ABSTRACT
Flaviviruses pose a significant threat to both animals and humans. Recently, a novel flavivirus, duck Tembusu virus (DTMUV), was identified to be the causative agent of a serious duck viral disease in Asia. Its rapid spread, expanding host range, and uncertain transmission routes have raised substantial concerns regarding its potential threats to nonavian hosts, including humans. Here, we demonstrate that DTMUV is not pathogenic for nonhuman primates and is highly sensitive to mammal type I interferon (IFN) signaling. In vitro assays demonstrated that DTMUV infected and replicated efficiently in various mammalian cell lines. Further tests in mice demonstrated high neurovirulence and the age-dependent neuroinvasiveness of the virus. In particular, the inoculation of DTMUV into rhesus monkeys did not result in either viremia or apparent clinical symptoms, although DTMUV-specific humoral immune responses were detected. Furthermore, we revealed that although avian IFN failed to inhibit DTMUV in avian cells, DTMUV was more sensitive to the antiviral effects of type I interferon than other known human-pathogenic flaviviruses. Knockout of the type I IFN receptor in mice caused apparent viremia, viscerotropic disease, and mortality, indicating a vital role of IFN signaling in protection against DTMUV infection. Collectively, we provide direct experimental evidence that this novel avian-origin DTMUV possesses a limited capability to establish infection in immunocompetent primates due to its decreased antagonistic activity in the mammal IFN system. Furthermore, our findings highlight the potential risk of DTMUV infection in immunocompromised individuals and warrant studies on the cross-species transmission and pathogenesis of this novel flavivirus.
IMPORTANCE Mosquito-borne flaviviruses comprise a large group of pathogenic and nonpathogenic members. The pathogenic flaviviruses include dengue, West Nile, and Japanese encephalitis viruses, and the nonpathogenic flaviviruses normally persist in a natural cycle and rarely cause disease in humans. A novel flavivirus, DTMUV (also known as duck egg drop syndrome flavivirus [DEDSV]) was identified in 2012 in ducks and then rapidly spread to several Asian countries. This new flavivirus was then shown to infect multiple avian species, resulting in neurological symptoms with unknown routes of transmission. There is public concern regarding its potential transmission from birds to humans and other nonavian hosts. Our present study shows that the mammalian IFN system can efficiently eliminate DTMUV infection and that the emergence of severe DTMUV-associated disease in mammals, especially humans, is unlikely. Currently, DTMUV infection mostly affects avian species.
INTRODUCTION
The genus Flavivirus of the family Flaviviridae contains emerging and reemerging enveloped RNA viruses that cause serious diseases in humans and animals, including dengue virus (DENV), yellow fever virus (YFV), Japanese encephalitis virus (JEV), West Nile virus (WNV), and tick-borne encephalitis virus (TBEV). A group of over 70 different species distributed throughout the world has been identified within this genus. The majority of the known flaviviruses are zoonotic pathogens that are transmitted to humans or other animals by mosquitoes or ticks. These viruses are able to cause a range of distinct clinical diseases in humans with from asymptomatic to severe manifestations, including life-threatening vascular leakage and hemorrhage (DENV and YFV) and lethal encephalitis (WNV, JEV, and TBEV). However, some flaviviruses, such as cell fusing agent virus, Culex flavivirus, and Kamiti River virus, generally persist in nature and are rarely linked to mammal or human diseases (1).
In 2010, a severe duck egg drop syndrome (DEDS) epidemic suddenly emerged in southeast China and quickly spread throughout the country (2, 3). Very recently, DEDS outbreaks in ducks have been reported in Malaysia and Thailand (4, 5). The causative agent of DEDS was identified to be a new member of the genus Flavivirus called duck Tembusu virus (DTMUV) or duck egg drop syndrome flavivirus (DEDSV) (2, 6, 7). To date, over 10 million shelducks have been infected, and approximately 1 million have died, thus causing huge economic losses in duck farming (8). In addition to egg drop syndrome, the DTMUV-infected ducks developed acute anorexia, retarded growth, and neurological dysfunction, with the morbidity rate being 90% to 100% and the mortality rate being 10% to 30% (9). Moreover, DTMUV was shown to expand its host range to multiple avian species, including chickens, sparrows, and geese (10–12). The transmission of DTMUV remains largely unknown. Mosquitoes are likely involved in the transmission cycle of DTMUV because the viruses have been isolated from various species of mosquitoes (13). Recently, a combined epidemiological and experimental investigation demonstrated the airborne transmission of DTMUV (14).
As with other flaviviruses, DTMUV has an approximately 11-kb positive-sense single-stranded RNA genome that contains a single open reading frame that encodes three structural proteins (the capsid [C], premembrane [prM], and envelope [E] proteins) and seven nonstructural (NS) proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5), flanked by the 5′ and 3′ untranslated regions (UTRs). The structural proteins are engaged in cellular attachment, membrane fusion, and virion assembly, and the nonstructural proteins are involved in viral replication and the counteraction of host immunity in a mechanism similar to that of other known pathogenic flaviviruses. Most flaviviruses are potent in antagonizing interferon (IFN)-induced signaling through the JAK-STAT pathway with a complex mechanism mediated by their nonstructural proteins (15–19), which are responsible for cross-species transmission and pathogenesis in vivo.
Phylogenetic analyses of the whole polyproteins of flaviviruses showed that the DTMUV isolates cluster within the clade of mosquito-borne flaviviruses (2, 20). Given its close relation to other human-pathogenic flaviviruses, its uncertain route of transmission, the severe disease outcome in DTMUV-infected animals, and its wide geographic distribution, considerable concerns have been raised regarding the possibility of DTMUV's emergence in humans and other nonavian animals, leading to an epidemic of a new zoonosis. Although no cases of DTMUV-related human disease have been reported, a recent study identified DTMUV-specific antibodies and viral RNA in duck industry workers in China (21).
Here, we first investigated the susceptibility of various mammalian cell lines to DTMUV and systematically characterized its virulence and pathogenicity in mice and rhesus monkeys. Furthermore, we elucidated the role of type I IFN signaling in inhibiting DTMUV replication in vitro compared with the role of type I IFN signaling in inhibiting a representative pathogenic flavivirus, JEV, and in controlling DTMUV infection in vivo. Based on the results that DTMUV failed to cause viremia or any clinical manifestation in nonhuman primates and that DTMUV was more sensitive to the antiviral action of host IFN than other known human-pathogenic flaviviruses, we clearly ruled out the possibility that DTMUV is a zoonotic pathogen for humans.
MATERIALS AND METHODS
Ethics statement.
All animal experiments were performed in strict accordance with the guidelines of the Chinese Regulations of Laboratory Animals (Ministry of Science and Technology of the People's Republic of China) and the Laboratory Animal Requirements of Environment and Housing Facilities (GB 14925-2010, National Laboratory Animal Standardization Technical Committee). All procedures were performed under sodium pentobarbital anesthesia by trained technicians, and all efforts were made to ensure the welfare and minimize the suffering of the animals in accordance with the recommendations of The Weatherall Report on the Use of Non-Human Primates (22). The experimental protocols were approved by the Animal Experiment Committee of the Beijing Institute of Microbiology and Epidemiology, Beijing, China (IME number 2013011).
Cells and viruses.
All cell lines were purchased from the American Type Culture Collection (ATCC) and were grown at 37°C in 5% CO2. BHK-21, Vero, HeLa, HepG2, DF-1, and SH-SY5Y cells were propagated in Dulbecco's minimal essential medium (DMEM; Life Technologies) supplemented with 10% fetal bovine serum (FBS) and with 100 U/ml penicillin and 100 μg/ml streptomycin (PS). A549 cells were cultured in RPMI 1640 medium (Life Technologies) supplemented with 10% FBS and PS. DTMUV strain BYD-1 (2) and JEV strain SA14 (23) were prepared in BHK-21 cells, followed by the titration of the viruses by a standard plaque assay in BHK-21 cells.
