ABSTRACT
The specificity of encapsidation of C-cluster enteroviruses depends on an interaction between capsid proteins and nonstructural protein 2CATPase. In particular, residue N252 of poliovirus 2CATPase interacts with VP3 of coxsackievirus A20, in the context of a chimeric virus. Poliovirus 2CATPase has important roles both in RNA replication and encapsidation. In this study, we searched for additional sites in 2CATPase, near N252, that are required for encapsidation. Accordingly, segments adjacent to N252 were analyzed by combining triple and single alanine mutations to identify residues required for function. Two triple alanine mutants exhibited defects in RNA replication. The remaining two mutations, located in secondary structures in a predicted three-dimensional model of 2CATPase, caused lethal growth phenotypes. Most single alanine mutants, derived from the lethal variants, were either quasi-infectious and yielded variants with wild-type (wt) or temperature-sensitive (ts) growth phenotypes or had a lethal growth phenotype due to defective RNA replication. The K259A mutation, mapping to an α helix in the predicted structure of 2CATPase, resulted in a cold-sensitive virus. In vivo protein synthesis and virus production were strikingly delayed at 33°C relative to the wt, suggesting a defect in uncoating. Studies with a reporter virus indicated that this mutant is also defective in encapsidation at 33°C. Cell imaging confirmed a much-reduced production of K259A mature virus at 33°C relative to the wt. In conclusion, we have for the first time linked a cold-sensitive encapsidation defect in 2CATPase (K259A) to a subsequent delay in uncoating of the virus particle at 33°C during the next cycle of infection.
IMPORTANCE Enterovirus morphogenesis, which involves the encapsidation of newly made virion RNA, is a process still poorly understood. Elucidation of this process is important for future drug development for a large variety of diseases caused by these agents. We have previously shown that the specificity of encapsidation of poliovirus and of C-cluster coxsackieviruses, which are prototypes of enteroviruses, is dependent on an interaction of capsid proteins with the multifunctional nonstructural protein 2CATPase. In this study, we have searched for residues in poliovirus 2CATPase, near a presumed capsid-interacting site, important for encapsidation. An unusual cold-sensitive mutant of 2CATPase possessed a defect in encapsidation at 37°C and subsequently in uncoating during the next cycle of infection at 33°C. These studies not only reveal a new site in 2CATPase that is involved in encapsidation but also identify a link between encapsidation and uncoating.
INTRODUCTION
Protein 2CATPase is a highly conserved nonstructural protein of the Picornaviridae, a family of plus-strand viruses that cause a wide range of important diseases in both humans and animals. 2CATPase maps roughly to the center of the polyprotein of poliovirus (PV) (Fig. 1A). Genetic, drug inhibition, and biochemical studies have identified multiple functions of this viral polypeptide, such as uncoating (1), host cell membrane rearrangement (2–4), RNA replication (5–8), and morphogenesis (9-13). During the last few years, we have concentrated our efforts on deciphering the role of 2CATPase in enterovirus assembly. We first provided evidence that the specificity of encapsidation is determined by an interaction between protein 2CATPase and capsid proteins rather than by an RNA encapsidation signal and RNA-protein interaction (10, 11). In a PV/coxsackievirus A20 (CAV20) chimera, the interaction site was identified to be between N252 of PV 2CATPase and E180 of CAV20 capsid protein VP3 (10). Subsequently, temperature-sensitive (ts) and quasi-infectious (qi) variants derived from alanine scanning mutagenesis of the PV 2CATPase polypeptide revealed other sites near the C terminus of the polypeptide that are involved in encapsidation (12, 13). In addition, suppressor mutations of one of these alanine mutants in 2CATPase mapped to capsid protein VP1 or VP3, confirming the importance of the 2CATPase-capsid interactions for the formation of mature poliovirus particles (12). Encapsidation is difficult to study because this process is tightly linked with viral translation and RNA replication (14, 15). Additional conditional defective ts 2CATPase mutants would be useful in further enhancing our understanding of the role of this domain in this complex process.
FIG 1.
Poliovirus genome organization and functional motifs in the PV 2CATPase protein. (A) Poliovirus RNA contains a long 5′ nontranslated region (5′NTR), a single open reading reading frame, a short 3′NTR, and a poly(A) tail. (B) The locations of the known functional domains of the 2CATPase protein are illustrated. (C) Previously identified mutations in 2CATPase involved in encapsidation or uncoating are shown in detail. These include the hydantoin-resistant mutations (9), N252, the capsid-interacting site in the PV/CAV chimera (10), the residues involved in encapsidation derived from alanine scanning mutagenesis (12, 13), and the mutations leading to an uncoating defect in a mutant containing a nearby linker insertion (1).
Encapsidation is the last step in the viral replicative cycle, providing to newly synthesized genomes a protective protein coat that, in turn, is required for a virion's attachment to and penetration into a new host cell. Attachment and penetration lead to uncoating of the genome, a complex process involving structural alterations to the viral capsid and finally the release of infectious genomic RNA into the cytoplasm. With poliovirus, uncoating begins with the loss of VP4 from the capsid, followed by the loss of VP2 (Fig. 1), and finally the dissociation of VP1/VP3 and the viral RNA (16–20).
The RNA genome of PV is about 7,500 nucleotides (nt) long and encodes a polyprotein with one structural domain (P1) and two nonstructural domains (P2 and P3) (Fig. 1A). The polyprotein is processed into precursor and mature proteins by viral proteinases 3Cpro/3CDpro and 2Apro (21–23). In poliovirus, 2CATPase is 329 amino acids long, and based on amino acid sequence analyses, it is classified as a member of the superfamily III helicases, which form hexameric ring structures (24). Such proteins contain three conserved motifs, two of which are typical nucleoside triphosphate (NTP)-binding motifs (A+B) and the third one (C), downstream of motif B, contains an invariant asparagine preceded by a stretch of hydrophobic residues, but its exact function is unknown (Fig. 1B). Downstream of motif C is residue N252, which is involved in the interaction with VP3 in a PV/CAV20 chimera (10). Poliovirus 2CATPase possesses ATPase activity in vitro (25–27), which is inhibited by guanidine hydrochloride (GnHCl) (27), a specific inhibitor of enterovirus RNA replication (28). Numerous attempts to discover helicase activity have failed in the past, although recently an RNA chaperone-type activity was reported to be associated with the 2CATPase protein of EoV, a picorna-like virus (29). Near its N terminus, the PV protein contains an amphipathic helix (7), an RNA binding domain (30), a membrane binding domain (31), and an oligomerization domain (Fig. 1B) (32). The central and C-terminal domains of the protein possess serpin (serine protease inhibitor) motifs, and accordingly 2CATPase inhibits the proteinase activity of 3Cpro both in vitro and in vivo (33). Near the C terminus, the protein contains another amphipathic helix (8) and a cysteine-rich Zn2+ binding domain (34). The protein has the ability to oligomerize through sequences near its N terminus (32) and to interact with viral proteins 2B/2BC, 3A/3AB, 3Cpro, and VP3 (10, 33, 35, 36) and cellular protein reticulon 3 (37). The 2BC precursor of 2CATPase also interacts with cellular protein valosin-containing protein (VCP)/p97 (38). A small RNA hairpin cre in the coding sequence of 2CATPase (Fig. 1B) serves as the template for the uridylylation of VPg during RNA synthesis (39, 40), but the cre function is totally independent of the 2CATPase function.
The initial observation that linked 2CATPase to encapsidation came from experiments with hydantoin, a drug that inhibits virus growth at the stage of encapsidation (9). Drug-resistant variants were identified that mapped to the N-terminal and central portions of the polypeptide (Fig. 1C), but there was no clear correlation between these sites and other known motifs (9, 12). Early genetic studies also indicated the involvement of 2CATPase in encapsidation. A ts mutant with an insertion in 2CATPase yielded suppressor mutations that resulted in a cold-sensitive uncoating defect (1). This finding suggested that 2CATPase has a role in determining some aspect of virion structure. Our charged-to-alanine scanning mutagenesis, noted above, has revealed that residues K279 and R280 near the C terminus of the protein in a Zn2+ binding domain are also required for encapsidation (12). In addition, we identified a ts mutant (C272A H273A), also in the Zn2+ binding domain of the protein, defective in encapsidation (13). The delayed growth kinetics of this virus suggested the possibility of an additional uncoating defect.
In this study, we used alanine mutagenesis of amino acids near N252, the presumed 2CATPase-VP3 interaction site within a variable segment of the 2CATPase polypeptide (10), with the aim of identifying additional residues in this domain that are involved in encapsidation. Most of these mutations introduced conservative amino acid changes with the replacement of hydrophobic residues with alanine. We started with triple alanine mutations, and from the lethal mutations, we selected single amino acid changes for further analyses. It should be noted such an approach, combining triple and single alanine scanning mutagenesis, had been used in the past successfully to identify essential residues in the NS5A protein of hepatitis C virus (HCV) (41). Using this approach, we identified two lethal triple alanine mutants and two others with ts or quasi-infectious growth phenotypes that were defective in RNA replication. Single residues when changed to alanine also conferred wild-type (wt)-like, lethal, ts or quasi-infectious growth phenotypes. The most interesting mutant (K259A) was cold sensitive in growth, and it possessed an encapsidation defect, which resulted in a severe delay in uncoating during the next cycle of virus infection.
MATERIALS AND METHODS
Plasmids.
Plasmid pT7PVM contains the full-length infectious cDNA of PV1(M). pT7R-Luc-PPP is an infectious Renilla luciferase reporter virus construct in which the 311-amino-acid-long R-Luc polypeptide is expressed as an N-terminal fusion of the PV polyprotein (10). pT7F-Luc PP is a replicon firefly luciferase (F-Luc) construct in which the structural P1 domain is replaced with F-Luc coding sequences in the pT7PVM background.
