information
Brandt W. Meixell, Email: bmeixell@usgs.gov
Todd W. Arnold, Email: arnol065@umn.edu
Mark S. Lindberg, Email: mslindberg@alaska.edu
Matthew M. Smith, Email: mmsmith@usgs.gov
Jonathan A. Runstadler, Email: jrun@mit.edu
Andrew M. Ramey, Email: aramey@usgs.gov
References
- 1.Valkiūnas G. Avian malaria parasites and other haemosporidia. Boca Raton: CRC Press; 2005. [Google Scholar]
- 2.Bennett GF, Peirce MA, Ashford RW. Avian Haematozoa: mortality and pathogenicity. J Nat Hist. 1993;27:993–1001. doi: 10.1080/00222939300770621. [DOI] [Google Scholar]
- 3.Atkinson CT, Lease JK, Dusek RJ, Samuel MD. Prevalence of pox-like lesions and malaria in forest bird communities on leeward Mauna Loa Volcano, Hawaii. Condor. 2005;107:537–46. doi: 10.1650/0010-5422(2005)107[0537:POPLAM]2.0.CO;2. [DOI] [Google Scholar]
- 4.Warner RE. The role of introduced diseases in the extinction of the endemic Hawaiian avifauna. Condor. 1968;70:101–20. doi: 10.2307/1365954. [DOI] [Google Scholar]
- 5.van Riper IIIC, van Riper SG, Goff ML, Laird M. The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecol Monogr. 1986;56:327. doi: 10.2307/1942550. [DOI] [Google Scholar]
- 6.Palinauskas V, Valkiūnas G, Bolshakov CV, Bensch S. Plasmodium relictum (lineage P-SGS1): effects on experimentally infected passerine birds. Exp Parasitol. 2008;120:372–80. doi: 10.1016/j.exppara.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Fallis AM, Bennett GF. On the epizootiology of infections caused by Leucocytozoon simondi in Algonquin Park, Canada. Can J Zool. 1966;44:101–12. doi: 10.1139/z66-007. [DOI] [Google Scholar]
- 8.Khan RA, Fallis AM. Comparison of infections with Leucocytozoon simondi in black ducks (Anas rubripes), mallards (Anas platyrhynchos) and white Pekins (Anas bochas) Can J Zool. 1968;46:773–80. doi: 10.1139/z68-107. [DOI] [PubMed] [Google Scholar]
- 9.Dimitrov D, Palinauskas V, Iezhova TA, Bernotiene R, Ilgunas M, Bukauskaite D, Zehtindjiev P, Ilieva M, Shapoval AP, Bolshakov CV, et al. Plasmodium spp. an experimental study on vertebrate host susceptibility to avian malaria. Exp Parasitol. 2015;148:1–16. [DOI] [PubMed]
- 10.Garamszegi LZ. Climate change increases the risk of malaria in birds. Glob Chang Biol. 2011;17:1751–9. doi: 10.1111/j.1365-2486.2010.02346.x. [DOI] [Google Scholar]
- 11.Durrant KL, Beadell JS, Ishtiaq F, Graves GR, Olson SL, Gering E, Peirce M, Milensky CM, Schmidt BK, Gebhard C. Avian hematozoa in South America: a comparison of temperate and tropical zones. Ornithol Monogr. 2006;60:98–111. doi: 10.1642/0078-6594(2006)60[98:AHISAA]2.0.CO;2. [DOI] [Google Scholar]
- 12.Greiner EC, Bennett GF, White EM, Coombs RF. Distribution of the avian hematozoa of North America. Can J Zool. 1975;53:1762–87. doi: 10.1139/z75-211. [DOI] [PubMed] [Google Scholar]
- 13.Ramey AM, Reed JA, Schmutz JA, Fondell TF, Meixell BW, Hupp JW, Ward DH, Terenzi J, Ely CR. Prevalence, transmission, and genetic diversity of blood parasites infecting tundra-nesting geese in Alaska. Can J Zool. 2014;92:699–706. doi: 10.1139/cjz-2014-0041. [DOI] [Google Scholar]