Cell viability assay.
Cells seeded in 96-well tissue culture dishes were infected with DTMUV at a multiplicity of infection (MOI) of 0.01. The infected cells were examined twice a day by light microscopy for the development of a cytopathic effect (CPE). At 48 h postinfection, cell viability was determined using a CellTiter 96 nonradioactive cell proliferation assay (Promega) according to the manufacturer's protocol. The percent CPE was calculated according to the following formula: (OD490 of infected cells/OD490 of uninfected cells) × 100, where OD490 is the optical density at 490 nm. To evaluate cell death, the infected cells were fixed with formaldehyde and stained with crystal violet. The absorbance was measured at a wavelength of 620 nm.
Growth kinetics.
Viral growth curves were performed by inoculating confluent cells in a 12-well plate at an MOI of 0.01. Cell supernatants were collected at successive 24-h intervals postinfection. The yields of progeny virus in each sample were then quantitated by plaque assay of BHK-21 cells. Briefly, BHK-21 cells were infected with a 10-fold serial dilution of viruses. The plates were incubated at 37°C for 1 h with gentle rocking every 15 min. The supernatant was removed, and the cells were overlaid with 1% agarose (Promega) in DMEM containing 2% FBS. After further incubation at 37°C for 4 days, the cells were fixed with formaldehyde and stained with crystal violet to visualize the plaques.
IFA.
Indirect immunofluorescence assays (IFA) were performed as follows. Briefly, 90% confluent cells grown in culture dishes containing a 1-cm2 coverslip were infected with viruses at an MOI of 0.01. At 48 h postinfection, the cells seeded on the coverslips were fixed with acetone, incubated with primary antibodies (mouse antiserum against DTMUV), and then incubated with secondary antibodies conjugated to Alexa Fluor 488 (Invitrogen) in phosphate-buffered saline (PBS). Fluorescent cells were examined using a fluorescence microscope (Olympus).
Nuclear translocation of p-STAT1.
To examine virus protein expression and STAT1 phosphorylation in cells, Vero cells were infected with DTMUV or JEV at an MOI of 1. At 6 h postinfection, the cells were treated with complete DMEM as a control or with complete DMEM containing alpha A/D IFN (IFN-αA/D; 1,000 U/ml; Life Technologies) at 37°C for 30 min. The cells were fixed in methanol, rinsed twice in PBS, and then incubated with rabbit anti-phospho-STAT1 (p-STAT1) antibodies and mouse antiserum specific for DTMUV or JEV. The cells were washed in PBS and incubated with the corresponding secondary antibodies and DAPI (4′,6-diamidino-2-phenylindole) for 1 h at 37°C. Images were captured using a fluorescence microscope (Olympus).
IFN antiviral assay.
BHK-21 cells grown in 96-well plates were treated with medium containing various doses of IFN-αA/D (0, 100, or 1,000 U). After 6 h of incubation, the cells were washed with PBS and infected with DTMUV or JEV at an MOI of 1. Then, the unbound virus was removed from the cells by gentle washing with PBS. At 24 h postinfection, the supernatants were collected and the viral titers were determined by plaque assay in BHK-21 cells. Cell viability was determined as described above according to the manufacturer's instructions.
DF-1 cells grown in 96-well plates were treated with medium containing various concentrations of chicken IFN-α (0, 100, or 1,000 μg; Abcam). After 6 h of incubation, the cells were washed with PBS and infected with DTMUV at an MOI of 1. The supernatants were collected, and the viral titers were determined by plaque assay in BHK-21 cells at 24 h postinfection.
IFN-stimulated response element (ISRE) reporter assay.
BHK-21 cells were transfected with a pISRE-Luc reporter plasmid (Clontech) or a Renilla luciferase (Luc) plasmid (pRL-TK; Promega) using the Lipofectamine 3000 reagent in Opti-MEM medium (Life Technology, USA). At 24 h posttransfection, the cells were infected with DTMUV or JEV at an MOI of 1 and incubated for another 24 h. The supernatants were replaced with fresh medium containing 100 U of IFN-αA/D. After treatment for 24 h, the cell lysates were harvested and the luciferase assay was performed using a dual-luciferase assay kit according to the manufacturer's instructions (Promega).
Mouse experiments. (i) BALB/c mice.
For the neurovirulence tests, 1-day-old or 3-week-old BALB/c mice were inoculated intracerebrally (i.c.) with various dilutions of DTMUV. For neuroinvasiveness tests, groups of female BALB/c mice were inoculated intraperitoneally (i.p.) with the dose of DTMUV indicated below. Mock-infected mice received PBS. Animals were monitored daily over a 21-day period of observation for the appearance of symptoms. For the study of viral replication in the mouse brain, 3-week-old mice were infected i.c. with 100 PFU of DTMUV. Two or three animals were euthanized on days 2, 4, and 6 postinoculation (p.i.). The brains were collected and homogenized in DMEM, and the viral titer in the supernatant was determined by plaque assay on BHK-21 cells.
(ii) 129/Sv/Ev (WT) mice and IFN-α/βR−/− mice.
IFN-α/β receptor-deficient (IFN-α/βR−/−) mice were kindly provided by Qi-Bin Leng (Shanghai Institute for Pasteur, CAS, China). For the survival study, groups of 3- to 4-week-old female wild-type (WT) mice or IFN-α/βR−/− mice were i.p. inoculated with the doses of DTMUV indicated below and monitored daily for 21 days to assess morbidity and mortality. Blood was collected, and sera were recovered, aliquoted, and stored at −80°C. The liver, spleen, kidney, and brain were dissected, weighed, and homogenized in PBS at 2, 4, and 6 days p.i. The titers of the virus stocks in the supernatants and viruses in serum were determined by plaque assay on BHK-21 cells.
(iii) Histopathological analysis.
Four-week-old female IFN-α/βR−/− mice were inoculated with 105 PFU of DTMUV or PBS by the i.p. route. At 6 days p.i., the brains, livers, and spleens of the mice were collected, fixed with perfusion fixative (formaldehyde) for 48 h, and processed according to standard histological assays. All sections from each tissue were stained with hematoxylin and eosin (H&E).
Monkey experiments. (i) Inoculation of rhesus monkeys.
Three 2-year-old monkeys were prescreened and found to be negative for IgG antibodies against DTMUV by enzyme-linked immunosorbent assay (ELISA). Animals were inoculated subcutaneously (s.c.) with 105 PFU of DTMUV. Blood was collected from each rhesus monkey daily p.i. for 10 days to detect viremia. For the IgM test, blood samples were collected before inoculation (day 0) and then on days 5, 10, 14, and 28 p.i. Blood samples for the IgG and neutralizing antibody tests were collected before inoculation (day 0) and then on days 14 and 28 p.i. Blood samples were collected on days 0, 5, 8, and 14 p.i. for blood biochemistry and hematology analysis. Additionally, the body temperature of the inoculated animals was measured daily for 10 days p.i.
(ii) Viremia determination.
Viremia in rhesus monkeys was measured by quantitative real-time reverse transcriptase PCR (qRT-PCR) as previously described (24). DTMUV was used to generate a standard curve, and the number of infectious RNA transcripts per reaction corresponded to the known number of PFU per reaction, expressed as the equivalent number of PFU (eq PFU). The detection limit was 0.32 log10 eq PFU/ml (25). Briefly, total RNA was extracted from 200 μl of monkey serum using a PureLink RNA minikit (Life Technology, USA) according to the manufacturer's instructions. qRT-PCR was performed (one-step SYBR primer script plus RT-PCR kit; Takara) with primers DTMUV-F (CCAGCACTCCTATTACAGA) and DTMUV-R (TTAAGAACACCGCCACTA). The 25-μl reaction mixtures were set up with a 0.5 μM concentration of each primer and 5 μl of RNA. The thermocycling programs consisted of 42°C for 5 min, 95°C for 10 s, and 40 cycles of 95°C for 5 s and 60°C for 30 s.