Site-directed mutagenesis.
The standard site-directed mutagenesis protocol was used to obtain the desired mutations for both the triple alanine and single alanine mutants of PV 2CATPase. In each case, the desired residues were replaced with alanine by changing the corresponding codons. An XhoI/HpaI fragment, spanning parts of the 2CATPase and 3A coding regions, was used as the template for site-directed mutagenesis. The mutated sites and corresponding codon changes are summarized in Tables 1 and 2. After sequencing analysis, the designed 2CATPase mutations were independently subcloned into pT7PVM or the Renilla luciferase reporter virus plasmid (R-Luc-PPP) for K259A.
TABLE 1.
Summary of the growth phenotypes of triple alanine mutants surrounding N252, a capsid-interacting site in poliovirus 2CATPase
| Mutant | Mutations in 2CATPase | Time of full CPE (growth phenotype) ata: |
||
|---|---|---|---|---|
| 33°C | 37°C | 39.5°C | ||
| FMI/AAA | F244A, M246A, I248A | |||
| QVM/AAA | Q249A, V250A, M251A | Transfection (qi ts) | Transfection (qi ts) | Passage 2 (ts) |
| EYS/AAA | E253A, Y254A, S255A | Transfection (ts) | Passage 2 (ts) | |
| GKL/AAA | G258A, K259A, L260A | |||
ts, temperature sensitive; qi, quasi-infectious.
TABLE 2.
Summary of the growth phenotypes of individual amino acid mutants of nonviable FMI/AAA and GKL/AAA mutants
| Mutant | Nucleotide |
Time of full CPE | Growth phenotype of alanine mutantb | Varianta | Growth phenotype of variant mutantb | |
|---|---|---|---|---|---|---|
| wt | Alanine mutanta | |||||
| F244A | TTC | gcC | Nonviable | |||
| M246A | ATG | gcc | Transfection | Viable | gTc(valine) | ts |
| I248A | ATT | gcc | Transfection | qi | gTc(valine) | ts |
| K259A | AAA | gcA | Transfection | cs | gcA | cs |
| L260A | TTG | gca | Passage 1 | qi | gTa(valine) | wt-like |
| G258A | GGG | Gcc | Transfection | Viable | Gcc | wt-like |
Lowercase letters indicate changed nucleotides.
ts, temperature sensitive; cs, cold sensitive; qi, quasi-infectious.
RNA transcription.
The wt and mutant plasmid DNAs of pT7PVM were linearized with EcoRI, and the pT7R-Luc-PPP plasmids were linearized with PvuII. The linearized plasmids were transcribed with T7 RNA polymerase.
RNA transfections.
RNA transcripts (3 to 10 μg) were transfected into 35-mm-diameter HeLa R19 cell monolayers by the DEAE-dextran method, as previously described (42), and incubated at the indicated temperatures. Two days posttransfection at 37 and 39.5°C or 3 days after transfection at 33°C, viruses, if any, were harvested. Full cytopathic effect (CPE) was defined as the stage where 90% to 95% of the cells displayed CPE. Plates of monolayer cells that did not show signs of CPE were freeze-thawed three times and centrifuged to remove cell debris from the virus supernatant. Fresh monolayers were independently inoculated with these supernatants. Titers (PFU per milliliter) and phenotypes of all viable viruses that displayed CPE at 33°C were determined for viruses plaqued at 33, 37, and 39.5°C. The genotypes of viable viruses were confirmed by reverse transcription-PCR (RT-PCR) and sequencing analyses.
In vitro translation.
Cytoplasmic extracts were prepared using HeLa S3 suspension cells, as described before (42). In vitro RNA translations were performed in the presence of Tran35S-label at 34°C overnight (42). Viral proteins were separated by SDS-PAGE (12.5% acrylamide), and the protein bands were visualized by autoradiography.
Plaque assays.
Plaque assays were performed on HeLa R19 monolayers using 0.6% tragacanth gum. After 72 h of incubation at 33°C or 48 h of incubation at 37 or 39.5°C, the viral plaques were developed with 1% crystal violet (42).
RT-PCR and sequencing analysis of isolated viral RNA from purified plaques.
Total RNA was extracted from 200 μl lysate with 800 μl TRIzol reagent (Invitrogen) and reverse transcribed into cDNA using SuperScript III reverse transcriptase (Invitrogen). PCR products were generated using high-fidelity Fusion polymerase (Finnzyme). PCR products were purified and further sequenced.
qRT-PCR.
Viral RNA was quantitated by one-step real-time quantitative reverse transcription-PCR (qRT-PCR). HeLa cells were infected with wt PV or with viable triple alanine mutants (QVM/AVA and EYS/AAA) for 0, 2, 4, and 8 h. The infected cells were washed with phosphate-buffered saline (PBS), and total RNAs were extracted with TRIzol reagent (Invitrogen). Two hundred to 300 ng of total cellular RNAs was used to perform one-step qRT-PCR (Quanta Biosciences) with primers binding to the P1 structural region. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) RNA was used as an internal control.
Luciferase assays.
Monolayer HeLa R19 cells were independently transfected with 3 to 5 μg of R-Luc-PPP reporter virus transcript RNAs derived from cDNAs linearized with PvuI. The transfected cells were incubated at 33, 37, and 39.5°C overnight in Dulbecco's modified Eagle's medium (DMEM) with 2% (vol/vol) bovine calf serum (BCS) in the presence and absence of 2 mM GnHCl. Luciferase activity was determined on the cell supernatants after three freeze-thawing steps. Cell supernatant (100 μl) was mixed with 20 μl Renilla luciferase (R-Luc) assay reagent (Promega luciferase assay system; catalog no. E2810), and R-Luc activity was measured in an Optocom I luminometer (MGM Instruments, Inc.). Two hundred fifty microliters of cell supernatants from transfections in the absence of GnHCl was passaged once in the presence and absence of 2 mM GnHCl. Luciferase activity was determined in the supernatants after an overnight incubation. The R-Luc ratio was calculated as luciferase activity without GnHCl (−GnHCl) divided by luciferase activity with GnHCl (+GnHCl) in either transfection or infection.
Immunofluorescence cell imaging.
HeLa R19 cells were infected with wt or K259A virus at a multiplicity of infection (MOI) of 5 and incubated at 37°C for 4 h, 35°C for 5 h, or 33°C for 6 h. Cells were fixed with 4% paraformaldehyde for 15 min at room temperature. Then the cells were permeabilized with 0.2% saponin and probed for mature virus with A12 primary antibody, which exclusively recognizes PV mature virus (43), and secondary Alexa Fluor 488-conjugated antibody. The localization of 2CATPase was determined in the same cell using monoclonal 2CATPase antibody and an Alexa Fluor 555-conjugated secondary antibody. Images were taken by Nikon's superresolution three-dimensional (3D)-structured illumination microscopy (SIM).
Transmission electron microscopy.
PV 2C wt and K259A viruses, grown at 37°C on HeLa R19 cells, were purified by CsCl density gradients. The recovered viruses were dialyzed in PBS overnight and were fixed in 5% glutaraldehyde grade 1 solution for 5 min. Fixed viruses were transferred to an imaging grid and stained with uranyl acetate. Viruses were visualized with a transmission electron microscope (FEI Tecnai).
Western blot analyses.
Monolayer HeLa R19 cells were infected at an MOI of 5 with wt or K259A viruses and incubated at 33, 37, and 39.5°C. The growth media were aspirated at 3, 5, 6.5, and 8 h, respectively, and lysed with detergent. Cell lysates from the various time points were run on an SDS-PAGE gel and transferred to a nitrocellulose membrane. 2CATPase protein and VP3 (capsid protein) were detected using primary mouse monoclonal and rabbit polyclonal antibodies, respectively.
RESULTS
Studies of the role of 2CATPase in PV encapsidation have been hindered by the multifunctional nature of the protein (see reference 12 and references therein) as well as by the stringent dependence in cis of translation > RNA replication > assembly (12, 14, 15, 42). Since 2CATPase plays an essential role in RNA replication, a step prior to particle assembly, mutations in 2CATPase leading to RNA replication defects will also prevent proper encapsidation. Our previous genetic studies with a CAV20/PV chimera indicated the importance of residue N252 in PV 2CATPase for an interaction with CAV20 capsid protein VP3, an interaction required for encapsidation (10). Unexpectedly, in the context of the PV polyprotein, N252 is not important for encapsidation because its replacement with A252, G252, S252, or D252 had no effect on virus growth (data not shown). We, therefore, extended the genetic analysis of assembly by introducing multiple mutations into the region surrounding the flexible domain harboring N252 (Fig. 2B and E). We speculate that in the PV background one or more residues in the vicinity of N252, in or near the flexible domain, might have a similar function in encapsidation as N252 in the context of the CAV20/PV chimera. In this study, we used alanine mutagenesis of selected residues to try to answer this question.
FIG 2.