- 14.Ramey AM, Schmutz JA, Reed JA, Fujita G, Scotton BD, Casler B, Fleskes JP, Konishi K, Uchida K, Yabsley MJ. Evidence for intercontinental parasite exchange through molecular detection and characterization of haematozoa in northern pintails (Anas acuta) sampled throughout the North Pacific Basin. Int J Parasitol Parasites Wildl. 2015;4:11–21. doi: 10.1016/j.ijppaw.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett GF, MacInnes CD. Blood parasites of geese of the McConnell River, NWT. Can J Zool. 1972;50:1–4. doi: 10.1139/z72-001. [DOI] [PubMed] [Google Scholar]
- 16.Deviche P, Greiner EC, Manteca X. Seasonal and age-related changes in blood parasite prevalence in Dark-eyed Juncos (Junco hyemalis, Aves, Passeriformes) J Exp Zool. 2001;289:456–66. doi: 10.1002/jez.1027. [DOI] [PubMed] [Google Scholar]
- 17.Loiseau C, Harrigan RJ, Cornel AJ, Guers SL, Dodge M, Marzec T, Carlson JS, Seppi B, Sehgal RN. First evidence and predictions of Plasmodium transmission in Alaskan bird populations. PLoS One. 2012;7:e44729. doi: 10.1371/journal.pone.0044729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stabler RM, Kitzmiller NJ, Ellison LN, Holt PA. Hematozoa from the Alaskan spruce grouse, Canachites canadensis. J Parasitol. 1967;53:233–4. doi: 10.2307/3276566. [DOI] [PubMed] [Google Scholar]
- 19.Bennett GF, Nieman DJ, Turner B, Kuyt E, Whiteway M, Greiner EC. Blood parasites of prairie anatids and their implication in waterfowl management in Alberta and Saskatchewan. J Wildl Dis. 1982;18:287–96. doi: 10.7589/0090-3558-18.3.287. [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson LC, Handel CM, Van Hemert C, Loiseau C, Sehgal RN. Avian malaria in a boreal resident species: long-term temporal variability, and increased prevalence in birds with avian keratin disorder. Int J Parasitol. 2016;46:281–90. doi: 10.1016/j.ijpara.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Oakgrove KS, Harrigan RJ, Loiseau C, Guers S, Seppi B, Sehgal RN. Distribution, diversity and drivers of blood-borne parasite co-infections in Alaskan bird populations. Int J Parasitol. 2014;44:717–27. doi: 10.1016/j.ijpara.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Bennett GF, Montgomerie R, Seutin G. Scarcity of haematozoa in birds breeding on the Arctic tundra of North America. Condor. 1992;94:289–92. doi: 10.2307/1368821. [DOI] [Google Scholar]
- 23.Kaufman DS, Schneider DP, McKay NP, Ammann CM, Bradley RS, Briffa KR, Miller GH, Otto-Bliesner BL, Overpeck JT, Vinther BM, et al. Recent warming reverses long-term Arctic cooling. Science. 2009;325:1236–9. doi: 10.1126/science.1173983. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Rodriguez A, de la Hera I, Fernandez-Gonzalez S, Perez-Tris J. Global warming will reshuffle the areas of high prevalence and richness of three genera of avian blood parasites. Glob Chang Biol. 2014;20:2406–16. doi: 10.1111/gcb.12542. [DOI] [PubMed] [Google Scholar]
- 25.Zamora-Vilchis I, Williams SE, Johnson CN. Environmental temperature affects prevalence of blood parasites of birds on an elevation gradient: implications for disease in a warming climate. PLoS One. 2012;7:e39208. doi: 10.1371/journal.pone.0039208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett GF, Bishop MA. Change in status of haematozoan infections in wild passeriforms sampled in successive years. Proc Zool Soc (Calcutta) 1990;43:9–18. [Google Scholar]