(iii) Humoral immune response.
Serum IgM and IgG antibodies against DTMUV were detected by ELISA using formaldehyde-inactivated DTMUV as an antigen source. Serial 2-fold dilutions of serum were tested in duplicate. Neutralizing antibody titers were determined by a constant virus-serum dilution 50% plaque reduction neutralization test (PRNT50) as previously described (24). Briefly, serial 2-fold dilutions of inactivated serum were mixed with equal volumes of DTMUV in DMEM supplemented with 2% FBS. After incubation at 37°C for 1 h, virus-antibody mixtures were added to plates containing BHK-21 cells. The concentration of infectious virus was determined using the plaque assay described above. The endpoint neutralization titer was calculated using the method described by Reed and Muench (26).
Statistical analysis.
For the in vitro experiments, an unpaired two-tailed t test was used to determine statistically significant differences. For survival analysis, Kaplan-Meier survival curves were analyzed by a log-rank test. All data were analyzed using the standard GraphPad Prism software, version 5.0.
RESULTS
Mammalian cell lines are permissive and susceptible to DTMUV infection.
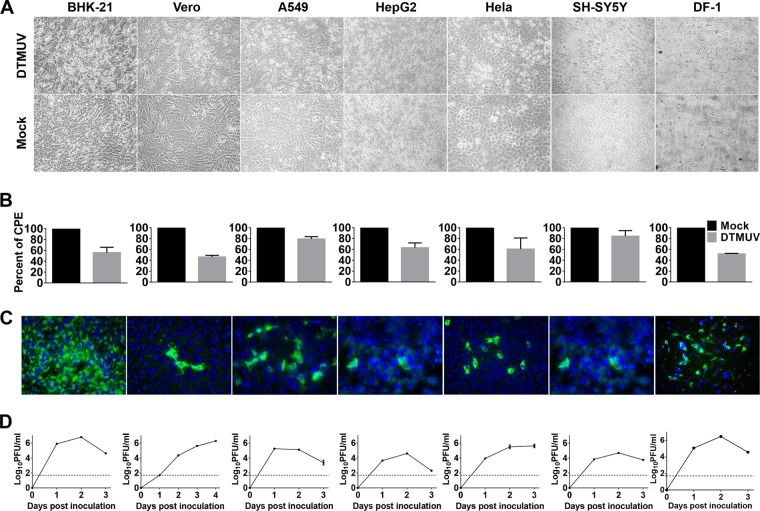
To examine whether DTMUV is capable of replicating in mammalian cells, a panel of mammalian cell lines derived from hamsters (BHK-21), monkeys (Vero), and humans (A549, HeLa, and SH-SY5Y) was subjected to DTMUV infection. Cell morphology results showed that a noticeable cytopathic effect (CPE), characterized by cell shrinkage, rounding, syncytia, and detachment, was observed in all the infected mammalian cells (Fig. 1A). Then, the viability of the DTMUV-infected cells was measured. Generally, as shown in Fig. 1B, viral infection reduced the viability of the virus-infected cells by approximately 17 to 55% at 48 h postinfection compared to that of the mock-infected cells, with the greatest loss of viability being for the DTMUV-infected Vero and BHK-21 cells. IFA results with anti-DTMUV antiserum showed that DTMUV-specific proteins were detected in all infected cells (Fig. 1C). Furthermore, DTMUV grew well to high titers and reached peak titers before 48 h postinfection in most cell lines tested, with the highest peak titer being 106.79 PFU/ml in BHK-21 cells (Fig. 1D). Notably, efficient DTMUV infection of SH-SY5Y human neuroblastoma cells was observed, suggesting a potential association with the neurotropic phenotype of DTMUV in avian species. As expected, DTMUV replicated rapidly in chicken embryo fibroblast DF-1 cells, resulting in a drastic CPE at 48 h postinfection (Fig. 1). Taken together, these results clearly demonstrate that DTMUV possesses the capacity to infect and cause a CPE in a wide spectrum of mammalian cell lines.
FIG 1.
DTMUV possesses the ability to infect a broad spectrum of mammalian cells. (A) CPE caused by DTMUV infections in various cells. The cells were infected with DTMUV at an MOI of 0.01 and examined by light microscopy at 48 h postinfection. (B) Cell viability was determined by the CellTiter 96 nonradioactive cell proliferation assay, and the percent CPE was calculated accordingly. Each assay was performed in triplicate. (C) IFA results for DTMUV-infected cells. (D) Viral growth in different cells. Viral yields in supernatants were quantitated by plaque assay on BHK-21 cells. Dotted lines, limits of detection of the plaque assay.
DTMUV exhibits neurovirulence and age-dependent neuroinvasiveness in mice.
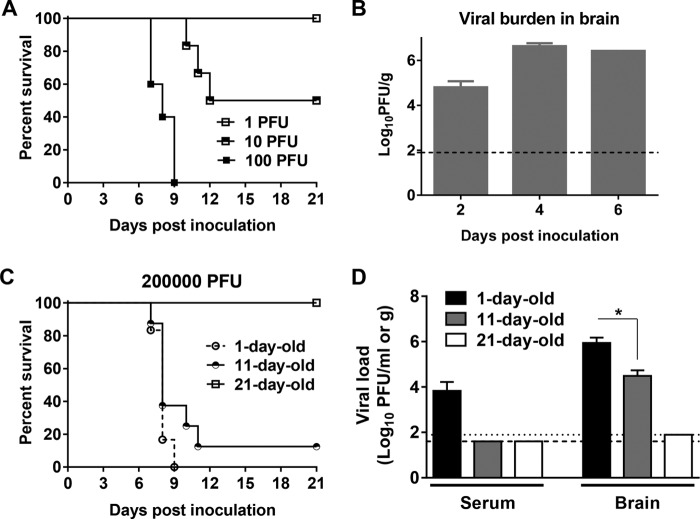
To further determine whether DTMUV could replicate in mammalian hosts and whether it possesses pathogenicity in mammalian hosts, we assessed DTMUV infection of mice and the pathogenicity of DTMUV in mice. Because many flaviviruses exhibit a neurotropic phenotype in mice, we initially examined the neurovirulence of DTMUV in 3-week-old mice. As shown in Fig. 2A, 10 PFU of DTMUV was sufficient to result in 50% mortality, with an average survival time (AST) of 11 days, but neither morbidity nor mortality was observed in mice following inoculation with 1 PFU. Following infection with 100 PFU, all mice showed severe symptoms by day 5, including hunching, fur ruffling, and greatly reduced activity, and finally died within 7 to 9 days. To extend these findings, the viral burden in mouse brains was also determined by plaque assay. As shown in Fig. 2B, a high viral load (104.80 PFU/g) was detected at day 2, and then the viral load peaked at 106.62 PFU/g at day 4 and declined slightly to 106.42 PFU/g at day 6 p.i. This finding suggests the efficient replication of DTMUV in the mouse brain, which was likely to result in mortality upon i.c. inoculation. Additionally, suckling mice were highly susceptible to DTMUV i.c. infection, with the 50% lethal dose (LD50) being 0.7 PFU (Table 1), and developed typical neurological symptoms. These observations reveal a DTMUV phenotype consisting of a high degree of neurovirulence in mice.
FIG 2.
DTMUV exhibits high neurovirulence and age-dependent neuroinvasiveness in mice. (A) Neurovirulence test in adult mice. Groups of 3-week-old female BALB/c mice (n = 5 to 6) were inoculated i.c. with various concentrations of DTMUV. Mock-infected mice received PBS. Animals were monitored daily for the appearance of symptoms during the 21-day period of observation. (B) Viral load in the mouse brain. Three-week-old female BALB/c mice were infected i.c. with 100 PFU of DTMUV. Two or three animals were euthanized on days 2, 4, and 6 p.i. Brains were collected and homogenized in DMEM. The viral titer in each supernatant was determined by plaque assay on BHK-21 cells. (C) Neuroinvasiveness tests in mice at different ages. Groups of 1-day-old, 11-day-old, and 21-day-old female BALB/c mice were inoculated i.p. with 100 μl of dilutions containing the indicated dose of DTMUV. Animals were monitored daily for the appearance of symptoms during the 21-day period of observation. (D) Viral loads in the serum and brain of mice determined by plaque assay. Dotted and dashed lines, limits of detection of the respective assays. Statistically significant differences are indicated. *, P < 0.05.