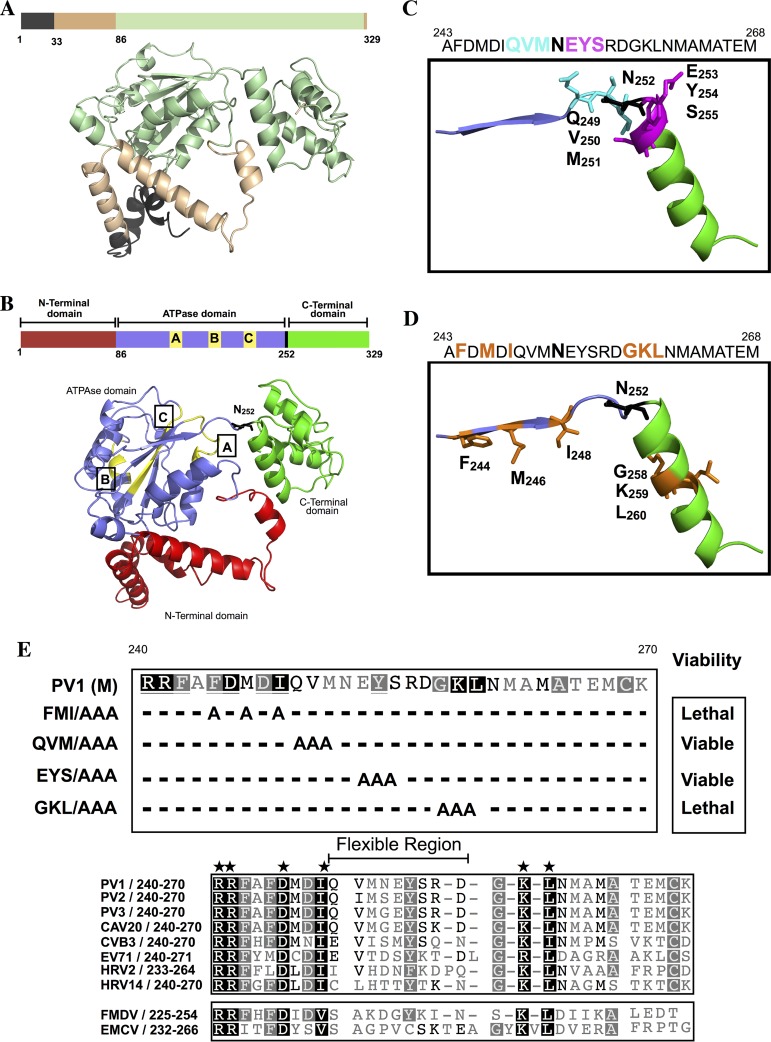
Phyre2-predicted model of the PV 2CATPase polypeptide structure and the locations of the FMI/AAA and GKL/AAA mutations on the structure. (A) The amino acid sequence of PV1(M) 2CATPase was submitted to the Phyre2 online server to generate a 3D structural model (Materials and Methods). The structure of the N-terminal domain, highlighted in black, is highly unreliable. Sequences highlighted in gold are predicted with good confidence. The 2CATPase structure shown in light green is predicted with 100% confidence. (B) The predicted three domains in the 2CATPase structure. The N-terminal domain (red) consists of helical structures, the central helicase domain (blue) contains the NTP binding/helicase boxes A, B, and C, and the C-terminal domain (green) consist mostly of helical structures. The flexible region between the central and C-terminal domains contains residue N252. (C and D) Locations of the triple alanine mutations on the predicted 2CATPase structure are illustrated. The QVM residues are in a flexible domain, while the EYS residues are partly in an α-helical structure. The FMI residues are predicted to be located in a β sheet, and the GKL residues are in an α-helix structure. (E) Alignment of picornavirus 2CATPase proteins surrounding residues N252. (Top) The locations of the triple alanine mutants in the 2CATPase polypeptide are shown. The growth phenotypes of the mutants are also indicated. (Below) The amino acid sequence of picornavirus 2CATPase proteins, surrounding N252, is shown. The highly conserved residues are indicated by stars over a black background, and the less conserved residues are shown with a gray background. Dashes indicate the absence of certain residues in the sequences alignment. The size and location of the flexible region are also indicated. The enterovirus 2CATPase proteins are boxed to separate them from the more distantly related picornaviruses (FMDV, Aphtovirus genus; EMCV [encephalomyocarditis virus], Cardiovirus genus).
2CATPase three-dimensional structure prediction.
The propensity of PV 2CATPase to bind to membranes has thus far prevented the purification of the polypeptide in a soluble form in quantities sufficient to determine its three-dimensional structure. Only the glutathione S-transferase (GST)- and maltose binding protein (MBP)-tagged full-length PV 2CATPase polypeptides or the protein anchored to small nanodisk membrane bilayers have been obtained in soluble forms (27, 32, 44). In an attempt to obtain information about the location of the mutations that we have selected for analysis in the structure of the PV 2CATPase polypeptide, we used Phyre2, a protein homology/analogy recognition engine (45). The 2CATPase structure was modeled after chain C of the cellular transport protein VCP/p97 in a complex with ADP (46). VCP/p97 is a cellular ATPase that belongs to the class I AAA+ family (47). The server predicted a 2CATPase structure for 92% of the residues with greater than 90% confidence in the modeled polypeptide (Fig. 2A). The N-terminal segment, highlighted in black, is highly unreliable, while sequences highlighted in light brown can be predicted with good confidence (Fig. 2A). The remainder of the sequence, shown in light green, is predicted with 100% confidence.
The predicted 2CATPase model can be subdivided into three structured domains (N-terminal, central helicase, and C-terminal) connected by flexible regions (Fig. 2B). The N-terminal domain (red) contains helical regions. It should be noted that within this region the removal of the amphipathic helix (residues 1 to 33), which affects membrane binding, was found to be sufficient for the production of truncated soluble foot-and-mouth disease virus (FMDV) 2CATPase protein (48). The central domain (blue) contains the conserved NTP binding boxes (yellow) A, box B of all helicases, and box C, specific for SF3 helicases (24). Phyre2 predicted the structure of this domain with 100% confidence to contain both α helices and β-sheet secondary structures. Residue N252 is located in a flexible region of the polypeptide between the central ATPase/helicase and C-terminal (green) domains of the polypeptide (Fig. 2B). The C-terminal domain contains the cysteine rich Zn2+ binding domain that is already known to be involved in encapsidation (12, 13).
In the flexible domain of the polypeptide, the amino acid sequences are not conserved among picornavirus 2CATPase proteins (Fig. 2E). This has led us to conclude that the role of N252 residue in C-cluster enterovirus assembly (1) is unique for the chimeric polyprotein consisting of CAV20 capsid and the poliovirus P2 domain. We speculate that in the poliovirus background, one or more residues in the vicinity of N252, in or near the flexible domain, might have a function similar to N252 in encapsidation.
Construction of triple alanine mutants near residue N252 and their growth phenotypes.
Since we targeted all clusters of charged residues in 2CATPase for alanine mutagenesis in our previous work (12), in this study we selected triplet hydrophobic amino acids or single charged residues near N252 for analysis that have not yet been previously analyzed. We designed and constructed four triple alanine mutants—two just upstream of N252 (F244A M246A I248A [FMI/AAA] and Q249A V250A M251A [QVM/AAA]) and two just downstream of N252 (E253A Y254A S255A [EYS/AAA] and G258A K259A L260A [GKL/AAA]), respectively (Table 1; Fig. 3A). The triple alanine mutant FMI/AAA is predicted to be located in a β sheet, with QVM/AAA in the flexible region just upstream of N252 (Fig. 2C and D). The domain downstream of N252 is predicted to contain the other two triple mutants, EYS/AAA and GKL/AAA, which are either partly or fully within a helical region (Fig. 2C and D).
FIG 3.
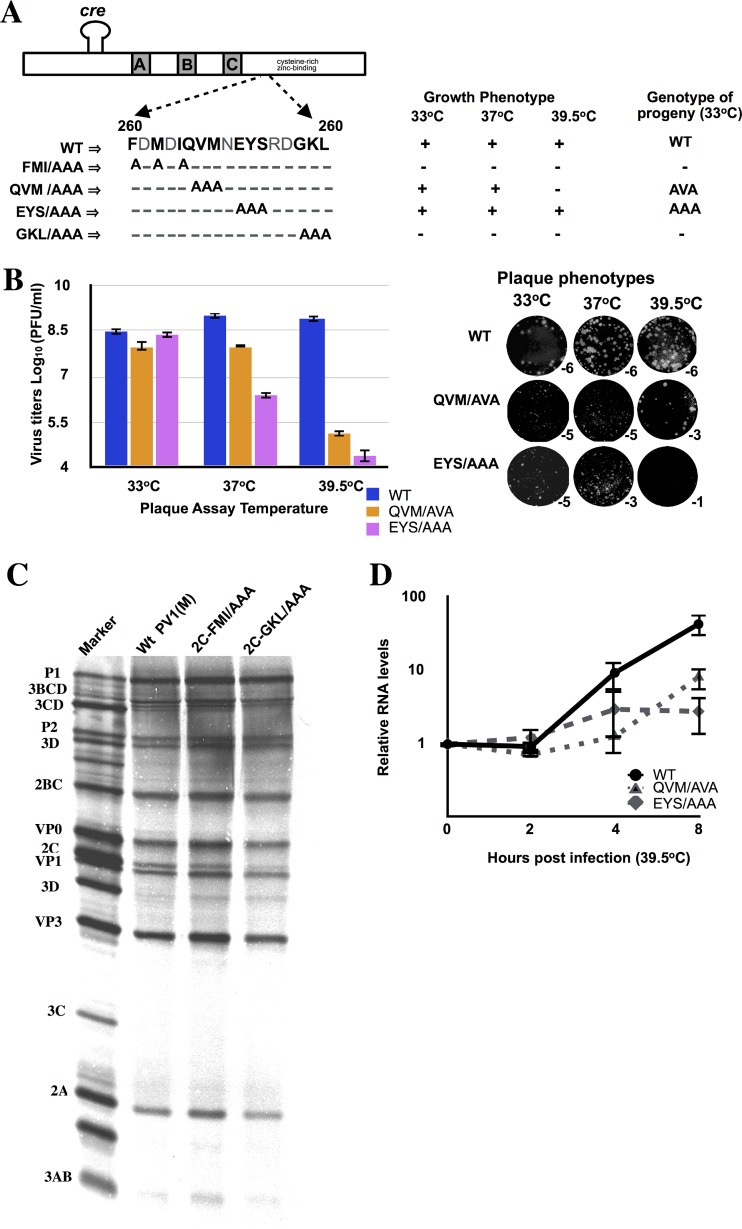
Characterization of PV 2CATPase triple alanine mutants. (A) Growth properties of mutants at different temperatures. The locations of N252 and of the triple alanine mutants on the 2CATPase sequence are illustrated on top. RNA transcripts of wt and mutant constructs were transfected into HeLa R19 monolayers and incubated at 33, 37, and 39.5°C for 48 h or until CPE (Materials and Methods). Freeze-thawed supernatants were used for passage on fresh HeLa R19 monolayers. The genotypes of recovered progeny viruses from 33°C passages are indicated. (B) Virus titers and plaque phenotypes of viable mutants QVM/AVA and EYS/AAA. Viruses derived from 33°C transfections were plaqued at 33, 37, and 39.5°C, and the titers were determined (Materials and Methods). The plaque phenotypes of the viruses at different temperatures are shown at the indicated dilutions. The lysates derived from the 33°C transfections were passaged 10 times, and the sequences of the full-length genomes of the progeny were determined. No additional genetic changes were observed. (C) In vitro translation of wt and mutant transcript RNAs. Transcript RNAs of the wt and of the lethal mutants were translated in HeLa cell extracts (Materials and Methods). (D) RNA levels in HeLa cells infected with mutant EYS/AAA and variant QVM/AVA. HeLa cells were infected at an MOI of 5 with wt and mutant viruses obtained from 33°C transfections, and the titer was determined at 37°C. The infected cells were harvested at various time points after infection, and total RNA was isolated from the lysates. RNA levels were determined by qPCR, as described in Materials and Methods.