- 27.Van Hemert C, Pearce JM, Handel CM. Wildlife health in a rapidly changing North: focus on avian disease. Front Ecol Environ. 2014;12:548–56. doi: 10.1890/130291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett GF. On some ornithophilic blood-sucking Diptera in Algonquin Park, Ontario, Canada. Can J Zool. 1960;38:377–89. doi: 10.1139/z60-042. [DOI] [Google Scholar]
- 29.Anderson JR, DeFoliart GR. Feeding behavior and host preferences of some black flies (Diptera: Simuliidae) in Wisconsin. Ann Entomol Soc Am. 1961;54:716–29. doi: 10.1093/aesa/54.5.716. [DOI] [Google Scholar]
- 30.Ricklefs RE. Embryonic development period and the prevalence of avian blood parasites. Proc Natl Acad Sci U S A. 1992;89:4722–5. doi: 10.1073/pnas.89.10.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medeiros MC, Hamer GL, Ricklefs RE. Host compatibility rather than vector-host-encounter rate determines the host range of avian Plasmodium parasites. Proc Biol Sci. 2013;280:20122947. doi: 10.1098/rspb.2012.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arriero E, Moller AP. Host ecology and life-history traits associated with blood parasite species richness in birds. J Evol Biol. 2008;21:1504–13. doi: 10.1111/j.1420-9101.2008.01613.x. [DOI] [PubMed] [Google Scholar]
- 33.Palinauskas V, Valkiūnas G, Bolshakov CV, Bensch S. Plasmodium relictum (lineage SGS1) and Plasmodium ashfordi (lineage GRW2): the effects of the co-infection on experimentally infected passerine birds. Exp Parasitol. 2011;127:527–33. doi: 10.1016/j.exppara.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Van Rooyen J, Lalubin F, Glaizot O, Christe P. Avian haemosporidian persistence and co-infection in great tits at the individual level. Malar J. 2013;12:40. doi: 10.1186/1475-2875-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cox FE. Concomitant infections, parasites and immune responses. Parasitology. 2001;122(Suppl):S23–38. doi: 10.1017/S003118200001698X. [DOI] [PubMed] [Google Scholar]
- 36.Medeiros MC, Anderson TK, Higashiguchi JM, Kitron UD, Walker ED, Brawn JD, Krebs BL, Ruiz MO, Goldberg TL, Ricklefs RE, et al. An inverse association between West Nile virus serostatus and avian malaria infection status. Parasit Vectors. 2014;7:415. doi: 10.1186/1756-3305-7-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ludford CG, Purchase HG, Cox HW. Duck infectious anemia virus associated with Plasmodium lophurae. Exp Parasitol. 1972;31:29–38. doi: 10.1016/0014-4894(72)90044-6. [DOI] [PubMed] [Google Scholar]
- 38.Stallknecht DE, Brown JD, Swayne DE. Ecology of avian influenza in wild birds. In: Swayne DE, editor. Avian influenza. 2008. pp. 43–58. [Google Scholar]
- 39.Kuiken T. Is low pathogenic avian influenza virus virulent for wild waterbirds? Proc R Soc B. 2013;280:20130990. doi: 10.1098/rspb.2013.0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shutler D, Clark RG, Rutherford ST, Mullie A. Blood parasites, clutch volume, and condition of Gadwalls and Mallards. J Avian Biol. 1999;30:295–301. doi: 10.2307/3677355. [DOI] [Google Scholar]
- 41.Lachish S, Knowles SC, Alves R, Wood MJ, Sheldon BC. Fitness effects of endemic malaria infections in a wild bird population: the importance of ecological structure. J Anim Ecol. 2011;80:1196–206. doi: 10.1111/j.1365-2656.2011.01836.x. [DOI] [PubMed] [Google Scholar]
- 42.Marzal A. Recent advances in studies on avian malaria parasites. In: Okwa OO, editor. Malaria Parasites. Rijeka, Croatia: Intech; 2012. p. 135–158.