TABLE 1.
Survival data for suckling mice following i.c. inoculation with DTMUVa
| Inoculum | Dose (no. of PFU/mouse) | % mortality (no. of mice dead/no. tested) | AST (day) |
|---|---|---|---|
| DTMUV | 10 | 100 (5/5) | 8.2 |
| DTMUV | 1 | 60 (3/5) | 8.0 |
| DTMUV | 0.1 | 0 (4/4) | NA |
| PBS | NA | 0 (0/5) | NA |
One-day-old suckling BALB/c mice were i.c. inoculated with the indicated dose of DTMUV or PBS as a control and monitored daily for 21 days to assess morbidity and mortality. The LD50 was 0.7 PFU. NA, not applicable.
Next, we attempted to assess the neuroinvasiveness of DTMUV in mice upon i.p. inoculation. After inoculation with 200,000 PFU of DTMUV, 1-day-old suckling mice developed paralysis and rapidly succumbed to infection with 100% lethality and an AST of 8 days (Fig. 2C). This result suggests that 1-day-old mice are highly susceptible to DTMUV infection by the i.p. route. Eleven-day-old mice displayed similar neurological manifestations following inoculation with the same dose of DTMUV and exhibited 90% mortality with an AST of 8.8 days (Fig. 2C). In contrast, neither morbidity nor mortality was observed in 21-day-old mice following inoculation with 200,000 PFU of the virus (Fig. 2C). To further confirm the neuroinvasiveness of the virus, we assessed the viral burden in the serum and brain on day 6 after i.p. inoculation. As shown in Fig. 2D, obvious viremia (103.83 PFU/ml) was detected in 1-day-old mice, whereas no detectable viremia was observed in 11- or 21-day-old mice. One-day-old mice had a significantly higher viral burden in the brain than did 11-day-old mice (105.94 PFU/g versus 104.49 PFU/g) (Fig. 2D). In comparison, infectious DTMUV was not detected in the brains of 21-day-old mice (Fig. 2D). These findings indicate that DTMUV exhibits age-dependent neuroinvasiveness in mice following peripheral infection.
DTMUV inoculation fails to cause any viremia or clinical symptoms in rhesus monkeys.
Next, we tested the pathogenicity of DTMUV in rhesus monkeys based on the fact that nonhuman primates have been utilized as a model of pathogenesis for a variety of pathogenic flaviviruses. Following s.c. inoculation with 105 PFU of DTMUV, none of the infected animals developed any clinical or physiological changes. The body temperature of each inoculated animal was observed for 10 days p.i., and no fever over 38°C was recorded (data not shown). Specifically, no viremia was observed in any inoculated monkeys from 1 to 10 days p.i. (Table 2). Various blood parameters and blood chemistries were assessed over time after DTMUV inoculation. No obvious difference from normal values was detected, and the values that varied remained within the normal range during the observation period (data not shown).
TABLE 2.
Viremia and neutralizing antibody titer in rhesus monkeys inoculated with DTMUVa
| Monkey no. | Neutralizing antibody titer on day p.i.b: |
||
|---|---|---|---|
| 0 | 14 | 28 | |
| 10R0469 | <10.0 | 15.9 | 36.5 |
| 11R0102 | <10.0 | 18.6 | 70.1 |
| 0807087 | <10.0 | 19.3 | 29.7 |
| GMTc | <10.0 | 17.9 | 42.4 |
Serum was collected on days 1 to 10, and the peak viral titer was the detection limit of 0.32 log10 eq PFU/ml.
The data represent the reciprocal titer against DTMUV determined by the PRNT50. The lower limit of detection was 10.0.
GMT, geometric mean neutralizing antibody titer.
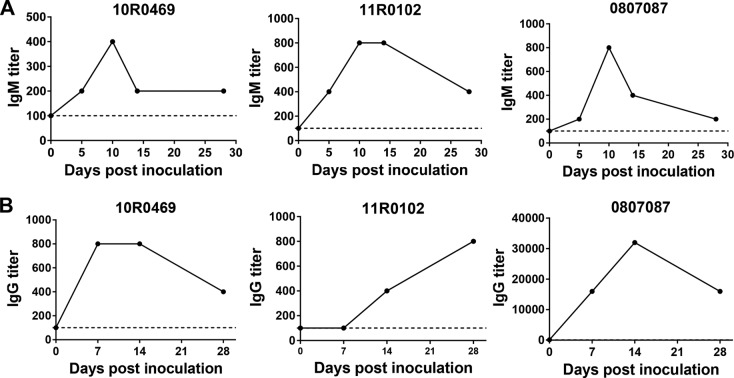
We further determined whether DTMUV-specific humoral immune responses were induced in inoculated animals. Despite the absence of measurable viremia, anti-DTMUV IgM was first detected on day 5 p.i., and titers further rapidly increased on day 10 p.i. (Fig. 3A). Anti-DTMUV IgG was detectable on day 10 p.i., and, similar to IgM levels, the IgG levels increased over time (Fig. 3B). All of the inoculated monkeys developed neutralizing antibody responses with geometric mean titers of 17.9 on day 14 p.i. and 42.4 on day 28 p.i. (Table 2). Collectively, these observations demonstrate that the inoculation of DTMUV fails to cause any viremia or clinical symptoms in rhesus monkeys, even though the humoral immune response can be induced by limited replication.
FIG 3.
DTMUV inoculation elicits virus-specific humoral immune responses in rhesus monkeys. (A) DTMUV-specific IgM kinetics in serum. Blood samples were collected at the indicated times and subjected to ELISA using formaldehyde-inactivated DTMUV as an antigen source, as described in Materials and Methods. (B) DTMUV-specific IgG kinetics in serum. Blood samples were collected at the indicated times and subjected to ELISA.
DTMUV displays enhanced sensitivity to a mammalian type I IFN response.
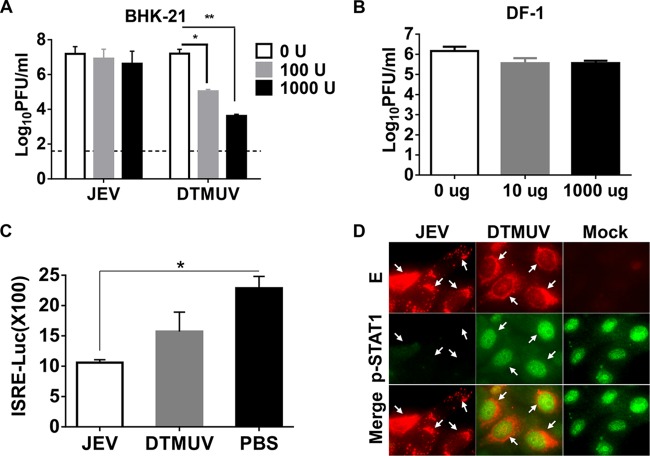
The mammal IFN system has been well demonstrated to play critical roles in controlling flavivirus infection; here, we aimed to understand the role of host IFN signaling in the absence of viremia or symptoms of DTMUV infection in mammals. We first examined the antiviral effect of IFN on DTMUV replication in mammalian cells in comparison with the effect of IFN on another neurological flavivirus, JEV. As shown in Fig. 4A, IFN-αA/D treatment resulted in a significant decrease in the level of production of DTMUV compared with that obtained after mock treatment in a dose-dependent manner, whereas the level of JEV production was only slightly affected, as expected. Furthermore, we observed an inhibitory effect of chicken IFN-α on DTMUV, and the results showed that the production of DTMUV was not affected by chicken IFN-α (Fig. 4B), suggesting that DTMUV is an effective IFN antagonist in avian cells. These results suggest that although DTMUV can antagonize avian IFN signaling, it is sensitive to the antiviral effects of IFN in mammalian cells.