To compare the growth phenotypes of the triple alanine mutants with that of the wt virus, we transfected RNA transcripts into HeLa R19 cells. The transfected cells were incubated at 33, 37, or 39.5°C for about 72 h or until full cytopathic effect (CPE) developed. Two constructs (FMI/AAA and GKL/AAA) produced no progeny even after 10 passages on fresh HeLa cells at all 3 temperatures (Table 1; Fig. 2E and 3A). One mutant (EYS/AAA) exhibited wt-like growth at 33°C, but at 37°C progeny was produced only after two passages on fresh HeLa cells (Table 1; Fig. 3A). The second viable mutant (QVM/AAA) was defective in growth at 39.5°C but produced progeny at 33 or 37°C (Table 1; Fig. 3A). The titers of lysates of the two viable viruses, obtained from 33°C transfections, were determined by plaque assays at 33, 37, or 39.5°C. As shown in Fig. 3B, virus titers at 37 and 39.5°C were significantly lower with both mutants than what was obtained with the wt virus. The QVM/AVA variant and the EYS/AAA virus exhibited mostly tiny plaques at all temperatures tested (Fig. 3B).
Viral RNAs were extracted from lysates of the two viable triple alanine mutants grown at different temperatures and were subjected to RT-PCR and full-length genome sequencing. The results indicated that QVM/AAA is quasi-infectious (49): progeny viruses isolated from HeLa cell lysates were always found to be genetic variants. The variant produced had a single nucleotide reversion QVM/AVA. No nucleotide substitutions were observed with the EYS/AAA mutant (Fig. 3A).
Nonviable triple alanine mutants exhibit normal protein synthesis.
To rule out the possibility that the lethal growth phenotypes of the FMI/AAA and GKL/AAA mutants were due to a defect in protein translation or polyprotein processing, we translated RNA transcripts of these mutants in HeLa cell extracts (42). After incubation for 8 h at 34°C, the samples were analyzed by SDS-PAGE. As shown in Fig. 3C, both mutants exhibited normal protein synthesis and polyprotein processing profiles. Surprisingly, the amino acid substitutions with alanine did not influence the migration of the 2CATPase-related polypeptides compared to wt translation patterns.
Triple alanine ts mutants are defective in RNA replication at 39.5°C.
The two ts triple mutants EYS/AAA and the partial revertant of QVM/AAA (QVM/AVA) were further analyzed to test for defects in RNA replication. Viruses grown at 33°C were used to infect HeLa cells at 39.5°C, and aliquots were taken at various times postinfection. Plus-strand RNA levels in lysates of cells infected with these viruses were measured by qPCR. Both mutants exhibited a severe defect in RNA replication at 39.5°C compared to the wt virus (Fig. 3D). Since encapsidation is dependent upon RNA replication (15, 42), these mutants were not further analyzed for any additional encapsidation defects.
Construction and growth properties of single alanine mutants.
Triple alanine mutants that displayed a lethal growth phenotype (FMI/AAA and GKL/AAA) were subsequently scanned by single alanine mutagenesis to identify the specific residues responsible for the growth defect. Of the amino acids contained within FMI and GKL, six (F244A, M246A, I248A, G258A, K259A, and L260A) were mutated to alanine (Fig. 4A; Table 2). RNA transcripts of the six alanine mutant cDNAs were transfected into HeLa cells at 37°C, and their growth phenotypes were examined. Only one of the mutants (G258A) grew like the wt virus. The F244A mutant was not viable and did not produce any progeny after several passages on HeLa cells. The lethal growth phenotype was not due to a defect in translation or protein processing as shown by in vitro translation of mutant RNA transcripts in HeLa cell extracts (data not shown). qPCR analysis of viral RNA levels in infected cells indicated a defect in RNA replication (data not shown), and therefore the mutant was not further analyzed.
FIG 4.
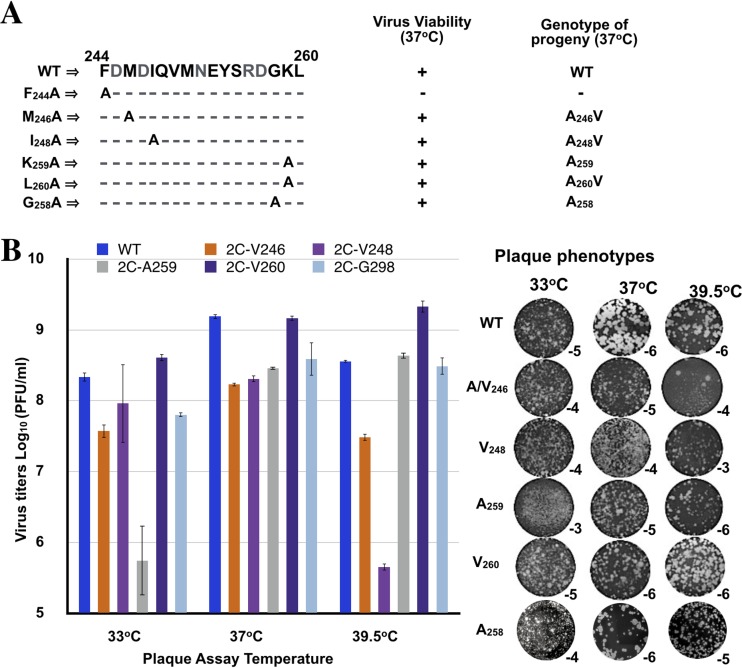
Growth phenotypes of single alanine mutants. (A) Virus viability and the genotype of progeny at 37°C. Single alanine mutants derived from the two nonviable triple amino acid mutants FMI/AAA and GKL/AAA were generated. They were tested for viability at 37°C, and the genotypes of the progeny were determined. (B) Virus titers and plaque phenotypes of single alanine mutants. Titers of viruses grown at 37°C were determined at 33, 37, and 39.5°C by plaque assay (Materials and Methods). The plaque phenotypes are shown on the right panel. Subscripts indicate the virus dilution at which the image was taken. It should be noted that the mixed plaque sizes seen with the lysate derived from M246A (39.5°C) presumably contain a mixture of the original alanine mutant (small) and of the V variant (large). Lysates derived from 37°C transfections were passaged 4 times, and the sequences of the full-length genomes were determined. No additional genetic changes were observed.
Two of the mutants (I248A and L260A) were quasi-infectious and produced A→V variants during transfection or first passage, an observation indicating that the original alanine residues at these positions were nonfunctional (Fig. 4A; Table 2). Although the M246A virus was viable, it mutated to a V during transfection, yielding an M246V variant. Titers of viruses derived from 37°C transfections were determined by plaque assay at 33, 37, and 39.5°C (Fig. 4B). The I248V variant was ts with a particularly strong growth defect at 39.5°C but less prominent at 33 or 37°C. The M246V variant exhibited mildly lower titer than the wt at all three temperatures tested, while the L260V variant grew nearly as well as the wt virus. The plaque sizes of the single alanine mutants or the valine variants were somewhat smaller at 37 or 39.5°C than that of the wt (Fig. 4B).
The last of the single mutants, K259A, produced progeny during transfection, and our preliminary studies indicated that it was cold sensitive. Importantly, it retained its original alanine mutation genotype even after several passages at 33°C (Fig. 4A and B). It should be noted that from the only two other cold-sensitive mutants of poliovirus known so far, one had a mutation in viral protein 3A with a defect in viral RNA synthesis (50), and the other was shown to be defective in uncoating due to a possibly defect in virion structure (1). This uncoating cold-sensitive mutant contained a linker insertion (4 amino acids) between residues 255 and 256, just upstream of K259, and two secondary mutations at M293V and R295K that were obtained after passaging at 39.5°C (1). Notably, a single N140S change was able to suppress the cold-sensitive growth phenotype, suggesting an interaction between the C-terminal domain of the polypeptide and a domain between boxes A and B of the NTP binding domain. We have similarly postulated an interaction between these domains from our previous alanine scanning analyses (12, 13). Based on these previous genetic studies with a linker insertion in 2CATPase, our K259A mutant appeared to be a good candidate in the search for a possible encapsidation/uncoating defect.