- 43.Asghar M, Hasselquist D, Hansson B, Zehtindjiev P, Westerdahl H, Bensch S. Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science. 2015;347:436–8. doi: 10.1126/science.1261121. [DOI] [PubMed] [Google Scholar]
- 44.Merino S, Moreno J, Sanz JJ, Arriero E. Are avian blood parasites pathogenic in the wild? A medication experiment in blue tits (Parus caeruleus) Proc Biol Sci. 2000;267:2507–10. doi: 10.1098/rspb.2000.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shutler D, Lowe AG, Robinson SR. Relationships between circulating leucocytes and Leucocytozoon simondi in mallard, Anas platyrhynchos, ducklings. Comp Biochem Physiol A Mol Integr Physiol. 2010;156:46–9. doi: 10.1016/j.cbpa.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 46.Shutler D, Ankney CD, Dennis DG. Could the blood parasite Leucocytozoon deter mallard range expansion? J Wild Manage. 1996;60:569–80. doi: 10.2307/3802074. [DOI] [Google Scholar]
- 47.Petrula MJ. Nesting ecology of ducks in interior Alaska. M.Sc. thesis. Fairbanks: University of Alaska Fairbanks; 1994. [Google Scholar]
- 48.Walker J, Lindberg MS, MacCluskie MC, Petrula MJ, Sedinger JS. Nest survival of scaup and other ducks in the boreal forest of Alaska. J Wild Manage. 2005;69:582–91. doi: 10.2193/0022-541X(2005)069[0582:NSOSAO]2.0.CO;2. [DOI] [Google Scholar]
- 49.Sharp DE, Lokemoen JT. A decoy trap for breeding-season mallards in North Dakota. J Wild Manage. 1987;51:711–5. doi: 10.2307/3801731. [DOI] [Google Scholar]
- 50.Dill HH, Thornsberry WH. A cannon-projected net trap for capturing waterfowl. J Wild Manage. 1950;14:132–7. doi: 10.2307/3796320. [DOI] [Google Scholar]
- 51.Hunt GS, Dahlka KJ. Live trapping of diving ducks. J Wild Manage. 1953;17:92–5. doi: 10.2307/3796814. [DOI] [Google Scholar]
- 52.Hochbaum HA. Sex and age determination of waterfowl by cloacal examination. Trans N A Wildlife Conf. 1942;7:299–307. [Google Scholar]
- 53.Carney SM. Species, age and sex identification of ducks using wing plumage. Washington DC: US Fish and Wildlife Service; 1992. [Google Scholar]
- 54.Dzubin A, Cooch EG. Measurements of geese: general field methods. Sacramento: California Waterfowl Association; 1992. [Google Scholar]
- 55.Ramey AM, Fleskes JP, Schmutz JA, Yabsley MJ. Evaluation of blood and muscle tissues for molecular detection and characterization of hematozoa infections in northern pintails (Anas acuta) wintering in California. Int J Parasitol Parasites Wildl. 2013;2:102–9. doi: 10.1016/j.ijppaw.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hellgren O, Waldenström J, Bensch S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J Parasitol. 2004;90:797–802. doi: 10.1645/GE-184R1. [DOI] [PubMed] [Google Scholar]
- 57.Reeves AB, Smith MM, Meixell BW, Fleskes JP, Ramey AM. Genetic diversity and host specificity varies across three genera of blood parasites in ducks of the Pacific Americas flyway. PLoS One. 2015;10:e0116661. doi: 10.1371/journal.pone.0116661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kerr KC, Stoeckle MY, Dove CJ, Weigt LA, Francis CM, Hebert PD. Comprehensive DNA barcode coverage of North American birds. Mol Ecol Notes. 