FIG 4.
DTMUV is sensitive to the type I IFN response in mammalian cells. (A) Antiviral effects of IFN-α in mammalian cells. BHK-21 cells were treated with medium containing various doses of IFN-αA/D (0, 100, or 1,000 U) and then infected with DTMUV or JEV at an MOI of 1. The supernatants were collected, and the viral titers were determined by plaque assay in BHK-21 cells at 24 h postinfection. Statistically significant differences are indicated. *, P < 0.05; **, P < 0.01. (B) Antiviral effects of IFN-α in avian cells. DF-1 cells treated with the indicated concentrations of avian IFN-αA/D were subjected to DTMUV infection, and viral titers were then determined by plaque assay at 24 h postinfection. (C) ISRE reporter assays. BHK-21 cells were transfected with the corresponding reporter plasmids and then infected with DTMUV or JEV in the presence of 100 U IFN-αA/D. A luciferase assay was performed using a dual-luciferase assay kit, and the results were calculated accordingly. Statistically significant difference is indicated. *, P < 0.05. (D) Nuclear translocation assay of p-STAT1. Vero cells infected with DTMUV or JEV were then subjected to IFN-αA/D (1,000 U/ml) treatment. The cells were fixed and immunostained by using rabbit anti-p-STAT1 (green) and mouse antiserum specific for DTMUV or JEV (red). Arrows, examples of viral proteins and/or nuclear staining of p-STAT1.
We next examined by an ISRE reporter assay the effect of DTMUV infection on IFN signaling in mammalian cells. As shown in Fig. 4C, JEV infection significantly decreased IFN-stimulated luciferase activity in BHK-21 cells, while DTMUV infection failed to do so. Furthermore, the nuclear localization of p-STAT1 in response to IFN-α treatment was not detected in JEV-infected cells. In contrast, p-STAT1 was translocated to the nucleus following IFN treatment in DTMUV-infected cells (Fig. 4D), which suggested the absence of IFN-antagonistic activity of DTMUV. These findings indicate that DTMUV exhibits high sensitivity to the antiviral effects of mammalian IFN signaling due in part to the reduced capacity to block the nuclear translocation of p-STAT1.
IFN signaling is critical for protection against DTMUV infection in mice.
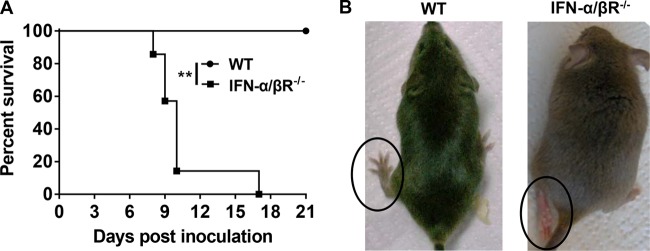
To further clarify the role of IFN signaling in vivo, WT mice or IFN-α/βR−/− mice were i.p. inoculated with various doses of DTMUV, and the morbidity and mortality were recorded. As expected, WT mice infected with even high doses of 106 PFU of DTMUV did not show any signs of illness, and all mice survived during the observation period (Fig. 5A). In contrast, IFN-α/βR−/− animals inoculated with 106 PFU of the virus manifested neurologic disease, such as a hunchback posture or limb paralysis (Fig. 5B), and all succumbed to the DTMUV infection within 17 days. The LD50 of the virus in WT mice was >106 PFU, while the LD50 in IFN-α/βR−/− mice was <104 PFU, a difference in susceptibility of at least 100-fold (data not shown). These data suggest that the absence of type I IFN renders mice more susceptible to DTMUV-induced disease.
FIG 5.
Susceptibility of WT and IFN-α/βR−/− mice. (A) Survival curves for mice infected with DTMUV. Three- to 4-week-old female WT (n = 5) or IFN-α/βR−/− mice (n = 7) were i.p. inoculated with 106 PFU of DTMUV and monitored daily for 21 days. Survival differences were judged by the log-rank test. Statistically significant difference is indicated. **, P < 0.01. (B) Clinical symptoms developed in IFN-α/βR−/− mice infected with DTMUV. The IFN-α/βR−/− mice showed hind limb paralysis.
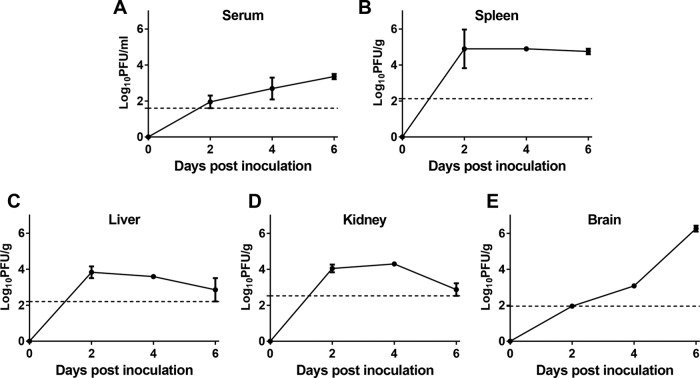
To further understand how type I IFN deficiency impacts the control of DTMUV infection, WT and IFN-α/βR−/− mice were infected i.p. with 106 PFU of the virus. The viral loads in the serum, peripheral tissues (spleen, kidney, and liver), and brain were measured using a plaque assay at early (day 2 p.i.), middle (day 4 p.i.), and late (day 6 p.i.) stages of infection. As expected, no infectious virus was detected in any tissue in the WT animals throughout the observation period (data not shown). In contrast, obvious viremia and the rapid dissemination of DTMUV to the peripheral tissues were observed in the IFN-α/βR−/− mice during early stages of infection. As shown in Fig. 6A, infectious virus was detected in serum at day 2 p.i., and the titer steadily increased over the course of infection, with a peak titer of 103.35 PFU/ml occurring at day 6 p.i. The viral load in the spleens of the IFN-α/βR−/− mice attained a peak titer of 104.90 PFU/g at day 2 p.i. and remained at the same level at day 6 p.i. (Fig. 6B). Additionally, the spleen samples from DTMUV-infected mice were enlarged compared to those from the uninfected control mice (data not shown). The livers and kidneys of IFN-α/βR−/− mice harbored high viral loads (103.83 PFU/g and 104.29 PFU/g, respectively). These levels were maintained between days 2 and 4 p.i. and steadily declined to the limit of detection at day 6 p.i. (Fig. 6C and D). Notably, although the viral load in the brain was below the detection limit at day 2 p.i., DTMUV became detectable in the brain by day 4 p.i. and rapidly increased to a high level at day 6 p.i., when virus titers were over 3 log units higher than those on day 4 p.i. (Fig. 6E).
FIG 6.
Viremia and tissue distribution of DTMUV in IFN-α/βR−/− mice. Blood (A), spleen (B), liver (C), kidney (D), and brain (E) were collected at the indicated times p.i., and viral titers were determined by plaque assay on BHK-21 cells. Dashed lines, limits of detection of the respective assays.