The PV 2CATPase K259A mutant exhibits a delay in growth and protein synthesis at 35 and 33°C.
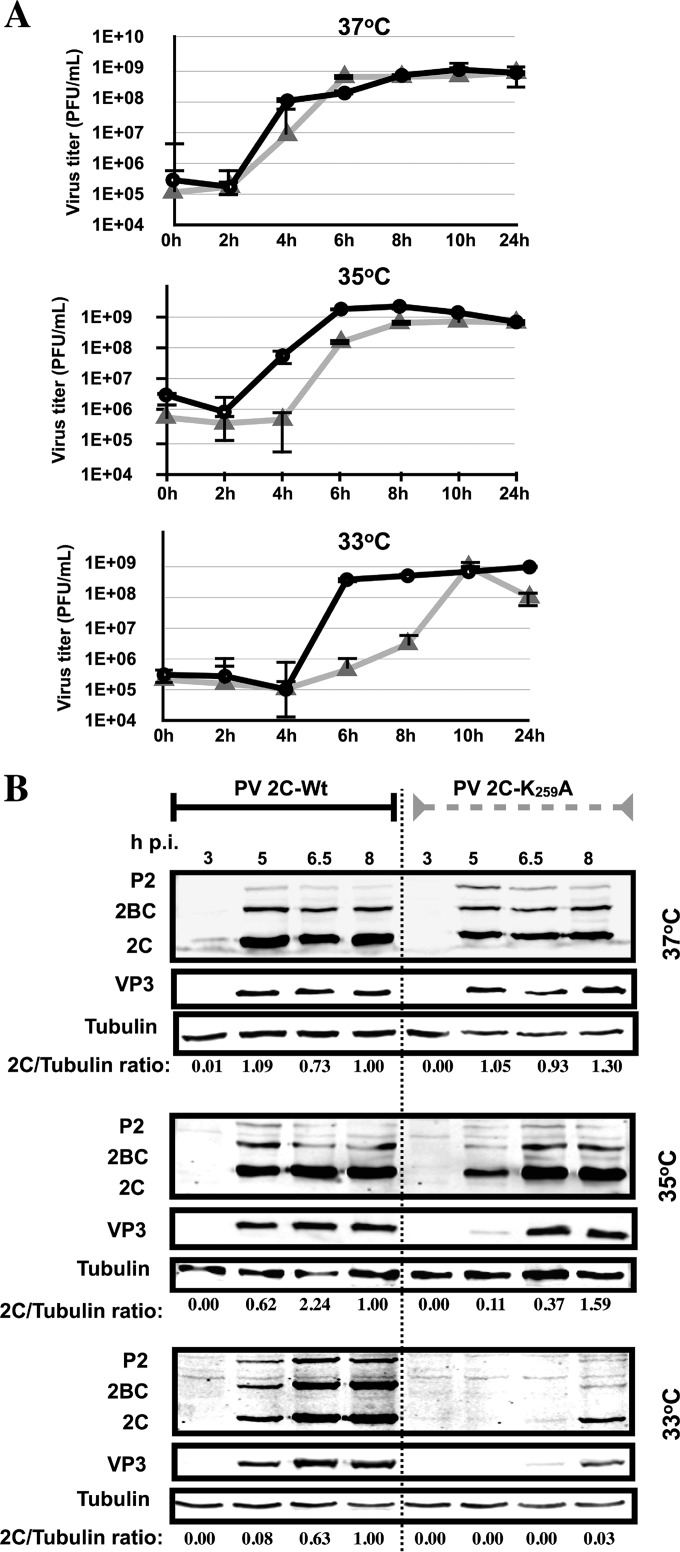
To learn more about the cold-sensitive phenotype of this mutant, viruses grown at 37°C were used to infect HeLa cells (MOI of 5) at 37, 35, or 33°C. Compared to the wt virus, the cold-sensitive mutant exhibited increasingly longer delays in growth when the temperature of the infection was reduced from 37°C to 35°C and then to 33°C, respectively (Fig. 5A). It took 10 h postinfection for the virus titer of PV 2CATPase K259A to catch up with the titer produced by the wt virus at 6 h postinfection (Fig. 5A). These results suggested a defect at an early step of infection with the PV 2CATPase K259A virus, possibly in uncoating.
FIG 5.
The 2CATPase K259A mutant is delayed in virus production and protein synthesis at the restrictive temperatures (35 and 33°C). (A) Growth curves of wt and K259A mutant polioviruses at the permissive (37°C) and restrictive (35 and 33°C) temperatures. HeLa cells were infected at an MOI of 5 with viruses derived from 37°C transfections. The titer of the viral progeny was determined by plaque assay at different times postinfection (Materials and Methods). Black lines indicate growth curves at 37°C, and gray lines indicate growth curves at 33°C. (B) Protein synthesis by the wt and K259A mutant measured by Western analysis. HeLa cells were infected at either 33, 35, or 37°C at an MOI of 5 with viruses derived from 37°C transfections. The infected cells were isolated at various times postinfection and lysed. The level of 2CATPase-related proteins and of capsid protein VP3 were measured by Western analysis using a monoclonal antibody to 2CATPase and a polyclonal antibody to VP3, respectively, as described in Materials and Methods. Tubulin was used as a loading control. The experiment was carried out three times.
Since in the viral life cycle protein synthesis follows immediately after uncoating, we reasoned that the K259A mutant might also exhibit a delay in protein synthesis at 33 and 35°C compared to the wt virus. Virus grown at 37°C was used to infect HeLa cells at different temperatures at an MOI of 5 (Fig. 5B). At various times postinfection, lysates were analyzed on SDS-polyacrylamide gels, and the 2CATPase-related proteins were identified by Western analysis using an antibody to 2CATPase. Relative to the wt virus, there was a small delay in protein synthesis by the mutant at 37°C, and there were increasingly longer delays when the temperature was reduced from 37°C to 35 and 33°C, respectively (Fig. 5B). The results parallel the growth kinetics of the mutant virus at 33, 35, and 37°C compared to the wt virus and provide support for our hypothesis that the cold-sensitive mutant is defective in uncoating at the restrictive temperatures. In agreement with these experiments, we observed no difference in the kinetics of RNA replication in experiments where RNA transfection, instead of infection, initiated the replication cycle of an F-Luc replicon at 33°C (Fig. 6B).
FIG 6.
The K259A 2CATPase mutant possesses an encapsidation defect at 33°C. (A) Genome structure of a Renilla luciferase (R-Luc) reporter virus (R-Luc-PPP). The R-Luc gene was fused between the 5′NTR and P1 structural proteins, flanked by a 3CDpro cleavage site. Wild-type and mutant K259A 2CATPase reporter virus RNA transcripts were transfected into HeLa cells at 33 or 37°C, in both the absence and presence of GnHCl (Materials and Methods). R-Luc assays were performed at 8 h posttransfection. Aliquots of the lysates from the transfections were used to infect fresh HeLa cells, in both the absence and presence of GnHCl. R-Luc assays were performed at 8 h postinfection. R-Luc ratios were calculated by dividing the raw R-Luc values in the absence by the R-Luc values in the presence of GnHCl. (B) The genome structure of firefly luciferase PV1(M) replicons used in the experiment is shown above. A time course of RNA replication was measured with the wt and the mutant 2CATPase K259A mutant using F-Luc replicons. Monolayer HeLa R19 cells were transfected with 3 to 5 μg of firefly Renilla luciferase replicon transcript RNAs. Transfected cells were incubated for 2, 4, 6, and 8 h at 33°C in the presence or absence of 2 mM GnHCl. Luciferase activity was determined on the cell supernatants after three freeze-thawing steps. F-Luc activity with the wt virus is taken as 100%. The experiment was carried out three times.
The PV 2CATPase K259A mutant is defective in encapsidation at 33 and 35°C.
The delayed growth and protein synthesis by the K259A mutant at 33°C suggested the possibility of a defect at some stage of uncoating. However, since 2CATPase is not a part of the virus particle and is synthesized after viral entry and uncoating, a direct role for this protein in this process can be ruled out. To decipher the mechanism by which a mutation in 2CATPase might affect uncoating, as we have hypothesized, we tested for defects in RNA replication and encapsidation using an R-Luc reporter virus (Fig. 6A). In this construct, R-Luc is fused to the N terminus of the poliovirus polyprotein (10). After infection, the chimera synthesizes the polyprotein from which the N-terminal R-Luc reporter protein is cleaved by 3CDpro, after which it signals the extent of protein translation and RNA replication (10). We used T7 RNA transcripts of the chimeric virus constructs (wt and K259A mutant) and transfected these into HeLa R19 cells at different temperatures in the absence and presence of guanidine hydrochloride (GnHCl), a potent inhibitor of PV RNA replication (28). Luciferase activity was measured 16 h posttransfection. In the presence of GnHCl, the R-Luc activity measures translation of the input RNA, while in the absence of the drug, the R-Luc signal represents both translation and RNA replication. To gauge encapsidation, cell lysates from transfections made in the absence of GnHCl were then passaged to fresh HeLa cells, and R-Luc activity was measured 8 h postinfection. Only virions that were assembled during the first incubation will be able to infect the second set of HeLa cells. The results indicate that at 37°C, both RNA replication and encapsidation of the K259A mutant nearly matched the level observed with the wt construct (Fig. 6A). At 35°C, passaging to new HeLa cells reduced the R-Luc signal for both the wt and mutant constructs, although the decrease was more pronounced with the mutant, an observation suggesting a small encapsidation defect relative to the wt. However, at 33°C, the mutant exhibited a small (∼2-fold) reduction in RNA replication but a striking 25-fold decrease in R-Luc signal that measures encapsidation. We consider it highly unlikely that such a small decrease in RNA replication would result in such a very large defect in encapsidation. Therefore, we conclude that the K259A mutant is severely defective in encapsidation at 33°C.