2007;7:535–43. doi: 10.1111/j.1471-8286.2007.01670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Runstadler JA, Happ GM, Slemons RD, Sheng Z-M, Gundlach N, Petrula M, Senne D, Nolting J, Evers DL, Modrell A, et al. Using RRT-PCR analysis and virus isolation to determine the prevalence of avian influenza virus infections in ducks in Minto Flats State Game Refuge, Alaska, during August 2005. Arch Virol. 2007;152:1901–10. doi: 10.1007/s00705-007-0994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown JD, Stallknecht DE, Berghaus RD, Luttrell MP, Velek K, Kistler W, Costa T, Yabsley MJ, Swayne D. Evaluation of a commercial blocking enzyme-linked immunosorbent assay to detect avian influenza virus antibodies in multiple experimentally infected avian species. Clin Vaccine Immunol. 2009;16:824–9. doi: 10.1128/CVI.00084-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramey AM, Ely CR, Schmutz JA, Pearce JM, Heard DJ. Molecular detection of hematozoa infections in tundra swans relative to migration patterns and ecological conditions at breeding grounds. PLoS One. 2012;7:e45789. doi: 10.1371/journal.pone.0045789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McClintock BT, Nichols JD, Bailey LL, MacKenzie DI, Kendall W, Franklin AB. Seeking a second opinion: uncertainty in disease ecology. Ecol Lett. 2010;13:659–74. doi: 10.1111/j.1461-0248.2010.01472.x. [DOI] [PubMed] [Google Scholar]
- 63.MacKenzie DI, Nichols JD, Royle JA, Pollock KH, Bailey LL, Hines JE. Occupancy estimation and modeling: inferring patterns and dynamics of species occurrence. San Diego: Academic; 2006. [Google Scholar]
- 64.White GC, Burnham KP. Program MARK: survival estimation from populations of marked animals. Bird Study. 1999;46:S120–39. doi: 10.1080/00063659909477239. [DOI] [Google Scholar]
- 65.Arnold TW. Uninformative parameters and model selection using Akaike’s Information Criterion. J Wild Manage. 2010;74:1175–8. doi: 10.2193/2009-367. [DOI] [Google Scholar]
- 66.Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information-theoretic approach. 2. New York: Springer; 2002. [Google Scholar]
- 67.Valkiūnas G, Bairlein F, Iezhova TA, Dolnik OV. Factors affecting the relapse of Haemoproteus belopolskyi infections and the parasitaemia of Trypanosoma spp. in a naturally infected European songbird, the blackcap, Sylvia atricapilla. Parasitol Res. 2004;93:218–22. doi: 10.1007/s00436-004-1071-2. [DOI] [PubMed] [Google Scholar]
- 68.Herman CM. Blood parasites of North American waterfowl. Trans N Am Wildl Nat Resour Conf. 1968;33:348–59. [Google Scholar]
- 69.Lachish S, Gopalaswamy AM, Knowles SCL, Sheldon BC. Site-occupancy modelling as a novel framework for assessing test sensitivity and estimating wildlife disease prevalence from imperfect diagnostic tests. Methods Ecol Evol. 2012;3:339–48. doi: 10.1111/j.2041-210X.2011.00156.x. [DOI] [Google Scholar]
- 70.Royle JA, Nichols JD. Estimating abundance from repeated presence-absence data or point counts. Ecology. 2003;84:777–90. doi: 10.1890/0012-9658(2003)084[0777:EAFRPA]2.0.CO;2. [DOI] [Google Scholar]
- 71.Beaudoin RL, Applegate JE, Davis DE, McLean RG. A model for the ecology of avian malaria. J Wildl Dis. 1971;7:5–13. doi: 10.7589/0090-3558-7.1.5. [DOI] [PubMed] [Google Scholar]
- 72.Bennett GF, Cameron M. Seasonal prevalence of avian hematozoa in passeriform birds of Atlantic Canada. Can J Zool. 1974;52:1259–64. doi: 10.1139/z74-167. [DOI] [PubMed] [Google Scholar]