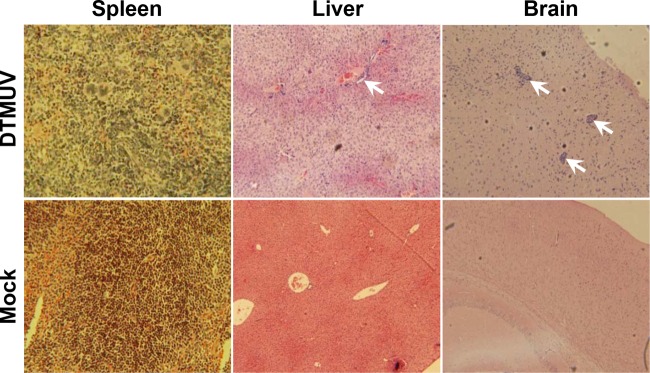
To further understand the basis for the increased mortality in IFN-α/βR−/− mice, we examined visceral organs and brains for histopathological changes following infection and compared them to those of WT mice. Histological analysis of the spleens and livers from IFN-α/βR−/− mice showed increased cellular infiltrates and tissue destruction after DTMUV infection compared to the findings for the spleens and livers from WT mice (Fig. 7). Additionally, the brain histopathology correlated well with the level of viral replication. A severe histopathology, including perivascular and parenchymal mononuclear inflammatory cell infiltration as well as neuronal degeneration, were observed in DTMUV-infected IFN-α/βR−/− mice (Fig. 7). Collectively, these observations provide solid evidence that IFN-α/β signaling strongly restricts the replication and dissemination of DTMUV in adult mice.
FIG 7.
Histopathological assays of major target organs in DTMUV-infected IFN-α/βR−/− mice. Four-week-old female IFN-α/βR−/− mice were inoculated by the i.p. route with 105 PFU of DTMUV or PBS as a control. At 6 days p.i., the brains, livers, and spleens of mice were collected, fixed with perfusion fixative (4% formaldehyde) for 48 h, and processed according to standard histological methods. All sections from each tissue were stained with H&E. Arrows, perivascular and parenchymal mononuclear inflammatory cell infiltration and neuronal degeneration.
DISCUSSION
The fact that flaviviruses pose a significant threat to public health emphasizes the urgent necessity to determine whether the new flavivirus DTMUV has emerged as a human pathogen. In this study, we demonstrate that DTMUV possesses the ability to infect a spectrum of mammalian cell lines and exhibits neurovirulence and age-related neuroinvasiveness in mice. We further show that viral replication is highly restricted in nonhuman primates and fails to cause any viremia or clinical manifestations. Finally, we reveal that DTMUV is sensitive to host type I IFN signaling due to the attenuated capacity of DTMUV to block the nuclear translocation of p-STAT1. These findings provide solid experimental evidence that DTMUV is unlikely to emerge as a human pathogen for the time being.
The current knowledge of DTMUV infection derives from avian research, and our and previous studies have shown that DTMUV replicates well in avian cells (12). To our surprise, DTMUV isolated from ducks possesses the capacity to infect a panel of cell lines derived from mammals, including mice, nonhuman primates, and humans. Mosquito-borne flaviviruses can generally replicate well in a wide spectrum of vertebrate cell lines, including BHK-21, Vero, A549, and HepG2 cells (27, 28). Although cell lines are not ideal surrogates for in vivo infections, observations thereof are indicative of the capability of flavivirus DTMUV to replicate in mammals. Of note, the production of DTMUV in human neuroblastoma SH-SY5Y cells suggests the neurotropic potential of DTMUV in mammals.
Mice are widely used as an animal model to study flavivirus pathogenicity and antiviral tests (29–31). To this end, we systematically characterized the neurovirulence and neuroinvasiveness of DTMUV in mice. DTMUV was demonstrated to have the typical neurovirulence phenotype in adult mice, and robust replication in the mouse brain was observed. This finding was anticipated, as the majority of members within the Flavivirus genus, including WNV (32), JEV (33), YFV (34), and TBEV (35), exhibit high levels of neurovirulence in immunocompetent adult mice. Of note, DTMUV displays an age-dependent attenuation of neuroinvasiveness in mice. This finding was further confirmed by the observation of DTMUV replication in the suckling mouse brain and the absence of DTMUV in adult mouse brains following i.p. inoculation (Fig. 2D). Indeed, a number of physiological variables differentiate neonatal and adult hosts, including the relative proportion of tissue fibroblasts and the maturity and effectiveness of the innate immune system (36). The age dependence of disease severity has been observed in mice infected with members of the genus Alphavirus, such as Chikungunya virus (37) and Sindbis virus (38). A study on La Crosse virus also showed that peripheral IFN responses were significantly reduced in young mice compared to adults, which suggested that the ability of type I IFN to respond to virus infection develops as the animal matures (39). In comparison, the phenomenon of age-dependent resistance to viral invasion of animal brains is rarely reported for flaviviruses. This finding could be due to the difference between the neonatal and adult hosts with respect to the maturity and effectiveness of their innate immunity. Especially, considering the link between rodents and duck farming, the pathogenicity of DTMUV in mice encourages the further study of the possibility that rodents could be reservoir hosts of DTMUV.
One of the most important findings of our work is that DTMUV exhibits restricted replication in rhesus monkeys with the absence of viremia, despite the induction of humoral immune responses. Studies with nonhuman primates with an immune system that is more similar to that of humans may provide direct evidence of DTMUV infection in humans. It is well established that nonhuman primates are susceptible to infection with multiple pathogenic flaviviruses. Viremia is one of the major clinical manifestations of neurotropic flavivirus infection. Studies with rhesus monkeys demonstrated that the animals developed viremia following peripheral inoculation with WNV (40), JEV (41), TBEV (42), and DENV (43). In addition, previous reports have shown a direct correlation between high levels of viremia and the severity of the disease caused by viral infection (44, 45). In our study, following s.c. inoculation with 105 PFU of DTMUV, none of the rhesus monkeys became viremic (Table 2) or displayed any obvious clinical outcomes. Blood chemistry and hematology results were within normal ranges. The level of viremia is vital and allows estimation of the probability of virus penetration into the central nervous system and visceral organs (46). The absence of viremia in the DTMUV-inoculated monkeys is likely due to its attenuated virulence phenotype. Nevertheless, DTMUV infection resulted in humoral immune responses in terms of the appearance of DTMUV-specific IgM and IgG antibodies in rhesus monkeys (Fig. 3A and B) as well as high levels of neutralizing antibodies against the virus (Table 2). These data provide solid evidence of the restricted replication of DTMUV in monkeys with the absence of viremia.
The age-related neuroinvasiveness of DTMUV in mice and restricted viral replication in monkeys prompted us to examine the sensitivity of DTMUV to type I IFN in vitro and in vivo. Experiments in avian DF-1 cells showed that IFN-α failed to inhibit DTMUV replication. However, in mammalian cells, DTMUV exhibited high levels of sensitivity to the antiviral effects of IFN-α compared to that of JEV (Fig. 4). Furthermore, animal studies in IFN-α/βR−/− and WT mice demonstrated the critical role of the IFN-α/β signaling pathway in systemically restricting DTMUV infection. In contrast, to successfully establish infection, several human-pathogenic flaviviruses, including DENV, WNV, JEV, YFV, and TBEV, are able to utilize various mechanisms to antagonize the host IFN response (15–19, 47). Our preliminary results showed that DTMUV failed to block the nuclear translocation of p-STAT1, although the molecular mechanism deserves further exploration. Of note, the observation of age-dependent neuroinvasiveness in immunocompetent mice and high pathogenicity in IFN-α/βR−/− mice highlights the potential risk of development of severe DTMUV infection in children and immunocompromised patients. Additionally, IFN therapy might be a potential treatment of DTMUV infections in mammals due to its high sensitivity to the antiviral effects of IFN.
Currently, all human-pathogenic flaviviruses are zoonotic agents. In nature, these viruses maintain a life cycle in vertebrates and/or arthropods, but accidental introduction into a new life cycle in humans results in unexpected diseases (48). The ability of the virus to cross the species barrier is a complex multifactorial process, and our understanding of the molecular basis of the pathogenesis of severe disease remains inadequate. In our study, we provide experimental evidence that DTMUV remains an avian virus and the risk of human diseases caused by DTMUV is low. Compared with other known human-pathogenic flaviviruses, DTMUV loses the ability to block the nuclear translocation of p-STAT1 induced by IFN, which shows enhanced sensitivity to the antiviral effects of IFN signaling in mammals. These findings also raise the possibility of DTMUV-associated disease in immunodeficient patients. Additionally, our study, together with previous findings related to DTMUV, highlights the urgent need to deepen our understanding of the molecular basis of the cross-species transmission of flaviviruses.