We also carried out a more detailed analysis of the kinetics of RNA replication with the wt and K259A mutant using F-Luc replicons (Fig. 6B). The RNA of the replicons was directly transfected into HeLa cells at 33°C, and F-Luc activity was measured at the indicated time points. Again, the experiment was carried out with or without the GnHCl inhibitor. Under these conditions, where uncoating is not involved, the kinetics of early RNA replication by the wt and mutant were essentially identical. Only at 8 h posttransfection did we observe a 2-fold reduction in RNA levels, confirming the results obtained by the R-Luc experiments (Fig. 6).
Immunofluorescence imaging shows inhibition of mature virus production with the K259A mutant at 33°C.
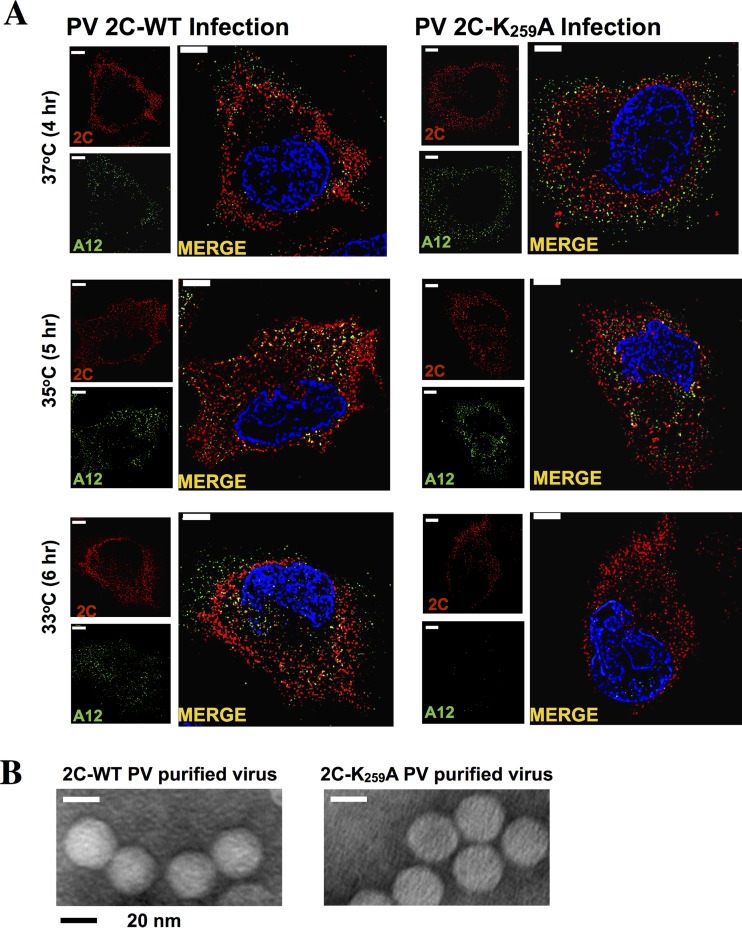
The inhibition of encapsidation, resulting in decreased amounts of infectious virus at 33°C (Fig. 6A), was supported by immunofluorescence imaging (Fig. 7A). PV 2CATPase K259A virus, grown at 37°C, was used to infect HeLa cells at 37, 35, and 33°C at an MOI of 5, and incubation continued for 4, 5, and 6 h, respectively, at the same temperatures. Infected cells were probed with monoclonal antibodies to 2CATPase and A12 antibodies, the latter recognizing mature virus (51). As shown in Fig. 7A, both the localization and estimated quantity of mature virus are comparable for the two viruses at 37 and 35°C. However, at 33°C there is a strong reduction in the amount of mature virus present in K259A-infected cells compared to the amount in cells infected with the wt virus. Notably, there are differences in the localization of 2CATPase in the wt and mutant virus-infected cells. In wt virus-infected cells, 2CATPase localizes in the perinuclear region of the cell, while with the mutant, this protein is primarily in the cytoplasm. Taken together, our results clearly indicate a relationship between the encapsidation defect of the K259A mutant at 33°C, the production of mature virus, and a delay in uncoating and protein synthesis during the next cycle of virus growth. It should be noted that an electron microscopic analysis of purified wt and K259A viral particles grown at 37°C did not reveal any detectable differences between the two viruses (Fig. 7B).
FIG 7.
Immunofluorescence imaging of PV 2C wt and PV 2C K259A mutant virus-infected HeLa cells. HeLa cells were infected with wt or PV 2C K259A virus at an MOI of 5. Cells were incubated for 4 (37°C), 5 (35°C), or 6 (33°C) h and were fixed with paraformaldehyde. Infected cells were probed with primary antibody against 2CATPase and mature virus (monoclonal antibody A12), followed by Alexa Fluor 555 (red)- and 488 (green)-conjugated antibodies, respectively. The cell nucleus was stained with DAPI (4′,6-diamidino-2-phenylindole), shown in blue. (B) Electron microscopy of purified wt and K259A 2CATPase mutant viruses. Wild-type and mutant viruses were grown at 37°C and purified on cesium chloride density gradients (see Materials and Methods).
Our previous studies provided strong genetic evidence that in a CAV20-PV chimera (constructed of CAV20 P1 and PV P2 and -3 and designated CPP), the CAV20 VP3 “communicates” with PV 2CATPase, an event required for assembly (1). We were able to support this assertion by coprecipitation experiments of in vitro-synthesized CAV20 VP3 and PV 2CATPase (1). Attempts to achieve similar results with PV VP3 and PV 2CATPase K259A have failed: we have observed coprecipitation of PV VP3 with both the wt and the mutant PV 2CATPase proteins (data not shown). We speculate that since the mutations in the viral 2CATPases of the polyproteins CPP and PPP (2CATPase N252S and K259A, respectively) map to very different positions in the proposed 2CATPase structure (hinge versus helix) (Fig. 2), they are differently exposed and available for potential interaction under the conditions of these experiments. On the other hand, in vivo, the interaction between capsid precursors and the replication complex involves very large, high-molecular-weight entities that may present the communicating residues in CPP or in PPP for binding in different ways.
DISCUSSION
This study provides genetic evidence that a single amino acid replacement in a PV nonstructural protein (2CATPase K259A) generates variant virions with an unusual functional phenotype at 37°C: these virions that were assembled with wt proteins can infect cells at physiological temperature with wt efficiency, but they are defective in their ability to initiate infection at 33°C. We must conclude, therefore, that at 37°C the mutant nonstructural viral protein 2CATPase directed the formation of faulty PV virions.
Previous drug inhibition (9) and genetic experiments have led to the surprising observation that poliovirus 2CATPase was essential not only in genome replication but also in virion assembly (10–13). More surprising was the realization that an RNA packaging signal is not involved in poliovirus assembly (11), unlike with numerous other plus-strand RNA viruses (see, for example, references 52, 53, 54, and 55). Uniquely, the specificity of assembly results from an interaction of the nonstructural poliovirus protein 2CATPase with cognate capsid protein VP1 and/or VP3, or with capsid protein VP3 of C-cluster coxsackieviruses (10). Moreover, mutations influencing PV assembly seem to map to different parts of the 2CATPase polypeptide (Fig. 1C). A particularly powerful tool to deduce the role of PV 2CATPase in encapsidation was alanine scanning mutagenesis, implicating residues K279 and R280 and C272 and H273 within the C-terminal Zn2+ binding domain (residues 269 to 286) in encapsidation and/or uncoating (12, 13, 34). Suppressor variants of the K279A R280A mutant indicated that this site interacts with both capsid proteins VP1 and VP3, possibly in the context of one or more capsid precursors or the fully assembled capsid (12). On the other hand, suppressor variants of the C272A H273A mutant revealed an interaction with an upstream segment of 2CATPase that is located between boxes A and B of the NTP binding domain (12, 13). The K259 residue in poliovirus 2CATPase adds to the previously discovered locations near the C terminus of the polypeptide that are involved in encapsidation/uncoating. This domain extends from residue N252 to the M293 and K295 suppressor mutations of a linker insertion at residues 255 and 256 of the cold-sensitive mutant of Li and Baltimore (1).
Originally, the aim of our studies was to search for and identify one or more sites in protein 2CATPase near amino acid N252 that could be implicated in encapsidation. N252 was identified to be essential in the assembly of a CAV20/PV chimera constructed to contain the CAV20 capsid followed by poliovirus P2/P3 (10). N252 maps to a variable flexible region within PV 2CATPase (Fig. 2B to D), a site presumed suitable for an interaction between 2CATPase and a capsid polypeptide. To our surprise, we realized, however, that the asparagine at residue 252 of 2CATPase is not conserved among enteroviruses, not even between three poliovirus serotypes (Fig. 2E) or in CAV20, the capsid donor of the PV/CAV20 chimera. We speculated, therefore, that residues in the variable flexible region other than N252 or in addition to N252 might play a role as the presumed capsid-interacting site involved in particle assembly.
We first introduced triple alanine mutations into PV 2CATPase near N252 (Fig. 2B to D). We selected mutants for analysis that fell either into highly structured or flexible domains. We identified two triple alanine mutants (FMI/AAA and GKL/AAA) that possessed lethal growth phenotypes at all temperatures tested (33, 37, and 39.5°C). These mutations are predicted to be located in a β sheet and a helical domain, respectively, of the 2CATPase structural model. In addition, we observed two mutants that were ts and/or quasi-infectious and exhibited normal protein translation and processing profiles but were defective in RNA replication (EYS/AAA and QVM/AAA). These mutations fell into a partly or fully flexible stretch of residues in the predicted 2CATPase structure. Since the processes of encapsidation and RNA replication are linked, we cannot exclude the possibility that these mutants also had encapsidation defects, independent of RNA replication. It should be noted that an E253G change in PV 2CATPase was previously shown to yield a small-plaque virus, to prevent secretion inhibition in tissue culture cells (56), and to produce a valosin-containing protein (VCP)-knockdown-resistant PV mutant (38). Whether the E253A substitution, within the context of the EYS mutation, would cause similar defects is not yet known.