- 73.McCurdy DG, Shutler D, Mullie A, Forbes MR. Sex-biased parasitism of avian hosts: relations to blood parasite taxon and mating system. Oikos. 1998;82:303–12. doi: 10.2307/3546970. [DOI] [Google Scholar]
- 74.Valkiūnas G, Iezhova TA, Loiseau C, Sehgal RN. Nested cytochrome b polymerase chain reaction diagnostics detect sporozoites of hemosporidian parasites in peripheral blood of naturally infected birds. J Parasitol. 2009;95:1512–5. doi: 10.1645/GE-2105.1. [DOI] [PubMed] [Google Scholar]
- 75.Williams NA, Calverley BK, Mahrt JL. Blood parasites of mallard and pintail ducks from central Alberta and the Mackenzie Delta, Northwest Territories. J Wildl Dis. 1977;13:226–9. doi: 10.7589/0090-3558-13.3.226. [DOI] [PubMed] [Google Scholar]
- 76.Hohman WL, Ankney CD, Gordon DH, Batt B, Afton A, Anderson M, Ankney C, Johnson D, Kadlec J, Krapu G. Ecology and management of postbreeding waterfowl. In: Batt B, editor. Ecology and management of breeding waterfowl. Minneapolis: University of Minnesota Press; 1992. pp. 128–89. [Google Scholar]
- 77.Bennett GF, Turner B, Holton G. Blood parasites of trumpeter swans, Olor buccinator (Richardson), from Alberta. J Wildl Dis. 1981;17:213–5. doi: 10.7589/0090-3558-17.2.213. [DOI] [PubMed] [Google Scholar]
- 78.Hamilton WD, Zuk M. Heritable true fitness and bright birds: a role for parasites? Science. 1982;218:384–7. doi: 10.1126/science.7123238. [DOI] [PubMed] [Google Scholar]
- 79.Tella JL, Blanco G, Forero MG, Gajon A, Donazar JA, Hiraldo F. Habitat, world geographic range, and embryonic development of hosts explain the prevalence of avian hematozoa at small spatial and phylogenetic scales. Proc Natl Acad Sci U S A. 1999;96:1785–9. doi: 10.1073/pnas.96.4.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baldassarre G. Ducks, geese, and swans of North America. Baltimore: Johns Hopkins University Press; 2014. [Google Scholar]
- 81.Deviche P, Greiner EC, Manteca X. Interspecific variability of prevalence in blood parasites of adult passerine birds during the breeding season in Alaska. J Wildl Dis. 2001;37:28–35. doi: 10.7589/0090-3558-37.1.28. [DOI] [PubMed] [Google Scholar]
- 82.Telfer S, Lambin X, Birtles R, Beldomenico P, Burthe S, Paterson S, Begon M. Species interactions in a parasite community drive infection risk in a wildlife population. Science. 2010;330:243–6. doi: 10.1126/science.1190333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dijk JG, Fouchier RA, Klaassen M, Matson KD. Minor differences in body condition and immune status between avian influenza virus‐infected and noninfected mallards: a sign of coevolution? Ecol Evol. 2015;5:436–49. doi: 10.1002/ece3.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shutler D, Ankney CD, Mullie A. Effects of the blood parasite Leucocytozoon simondi on growth rates of anatid ducklings. Can J Zool. 1999;77:1573–8. doi: 10.1139/z99-121. [DOI] [Google Scholar]
- 85.Smith MM, Schmutz J, Apelgren C, Ramey AM. A real-time, quantitative PCR protocol for assessing the relative parasitemia of Leucocytozoon in waterfowl. J Microbiol Methods. 2015;111:72–7. doi: 10.1016/j.mimet.2015.01.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data presented in this paper are publicly available via the USGS Alaska Science Center (DOI:10.5066/F7QJ7FD2).