ACKNOWLEDGMENTS
We thank Jing-Liang Su (Chinese Agriculture University, China) for providing DF-1 cells and Qi-Bin Leng (Shanghai Institute for Pasteur, CAS, China) for providing the IFN-α/βR−/− mice.
This work was supported by the National Basic Research Project (grant no. 2012CB518904), the Beijing Nova Program (grant no. 2016110 and no. 2010B041), and the National Natural Science Foundation (NSFC) of China (grant no. 31470265 and 31270974). C.-F.Q. was supported by the Excellent Young Scientist Program from NSFC of China (grant no. 81522025) and the Newton Advanced Fellowship from the Academy of Medical Sciences, United Kingdom, and NSFC of China (grant no. 81661130162).
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Bolling BG, Weaver SC, Tesh RB, Vasilakis N. 2015. Insect-specific virus discovery: significance for the arbovirus community. Viruses 7:4911–4928. doi: 10.3390/v7092851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu P, Lu H, Li S, Moureau G, Deng YQ, Wang Y, Zhang L, Jiang T, de Lamballerie X, Qin CF, Gould EA, Su J, Gao GF. 2012. Genomic and antigenic characterization of the newly emerging Chinese duck egg-drop syndrome flavivirus: genomic comparison with Tembusu and Sitiawan viruses. J Gen Virol 93:2158–2170. doi: 10.1099/vir.0.043554-0. [DOI] [PubMed] [Google Scholar]
- 3.Burke CW, Mason JN, Surman SL, Jones BG, Dalloneau E, Hurwitz JL, Russell CJ. 2011. Illumination of parainfluenza virus infection and transmission in living animals reveals a tissue-specific dichotomy. PLoS Pathog 7:e1002134. doi: 10.1371/journal.ppat.1002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Homonnay ZG, Kovacs EW, Banyai K, Albert M, Feher E, Mato T, Tatar-Kis T, Palya V. 2014. Tembusu-like flavivirus (Perak virus) as the cause of neurological disease outbreaks in young Pekin ducks. Avian Pathol 43:552–560. doi: 10.1080/03079457.2014.973832. [DOI] [PubMed] [Google Scholar]
- 5.Thontiravong A, Ninvilai P, Tunterak W, Nonthabenjawan N, Chaiyavong S, Angkabkingkaew K, Mungkundar C, Phuengpho W, Oraveerakul K, Amonsin A. 2015. Tembusu-related flavivirus in ducks, Thailand. Emerg Infect Dis 21:2164–2167. doi: 10.3201/eid2112.150600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wan C, Huang Y, Fu G, Shi S, Cheng L, Chen H. 2012. Complete genome sequence of avian Tembusu-related virus strain WR isolated from White Kaiya ducks in Fujian, China. J Virol 86:10912. doi: 10.1128/JVI.01582-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Yuan X, Li Y, Yu K, Yang J, Xu H, Zhang Y, Liao M, Qin Z. 2011. Rapid detection of newly isolated Tembusu-related flavivirus by reverse-transcription loop-mediated isothermal amplification assay. Virol J 8:553. doi: 10.1186/1743-422X-8-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan L, Yan P, Zhou J, Teng Q, Li Z. 2011. Establishing a TaqMan-based real-time PCR assay for the rapid detection and quantification of the newly emerged duck Tembusu virus. Virol J 8:464. doi: 10.1186/1743-422X-8-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan P, Zhao Y, Zhang X, Xu D, Dai X, Teng Q, Yan L, Zhou J, Ji X, Zhang S, Liu G, Zhou Y, Kawaoka Y, Tong G, Li Z. 2011. An infectious disease of ducks caused by a newly emerged Tembusu virus strain in mainland China. Virology 417:1–8. doi: 10.1016/j.virol.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Chen S, Wang S, Li Z, Lin F, Cheng X, Zhu X, Wang J, Chen S, Huang M, Zheng M. 2014. Isolation and characterization of a Chinese strain of Tembusu virus from Hy-Line Brown layers with acute egg-drop syndrome in Fujian, China. Arch Virol 159:1099–1107. doi: 10.1007/s00705-013-1931-0. [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Han K, Zhao D, Liu Y, Zhang J, Niu H, Zhang K, Zhu J, Wu D, Gao L, Li Y. 2013. Identification and molecular characterization of a novel flavivirus isolated from geese in China. Res Vet Sci 94:774–780. doi: 10.1016/j.rvsc.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Liu M, Chen S, Chen Y, Liu C, Chen S, Yin X, Li G, Zhang Y. 2012. Adapted Tembusu-like virus in chickens and geese in China. J Clin Microbiol 50:2807–2809. doi: 10.1128/JCM.00655-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang Y, Diao Y, Chen H, Ou Q, Liu X, Gao X, Yu C, Wang L. 2015. Isolation and genetic characterization of a Tembusu virus strain isolated from mosquitoes in Shandong, China. Transbound Emerg Dis 62:209–216. doi: 10.1111/tbed.12111. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Shi Y, Liu Q, Wang Y, Li G, Teng Q, Zhang Y, Liu S, Li Z. 2015. Airborne transmission of a novel Tembusu virus in ducks. J Clin Microbiol 53:2734–2736. doi: 10.1128/JCM.00770-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones M, Davidson A, Hibbert L, Gruenwald P, Schlaak J, Ball S, Foster GR, Jacobs M. 2005. Dengue virus inhibits alpha interferon signaling by reducing STAT2 expression. J Virol 79:5414–5420. doi: 10.1128/JVI.79.9.5414-5420.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laurent-Rolle M, Morrison J, Rajsbaum R, Macleod JM, Pisanelli G, Pham A, Ayllon J, Miorin L, Martinez-Romero C, tenOever BR, Garcia-Sastre A. 2014. The interferon signaling antagonist function of yellow fever virus NS5 protein is activated by type I interferon. Cell Host Microbe 16:314–327. doi: 10.1016/j.chom.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashour J, Laurent-Rolle M, Shi PY, Garcia-Sastre A. 2009. NS5 of dengue virus mediates STAT2 binding and degradation. J Virol 83:5408–5418. doi: 10.1128/JVI.02188-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurent-Rolle M, Boer EF, Lubick KJ, Wolfinbarger JB, Carmody AB, Rockx B, Liu W, Ashour J, Shupert WL, Holbrook MR, Barrett AD, Mason PW, Bloom ME, Garcia-Sastre A, Khromykh AA, Best SM. 2010. The NS5 protein of the virulent West Nile virus NY99 strain is a potent antagonist of type I interferon-mediated JAK-STAT signaling. J Virol 84:3503–3515. doi: 10.1128/JVI.01161-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin RJ, Chang BL, Yu HP, Liao CL, Lin YL. 2006. Blocking of interferon-induced Jak-Stat signaling by Japanese encephalitis virus NS5 through a protein tyrosine phosphatase-mediated mechanism. J Virol 80:5908–5918. doi: 10.1128/JVI.02714-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yun T, Zhang D, Ma X, Cao Z, Chen L, Ni Z, Ye W, Yu B, Hua J, Zhang Y, Zhang C. 2012. Complete genome sequence of a novel flavivirus, duck Tembusu virus, isolated from ducks and geese in china. J Virol 86:3406–3407. doi: 10.1128/JVI.07132-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Y, Gao X, Diao Y, Feng Q, Chen H, Liu X, Ge P, Yu C. 2013. Tembusu virus in human, China. Transbound Emerg Dis 60:193–196. doi: 10.1111/tbed.12085. [DOI] [PubMed] [Google Scholar]
- 22.The Royal Society. 2005. The Weatherall report on the use of non-human primates. The Royal Society, London, United Kingdom. [Google Scholar]
- 23.Ye Q, Li XF, Zhao H, Li SH, Deng YQ, Cao RY, Song KY, Wang HJ, Hua RH, Yu YX, Zhou X, Qin ED, Qin CF. 2012. A single nucleotide mutation in NS2A of Japanese encephalitis-live vaccine virus (SA14-14-2) ablates NS1′ formation and contributes to attenuation. J Gen Virol 93:1959–1964. doi: 10.1099/vir.0.043844-0. [DOI] [PubMed] [Google Scholar]