To identify the specific residues responsible for the lethal growth phenotypes of the triple alanine mutants, we scanned them by single alanine mutagenesis. The mutants included the F244A, I248A, G258A, K259A, and L260A residues that are highly conserved in picornavirus 2CATPase proteins and M246, which is less conserved (Fig. 2E). Mutant F244A was nonviable and had a severe defect in RNA replication (data not shown). Interestingly, our previous alanine mutagenesis of positively charged residues R240/R241 and D245/D247, in close vicinity to F244, also resulted in lethal growth phenotypes and severe replication defects (12). Three of the single alanine mutants (M246A, I248A, and L260A) were quasi-infectious and produced variants that had either wt or ts growth phenotypes. In all cases (M246A, I246A, and L260A), the variants contained an exchange of a moderately hydrophobic residue, alanine, with a more hydrophobic and larger amino acid, valine. The original residues, M246, I246, and L260, are also strongly hydrophobic and larger than the alanines that replaced them. It should also be noted that reversion to the original genotypes would have required two simultaneous nucleotide substitutions, while the A→V changes occurred with the replacement of a single nucleotide.
The last single alanine mutant, K259A, was cold sensitive and exhibited a delay in growth and in protein synthesis at the restrictive temperatures, 35°C and, particularly, 33°C. Our experiment with a reporter virus indicated a specific defect in encapsidation at these temperatures. Although this defect was not detectable at 37°C with this assay, an abnormality in virion structure, presumably resulting from imperfect encapsidation at this temperature, could be inferred from the observation that mutant viruses grown at 37°C were strongly delayed both in growth and in protein synthesis when used for infections and growth at 35 or 33°C (Fig. 5A and B). The 2CATPase K259A mutation alone did not confer such phenotypes onto a replicon (Fig. 6B), which pinpointed the defect to the virion structure of PV grown at 37°C. Immunofluorescence imaging of cells infected with the wt and mutant viruses confirmed the nearly total lack of mature virus production by the mutant at 33°C 6 h postinfection (Fig. 7). In addition, the imaging experiments revealed differences in the localization of 2CATPase with the wt and the mutant viruses at 33°C. While the wt virus exhibited 2CATPase localization in the perinuclear region of the cell, the mutant protein was primarily in the cytoplasm.
As mentioned above, the asparagine at position 252 is not conserved in an alignment of picornavirus 2CATPase proteins and even in different poliovirus serotypes (Fig. 2E). Not surprisingly, the N252 in PV1(M) can be replaced with A, G, S, or D without any deleterious effect on viral growth (10; unpublished data). Some residues in the immediate vicinity of N252 that were included in our mutational analyses (M246, Q249, M251, E253, and S255) are also poorly conserved (Fig. 2E). However, we suggest that the growth phenotypes of the mutants correlated with the extent of conservation of amino acids within this domain of the protein. Accordingly, the two mutants with the highest conservation (FMI/AAA, GKL/AAA) exhibited lethal phenotypes, while mutants (EYS/AAA and QVM/AAA) that were either ts or quasi-infectious, respectively, contained substitutions in the variable region.
With every replicative cycle, RNA viruses replicate their genomes with the astounding error rate of 10−4, and indeed, most RNA viruses have not developed any proofreading and editing functions. This phenomenon, for which RNA viruses are called quasispecies (57), is of great advantage for these infectious agents, but there is also a disadvantage: RNA viruses live under conditions of genetic austerity, e.g., their genome is very small. High error rates at every step of replication can be a burden, but as Kirkegaard realized first (14), PV has evolved to possess a powerful proofreading mechanism because of the stringent dependence of individual steps in replication in cis (12, 15, 38, 42, 58). In addition, Hogle has added that uncoating and encapsidation are linked since the normal release of the genome depends upon correctly assembled virion particles (20). The 2CATPase K259A mutant described here fits well into this proofreading scheme at the steps of encapsidation/uncoating.
Our studies reported here identify K259 as a residue critical for virion assembly and the subsequent step of uncoating during the next cycle of infection. Although many unanswered questions still exist about the roles of 2CATPase in morphogenesis and uncoating, these results together with those from our previous alanine scanning experiments demonstrate the usefulness of genetic analyses with ts and quasi-infectious variants for the identification of residues important for these processes.
ACKNOWLEDGMENTS
We thank Steffen Mueller for the plasmid of the R-Luc reporter virus, Kostya Chumakov for monoclonal antibody A12, and Sarah Georges for expert technical assistance.
REFERENCES
- 1.Li J-P, Baltimore D. 1990. An intragenic revertant of a poliovirus 2C mutant has an uncoating defect. J Virol 64:1102–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho MW, Teterina N, Egger D, Bienz K, Ehrenfeld E. 1994. Membrane rearrangement and vesicle induction by recombinant poliovirus 2C and 2BC in human cells. Virology 202:129–145. doi: 10.1006/viro.1994.1329. [DOI] [PubMed] [Google Scholar]
- 3.Aldabe R, Carrasco L. 1995. Induction of membrane proliferation by poliovirus proteins 2C and 2BC. Biochem Biophys Res Commun 206:64–76. doi: 10.1006/bbrc.1995.1010. [DOI] [PubMed] [Google Scholar]
- 4.Teterina NL, Kean KM, Gorbalenya AE, Agol VI, Girard M. 1992. Analysis of the functional significance of amino acid residues in the putative NTP-binding pattern of the poliovirus 2C protein. J Gen Virol 73:1977–1986. [DOI] [PubMed] [Google Scholar]
- 5.Barton DJ, Flanegan JB. 1997. Synchronous replication of poliovirus RNA: initiation of negative-strand RNA synthesis requires the guanidine-inhibited activity of protein 2C. J Virol 71:8482–8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li JP, Baltimore D. 1988. Isolation of poliovirus 2C mutants defective in viral RNA synthesis. J Virol 62:4016–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paul AV, Molla A, Wimmer E. 1994. Studies of a putative amphipathic helix in the N-terminus of poliovirus protein 2C. Virology 199:188–199. doi: 10.1006/viro.1994.1111. [DOI] [PubMed] [Google Scholar]
- 8.Teterina NL, Gorbalenya AE, Egger D, Bienz K, Ehrenfeld E. 1997. Poliovirus 2C protein determinants of membrane binding and rearrangements in mammalian cells. J Virol 71:8962–8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vance LM, Moscufo N, Chow M, Heinz BA. 1997. Poliovirus 2C region functions during encapsidation of viral RNA. J Virol 71:8759–8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Wang C, Mueller S, Paul AV, Wimmer E, Jiang P. 2010. Direct interaction between two viral proteins, the nonstructural protein 2CATPase and the capsid protein VP3, is required for enterovirus morphogenesis. PLoS Pathog 6:e1001066. doi: 10.1371/journal.ppat.1001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang P, Liu Y, Ma HC, Paul AV, Wimmer E. 2014. Picornavirus morphogenesis. Microbiol Mol Biol Rev 78:418–437. doi: 10.1128/MMBR.00012-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, Jiang P, Sand C, Paul AV, Wimmer E. 2012. Alanine scanning of poliovirus 2CATPase reveals new genetic evidence that capsid protein/2CATPase interactions are essential for morphogenesis. J Virol 86:9964–9975. doi: 10.1128/JVI.00914-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, Ma HC, Wimmer E, Jiang P, Paul AV. 2014. A C-terminal, cysteine-rich site in poliovirus 2CATPase is required for morphogenesis. J Gen Virol 95:1255–1265. doi: 10.1099/vir.0.062497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novak JE, Kirkegaard K. 1994. Coupling between genome translation and replication in an RNA virus. Genes Dev 8:1726–1737. doi: 10.1101/gad.8.14.1726. [DOI] [PubMed] [Google Scholar]
- 15.Nugent CI, Johnson KL, Sarnow P, Kirkegaard K. 1999. Functional coupling between replication and packaging of poliovirus replicon RNA. J Virol 73:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koch F, Koch G. 1985. The molecular biology of poliovirus. Springer, New York, NY. [Google Scholar]
- 17.Fenwick ML, Wall MJ. 1973. Factors determining the site of synthesis of poliovirus proteins: the early attachment of virus particles to endoplasmic membranes. J Cell Sci 13:403–413. [DOI] [PubMed] [Google Scholar]
- 18.Lonberg-Holm K, Gosser LB, Kauer JC. 1975. Early alteration of poliovirus in infected cells and its specific inhibition. J Gen Virol 27:329–342. doi: 10.1099/0022-1317-27-3-329. [DOI] [PubMed] [Google Scholar]
- 19.De Sena J, Torian B. 1980. Studies on the in vitro uncoating of poliovirus. III. Roles of membrane-modifying and -stabilizing factors in the generation of subviral particles. Virology 104:149–163. [DOI] [PubMed] [Google Scholar]
- 20.Hogle JM. 2002. Poliovirus cell entry: common structural themes in viral cell entry pathways. Annu Rev Microbiol 56:677–702. doi: 10.1146/annurev.micro.56.012302.160757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toyoda H, Nicklin MJ, Murray MG, Anderson CW, Dunn JJ, Studier FW, Wimmer E. 1986. A second virus-encoded proteinase involved in proteolytic processing of poliovirus polyprotein. Cell 45:761–770. doi: 10.1016/0092-8674(86)90790-7. [DOI] [PubMed] [Google Scholar]
- 22.Ypma-Wong MF, Filman DJ, Hogle JM, Semler BL. 1988. Structural domains of the poliovirus polyprotein are major determinants for proteolytic cleavage at Gln-Gly pairs. J Biol Chem 263:17846–17856. [PubMed] [Google Scholar]
- 23.Wimmer E, Hellen CU, Cao X. 1993. Genetics of poliovirus. Annu Rev Genet 27:353–436. doi: 10.1146/annurev.ge.27.120193.002033. [DOI] [PubMed] [Google Scholar]
- 24.Gorbalenya AE, Koonin EV. 1993. Helicases: amino acid sequence comparisons and structure-function relationships. Curr Opin Struct Biol 3:419–429. doi: 10.1016/S0959-440X(05)80116-2. [DOI] [Google Scholar]
- 25.Mirzayan C, Wimmer E. 1994. Biochemical studies on poliovirus polypeptide 2C: evidence for ATPase activity. Virology 199:176–187. doi: 10.1006/viro.1994.1110. [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez PL, Carrasco L. 1993. Poliovirus protein 2C has ATPase and GTPase activities. J Biol Chem 268:8105–8110. [PubMed] [Google Scholar]
- 27.Pfister T, Wimmer E. 1999. Characterization of the nucleoside triphosphatase activity of poliovirus protein 2C reveals a mechanism by which guanidine inhibits poliovirus replication. J Biol Chem 274:6992–7001. doi: 10.1074/jbc.274.11.6992. [DOI] [PubMed] [Google Scholar]
- 28.Pincus SE, Diamond DC, Emini EA, Wimmer E. 1986. Guanidine-selected mutants of poliovirus: mapping of point mutations to polypeptide 2C. J Virol 57:638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng Z, Yang J, Xia H, Qiu Y, Wang Z, Han Y, Xia X, Qin CF, Hu Y, Zhou X. 2013. The nonstructural protein 2C of a picorna-like virus displays nucleic acid helix destabilizing activity that can be functionally separated from its ATPase activity. J Virol 87:5205–5218. doi: 10.1128/JVI.00245-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodríguez PL, Carrasco L. 1995. Poliovirus protein 2C contains two regions involved in RNA binding activity. J Biol Chem 270:10105–10112. doi: 10.1074/jbc.270.17.10105. [DOI] [PubMed] [Google Scholar]
- 31.Echeverri A, Banerjee R, Dasgupta A. 1998. Amino-terminal region of poliovirus 2C protein is sufficient for membrane binding. Virus Res 54:217–223. doi: 10.1016/S0168-1702(98)00016-1. [DOI] [PubMed] [Google Scholar]
- 32.Adams P, Kandiah E, Effantin G, Steven AC, Ehrenfeld E. 2009. Poliovirus 2C protein forms homo-oligomeric structures required for ATPase activity. J Biol Chem 284:22012–22021. doi: 10.1074/jbc.M109.031807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerjee R, Weidman MK, Echeverri A, Kundu P, Dasgupta A. 2004. Regulation of poliovirus 3C protease by the 2C polypeptide. J Virol 78:9243–9256. doi: 10.1128/JVI.78.17.9243-9256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfister T, Jones KW, Wimmer E. 2000. A cysteine-rich motif in poliovirus protein 2CATPase is involved in RNA replication and binds zinc in vitro. J Virol 74:334–343. doi: 10.1128/JVI.74.1.334-343.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin J, Liu Y, Wimmer E, Paul AV. 2007. Complete protein linkage map between the P2 and P3 non-structural proteins of poliovirus. J Gen Virol 88:2259–2267. doi: 10.1099/vir.0.82795-0. [DOI] [PubMed] [Google Scholar]
- 36.Cuconati A, Xiang W, Lahser F, Pfister T, Wimmer E. 1998. A protein linkage map of the P2 nonstructural proteins of poliovirus. J Virol 72:1297–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang WF, Yang SY, Wu BW, Jheng JR, Chen YL, Shih CH, Lin KH, Lai HC, Tang P, Horng JT. 2007. Reticulon 3 binds the 2C protein of enterovirus 71 and is required for viral replication. J Biol Chem 282:5888–5898. doi: 10.1074/jbc.M611145200. [DOI] [PubMed] [Google Scholar]
- 38.Arita M, Wakita T, Shimizu H. 2012. Valosin-containing protein (VCP/p97) is required for poliovirus replication and is involved in cellular protein secretion pathway in poliovirus infection. J Virol 86:5541–5553. doi: 10.1128/JVI.00114-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodfellow I, Chaudhry Y, Richardson A, Meredith J, Almond JW, Barclay W, Evans DJ. 2000. Identification of a cis-acting replication element within the poliovirus coding region. J Virol 74:4590–4600. doi: 10.1128/JVI.74.10.4590-4600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paul AV, Rieder E, Kim DW, van Boom JH, Wimmer E. 2000. Identification of an RNA hairpin in poliovirus RNA that serves as the primary template in the in vitro uridylylation of VPg. J Virol 74:10359–10370. doi: 10.1128/JVI.74.22.10359-10370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tellinghuisen TL, Foss KL, Treadaway JC, Rice CM. 2008. Identification of residues required for RNA replication in domains II and III of the hepatitis C virus NS5A protein. J Virol 82:1073–1083. doi: 10.1128/JVI.00328-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molla A, Paul AV, Wimmer E. 1991. Cell-free, de novo synthesis of poliovirus. Science 254:1647–1651. doi: 10.1126/science.1661029. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y-H, Du W, Hagemeijer MC, Takvorian PM, Pau C, Cali A, Brantner CA, Stempinski ES, Connelly PS, Ma H-C, Jiang P, Wimmer E, Altan-Bonnet G, Altan-Bonnet N. 2015. Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cell 160:619–630. doi: 10.1016/j.cell.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Springer CL, Huntoon HP, Peersen OB. 2013. Polyprotein context regulates the activity of poliovirus 2CATPase bound to bilayer nanodiscs. J Virol 87:5994–6004. doi: 10.1128/JVI.03491-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelley LA, Sternberg MJE. 2009. Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc 4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 46.Davies JM, Brunger AT, Weis WI. 2008. Improved structures of full-length p97, an AAA ATPase: implications for mechanisms of nucleotide-dependent conformational change. Structure 16:715–726. doi: 10.1016/j.str.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 47.White SR, Lauring B. 2007. AAA+ ATPases: achieving diversity of function with conserved machinery. Traffic 8:1657–1667. doi: 10.1111/j.1600-0854.2007.00642.x. [DOI] [PubMed] [Google Scholar]
- 48.Sweeney TR, Cisnetto V, Bose D, Bailey M, Wilson JR, Zhang X, Belsham GJ, Curry S. 2010. Foot-and-mouth disease virus 2C is a hexameric AAA+ protein with a coordinated ATP hydrolysis mechanism. J Biol Chem 285:24347–24359. doi: 10.1074/jbc.M110.129940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gmyl AP, Pilipenko EV, Maslova SV, Belov GA, Agol VI. 1993. Functional and genetic plasticities of the poliovirus genome: quasi-infectious RNAs modified in the 5′-untranslated region yield a variety of pseudorevertants. J Virol 67:6309–6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berstein HD, Baltimore D. 1988. Poliovirus mutant that contains a cold-sensitive defect in viral RNA synthesis. J Virol 62:2922–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Z, Fischer ER, Kouiavskaia D, Hansen BT, Ludtke SJ, Bidzhieva B, Makiya M, Agulto L, Purcell RH, Chumakov K. 2013. Cross-neutralizing human anti-poliovirus antibodies bind the recognition site for cellular receptor. Proc Natl Acad Sci U S A 110:20242–20247. doi: 10.1073/pnas.1320041110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tzeng WP, Matthews JD, Frey TK. 2006. Analysis of rubella virus capsid protein-mediated enhancement of replicon replication and mutant rescue. J Virol 80:3966–3974. doi: 10.1128/JVI.80.8.3966-3974.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Subba-Reddy CV, Yunus MA, Goodfellow IG, Kao CC. 2012. Norovirus RNA synthesis is modulated by an interaction between the viral RNA-dependent RNA polymerase and the major capsid protein, VP1. J Virol 86:10138–10149. doi: 10.1128/JVI.01208-12. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.D'Souza V, Summers MF. 2005. How retroviruses select their genomes. Nat Rev Microbiol 3:643–655. doi: 10.1038/nrmicro1210. [DOI] [PubMed] [Google Scholar]
- 55.Rao ALN. 2006. Genome packaging by spherical plant RNA viruses. Annu Rev Phytopathol 44:61–87. doi: 10.1146/annurev.phyto.44.070505.143334. [DOI] [PubMed] [Google Scholar]
- 56.Burgon TB, Jenkins JA, Deitz SB, Spagnolo JF, Kirkegaard K. 2009. Bypass suppression of small-plaque phenotypes by a mutation in poliovirus 2A that enhances apoptosis. J Virol 83:10129–10139. doi: 10.1128/JVI.00642-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Domingo E, Sheldon J, Perales C. 2012. Viral quasispecies evolution. Microbiol Mol Biol Rev 76:159–216. doi: 10.1128/MMBR.05023-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verlinden Y, Cuconati A, Wimmer E, Rombaut B. 2000. The antiviral compound 5-(3,4-dichlorophenyl) methylhydantoin inhibits the post-synthetic cleavages and the assembly of poliovirus in a cell-free system. Antiviral Res 48:61–69. doi: 10.1016/S0166-3542(00)00119-4. [DOI] [PubMed] [Google Scholar]