- 24.Li XF, Deng YQ, Yang HQ, Zhao H, Jiang T, Yu XD, Li SH, Ye Q, Zhu SY, Wang HJ, Zhang Y, Ma J, Yu YX, Liu ZY, Li YH, Qin ED, Shi PY, Qin CF. 2013. A chimeric dengue virus vaccine using Japanese encephalitis virus vaccine strain SA14-14-2 as backbone is immunogenic and protective against either parental virus in mice and nonhuman primates. J Virol 87:13694–13705. doi: 10.1128/JVI.00931-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rumyantsev AA, Goncalvez AP, Giel-Moloney M, Catalan J, Liu Y, Gao QS, Almond J, Kleanthous H, Pugachev KV. 2013. Single-dose vaccine against tick-borne encephalitis. Proc Natl Acad Sci U S A 110:13103–13108. doi: 10.1073/pnas.1306245110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed LJ, Muench H. 1938. A simple method of estimating fifty percent endpoints. Am J Hyg 27:493–497. [Google Scholar]
- 27.Blitvich BJ, Firth AE. 2015. Insect-specific flaviviruses: a systematic review of their discovery, host range, mode of transmission, superinfection exclusion potential and genomic organization. Viruses 7:1927–1959. doi: 10.3390/v7041927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diamond MS, Roberts TG, Edgil D, Lu B, Ernst J, Harris E. 2000. Modulation of dengue virus infection in human cells by alpha, beta, and gamma interferons. J Virol 74:4957–4966. doi: 10.1128/JVI.74.11.4957-4966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holbrook MR, Aronson JF, Campbell GA, Jones S, Feldmann H, Barrett AD. 2005. An animal model for the tickborne flavivirus—Omsk hemorrhagic fever virus. J Infect Dis 191:100–108. doi: 10.1086/426397. [DOI] [PubMed] [Google Scholar]
- 30.Meier KC, Gardner CL, Khoretonenko MV, Klimstra WB, Ryman KD. 2009. A mouse model for studying viscerotropic disease caused by yellow fever virus infection. PLoS Pathog 5:e1000614. doi: 10.1371/journal.ppat.1000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zellweger RM, Shresta S. 2014. Mouse models to study dengue virus immunology and pathogenesis. Front Immunol 5:151. doi: 10.3389/fimmu.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pletnev AG, Putnak R, Speicher J, Wagar EJ, Vaughn DW. 2002. West Nile virus/dengue type 4 virus chimeras that are reduced in neurovirulence and peripheral virulence without loss of immunogenicity or protective efficacy. Proc Natl Acad Sci U S A 99:3036–3041. doi: 10.1073/pnas.022652799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song BH, Yun GN, Kim JK, Yun SI, Lee YM. 2012. Biological and genetic properties of SA(1)(4)-14-2, a live-attenuated Japanese encephalitis vaccine that is currently available for humans. J Microbiol 50:698–706. doi: 10.1007/s12275-012-2336-6. [DOI] [PubMed] [Google Scholar]
- 34.Guirakhoo F, Zhang ZX, Chambers TJ, Delagrave S, Arroyo J, Barrett AD, Monath TP. 1999. Immunogenicity, genetic stability, and protective efficacy of a recombinant, chimeric yellow fever-Japanese encephalitis virus (ChimeriVax-JE) as a live, attenuated vaccine candidate against Japanese encephalitis. Virology 257:363–372. doi: 10.1006/viro.1999.9695. [DOI] [PubMed] [Google Scholar]
- 35.Pletnev AG, Men R. 1998. Attenuation of the Langat tick-borne flavivirus by chimerization with mosquito-borne flavivirus dengue type 4. Proc Natl Acad Sci U S A 95:1746–1751. doi: 10.1073/pnas.95.4.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy O. 2007. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol 7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 37.Couderc T, Chretien F, Schilte C, Disson O, Brigitte M, Guivel-Benhassine F, Touret Y, Barau G, Cayet N, Schuffenecker I, Despres P, Arenzana-Seisdedos F, Michault A, Albert ML, Lecuit M. 2008. A mouse model for Chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog 4:e29. doi: 10.1371/journal.ppat.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryman KD, Klimstra WB, Nguyen KB, Biron CA, Johnston RE. 2000. Alpha/beta interferon protects adult mice from fatal Sindbis virus infection and is an important determinant of cell and tissue tropism. J Virol 74:3366–3378. doi: 10.1128/JVI.74.7.3366-3378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor KG, Woods TA, Winkler CW, Carmody AB, Peterson KE. 2014. Age-dependent myeloid dendritic cell responses mediate resistance to La Crosse virus-induced neurological disease. J Virol 88:11070–11079. doi: 10.1128/JVI.01866-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ratterree MS, Gutierrez RA, Travassos da Rosa AP, Dille BJ, Beasley DW, Bohm RP, Desai SM, Didier PJ, Bikenmeyer LG, Dawson GJ, Leary TP, Schochetman G, Phillippi-Falkenstein K, Arroyo J, Barrett AD, Tesh RB. 2004. Experimental infection of rhesus macaques with West Nile virus: level and duration of viremia and kinetics of the antibody response after infection. J Infect Dis 189:669–676. doi: 10.1086/381461. [DOI] [PubMed] [Google Scholar]
- 41.Dean CH, Alarcon JB, Waterston AM, Draper K, Early R, Guirakhoo F, Monath TP, Mikszta JA. 2005. Cutaneous delivery of a live, attenuated chimeric flavivirus vaccine against Japanese encephalitis (ChimeriVax)-JE) in non-human primates. Hum Vaccin 1:106–111. doi: 10.4161/hv.1.3.1797. [DOI] [PubMed] [Google Scholar]
- 42.Pletnev AG, Bray M, Hanley KA, Speicher J, Elkins R. 2001. Tick-borne Langat/mosquito-borne dengue flavivirus chimera, a candidate live attenuated vaccine for protection against disease caused by members of the tick-borne encephalitis virus complex: evaluation in rhesus monkeys and in mosquitoes. J Virol 75:8259–8267. doi: 10.1128/JVI.75.17.8259-8267.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guirakhoo F, Weltzin R, Chambers TJ, Zhang ZX, Soike K, Ratterree M, Arroyo J, Georgakopoulos K, Catalan J, Monath TP. 2000. Recombinant chimeric yellow fever-dengue type 2 virus is immunogenic and protective in nonhuman primates. J Virol 74:5477–5485. doi: 10.1128/JVI.74.12.5477-5485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA, Nisalak A. 2000. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis 181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 45.Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, Green S, Vaughn DW, Nisalak A, Ennis FA, Rothman AL. 2002. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis 186:1165–1168. doi: 10.1086/343813. [DOI] [PubMed] [Google Scholar]
- 46.Chambers TJ, Diamond MS. 2003. Pathogenesis of flavivirus encephalitis. Adv Virus Res 60:273–342. doi: 10.1016/S0065-3527(03)60008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Best SM, Morris KL, Shannon JG, Robertson SJ, Mitzel DN, Park GS, Boer E, Wolfinbarger JB, Bloom ME. 2005. Inhibition of interferon-stimulated JAK-STAT signaling by a tick-borne flavivirus and identification of NS5 as an interferon antagonist. J Virol 79:12828–12839. doi: 10.1128/JVI.79.20.12828-12839.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ashraf U, Ye J, Ruan X, Wan S, Zhu B, Cao S. 2015. Usutu virus: an emerging flavivirus in Europe. Viruses 7:219–238. doi: 10.3390/v7010219. [DOI] [PMC free article] [PubMed] [Google Scholar]